Abstract
Background
Currently dual-energy X-ray absorptiometry (DXA) and phantom-based quantitative computed tomography (PB-QCT) have been utilized to diagnose osteoporosis widely in clinical practice. While traditional phantom-less QCT (PL-QCT) is limited by the precision of manual calibration using body tissues, such as fat and muscle.
Objective
The aim of this study is to validate the accuracy and precision of one newly-developed automatic PL-QCT system to measure spinal bone mineral density (BMD) and diagnose osteoporosis.
Methods
A total of 36 patients were enrolled for comparison of BMD measurement between DXA and QCT. CT images of 63 patients were analyzed by both PB-QCT and newly developed automatic PL-QCT system, then the BMD results generated by the automatic PL-QCT were utilized to diagnose osteoporosis. The diagnostic outcomes were compared with that of DXA and PB-QCT to assess the performance of the new system.
Results
BMD test results showed that the automatic PL-QCT system had higher precision than previous studies performed with QCT, while maintaining similar capability to diagnose osteoporosis as DXA and PB-QCT. Area under curve (AUC) result of PL-QCT was larger than 0.8 for predicting spine DXA T-score in receiver operating characteristic (ROC) analysis. Pearson correlation analysis (r = 0.99) showed strong linear correlation and Bland-Altman analysis (bias = 3.0mg/cc) indicated little difference between the two methods. The precision result (CV = 0.89%) represented good reproducibility of the new system.
Conclusion
The traditional PL-QCT system has relatively low reproducibility due to the manual selection of the region of interest (ROI) of body tissues. Automatic selection of ROI in this new system makes the BMD testing more convenient and improves precision significantly. Compared with traditional BMD measurement methods, the automatic PL-QCT system had higher precision in accurate diagnosis of osteoporosis with great potential in translational research and wide clinical application.
Translational potential statement
With high accuracy and precision, the automatic PL-QCT system could serve as an opportunistic screening tool for osteoporosis patients in the future. It could also facilitate related researches by providing more reliable data collection, both retrospectively and longitudinally.
Keywords: Bone mineral density, DXA, Osteoporosis, Phantom-less QCT, Spine
1. Introduction
Osteoporosis is one of the most troublesome diseases that threaten millions of the elderly in the world. It is estimated that this disease has become a major health problem for more than 200 million people all around the world [1]. Osteoporotic fracture in spine is the most common complication of osteoporosis [2]. Moreover, hip fracture causes the highest mortality and morbidity for patients due to the prolonged immobilization [3,4]. In China, it is estimated that the medical cost of osteoporosis-related fractures will be around 19 billion US dollars in 2035 [5]. This disease will similarly cause huge economic burden in many other countries all around the world. Osteoporotic fracture can be prevented by opportunistic screening of osteoporosis for the elderly followed by a variety of treatments, including medication and bracing. However, the screening rate of osteoporosis is relatively low in China. Dual X-ray Absorptiometry (DXA), quantitative computed tomography (QCT) and quantitative ultrasound system (QUS) [6,7] are three main types of technologies applied in the bone mineral density (BMD) measurement currently. DXA has been widely used to measure BMD and diagnose osteoporosis in many countries [8,9]. Nowadays, DXA is the golden standard for BMD measurement for clinicians [10]. World Health Organization recommends the T-score of DXA testing results as the standard to diagnose osteoporosis [11]. However, DXA examines the areal BMD rather than the volumetric BMD, resulting in its inaccuracy in testing the local bone quality and predicting the risk of osteoporotic fracture [12,13].
QCT was originally invented by Ben Arnold in 1980s [14]. This technology can separate cortical and trabecular bone, facilitating the analysis of localized BMD. Besides, it also provides accurate volumetric BMD results measured in units of mg/cm3 rather than g/cm2. Osteophyte or aortic calcification will not influence the QCT testing results [15]. Conventionally, a phantom was placed below the patient body for standardizing purpose. However, it has a great impact on X-ray penetration due to beam hardening and scatter effect [16,17]. Wang L et al. have carried out one clinical study to validate the accuracy and reproducibility of the asynchronous phantom-based QCT(PB-QCT) method [18], which led to the professional consensus, that the diagnosis standard for osteoporosis using QCT is absolute BMD value less than 80 mg/cm3 [[19], [20]]. Asynchronous QCT bone health assessment extends the utilization of CT images for BMD measurement. It eliminates the variability of synchronous calibration method and improves the precision of BMD measurement in short-term [21,22]. The major disadvantage of asynchronous QCT is that scanner instability that occurs between phantom calibrations cannot be detected nor corrected [23].
Phantom-less QCT (PL-QCT) can be utilized to avoid this problem due to its calibration method using body tissue (such as fat and muscle) rather than one external solid phantom [24,25]. In addition, due to the advantage of using no phantom in the examination procedure, retrospective studies involving BMD measurement are practicable using previous CT images that were not initially scanned for BMD testing. This will greatly increase the number of testing results included in related studies and make the statistical analysis more convincing. Another useful application is that low-dose CTs scanned for lung-cancer screening can also be utilized for BMD measurement [26]. However, one of the disadvantages of PL-QCT is the relatively lower precision compared with PB-QCT. It was reported that the precision of PB-QCT BMD measurement was 1.1–2.9 times better than PL-QCT system [17,27]. This is one of the major reasons why the clinical application of PL-QCT is limited nowadays.
