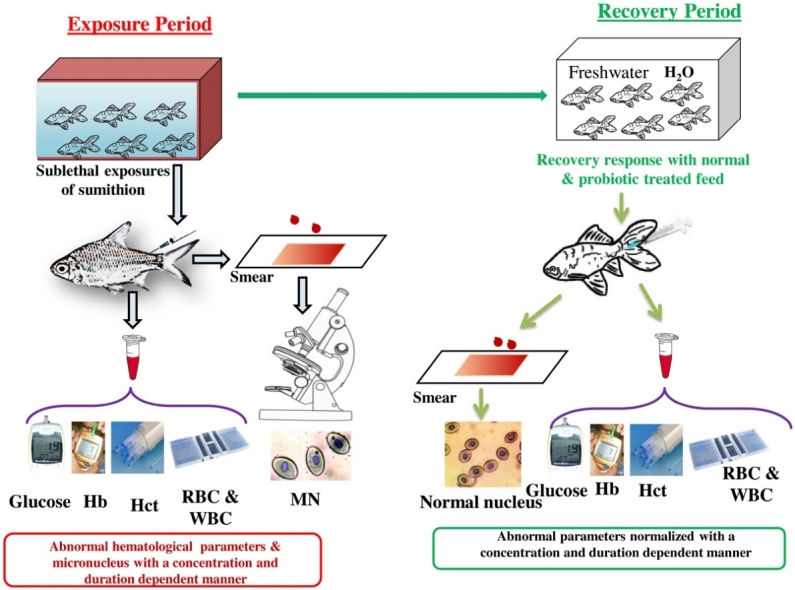
Graphical abstract
Keywords: Hematoxicity, Sublethal, LC50, Probiotic, In vivo exposures, Recovery
Highlights
-
•
Sumithion induced hematoxicity and their recovery pattern in Barbonymus gonionotus was measured.
-
•
Sumithion induces severe formation and prevalence of micronuclei and significantly (p< 0.05) increased white blood cells.
-
•
Blood glucose, red blood cell, hematocrit, and hemoglobin were found to be decreased after the exposure of sumithion.
-
•
In recovery experiment, the recovery rate was significantly higher in probiotic treated groups than other treatments.
-
•
Fish micronucleus and hematology is an effective biomarker for toxicity test of an organophosphate.
Abstract
The experiment was conducted to clarify sumithion induced hematoxicity in silver barb (Barbonymus gonionotus) through in vivo exposures (25 % and 50 % of LC50 of sumithion) and subsequent recovery patterns using normal and probiotic treated feed were also assessed. Three treatments each incorporating three replications were used in the experiment for different days (1, 7, 14, 21, and 28). Treatment T1 was control (0 mg/L), and two concentrations, such as 2.61 mg/L (25 % of 96 h LC50), 5.21 mg/L (50 % of 96 h LC50) were used as Treatment T2 and T3, respectively. After 28 days of exposure to pesticide half of the fishes of T2 and T3 were reared in sumithion free water with normal (T2N, T3N) and probiotic treated feed (T2P, T3P). The median lethal concentration (50 %) for 96 h was 10.42 mg/L. In pesticide-treated groups, values of each hematological parameter (blood glucose, red blood cell, hematocrit, and hemoglobin) decreased but prevalence and severity of micronucleus and white blood cells increased significantly (p< 0.05) with concentration and time duration. Other blood indices including mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH) and mean corpuscular hemoglobin concentration (MCHC) were correspondingly changed in comparison to the control. In the recovery experiment, the silver barb recovered spontaneously, but the recovery rate was significantly higher in probiotic treated groups than normally treated groups in time and duration reliant fashion. In conclusion, persistent sublethal dosages of sumithion caused hematological abnormalities in silver barb. Probiotic supplement can recover the damage but only 28 days of recovery is not enough to recover the total alterations.
1. Introduction
Pesticides are chemical components used to destroy or control pest to meet the growing demand for foods [1]. It was estimated that nearly one-third of the products in agriculture are produced by using pesticides [2]. In 2013, Greenpeace reported that plants did not absorb 70 % of pesticide used in China, but instead seeped into the soil and groundwater [3]. However, indiscriminate overdose and overtime use of pesticides have polluted the environment [[4], [5], [6]]. Among the different types of pesticides, organophosphates (sumithion) have become one of the widely used classes of pesticides in the agricultural region worldwide [7,8]. These pesticides introduced into aquatic environments through surface runoff may cause severe hampers to fish health [9,10]. Therefore, monitoring the impact of these pesticides is essential.
Hematological indices indicate what is happening in the body of fish exposed to pesticide. In some cases, intensity of damage due to exposure to pesticides can be recognize by abnormality levels in some biochemical parameters in the blood [11]. Therefore, hematology is a widely used diagnostic tool to investigate physiological alterations caused by disease or other factors [12]. Besides, understanding recovery pattern after exposure to pesticides are also important in risk assessment. Information on time required to recover completely can help improve and maintain fish health and indirectly human health [13].
Changes in structure and irregularities in erythrocyte are thought to be key indicators of oxidative stress [14,15]. Micronuclei, or additional nuclear bodies of chromosomal fragments, also reveal pesticide-induced physical and chemical changes. As a result, micronuclei are thought to be the best indicator for DNA damage or genetic changes [[16], [17], [18]].
Dietary probiotic supplementation in fish improves growth performance, immune response and the disease resistance [19,20]. Recent studies have shown that probiotic prevents systemic toxin absorption [21] and breakdown pesticides through bioremediation process in environmentally contaminated soil and water [22,23]. However, there is little information on effect of probiotic bacteria in reducing negative consequences of chronic pesticide exposure in fish in vivo. Therefore, the experiment was outlined to know the toxicological effect of organophosphorus pesticides (sumithion) on hematological parameters and its recovery rate using the regular and probiotic treated feed. Silver barb B. gonionotus was selected because it is ecologically and commercially important and similar to local barb species.
2. Materials and methods
2.1. Experiment 1: toxicity assay
The acute toxicity test on Silver barb (B. gonionotus) was done with selected organophosphorus pesticide sumithion 50EC (Bangladesh, Shetu Agro Industries Limited) collected from an authorized dealer of the pesticide. Healthy and homogenous size B. gonionotus were transported from a commercial fish farm and housed in a plastic tank. Fishes with an average weight of 5.9 ± 3.61 g and standard length of 8.11 ± 1.44 cm were allowed to acclimatize for two weeks in the laboratory conditions to remove the unhealthy fishes. Then total 480 individuals were assigned randomly. The fishes were given commercial dry pellets (38 % protein) twice a day. The tank's water was changed in every alternative two days. The fishes were deemed to be well suited to laboratory environment during acclimatization phase of 14 days when mortality less than 1% was recorded. Fish of both sexes were used.
2.2. Preparation of test solution
The stock solution of sumithion was quantified by a micropipette (Model E-MILB5700, England) according to the EC% component. The desired pesticide concentrations were carefully poured into 30 L of de-chlorinated tap water. To ensure complete mixing, the water was gently stirred. The fish were treated with sumithion 50EC to observe the LC50, sub-lethal dose, mortality and symptoms of intoxication.
