Highlights
-
•
Tract volume and number of tracts are reduced in the left slMFB.
-
•
Those microstructural alterations are related to depression severity and anhedonia.
-
•
There is increased VTA-PFC functional connectivity in depression.
-
•
Those increases are more pronounced in patients with severe anhedonia.
-
•
Our results extend pathophysiological models of anhedonia in depression.
Keywords: Anhedonia, Depression, Diffusion tensor imaging, Functional MRI, Medial forebrain bundle, Reward system
Abstract
The ventral tegmental area (VTA), nucleus accumbens (NAcc), and prefrontal cortex (PFC) are essential for experiencing pleasure and initiating motivated behaviour. The VTA, NAcc, and PFC are connected through the medial forebrain bundle (MFB). In humans, two branches have been described: an infero-medial branch (imMFB) and a supero-lateral branch (slMFB). This study aimed to explore the associations between structural connectivity of the MFB, functional connectivity (FC) of the VTA, anhedonia, and depression severity in patients with depression. Fifty-six patients with unipolar depression and 22 healthy controls matched for age, sex, and handedness were recruited at the University Hospital of Psychiatry and Psychotherapy in Bern, Switzerland. Diffusion-weighted imaging and resting-state functional magnetic resonance imaging scans were acquired. Using manual tractography, the imMFB and slMFB were reconstructed bilaterally for each participant. Seed-based resting-state FC was computed from the VTA to the PFC. Hedonic tone was assessed using the Fawcett-Clark Pleasure Scale. We identified reduced tract volume and reduced number of tracts in the left slMFB. There was an increase in FC between the VTA and right medial PFC in patients with depression. Depression severity was associated with reduced tract volume and fewer tracts in the left slMFB. Reduced hedonic tone was associated with reduced tract volume. Conversely, reduced hedonic tone was associated with increased FC between the VTA and the PFC. In conclusion, our results suggest reduced structural connectivity of the slMFB in patients with depression. Increases in FC between the VTA and PFC may be associated with anhedonia or compensatory hyperactivity.
1. Introduction
Anhedonia is a core feature of depression that has been associated with structural and functional alterations of the reward system (Bracht et al., 2015b, Keedwell et al., 2005, Nestler and Carlezon, 2006). The ventral tegmental area (VTA), nucleus accumbens (NAcc), and orbitofrontal cortex (OFC) are central relay stations of the reward system. They mediate pleasure and reward-seeking behaviour (Nestler and Carlezon, 2006). The medial forebrain bundle (MFB) connects the core regions of the reward system (Nieuwenhuys et al., 1982). The most comprehensive work on the anatomy stems from histological brain sections in rats. Its findings revealed that tiny unmyelinated fibres connect the VTA to the NAcc and traverse the lateral hypothalamus to precede to the forebrain (Geeraedts et al., 1990). Using diffusion tensor imaging (DTI)-based tractography, the MFB of human patients was reconstructed for the first time in a case report (Coenen et al., 2009). This work was followed by a comprehensive anatomical and conceptual study suggesting that in humans, the MFB has two branches; the infero-medial branch (imMFB), which corresponds to the MFB described in rodents and travels through the lateral hypothalamus, approaching the nearby NAcc, and the supero-lateral branch (slMFB), which projects through the anterior limb of the internal capsule to connect the VTA with the prefrontal cortex (PFC) (Coenen et al., 2012). The slMFB branch may stem from the massive phylogenetic development of PFC in humans (Coenen et al., 2012). Because of the dense corticofugal and corticopetal projection pathways connecting the VTA to the PFC, the slMFB has also been referred to as the projection pathway of the VTA (vtaPP = slMFB), (Coenen et al., 2020, Coenen et al., 2018, Fenoy et al., 2021). In this work, we focus on both the imMFB and the slMFB and will for better readability stick with the term MFB (imMFB and slMFB), as we have done in our previous work (Bracht et al., 2015a).
Converging evidence suggests that the MFB plays a core role in experiencing pleasure and motivated behaviour (Coenen et al., 2011, Nestler and Carlezon, 2006). This assumption is supported by a tractography study of the slMFB in unipolar depression. The study suggests that reduced fractional anisotropy (FA), the most commonly used diffusion magnetic resonance imaging (MRI) based measure to characterise white matter microstructure (Basser and Pierpaoli, 1996), between the VTA and the PFC (OFC and dorsolateral prefrontal cortex [dlPFC]) underlies melancholic features, including anhedonia (Bracht et al., 2014). This finding was extended by a recent tractography study that found reduced tract length of the slMFB in bipolar disorder. Again, the findings were observed in a subgroup of patients with melancholic features (Denier et al., 2020). In addition, hedonic tone, the capacity to derive pleasure from rewarding experiences, was associated with the FA of slMFB in healthy women with or without a history of depression (Bracht et al., 2015a). There is less evidence for structural alterations of the imMFB in depression. No alterations were found while comparing never-depressed with remitted depressed women using tractography (Bracht et al., 2015a) and patients with unipolar depression with healthy controls using a region of interest (ROI) approach (Blood et al., 2010). However, these studies were limited in their sample size, which motivated us to investigate the role of the imMFB in depression pathophysiology in a larger sample.
Event-related functional MRI (fMRI) studies have demonstrated repeatedly the relevance of the VTA, NAcc, and PFC for reward processing e.g. (D'Ardenne et al., 2008, Keedwell et al., 2005, Kringelbach, 2005, Wacker et al., 2009). Resting-state fMRI extends such analyses and provides information on the strength of functional connectivity (FC) between spatially distributed brain regions at rest, giving a basis for neural networks (van den Heuvel and Hulshoff Pol, 2010). Several resting-state FC studies in depression have identified distinct networks with both increased and decreased FC (Mulders et al., 2015). However, only a few studies have investigated seed-based resting-state FC from the VTA, leading to diverging results showing negative results (Anand et al., 2018) or different localisations of significant clusters with either increased or decreased FC (Nakamura et al., 2020, Wagner et al., 2017). One study found increased FC in unipolar depression between VTA and dlPFC (Wagner et al., 2017), which are regions that are structurally connected through the slMFB. Increases in FC between the VTA and the anterior cingulate cortex (ACC), medial PFC, and dlPFC were also found in a small sample of patients with depression using 7-Tesla MRI (Morris et al., 2019). Increases in FC between the VTA and ACC were associated with more pronounced anhedonia (Morris et al., 2019). These findings are in line with established rodent models of depression, linking VTA hyperactivity to anhedonia and reduction in goal-directed behaviour (Cao et al., 2010, Friedman et al., 2016, Han and Nestler, 2017). Therefore, hyperactivity of the VTA may represent a functional correlation of anhedonia in depression. However, conflicting results of resting-state FC studies warrant further investigation of this research question (Anand et al., 2018, Nakamura et al., 2020, Wagner et al., 2017).
This study aimed to extend knowledge on the associations between structural connectivity of the MFB (imMFB and slMFB), FC of the VTA, anhedonia, and depression severity. First, we explored microstructural and macrostructural alterations of the white matter of imMFB/slMFB in unipolar depression. We hypothesised reduced structural connectivity of the imMFB/slMFB in unipolar depression (Bracht et al., 2015a, Denier et al., 2020). Second, we investigated alterations in the VTA and NAcc FC. We hypothesised increased FC between the VTA and PFC in depression (Friedman et al., 2016, Morris et al., 2019, Wagner et al., 2017). Third, we hypothesised that decreased MFB structural connectivity and increased VTA FC are associated with hedonic tone and depression severity (Bracht et al., 2014, Morris et al., 2019).