Automatic selection of suitable fat and muscle region of interest (ROI) enhances the reproducibility of BMD measurement results of PL-QCT. The application of automatic selection of ROI, along with the user-friendly operation procedures in the QCT system, would facilitate efficient clinical practice of diagnosing osteoporosis. This study is aimed to validate the accuracy and precision of one newly-developed automatic PL-QCT system for BMD measurement and osteoporosis screening. BMD measurement of the lumbar spine was used to investigate the difference in the detection rate of osteoporosis for the same group of patients. Possible reasons were discussed to explain the difference between these BMD measurement methods. The main objectives of this study include: 1. to validate the accuracy and the precision of the newly-developed automatic PL-QCT system; 2. to compare the diagnostic efficacy of automatic PL-QCT system with DXA and PB-QCT system based on BMD measurement results.
2. Material and methods
2.1. Newly developed automatic PL-QCT technique
One novel PL-QCT system was recently developed and expected to be applied in precise volumetric BMD measurement. This new system utilizes the subcutaneous fat and paraspinal muscle as the internal calibration references and the automatic selection of ROI technology was firstly applied to calibrate the BMD measurement results. Automatic selection of fat and muscle ROI is the key step that improves the precision of BMD results, which solves one major problem of the traditional PL-QCT. To accurately select body tissues ROI from CT scans, several steps were performed to achieve this goal.
Firstly, the Hounsfield unit (HU) scale is utilized to achieve coarse segmentation of different body tissues in CT scans, which provides critical contextual cues for ROI localization. A tissue-specific HU thresholding is carried out on a selected axial plane of a CT scan to separate one type of body tissue from the others in the form of a binary mask M:
The determination of the HU range was based on clinical practices and experts' observations of spinal CT scans: fat () and muscle () (Fig. 1 a).
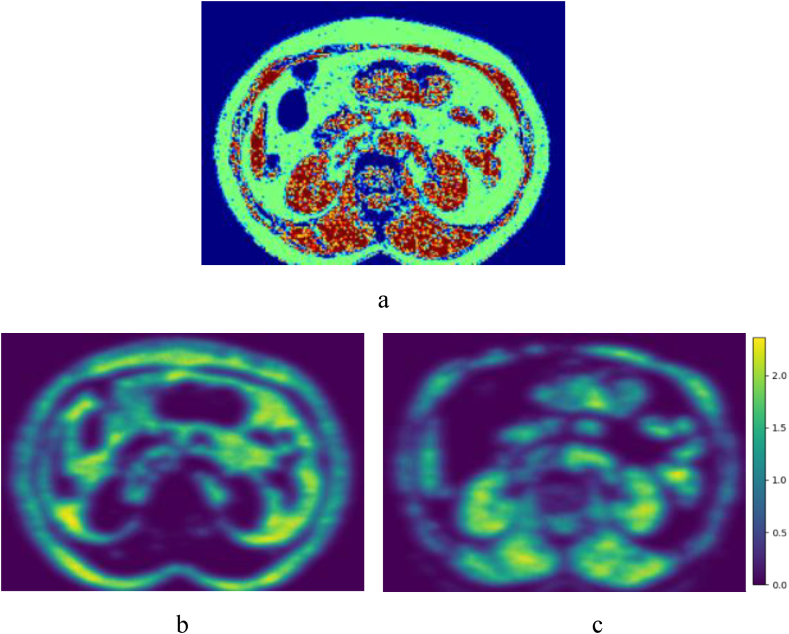
Figure 1.
Example of a CT scan processed by the new PL-QCT system. a. Green region represents fat, red region represents muscle and blue region represents others. b. Convolution map of fat. c. Convolution map of muscle.
Secondly, the two-dimensional convolution operation to efficiently perform filtering. Specifically, ROI is expressed in the form of a binary kernel (convolution matrix):
where x and y are 2D coordinates and the kernel is of length (X, Y).
A kernel pyramid is constructed to increase spatial robustness towards region boundaries. Specifically, a three-level kernel pyramid is introduced, which consists of kernels spatially scaled by 0.5, 1.0. 1.5 respectively. The resulting convolution map at location (x, y) is therefore given as the sum of three convolution operations:
where “∗” is the convolution operation. The convolution map C measures the degree of spatial continuity at any given location since the higher C (x, y) is, the smaller non-tissue area the ROI centered at this location contains (Fig. 1 b and c).
Thirdly, a distance-based factor was further proposed to scale the convolution value by at each different location. Priority is given to locations that are spatially closer to the vertebrae location provided by the user. The scaled convolution map at location (x, y) is given by:
Where is a scale constant that controls the fall-off of the distance-based factor. Finally, the optimal ROI location (x∗, y∗) computed by the proposed automatic method is given by:
3. Patient population
3.1. Patients collected for comparison between DXA and QCT
A total of 36 patients with both DXA and CT scanning were retrospectively collected from the patient database in Sun Yat-sen Memorial hospital. The time periods between DXA and CT scanning for included patients are all less than one month, which ensures that the BMD value of the same patient does not change significantly between the DXA and CT diagnosis time point. The CT images of them were utilized to perform the asynchronous PB-QCT and PL-QCT analysis.