2.3. Static bioassays
All the glass aquaria used for the study were rinsed and filled up with de-chlorinated tap water before to the commencement of the experiment. For range finding, ten fish were exposed in a fish tank containing 30 L of water to a range of sequential concentrations (2, 3.5, 4.5, 20 mg/L) of sumithion. Mortalities were recorded (24, 48, 72 and 96 h) and dead fishes were removed as quickly as possible.
To estimate the lethal concentrations of sumithion, a static bioassay was accomplished using the standard approach. There were nine groups in the study including one control group with replicates. Each group was treated to various measures of pesticide like 4.5, 6.5, 7.5, 9.5, 11.5, 12, 15 and 17 mg/L for 4 days. Pesticide-free medium was maintained in case of control group. To achieve a homogenous concentration of the toxic chemical, the aquarium was aerated for 2 h. Then 10 fish were chosen at random and transferred to each test aquarium without being stressed. The fish were not fed for 24 h before to the toxicity test. Fish were considered dead when there was no visible movement. Dead fishes were immediately removed and mortality was recorded. Results up to three and six hours after the start of the test are important. Probit analysis was undertaken to determine the LC10, LC50, and LC100 values for corresponding time period.
2.4. Experiment 2: Sub-lethal test of sumithion 50EC compounds on experimental fishes
According to the findings of the 96 h LC50 of sumithion pesticide, 350 silver barb were subjected to a dosage of 25 % and 50 % value of the LC50 of the sumithion for 28 days. Each treatment incorporated double replication along with a control group. The toxicant was applied to each aquarium in the proper levels, except the control tank. The fish were fed a pelleted meal with a crude protein content of 38 % at a rate of 3% body weight. To maintain homogeneity of toxicant, level of dissolved oxygen and to minimize ammonia level, both sumithion and water were exchanged every two days’ interlude. Six fishes from each group were sampled at 7 days’ intervals until the end of the study period of 28 days. No mortalities occurred in any group during the experimental period.
2.5. Estimation of micronucleus
The fish collected from the aquaria were mopped with tissue paper to remove slime from the external surface. Blood samples collected with a disposable syringe from the tail portion of the body were spread onto a glass slides and dried for 10 min at room temperature. The slide was then placed into methanol for 10 min, stained with 5% Giemsa stain and finally cleaned with tap water. The dried and mounted glass slides with DPX were then observed under a microscope (MICROS, MCX100) using the x1000 magnification. For each individual fish, a minimum of three glass slides were put up, with 2000 cells counted from each glass slide. No less than five fishes were examined in each group. Coding and blind scoring were performed on the best cell with unblemished cellular and nuclear films.
2.6. Experiment 3: Effect of sumithion compounds on blood biochemical parameters
For haematology studies a week after the fishes were exposed to sumithion compounds, three fishes from each test aquarium were sacrificed for the hematological assessment. Fish were caught with a hand net and by covering the head with a towel blood samples were obtained from the caudal vein using a micropipette without the use of anesthetics. To eliminate stress effects and reduce an inaccuracy in normal blood values, the complete blood collection operation took less than 1 min/fish. The blood was gently poured into sterilized eppendorf tubes containing anticoagulant (ethylene diamine tetra-acetic acid, EDTA) until a final concentration of 5 mg EDTA per cm3 blood was achieved.
Blood glucose, Hematocrit (Hct, %), hemoglobin (Hb, g dl-1), white blood cells (WBC), and red blood cells (RBC) were determined by using the method of Blaxhall and Daisley [24]. Mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH) and mean corpuscular hemoglobin concentration (MCHC) were calculated by using the following formula:
| MCHC = (Hb ÷ PCV) x 100; |
| MCV = (PCV ÷ RBC) x 10; |
| MCH (pg) = (Hb x 10) ÷ RBC |
2.7. Experiment 4: Determination of the recovery pattern of sumithion toxicity in blood biochemical parameters
2.7.1. Experimental feed preparation
A commercial probiotic (PROCID) containing mainly Bacillus and Lactobacillus sp. (22 × 109 CFU/g) was collected from a well-recognized pharmaceutical company (Eon group, Bangladesh). Advantage of these probiotic is that they are able to survive in the pelletization process. Probiotic mixed feed was prepared by grinding commercial fish feed (Mega Fish Feed Ltd.) containing 38 % protein. Then 1% probiotics were mixed with that grinded feed and homogenous doughy matter was prepared by adding water. The dough was processed through a meat chopper (Brand-Filizola) to produce 2 mm diameter pellets, which were then sun-dried for two days. Then they were stored in plastic containers at refrigerator for further use. Feeding was done twice a day with 3% body weight.
2.7.2. Recovery pattern
After exposure to 25 % and 50 % of LC50 sumithion for 28 days, treated fish were transmitted in separate freshwater aquaria (without sumithion) for another 28 days to observe recovery pattern. Sampling were done at 7 days interval (i.e. 7, 14, 21 and 28 d) with daily renewal of water and feeding. During the experimentation, feeding of fish were done after changing water on alternate day. Each treatment (T2 and T3) were subdivided into two groups (i.e. T2N and T2P) according to feed supplied. In treatment T2N and T3N normal feeding was done but in treatment T2P and T3P 1% probiotic treated feed was used to observe the recovery rate. At least 6 fishes were sampled from each aquarium for recovery assessment. Continuous aeration system was applied for sufficient dissolved oxygen.
2.8. Water quality parameters
Water temperature and water chemistry characteristics of dissolved oxygen and pH were monitored according to APHA (1995) in all test aquaria every day. The temperature, dissolved oxygen and pH were measured by DO meter (Model 2020, UK) and pH meter (Model YSI 58, USA), respectively.
2.9. Statistical analysis
Statistical analysis was carried out using the one-way ANOVA to find out dissimilarities between treatments means at significance rate of P < 0.05. The standard errors of treatment means were also determined. All statistics were performed using SPSS 16.0 version.
3. Results
3.1. Static bioassay - study of lethal concentration
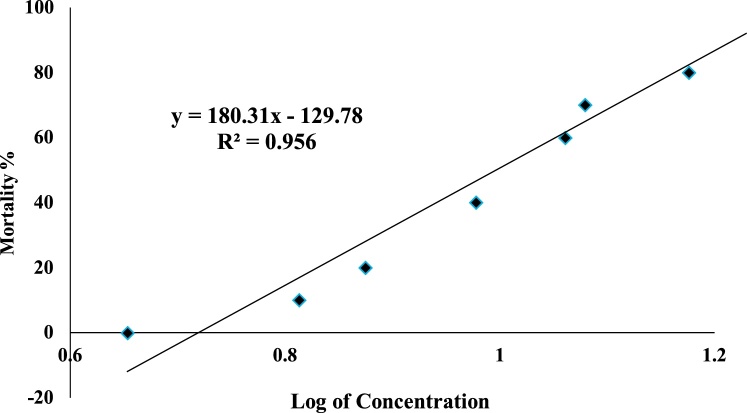
Based on the range finding test, the median lethal concentration (LC50) of B. gonionotus was to be between 4.5 mg/L and 20 mg/L of sumithion. A median lethal toxicity investigation was conducted with sumithion concentrations ranging from 4.5–17 mg/L. The study showed that practically there was no mortality noticed up to a concentration of 4.5 mg/L. The subjection of fish to 96 h of sumithion at a dose of 6.5 mg/L resulted in 10 % death, while at a quantity of 17 mg/L, 100 % death was recorded. According to the probit study, the fatal concentration for 50 percent fish mortality at 96 h was 10.42 mg/L (Table 1 and Fig. 1).
