Abstract
Mentalizing, or the ability to understand the mental states and intentions of others, is an essential social cognitive function that children learn and continue to cultivate into adolescence. While most typically developing children acquire sufficient mentalizing skills, individual differences in mentalizing persist throughout childhood and are likely influenced by a combination of cognitive functioning, the social environment, and biological factors. DNA methylation of the oxytocin receptor gene (OXTRm) impacts gene expression and is associated with increased brain activity in mentalizing regions during displays of animacy in healthy young adults. The establishment, fine-tuning, and implications of such associations in the context of broader social functioning remain unclear. Using a developmental neuroimaging epigenetic approach, we investigated the contributions of OXTRm to individual variability in brain function during animate motion perception in middle childhood. We find that higher levels of OXTRm are associated with increased neural responses in the left temporo-parietal junction and inferior frontal gyrus. We also find a positive association between neural activity in LTPJ and social skills. These findings provide evidence of epigenetic influence on the developing child brain and demonstrate that variability in neural social perception in childhood is multifaceted with contributions from individual social experience and the endogenous oxytocin system.
Keywords: Oxytocin, Middle childhood, FMRI, Mentalizing, Epigenetics
Highlights
-
•
Variation in oxytocin receptor (OXTR) DNA methylation is associated with neural function during the perception of animacy.
-
•
OXTR DNA methylation and social skills are positively associated with BOLD response in the left temporo-parietal junction.
-
•
The variance explained by OXTR DNA methylation and social skills are unique.
-
•
OXTR DNA methylation is positively associated with BOLD response in the left inferior frontal gyrus.
1. Introduction
Middle childhood, often defined as the period between 6 and 12 years of age, is a time of extensive cognitive, social, and emotional development (Skuse, 2017, Del Giudice, 2014). During this time, children develop complex reasoning skills and display substantial changes in self-regulation, executive function, and peer interactions (Bigelow, 1977). Understanding of mental states, referred to as a theory of mind, improves into middle childhood and is essential for successful navigation of the complex social world (Miller, 2009, Miller et al., 2018). Individual differences in theory of mind in preschool have been associated with variability in socioeconomic status (Hughes and Ensor, 2005), cognitive skills (Carlson et al., 2002, Lecce et al., 2017) and language (Hughes and Ensor, 2007); these differences in preschool are well documented, and are predictive of teacher-rated social competence in elementary school (Devine et al., 2016). While less studied at older ages, theory of mind continues to develop in middle and late childhood, with evidence from cross-sectional and longitudinal studies demonstrating significant individual differences in the skill of mental attribution, or mentalizing. Variability in the magnitude of neural activity within key mentalizing regions during theory of mind tasks has also been linked to social cognition behaviors in children (Mukerji et al., 2019). Taken together, individual differences in mentalizing ability that persist through middle childhood are likely impacted by complex interactions between cognitive skills, the social environment, and biological influence that help shape the development of a child’s ability to effectively reason about the mental states of others (Lecce et al., 2017, Devine et al., 2016, Lecce et al., 2011, Banerjee et al., 2011, Tucker-Drob and Briley, 2014).
One important biological modulator of social and emotional processing is the hormone and neuromodulator oxytocin. Intranasal delivery of oxytocin enhances activity of neural circuits associated with social reward and social attention in children with Autism Spectrum Disorder (ASD) (Gordon, 2016, Gordon, 2013), improving their social cognitive function and promoting sociality (Anagnostou, 2014). Intranasal oxytocin administration also improves mentalizing and emotion recognition accuracy in individuals with and without ASD (Guastella, 2010, Feeser, 2015). In children, increased plasma oxytocin concentration is associated with improved performance on theory of mind tasks and in social communication skills (Parker, 2014), as well as increased social reciprocity (Feldman et al., 2013). In children with ADHD, higher oxytocin serum levels are associated with lower impulsivity and aggression and increased empathy (Demirci et al., 2016). Though some reports have shown intriguing relationships establishing links between oxytocin levels and specific social abilities, replication has proven difficult and the majority of studies have mixed results (McCullough et al., 2013). These inconsistencies likely occur because the action of oxytocin is dependent upon the expression level of its receptor (OXTR), and variability in the expression of the OXTR gene plays a major role in the function of the endogenous oxytocin system (Perkeybile, 2019).