2. Methods
2.1. Participants
We recruited 56 patients with unipolar depression at the University Hospital of Psychiatry and Psychotherapy in Bern. Inclusion criteria were age between 18 and 65 years and a current depressive episode. The exclusion criteria were psychiatric comorbidities as assessed using the Mini International Neuropsychiatric Interview (MINI) (Sheehan et al., 1998). Personality disorders were screened using the Structured Clinical Interview for DSM-IV Axis II (SCID-II) (Wittchen et al., 1997). Diagnosis of a current depressive episode according to DSM-IV was made by the treating psychiatrist and confirmed using MINI. Depression severity was assessed using the 21-item Hamilton Rating Scale for Depression (HAMD) (Hamilton, 1967) and the 21-item Beck Depression Inventory (BDI-II) (Beck et al., 1996). The Fawcett–Clark Pleasure Scale (FCPS), a questionnaire that measures the intensity of pleasurable responses to enjoyable situations, was used to quantify hedonic tone (Fawcett et al., 1983). Handedness was assessed using the Edinburgh Handedness Inventory (Oldfield, 1971).
Twenty-two healthy controls were matched for age, sex, and handedness. The inclusion criteria were the absence of any present or past psychiatric disorder as assessed using MINI and SCID-II screening questionnaires. Further exclusion criteria for all participants were claustrophobia or contraindications for MRI. All patients provided informed written consent. The study was approved by the local national ethics committee (KEK-number: 2017-00731).
2.2. MRI data acquisition
Structural and functional MRI data were acquired using a 3-Tesla Magnetom Prisma scanner (Siemens, Erlangen, Germany) and a 64-channel head and neck coil at the University Hospital of Bern. For acquisition of high-contrast T1-weighted images, we used a bias-field-corrected MP2RAGE sequence with two gradient echo images (INV1 and INV2) and a T1-weighted image (UNI). Parameters of the MP2RAGE sequence were: 256 Slices, FOV = 256 × 256, 256 × 256 matrix, 1 × 1 × 1 mm3 isotropic resolution, TR = 5000 ms, TE = 2.98 ms, TI = 700 ms and T2 = 2500 ms. Diffusion-weighted images (DWIs) with 64 non-collinear directions were acquired using a spin-echo echo-planar sequence. DWI parameters were: 64 × b = 1000 s/mm2, 1 × b = 0 s/mm2, 60 slices, FOV = 269 × 269, 128 × 128 matrix, 2.2 × 2.2 × 2.2 mm3 isotropic resolution, TR = 6200 ms, and TE = 69 ms. A continuous resting-state fMRI scan with condition ‘eyes closed’ was acquired by echo planar imaging (EPI) with the following parameters: 480 volumes with 48 slices per volume, FOV = 230 × 230, 94 × 94 matrix, 2.4 × 2.4 × 2.4 mm3 isotropic resolution, TR = 1000 ms, and TE = 30 ms.
2.3. Data analyses
2.3.1. Diffusion-weighted MRI
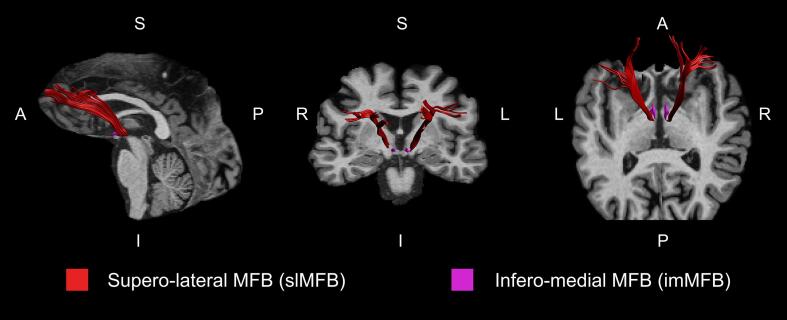
We used FSL 6.0 (http://www.fmrib.ox.ac.uk/fsl/) and FSL-BET for robust brain extraction (-R option). Owing to the noisy background of MP2RAGE UNI images, we used INV2 images as input and applied a derived binary mask to the UNI image to obtain the extracted brain. Diffusion-weighted MRI scans were processed using ExploreDTI 4.8.6 (Leemans et al., 2009). First, we performed a correction for subject motion by co-registering the DWIs to the b0 image (Leemans and Jones, 2009). Using the realignment motion parameters, we calculated the mean framewise displacement (FD) for each subject. The groups did not differ significantly in mean FD (patients: 5.9 ± 1.8; controls: 5.6 ± 1.7; T = 0.74, p = 0.46). Second, an EPI correction was performed to correct for eddy current distortions and field inhomogeneities warping the motion-corrected DWIs to the brain-extracted MP2RAGE image (Wu et al., 2008). This resulted in DWIs being localised in the same undistorted native space as the MP2RAGE images. Depending on the ROI, native space DWIs or MP2RAGE images were used for ROI delineation. Whole-brain deterministic tractography was performed by applying a diffusion tensor model (Basser et al., 1994). The following termination criteria were used: fractional anisotropy (FA) < 0.2 and angle threshold > 45°. ROIs were delineated as described in our previous work (Bracht et al., 2021, Bracht et al., 2019, Denier et al., 2020). For both pathways (imMFB and slMFB), the VTA was encircled on a DWI horizontal section (see Supplementary Fig. S1. The anatomical borders of the VTA are the red nucleus (posterior), substantia nigra (lateral), and mammillary bodies (anterior) (Nieuwenhuys et al., 2008). For reconstruction of the slMFB, a second ROI was delineated on the coronal MP2RAGE image section surrounding the anterior limb of the internal capsule at the height of the NAcc surrounding the caudate and putamen (see Supplementary Fig. S2. For reconstruction of the imMFB, a second ROI was drawn one section above the ROI of the VTA on a horizontal section of the MP2RAGE image surrounding the hypothalamus (see Supplementary Fig. S3, to capture fibres that proceed through the lateral hypothalamus and travel towards the nearby NAcc (Bracht et al., 2015a). In line with previous publications, only segments dorsal to the VTA were considered (see Fig. 1 (Bracht et al., 2015a, Denier et al., 2020). The following microstructural and macrostructural tract properties were computed separately for both hemispheres of each tract (slMFB and imMFB): FA, mean diffusivity (MD), radial diffusivity (RD), axial diffusivity (AD) (microstructure), and tract volume, number of tracts and tract length (geometric properties).
Fig. 1.
Visualisation of the slMFB and imMFB. The imMFB (magenta) is located medial to the slMFB (red) and travels through the lateral hypothalamus. The slMFB proceeds through the lateral part of the anterior limb of the internal capsule and connects the VTA to the prefrontal cortex. L: left; R: right; A: anterior; P: posterior; I: inferior; S: superior. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
2.3.2. Resting-state functional MRI
We analysed resting-state fMRI using the CONN 20b toolbox (Whitfield-Gabrieli and Nieto-Castanon, 2012). Pre-processing steps included realignment and co-registration of EPI volumes to MP2RAGE, segmentation and normalisation to the MNI space, and smoothing using an FWHM kernel of 8 × 8 × 8 mm. We applied band-pass filtering (0.008–0.09 Hz) to remove physiological signals and regress nuisance variables of each of the five time series within segmented white matter and cerebrospinal fluid and 12 realignment parameters. Scrubbing of outlier volumes with global BOLD signal or FD higher than the 95th percentile was performed using the Artefact Detection Tools (ART) toolbox implemented in CONN. Additionally, for every subject, we computed the mean FD of the motion parameters and mean-DVARS, which is the spatial root mean square of the BOLD signal after temporal differencing (Afyouni and Nichols, 2018). For further analyses, we excluded subjects with values (mean-FD and mean-DVARS) higher than two standard deviations above the mean. We excluded seven subjects (six patients, one control group). There was no group difference in mean-DVARS (patients: 0.16 ± 0.06; controls 0.16 ± 0.04; T = 0.019, p = 0.985); however, mean-FD (patients: 0.15 ± 0.05; controls 0.12 ± 0.04; T = 2.734, p = 0.008) exhibited a difference.