Table 1.
Lethal toxicity value of sumithion at 96 h in B. gonionotus.
| Probability | 95 % Confidence Limits for concentration |
95 % Confidence Limits for log(concentration)a |
||||||
|---|---|---|---|---|---|---|---|---|
| Estimate | Lower Bound | Upper Bound | Estimate | Lower Bound | Upper Bound | |||
| PROBIT | 0.01 | 4.437 | 2.226 | 5.841 | 0.647 | 0.347 | 0.767 | |
| 0.1 | 6.511 | 4.396 | 7.740 | 0.814 | 0.643 | 1.889 | ||
| 0.2 | 7.653 | 5.795 | 8.806 | 0.884 | 0.763 | 0.945 | ||
| 0.3 | 8.598 | 6.993 | 9.772 | 0.934 | 0.845 | 0.990 | ||
| 0.4 | 9.497 | 8.092 | 10.839 | 0.978 | 0.908 | 1.035 | ||
| 0.5 | 10.422 | 9.109 | 12.159 | 1.018 | 0.959 | 1.085 | ||
| 0.6 | 11.438 | 10.069 | 13.891 | 1.058 | 1.003 | 1.143 | ||
| 0.7 | 12.635 | 11.043 | 16.256 | 1.102 | 1.043 | 1.211 | ||
| 0.8 | 14.195 | 12.165 | 19.762 | 1.152 | 1.085 | 1.296 | ||
| 0.9 | 16.682 | 13.775 | 26.169 | 1.222 | 1.139 | 1.418 | ||
| 0.99 | 24.480 | 18.192 | 51.862 | 1.389 | 1.260 | 1.715 | ||
Logarithm base = 10.
Fig. 1.
Graph showing linear transformation and the relationship of probit of log concentration of sumithion used to determine LC50.
3.2. Water quality parameters
The water quality parameters (temperature, dissolved oxygen and pH) were monitored during the recovery and exposure period of sumithion 50EC at various concentrations as well as with control (Table 2). Temperature values were almost uniform in all the treatments throughout the experiment. pH values increased with increasing concentrations of sumithion. Dissolved oxygen values tended to decrease with increasing the level of the test chemicals. During recovery period pH values and dissolved oxygen values were also almost uniform. However, values between treatments were not significantly different (P > 0.05) in recovery and exposure period. Continuous aeration system was applied during recovery assessment. Again, normal and probiotic feeds were supplied.
Table 2.
Water quality parameters (Mean ± SD) during exposure period of malathion.
| Treatments | Temperature (°C) | pH | Dissolved oxygen (mg/l) |
|---|---|---|---|
| Exposure Period | |||
| T1 (0 mg/L) | 25.58 ± 0.67 | 7.50 ± 0.06 | 5.18 ± 0.09 |
| T2 (2.61 mg/L) | 25.55 ± 0.65 | 7.68 ± 0.12 | 4.90 ± 0.13 |
| T3 (5.21 mg/L) | 25.6 ± 0.69 | 7.86 ± 0.09 | 4.70 ± 0.17 |
| Recovery Period | |||
| T1 (0 mg/L) | 25.60 ± 0.50 | 7.51 ± 0.05 | 5.15 ± 0.19 |
| T2N (2.61 mg/L) | 25.63 ± 0.33 | 7.58 ± 0.06 | 5.03 ± 0.19 |
| T2P (2.61 mg/L) | 25.57 ± 0.31 | 7.55 ± 0.03 | 5.04 ± 0.11 |
| T3N (5.21 mg/L) | 25.81 ± 0.70 | 7.56 ± 0.04 | 5.08 ± 0.24 |
| T3P (5.21 mg/L) | 25.77 ± 0.64 | 7.51 ± 0.02 | 5.01 ± 0.17 |
3.3. Effects of sumithion on micronucleus (MN) formation
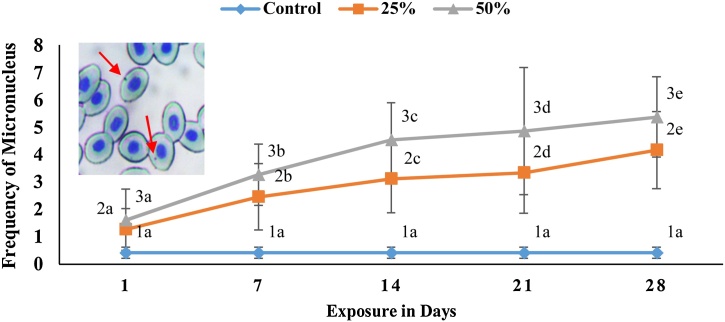
In pesticide free waters, rate of micronuclei was nearly zero (0.42 ± 0.21), but a significant rise (p < 0.05) in occurrence of MN was recorded in a concentration and duration dependent manner in fishes that have been subjected to sublethal doses 25 % and 50 % of LC50 of sumithion compared to the control group. The occurrence of MN was 1.27 ± 0.76, 2.46 ± 1.21, 3.11 ± 1.23, 3.34 ± 1.47, 4.17 ± 1.41 for 1, 7, 14, 21 and 28 days, respectively at 25 % concentration. Similarly, at 50 % concentrations, micronuclei abnormality was 1.61 ± 1.13, 3.27 ± 1.12, 4.54 ± 1.37, 4.87 ± 2.33, 5.38 ± 1.47 for 1, 7, 14, 21 and 28 days of exposure, respectively. At higher concentrations, there was a three to five-fold increase in the frequency of MN, indicating pesticide genotoxicity (Fig. 2).
Fig. 2.
Micronuclei (MN) of silver barb exposed to different sublethal concentrations of sumithion. Values with different numeric superscripts differ significantly (p < 0.05) between concentrations within duration in MN. Values with different alphabet superscripts differ significantly (p < 0.05) between duration within concentration in MN. All values expressed as mean ± SD. Photograph in the inset is showing MN (arrow).
3.4. Effects of sumithion on hematological parameters
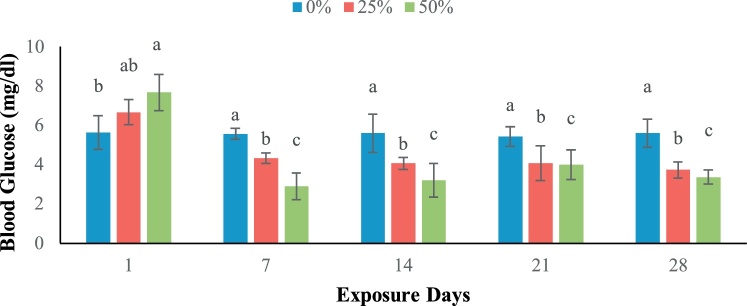
Different hematological parameters were measured at 1, 7, 14, 21 and 28 days later of the subjection of different sub-lethal concentration of sumithion (Table 3). The blood glucose level significantly reduced at 7, 14, 21 and 28 days of exposure in treatment T2 and T3 (Fig. 3).
