Abstract
Chinese rice wine (Huangjiu) has a long history and has been popular in China for thousands of years. During Huangjiu fermentation, many kinds of microorganisms are involved and hundreds of metabolites are produced, which form its unique aroma. The composition of the Huangjiu microbiome has a strong influence on the flavor and quality of the final consumer product. Therefore, this review summarizes the research progress on the main components, quality control indicators and flavor compounds, as well as potential hazards during the fermentation of Huangjiu. The influence of the dominant microbial species on the Huangjiu fermentation, the sensory qualities and the formation of harmful substances are discussed. This helps to provide a theoretical basis for modification of the Huangjiu microbiome to improve control of the fermentation process and the overall sensory quality of the final product.
Keywords: Chinese rice wine (Huangjiu), Flavor compounds, Microorganism, Fermentation process, Potential hazards, Quality control
Graphical abstract

Highlights
-
•
The microbial community is high diversity and complexity in Huangjiu.
-
•
Huangjiu fermentation process is closely related to production of flavor substances.
-
•
The dominant microbial have a greater impact on the quality of Huangjiu.
-
•
There is a relationship of cooperation and competition between microbial communities.
1. Introduction
Chinese rice wine (Huangjiu) is an alcoholic beverage fermented from rice, glutinous rice, or millet, with wheat Qu, or yeast as a starter. Well-known types include the famous Shaoxing rice wine, Ji Mo Lao Jiu and Hong Qu Glutinous Rice Wine; production is widely distributed in Zhejiang, Shandong and the Yangtze River Delta region (Lv et al., 2013b; Xu et al., 2015). Huangjiu is one of the three most popular alcoholic beverages in the world (i.e., beer, grape wine and rice wine). A traditional alcoholic beverage, rice wine has been popular in China, because of its unique flavor, low alcohol content and abundant nutritional components, such as amino acids, peptides, vitamins and oligosaccharides (Ren et al., 2019). According to the National Bureau of Statistics, China's rice wine production was 3.53 million kiloliters in 2019 and China is a major exporter of Huangjiu.
China is one of the oldest civilizations in the world and Huangjiu was the first wine to be produced in China, with a history going back more than 5000 years; a Chinese cultural treasure and a precious scientific heritage. There are many varieties of Huangjiu in China, such as Fujian Lao Jiu, Shaoxing rice wine, Shanghai Lao Jiu and Shandong Ji Mo Lao Jiu (Lv et al., 2012; Song et al., 2019). Shaoxing Huangjiu appears to be the original variety produced (Liu et al., 2015) and was recorded in writings from the Spring and Autumn (770–476 BCE) and Warring States (475–221 BCE) periods (Cai et al., 2018). The unique flavor and aroma of Huangjiu have been developed over thousands of years and it is exported worldwide (Wang et al., 2014a). The use of Qu (starter cultures) in winemaking is a feature of Huangjiu. The use of starter cultures originated in China and spread to other Asian countries (Park et al., 2016) and has great significance because: (1) Qu is an important source of brewing strains; many excellent strains has been isolated from Qu, laying the foundation for the production of pure-strain starters; (2) Qu contains many different saccharification and health-beneficial strains and has the functions of conversion of grain-starch into glucose and then fermentation to produce alcohol; (3) The microorganisms in Qu can produce a variety of enzymes, such as saccharification enzymes and esterase, which have a unique effect on flavor formation; (4) The process of solid Qu production is simple and the Qu is very stable during storage, which is convenient for industrial production; (5) The development and application of Qu has had a profound impact on the production and development of fermented foods in China (Mo et al., 2010). Huangjiu is not just a pleasant low alcohol beverage, it is reported to have as good, or better health-promoting properties, compared with red grape wine. Huangjiu contains oligosaccharides, phenolic compounds and mineral components, which confer health-promoting properties, such as reducing cholesterol, antioxidant activity and anti-aging effects (He et al., 2013; Lv et al., 2015b). Huangjiu has an important place in Chinese culture and the everyday life of the Chinese people.
With the great improvement in living standards in China in recent decades, food safety has become of increasing importance. Nitrogen-containing toxins produced by microbial metabolism, such as biogenic amines (BAs) and ethyl carbamate (EC) have been detected frequently during fermentation of Huangjiu (Liu et al., 2018a; Xia et al., 2018b). EC is the greatest hazard, because of its demonstrated mutagenicity and carcinogenicity in mice, rats and monkeys (Weber and Sharypov, 2009; Wu et al., 2014). The carcinogenicity of EC has been confirmed by molecular biological studies. Consumer demand for improved food quality and safety has focused attention on the relationship between the Huangjiu microbiome and metabolome (Li et al., 2014; Yan et al., 2019). This review discusses progress in research aimed at reducing or eliminating harmful substances from Huangjiu, with the aim of improving understanding of this field and providing a reference for improving the safety of other fermented products.
2. Huangjiu fermentation process
The process of high temperature starter production and low temperature wine-making originated in ancient China, has been developed and refined over thousands of years and continues to this day (Wu et al., 2014). The Huangjiu brewing process can be divided into two main stages, pre-fermentation and post-fermentation. The pre-fermentation is carried out at a low temperature, after mixing the raw materials with the starter culture and is the key step which determines the quality of the final product (Liu et al., 2014).
2.1. Main raw materials and key ingredients of Huangjiu fermentation
After thousands of years of development, there is a wide choice of raw materials available, but those used for brewing Huangjiu are still grains (rice, glutinous rice, millet), yeast starters and wheat Qu (Chen and Xu, 2014). The yeast starter, a mixture of S. cerevisiae and bacteria, is the most important component of the rice wine fermentation process. The yeast are naturally fermented and grown on a solid substrate, such as rice flour and some herbs, and S. cerevisiae is the main producer of ethanol (Cai et al., 2018). Wheat Qu is another key component of Huangjiu brewing. It contains various microorganisms, such as Mucor, Bacillus, Aspergillus, Rhizopus and Lactobacillus, grown on wheat as a solid substrate, which produce many enzymes, including amylase, protease and esterase, which saccharify the grain starch to glucose and produce ethanol from it (Lai et al., 2019; Ren et al., 2019). Wheat Qu is similar to koji, used in Japanese sake production and contributes to the product color, and the formation of the unique aroma and flavor of Huangjiu. Therefore, the wheat Qu is a vital part of Huangjiu production and its quality has a great influence on the quality of the final product (Zhang et al., 2012). The yeast starter and the wheat Qu are mixed under appropriate conditions of temperature and humidity, then the fermentation process begins, resulting in a low concentration alcoholic beverage (Wang et al., 2014b). Therefore, the microbiome composition has a major influence on the functions of the wheat Qu and yeast starter, as well as the sensory attributes and quality of the final product.
2.2. Typical brewing techniques for Huangjiu
The semi-solid open fermentation process is typical of Huangjiu production (Fig. 1). Unlike the liquid brewing process used for beer and grape wine, there is no sterilization procedure. Wheat Qu supplies a wide range of microorganisms and the fermentation is carried out in open fermenter trays (Liu et al., 2016).
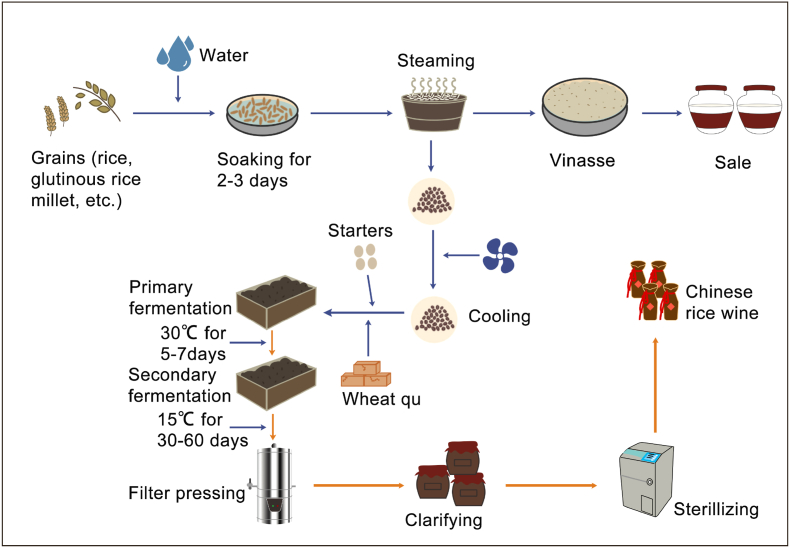
Fig. 1.
Typical Huangjiu fermentation process.
The grains used for Huangjiu fermentation are glutinous rice, rice, or millet. It is soaked in water for 2–3 days, then steamed and cooled. Wheat Qu, starters and the cooled rice are mixed in a specific ratio and water is added. After fermentation at 30 °C for 5–7 days, the mash is opened to lower the temperature to 15 °C, then maintained at this temperature for 1–2 months until the fermentation is complete. The fermented liquid is filtered, clarified, sterilized and aged to obtain the final product.
This process has two main steps. The first step is raw material pretreatment, soaking the rice in water, then steaming it. Soaking is the most important step, because it directly determines the quality of the final Huangjiu and has been used for hundreds of years. In the traditional Huangjiu soaking process, the rice soaking time was longer (Ai et al., 2017), needing 16–20 days for water absorption, main component decomposition and water acidity increase. The rice is steamed after soaking.
The second part is the fermentation, in which wheat Qu and yeast starter is mixed with the steamed rice and the temperature is controlled for up to 2 months (Wei et al., 2017). The brewing process is mainly controlled by experienced technicians, automated instrument control is still rare. The manual control method has the advantage that it allows each batch of Huangjiu to have a different flavor (Liu et al., 2014). Although traditional craftsmanship has been successful for centuruss, the increasing consumer demand for improved quality and safety mean that a more scientific approach to process control is needed.