3.2. Patients collected for comparison between PB-QCT and automatic PL-QCT
A total of 63 patients were enrolled in this study and the age of these patients range from 20 to 90. 25.4% of the patients are men and 74.6% are women. The basic information of patients including gender, age, weight and height were collected by clinicians in Sun Yat-Sen Hospital. The basic information of the patients is exhibited in the Table 1. Patients who were treated with internal fixation, had implants in the spine or had previous vertebral fractures were excluded from this study. Vertebrae with inhomogeneous BMD distribution were not included as well.
Table 1.
Basic information of patients with CT scanning.
| Basic Information | Male (n = 16) | Female (n = 47) | Total Subjects (n = 63) |
|---|---|---|---|
| Age(years) | 51.7518.22 | 64.159.04 | 61.00 ± 13.01 |
| Height(m) | 1.70 | 1.59 | |
| Weight(kg) | 12.83 | 8.43 | 63.63 |
| BMI() | .33 | 24.58 | 24.62 |
| Osteoporosis(PB-QCT) | 2/16 | 24/47 | 26/63 |
| Osteoporosis(PL-QCT) | 3/16 | 22/47 | 25/63 |
This retrospective study was approved by the Ethics Committee in Sun Yat-Sen Hospital.
4. DXA acquisition
The BMD results of the lumbar spine (L1-L4) was obtained by a DXA scan (Lunar Prodigy, GE Lunar, Madison, WI, USA) for potential osteoporotic patients. All the DXA scans were performed on the same DXA system. For ROI selection, the anteroposterior images of L1-L4 vertebra were used for spine BMD measurement. All evaluable vertebrae (2 at least) were included except for those affected by local structural change. The BMD of each subject was expressed as a T-score (expressed as g/cm2 and standard deviation scores), which shows the amount of bone present compared to a young adult of the same gender with peak bone mass.
5. PB-QCT analysis
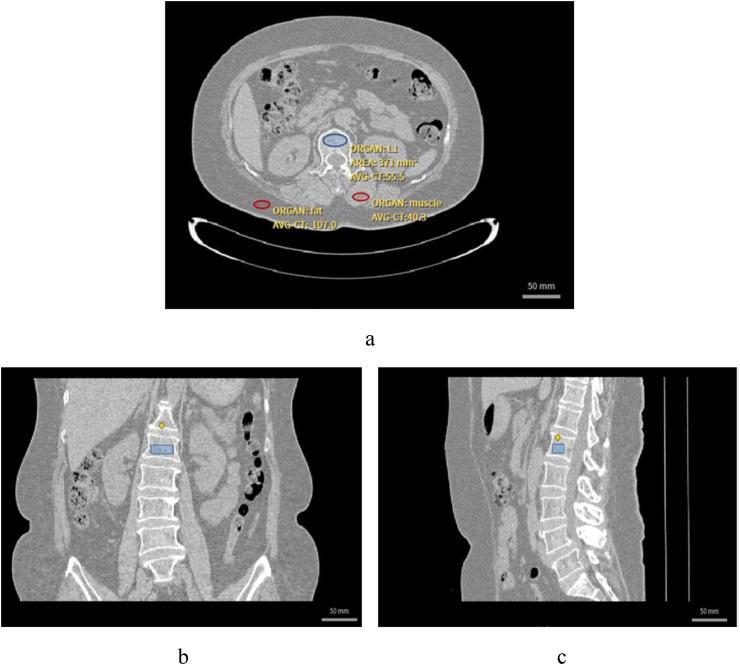
CT scans for this study were acquired on a third-generation dual-source DECT scanner (SOMATOM Force, Siemens Healthcare, Erlangen, Germany). All the QCT scans utilized in this study were performed on the same CT system. The scanning parameters of CT are set at the values of 120 KVp, 150mAs/slice, 3-mm slice thickness and 512 512 matrix. The standard reconstruction kernel is soft tissue kernel. The CT images analyzed by asynchronous PB-QCT were collected. The SFOV is about 500 mm and table height is around 150 cm (see Supplementary Material 1). Two or three vertebrae were analyzed for each patient, and L1-L3 were chosen as priority. If vertebral fracture exists in these vertebrae, other vertebra from T12-L5 can be alternative options. ROI for each vertebra was selected as elliptical shape with limited range of adjustment in the PB-QCT analysis (Fig. 2). The BMD reports were collected, which include patients' basic information and BMD measurement results. In contrast to conventional QCT method, the asynchronous QCT method does not require the phantom to be simultaneously scanned with patients [21]. However, it needs to be calibrated by quality assurance phantom periodically, once a month in general. The asynchronous PB-QCT analysis were performed on the Mindways QCT (USA) system. The phantom used in this study is the Model 4 CT standard phantom of Mindways.
Figure 2.
ROI selection of L1 vertebra, fat and muscle in the cross section of CT image. a. cross section of the vertebral ROI. b. coronal plane. c. sagittal plane of the vertebral ROI.