Variability in OXTR gene expression is regulated in part by DNA methylation, an epigenetic modification that involves the addition of a methyl group typically to cytosine-guanine dinucleotide pairs (CpG). Increased DNA methylation at specific CpG sites has been associated with reduced expression of OXTR in human cortex (Gregory, 2009). OXTR DNA methylation (OXTRm) at these sites is positively associated with autism (Gregory, 2009), callous unemotional traits (Dadds, 2014), and stronger neural response during social perception in adults (Jack et al., 2012) and infants (Krol et al., 2019a). There is convincing evidence that peripheral measurement of OXTRm may be used as a marker of the activity and DNA methylation state of this gene in animal model systems (Perkeybile, 2019) and the human brain (Gregory, 2009). Additionally, recent work has demonstrated OXTRm is robust to cell type differences (Puglia et al., 2018) and that blood and saliva derived methylation levels are highly correlated in humans, allowing non-invasive collection in developmental populations (Krol et al., 2019a).
Our group was the first to assess the role of OXTRm in functional differences of social perception in healthy young adults using a classic Heider and Simmel animate task adapted by Castelli (Jack et al., 2012, Castelli et al., 2000) in which geometric shapes move in a random fashion or in goal-directed animate motion. This task reliably recruits brain structures considered to be important in mentalizing even without instruction to pay attention to social contingencies. This adult study revealed a positive correlation where higher levels of OXTRm were associated with increased blood oxygenation level dependent (BOLD) response in the anterior cingulate cortex (ACC) and superior temporal gyrus (STG) during the perception of animate motion. However, the implications of the observed positive associations between OXTRm and neural processing in the context of individual differences in social behavior and social functioning remain unclear.
A developmental approach is particularly well suited for untangling the complex interplay between behavioral, epigenetic, and neural activity during mental attribution tasks. Children between 6 and 12 years old recruit brain regions consistent with adult networks during mentalizing tasks including the temporo-parietal junction (TPJ), medial prefrontal cortex (mPFC), posterior cingulate cortex (PCC) and superior temporal sulcus (STS), but considerable shifts within the mentalizing network occur during development (Kobayashi et al., 2007, Saxe et al., 2009, Sommer, 2010). For example, there is evidence of age-related increases in bilateral TPJ activation from 5 to 11 years, suggesting fine tuning and selectivity of this region (Gweon et al., 2012). Individual differences in neural maturation and fine tuning of these regions may result in enhanced variability in mentalizing skills observed at this period in development. By investigating the neural activity which scaffolds mentalizing skills in development, we may better understand the implication of a positive association between OXTRm and these regions in adults who have already mastered mentalizing abilities. In the current experiment, we capitalized on the profound development in mentalizing skills and the heightened variability in these skills that occurs as children transition into middle childhood to investigate how OXTRm contributes to individual variability in neural function supporting social perception.
While mentalizing skills typically improve with age, in the present study we first hypothesized that in children between 5 and 11 years old, OXTRm would be associated with variability in neural function within the mentalizing network. We previously published a positive association between OXTRm and BOLD activity in mentalizing regions in young adults that suggested greater recruitment of these areas may be necessary for intensive processing of social contingencies but we lacked behavioral data to support this hypothesis. In the current study, we include parent reported social skills to better understand the associations between OXTRm and neural activity during animate social perception. We further hypothesized that an association between social skills and neural function in mentalizing regions would be inverse from an association between OXTRm and neural function as individuals with higher methylation levels would presumably have less sensitivity to endogenous oxytocin and would therefore exhibit fewer social skills. Because individual differences in mentalizing abilities have been linked to a variety of demographic and cognitive functioning variables we wanted to ensure that any variance explained by OXTRm in neural function was unique. We primarily controlled for chronological age in all analyses given the strong associations between age and theory of mind, and then subsequently stepwise tested for associations with biological sex and parental education as additional control variables.