3. Main components during the fermentation of Huangjiu
Huangjiu fermentation is essentially a mixed fermentation with many strains of mold, yeast and bacteria. As the fermentation progresses and the environment changes, there is a continuous succession of dominant microbes and strains, during which fungi and bacteria secrete various enzymes (such as amylase, protease and esterase) to hydrolyze starch and protein (Lv et al., 2015a), yeast convert sugar to ethanol, and acetic acid bacteria (AAB) and lactic acid bacteria (LAB) produce organic acids to lower the pH (Wang et al., 2014a). During the fermentation the wide diversity of microorganisms produces sugar, ethanol, organic acids, aromas component and nutrients, forming the unique sensory attributes of Huangjiu (Liu et al., 2016) and determine the fermentation speed, flavor and qualities (Lv et al., 2017).
3.1. Main quality control indicators of Huangjiu
The microbial metabolism and biochemical changes during brewing of Huangjiu are very complex, so to help control the quality of finished Huangjiu, the National Bureau of Standards has set a series of standards that must be implemented during the production process. The general quality indicators for controlling Huangjiu products as the following.
-
(1)
Sensory indicators (Table 1). These include the appearance, aroma, taste and style of Huangjiu (GB/T 13,662-2018). The judging criteria mainly apply to the sensory evaluation method and refers to the analysis and evaluation of the color, aroma, taste and style characteristics of the wine sample by human tasters (Yu et al., 2015). This approach is rapid and relatively accurate, but only semi-quantitative, relying on subjective human judgement, which can be influenced by factors, such as region, ethnicity and personal tastes and preferences.
-
(2)
Physicochemical indicators (Table 1). These involve conventional chemical analysis methods for measurement of total sugar, alcohol content, total acid, non-sugar solids, amino nitrogen, pH, benzoic acid and calcium oxide in Huangjiu (GB/T 13,662-2018). The most common assays used are total sugar, alcohol and total acid content (Yang et al., 2017).
Table 1.
Main components of Huangjiu.
| Quality control indicator | Main flavor compounds | Main hazards | Reference |
|---|---|---|---|
| Sensory indicators | Esters: Ethyl acetate, Ethyl octanoate, Ethyl decanoate, Isoamyl acetate, 2-Phenethyl acetate, ethyl butyrate, ethyl hexanoate | EC | (Xia et al., 2018a; Zhou et al., 2017) |
| Appearance | Histamine | Zhang et al. (2019) | |
| Aroma | Tyramine | Li et al. (2019b) | |
| Taste | Putrescine | Li et al. (2019a) | |
| Style | Higher alcohols | Zhang et al. (2015) | |
| Physicochemical indicators | Alcohols: Ethanol, 1-Propanol | ||
| Total sugar | Hexyl alcohol | ||
| Alcohol content, | Isoamyl alcohol | ||
| Total acid, | Phenylethyl alcohol | ||
| Non-sugar solids, | Aldehydes: Benzaldehyde, Phenylacetaldehyde, Decanal | ||
| Amino nitrogen, | |||
| β-Phenylethanol | |||
| pH |
The composition of Huangjiu including the main quality control indicators (including both sensory and physicochemical indicators), the main flavor compounds and the main hazards (hazards shown in bold). The main flavor compounds are esters, alcohols and aldehydes, and the table lists the more abundant individual compounds. The hazardous compounds are ethyl carbamate (EC), biogenic amines (BAs) and higher alcohols (such as 1-methyl-cyclohexanol, 3-Methyl-1-butanol, etc).
The total sugar content is one of the important indicators to determine the grade of Huangjiu. The total sugar content is measured by complete acid hydrolysis of non-reducing sugars into reducing sugars, which are then determined by the Lane Eynon method, potassium ferrocyanide titration, anthrone colorimetric assay, or 3,5-dinitrosalicylic acid colorimetry (GB/T 13,662-2018).
Alcohol content is another important indicator of Huangjiu product quality, so all Huangjiu manufacturers attach great importance to accurate determination of alcohol content. However, according to the measurement method in the national standard GB/T13662-2018, the stability of the measured alcohol content is often affected by differences in season, climate, temperature and measuring instrument. In recent years, near infrared spectroscopy has increasingly been applied to determination of physicochemical quality indicators (Qin et al., 2016; Teixeira dos Santos et al., 2018).
Total acid refers to the carboxylic acids formed during fermentation, including volatile acids (such as acetic acid, butyric acid) and non-volatile acids (such as lactic acid, tartaric acid, succinic acid). Acid-base titration is usually used to determine the total acid content of Huangjiu (GB/T 5517-2010, China) (Xu et al., 2018).
3.2. Main flavor compounds in Huangjiu
The improvement in Chinese standards of living has increased consumer demand for high quality Huangjiu, stimulating research interest in understanding its characteristic flavor and aroma compounds (Ren et al., 2019). The aroma of Huangjiu is an important determinant of its sensory quality and consumer preference. Huangjiu is a complex mixture containing hundreds of flavor compounds, both volatile and non-volatile. The volatile compounds are derived from the raw materials and metabolites of the microorganisms in different brewing processes, whereas the non-volatile compounds are mainly derived from enzymic hydrolysis of the rice starch (Fig. 2) (Son et al., 2018). However, flavor differences may be caused by a variety of factors, such as differences in the raw materials, starters, fermentation and aging process (Chen et al., 2020; Xu et al., 2018).
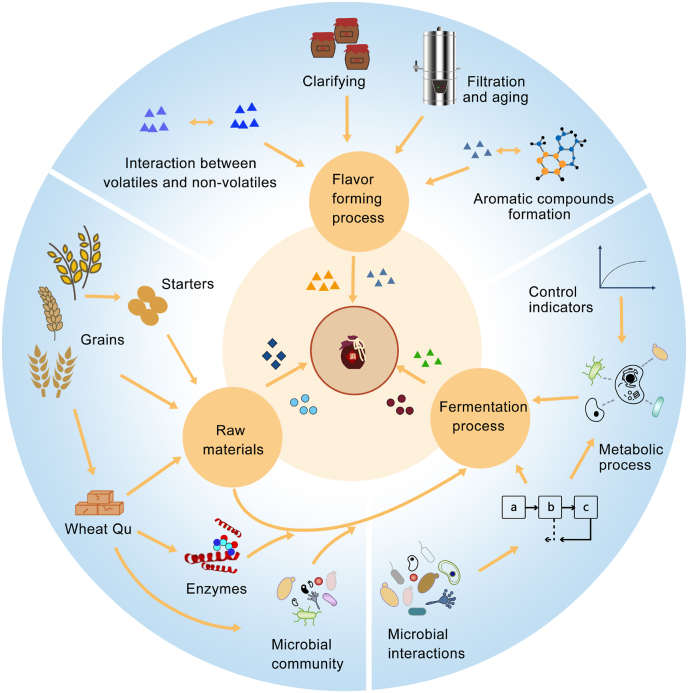
Fig. 2.
Correlation between the factors influencing Huangjiu flavor formation.
The figure shows the influence of the raw materials, microbial species and their metabolic processes on the flavor of Huangjiu, and the correlations between the main influencing factors. The raw materials for the fermentation are rice, wheat Qu and starter, which provide microorganisms, enzymes, flavors and their precursors. During the fermentation process, the dynamics of the microbiome and the metabolites produced are continually changing, and the non-volatile flavor compounds may be precursors of aroma volatiles.
The flavor compounds, especially the volatile aroma components, are the main determinants of the style and quality of Huangjiu (Chen et al., 2019). The increased research interest in Huangjiu aroma has resulted in the application of a variety of high precision instruments to detect and analyze Huangjiu aroma components, which are mostly alcohols and esters (Table 1). More than 100 flavor compounds have been identified in more than 10 categories, including aldehydes, acids, proteins, monosaccharides, polysaccharides, amino acids and vitamins. The volatile compounds are composed of a variety of trace components, including alcohols, esters, acids, aldehydes and various heterocyclic compounds (Chen et al., 2013; Yu et al., 2019). The alcohols include ethanol, methanol, n-propanol and n-butanol, which are both fragrant and tasteful, enhance the sweetness and flavor of the Huangjiu, and are the precursors of esters. The esters include ethyl lactate, ethyl acetate, ethyl succinate and ethyl formate, and contribute to the flavor (Hu et al., 2019). The acids include butyric, propionic, acetic and caproic acids (Xu et al., 2018) and greatly influence the flavor and aging of Huangjiu, giving rise to the saying “no acid and no taste”. Although the content of volatile aroma compounds is very low, they are vital flavor components and of great significance for controlling the quality and improving the flavor of Huangjiu (Xiang et al., 2019).
Black glutinous rice wine (BGRW) is a famous traditional Huangjiu in Guizhou province. Zhao et al. used HS-SPME/GC-MS to analyze the content of volatile flavor compounds during BGRW fermentation. A total of 43 compounds was detected, including 17 esters, seven alcohols, six alkanes, four acids and three aldehydes; these flavor volatiles changed dynamically during the fermentation (Zhao et al., 2020a). Wang et al. conducted a sensory-oriented analysis of the main aroma components of Shanxi Huangjiu. A total of 106 compounds were separated and identified by gas chromatography-olfactory combined with aroma extract dilution analysis and odor specific magnitude estimation (Wang et al., 2020).
Analysis of the flavor volatiles in the representative Shaoxing Huangjiu found 64 compounds, including 33 esters, 13 alcohols, phenols and aldehydes, four ketones, nitrogen-containing compounds and naphthalenes, three alkanes and one sulfide. Among these volatile substances, esters were most abundant, 11 species of which were identified for the first time, including ethyl acetate (84% of the total ester content), diethyl succinate, ethyl hexanoate, ethyl tetradecanoate, ethyl palmitate, ethyl oleate and ethyl linoleate; esters impart fruity and floral flavors to the Huangjiu. In addition, 13 alcohols (not including ethanol) were found, alcohols are one of the most important flavor components in fresh Huangjiu, with isobutanol, isoamyl alcohol and phenylethyl alcohol as major components (Liu et al., 2015; Wang et al., 2014a).