The midbrain seed region encapsulated bilateral VTA, and was defined as a spherical volume with a 3-mm radius (152 mm3) centred at MNI coordinates x = 0, y = −16, z = −7, which is in accordance with previous studies (Gu et al., 2010, Hadley et al., 2014, Zhang et al., 2015).
To examine the FC of the VTA to the PFC, we generated a PFC mask using the Talairach Daemon atlas implemented in the WFU PickAtlas 3.0 (Maldjian et al., 2003). Seed-based FC of the VTA to the PFC was compared between patients and controls. We performed between-group analyses with age, sex, and mean-FD, and mean-DVARS as covariates with a voxel threshold of p < 0.001 and with a family-wise error (FWE) correction of p < 0.05. To further explore the FC of VTA and its possible association with clinical symptoms, we extracted values of significant clusters localised in the PFC, our hypothesis-driven area of interest.
2.4. Statistical analyses
2.4.1. Structural connectivity of the MFB
Based on the results of previous tractography studies of the slMFB, we expected an effect size of f = 0.4 for the between-factor group (patients vs. controls) (Bracht et al., 2014). Using the program G*Power (Faul et al., 2007) with an effect size of f = 0.4, alpha = 0.05, beta = 0.8, number of groups = 2, numerator df = 1, number of groups = 2, and number of covariates = 2, results of a power analysis suggest a required total sample size of n = 52 participants. The total number of participants (n = 78) exceeded the required number because patients in this study participated in a larger ongoing observational longitudinal study investigating remission plasticity. All patient data were derived from the baseline assessments of this study.
The Statistical Package for Social Sciences SPSS 27.0 (SPSS, Inc., Chicago, IL, USA) was used for data analyses. Age was compared using a two-sample t-test. Sex and handedness were compared using test. Four separate mixed-model analyses of covariance (ANCOVAs) with the between-subject factor group (patients vs. controls), the within-subject factor hemisphere (left vs. right), and the four dependent variables (FA, tract volume, number of tracts, and tract length) were calculated for the slMFB and imMFB. In case of a significant group × hemisphere interaction, separate ANCOVAs controlling for age and sex were calculated for each hemisphere for the respective modalities. We applied a two-tailed level of significance and Bonferroni correction for the number of tests performed for each tract, resulting in a level of significance of p < 0.0125 (0.05/4). Effect sizes were reported using η2 (Olejnik and Algina, 2003). To complement our analysis of white matter microstructure, additional exploratory ANCOVAs controlling for age and sex were performed to compare the diffusion measures MD, RD, and AD.
2.4.2. Associations with anhedonia and depression severity
We explored associations between hedonic tone (FCPS total score) and depression severity (HAMD total score) with the measures of MFB structural connectivity and VTA FC (extracted from the significant cluster localised in the PFC) that differed significantly between groups. Given the lack of variation in HAMD scores in the control group, correlations between HAMD scores and further variables were limited to the patient group. Correlations with the FCPS total scores were calculated across both groups. We applied a two-tailed significance level of p < 0.05.
3. Results
Patients and controls did not differ in terms of age, sex, and handedness. Patients had a mean score of 21.69 ± 5 on the HAMD-21 (range 10–35, median 22), suggesting moderate to severe depression (see Supplementary Fig. S4. All but nine patients were on antidepressant medication at the time of the MRI scan. Eight percent of the patients had a history of electroconvulsive therapy (ECT), and 7% had a history of repetitive transcranial magnetic stimulation (rTMS) treatment. The demographics of patients and controls are displayed in Table 1.
Table 1.
Demographics.
| Patients (n = 56) | Controls (n = 22) | P Value | |
|---|---|---|---|
| Age (years) | 43.64 ± 12 | 42.91 ± 13 | P = 0.82 |
| Gender (male) | 55% | 54% | P = 0.95 |
| Handedness (right, left, ambidextrous,) | (83%, 11%, 6%) | (90%, 5%, 5%) | P = 0.67 |
| HAMD 21 items total score | 21.69 ± 5 | 0.58 ± 1 | P < 0.001*** |
| BDI total score | 28.60 ± 9 | 1.48 ± 2 | P < 0.001*** |
| FCPS total score | 91.96 ± 21 | 124.38 ± 16 | P < 0.001*** |
| Duration of episode (months) | 12.00 ± 11 | N/A | N/A |
| Number of episodes | 3.43 ± 3 | N/A | N/A |
| SSRI | 18 % | N/A | N/A |
| Dual antidepressants | 38 % | N/A | N/A |
| Tricyclic antidepressants | 23% | N/A | N/A |
| Lithium | 35% | N/A | N/A |
*** P < 0.001
3.1. Structural connectivity of the MFB
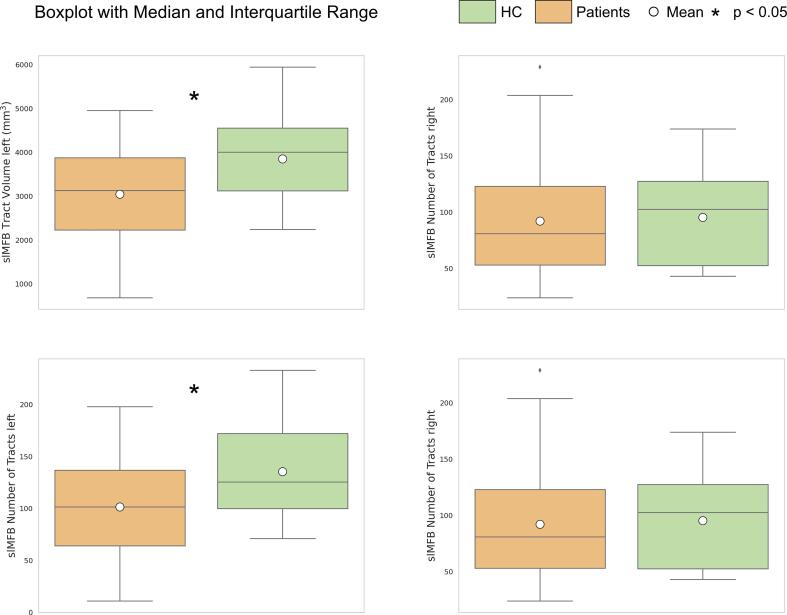
Patients had reduced tract volume (patients: 1943 ± 650 mL, controls 2400 ± 585 mL, F (1, 74) = 9.00, p = 0.004, η2 = 0.108) and fewer tracts (patients: 96 ± 47, controls: 128 ± 48, F (1, 74) = 7.619, p = 0.007, η2 = 0.093) in the left slMFB compared with controls. Tract volume and number of tracts of the right slMFB did not differ between the two groups (see Fig. 2. There were no differences in the slMFB regarding tract length or FA (see Supplementary Fig. S5 and S6. Patients and controls did not differ in FA, tract volume, number of tracts, and tract length of the imMFB (see Table 2, Table 3, and Supplementary Fig. S7 and S8. There were no significant differences in the imMFB or slMFB in our exploratory analysis of further diffusion properties (see Supplementary Tables S1 and S2, and Supplementary Fig. S6 and S8.
Fig. 2.
Group comparison of tract volume and number of tracts of the slMFB. Boxplots and interquartile ranges are displayed for patients (orange) and controls (green) for the left and right slMFB. Patients had reduced tract volume and fewer tracts in the left slMFB compared to controls. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Table 2.