Table 3.
Effects of sub−lethal exposure of sumithion on hematology (Means ± SD) at different time intervals in Silver barb. Different superscript alphabets are significantly different at P<0.05.
| Parameter | Treatments | Exposure time (days) |
||||
|---|---|---|---|---|---|---|
| 1 | 7 | 14 | 21 | 28 | ||
| Hemoglobin | T1 | 11.67 ± 0.45a | 12.10 ± 0.24 a | 11.93 ± 0.23 a | 11.97 ± 0.29 a | 11.73 ± 0.19 a |
| T2 | 11.53 ± 0.60 ab | 11.33 ± 0.31 b | 10.83 ± 0.29 b | 10.13 ± 0.19 b | 9.30 ± 0.15 b | |
| T3 | 10.90 ± 0.24 b | 10.23 ± 0.52 c | 10.03 ± 0.34 c | 9.07 ± 0.14 c | 7.97 ± 0.27 c | |
| Hematocrit/PCV | T1 | 46.67 ± 0.85 a | 43.05 ± 0.21 a | 45.14 ± 0.25 a | 44.44 ± 2.46 a | 44.31 ± 3.56 a |
| T2 | 46.52 ± 1.15 a | 42.47 ± 0.73 a | 38.76 ± 0.90 b | 35.58 ± 0.92 b | 31.00 ± 1.31 b | |
| T3 | 44.33 ± 1.38 c | 33.91 ± 1.37 c | 27.37 ± 1.79 c | 24.88 ± 1.75 c | 22.19 ± 2.19 c | |
| RBCs (x 106/mm3) | T1 | 5.68 ± 0.22 a | 5.50 ± 0.45 a | 5.46 ± 0.46 a | 5.71 ± 0.08 a | 5.66 ± 0.10 a |
| T2 | 5.27 ± 0.30 ab | 4.84 ± 0.49 b | 4.53 ± 0.24 b | 3.96 ± 0.57 b | 3.27 ± 0.41 b | |
| T3 | 5.14 ± 0.34 b | 4.11 ± 0.11 c | 3.23 ± 0.10 c | 2.34 ± 0.06 c | 2.07 ± 0.04 c | |
| WBCs (×104/mm3) | T1 | 3.01 ± 0.16 a | 2.96 ± 0.24 c | 3.00 ± 0.14 c | 2.92 ± 0.24 a | 2.94 ± 0.06 c |
| T2 | 3.08 ± 0.14 a | 3.62 ± 0.19 b | 3.80 ± 0.61 b | 4.31 ± 0.21 b | 4.42 ± 0.25 b | |
| T3 | 3.03 ± 0.15 a | 4.37 ± 0.25 a | 4.44 ± 0.18 a | 5.16 ± 0.24 a | 5.67 ± 0.11 a | |
| MCV (μm3) | T1 | 82.27 ± 4.71 a | 78.69 ± 6.94 b | 83.19 ± 7.51 a | 77.75 ± 3.36 c | 78.34 ± 6.80 b |
| T2 | 88.55 ± 6.24 a | 88.45 ± 8.45 b | 85.66 ± 3.76 a | 91.04 ± 9.89 b | 96.44 ± 14.86 a | |
| T3 | 86.57 ± 5.59 a | 82.50 ± 2.36 a | 84.87 ± 7.16 a | 106.33 ± 7.17 a | 107.47 ± 12.40 a | |
| MCH (pg) | T1 | 20.54 ± 0.74 a | 22.13 ± 2.18 a | 21.96 ± 1.53 b | 20.95 ± 0.51 c | 20.74 ± 0.66 c |
| T2 | 21.99 ± 2.32 a | 23.62 ± 2.51 a | 23.94 ± 1.09 b | 25.99 ± 3.42 b | 28.83 ± 3.53 b | |
| T3 | 21.30 ± 1.55 a | 24.89 ± 0.90 a | 31.09 ± 1.53 a | 38.76 ± 0.79 a | 38.55 ± 2.10 a | |
| MCHC (%) | T1 | 25.01 ± 1.28 a | 28.11 ± 0.42 b | 26.44 ± 0.53 b | 26.97 ± 1.10 b | 26.59 ± 1.72 c |
| T2 | 24.79 ± 1.19 a | 26.68 ± 0.35 c | 27.96 ± 1.12 b | 28.49 ± 0.83 b | 30.04 ± 1.29 b | |
| T3 | 24.60 ± 0.41 a | 30.17 ± 0.32 a | 36.72 ± 1.26 a | 36.61 ± 2.93 a | 36.09 ± 2.40 a | |
Fig. 3.
Blood glucose level in different exposure periods.
In the present study, the Hb, Hct (%)/PCV, RBC were also significantly decreased (P < 0.05) at 7, 14, 21 and 28 days of exposure time in two concentrations (T2 and T3) compared to control group (T1). The WBC significantly increased (P < 0.05) during toxicity of sumithion in a time dependent manner in both T2 and T3 treatments compared to T1 (Control). Other hematological indices including MCV and MCH increased significantly (P < 0.05) from 1, 7, 14, 21, 28 days of exposure in T2 and T3. MCHC values decreased initially but at 14, 21, and 28 days MCHC values was increased in T2 and T3 during experimental period of 28 days.
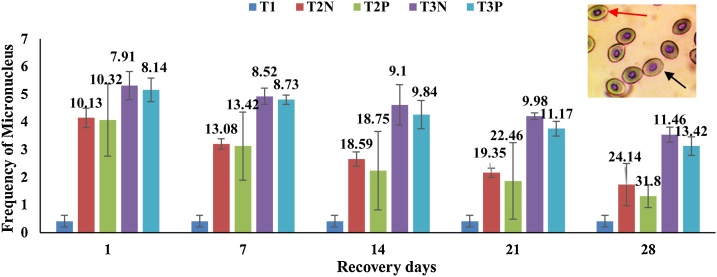
3.5. Recovery response of MN induced by sumithion
The data of the recovery responses of MN are presented in Fig. 4. The value of MN content reduced at a time dependent manner in all the treatments during the recovery period. The recovery rate at different concentration of sumithion varied significantly (p < 0.05). The results showed that the recovery rate is higher at 25 % concentration level of sumithion than 50 % concentration. Again the recovery rate is higher at probiotic treated feed compared to normal feed. The treatment T2P has a higher healing rate than the 50 % concentration. Fishes recovered partially and the recovery rate is highest at 28 days at a concentration of 25 %, according to the results (Fig. 4).
Fig. 4.
Recovery responses of MN induced by sumithion (Means ± SD) at different time intervals in B. gonionotus. Data label showing recovery rates (%) at different time intervals. Photograph in the inset is showing normal nuclei (black arrow) and micronuclei (red arrow) (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article).