Liu et al. (2018b) compared Hong Qu glutinous Huangjiu made with different starters. Huangjiu fermented with Wuyi Qu (WYQ) and Gutian Qu (GTQ) as fermenting starters, contained 66 identified volatile components, including 13 esters, 6 acids, 10 alcohols, 14 aldehydes and ketones, 5 lactones, 4 alkanes, plus cycloalkanes, aromatic compounds and nitrogen compounds. However, the volatile composition of WYQ- and GTQ-based Huangjiu had almost no similarities. The main aroma components of Hong Qu glutinous Huangjiu were volatile esters and alcohols, including ethyl butyrate, ethyl acetate, ethyl isobutyrate, ethyl octanoate, ethyl hexanoate, ethanol, hexanol, butyric acid, octanoic acid, caproic acid, hexanal, 2-heptanone, 2-nonanone. Their relative content was more than 1%, accounting for 86–94% of the total peak area (Huang et al., 2018). Yang et al. used different pure yeast starters (S. cerevisiae BR 30 and S. cerevisiae FC 15 and a mixture) for Huangjiu production. A total of 57 major flavor compounds was identified, including 28 esters, 13 alcohols, 8 aldehydes, 2 phenols, 2 ketones, and 4 acids. In the initial fermentation process, 35 volatile flavor compounds were identified in FC 15 Huangjiu, BR 30 Huangjiu contained 40 volatile compounds, and mixed Huangjiu contained 35 species. The main difference between volatile substances in FC 15 and BR 30 Huangjiu was the amount and content of alcohols and esters. Principal component analysis (PCA) indicated that diethyl succinate was the main flavor compound. Short-chain fatty acid ethyl esters were the main flavor compounds during fermentation and long-chain fatty acid ethyl esters were more important after clarification/sterilization. Sensory evaluation found that FC 15 Huangjiu had a strong alcohol aroma and BR 30 Huangjiu had a strong cereal-aroma (Yang et al., 2017). These findings indicated that the flavor and taste of Huangjiu could be modified by several factors, such as different raw materials, starter cultures, production regions and brewing processes.
In addition, studies have shown that differences in brewing materials will significantly affect the quality and flavor of Huangjiu. Years of practice has established the superiority of making Huangjiu with glutinous rice. The content of amylopectin in glutinous rice is higher and it is easier to be digested by enzymes. Microbes use the metabolites produced by the protein in glutinous rice to increase the flavor of rice wine (Zhu et al., 2020). Black glutinous rice is used for brewing black glutinous rice wine (BGRW) because of its high content of protein, fat, carbohydrates, phenols, flavonoids and anthocyanins. Therefore, black glutinous rice, as the fermentation material of BGRW, meets consumers' increasing demand for healthy products with its unique quality and flavor (Jiang et al., 2020).
3.3. Production of hazardous compounds during Huangjiu fermentation
The fermentation of Huangjiu is accomplished by a wide variety of microorganisms from the starter cultures and wheat Qu; however, because of the open fermentation process, contamination by pathogenic bacteria can occur (Kim et al., 2018) and their metabolites, such as ethyl carbamate (EC), biogenic amines (BAs), formaldehyde, aflatoxins and higher alcohols are potentially hazardous (Xia et al., 2018a; Zhang et al., 2015; Zhou et al., 2017). During Huangjiu fermentation, the main hazards known are EC and BAs (Table 1) (Xia et al., 2018b); EC is found in many fermented foods and alcoholic beverages (Wang et al., 2014b) and the recent detection of high concentrations of EC in alcoholic beverages has generated widespread concern. Because animal experiments revealed the potential mutagenicity and carcinogenicity of EC, it has been classified as a Class 2A carcinogen by the International Agency for Research on Cancer (IARC), part of the WHO (Chen et al., 2017; Fang et al., 2019). EC is mainly formed in Huangjiu by the spontaneous reaction of urea and ethanol. Various methods have been tried to reduce the EC content in alcoholic beverages, including physical, chemical and enzymatic methods, as well as metabolic process modification (Yang et al., 2015; Zhang et al., 2017; Zhao et al., 2013). Zhou et al. investigated the effect of bamboo leaf extract (BLE) on the formation of EC by adding BLE to three different Huangjiu fermentation starters (S. cerevisiae, S. cerevisiae/Lactobacillus brevis, and Chinese yeast). BLE inhibited the reaction between urea/citrulline and ethanol in Huangjiu fermented by various microorganisms (Zhou et al., 2020).
During Huangjiu fermentation, bacteria produce various metabolites, which contribute to nutritional value and flavor, however, they can also produce harmful substances, such as BAs, which are mainly produced by lactic acid bacteria (LABs) (Liu et al., 2016). BAs are low molecular weight nitrogen-containing compounds that have been identified as harmful and are found in a variety of foods, including aqua-cultured fish, dairy products, meat, and wine (Niu et al., 2019). An appropriate human intake of biogenic amines promotes growth and metabolism, and scavenges free radicals, but excessive intake causes a variety of health problems, such as headache, hypotension, and palpitations, which are harmful to the nervous and cardiovascular systems (Zhang et al., 2019). Common biogenic amines are tyramine (TYR), histamine (HIS), tryptamine (TRP), phenylethylamine (PHE), cadaverine (CAD) and putrescine (PUT), formed from tyrosine, histidine, tryptophan, lysine, phenylalanine and ornithine, respectively (Fig. 3B) (Xia et al., 2018b; Zhang et al., 2019). Of the BAs, HIS is the most toxic, causing headache, digestive disorder, neurotoxicity, and low blood pressure. TYR stimulates the release of norepinephrine, leading to an increase in arterial blood pressure (Li et al., 2019b). CAD and PUT inhibit the effects of HIS and TYR on metabolic enzymes, and CAD and PUT can also react with nitrite to produce carcinogenic nitrosamines (Zhang et al., 2019). Moreover, CAD has a synergistic effect which increases HIS toxicity (Lyons et al., 1983). The presence of BAs is usually considered to be a sign of poor Huangjiu quality and a poorly controlled brewing process, so the content of biogenic amines can be limited by careful process control.
Fig. 3.
Metabolic pathways that produce ethyl carbamate in S. Cerevisiae (A). AR: arginase, UCA: urea carboxylase, AH: allophanate hydrolase. Precursors and enzymes that form biogenic amines (B).
Based on the underlying mechanism of BAs formation, several strategies have been established to control the accumulation of BAs in food. Physical methods include cold treatment, hydrostatic pressure, irradiation to inhibit its microbial activity and destroy the cells of the production strain (Naila et al., 2010). However, these physical technologies have a certain impact on the growth of microorganisms, which will affect the flavor and quality of the final product. Another way to control the formation of BAs is to regulate protein catabolism by using specific microorganisms with low decarboxylase and significant amine oxidase activity. For example, Lactobacillus plantarum J16 and Pediococcus acidilactici CECT 5930 isolated by Callejon et al. have laccase and copper oxidase, which can degrade amines in wine (Callejón et al., 2014). Dehaut et al. demonstrated that inoculating fermented anchovies with Staphylococcus xylosus can reduce the total BAs content by 16%, especially the content of PUT, CAD and TYR (Dehaut et al., 2014). The above results indicate that the use of microbial methods to degrade BAs in fermented foods is effective and safe.
4. Major microorganisms present in Huangjiu fermentation
Currently, Huangjiu fermentation is mainly carried out by traditional fermentation methods, which have been used with little change for centuries. However, there is regional variation in the microbial composition of Huangjiu starters. The microbial diversity in traditional Huangjiu fermentation, including Huangjiu starters and wheat Qu has been analyzed. In the representative Shaoxing Huangjiu fermentation process (Zhang et al., 2012), S. cerevisiae was the predominant yeast and the 10 most abundant bacterial genera were Bacillus, Lactococcus, Leuconostoc, Staphylococcus, Pseudomonas, Weissella, Thermoactinomyces, Saccharopolyspora, Enterobacter and Lactobacillus (Liu et al., 2015). Hong Qu glutinous rice Huangjiu, is usually made using WYQ and GTQ as starters (Liu et al., 2018b; Lv et al., 2012). The 10 genera with the highest relative abundance in the starters were Burkholderia, Ochrobactrum, Bacillus, Staphylococcus, Erwinia, Agrobacterium, Klebsiella, Shewanella, Lactococcus, and Acinetobacter. Bacillus and Burkholderia were the most abundant species in WYQ, with relative abundances of about 45% and 8%, respectively. Burkholderia was the most abundant species in GTQ, with a relative abundance of 72% (Liu et al., 2018b; Lv et al., 2013a, Lv et al., 2013b). The 10 most abundant fungal species identified from the Unite database were identified as Monascus purpureus, Aspergillus sp., Saccharomyces sp., Agaricomycetes sp., Aspergillus flavus, Eurotiomycetes sp., Rhizopus microsporus, Rhizopus oryzae, Aspergillus niger and Fusarium pseudensiforme. M. purpureus was the most abundant in WYQ and GTQ, accounting for 56 ± 16.0% and 98 ± 0.8%, respectively (Liu et al., 2018b). The bacterial composition of 20 rice wine koji (a type of starter culture) from the Xiaogan area in Hubei province and Dazhu area in Sichuan province were also analyzed. Rice wine koji was mainly composed of Weissella, Lactobacillus, Lactococcus, Bacillus, Enterococcus and Cronobacter (Zhao et al., 2020b).
In other Asian countries rice wine fermentation, such as Japanese sake fermentation, is dominated by Saccharomyces cerevisiae, koji mold (mainly Aspergillus oryzae) and LAB (Tsuji et al., 2018). The main fungi in the fermentation of traditional Indian rice wine are Mucor circinelloides, Rhizopus delemar, and Meyerozyma guilliermondii,Aspergillus sp., Saccharomyces cerevisiae, Ethanol producers i.e.., Candida glabrata, Wickerhamomyces ciferrii, Debaryomyces hansenii, Ogataea parapolymorpha, and Dekkera bruxellensis. The LABs include Weissella cibaria, Lactobacillus plantarum, Lactococcus lactis, Leuconostoc lactis, Lactobacillus brevis, Weissella para mesenteroides, Leuconostoc pseudomesenteroides (Bora et al., 2016; Jeyaram et al., 2008). In summary, the Huangjiu fermentation microbiome is mainly derived from the starter and the saccharifying agent, so the use of different starters allows considerable control over the microbiome composition and the course of the fermentation.