Structural connectivity of slMFB and imMFB.
| Supero-lateral medial forebrain bundle | |||
|---|---|---|---|
| Measure | Group | Hemisphere | Group × hemisphere |
| FA | F(1,74) = 0.06, p = 0.805 | F(1,74) = 1.92, p = 0.169 | F(1,74) = 7.14 , p = 0.009* |
| Tract length | F(1,74) = 0.52, p = 0.472 | F(1,74) = 2.81, p = 0.098 | F(1,74) = 0.80, p = 0.375 |
| Tract volume | F(1,74) = 5.18, p = 0.026* | F(1,74) = 3.51 , p = 0.065 | F(1,74) = 4.52 , p = 0.037* |
| Number of tracts | F(1,74) = 3.61, p = 0.061 | F(1,74) = 7.73, p = 0.007 | F(1,74) = 5.67, p = 0.020* |
| Infero-medial medial forebrain bundle | |||
| Measure | Group | Hemisphere | Group × hemisphere |
| FA | F(1,74) = 2.28, p = 0.135 | F(1,74) = 1.03, p = 0.314 | F(1,74) = 2.39, p = 0.127 |
| Tract length | F(1,74) = 0.82, p = 0.368 | F(1,74) = 1.60, p = 0.209 | F(1,74) = 0.05, p = 0.831 |
| Tract volume | F(1,74) = 0.00, p = 0.975 | F(1,74) = 0.14, p = 0.711 | F(1,74) = 8.75, p = 0.004* |
| Number of tracts | F(1,74) = 0.00, p = 0.993 | F(1,74) = 0.78, p = 0.379 | F(1,74) = 4.69, p = 0.034* |
ANCOVAs controlling for age and gender for measures of structural connectivity * P < 0.05; ** p < 0.01.
Table 3.
Structural connectivity of slMFB and imMFB for separate hemispheres.
| Supero-lateral medial forebrain bundle | ||
|---|---|---|
| Measure | Left | Right |
| FA | F(1,74) = 1.54, p = 0.219 | F(1,74) = 0.66, p = 0.420 |
| Tract volume | F(1,74) = 9.00, p = 0.004** | F(1,74) = 0.63, p = 0.432 |
| Number of tracts | F(1,74) = 7.62, p = 0.007* | F(1,74) = 0.25, p = 0.618 |
| Infero-medial medial forebrain bundle | ||
| Measure | Left | Right |
| Tract volume | F(1,74) = 2.73, p = 0.102 | F(1,74) = 2.88, p = 0.094 |
| Number of tracts | F(1,74) = 1.18, p = 0.280 | F(1,74) = 2.39, p = 0.126 |
ANCOVAs controlling for age and gender for measures of structural connectivity with significant group × hemisphere interactions. * P < 0.05; ** p < 0.01.
3.2. Functional connectivity of the VTA and the NAcc
There were significant group differences with increased FC in patients between the VTA and the PFC in a cluster localised in the right medial PFC bordering the OFC (AAL: frontal superior medial; peak [MNI]: 2, 54, 08; p-FWE = 0.026; cluster size: 95 voxels). Results are displayed using the applied voxel threshold of p < 0.001 and for visualisation purposes of p < 0.01 (see Fig. 3.
Fig. 3.
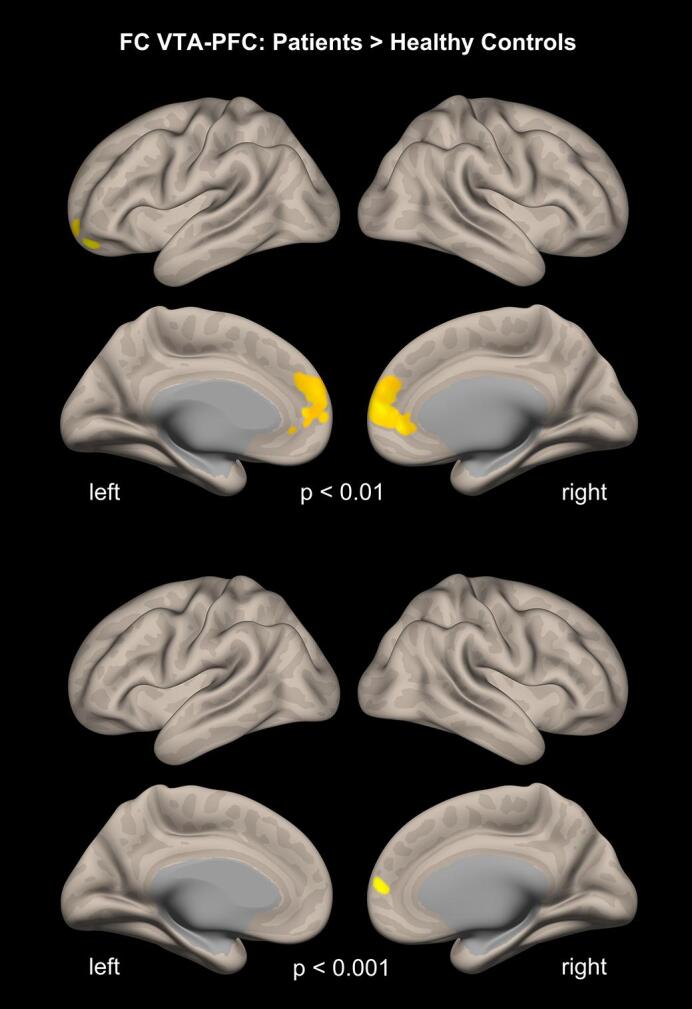
Resting state functional connectivity of the VTA to the PFC. A higher seed-based resting-state FC from the VTA to the PFC was observed in the patients. Results are displayed using voxel thresholds of p < 0.001 and for visualisation purposes of p < 0.01. A false discovery rate (FWE) correction of p < 0.05 was used.
3.3. Associations between hedonic tone and depression severity and measures of structural and functional connectivity
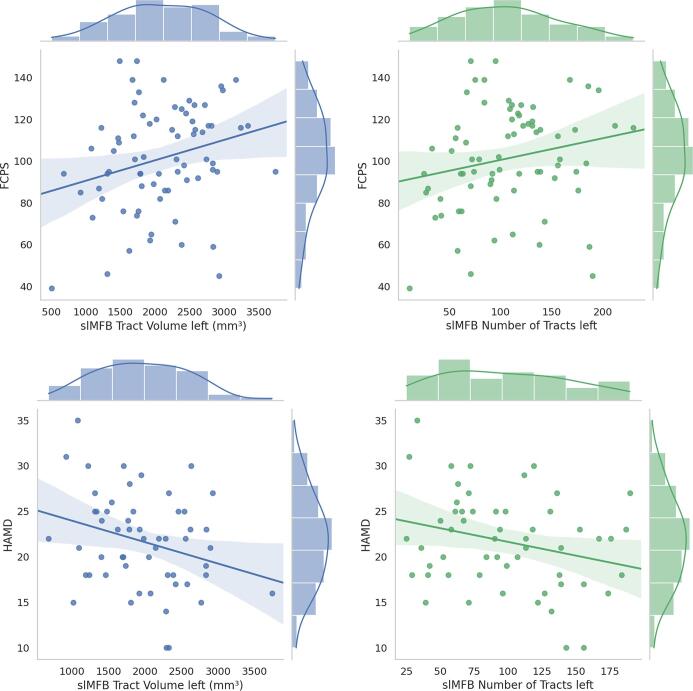
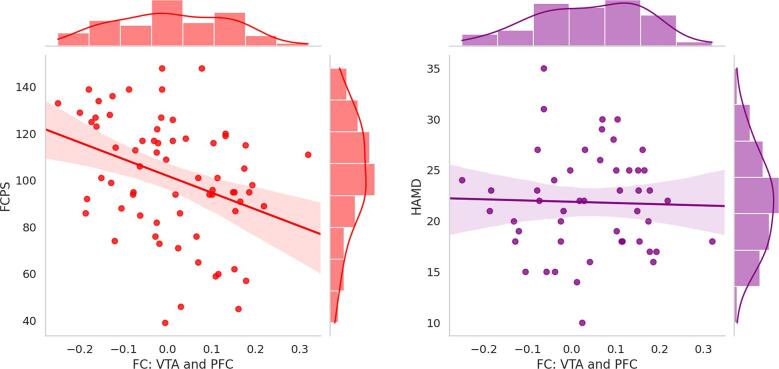
There was a positive correlation between hedonic tone and the tract volume of the left slMFB (r = 0.259, p = 0.027), and a non-significant trend for a positive correlation between hedonic tone and the number of tracts of the left slMFB (r = 0.205, p = 0.082). Conversely, there was a negative correlation between hedonic tone and VTA FC values derived from the cluster localised in the right medial PFC (r = −0.351, p = 0.003). Depression severity was negatively correlated with both tract volume (r = -0.283, p = 0.036) and number of tracts (r = -0.268, p = 0.049) of the left slMFB, but not with VTA-PFC FC (r = −0.029, p = 0.841) (see Fig. 4, Fig. 5.