3.6. Hematological parameter after recovery assessment
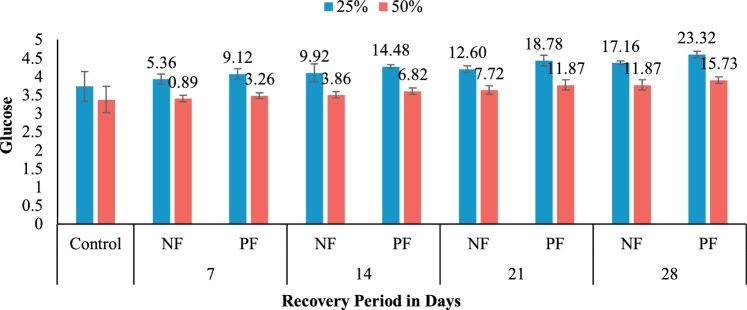
After 28 days of treatment, fishes were released in pesticide free environment. Hematological parameters like blood glucose, haemoglobin, hematocrit, RBC, WBC were observed (Table 4). Those were gradually changed due to the removal of pesticides. Blood glucose were increased but not reach to normal condition (Fig. 5). Hemoglobin, Hematocrit and RBC shown the similar changes like blood glucose. But in contrast, WBC was reduced from high level to normal level. Again the fishes which treated with probiotics were recover more than fishes which were treated with normal feeds. The highest recovery rate of glucose was 23.32 % (4.60 ± 0.09) at 28 days’ recovery period in T2P and the lowest recovery rate was observed 0.89 % (3.40 ± 0.09) after 7 days of recovery period in T3N. Probiotic reduce the bioaccumulation of pesticide in vivo.
Table 4.
Recovery pattern of Silver barb (Means ± SD) at different time intervals (T2: 25 % of LC50; T3: 50 % of LC50). Different superscript alphabets are significantly different at P<0.05.
| Parameter | Treatments | After 28 days exposure | Exposure time to pesticide free water in days (Percentage Recovery rate) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 7 |
14 |
21 |
28 |
|||||||
| Normal Feed | Probiotic Feed | Normal Feed | Probiotic Feed | Normal Feed | Probiotic Feed | Normal Feed | Probiotic Feed | |||
| Hemoglobin | T2 | 9.30 ± 0.15a | 9.70 ± 0.09b | 9.80 ± 0.09b | 9.95 ± 0.21b | 10.20 ± 0.09c | 10.27 ± 0.14b | 10.50 ± 0.09c | 10.60 ± 0.09b | 10.83 ± 0.05c |
| (4.13 %) | (5.38 %) | (6.99 %) | (9.68 %) | (10.43 %) | (12.9 %) | (13.98 %) | (16.45 %) | |||
| T3 | 7.97 ± 0.27x | 8.20 ± 0.09y | 8.33 ± 0.05z | 8.35 ± 0.10y | 8.50 ± 0.09z | 8.65 ± 0.10y | 8.83 ± 0.22y | 8.90 ± 0.18y | 9.07 ± 0.14y | |
| (2.89 %) | (4.52 %) | (4.77 %) | (6.65 %) | (8.53 %) | (10.79 %) | (11.67 %) | (13.8 %) | |||
| Hematocrit/PCV | T2 | 31.00 ± 1.31a | 33.17 ± 0.75b | 34.17 ± 0.68c | 34.83 ± 0.92b | 36.74 ± 1.05c | 35.99 ± 0.88b | 38.34 ± 0.77c | 37.36 ± 1.07b | 40.19 ± 1.26c |
| (7.00 %) | (10.23 %) | (12.35 %) | (18.52 %) | (16.1 %) | (23.68 %) | (20.52 %) | (29.65 %) | |||
| T3 | 22.19 ± 2.19x | 23.28 ± 0.95xy | 23.63 ± 1.00y | 24.31 ± 0.55y | 25.26 ± 0.80z | 25.39 ± 0.63y | 26.59 ± 0.86z | 26.12 ± 1.05y | 27.45 ± 0.81z | |
| (4.91 %) | (6.49 %) | (9.55 %) | (13.84 %) | (14.42 %) | (19.83 %) | (17.71 %) | (23.7 %) | |||
| RBCs (x 106/mm3) | T2 | 3.27 ± 0.41a | 3.44 ± 0.05b | 3.57 ± 0.09c | 3.56 ± 0.07b | 3.75 ± 0.09c | 3.70 ± 0.05b | 4.02 ± 0.04c | 3.94 ± 0.07b | 4.28 ± 0.06c |
| (5.21 %) | (9.17 %) | (8.87 %) | (14.685) | (13.15 %) | (22.94 %) | (20.49 %) | (30.89 %) | |||
| T3 | 2.07 ± 0.04x | 2.13 ± 0.04y | 2.19 ± 0.06y | 2.21 ± 0.02y | 2.27 ± 0.03z | 2.29 ± 0.05y | 2.44 ± 0.02z | 2.44 ± 0.05y | 2.59 ± 0.04z | |
| (2.91 %) | (5.81 %) | (6.76 %) | (9.66 %) | (10.63 %) | (17.87 %) | (17.87 %) | (25.12 %) | |||
| WBCs (×104/mm3) | T2 | 4.42 ± 0.25a | 4.28 ± 0.03b | 4.21 ± 0.08b | 4.13 ± 0.07b | 3.92 ± 0.16c | 3.96 ± 0.17b | 3.73 ± 0.04c | 3.73 ± 0.08b | 3.63 ± 0.09c |
| (-3.17 %) | (-4.75 %) | (-6.56 %) | (-11.31 %) | (-10.41 %) | (-15.61 %) | (-15.61 %) | (-17.87 %) | |||
| T3 | 5.67 ± 0.11x | 5.60 ± 0.07xy | 5.54 ± 0.12y | 5.49 ± 0.08y | 5.41 ± 0.05z | 5.33 ± 0.04y | 5.22 ± 0.03z | 5.13 ± 0.13y | 5.01 ± 0.15y | |
| (-1.23 %) | (-2.29 %) | (-3.17 %) | (-4.59 %) | (-6.01 %) | (-7.94 %) | (-7.94 %) | (-11.64 %) | |||
Values are mean ± SD. Different superscript alphabets show significant difference (p< 0.05).
Fig. 5.
Blood Glucose level in recovery period. NF = Normal Feed, PF = Probiotic treated Feed. Data label showing recovery rates (%) at different time intervals.
4. Discussion
The LC50 of the organophosphorus pesticide, sumithion was found 10.42 mg/L for B. gonionotus. Acute toxicity effects of another organophosphorus pesticide, Diazinon on Swordtail (Xiphophorus helleri) was found 2.87 mg/L [25]. The determined half lethal concentration (LC50) of diazinon for Clarias gariepinus was 5.98 mg/L at 96 h [26]. The 96 h LC50 values of sumithion on Heteropneustes fossilis larvae was estimated to be 6.782 mg/L [10]. Sumithion's median fatal concentration (96 -h LC50) for striped catfish fingerlings was 5.886 mg/l [27]. These results suggest that the difference in LC50 of pesticide is a function of size and species of the fish. Insecticides can alter a fish's hematological profile, which can be utilized as a biomarker for pollution monitoring [28]. Present findings showed that sumithion had some effects on the hematological parameters of Silver barb. Although the LC50 dose of sumithion was 10.42 mg/L, it is shown that the 25 % and 50 % of the LC50 dose examined in this study also causes essential changes in the hematological properties of the Silver barb.