4.1. Main research methods used to determine the composition of the Huangjiu microbiome
The Huangjiu microbiome is hugely diverse and can contain hundreds, or even thousands of microbial species and strains (Mo et al., 2010). To study the microbial composition of Huangjiu, the available methods include traditional culture and non-culture methods, genomics methods and flow cytometry (FCM) (Laplace Builhé, Hahne, Hunger, Tirilly and Drocourt, 1993; Longin et al., 2017). The conventional microbiological methods use different growth media for separation and counting and can distinguish the composition of different microbial populations and quantify isolated cultures, however, this method is time-consuming and laborious, with low reliability. Non-culture methods include deformation gradient gel electrophoresis (DGGE), real-time fluorescent quantitative PCR (qPCR) and fluorescence in situ hybridization (FISH) (Table 2) (Wei et al., 2018). These methods can detect dynamic changes in microbial composition during the fermentation, but only the more abundant species. For example, the DGGE method cannot detect species with a population density lower than 103 CFU/mL and the qPCR method is limited to the quantification of a few target species, which prevents a comprehensive understanding of the microbiome (Wei et al., 2018).
Table 2.
Research methods, classification and functions of main microorganisms in Huangjiu.
| Research method | Classification | Affect flavor | Safety risk |
|---|---|---|---|
| New methods | Moulds: Rhizopus oryzae, Mucor circinelloides, Mucor indicus, Rhizopus microspores, Aspergillus oryzae, Aspergillus flavus, Monascus purpureus | Moulds: Mucor indicus, Rhizopus oryzae | |
| Metagenomic sequencing | |||
| High-throughput sequencing (HTS) | |||
| 454 Pyrosequencing | Yeast: Saccharomyces cerevisiae, Saccharomycopsis fibuligera, Pichia guilliermondii, Wickerhamomyces anomalus, Candida glabrata, Pichia fabianii | Yeast: Saccharomyces cerevisiae, Pichia guilliermondii, Wickerhamomyces anomalus | Yeast: Saccharomyces cerevisiae |
| Flow cytometry (FCM) | |||
| Traditional methods | |||
| Restriction fragment length polymorphism (RFLP) | |||
| Random amplified polymorphic analysis (RAPD) | Bacteria: Janthinobacterium lividum, Lactobacillus plantarum, Weissella soli, Leuconostoc mesenteroides, Lactobacillus brevis, Pediococcus pentosaceus, Lactococcus lactis subsp. lactis, Pediococcus acidilactici, Bacillus subtilis, Bacillus aryabhattai and Bacillus megaterium ra | Bacteria: Lactobacillus plantarum, Aspergillus niger, Lactobacillus crispatus, Lactobacillus acidipiscis, Lactobacillus alimentarius occus | Bacteria: Pediococcus parvulus, Lactobacillus hilgardii, Enterococcus faecalis, Lactobacillus brevis, Lactococcus lactis, Staphylococcus xylosus |
| Denaturing gradient gel electrophoresis (DGGE) | |||
| Real-time fluorescence PCR | |||
| Fluorescence in situ hybridization (FISH) |
Currently, the most widely used methods are metagenomic sequencing and high-throughput sequencing (HTS) (Fig. 4). Metagenomics, first proposed by Handelman, is a method to study directly, the whole genome information contained in the microbiome (Handelsman et al., 1998). Kevin et al. (Chen and Pachter, 2005) defined metagenomics as “bypassing the isolation and cultivation of microbial individuals and applying genomics techniques to study microbial communities in the natural environment”. It avoids the isolation and cultivation of individual species and provides a way to study the microorganisms that cannot be isolated and cultured. It more accurately reflects the diversity and interactions of microbial species and strains, even at the molecular level, and the metabolic pathways and cellular gene prediction functions (Tringe and Rubin, 2005). Therefore, the metagenomic method can be used to reveal microbial diversity and provide a deeper understanding of the metabolic capacity of the microbiome (Liu et al., 2019; Wei et al., 2018). Generally, after extracting the total DNA from the samples, sequencing and bioinformatics analysis are performed (Wei et al., 2018) and metagenomic sequencing has been widely used to study microbial populations and functions in fermented foods (De Filippis, Parente and Ercolini, 2017; Liu et al., 2019), such as Huangjiu (Wang et al., 2014a), soy sauce (Wang et al., 2017), dry sausages (Hu et al., 2021), traditional Korean bean paste and kimchi (Jeong et al., 2014; Jung et al., 2011), and traditional fermented fish sauce (Shen et al., 2021). These studies used metagenomic sequencing to provide a theoretical basis for analyzing the relationship between microbial populations and specific flavors in these fermented foods.
Fig. 4.
Summary of metagenomic and high-throughput sequencing workflow.
Identification of microbial species in Huangjiu, by metagenomics and high-throughput sequencing. The general sequencing steps are: (I) collecting samples, DNA extraction and detection; (II) library construction and quality inspection; (III) sequence pretreatment and assembly; (IV) final bioinformatics analysis and validation.
HTS brought about a revolutionary change to conventional sequencing and can sequence hundreds of thousands to millions of DNA molecules at a time. HTS can construct the transcriptome of a species and provide a detailed analysis of the whole genome, also known as “deep sequencing” (Cao et al., 2017). By providing a large amount of detailed and low cost data, HTS has made it possible to understand microbial diversity on a larger scale (Wei et al., 2018). HTS has been widely used in the analysis of microbiome composition and dynamic compositional changes for both culturable and non-culturable microorganisms, for example, 454 pyrosequencing which can accurately characterize microbial diversity in complex environmental ecosystems, including food samples (Portillo and Mas, 2016). In recent years, HTS technology has been used to determine the microbial diversity in wines. The use of the bacterial 16S rRNA gene and the fungal ITS1-5.8S rRNA-ITS2 gene is considered the gold standard for assessing microbiome diversity (Morgan et al., 2017). HTS technology has had a profound impact on the field of microbiology, especially for the sequencing of genomes to identify microbial isolates, overcoming the limitations of dependence on microbial culture. Therefore, HTS will be used in the future, in combination with other methods to determine the specific role of bacteria in foods and improve understanding of microbial changes in food (Ercolini, 2013).
4.2. Classification of key microorganisms in the Huangjiu fermentation
Microbial classification is based on each species’ morphological characteristics, physiological and biochemical characteristics, and ribosomal ribonic acid (rRNA) (Table 2). Conventional microbial classifications rely on the morphological characteristics of colonies and tests for identifying specific species, but these methods can only detect clearly visible differences (Hibbett et al., 2016; Juste et al., 2014), so microbial diversity has always been a problem in taxonomy. With the development of next-generation sequencing technology, many microorganisms that cannot be cultured have been discovered and classified (Lv et al., 2013b; Tsai et al., 2019). HTS and metagenomic sequencing technologies have become the main techniques used to classify microorganisms in rice wine. The analysis of marker gene sequences is a key step in sequence-based classification methods of microbial taxonomy (Hibbett et al., 2016); generally, 16S rRNA sequences are used to classify bacteria and ITS region sequences to classify fungi, because these gene sequences are highly conserved. Sequence analysis has revealed that the microbiome of rice wine is composed of yeast (mainly Saccharomyces cerevisiae), moulds (mainly A. oryzae, R. oryzae, M. purpureus) and bacteria (mainly Bacillus and LAB, including Lactobacillus plantarum, Lactobacillus brevis, Pediococcus pentosaceus and Weissella soli) (Huang et al., 2018; Lv et al., 2013a, Lv et al., 2013b). Most of the major microbial species in Huangjiu fermentation have now been identified and classified.
4.3. Key microorganisms affecting the flavor of Huangjiu
The complex mixture of flavor compounds in Huangjiu, which contains hundreds of substances, such as esters, alcohols, aldehydes, organic acids and amino acids, is derived from microbial metabolites of the ingredients (Liu et al., 2019; Wang et al., 2014a). During Huangjiu fermentation, the microorganisms form a complex symbiotic relationship, thus forming the unique flavor. Yang et al. used different starters to study the relationship between flavor development and microbial metabolism in Chinese Huangjiu; S. cerevisiae was the main ethanol-producer and there were significant differences in the types and quantities of alcohols and esters produced by different S. cerevisiae strains (Yang et al., 2018; Yang et al., 2017). Wang et al. studied the bacteria and flavor characteristics of Shaoxing Huangjiu; Lactobacillus and Bacillus were the main acid-producing bacteria and contribute to the development of Huangjiu flavor (Wang et al., 2014a). To evaluate the effect of bacteria on the formation of volatile compounds, the relationship between microbiome composition and volatile compound formation during fermentation was analyzed by partial least squares regression; Pseudomonas, Bacillus, Lactococcus and Thermoactinomyces all participated in the biosynthesis of volatile compounds (Table 2).
Huang et al. explored the relationship between the microbiome and flavor biosynthesis, using different starters to brew Hong Qu Huangjiu; the abundance of Lactobacillus plantarum, Lactobacillus alimentarius, Lactobacillus brevis, Aspergillus correlated positively with production of acidic compounds (such as 2-methylvaleric acid, 2-methylpropionic acid, phenylacetic acid and 2-heptenoic acid) and Candida sp., Pichia sp., Monascus purpureus, Lactobacillus alimentarius, Lactobacillus brevis correlated positively with production of esters with fruit and floral aromas (such as ethyl butyrate, ethyl stearate, N-caproic acid vinyl ester and phenethyl acetate) (Huang et al., 2019). Amino acids are important nutrients and flavor precursors in Huangjiu. They provide a nitrogen source for the growth of microorganisms during the fermentation process and contribute to the quality. Principal components analysis and hierarchical clustering analysis revealed that the bitter amino acids were positively correlated with Pediococcus, Saccharomyces, Lactobacillus, Monascus, and Halomonas in Hong Qu glutinous rice Huangjiu (Liang et al., 2020). These findings indicate that the microbiome of Huangjiu is instrumental in the formation of its unique flavor profile.