Fig. 4.
Correlations between structural connectivity, hedonic tone, and depression severity. FCPS, Fawcett–Clark Pleasure Scale; HAMD, Hamilton Rating Scale for Depression; slMFB, supero-lateral medial forebrain bundle.
Fig. 5.
Correlations between functional connectivity, hedonic tone, and depression severity. FCPS, Fawcett–Clark Pleasure Scale; HAMD, Hamilton Rating Scale for Depression; FC, functional connectivity; VTA, ventral tegmental area; PFC, prefrontal cortex.
4. Discussion
This is the first study to combine analyses of FC in the VTA with tractography of the MFB to explore their role in anhedonia and depression severity in unipolar depression. We identified reduced tract volume and fewer tracts in the left slMFB in unipolar depression. In addition, we found increase in FC between the VTA and right medial PFC. Reduced tract volume and fewer tracts in the left slMFB were associated with increased depression severity. Similarly, a low hedonic tone (anhedonia) was associated with decreased tract volume. Conversely, low hedonic tone was associated with increase in FC between the VTA and right medial PFC.
Comparing the white matter microstructure and tract geometry of the MFB, we found alterations in structural connectivity in the slMFB, but not in the imMFB, highlighting the specific role of the slMFB in depression pathophysiology (Bracht et al., 2015a, Coenen et al., 2012, Denier et al., 2020). We identified a volume reduction of the left slMFB, showing a medium to large effect size (η2 = 0.108). Our results suggest that the identified reduction in tract volume stems from a reduced number of tracts (tract length did not differ significantly between groups). Due to the lack of specificity of diffusion-weighted measures regarding biological sub-compartments, we can only speculate about its biological correlates. It is possible that white matter atrophy or loss of neurones may drive the reduction in tract volume and number of tracts. This assumption is in line with both post-mortem findings of depression and animal models of stress (Banasr et al., 2011, Pittenger and Duman, 2008, Tham et al., 2011). Another explanation is that subtle FA reductions at the margins of the tracts (e.g., due to reduced integrity of myelin) led to a reduced number of reconstructed fibre tracts. However, in contrast to tract volume and number of tracts, none of the diffusion properties (FA, MD, RD, and AD) that were averaged across the entire tract differed between groups.
Our negative finding of diffusion properties is in line with a previous tractography study comparing the FA of the slMFB between never-depressed women and women with depression remittance (Bracht et al., 2015a). Furthermore, in contrast to the findings of reduced FA in the slMFB in unipolar melancholic depression (Bracht et al., 2014), a previous tractography study in bipolar depression did not find alterations in FA in the slMFB, even though tract length was reduced in the patient group (Denier et al., 2020). Thus, it is possible that geometric tract measures (e.g., tract volume, number of tracts, and tract length) and mean diffusion properties have the potential to identify distinct and independent alterations of tract structural connectivity (Denier et al., 2020, Kubicki et al., 2019). Therefore, future studies should incorporate both measures in their analyses (Bracht et al., 2021). Furthermore, additional research is warranted to advance our understanding of alterations in the structural connectivity of the slMFB that are specific for depression subtypes, or dependent on chronicity or psychopathology (e.g., melancholia) (Bracht et al., 2015b).
There were no group differences in the measures of white matter microstructure or measures of fibre geometry in imMFB. This is in line with an ROI study investigating FA of imMFB in unipolar depression (Blood et al., 2010) and a tractography study in women with depression remittance (Bracht et al., 2015a). In addition, a tractography study in unipolar depression investigating FA of the adjacent nigrostriatal tract did not yield significant group differences, and there were no alterations in FA in patients with unipolar melancholic depression in a segment incorporating the imMFB (VTA-NAcc segment), even though FA was reduced in segments of the slMFB (VTA-OFC and VTA-dlPFC segments) (Bracht et al., 2014). Thus, additional studies are needed to explore the role of the imMFB (MacNiven et al., 2020). Ideally, future studies should apply advanced tractography methods to improve the reconstruction of this small pathway (Guo et al., 2021).
Decreases in tract volume and number of tracts of the left slMFB were associated with increased depression severity and decreased hedonic tone. This suggests that anhedonia may stem from reduced structural connectivity of the slMFB, which is in line with previous findings by our group (Bracht et al., 2014, Denier et al., 2020). In contrast to the decrease in slMFB structural connectivity, we found increase in FC between the VTA and a cluster localised in the right medial PFC in close proximity to the medial OFC in unipolar depression. The identified increase in FC were more pronounced in patients with more severe anhedonia. This is in line with the findings of a 7-Tesla VTA FC study in patients with unipolar depression (Morris et al., 2019). This is also consistent with animal models linking VTA hyperactivity to depression symptomatology (Cao et al., 2010, Friedman et al., 2016, Han and Nestler, 2017). The medial OFC plays an essential role in experiencing pleasure (Kringelbach, 2005) and may therefore be implicated in the pathophysiology of depression (Rolls et al., 2020). Its activity reflects a representation of rewards, which in turn contributes to decision-making processes that involve more anterior regions, such as the medial PFC (Rolls, 2019). Our finding of increased FC between the VTA and right medial PFC may therefore reflect compensatory hyperactivity in patients with severe anhedonia. Hyperactivity of the medial PFC may normalise following successful antidepressant treatment (Drevets, 2007). This suggests a putative role for medial PFC activity as a marker of depression status. Additional longitudinal studies are required to investigate this hypothesis.
A compensatory mechanism of the slMFB in severe treatment-resistant depression has been suggested, based on observations that deep brain stimulation (DBS) of the slMFB leads to rapid clinical improvements in patients with treatment-resistant depression (Coenen et al., 2019, Fenoy et al., 2016, Fenoy et al., 2018, Schlaepfer et al., 2013). The efficacy of tractography-guided stimulation of the slMFB may stem from the activation of corticofugal glutamatergic pathways from the OFC to the VTA, which in turn activates corticopetal dopaminergic projections from the VTA to the PFC (via the slMFB and/or the imMFB) (Fenoy et al., 2021). Our finding of increased FC between the VTA and the PFC in severely depressed unipolar patients indirectly supports this model, assuming a compensatory mechanism in this patient subgroup. It is also possible that DBS of the slMFB induces normalisation of pathological hyperactivation of the VTA, a process that has been linked to reductions in anhedonia and depressive symptoms in animal models (Cao et al., 2010, Friedman et al., 2016).
This study has several limitations that must be considered. First, the general limitations of tractography apply (e.g., no conclusions can be drawn on the directionality of fibre tracts [afferent or efferent fibres] and interconnections [mono- or polysynaptic connections] cannot be resolved, and no statements on neurotransmitters [e.g., dopaminergic or glutamatergic connections] can be made). Second, resting-state fMRI acquisition is subject to several limitations, such as differences in subjective experiences and motion artefacts (Weinberger and Radulescu, 2016). To reduce these limitations, all participants were instructed not to ruminate during resting-state fMRI data acquisition. To minimise motion artefacts, we excluded outliers of mean-DVARS and mean-FD. We performed scrubbing of outlier volumes regarding global BOLD signal or FD, and FC group comparisons were controlled for mean-DVARS and mean-FD. Third, our sample consisted of mainly moderate to severely depressed patients with a history of several depressive episodes and a chronic course. Thus, the results cannot be generalised to patients with less chronic depression (e.g., patients with first episode). Fourth, even though the lifetime prevalence of depression is higher in women, the sex distribution of our sample was even. This may be due to the exclusion of patients with comorbidities that are more frequent in women such as anxiety disorders (McLean et al., 2011)) and because the sex gap diminishes with age and chronicity of depression (Eaton et al., 2008, Kessler et al., 1993). Fifth, the vast majority of our patients were medicated, including a high percentage of patients receiving lithium. Thus, our results may have been influenced by the impact of medication on white matter microstructure (Seiger et al., 2021). However, a recent longitudinal multisite study did not find an impact of selective serotonin reuptake inhibitor (SSRI) intake on diffusion properties (Davis et al., 2019). Furthermore, lithium intake has been suggested to preserve the integrity of the white matter microstructure (Espanhol and Vieira-Coelho, 2021). Therefore, such an impact would mitigate rather than strengthen our finding of reduced structural connectivity of the left slMFB. Finally, lithium intake may affect MRI signals. However, while such an impact on voxel-based morphometry is discussed (Vernon and Hajek, 2013), this may not apply to volumetric measures of white matter (Cousins et al., 2013).