6. Automatic PL-QCT analysis
Vertebrae with fracture were diagnosed and excluded from this study. The fractured vertebrae were identified according to the Genant Semi-quantitative Classification Method [28]. Fractured vertebrae of Grade 1 or higher level will not be analyzed due to the inaccurate results of BMD. PB-QCT analysis of all patients was performed on the Mindways software by the same operator. PL-QCT analysis was performed by two operators respectively. The spine BMD were analyzed by both PL-QCT and PB-QCT and comparative analysis was conducted. The PL-QCT system utilized in this study selects ROI automatically, which eliminates the influence of manual operation and thus enhances the BMD testing consistency. The trabecular BMD is calibrated by the CT value of paraspinal muscle and subcutaneous fat ROI in PL-QCT.
7. Statistical analysis
7.1. Comparison between DXA and QCT
BMD results of DXA for 36 patients have also been collected to conduct a comparative analysis with QCT results. The diagnostic efficacy of different types of tools including DXA, PB-QCT and automatic PL-QCT were compared. Percentages of normal/osteopenia/osteoporosis patients are respectively calculated according to BMD results tested by different devices. Osteoporosis detection rate is one of the key indicators that measure BMD testing performance of different devices. Since DXA was widely applied in the clinical practice as the golden standard for the diagnosis of osteoporosis, diagnosis made by DXA spine BMD results was utilized as the reference in the ROC analysis of comparison between DXA and QCT.
7.2. Comparison between PB-QCT and automatic PL-QCT
7.2.1. Accuracy and precision analysis
The degree of agreement level between PL-QCT and PB-QCT is determined by linear regression. The accuracy result is assessed by systematic bias value between BMD results of the two QCT methods. The confidence interval of 95% has been applied in analysis. Intraobserver variability were analyzed by the results of Root Mean Square of Standard Deviation (RMSSD) and accuracy is assessed by Bland-Altman analysis [18]. Intraobserver analysis means that one operator analyzed the same group of CT images at two different time points.
Coefficient of variation (CV) is another important statistical index to measure the reproducibility of BMD results. CV is calculated as the following equation:
Since the T-score is not applicable to spine QCT analysis, the absolute BMD value is utilized to perform accuracy and precision analysis. The statistical significance level was defined as p<0.05. All the statistical analysis was done by the MedCalc Statistical Software version 19.0.4 (MedCalc Software bv, Ostend, Belgium)
8. Results
8.1. Comparison between DXA and QCT systems
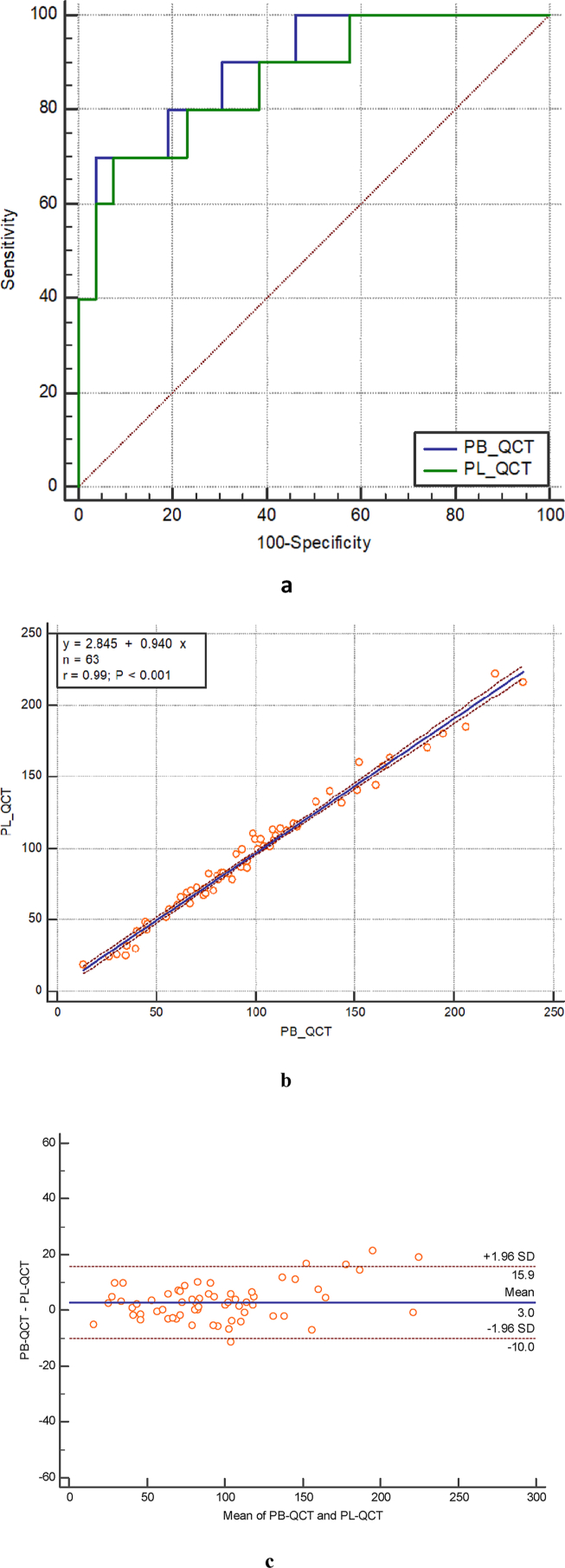
From the ROC analysis, based on the spine DXA diagnosis standard, the AUC of PB-QCT and automatic PL-QCT were relatively 0.89 and 0.87 (Fig. 3 a and Supplementary Material 2). PB-QCT and automatic PL-QCT had similar osteoporosis detection capability according to the ROC analysis results. Of automatic PL-QCT, the Youden Index J is 0.6213. The sensitivity and specificity were 70.00% and 92.31% respectively (Supplementary Material 3).