4.4. Key microorganisms affecting the safety of Huangjiu
As discussed above, the main potential hazards in Huangjiu are ethyl carbamate (EC) and biogenic amines (BAs). The metabolic pathways that produce these toxins are discussed below. In the industrial fermentation process, S. cerevisiae is the main producer of ethanol and flavor substances, but it also produces EC. In the Huangjiu fermentation, the precursors of EC are urea and ethanol, both of which can be produced by S. cerevisiae (Table 2) (Wei et al., 2020). Urea is mainly produced by the arginase degradation pathway, which converts arginine to ornithine. When the urea concentration is high, it becomes toxic to S. cerevisiae, so it is excreted to the outside of the cell (Fig. 3A).
BAs are produced by decarboxylation of the corresponding amino acids by LABs. In rice wine, histamine, the most toxic BA, is synthesized by decarboxylation of histidine by Pediococcus parvulus and Lactobacillus hilgardii (Landete et al., 2005). Putrescine is biosynthesized via the agmatine deamination pathway by Enterococcus faecalis, Enterococcus durans and Enterococcus faecium (Ladero et al., 2012). Lactococcus lactis can produce putrescine in dairy products, and its formation can be inhibited by glucose and lactose (del Rio et al., 2015). Tyramine is produced by decarboxylation of tyrosine, by Lactobacillus brevis IOEB 9809 (Moreno-Arribas and Lonvaud-Funel, 2001).Luo et al. used the gene predictive function method to calculate the relative microbial contributions to amino acid decarboxylase production during Huangjiu fermentation; Citrobacter, Acinetobacter, Lactobacillus, Exiguobacterium, Bacillus, Pseudomonas, and Enterobacter spp. were the main contributors (Luo et al., 2020). Therefore, the selection of safe microbial starters is of great importance to minimizing toxin production during Huangjiu fermentation.
4.5. Genetic breeding of key microorganisms to improve Huangjiu fermentation
Microbial genetic breeding is an effective method to engineer a strain with specific production characteristics, to improve the yield and quality of a fermentation product. Genetic breeding has been applied to animals, plants and microorganisms. Among the microorganisms in Huangjiu fermentation, the strain most needing genetic breeding is S. cerevisiae. In recent years, the breeding program for yeast has mainly focused on two methods, mutation breeding and cross breeding (Naumov et al., 2006). S. cerevisiae is a diploid yeast with a high degree of clonal reproduction. However, the internal genetic variation of S. cerevisiae is shaped by the complex historical effects of past cross-breeding and incorporation of exogenous genes; morphological changes are independent of population structure, geographic origin and source environment, suggesting that morphological differentiation occurs more rapidly within species. This suggests that rapid and unpredictable phenotypic changes can be detected by high dimensional phenotypes after each breeding step (Zheng et al., 2013). High dimensional, single-cell and morphological phenotypes were used to study the phenotype and differentiation of 27 sake yeasts, to elucidate their genetic breeding processes. Different breeding approaches indicate that crossbreeding of cell morphological changes has a greater effect than mutation breeding. In addition, morphological analysis showed that some sake yeast strains had strong morphological heterogeneity (Ohnuki et al., 2017).
4.6. The relationship between microbes in Huangjiu fermentation
The interaction between microbial is an important factor affecting the structure of microbial (Zhao et al., 2020a). Any complex microbiome is a dynamic equilibrium between species, involving extensive competition, but also some cooperation, for example, if one species can metabolize a waste product from another. There are two ways for organisms to compete, exploitative competition, i.e., indirect competition over consumption of limited resources, and disruptive competition, involving direct conflict with competitors, for example by producing toxins to attack them (France and Remold, 2016). Microbial community is unevenly distributed in the environment and exhibit biogeographical patterns. In large organisms, these biogeographic patterns are determined by biological factors such as competition between species, predation and parasitism, as well as abiotic factors such as temperature range, nutrients, and biodistribution (France and Remold, 2016). Studies have shown that in the process of Huangjiu fermentation, not only bacteria have a mutual influence relationship, but also fungi and fungi, bacteria and fungi have mutual influence. There is a cooperative or competitive relationship between them. This makes researchers believe that the cooperation or competition between microbial communities has a significant contribution to enriching the flavor of rice wine (Liang et al., 2020; Zhao et al., 2020a).
5. Conclusions and perspectives
The Huangjiu fermentation microbiome and its metabolites are critical to the formation of flavor and aroma compounds and the unique sensory characteristics of the final product. Recent studies have revealed that the relationship between microbial metabolism and flavor compounds. The main research effort has been on the influence of the predominant microbial species on fermentation and the relationship between these species and potentially hazardous compounds in Huangjiu. However, there is less knowledge and understanding of the contribution from microorganisms with relatively low abundances. Future work should aim to adopt a multi-omics approach to improve understanding of Huangjiu fermentation. This approach may identify biological pathways for the formation of specific flavor aroma compounds, which is relevant, not only to Huangjiu research, but also to production of other fermented foods.
CRediT authorship contribution statement
Shufang Tian: arranged the text contents, literatures, Writing – original draft. Weizhu Zeng: Data curation, created the tables. Fang Fang: Supervision, Validation. Jingwen Zhou: Supervision, Funding acquisition, Writing – review & editing. Guocheng Du: Supervision, Validation, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by The National Key Research and Development Program of China (2017YFC1600403, 2018YFC1604102), the Foundation for Innovative Research Groups of the National Natural Science Foundation of China (32021005), the National Science Fund for Excellent Young Scholars (21822806), the Fundamental Research Funds for the Central Universities (JUSRP51701A), the National First-class Discipline Program of Light Industry Technology and Engineering (LITE2018-08), the Distinguished Professor Project of Jiangsu Province, and the 111 Project (111-2-06).
Contributor Information
Jingwen Zhou, Email: zhoujw1982@jiangnan.edu.cn.
Guocheng Du, Email: gcdu@jiangnan.edu.cn.
References
- Ai q., Xu X., Jin Z. Research progress on the brewing techniques of new-type rice wine. Food Chem. 2017;215:508–515. doi: 10.1016/j.foodchem.2016.08.014. [DOI] [PubMed] [Google Scholar]
- Bora S.S., Keot J., Das S., Sarma K., Barooah M. Metagenomics analysis of microbial communities associated with a traditional rice wine starter culture (Xaj-pitha) of Assam, India. 3 Biotech. 2016;6(2) doi: 10.1007/s13205-016-0471-1. 153-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai H., Zhang T., Zhang Q., Luo J., Cai C., Mao J. Microbial diversity and chemical analysis of the starters used in traditional Chinese sweet rice wine. Food Microbiol. 2018;73:319–326. doi: 10.1016/j.fm.2018.02.002. [DOI] [PubMed] [Google Scholar]
- Callejón S., Sendra R., Ferrer S., Pardo I. Identification of a novel enzymatic activity from lactic acid bacteria able to degrade biogenic amines in wine. Appl. Microbiol. Biotechnol. 2014;98(1):185–198. doi: 10.1007/s00253-013-4829-6. [DOI] [PubMed] [Google Scholar]
- Cao Y., Fanning S., Proos S., Jordan K., Srikumar S. A review on the applications of next generation sequencing technologies as applied to food-related microbiome studies. Front. Microbiol. 2017;8:1829. doi: 10.3389/fmicb.2017.01829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C., Liu Y., Tian H.X., Ai L.Z., Yu H.Y. Metagenomic analysis reveals the impact of JIUYAO microbial diversity on fermentation and the volatile profile of Shaoxing-jiu. Food Microbiol. 2020;86:103326. doi: 10.1016/j.fm.2019.103326. [DOI] [PubMed] [Google Scholar]
- Chen D.W., Ren Y.P., Zhong Q.D., Shao Y., Zhao Y.F., Wu Y.N. Ethyl carbamate in alcoholic beverages from China: levels, dietary intake, and risk assessment. Food Control. 2017;72:283–288. doi: 10.1016/j.foodcont.2015.10.047. [DOI] [Google Scholar]