In summary, our study provides additional evidence for the core role of the slMFB in the pathophysiology of depression (Bracht et al., 2014, Denier et al., 2020). Our results point to reduced structural connectivity of the left slMFB in unipolar depression, contributing to anhedonia and depression severity. Increase in FC between the VTA and PFC may represent a physiological marker of anhedonia, a dysregulated top-down regulation, or a compensatory mechanism for anhedonia in severe depression. Our results shed further light on the role of the human MFB in depression pathophysiology, which is important for understanding why DBS of the slMFB is effective in treatment-resistant depression (Fenoy et al., 2021, Schlaepfer et al., 2013).
Funding
This work was supported by the Robert Enke Foundation and the Novartis Foundation for Medical Biological Research (#19C203) and the Swiss Life Foundation (to Tobias Bracht). Tobias Bracht received a grant from the Adrian et Simone Frutiger Foundation.
CRediT authorship contribution statement
Tobias Bracht: Conceptualization, Funding acquisition, Writing – original draft, Writing – review & editing. Nicolas Mertse: Writing – review & editing. Sebastian Walther: Conceptualization, Funding acquisition, Writing – review & editing. Karin Lüdi: Writing – review & editing. Sigrid Breit: Writing – review & editing. Andrea Federspiel: Writing – review & editing. Roland Wiest: Writing – review & editing. Niklaus Denier: Conceptualization, Writing – original draft, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2022.102961.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Afyouni S., Nichols T.E. Insight and inference for DVARS. Neuroimage. 2018;172:291–312. doi: 10.1016/j.neuroimage.2017.12.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand A., Jones S.E., Lowe M., Karne H., Koirala P. Resting State Functional Connectivity of Dorsal Raphe Nucleus and Ventral Tegmental Area in Medication-Free Young Adults With Major Depression. Front. Psychiatry. 2018;9 doi: 10.3389/fpsyt.2018.00765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banasr M., Dwyer J.M., Duman R.S. Cell atrophy and loss in depression: reversal by antidepressant treatment. Curr. Opin. Cell Biol. 2011;23(6):730–737. doi: 10.1016/j.ceb.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basser P.J., Mattiello J., Lebihan D. Estimation of the effective self-diffusion tensor from the NMR spin echo. J. Magn. Reson. B. 1994;103(3):247–254. doi: 10.1006/jmrb.1994.1037. [DOI] [PubMed] [Google Scholar]
- Basser P.J., Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J. Magn. Reson. B. 1996;111(3):209–219. doi: 10.1006/jmrb.1996.0086. [DOI] [PubMed] [Google Scholar]
- Beck, A.T., Steer, R.A., Brown, G., 1996. Beck Depression Inventory -II. APA PsyTests.
- Blood A.J., Iosifescu D.V., Makris N., Perlis R.H., Kennedy D.N., Dougherty D.D., Kim B.W., Lee M.J., Wu S., Lee S., Calhoun J., Hodge S.M., Fava M., Rosen B.R., Smoller J.W., Gasic G.P., Breiter H.C., Bartolomucci A. Microstructural abnormalities in subcortical reward circuitry of subjects with major depressive disorder. PLoS ONE. 2010;5(11):e13945. doi: 10.1371/journal.pone.0013945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bracht T., Doidge A.N., Keedwell P.A., Jones D.K. Hedonic tone is associated with left supero-lateral medial forebrain bundle microstructure. Psychol. Med. 2015;45(4):865–874. doi: 10.1017/S0033291714001949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bracht T., Horn H., Strik W., Federspiel A., Schnell S., Höfle O., Stegmayer K., Wiest R., Dierks T., Müller T.J., Walther S. White matter microstructure alterations of the medial forebrain bundle in melancholic depression. J. Affect. Disord. 2014;155:186–193. doi: 10.1016/j.jad.2013.10.048. [DOI] [PubMed] [Google Scholar]
- Bracht T., Linden D., Keedwell P. A review of white matter microstructure alterations of pathways of the reward circuit in depression. J. Affect. Disord. 2015;187:45–53. doi: 10.1016/j.jad.2015.06.041. [DOI] [PubMed] [Google Scholar]
- Bracht T., Soravia L., Moggi F., Stein M., Grieder M., Federspiel A., Tschumperlin R., Batschelet H.M., Wiest R., Denier N. The role of the orbitofrontal cortex and the nucleus accumbens for craving in alcohol use disorder. Transl. Psychiatry. 2021;11:267. doi: 10.1038/s41398-021-01384-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bracht T., Viher P.V., Stegmayer K., Strik W., Federspiel A., Wiest R., Walther S. Increased structural connectivity of the medial forebrain bundle in schizophrenia spectrum disorders is associated with delusions of paranoid threat and grandiosity. Neuroimage Clin. 2019;24:102044. doi: 10.1016/j.nicl.2019.102044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J.-L., Covington H.E., Friedman A.K., Wilkinson M.B., Walsh J.J., Cooper D.C., Nestler E.J., Han M.-H. Mesolimbic dopamine neurons in the brain reward circuit mediate susceptibility to social defeat and antidepressant action. J. Neurosci. 2010;30(49):16453–16458. doi: 10.1523/JNEUROSCI.3177-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coenen V.A., Bewernick B.H., Kayser S., Kilian H., Boström J., Greschus S., Hurlemann R., Klein M.E., Spanier S., Sajonz B., Urbach H., Schlaepfer T.E. Superolateral medial forebrain bundle deep brain stimulation in major depression: a gateway trial. Neuropsychopharmacology. 2019;44(7):1224–1232. doi: 10.1038/s41386-019-0369-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coenen V.A., Honey C.R., Hurwitz T., Rahman A.A., McMaster J., Burgel U., Madler B. Medial forebrain bundle stimulation as a pathophysiological mechanism for hypomania in subthalamic nucleus deep brain stimulation for Parkinson's disease. Neurosurgery. 2009;64:1106–1114. doi: 10.1227/01.NEU.0000345631.54446.06. discussion 1114–1105. [DOI] [PubMed] [Google Scholar]
- Coenen V.A., Panksepp J., Hurwitz T.A., Urbach H., Mädler B. Human medial forebrain bundle (MFB) and anterior thalamic radiation (ATR): imaging of two major subcortical pathways and the dynamic balance of opposite affects in understanding depression. J. Neuropsychiatry Clin. Neurosci. 2012;24(2):223–236. doi: 10.1176/appi.neuropsych.11080180. [DOI] [PubMed] [Google Scholar]
- Coenen V.A., Schlaepfer T.E., Maedler B., Panksepp J. Cross-species affective functions of the medial forebrain bundle-implications for the treatment of affective pain and depression in humans. Neurosci. Biobehav. Rev. 2011;35(9):1971–1981. doi: 10.1016/j.neubiorev.2010.12.009. [DOI] [PubMed] [Google Scholar]