Figure 3.
Diagrams demonstrating comparison between DXA, PB-QCT and automatic PL-QCT. a ROC curves for predicting spine DXA T-score for osteoporosis with automatic PL-QCT and PB-QCT. b. Linear regression relationship between PB-QCT and automatic PL-QCT methods. c. Bland-Altman analysis for automatic PL-QCT and PB-QCT BMD measurement results.
The percentages of normal, osteopenia and osteoporosis patients were exhibited in the Table 2. Different diagnosis rate of osteoporosis was found between DXA and QCT systems.
Table 2.
The portion of patients (%) identified as normal/osteopenia/osteoporosis using different types of diagnosis tools including DXA (spine), PB-QCT and automatic PL-QCT.
| Methods | Normal | Osteopenia | Osteoporosis |
|---|---|---|---|
| DXA(spine) | 30.56%(11/36) | 41.67%(15/36) | 27.78%(10/36) |
| PB-QCT | 5.56%(2/36) | 38.89%(14/36) | 55.56%(20/36) |
| Automatic PL-QCT | 2.78%(1/36) | 41.67%(15/36) | 55.56%(20/36) |
For DXA, the T-score of spine were utilized to classify the different conditions of BMD.
•Normal: T-score > −1.0
•Osteopenia: −2.5 < T-score -1.0
•Osteoporosis: T-score -2.5
For PB-QCT and PL-QCT, the absolute BMD value was utilized to classify the different conditions of BMD.
•Normal: BMD >120 mg/cm3
•Osteopenia: 80mg/cm3
< BMD 120mg/cm3
•Osteoporosis: BMD ≤ 80mg/cm3
8.2. Comparison between PB-QCT and automatic PL-QCT system
8.2.1. Accuracy and precision
Summary for spinal BMD measurement results was shown in Table 3. The linear relationship between PL-QCT and PB-QCT was BMDPL-QCT = 0.94BMDPB-QCT + 2.845 mg/cm3. The Pearson r value of the linear regression equaled to 0.99, which showed one convincing linear relationship between them (Fig. 3 b). This regression demonstrated the similar BMD measurement capability of the two QCT methods.
Table 3.
Summary for spinal BMD measurement results by PB-QCT and automatic PL-QCT.
| Variables | Spine vBMD () |
|---|---|
| Number | 63 |
| Mean SD (phantom-based) | 90/07 ±48.48 |
| Mean SD (phantom-less) | 94.11 |
| Difference | 2.95 6.95 |
| 95% confidence interval | [1.30, 4.61] |
| t statistic | 3.56 |
| P | 0.6007 |
The accuracy analysis was conducted to compare the BMD results tested by PB-QCT and automatic PL-QCT. According to the results of Bland-Altman analysis, the bias between two methods was 3.0 mg/cm3 and showed no significant difference between the two methods. The limits of agreement ranged from −10.0 mg/cm3 to 15.9 mg/cm3 (Fig. 3 c).
8.2.2. Intraclass correlation (ICC) analysis
The ICC analysis result showed that the single measure and average measures results were both close to 0.99, which indicated good correlation between the two diagnosis methods (Table 4).
Table 4.
ICC analysis results of PB-QCT and automatic PL-QCT BMD measurements.
| Measures | Intraclass Correlation | 95% Confidence Interval |
|---|---|---|
| Single measures | 0.9903 | 0.9840 to 0.9941 |
| Average measures | 0.9951 | 0.9919 to 0.9970 |
Precision is another important parameter to examine the consistency of one testing method. As shown in Table 5, intraobserver variability was measured in this study. Intraobserver variability results in this study had shown that the automatic PL-QCT system could reach the coefficient of variation (CV) at about 0.89%, which was less than the PB-QCT system (CV >1%) reported by previous studies. Compared to other traditional PL-QCT, the precision of this automatic PL-QCT also has a better outcome. This ensured the diagnostic precision of this automatic PL-QCT system in future clinical application.
Table 5.
BMD measurement precision of PB-QCT and automatic PL-QCT.
9. Discussion
In this study, one newly developed automatic PL-QCT system has been validated to examine BMD and diagnose osteoporosis based on the retrospectively collected CT data. This automatic PL-QCT system utilizes coarse segmentation, tissue-specific thresholding and the two-dimensional convolution operation to efficiently perform filtering and selecting the optimal ROI location computed by the proposed automatic method. A series of algorithms make the automatic selection of fat and muscle ROI more accurate and thus improve the precision of PL-QCT BMD measurement.