- Chen K., Pachter L. Bioinformatics for whole-genome shotgun sequencing of microbial communities. PLoS Comput. Biol. 2005;1(2):106–112. doi: 10.1371/journal.pcbi.0010024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S., Wang C., Qian M., Li Z., Xu Y. Characterization of the key aroma compounds in aged Chinese rice wine by comparative aroma extract dilution analysis, quantitative measurements, aroma recombination, and omission studies. J. Agric. Food Chem. 2019;67(17):4876–4884. doi: 10.1021/acs.jafc.9b01420. [DOI] [PubMed] [Google Scholar]
- Chen S., Wang D., Xu Y. Characterization of odor-active compounds in sweet-type Chinese rice wine by aroma extract dilution analysis with special emphasis on sotolon. J. Agric. Food Chem. 2013;61(40):9712–9718. doi: 10.1021/jf402867m. [DOI] [PubMed] [Google Scholar]
- Chen S., Xu Y. Adaptive evolution of Saccharomyces cerevisiae with enhanced ethanol tolerance for Chinese rice wine fermentation. Appl. Biochem. Biotechnol. 2014;173(7):1940–1954. doi: 10.1007/s12010-014-0978-z. [DOI] [PubMed] [Google Scholar]
- De Filippis F., Parente E., Ercolini D. Metagenomics insights into food fermentations. Microbial. Biotechnol. 2017;10(1):91–102. doi: 10.1111/1751-7915.12421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehaut A., Himber C., Mulak V., Grard T., Krzewinski F., Le Fur B., Duflos G. Evolution of volatile compounds and biogenic amines throughout the shelf life of marinated and salted anchovies (Engraulis encrasicolus) J. Agric. Food Chem. 2014;62(32):8014–8022. doi: 10.1021/jf5021736. [DOI] [PubMed] [Google Scholar]
- del Rio B., Ladero V., Redruello B., Linares D.M., Fernández M., Martín M.C., Alvarez M.A. Lactose-mediated carbon catabolite repression of putrescine production in dairy Lactococcus lactis is strain dependent. Food Microbiol. 2015;48:163–170. doi: 10.1016/j.fm.2014.11.018. [DOI] [PubMed] [Google Scholar]
- Ercolini D. High-throughput sequencing and metagenomics: moving forward in the culture-independent analysis of food microbial ecology. Appl. Environ. Microbiol. 2013;79(10):3148. doi: 10.1128/AEM.00256-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang R., Zhou W., Chen Q. Ethyl carbamate regulation and genomic expression of Saccharomyces cerevisiae during mixed-culture yellow rice wine fermentation with Lactobacillus sp. Food Chem. 2019;292:90–97. doi: 10.1016/j.foodchem.2019.04.014. [DOI] [PubMed] [Google Scholar]
- France M.T., Remold S.K. Interference competition among household strains of Pseudomonas. Environ. Microbiol. 2016;72(4):821–830. doi: 10.1007/s00248-015-0652-1. [DOI] [PubMed] [Google Scholar]
- Handelsman J., Rondon M.R., Brady S.F., Clardy J., Goodman R.M. Molecular biological access to the chemistry of unknown soil microbes: a new frontier for natural products. Chem. Biol. 1998;5(10):245–249. doi: 10.1016/S1074-5521(98)90108-9. [DOI] [PubMed] [Google Scholar]
- He S., Mao X.Z., Liu P., Lin H., Du Z.Y., Lv N., Han J.C., Qiu C.F. Research into the functional components and antioxidant activities of North China rice wine (Ji Mo Lao Jiu) Int. J. Food Sci. Nutr. 2013;1(4):307–314. doi: 10.1002/fsn3.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibbett D., Abarenkov K., Kõljalg U., Öpik M., Chai B., Cole J., Wang Q., Crous P., Robert V., Helgason T., Herr J.R., Kirk P., Lueschow S., O’Donnell K., Nilsson R.H., Oono R., Schoch C., Smyth C., Walker D.M., Porras-Alfaro A., Taylor J.W., Geiser D.M. Sequence-based classification and identification of Fungi. Mycologia. 2016;108(6):1049–1068. doi: 10.3852/16-130. [DOI] [PubMed] [Google Scholar]
- Hu K., Jin G., Xu Y., Xue S., Qiao S., Teng Y., Tao Y. Enhancing wine ester biosynthesis in mixed Hanseniaspora uvarum/Saccharomyces cerevisiae fermentation by nitrogen nutrient addition. Food Res. Int. 2019;123:559–566. doi: 10.1016/j.foodres.2019.05.030. [DOI] [PubMed] [Google Scholar]
- Hu Y.Y., Wang H., Kong B.H., Wang Y., Chen Q. The succession and correlation of the bacterial community and flavour characteristics of Harbin dry sausages during fermentation. LWT - Food Sci. Technol. (Lebensmittel-Wissenschaft -Technol.) 2021;138:110689. doi: 10.1016/j.lwt.2020.110689. [DOI] [Google Scholar]
- Huang Z., Guo W., Zhou W., Li L., Xu J., Hong J., Liu H., Zeng F., Bai W., Liu B., Ni L., Rao P., Lv X. Microbial communities and volatile metabolites in different traditional fermentation starters used for Hong Qu glutinous rice wine. Food Res. Int. 2019;121:593–603. doi: 10.1016/j.foodres.2018.12.024. [DOI] [PubMed] [Google Scholar]
- Huang Z., Hong J., Xu J., Li L., Guo W., Pan Y., Chen S., Bai W., Rao P., Ni L., Zhao L., Liu B., Lv X. Exploring core functional microbiota responsible for the production of volatile flavour during the traditional brewing of Wuyi Hong Qu glutinous rice wine. Food Microbiol. 2018;76:487–496. doi: 10.1016/j.fm.2018.07.014. [DOI] [PubMed] [Google Scholar]
- Jeong D.W., Kim H.R., Jung G., Han S., Kim C.T., Lee J.H. Bacterial community migration in the ripening of Doenjang, a traditional Korean fermented soybean food. J. Microbiol. Biotechnol. 2014;24(5):648–660. doi: 10.4014/jmb.1401.01009. [DOI] [PubMed] [Google Scholar]
- Jeyaram K., Singh W.M., Capece A., Romano P. Molecular identification of yeast species associated with ‘Hamei’ — a traditional starter used for rice wine production in Manipur, India. Int. J. Food Microbiol. 2008;124(2):115–125. doi: 10.1016/j.ijfoodmicro.2008.02.029. [DOI] [PubMed] [Google Scholar]
- Jiang L., Su W., Mu Y., Mu Y. Major metabolites and microbial community of fermented black glutinous rice wine with different starters. Front. Microbiol. 2020;11 doi: 10.3389/fmicb.2020.00593. 593-593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung J.Y., Lee S.H., Kim J.M., Park M.S., Bae J.W., Hahn Y., Madsen E.L., Jeon C.O. Metagenomic analysis of kimchi, a traditional Korean fermented food. Appl. Environ. Microbiol. 2011;77(7):2264. doi: 10.1128/AEM.02157-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juste A., Malfliet S., Waud M., Crauwels S., De Cooman L., Aerts G., Marsh T.L., Ruyters S., Willems K., Busschaert P., Lievens B. Bacterial community dynamics during industrial malting, with an emphasis on lactic acid bacteria. Food Microbiol. 2014;39:39–46. doi: 10.1016/j.fm.2013.10.010. [DOI] [PubMed] [Google Scholar]
- Kim N.H., Jun S.H., Lee S.H., Hwang I.G., Rhee M.S. Microbial diversities and potential hazards of Korean turbid rice wines (makgeolli): multivariate analyses. Food Microbiol. 2018;76:466–472. doi: 10.1016/j.fm.2018.07.008. [DOI] [PubMed] [Google Scholar]
- Ladero V., Fernández M., Calles Enríquez M., Sánchez Llana E., Cañedo E., Martín M.C., Alvarez M.A. Is the production of the biogenic amines tyramine and putrescine a species-level trait in enterococci? Food Microbiol. 2012;30(1):132–138. doi: 10.1016/j.fm.2011.12.016. [DOI] [PubMed] [Google Scholar]
- Lai Q., Li Y., Wu Y., Ouyang J. The quality of rice wine influenced by the crystal structure of rice starch. J. Food Sci. Technol. 2019;56(4):1988–1996. doi: 10.1007/s13197-019-03667-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landete J.M., Ferrer S., Pardo I. Which lactic acid bacteria are responsible for histamine production in wine? J. Appl. Microbiol. 2005;99(3):580–586. doi: 10.1111/j.1365-2672.2005.02633.x. [DOI] [PubMed] [Google Scholar]
- Laplace Builhé C., Hahne K., Hunger W., Tirilly Y., Drocourt J.L. Application of flow cytometry to rapid microbial analysis in food and drinks industries. Biol. Cell. 1993;78(1‐2):123–128. doi: 10.1016/0248-4900(93)90122-U. [DOI] [PubMed] [Google Scholar]
- Li J., Zhou L., Feng W., Cheng H., Muhammad A.I., Ye X., Zhi Z. Comparison of biogenic amines in Chinese commercial soy sauces. Molecules. 2019;24(8):1522. doi: 10.3390/molecules24081522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Zou D., Ruan L., Wen Z., Chen S., Xu L., Wei X. Evaluation of the biogenic amines and microbial contribution in traditional Chinese sausages. Front. Microbiol. 2019;10 doi: 10.3389/fmicb.2019.00872. 872-872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Zhang W., Zheng D., Zhou Z., Yu W., Zhang L., Feng L., Liang X., Guan W., Zhou J., Chen J., Lin Z. Genomic evolution of Saccharomyces cerevisiae under Chinese rice wine fermentation. Genome Biol.evol. 2014;6(9):2516–2526. doi: 10.1093/gbe/evu201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Z., Lin X., He Z., Su H., Li W., Ren X. Amino acid and microbial community dynamics during the fermentation of Hong Qu glutinous rice wine. Food Microbiol. 2020;90:103467. doi: 10.1016/j.fm.2020.103467. [DOI] [PubMed] [Google Scholar]