2. Materials and methods
2.1. Experimental design
Ninety-six typically developing children between the ages of 5 and 11 years old were recruited from the local community for the current study. Families were identified by responding to flyers or by having previously signed up to be contacted for developmental research studies. Because population stratification can be a major issue for genetic association studies where an association could be due to systematic differences in allele frequencies across populations leading to spurious associations (Zhang, 2011), we aimed to recruit participants from similar geographic ancestry. While an imperfect measure of ancestry, we used parent report to recruit Caucasian children for this study. Additional exclusion criteria for this study included use of psychotropic medications and MRI contraindications. Informed written consent was obtained from the legal guardian of each child and participants independently assented to study participation for a protocol approved by the University of Virginia Institutional Review Board. Participation consisted of two study visits spaced about three weeks apart (M = 21.76 days, SD = 12.69). At the first visit, children completed a 15-minute mock scan to acclimate to the scanner environment and practice lying still. Participants who tolerated the mock scan were then scheduled for an MRI visit. Toleration of the scanning environment was determined by participants affect and comfort in the mock scan as well as their assent to participate in the real MRI scan. Participants who became distressed and did not tolerate the mock scan environment did not differ in sample characteristics from those who did in terms of age, sex, parental education, or social skills (all p > 0.05). Saliva was collected at time of scanning at the second visit. Families were compensated $50 at each visit for a total of $100 and the child received a toy at each visit. Seventy-six children participated in the MRI scan and saliva collection. One participant born at premature gestational age (26 weeks) with developmental delay was excluded from all analyses and an additional five participants were excluded because of excessive head motion (See Fig. S1). Epigenotyping from three participants failed pyrosequencing, resulting in a final sample of sixty-seven children (32 males, M = 8.15 y, SD = 1.57) with OXTRm and functional brain imaging data.
2.2. Parent-report demographic and behavioral data
Parent education was assessed via a brief demographic questionnaire to indicate the highest degree completed by the child’s caregiver(s) and in all instances degree completion of two caregivers were provided. For each participant, parent education was scored from 0 (8th grade) to 5 (PhD or other terminal degree) and summed for both caregivers to generate a parent education value (M = 6.55, SD = 2.09, range = 2–10). Between the two visits, caregivers were emailed a link to complete the Social Skills Improvement System rating scale (SSIS) (Gresham and Elliott, 2008) via Qualtrics (Qualtrics, 2019). The SSIS is a 79-item parent-report questionnaire designed to assess social skills and problem behaviors in children 3–18 years of age. Each item is rated on a 4-point Likert-type scale from 1 (never) to 4 (almost always). Seven caregivers of participants included in the MRI analysis did not complete the questionnaires.
2.3. Saliva Collection and Epigenotyping Procedures
Saliva was collected using CS-2 sponges in OG-250 collection kits from DNA Genotek (Ottawa, Canada) and stored at room temperature. Prior to DNA isolation, samples were incubated at 50 °C for 1 h and centrifuged at 1000 RPM for 10 min to recover all liquid from sponges. DNA was isolated following the manual purification protocol from DNA Genotek, resuspended in Hydration Solution (Qiagen, Hilden, Germany) and quantified using NanoDrop.
Two hundred nanograms of DNA were subjected to bisulfite treatment (Kit MECOV50, Invitrogen, Carlsbad, CA), which converts non-methylated cytosines to uracil and leaves methylated cytosines unmodified for downstream sequencing. A 116-base pair region of OXTR containing CpG Site − 924 was amplified via polymearase chain reaction (PCR) using 20 nanograms of bisulfite-converted DNA as a template, 0.2 µM primers TSL101F (5′-TTGAGTTTTGGATTTAGATAATTAAGGATT-3′) TSL101R (5′-biotin-AATAAAATACCTCCCACTCCTTATTCCTAA-3′) and using Pyromark PCR kits (Qiagen, Hilden, Germany). Samples were amplified in triplicate with the following cycling conditions: [Step 1: (95 °C/15 min)/1 cycle, Step 2: (94 °C/30 s, 56 °C/30 s, 72 °C/30 s)/50 cycles, Step 3: (72 °C/10 min)/1 cycle, Step 4: 4 °C hold]. Each PCR plate contained methylation standards (0% and 100% methylated), a positive control, and negative controls from bisulfite conversion and PCR. PCR amplification of a 116 bp product was confirmed by gel electrophoresis. DNA methylation for each sample was quantified by pyrosequencing (PyroMark Q24, Qiagen) using primer TSL101S (5′-AGAAGTTATTTTATAATTTTT-3′) and Pyromark Gold Q24 Reagents (Qiagen, Hilden, Germany). DNA collection for three samples was insufficient to obtain methylation levels. Interquartile outlier detection was used to identify three samples with high replicate variability. These samples were rerun to estimate deviant values and replicates that caused deviation for these samples were removed. Average mean deviation within replicates was ± 1.71%. Reported epigenotypes are an average of the three replicates.