Compared with DXA BMD results, this automatic PL-QCT system diagnoses osteoporosis according to different standards. From the ROC analysis results, automatic PL-QCT performs AUC of 0.87 when spine DXA BMD results was utilized as the osteoporosis diagnosis standard. Compared with DXA, the proportion of patients diagnosed with osteoporosis was relatively higher for PB-QCT and automatic PL-QCT, this might be due to the false negative cases caused by the osteophyte and vascular calcification in DXA measurements. Previous studies have also reported similar results regarding to the comparison between DXA and QCT [33,34]. Due to the false negative rate of DXA diagnostic results, some patients with osteoporosis may not receive timely treatment, which increases the risk of osteoporotic fractures. However, DXA is still the most widely used clinical tool for osteoporosis screening. This is related to the low cost of the instrument and patients' exposure to lower doses of X-ray radiation [35]. Further research including patients' follow-up needs to be conducted for the comparison between QCT and DXA.
QCT has also used in diagnosing osteoporosis clinically and this technology avoids miscalculation caused by osteophytes or vascular calcification. However, its diagnostic standard in clinical practice is not universal currently. This study had verified the accuracy and precision of automatic PL-QCT, which are two key indicators of validation applied in most clinical diagnosis studies. Through the analysis of linear regression and Bland-Altman analysis, this new system had similar accuracy with PB-QCT. In linear regression analysis, the r value was as high as 0.99, which indicated a strong correlation between two methods. PL-QCT and PB-QCT utilize different calibration methods, which are body compositions and external phantom respectively. From the abovementioned results, no significant difference was observed between the two types of methods. The systematic bias is close to 3.0mg/ , which indicates little difference between the mean BMD value tested by two methods. This difference lies in the range of (−10 mg/cm3, +10 mg/cm3), which is acceptable in similar research [27]. The accuracy of this automatic PL-QCT has been demonstrated through the Bland-Altman analysis, t-test, linear correlation and ICC analysis.
The size and position of fat and muscle ROI are important factors that influence the BMD calibration results for PL-QCT. Different amount of fat or muscle selected during each separate test may lead to the inconsistency of BMD results, thus traditional PL-QCT system can yield inconsistent results. This new system utilized automatic selection of fat and muscle ROI, which eliminated variation caused by the manual operation in this procedure. Moreover, the designed algorithm automatically recognizes the central position for the vertebra and selects the middle layer of the vertebral body for analysis. This ensured the consistency of BMD measurement and thus improved the precision since the same ROI was analyzed every single time. Previous studies also reported that comparing PB-QCT with PL-QCT, the instability of muscle and fat ROI selection was one major factor that influenced the BMD measurement stability. Therefore, BMD measurement using QCT is influenced by the inhomogeneity of body fat distribution [36]. The automatic PL-QCT system utilized in this study selects suitable fat and muscle ROI with normally distributed CT values automatically and can reduce the measurement error caused by manual operations.
As a diagnostic tool, PL-QCT screening for skeletal disorder can provide an appropriate, or even more precise, detection of osteoporosis. In a standard clinical setting, individuals at risk of osteoporosis would often receive a CT scan instead of DXA due to other conditions, in spite of DXA being the defining test. With this system, opportunistic screening using CT scans originally acquired for clinical reasons unrelated to musculoskeletal assessment is feasible for identifying potential osteoporosis patients over 50 years old, which is a viable alternative to traditional screening by DXA. Results in this study represented how PL-QCT would be clinically integrated. The goal of PL-QCT screening is complementing DXA as a diagnostic tool rather than replacing it, providing another method to identify individuals at risk of osteoporosis, as CT tests cover patients who may never have a DXA scan. Due to the disparity of DXA screening, application of PL-QCT to screen for people at high risk for osteoporotic fracture could serve as a means to provide prompt and appropriate clinical care.
10. Conclusion
PL-QCT is a powerful tool for osteoporosis screening in clinical practice. This study has demonstrated the accuracy and precision of one newly-developed automatic PL-QCT system. Compared with PB-QCT and traditional PL-QCT system, this automatic PL-QCT system with high precision has great potential in the assessment of BMD in the future. More BMD relevant clinical research can be conducted using this automatic PL-QCT technology. This will improve the prediction accuracy of osteoporosis and benefit the patients through early diagnosis.
Funding
This work was supported by Sun Yat-Sen University Clinical Research 5010 Program; Guangdong Basic and Applied Basic Research Foundation (2018A030313888); Shenzhen Science and Technology Funding (JCYJ20200109150420892); HKU-SZH Fund for Shenzhen Key Medical Discipline (SZXK2020084); Sanming Project of Medicine in Shenzhen “Team of Excellence in Spinal Deformities and Spinal Degeneration” (SZSM201612055) and Hong Kong RGC, JLFS/M-702/18.
Declaration of competing interest
The authors have no conflicts of interest relevant to this article
Acknowledgements
none.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jot.2021.11.008.
Contributor Information
Chun-Hai Li, Email: lichhai@mail.sysu.edu.cn.