- Liu D., Zhang H., Xiong W., Hu J., Xu B., Lin C., Xu L., Jiang L. BioMed research international; 2014. Effect of Temperature on Chinese Rice Wine Brewing with High Concentration Presteamed Whole Sticky Rice; p. 426929. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q., Yao X., Liang Q., Li J., Fang F., Du G., Kang Z. Molecular engineering of Bacillus paralicheniformis acid urease to degrade urea and ethyl carbamate in model Chinese rice wine. J. Agric. Food Chem. 2018;66(49):13011–13019. doi: 10.1021/acs.jafc.8b04566. [DOI] [PubMed] [Google Scholar]
- Liu S., Chen Q., Zou H., Yu Y., Zhou Z., Mao J., Zhang S. A metagenomic analysis of the relationship between microorganisms and flavor development in Shaoxing mechanized huangjiu fermentation mashes. Int. J. Food Microbiol. 2019;303:9–18. doi: 10.1016/j.ijfoodmicro.2019.05.001. [DOI] [PubMed] [Google Scholar]
- Liu S., Yu J., Wei X., Ji Z., Zhou Z., Meng X., Mao J. Sequencing-based screening of functional microorganism to decrease the formation of biogenic amines in Chinese rice wine. Food Control. 2016;64:98–104. doi: 10.1016/j.foodcont.2015.12.013. [DOI] [Google Scholar]
- Liu S.P., Mao J., Liu Y.Y., Meng X.Y., Ji Z.W., Zhou Z.L., Ai Lati A. Bacterial succession and the dynamics of volatile compounds during the fermentation of Chinese rice wine from Shaoxing region. World J. Microbiol. Biotechnol. 2015;31(12):1907–1921. doi: 10.1007/s11274-015-1931-1. [DOI] [PubMed] [Google Scholar]
- Liu Z.B., Wang Z.Y., Lv X.C., Zhu X.P., Chen L.L., Ni L. Comparison study of the volatile profiles and microbial communities of Wuyi Qu and Gutian Qu, two major types of traditional fermentation starters of Hong Qu glutinous rice wine. Food Microbiol. 2018;69:105–115. doi: 10.1016/j.fm.2017.07.019. [DOI] [PubMed] [Google Scholar]
- Longin C., Petitgonnet C., Guilloux-Benatier M., Rousseaux S., Alexandre H. Application of flow cytometry to wine microorganisms. Food Microbiol. 2017;62:221–231. doi: 10.1016/j.fm.2016.10.023. [DOI] [PubMed] [Google Scholar]
- Luo Y., Huang Y., Xu R.X., Qian B., Zhou J.W., Xia X.L. Primary and secondary succession mediate the accumulation of biogenic amines during industrial semidry Chinese rice wine fermentation. Appl. Environ. Microbiol. 2020;86(17):e01177–1120. doi: 10.1128/AEM.01177-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv X., Cai Q., Ke X., Chen F., Rao P., Ni L. Characterization of fungal community and dynamics during the traditional brewing of Wuyi Hong Qu glutinous rice wine by means of multiple culture-independent methods. Food Control. 2015;54:231–239. doi: 10.1016/j.foodcont.2015.01.046. [DOI] [Google Scholar]
- Lv X., Chen Z., Jia R., Liu Z., Zhang W., Chen S., Rao P., Ni L. Microbial community structure and dynamics during the traditional brewing of Fuzhou Hong Qu glutinous rice wine as determined by culture-dependent and culture-independent techniques. Food Control. 2015;57:216–224. doi: 10.1016/j.foodcont.2015.03.054. [DOI] [Google Scholar]
- Lv X., Huang R., Chen F., Zhang W., Rao P., Ni L. Bacterial community dynamics during the traditional brewing of Wuyi Hong Qu glutinous rice wine as determined by culture-independent methods. Food Control. 2013;34(2):300–306. doi: 10.1016/j.foodcont.2013.05.003. [DOI] [Google Scholar]
- Lv X., Huang X., Zhang W., Rao P., Ni L. Yeast diversity of traditional alcohol fermentation starters for Hong Qu glutinous rice wine brewing, revealed by culture-dependent and culture-independent methods. Food Control. 2013;34(1):183–190. doi: 10.1016/j.foodcont.2013.04.020. [DOI] [Google Scholar]
- Lv X., Huang Z., Zhang W., Rao P., Ni L. Identification and characterization of filamentous fungi isolated from fermentation starters for Hong Qu glutinous rice wine brewing. J. Appl. Microbiol. 2012;58(1):33–42. doi: 10.2323/jgam.2358.2333. [DOI] [PubMed] [Google Scholar]
- Lv X.C., Jiang Y.J., Liu J., Guo W.L., Liu Z.B., Zhang W., Rao P.F., Ni L. Evaluation of different PCR primers for denaturing gradient gel electrophoresis (DGGE) analysis of fungal community structure in traditional fermentation starters used for Hong Qu glutinous rice wine. Int. J. Food Microbiol. 2017;255:58–65. doi: 10.1016/j.ijfoodmicro.2017.05.010. [DOI] [PubMed] [Google Scholar]
- Lyons D.E., Beery J.T., Lyons S.A., Taylor S.L. Cadaverine and aminoguanidine potentiate the uptake of histamine in vitro in perfused intestinal segments of rats. Toxicol. Appl. Pharmacol. 1983;70(3):445–458. doi: 10.1016/0041-008x(83)90162-x. [DOI] [PubMed] [Google Scholar]
- Mo X., Xu Y., Fan W. Characterization of aroma compounds in Chinese rice wine Qu by solvent-assisted flavor evaporation and headspace solid-phase microextraction. J. Agric. Food Chem. 2010;58(4):2462–2469. doi: 10.1021/jf903631w. [DOI] [PubMed] [Google Scholar]
- Moreno-Arribas V., Lonvaud-Funel A. Purification and characterization of tyrosine decarboxylase of Lactobacillus brevis IOEB 9809 isolated from wine. FEMS (Fed. Eur. Microbiol. Soc.) Microbiol. Lett. 2001;195(1):103–107. doi: 10.1111/j.1574-6968.2001.tb10505.x. [DOI] [PubMed] [Google Scholar]
- Morgan H.H., du Toit M., Setati M.E. The grapevine and wine microbiome: insights from high-throughput amplicon sequencing. Front. Microbiol. 2017;8 doi: 10.3389/fmicb.2017.00820. 820-820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naila A., Flint S., Fletcher G., Bremer P., Meerdink G. Control of biogenic amines in food--existing and emerging approaches. J. Food Sci. 2010;75(7):139–150. doi: 10.1111/j.1750-3841.2010.01774.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naumov G.I., Naumova E.S., Kondratieva V.I. The use of hybridization in breeding of eukaryotic microorganisms. Genetika. 2006;42(11):1571–1576. doi: 10.1134/s1022795406110147. [DOI] [PubMed] [Google Scholar]
- Niu T., Li X., Guo Y., Ma Y. Identification of a lactic acid bacteria to degrade biogenic amines in Chinese rice wine and its enzymatic mechanism. Foods. 2019;8(8) doi: 10.3390/foods8080312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnuki S., Okada H., Friedrich A., Kanno Y., Goshima T., Hasuda H., Inahashi M., Okazaki N., Tamura H., Nakamura R., Hirata D., Fukuda H., Shimoi H., Kitamoto K., Watanabe D., Schacherer J., Akao T., Ohya Y. Phenotypic diagnosis of lineage and differentiation during sake yeast breeding. Genes Genomes Gene. 2017;7(8):2807–2820. doi: 10.1534/g3.117.044099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park K.H., Liu Z., Park C.S., Ni L. Microbiota associated with the starter cultures and brewing process of traditional Hong Qu glutinous rice wine. Food Sci. Biotechnol. 2016;25(3):649–658. doi: 10.1007/s10068-016-0115-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portillo M.d.C., Mas A. Analysis of microbial diversity and dynamics during wine fermentation of Grenache grape variety by high-throughput barcoding sequencing. LWT - Food Sci. Technol. (Lebensmittel-Wissenschaft -Technol.) 2016;72:317–321. doi: 10.1016/j.lwt.2016.05.009. [DOI] [Google Scholar]
- Qin O., Chen Q., Zhao J. Intelligent sensing sensory quality of Chinese rice wine using near infrared spectroscopy and nonlinear tools. Spectrochim. Acta Mol. Biomol. Spectrosc. 2016;154(5):42–46. doi: 10.1016/j.saa.2015.10.011. [DOI] [PubMed] [Google Scholar]
- Ren Q., Sun L., Wu H., Wang Y., Wang Z., Zheng F., Lu X., Xu J. The changes of microbial community and flavor compound in the fermentation process of Chinese rice wine using Fagopyrum tataricum grain as feedstock. Sci. Rep. 2019;9(1):3365. doi: 10.1038/s41598-019-40337-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y., Wu Y., Wang Y., Li L., Li C., Zhao Y., Yang S. Contribution of autochthonous microbiota succession to flavor formation during Chinese fermented Mandarin fish (Siniperca chuatsi) Food Chem. 2021;348:129107. doi: 10.1016/j.foodchem.2021.129107. [DOI] [PubMed] [Google Scholar]
- Son E.Y., Lee S.M., Kim M., Seo J.-A., Kim Y.-S. Comparison of volatile and non-volatile metabolites in rice wine fermented by Koji inoculated with Saccharomycopsis fibuligera and Aspergillus oryzae. Food Res. Int. 2018;109:596–605. doi: 10.1016/j.foodres.2018.05.008. [DOI] [PubMed] [Google Scholar]
- Song J., Luo J., Ma Z., Sun Q., Wu C., Li X. Quality and authenticity control of functional red yeast rice: a review. Molecules. 2019;24(10) doi: 10.3390/molecules24101944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira dos Santos C.A., Páscoa R.N.M.J., Porto P.A.L.S., Cerdeira A.L., González-Sáiz J.M., Pizarro C., Lopes J.A. Raman spectroscopy for wine analyses: a comparison with near and mid infrared spectroscopy. Talanta. 2018;186:306–314. doi: 10.1016/j.talanta.2018.04.075. [DOI] [PubMed] [Google Scholar]
- Tringe S.G., Rubin E.M. Metagenomics: DNA sequencing of environmental samples. Nat. Rev. Genet. 2005;6(11):805–814. doi: 10.1038/nrg1709. [DOI] [PubMed] [Google Scholar]