- Coenen V.A., Schlaepfer T.E., Sajonz B., Döbrössy M., Kaller C.P., Urbach H., Reisert M. Tractographic description of major subcortical projection pathways passing the anterior limb of the internal capsule. Corticopetal organization of networks relevant for psychiatric disorders. Neuroimage Clin. 2020;25:102165. doi: 10.1016/j.nicl.2020.102165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coenen V.A., Schumacher L.V., Kaller C., Schlaepfer T.E., Reinacher P.C., Egger K., Urbach H., Reisert M. The anatomy of the human medial forebrain bundle: Ventral tegmental area connections to reward-associated subcortical and frontal lobe regions. Neuroimage Clin. 2018;18:770–783. doi: 10.1016/j.nicl.2018.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousins D.A., Aribisala B., Nicol Ferrier I., Blamire A.M. Lithium, gray matter, and magnetic resonance imaging signal. Biol. Psychiatry. 2013;73(7):652–657. doi: 10.1016/j.biopsych.2012.09.029. [DOI] [PubMed] [Google Scholar]
- D'Ardenne K., McClure S.M., Nystrom L.E., Cohen J.D. BOLD responses reflecting dopaminergic signals in the human ventral tegmental area. Science. 2008;319(5867):1264–1267. doi: 10.1126/science.1150605. [DOI] [PubMed] [Google Scholar]
- Davis A.D., Hassel S., Arnott S.R., Harris J., Lam R.W., Milev R., Rotzinger S., Zamyadi M., Frey B.N., Minuzzi L., Strother S.C., MacQueen G.M., Kennedy S.H., Hall G.B. White Matter Indices of Medication Response in Major Depression: A Diffusion Tensor Imaging Study. Biol. Psychiatry Cogn. Neurosci. Neuroimaging. 2019;4(10):913–924. doi: 10.1016/j.bpsc.2019.05.016. [DOI] [PubMed] [Google Scholar]
- Denier N., Walther S., Schneider C., Federspiel A., Wiest R., Bracht T. Reduced tract length of the medial forebrain bundle and the anterior thalamic radiation in bipolar disorder with melancholic depression. J. Affect. Disord. 2020;274:8–14. doi: 10.1016/j.jad.2020.05.008. [DOI] [PubMed] [Google Scholar]
- DREVETS W.C. Orbitofrontal cortex function and structure in depression. Ann. N. Y. Acad. Sci. 2007;1121(1):499–527. doi: 10.1196/annals.1401.029. [DOI] [PubMed] [Google Scholar]
- Eaton W.W., Shao H., Nestadt G., Lee B.H., Bienvenu O.J., Zandi P. Population-based study of first onset and chronicity in major depressive disorder. Arch. Gen. Psychiatry. 2008;65(5):513. doi: 10.1001/archpsyc.65.5.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espanhol J.C.L., Vieira-Coelho M.A. Effects of lithium use on the white matter of patients with bipolar disorder - a systematic review. Nord. J. Psychiatry. 2021;76(1):1–11. doi: 10.1080/08039488.2021.1921264. [DOI] [PubMed] [Google Scholar]
- Faul F., Erdfelder E., Lang A.-G., Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods. 2007;39(2):175–191. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- Fawcett J., Clark D.C., Scheftner W.A., Gibbons R.D. Assessing anhedonia in psychiatric patients. Arch. Gen. Psychiatry. 1983;40:79–84. doi: 10.1001/archpsyc.1983.01790010081010. [DOI] [PubMed] [Google Scholar]
- Fenoy A.J., Quevedo J., Soares J.C. Deep brain stimulation of the “medial forebrain bundle”: a strategy to modulate the reward system and manage treatment-resistant depression. Mol. Psychiatry. 2021 doi: 10.1038/s41380-021-01100-6. [DOI] [PubMed] [Google Scholar]
- Fenoy A.J., Schulz P., Selvaraj S., Burrows C., Spiker D., Cao B.o., Zunta-Soares G., Gajwani P., Quevedo J., Soares J. Deep brain stimulation of the medial forebrain bundle: Distinctive responses in resistant depression. J. Affect. Disord. 2016;203:143–151. doi: 10.1016/j.jad.2016.05.064. [DOI] [PubMed] [Google Scholar]
- Fenoy A.J., Schulz P.E., Selvaraj S., Burrows C.L., Zunta-Soares G., Durkin K., Zanotti-Fregonara P., Quevedo J., Soares J.C. A longitudinal study on deep brain stimulation of the medial forebrain bundle for treatment-resistant depression. Transl. Psychiatry. 2018;8:111. doi: 10.1038/s41398-018-0160-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman A.K., Juarez B., Ku S.M., Zhang H., Calizo R.C., Walsh J.J., Chaudhury D., Zhang S., Hawkins A., Dietz D.M., Murrough J.W., Ribadeneira M., Wong E.H., Neve R.L., Han M.H. KCNQ channel openers reverse depressive symptoms via an active resilience mechanism. Nat. Commun. 2016;7:11671. doi: 10.1038/ncomms11671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geeraedts L.M.G., Nieuwenhuys R., Veening J.G. Medial forebrain bundle of the rat: III. Cytoarchitecture of the rostral (telencephalic) part of the medial forebrain bundle bed nucleus. J. Comp. Neurol. 1990;294(4):507–536. doi: 10.1002/cne.902940403. [DOI] [PubMed] [Google Scholar]
- Gu H., Salmeron B.J., Ross T.J., Geng X., Zhan W., Stein E.A., Yang Y. Mesocorticolimbic circuits are impaired in chronic cocaine users as demonstrated by resting-state functional connectivity. Neuroimage. 2010;53(2):593–601. doi: 10.1016/j.neuroimage.2010.06.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo F., Luca A., Parker G., Jones D.K., Viergever M.A., Leemans A., Tax C.M.W. The effect of gradient nonlinearities on fiber orientation estimates from spherical deconvolution of diffusion magnetic resonance imaging data. Hum. Brain Mapp. 2021;42(2):367–383. doi: 10.1002/hbm.25228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadley J.A., Nenert R., Kraguljac N.V., Bolding M.S., White D.M., Skidmore F.M., Visscher K.M., Lahti A.C. Ventral tegmental area/midbrain functional connectivity and response to antipsychotic medication in schizophrenia. Neuropsychopharmacology. 2014;39(4):1020–1030. doi: 10.1038/npp.2013.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton, M., 1967. Development of a rating scale for primary depressive illness. Br. J. Soc. Clin. Psychol. 6, pp. 278-296. [DOI] [PubMed]
- Han, M.H., Nestler, E.J., 2017. Neural Substrates of Depression and Resilience. Neurotherapeutics 14, 677-686. [DOI] [PMC free article] [PubMed]
- Keedwell, P.A., Andrew, C., Williams, S.C., Brammer, M.J., Phillips, M.L., 2005. The neural correlates of anhedonia in major depressive disorder. Biol. Psychiatry 58, 843-853. [DOI] [PubMed]
- Kessler R.C., McGonagle K.A., Swartz M., Blazer D.G., Nelson C.B. Sex and depression in the National Comorbidity Survey. I: Lifetime prevalence, chronicity and recurrence. J. Affect. Disord. 1993;29:85–96. doi: 10.1016/0165-0327(93)90026-g. [DOI] [PubMed] [Google Scholar]
- Kringelbach M.L. The human orbitofrontal cortex: linking reward to hedonic experience. Nat. Rev. Neurosci. 2005;6(9):691–702. doi: 10.1038/nrn1747. [DOI] [PubMed] [Google Scholar]
- Kubicki A., Leaver A.M., Vasavada M., Njau S., Wade B., Joshi S.H., Loureiro J., Hellemann G., Woods R.P., Espinoza R., Narr K.L. Variations in Hippocampal White Matter Diffusivity Differentiate Response to Electroconvulsive Therapy in Major Depression. Biol Psychiatry Cogn Neurosci Neuroimaging. 2019;4(3):300–309. doi: 10.1016/j.bpsc.2018.11.003. [DOI] [PubMed] [Google Scholar]
- Leemans, A., Jeurissen, B., Sijbers, J., Jones, D.K., 2009. ExporeDTI: a graphical toolbox for processing, analyzing and visualizing diffusion MR data. . Proceedings of the International Society for Magnetic Resonance in Medicine 17th Annual Meeting April 18-24, 3536 Honolulu Hawaii.