The development of micronuclei is a marker for chromosomal instability and cytotoxic/genotoxic events. The micronuclei of fish have been shown to be a useful tool for evaluating the genotoxic properties of pesticides found in the aquatic environment [[16], [17], [18]]. The current findings revealed a concentration and time-dependent increase in the occurrence of micronuclei in silver barb's erythrocytes that is in line with the findings of Ahmed [29]. In mice, for example, coexistence of cypermethrin and quinalphos can increase genotoxicity, and even quinalphos alone has been proven to cause considerable chromosome damage [30]. Rupa [31] demonstrated that quinalphos could induce chromosome breaks/fragments in mice and human lymphocytes. Therefore, in the current experiment MN demonstrated that excessive use of pesticide can result genetic damage in the silver barb.
In this study, recovery pattern showed the greatest drop in micronuclei creation and the strongest recovery response in T2P, which could imply DNA repair, loss of damaged cells, or both, as conferred by Grover [32]. This inverse relationship between application time and DNA damage could be due to the risk of xenobiotics irritating enzymatic operations involved in DNA damage activity [33]. The role of probiotic in micronucleus denegation is still unknown. The micronucleus frequency was substantially lower in probiotic-treated feed than in control feed in B. gonionotus, and it recovered significantly. This indicated that addition of probiotic could significantly reduce (p < 0.05) the genotoxicity.
In the present study, blood glucose level increased initially and then decreased significantly with the progressive increase of sumithion concentrations explains the stressful state of the fish when exposed to pesticides. This increase could be related to stressed fish's higher gluconeogenesis response in order to meet their higher energy needs [34]. Increased blood glucose levels represent a higher level of glucose transit maybe from the liver to muscle where high energy requirement was fulfilled due to rapid and irregular moves. The reduction in blood glucose level was perhaps due to the energy demanded to cope with energetic expenditures caused by pesticide [35,36].
The amount of hemoglobin in the blood of fish exposed to various sumithion concentrations reduced considerably. When common carp was exposed to sumithion [37] and malathion [38] it showed a comparable drop in Hb levels. In the present study the observed reduction in Hb levels in B. gonionotus could be attributed to pesticides disrupting the erythropoietic tissue which might be affected the viability of the cells which is corroborated by Mostakim et al. [39].
The decrease in packed cell volume PCV (%) or Hct (%) indicated that the fish was suffering from anaemia. Similar to the present findings, Chindah [40] discovered that different sub-lethal amounts of cypermethrin and chlorpyrifos reduced the value of Hct in Tilapia guineensis juveniles. Koprucu [41], Jayaprakash and Shettu [42] also reported similar observations.
Similar to Hb and Hct, the number of RBC was shown to be reduced in fish exposed to varied doses of sumithion, possibly due to hematopoietic system failure. A drop in RBC count is caused by erythropoiesis inhibition and a rise in the rate of erythrocyte degradation in the hematopoietic organs [43]. Development of hypoxic condition during the treatment can also be responsible for decreased RBC which intern leads to the destruction of RBC or due to unavailability of Hb content in cellular medium [44].
During the exposure period to sumithion, enhanced WBC count indicates that fish can overcome toxic stress by developing a defensive mechanism. In the presence of foreign substances, leucocytosis in fish stimulates immunological defense [45]. High WBC counts suggest damage to body tissues from infection, extreme physical stress, or even leukemia [46]. Increased WBC counts are linked to increased antibody synthesis, which aids in the recovery and survival of pesticide-exposed fish [37,38,43]. Other hematological indices including MCV, MCH and MCHC significantly increased in the experimental fish after 28 days of subjection to pesticide compared to the control group. Perturbations in these indices correspond with RBC, Hb, and Hct levels. Increase in MCV and MCH values indicate the symptoms of anaemia in animals [47].
Probiotics helped the fishes to recover rapidly than others because probiotic enhances the digestive process [48]. Recovery in T2P was higher than T3P, which might be due to the treatment T2 (25 % of LC50) had a lower dose of exposure than T3 (50 % of LC50). It was not possible to reach normal conditions. Because severe physiological alterations occurred due to pesticidal effects. But probiotics can metabolize organophosphate pesticides via phosphatase enzymes; again, it has organophosphate pesticide degrading ability [49]. Pesticides frequently enter the gastrointestinal tract and probiotics enhance gastrointestinal barrier function. Through bioremediation process probiotic bacteria can breakdown pesticides [22,23]. The gastrointestinal microbiota plays an important role in preventing systemic toxin absorption [21]. The adaptability of fish to toxic stress may have contributed to the moderate recovery of some hematological parameters during the recovery phase. However, in order to handle the stress and maintain normal physiology, a longer recovery period was required.
5. Conclusion
The findings indicated that the sumithion is moderately toxic to Silver barb at sub-lethal levels. The present study also suggests that probiotics help sumithion exposure fishes to recover their abnormalities. However, the fish's recuperation period (28 days) is insufficient to return the parameters to their normal state. The improvement in blood parameters of the test fish after being transplanted to pesticide-free freshwater implies that pesticides are gradually removed from the system. Probiotics work as a potential tool in contaminants remediation. As a result, the use of sumithion in agriculture and aquaculture must be carefully examined from an eco-physiological standpoint.
Data availability
Data will be made available on request.
Ethical statement
-
1)
that all authors have seen and approved the final version of the manuscript being submitted
-
2)
that the article is the authors' original work,
-
3)
the manuscript hasn't received prior publication and
-
4)
the manuscript isn't under consideration for publication elsewhere.
Declaration of Competing Interest
The authors declare that they have no conflict of interest.
Acknowledgement
This research was funded by University Grants Commission of Bangladesh (UGC).