2.4. Animacy stimuli
Stimuli were presented with PsychoPy (Peirce, 2009) using an LCD AVOTEC projector onto a screen located behind the subject’s head and viewed through an integrated head-coil mirror. As in Jack et al. (2012) participants passively viewed modified Heider and Simmel clips that engage a network of structures that support reasoning about others (Jack et al., 2012, Castelli et al., 2000). Eight ‘Animate’ animations and eight ‘Control’ animations were presented to subjects during scanning. Each animation featured three white geometric shapes (triangle, diamond, and circle) on a black background. The Animate condition involved goal-directed behavior such as playing hide and seek or dancing with one another while the Control animations showed the shapes moving around the screen in a random fashion with the same velocity and overall motion as the Animate condition. Each animation was approximately 16 s and Animate and Control animations were presented in alternating block design. Participants were instructed to simply observe the shapes as they moved along the screen.
2.5. Imaging procedures
Scanning was performed at the UVA Fontaine Research Park on a 3 T Siemens Prisma scanner with a 32 channel headcoil. Cushions were placed around the participants’ ears and forehead to minimize head movement during the scans. An experimenter stood by the participants’ feet to ensure that the participants remained awake and comfortable. If the experimenter noticed the participant move, they gently placed their hand on the participant’s leg to remind the participant to stay still.
T1-weighted high-resolution structural images were acquired using Siemens’ magnetization-prepared rapid-acquired gradient echo (MPRAGE) pulse sequence with the following specifications: echo time (TE) = 2.98 ms; repetition time (TR) = 2300 ms; flip angle (FA) = 9°; image matrix = 240 mm × 256 mm; slice thickness = 1 mm; 208 slices. Whole-brain functional images were acquired using the same parameters as the Adolescent Brain Cognitive Development protocol (Casey, 2018). The specifications for the T2 * weighted echo planar imaging (EPI) sequence sensitive to BOLD contrast were with the following specifications: TE = 30 ms; TR = 800 ms; FA = 52°; image matrix = 90 mm × 90 mm; slice thickness = 2.4 mm; slice gap = 2.4 mm; 332 slices.
2.6. fMRI preprocessing
Anatomical images were skull stripped using AFNI (Cox, 1996) and the results were manually corrected. Subsequent data preprocessing and analysis was carried out using FEAT (FMRI Expert Analysis Tool) Version 6.0, part of FSL (FMRIB’s Software Library) (Smith, 2004). The following pre-statistics processing was applied: motion correction using MCFLIRT (Jenkinson et al., 2002); spatial smoothing using a Gaussian kernel of full width half maximum 5 mm; grand-mean intensity normalization of the entire 4D dataset by a single multiplicative factor; highpass temporal filtering (Gaussian-weighted least-squares straight line fitting, with sigma = 50.0 s). Registration to the Montreal Neurologic Institute (MNI) Template standard space image was carried out using fMRIB’s software library linear registration tool (FLIRT) (Jenkinson et al., 2002). Registration from high resolution structural to standard space was then further refined using FSL’s nonlinear registration, FNIRT (Andersson et al., 2007). Head motion was quantified by an algorithm that quantifies the root mean square of the relative frame-wise displacement (FDRMS) between each volume of functional data (Smith, 2004). Participants with mean FDRMS greater than 0.15 mm were excluded from all analyses.
At the subject level, time-series statistical analysis was carried out using FSL's improved linear model (FILM) with motion parameters added to the model and local autocorrelation correction (Woolrich et al., 2001). Regressors for Animate and Control conditions were modeled by convolving the time course with a double-gamma hemodynamic response function (HRF) and with temporal filtering applied. An Animate > Control contrast was conducted and the contrast of parameter estimates (COPE) from this analysis for each individual was carried forward to higher-level analysis.