- Tsai M., Liu Y., Soo V., Chen C. A new genome-to-genome comparison approach for large-scale revisiting of current microbial taxonomy. Microorganisms. 2019;7(6):161. doi: 10.3390/microorganisms7060161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji A., Kozawa M., Tokuda K., Enomoto T., Koyanagi T. Robust domination of Lactobacillus sakei in microbiota during traditional Japanese sake starter Yamahai-moto fermentation and the accompanying changes in metabolites. Curr. Microbiol. 2018;75(11):1498–1505. doi: 10.1007/s00284-018-1551-8. [DOI] [PubMed] [Google Scholar]
- Wang H., Wei Q., Gui S., Feng Y., Zhang Y., Liu Y., Lu F. Metagenomic profiling of the bacterial community changes from koji to mash stage in the brewing of soy sauce. Pol. J. Microbiol. 2017;66(4):537–541. doi: 10.5604/01.3001.0010.7097. [DOI] [PubMed] [Google Scholar]
- Wang J., Yuan C., Gao X., Kang Y., Huang M., Wu J., Liu Y., Zhang J., Li H., Zhang Y. Characterization of key aroma compounds in Huangjiu from northern China by sensory-directed flavor analysis. Food Res. Int. 2020;134:109238. doi: 10.1016/j.foodres.2020.109238. [DOI] [PubMed] [Google Scholar]
- Wang P., Mao J., Meng X., Li X., Liu Y., Feng H. Changes in flavour characteristics and bacterial diversity during the traditional fermentation of Chinese rice wines from Shaoxing region. Food Control. 2014;44:58–63. doi: 10.1016/j.foodcont.2014.03.018. [DOI] [Google Scholar]
- Wang P.H., Sun J.Y., Li X.M., Wu D.H., Li T., Lu J., Chen J., Xie G.F. Contribution of citrulline to the formation of ethyl carbamate during Chinese rice wine production. Food Addit. Contam. 2014;31(4):587–592. doi: 10.1080/19440049.2013.878869. [DOI] [PubMed] [Google Scholar]
- Weber J.V., Sharypov V.I. Ethyl carbamate in foods and beverages: a review. Environ. Chem. Lett. 2009;7(3):233–247. doi: 10.1007/s10311-008-0168-8. [DOI] [Google Scholar]
- Wei T.Y., Jiao Z.H., Hu J.J., Lou H.H., Chen Q.H. Chinese yellow rice wine processing with reduced ethyl carbamate formation by deleting transcriptional regulator Dal80p in Saccharomyces cerevisiae. Molecules. 2020;25(16):3580. doi: 10.3390/molecules25163580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei X., Liu S., Yu J., Yu Y., Zhu S., Zhou Z., Hu J., Mao J. Innovation Chinese rice wine brewing technology by bi-acidification to exclude rice soaking process. J. Biosci. Bioeng. 2017;123(4):460–465. doi: 10.1016/j.jbiosc.2016.11.014. [DOI] [PubMed] [Google Scholar]
- Wei Y., Wu Y., Yan Y., Zou W., Xue J., Ma W., Wang W., Tian G., Wang L. High-throughput sequencing of microbial community diversity in soil, grapes, leaves, grape juice and wine of grapevine from China. PLoS One. 2018;13(3) doi: 10.1371/journal.pone.0193097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu P.G., Cai C.G., Shen X.H., Wang L.Y., Zhang J., Tan Y., Jiang W., Pan X.D. Formation of ethyl carbamate and changes during fermentation and storage of yellow rice wine. Food Chem. 2014;152:108–112. doi: 10.1016/j.foodchem.2013.11.135. [DOI] [PubMed] [Google Scholar]
- Xia Q., Yang C.J., Wu C.D., Zhou R.Q., Li Y.F. Quantitative strategies for detecting different levels of ethyl carbamate (EC) in various fermented food matrices: an overview. Food Control. 2018;84:499–512. doi: 10.1016/j.foodcont.2017.09.008. [DOI] [Google Scholar]
- Xia X.L., Luo Y., Zhang Q.W., Huang Y., Zhang B. Mixed starter culture regulates biogenic amines formation via decarboxylation and transamination during Chinese rice wine fermentation. J. Agric. Food Chem. 2018;66(25):6348–6356. doi: 10.1021/acs.jafc.8b01134.s001. [DOI] [PubMed] [Google Scholar]
- Xiang W., Xu Q., Zhang N., Rao Y., Zhu L., Zhang Q. Mucor indicus and Rhizopus oryzae co-culture to improve the flavor of Chinese turbid rice wine. J. Sci. Food Agric. 2019;99(12):5577–5585. doi: 10.1002/jsfa.9831. [DOI] [PubMed] [Google Scholar]
- Xu E., Long J., Wu Z., Li H., Wang F., Xu X., Jin Z., Jiao A. Characterization of volatile flavor compounds in Chinese rice wine fermented from enzymatic extruded rice. J. Food Sci. 2015;80(7):1476–1489. doi: 10.1111/1750-3841.12935. [DOI] [PubMed] [Google Scholar]
- Xu J., Wu H., Wang Z., Zheng F., Lu X., Li Z., Ren Q. Microbial dynamics and metabolite changes in Chinese Rice Wine fermentation from sorghum with different tannin content. Sci. Rep. 2018;8(1):4639. doi: 10.1038/s41598-018-23013-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan S., Chen X., Xiang X. Improvement of the aroma of lily rice wine by using aroma-producing yeast strain Wickerhamomyces anomalus HN006. Amb. Express. 2019;9(1):89. doi: 10.1186/s13568-019-0811-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Xia Y., Lin X., Wang G., Zhang H., Xiong Z., Yu H., Yu J., Ai L. Improvement of flavor profiles in Chinese rice wine by creating fermenting yeast with superior ethanol tolerance and fermentation activity. Food Res. Int. 2018;108:83–92. doi: 10.1016/j.foodres.2018.03.036. [DOI] [PubMed] [Google Scholar]
- Yang Y., Xia Y., Wang G., Yu J., Ai L. Effect of mixed yeast starter on volatile flavor compounds in Chinese rice wine during different brewing stages. Lwt-Food Science and Technology. 2017;78:373–381. doi: 10.1016/j.lwt.2017.01.007. [DOI] [Google Scholar]
- Yang Y.Q., Kang Z., Zhou J.L., Chen J., Du G.C. High-level expression and characterization of recombinant acid urease for enzymatic degradation of urea in rice wine. Appl. Microbiol. Biotechnol. 2015;99(1):301–308. doi: 10.1007/s00253-014-5916-z. [DOI] [PubMed] [Google Scholar]
- Yu H., Xie T., Xie J., Ai L., Tian H. Characterization of key aroma compounds in Chinese rice wine using gas chromatography-mass spectrometry and gas chromatography-olfactometry. Food Chem. 2019;293:8–14. doi: 10.1016/j.foodchem.2019.03.071. [DOI] [PubMed] [Google Scholar]
- Yu H., Zhao J., Li F., Tian H., Ma X. Characterization of Chinese rice wine taste attributes using liquid chromatographic analysis, sensory evaluation, and an electronic tongue. J. Chromatogr. B. 2015;997:129–135. doi: 10.1016/j.jchromb.2015.05.037. [DOI] [PubMed] [Google Scholar]
- Zhang B., Kong L., Cao Y., Xie G., Guan Z., Lu J. Metaproteomic characterisation of a Shaoxing rice wine “wheat Qu” extract. Food Chem. 2012;134(1):387–391. doi: 10.1016/j.foodchem.2012.02.057. [DOI] [Google Scholar]
- Zhang C., Qi Y., Ma H., Li W., Dai L., Xiao D. Decreased production of higher alcohols by Saccharomyces cerevisiae for Chinese rice wine fermentation by deletion of Bat aminotransferases. J. Ind. Microbiol. Biotechnol. 2015;42(4):617–625. doi: 10.1007/s10295-015-1583-z. [DOI] [PubMed] [Google Scholar]
- Zhang P., Du G.C., Zou H.J., Xie G.F., Chen J., Shi Z.P., Zhou J.W. Mutant potential ubiquitination sites in Dur3p enhance the urea and ethyl carbamate reduction in a model rice wine system. J. Agric. Food Chem. 2017;65(8):1641–1648. doi: 10.1021/acs.jafc.6b05348. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Zhang Y., Zhou Y., Li G., Yang W., Feng X. A review of pretreatment and analytical methods of biogenic amines in food and biological samples since 2010. J. Chromatogr. A. 2019:360361. doi: 10.1016/j.chroma.2019.07.015. [DOI] [PubMed] [Google Scholar]
- Zhao C., Su W., Mu Y.C., Jiang L., Mu Y. Correlations between microbiota with physicochemical properties and volatile flavor components in black glutinous rice wine fermentation. Food Res. Int. 2020;138:109800. doi: 10.1016/j.foodres.2020.109800. [DOI] [PubMed] [Google Scholar]
- Zhao X., Du G., Zou H., Fu J., Zhou J., Chen J. Progress in preventing the accumulation of ethyl carbamate in alcoholic beverages. Trends Food Sci. Technol. 2013;32(2):97–107. doi: 10.1016/j.tifs.2013.05.009. [DOI] [Google Scholar]
- Zhao X.X., Wang Y.R., Cai W.C., Yang M.J., Zhong X.D., Guo Z., Shan C.H. High-throughput sequencing-based analysis of microbial diversity in rice wine koji from different areas. Curr. Microbiol. 2020;77(5):882–889. doi: 10.1007/s00284-020-01877-9. [DOI] [PubMed] [Google Scholar]
- Zheng D., Liu T., Chen J., Zhang K., Li O., Zhu L., Zhao Y., Wu X., Wang P. Comparative functional genomics to reveal the molecular basis of phenotypic diversities and guide the genetic breeding of industrial yeast strains. Appl. Microbiol. Biotechnol. 2013;97(5):2067–2076. doi: 10.1007/s00253-013-4698-z. [DOI] [PubMed] [Google Scholar]
- Zhou W., Fang R., Chen Q. Effect of gallic and protocatechuic acids on the metabolism of ethyl carbamate in Chinese yellow rice wine brewing. Food Chem. 2017;233:174–181. doi: 10.1016/j.foodchem.2017.04.113. [DOI] [PubMed] [Google Scholar]
- Zhou W., Hu J., Zhang X., Chen Q. Application of bamboo leaves extract to Chinese yellow rice wine brewing for ethyl carbamate regulation and its mitigation mechanism. Food Chem. 2020;319:126554. doi: 10.1016/j.foodchem.2020.126554. [DOI] [PubMed] [Google Scholar]
- Zhu F., Li S., Guan X., Huang K., Li Q. Influence of vacuum soaking on the brewing properties of japonica rice and the quality of Chinese rice wine. J. Biosci. Bioeng. 2020;130(2):159–165. doi: 10.1016/j.jbiosc.2020.03.006. [DOI] [PubMed] [Google Scholar]