Yue Ding, Email: dingyue@mail.sysu.edu.cn.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Sozen T., Ozisik L., Basaran N.C. An overview and management of osteoporosis. Eur J Rheumatol. 2017;4(1):46–56. doi: 10.5152/eurjrheum.2016.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nazrun A.S., Tzar M.N., Mokhtar S.A., Mohamed I.N. A systematic review of the outcomes of osteoporotic fracture patients after hospital discharge: morbidity, subsequent fractures, and mortality. Therapeut Clin Risk Manag. 2014;10:937–948. doi: 10.2147/TCRM.S72456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brauer C.A., Coca-Perraillon M., Cutler D.M., Rosen A.B. Incidence and mortality of hip fractures in the United States. Jama. 2009;302(14):1573–1579. doi: 10.1001/jama.2009.1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnell O., Kanis J. Epidemiology of osteoporotic fractures. Osteoporos Int. 2005;16(2):S3–S7. doi: 10.1007/s00198-004-1702-6. [DOI] [PubMed] [Google Scholar]
- 5.Si L., Winzenberg T.M., Jiang Q., Chen M., Palmer A.J. Projection of osteoporosis-related fractures and costs in China: 2010-2050. Osteoporos Int. 2015;26(7):1929–1937. doi: 10.1007/s00198-015-3093-2. [DOI] [PubMed] [Google Scholar]
- 6.Malekzadeh M., Asadi M., Abbasi-Rad S., Abolghasemi J., Hamidi Z., Talebi M., et al. MDCT-QCT, QUS, and DXA in healthy adults: an intermodality comparison. Med J Islam Repub Iran. 2019;33:156. doi: 10.34171/mjiri.33.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee T.T.-Y., Lai K.K.-L., Cheng J.C.-Y., Castelein R.M., Lam T.-P., Zheng Y.-P. 3D ultrasound imaging provides reliable angle measurement with validity comparable to X-ray in patients with adolescent idiopathic scoliosis. Journal of Orthopaedic Translation. 2021;29:51–59. doi: 10.1016/j.jot.2021.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang L., Ran L., Zha X., Zhao K., Yang Y., Shuang Q., et al. Adjustment of DXA BMD measurements for anthropometric factors and its impact on the diagnosis of osteoporosis. J Archives of Osteoporosis. 2020;15(1):1–11. doi: 10.1007/s11657-020-00833-1. [DOI] [PubMed] [Google Scholar]
- 9.Alacreu E., Moratal D., Arana E. Opportunistic screening for osteoporosis by routine CT in Southern Europe. Osteoporos Int. 2017;28(3):983–990. doi: 10.1007/s00198-016-3804-3. [DOI] [PubMed] [Google Scholar]
- 10.Silva B.C., Broy S.B., Boutroy S., Schousboe J.T., Shepherd J.A., Leslie W.D. Fracture risk prediction by non-BMD DXA measures: the 2015 ISCD official positions part 2: trabecular bone score. J Clin Densitom. 2015;18(3):309–330. doi: 10.1016/j.jocd.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 11.World Health Organization . World Health Organization; 1994. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis : report of a WHO study group [meeting held in Rome from 22 to 25 June 1992]https://apps.who.int/iris/handle/10665/39142 [PubMed] [Google Scholar]
- 12.Kim K., Song S.H., Kim I.J., Jeon Y.K. Is dual-energy absorptiometry accurate in the assessment of bone status of patients with chronic kidney disease? Osteoporos Int. 2021:1–10. doi: 10.1007/s00198-020-05670-z. [DOI] [PubMed] [Google Scholar]
- 13.Xu X.-m., Li N., Li K., Li X.-Y., Zhang P., Xuan Y.-j., et al. Discordance in diagnosis of osteoporosis by quantitative computed tomography and dual-energy X-ray absorptiometry in Chinese elderly men. Journal of orthopaedic translation. 2019;18:59–64. doi: 10.1016/j.jot.2018.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Robertson A., Godavitarne C., Peters J. Bone quantification, Orthopaedics and Trauma. 2017;31(5):326–329. [Google Scholar]
- 15.Paggiosi M.A., Debono M., Walsh J.S., Peel N.F.A., Eastell R. Quantitative computed tomography discriminates between postmenopausal women with low spine bone mineral density with vertebral fractures and those with low spine bone mineral density only: the SHATTER study. Osteoporos Int. 2020;31(4):667–675. doi: 10.1007/s00198-020-05317-z. [DOI] [PubMed] [Google Scholar]
- 16.Toelly A., Bardach C., Weber M., Gong R., Lai Y., Wang P., et al. © Georg Thieme Verlag KG; 2017. Influence of contrast media on bone mineral density (BMD) measurements from routine contrast-enhanced MDCT datasets using a phantom-less BMD measurement tool, RöFo-Fortschritte auf dem Gebiet der Röntgenstrahlen und der bildgebenden Verfahren; pp. 537–543. [DOI] [PubMed] [Google Scholar]
- 17.Habashy A.H., Yan X., Brown J.K., Xiong X., Kaste S.C. Estimation of bone mineral density in children from diagnostic CT images: a comparison of methods with and without an internal calibration standard. Bone. 2011;48(5):1087–1094. doi: 10.1016/j.bone.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang L., Su Y., Wang Q., Duanmu Y., Yang M., Yi C., et al. Validation of asynchronous quantitative bone densitometry of the spine: accuracy, short-term reproducibility, and a comparison with conventional quantitative computed tomography. Sci Rep. 2017;7(1):6284. doi: 10.1038/s41598-017-06608-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Radiology A.C.o. American College of Radiology; Chicago, IL: 2008. ACR practice guideline for the performance of quantitative computed tomography (QCT) bone densitometry. [Google Scholar]