2.7. fMRI analysis
Group-level analysis of the data was conducted with FSL’s local analysis of mixed effects (FLAME) stage 1 and 2 (Beckmann et al., 2003, Woolrich et al., 2004). Z (Gaussianised T/F) statistic images were thresholded using clusters determined by Z > 2.3 (p < 0.01) and corrected cluster significance threshold of p < 0.05 (Worsley, 2012). We first conducted an exploratory ROI analysis targeting regions involved in mentalizing to identify regions in which Animate > Control activation was significantly associated with OXTRm. The mentalizing ROI mask was defined by a meta-analytic approach using Neurosynth.org (Tor D, 2011) feature set keyword “mentalizing”. Three regressors were included in the model: a group mean regressor, mean-centered OXTRm, and mean-centered age in months. Age was specifically included in this model as a control variable because of the wide age range in this study and well documented associations between mentalizing and age. Contrasts were computed testing for positive and negative linear associations between OXTRm and Animate > Control activation and between age and Animate > Control activation. Significant clusters associated with OXTRm regressor were registered to each participant’s native space and average Animate > Control Z statistic values for each individual were extracted. We next used the average Animate > Control BOLD activation in the significant ROI cluster from the OXTRm regressor for each individual to test for associations between parent reported social skills and BOLD activation. Stepwise linear regression analysis was conducted in R (Crawley, 2007, R Development Core Team, 2011) and model comparison was used to determine the best fitting model to predict BOLD activation in the significant cluster including OXTRm, social skills, age, sex, and parent education. Stepwise linear regression allows for identification of specific and unique variables that allows for a model that is flexible yet tailored enough to describe sample or the population well. An additional ROI analysis was conducted in FSL using OXTRm and all covariates, a group mean regressor, mean-centered OXTRm, mean-centered age in months, biological sex, and mean-centered parent education to confirm the primary association with OXTRm with the smaller sample size. Finally, we conducted an exploratory whole-brain analysis to identify any other regions in which Animate > Control activation was significantly associated with OXTRm while controlling for age.
3. Results
Sixty-seven typically developing Caucasian children aged 5–11 years old participated in this study. OXTRm was analyzed at CpG site − 924, a conserved CpG site previously shown to impact gene expression in a DNA methylation dependent manner (Perkeybile, 2019, Kusui, 2001). Saliva-derived DNA methylation at this site is highly correlated with blood-derived methylation and has been associated with individual differences in infant brain response to emotional faces at 7 months (Krol et al., 2019a) and behavioral temperament at 18 months (Krol et al., 2019b). At this site, OXTRm ranged from 44% to 77% methylated (M = 63.46, SD = 5.49) and a Shapiro-Wilk test showed no significant departure from normality (W = 0.97, p = 0.065). There were no significant associations of OXTRm with demographic control variables including child age (r(65) = 0.07, p = 0.57), sex (t(65) = 0.54, p = 0.595), or parent education (r(58) = 0.22, p = 0.089).
Sixty caregivers completed SSIS which assesses Social Skills and Problem Behaviors (Gresham and Elliott, 2008). Social Skills scores ranged from 59 to 129 (M=97.05, SD=14.69), and Problem Behavior scores ranged from 2 to 44 (M=19.68, SD=10.30); in neither domain was there a significant departure from normality (p > 0.05). There was no significant association between OXTRm and caregiver rated Social Skills (r(58) = 0.12, p = 0.351). There were also no significant associations of Social Skills with child age (r(58) = −0.01, p = 0.945), sex (t(58) = 1.88, p = 0.066), or parent education (r(58) = 0.02, p = 0.899). Data is similar for Problem Behaviors (all p-values > 0.05). See Fig. S2 for histograms of primary study constructs.