Handling Editor: DR. Aristidis Tsatsakis
References
- 1.Ullah S. 2015. Protective Role of Vitamin C against Cypermethrin Induced Toxicity in Labeo rohita (Ham.): Biochemical Aspects (Doctoral Dissertation, M. Phil Thesis, Department of Animal Sciences, Quaid-i-Azam University, Islamabad, Pakistan) [Google Scholar]
- 2.Zhang W.J., van der Werf W., Pang Y. A simulation model for vegetable-insect pest-insect nucleopolyhedrovirus epidemic system. J. Environ. Entomol. 2011;33(3):283–301. [Google Scholar]
- 3.Fan L.Y. 2017. China Founds Pesticide Office to Combat Pollution, Overuse. Retrived from https://www.sixthtone.com/news/1000987/china-founds-pesticide-office-to-combat-pollution%2C-overuse. Assessed November, 7, 2017. [Google Scholar]
- 4.Pimentel D. Environmental and economic costs of the application of pesticides primarily in the United States. Integrated Pest Management: Innovation-Dev. Process. 2009:89–111. doi: 10.1007/978-1-4020-8992-34. [DOI] [Google Scholar]
- 5.Liu L.H., Zhong L.Q., Li M.Q. An epidemiological review on pesticide poisoning in China. China Occupational Med. 2008;35(6):518–520. [Google Scholar]
- 6.Zhang W., Liu G., Ferrarini A. Situation and development of worldwide agri-environment: agricultural land uses, fertilizers consumption and carbon dioxide equivalent emissions. Environ. Skeptics and Critics. 2017;6(1):1–8. [Google Scholar]
- 7.Subburaj A., Jawahar P., Jayakumar N., Srinivasan A., Ahilan B. Acute toxicity bioassay of Malathion (EC 50%) on the fish, Oreochromis mossambicus (Tilapia) and associated histological alterations in gills. J. Entomol. Zool. Stud. 2018;6(1):103–107. [Google Scholar]
- 8.Aktar W., Sengupta D., Chowdhury A. Impact of pesticides use in agriculture: their benefits and hazards. Interdiscip. Toxicol. 2009;2(1):1–12. doi: 10.2478/v10102-009-0001-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arjmandi R., Tavakol M., Shayeghi M. Determination of organophosphorus insecticide residues in the rice paddies. Int. J. Environ. Sci. Technol. 2010;7(1):175–182. doi: 10.1007/BF03326129. [DOI] [Google Scholar]
- 10.Das T., Rahman M.M., Hossen M.S., Mollah M.F.A. Toxicity effects of sumithion on the breeding performance and viability of eggs, embryos and subsequent growth indices of Heteropneustes fossilis larvae. J. Bangladesh Agric. Univ. 2016;14(2):243–251. doi: 10.3329/jbau.v14i2.32700. [DOI] [Google Scholar]
- 11.Banaee M. InTech; 2013. Physiological dysfunction in fish after insecticides exposure. Insecticides–Development of Safer and more Effective Technologies; pp. 103–143. [DOI] [Google Scholar]
- 12.Sattanathan G., Amsath A., Senthilmurugan S., Tamizhazhagan V. Toxicity of copper oxychloride (fungicide) in Oreochromis mossambicus on haemato-immunological and biochemical alterations and recovery assessment by marine algae Chaetomorpha aerea. Int. J. Zoology Appl. Biosci. 2019;4:184–194. doi: 10.5281/zenodo.3478156. [DOI] [Google Scholar]
- 13.Adhikari S., Sarkar B., Chatterjee A., Mahapatra C.T., Ayyappan S. Effects of cypermethrin and carbofuran on certain hematological parameters and prediction of their recovery in a freshwater teleost, Labeo rohita (Hamilton) Ecotoxicol. Environ. Saf. 2004;58(2):220–226. doi: 10.1016/j.ecoenv.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 14.Celik A., Birgul M., Yusuf C., Askin A., Comelekoglu U. Induction of micronuclei by lambda-cyhalothrinin wistar rat bone marrow and gut epithelial cell. Mutation. 2005;20:125–129. doi: 10.1093/mutage/gei020. [DOI] [PubMed] [Google Scholar]
- 15.Hussain R., Mahmood F., Khan A., Javed M.T., Rehan S., Mehdi T. Cellular and biochemical effects induced by atrazine on blood of male Japanese quail (Coturnix japonica) Pestic. Biochem. Physiol. 2012;103(1):38–42. doi: 10.1016/j.pestbp.2012.03.001. [DOI] [Google Scholar]
- 16.Islam M.S., Khan M.M., Moniruzzaman M., Mostakim G.M., Rahman M.K. Recuperation patterns in fish with reference to recovery of erythrocytes in Barbonymus gonionotus disordered by an organophosphate. Int. J. Environ. Sci. Technol. 2019;16:7535–7544. doi: 10.1007/s13762-019-02425-0. [DOI] [Google Scholar]
- 17.Khan M.M., Moniruzzaman M., Mostakim G.M., Khan M.S.R., Rahmand M.K., Islam M.S. Aberrations of the peripheral erythrocytes and its recovery patterns in a freshwater teleost, silver barb exposed to profenofos. Environ. Pollut. 2018;234:830–837. doi: 10.1016/j.envpol.2017.12.033. [DOI] [PubMed] [Google Scholar]
- 18.Sadiqul I.M., Zannatul F., Tanvir A.N.M., Mostakim G.M., Khalilur R.M. Acute exposure to a quinalphos containing insecticide (convoy) causes genetic damage and nuclear changes in peripheral erythrocytes of silver barb, Barbonymus gonionotus. Environ. Pollut. 2016;219:949–956. doi: 10.1016/j.envpol.2016.09.066. [DOI] [PubMed] [Google Scholar]
- 19.Buruiana C.T., Profir A.G., Vizireanu C. Effects of probiotic Bacillus species in aquaculture–an overview. The Annals of the University Dunarea de Jos of Galati. Fascicle VI-Food Technol. 2014;38(2):9–17. [Google Scholar]
- 20.Hoseinifar S.H., Roosta Z., Hajimoradloo A., Vakili F. The effects of Lactobacillus acidophilus as feed supplement on skin mucosal immune parameters, intestinal microbiota, stress resistance and growth performance of black swordtail (Xiphophorus helleri) Fish Shellfish Immunol. 2015;42(2):533–538. doi: 10.1016/j.fsi.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 21.Bisanz J.E., Enos M.K., Mwanga J.R., Changalucha J., Burton J.P., Gloor G.B., Reid G. Randomized open-label pilot study of the influence of probiotics and the gut microbiome on toxic metal levels in Tanzanian pregnant women and school children. MBio. 2014;5:e01580–14. doi: 10.1128/mBio.01580-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barman D.N., Haque M.A., Islam S.M.A., Yun H.D., Kim M.K. Cloning and expression of ophB gene encoding organophosphorus hydrolase from endophytic Pseudomonas sp. BF1-3 degrades organophosphorus pesticide chlorpyrifos. Ecotoxicol. Environ. Saf. 2014;108:135–141. doi: 10.1016/j.ecoenv.2014.06.023. [DOI] [PubMed] [Google Scholar]
- 23.Yuan Y., Yang C., Song C., Jiang H., Mulchandani A., Qiao C. Anchorage of GFP fusion on the cell surface of Pseudomonas putida. Biodegradation. 2011;22:51–61. doi: 10.1007/s10532-010-9375-7. [DOI] [PubMed] [Google Scholar]
- 24.Blaxhall P.C., Daisley K.W. Routine haematological methods for use with fish blood. J. Fish Biol. 1973;5(6):771–781. doi: 10.1111/j.1095-8649.1973.tb04510.x. [DOI] [Google Scholar]