- Leemans A., Jones D.K. The B-matrix must be rotated when correcting for subject motion in DTI data. Magn. Reson. Med. 2009;61(6):1336–1349. doi: 10.1002/mrm.21890. [DOI] [PubMed] [Google Scholar]
- MacNiven K.H., Leong J.K., Knutson B. Medial forebrain bundle structure is linked to human impulsivity. Sci. Adv. 2020;6(38) doi: 10.1126/sciadv.aba4788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldjian J.A., Laurienti P.J., Kraft R.A., Burdette J.H. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19(3):1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- McLean C.P., Asnaani A., Litz B.T., Hofmann S.G. Gender differences in anxiety disorders: prevalence, course of illness, comorbidity and burden of illness. J. Psychiatr. Res. 2011;45(8):1027–1035. doi: 10.1016/j.jpsychires.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris L.S., Kundu P., Costi S., Collins A., Schneider M., Verma G., Balchandani P., Murrough J.W. Ultra-high field MRI reveals mood-related circuit disturbances in depression: a comparison between 3-Tesla and 7-Tesla. Transl. Psychiatry. 2019;9:94. doi: 10.1038/s41398-019-0425-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulders P.C., van Eijndhoven P.F., Schene A.H., Beckmann C.F., Tendolkar I. Resting-state functional connectivity in major depressive disorder: A review. Neurosci. Biobehav. Rev. 2015;56:330–344. doi: 10.1016/j.neubiorev.2015.07.014. [DOI] [PubMed] [Google Scholar]
- Nakamura, Y., Okada, N., Koshiyama, D., Kamiya, K., Abe, O., Kunimatsu, A., Okanoya, K., Kasai, K., Koike, S., 2020. Differences in Functional Connectivity Networks Related to the Midbrain Dopaminergic System-Related Area in Various Psychiatric Disorders. Schizophr Bull. [DOI] [PMC free article] [PubMed]
- Nestler E.J., Carlezon W.A. The mesolimbic dopamine reward circuit in depression. Biol. Psychiatry. 2006;59(12):1151–1159. doi: 10.1016/j.biopsych.2005.09.018. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuys R., Geeraedts L.M.G., Veening J.G. The medial forebrain bundle of the rat I. General introduction. J. Comp. Neurol. 1982;206(1):49–81. doi: 10.1002/cne.902060106. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuys R., Voogd J., van Huijzen C., editors. The Human Central Nervous System. Springer Berlin Heidelberg; Berlin, Heidelberg: 2008. [Google Scholar]
- Oldfield R.C. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Olejnik S., Algina J. Generalized eta and omega squared statistics: measures of effect size for some common research designs. Psychol. Methods. 2003;8(4):434–447. doi: 10.1037/1082-989X.8.4.434. [DOI] [PubMed] [Google Scholar]
- Pittenger C., Duman R.S. Stress, depression, and neuroplasticity: a convergence of mechanisms. Neuropsychopharmacology. 2008;33(1):88–109. doi: 10.1038/sj.npp.1301574. [DOI] [PubMed] [Google Scholar]
- Rolls E.T. The orbitofrontal cortex and emotion in health and disease, including depression. Neuropsychologia. 2019;128:14–43. doi: 10.1016/j.neuropsychologia.2017.09.021. [DOI] [PubMed] [Google Scholar]
- Rolls E.T., Cheng W., Feng J. The orbitofrontal cortex: reward, emotion and depression. Brain Commun. 2020;2:fcaa196. doi: 10.1093/braincomms/fcaa196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaepfer T.E., Bewernick B.H., Kayser S., Mädler B., Coenen V.A. Rapid effects of deep brain stimulation for treatment-resistant major depression. Biol. Psychiatry. 2013;73(12):1204–1212. doi: 10.1016/j.biopsych.2013.01.034. [DOI] [PubMed] [Google Scholar]
- Seiger, R., Gryglewski, G., Klobl, M., Kautzky, A., Godbersen, G.M., Rischka, L., Vanicek, T., Hienert, M., Unterholzner, J., Silberbauer, L.R., Michenthaler, P., Handschuh, P., Hahn, A., Kasper, S., Lanzenberger, R., 2021. The Influence of Acute SSRI Administration on White Matter Microstructure in Patients Suffering From Major Depressive Disorder and Healthy Controls. Int. J. Neuropsychopharmacol. 24, 542-550. [DOI] [PMC free article] [PubMed]
- Sheehan, D.V., Lecrubier, Y., Sheehan, K.H., Amorim, P., Janavs, J., Weiller, E., Hergueta, T., Baker, R., Dunbar, G.C., 1998. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatry 59 Suppl. 20, 22-33;quiz 34-57. [PubMed]
- Tham M.W., Woon P.S., Sum M.Y., Lee T.-S., Sim K. White matter abnormalities in major depression: evidence from post-mortem, neuroimaging and genetic studies. J. Affect. Disord. 2011;132(1-2):26–36. doi: 10.1016/j.jad.2010.09.013. [DOI] [PubMed] [Google Scholar]
- van den Heuvel M.P., Hulshoff Pol H.E. Exploring the brain network: a review on resting-state fMRI functional connectivity. Eur. Neuropsychopharmacol. 2010;20(8):519–534. doi: 10.1016/j.euroneuro.2010.03.008. [DOI] [PubMed] [Google Scholar]
- Vernon A.C., Hajek T. Effects of lithium on magnetic resonance imaging signal might not preclude increases in brain volume after chronic lithium treatment. Biol. Psychiatry. 2013;74(12):e39–e40. doi: 10.1016/j.biopsych.2012.12.028. [DOI] [PubMed] [Google Scholar]
- Wacker J., Dillon D.G., Pizzagalli D.A. The role of the nucleus accumbens and rostral anterior cingulate cortex in anhedonia: integration of resting EEG, fMRI, and volumetric techniques. Neuroimage. 2009;46(1):327–337. doi: 10.1016/j.neuroimage.2009.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner G., de la Cruz F., Kohler S., Bar K.J. Treatment Associated Changes of Functional Connectivity of Midbrain/Brainstem Nuclei in Major Depressive Disorder. Sci. Rep. 2017;7:8675. doi: 10.1038/s41598-017-09077-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger D.R., Radulescu E. Finding the Elusive Psychiatric “Lesion” With 21st-Century Neuroanatomy: A Note of Caution. Am. J. Psychiatry. 2016;173(1):27–33. doi: 10.1176/appi.ajp.2015.15060753. [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S., Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012;2(3):125–141. doi: 10.1089/brain.2012.0073. [DOI] [PubMed] [Google Scholar]
- Wittchen H.U., Zaudig M., Fydrich T. Hogrefe; Göttingen: 1997. Strukturiertes klinisches Interview für DSM-IV. [Google Scholar]
- Wu M., Chang L.C., Walker L., Lemaitre H., Barnett A.S., Marenco S., Pierpaoli C. Comparison of EPI distortion correction methods in diffusion tensor MRI using a novel framework. Med Image Comput. Comput. Assist. Interv. 2008;11:321–329. doi: 10.1007/978-3-540-85990-1_39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J.T., Ma S.S., Yip S.W., Wang L.J., Chen C., Yan C.G., Liu L., Liu B., Deng L.Y., Liu Q.X., Fang X.Y. Decreased functional connectivity between ventral tegmental area and nucleus accumbens in Internet gaming disorder: evidence from resting state functional magnetic resonance imaging. Behav. Brain Funct. 2015;11:37. doi: 10.1186/s12993-015-0082-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.