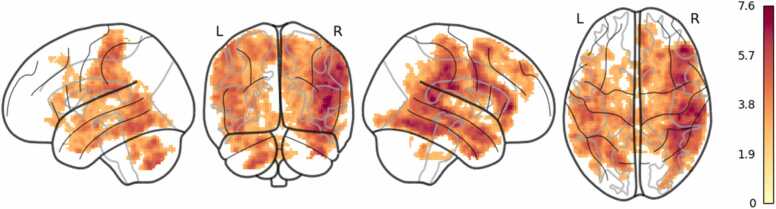
The main effect of Animate > Control condition was generally consistent with prior research with greater activity in lateral occipital regions, bilateral posterior superior temporal sulcus, and fusiform gyrus. Left lateral frontal regions including the inferior frontal gyrus and orbitofrontal cortex were also activated (See Fig. 1 and Table 1). Multiple comparisons were corrected for using the false discovery rate (FDR) q < 0.05 voxel significance level and spatial extent threshold (k) > = 10 contiguous voxels. For exploratory analyses, OXTRm was used as a regressor to predict individual variability in the processing of animate clips while controlling for age.
Fig. 1.
Response intensity of brain regions demonstrating significantly greater activity in the Animate > Control condition.
Table 1.
Main effect of Animate > Control local maxima statistics.
| Anatomical Region | Hem | x | y | z | Z |
|---|---|---|---|---|---|
| Superior Temporal Sulcus | R | 48 | -40 | 10 | 7.57 |
| Inferior Frontal Gyrus | R | 46 | 34 | 4 | 7.48 |
| Supramarginal Gyrus | R | 60 | -26 | 40 | 7.06 |
| Parietal Operculum Cortex | R | 54 | -22 | 22 | 7.05 |
| Precentral Gyrus | R | 46 | 10 | 24 | 6.91 |
| Fusiform Gyrus | R | 46 | 10 | 24 | 6.90 |
| Inferior Frontal Gyrus | L | -46 | 20 | 8 | 3.88 |
| Inferior Frontal Gyrus | L | -42 | 24 | 12 | 3.70 |
| Inferior Frontal Gyrus | L | -50 | 26 | 12 | 3.57 |
| Orbitofrontal Cortex | L | -28 | 30 | -12 | 3.56 |
| Inferior Frontal Gyrus | L | -46 | 30 | 2 | 3.54 |
| Orbitofrontal Cortex | L | -38 | 26 | -14 | 3.26 |
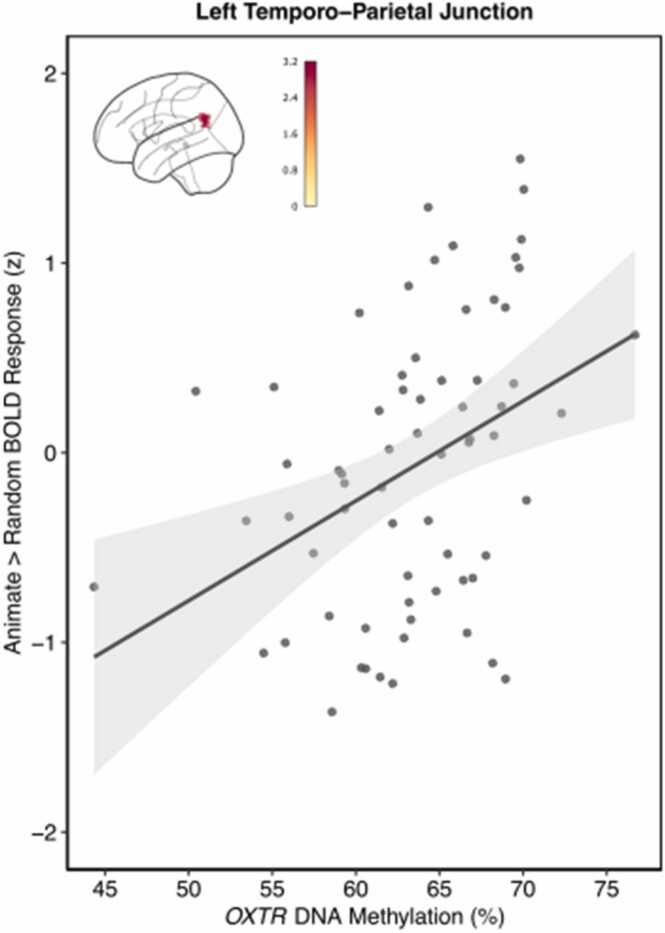
3.1. OXTR DNA methylation impacts Left Temporo-Parietal Junction response to animacy
We conducted an exploratory functional ROI analysis to assess the effect of OXTRm on activity within regions involved in mentalizing as defined by a meta-analytic approach using the Neurosynth.org feature set keyword “mentalizing”. This functional ROI analysis revealed a significant positive main effect of OXTRm on Animate > Control activity in the left temporo-parietal junction (TPJ) such that increased levels of methylation predicted increased BOLD activity (Fig. 2). Peak activity (Z = 3.21) for this cluster of voxels (k = 151) occurred at x = −46, y = −60, z = 24. The result from the functional ROI analysis including additional covariates of sex and parent education with the smaller sample size is similar and shown in S3.