- 25.Khalili M., Khaleghi S.R., Hedayati A. Acute toxicity test of two pesticides diazinon and deltamethrin, on swordtail fish (Xiphophorus helleri) Glob. Vet. 2012;8(5):541–545. [Google Scholar]
- 26.Bakhshwan S., Hamed H., Marzouk M., Hanna M. Some investigations on the clinical and biochemical alterations associated with dizinon toxicity in Clarias gariepinus. Egypt. J. Aquat. Biol. Fish. 2009;13(2):173–197. doi: 10.21608/ejabf.2009.2039. [DOI] [Google Scholar]
- 27.Islam S.M., Rahman M.A., Nahar S., Uddin M.H., Haque M.M., Shahjahan M. Acute toxicity of an organophosphate insecticide sumithion to striped catfish Pangasianodon hypophthalmus. Toxicol. Rep. 2019;6:957–962. doi: 10.1016/j.toxrep.2019.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kavitha P., Rao J.V. Sub-lethal effects of profenofos on tissue-specific antioxidative responses in a Euryhyaline fish, Oreochromis mossambicus. Ecotoxicol. Environ. Saf. 2009;72(6):1727–1733. doi: 10.1016/j.ecoenv.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 29.Ahmed M.K., Habibullah-Al-Mamun M., Hossain M.A., Arif M., Parvin E., Akter M.S., Khan M.S., Islam M.M. Assessing the genotoxic potentials of arsenic in tilapia (Oreochromis mossambicus) using alkaline comet assay and micronucleus test. Chemosphere. 2011;84:143–149. doi: 10.1016/j.chemosphere.2011.02.025. [DOI] [PubMed] [Google Scholar]
- 30.Chauhan L.K.S., Chandra S., Saxena P.N., Gupta S.K. In vivo cytogenetic effects of a commercially formulated mixture of cypermethrin and quinalphos in mice. Mutat. Res. 2005;587:120–125. doi: 10.1016/j.mrgentox.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 31.Rupa D.S., Reddy P.P., Reddy O.S. Cytogenetic effects of quinalphos in mice. Food Chem. Toxicol. 1991;29:115–117. doi: 10.1016/0278-6915(91)90165-4. [DOI] [PubMed] [Google Scholar]
- 32.Grover P., Banu B.S., Devi K.D., Begum S. In vivo genotoxic effects of mercuric chloride in rat peripheral blood leucocytes using comet assay. Toxicology. 2001;167:191–197. doi: 10.1016/s0300-483x(01)00469-3. [DOI] [PubMed] [Google Scholar]
- 33.Rank J., Jensen K. Comet assay on gill cells and hemocytes from the blue mussel Mytilus edulis. Ecotoxicol. Environ. Saf. 2003;54(3):323–329. doi: 10.1016/s0147-6513(02)00006-4. [DOI] [PubMed] [Google Scholar]
- 34.Winkaler E.U., Santos T.R., Machado-Neto J.G., Martinez C.B. Acute lethal and sublethal effects of neem leaf extract on the neotropical freshwater fish Prochilodus lineatus. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2007;145(2):236–244. doi: 10.1016/j.cbpc.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 35.Hori T.S.F., Avilez I.M., Inoue L.K., Moraes G. Metabolical changes induced by chronic phenol exposure in matrinxã Brycon cephalus (teleostei: characidae) juveniles. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2006;143(1):67–72. doi: 10.1016/j.cbpc.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 36.Abdel-Hameid N.A.H. Physiological and histopathological alterations induced by phenol exposure in Oreochromis aureus juveniles. Turk. J. Fish. Aquat. Sci. 2007;7(2):131–138. [Google Scholar]
- 37.Salam M.A., Sharmin S., Haque F., Shahjahan M. Acute toxicity of sumithion and its effects on liver morphology in common carp, cyprinus carpio. 5th International Conference on Environmental Aspects of Bangladesh. 2015:97–98. [Google Scholar]
- 38.Sharmin S., Salam M.A., Haque M.A., Shahjahan M. Toxicity bioassay of organophosphorous pesticide malathion in common carp, cyprinus carpio. Proceedings of 5th International Conference on Environmental Aspects of Bangladesh. 2015:99–100. [Google Scholar]
- 39.Mostakim G.M., Zahangir M.M., Mishu M.M., Rahman M.K., Islam M.S. Alteration of blood parameters and histoarchitecture of liver and kidney of silver barb after chronic exposure to quinalphos. J. Toxicol. 2015;3:1–8. doi: 10.1155/2015/415984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chindah A.C., Sikoki F.D., Vincent-Akpu I. Toxicity of Cypermethrin to Tilapia guineensis juveniles. J. Agricult. Biotechnol. Environ. 2004;2(2):60–66. [Google Scholar]
- 41.Koprucu S.S., Koprucu K., Ural M.Ş., Ispir U., Pala M. Acute toxicity of organophosphorous pesticide diazinon and its effects on behavior and some hematological parameters of fingerling European catfish (Silurus glanis L.) Pestic. Biochem. Physiol. 2006;86(2):99–105. doi: 10.1016/j.pestbp.2006.02.001. [DOI] [Google Scholar]
- 42.Jayaprakash C., Shettu N. Changes in the hematology of the freshwater fish, Channa punctatus (Bloch) exposed to the toxicity of deltamethrin. J. Chem. Pharm. Res. 2013;5(6):178–183. [Google Scholar]
- 43.Joshi P.K., Harish D., Bose M. Effect of lindane and malathione exposure to certain blood parameters in a fresh water teleost fish Clarias batrachus. Pollut. Res. 2002;21(1):55–57. [Google Scholar]
- 44.Chen X., Yin D., Hu S., Hou Y. Immunotoxicity of penta chlorophenol on macrophage immunity and IgM secretion of the crucian carp (Carassius auratus) Bull. Environ. Contam. Toxicol. 2004;73:153–160. doi: 10.1007/s00128-004-0407-z. [DOI] [PubMed] [Google Scholar]
- 45.John P.J. Alteration of certain blood parameters of freshwater teleost Mystus vittatus after chronic exposure to Metasystox and Sevin. Fish Physiol. Biochem. 2007;33(1):15–20. doi: 10.1007/s10695-006-9112-7. [DOI] [Google Scholar]
- 46.Verma A.K., Prakash S. Impact of arsenic on haematology, condition factor, hepatosomatic and gastrosomatic index of a fresh water cat fish, Mystus vittatus. Int. J. Biol. Sci. 2019;10(2):49–54. [Google Scholar]
- 47.Durrani A.Z., Kamal N. Identification of ticks and detection of blood protozoa in friesian cattle by polmerase chain reacton test and estimation of blood parameters in district Kasur, Pakistan. Trop. Anim. Health Prod. 2008;40:441–447. doi: 10.1007/s11250-007-9117-y. [DOI] [PubMed] [Google Scholar]
- 48.Schillinger U. Isolation and identification of lactobacilli from novel-type probiotic and mild yoghurts and their stability during refrigerated storage. Int. J. Food Microbiol. 1999;47(1–2):79–87. doi: 10.1016/S0168-1605(99)00014-8. [DOI] [PubMed] [Google Scholar]
- 49.Islam S.M., Math R.K., Cho K.M., Lim W.J., Hong S.Y., Kim J.M., Yun M.G., Cho J.J., Yun H.D. Organophosphorus hydrolase (OpdB) of Lactobacillus brevis WCP902 from kimchi is able to degrade organophosphorus pesticides. J. Agricult. Food Chem. 2010;58:5380–5386. doi: 10.1021/jf903878e. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.