- 20.Engelke K., Lang T., Khosla S., Qin L., Zysset P., Leslie W.D., et al. Clinical use of quantitative computed tomography (QCT) of the hip in the management of osteoporosis in adults: the 2015 ISCD official positions-Part I. J Clin Densitom. 2015;18(3):338–358. doi: 10.1016/j.jocd.2015.06.012. [DOI] [PubMed] [Google Scholar]
- 21.Brown J.K., Timm W., Bodeen G., Chason A., Perry M., Vernacchia F., et al. Asynchronously calibrated quantitative bone densitometry. J Clin Densitom. 2017;20(2):216–225. doi: 10.1016/j.jocd.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 22.Petraikin A.V., Nisovtsova L.A., Sergunova К.А., Akhmad E.S., Semenov D.S., Petryaykin F.A., et al. Accuracy of asynchronous quantitative computed tomography by phantom modelling. Radiology. 2019;6:78. [Google Scholar]
- 23.Engelke K. Quantitative computed tomography-current status and new developments. J Clin Densitom. 2017;20(3):309–321. doi: 10.1016/j.jocd.2017.06.017. [DOI] [PubMed] [Google Scholar]
- 24.Budoff M.J., Malpeso J.M., Zeb I., Gao Y.L., Li D., Choi T.-Y., et al. Measurement of phantomless thoracic bone mineral density on coronary artery calcium CT scans acquired with various CT scanner models. Radiology. 2013;267(3):830–836. doi: 10.1148/radiol.13111987. [DOI] [PubMed] [Google Scholar]
- 25.Michalski A.S., Besler B.A., Michalak G.J., Boyd S.K. CT-based internal density calibration for opportunistic skeletal assessment using abdominal CT scans. Med Eng Phys. 2020;78:55–63. doi: 10.1016/j.medengphy.2020.01.009. [DOI] [PubMed] [Google Scholar]
- 26.Pan Y., Shi D., Wang H., Chen T., Cui D., Cheng X., et al. Automatic opportunistic osteoporosis screening using low-dose chest computed tomography scans obtained for lung cancer screening. Eur Radiol. 2020;30(7):4107–4116. doi: 10.1007/s00330-020-06679-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mueller D.K., Kutscherenko A., Bartel H., Vlassenbroek A., Ourednicek P., Erckenbrecht J. Phantom-less QCT BMD system as screening tool for osteoporosis without additional radiation. Eur J Radiol. 2011;79(3):375–381. doi: 10.1016/j.ejrad.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 28.Genant H.K., Wu C.Y., Van Kuijk C., Nevitt M.C. Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res. 1993;8(9):1137–1148. doi: 10.1002/jbmr.5650080915. [DOI] [PubMed] [Google Scholar]
- 29.Boden S.D., Goodenough D.J., Stockham C.D., Jacobs E., Dina T., Allman R.M. Precise measurement of vertebral bone density using computed tomography without the use of an external reference phantom. J Digit Imag. 1989;2(1):31–38. doi: 10.1007/BF03168013. [DOI] [PubMed] [Google Scholar]
- 30.Gudmundsdottir H., Jonsdottir B., Kristinsson S., Johannesson A., Goodenough D., Sigurdsson G.J.O.I. Vertebral bone density in Icelandic women using quantitative computed tomography without. an external reference phantom. 1993;3(2):84–89. doi: 10.1007/BF01623378. [DOI] [PubMed] [Google Scholar]
- 31.Engelke K., Adams J.E., Armbrecht G., Augat P., Bogado C.E., Bouxsein M.L., et al. D.B.J.J.o.c.d. Hans Clinical use of quantitative computed tomography and peripheral quantitative computed tomography in the management of osteoporosis in adults: the 2007. ISCD Official Positions. 2008;11(1):123–162. doi: 10.1016/j.jocd.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 32.Bligh M., Bidaut L., White R.A., Murphy W.A., Jr., Stevens D.M., Cody D.D. Helical multidetector row quantitative computed tomography (QCT) precision. Acad Radiol. 2009;16(2):150–159. doi: 10.1016/j.acra.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 33.Li N., Li X.M., Xu L., Sun W.J., Cheng X.G., Tian W. Comparison of QCT and DXA: osteoporosis detection rates in postmenopausal women. Internet J Endocrinol. 2013;2013:895474. doi: 10.1155/2013/895474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Loffler M.T., Jacob A., Valentinitsch A., Rienmuller A., Zimmer C., Ryang Y.M., et al. Improved prediction of incident vertebral fractures using opportunistic QCT compared to DXA. Eur Radiol. 2019;29(9):4980–4989. doi: 10.1007/s00330-019-06018-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Damilakis J., Adams J.E., Guglielmi G., Link T.M. Radiation exposure in X-ray-based imaging techniques used in osteoporosis. Eur Radiol. 2010;20(11):2707–2714. doi: 10.1007/s00330-010-1845-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Formica C., Loro Maria-Luiza, Gilsanz Vicente, Ego Seeman Inhomogeneity in body fat distribution may result in inaccuracy in the measurement of vertebral bone mass. J Bone Miner Res. 1995;10(10):1504–1511. doi: 10.1002/jbmr.5650101011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.