Fig. 2.
Individuals with increased methylation of OXTR display elevated TPJ response to animate > control conditions. Mean Z statistic values are plotted against percent OXTRm for each participant (n = 67). Gray shading indicates 95% confidence interval.
3.2. Social skills predict the BOLD response in the Left TPJ
In order to better understand the association between OXTRm and left TPJ activity response to animacy, we investigated the possible association of child social skills within the left TPJ. We first conducted an exploratory analysis whereby the TPJ cluster was registered to subject space and the mean Z statistic value was extracted for each participant and correlated with scores from the SSIS social skills summary scale. Recall that caregivers of sixty children completed the questionnaire. Pearson correlation indicated that increased TPJ activity was related to higher social skills scores on the SSIS (r(58) = 0.29, p = 0.026). Demographic variables were tested as potentially confounding factors and revealed no significant effect or improvement to model fit.
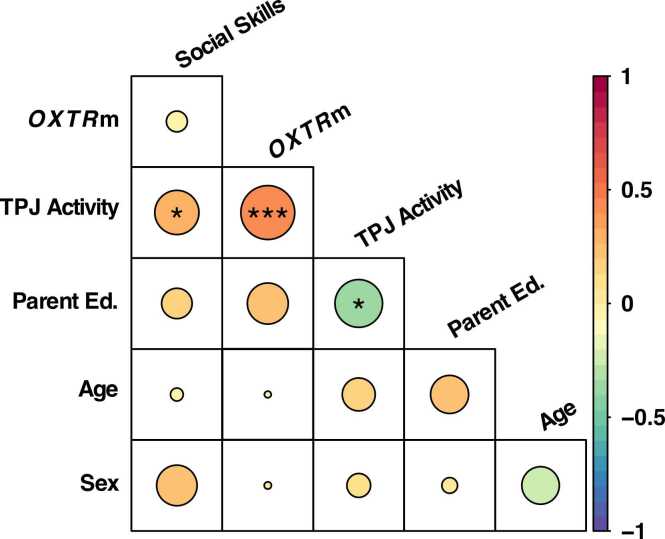
Given that social skills and OXTRm both were positively associated with increased TPJ activity in the perception of animacy but not associated with one another, we conducted stepwise linear regression analysis to determine the best fitting model for these data. Results indicate that the statistical association between OXTRm and social skills as predictors for TPJ activity increased when both were included in the model, F(2, 57) = 6.92, p = 0.002, adjusted R2 = 0.17. Additionally, we stepwise tested for demographic variables of age, sex, and parent education as confounding variables and results revealed parent education, but not age or sex, as a significant covariate of TPJ activity and improved model fit F(3, 56) = 7.06, p < 0.001, adjusted R2 = 0.24. The final predictive model showed the TPJ activity was positive relative to OXTRm (B = 0.055, p = 0.001) and social skills score (B = 0.012, p = 0.038), but negative relative to parent education (B = −0.103, p = 0.017). There was no evidence of interactions or mediation in the model. See Fig. 3 for partial correlation matrix of all variables and Table S1 for stepwise linear regression table.
Fig. 3.
Partial correlations between the six variables of interest. The correlation coefficients are represented by size of the circle and color-coded according to the color bar on the right. *p < 0.05, **p < 0.01, ***p < 0.001.
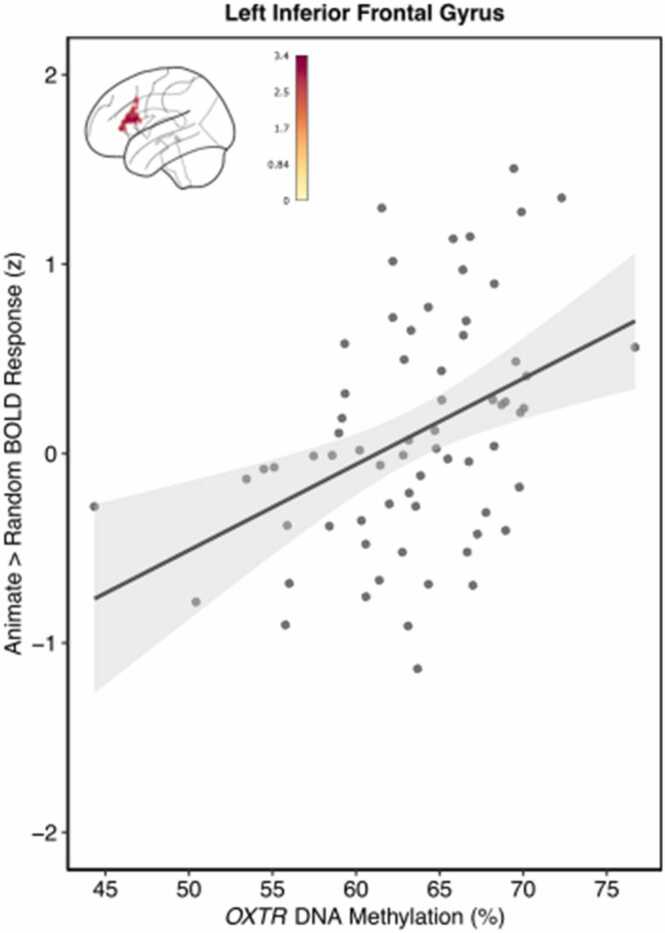
3.3. Exploratory whole brain analysis indicates OXTR DNA methylation influences the Left Inferior Frontal Gyrus
Finally, we performed an exploratory whole-brain analysis to identify potential associations between OXTRm and brain activity in regions not included in the mentalizing mask. This analysis revealed a significant positive main effect of OXTRm in the left inferior frontal gyrus (Fig. 4). Peak activity (Z = 3.36) for this cluster of voxels (k = 310) occurred at x = −56, y = 18, z = 16. While there was no linear association between age and Animate > Control activation in the IFG cluster, visual inspection of the data led us to test a non-linear inverted u-shape trend which was not significant.
Fig. 4.
Individuals with increased methylation of OXTR display elevated IFG response to animate > control conditions. Mean Z statistic values are plotted against percent OXTRm for each participant (n = 67). Gray shading indicates 95% confidence interval.
4. Discussion
We examined the association between the neural response to animate motion in regions of the brain involved in mentalizing and individual differences in the endogenous oxytocinergic system in middle childhood. Controlling for chronological age, we found that higher levels of OXTRm were associated with greater activity in the left temporal parietal junction, similar to findings in young adults (Jack et al., 2012). Our multiple regression demonstrated that in addition to OXTRm, children’s social skills were also a positive significant predictor of the BOLD response in the left TPJ, providing evidence that behavior may account for unique variance in individual differences in neural response to animate motion. These results suggest a complex interplay of social skills and biological influence in the development of children’s mentalizing abilities.
Our group has previously shown that OXTRm is positively associated with BOLD response to animate motion in healthy young adults in the left STG extending to the TPJ and dorsal ACC (Jack et al., 2012). However, the young adult study lacked additional phenotyping measures to characterize how the observed correlations were meaningful in the context of individual differences in overt social behavior. The primary motivation of the current study was to probe associations between OXTRm and neural systems supporting social perception during a developmental period characterized by significant changes in social cognition and behavior. By taking a developmental approach, we aimed to maximize the amount of variability due to differences in maturation and leverage this variance to explain the directionality of associations we found in young adults. In our child sample, we find a positive association of OXTRm and activity in the left TPJ that is similar to the findings in young adults. The consistency of this association in the left tempo-parietal areas in childhood and adulthood suggests that this association is established by middle childhood. While both the left TPJ and STG are consistently recruited in mentalizing and perspective taking tasks(Gallagher, 2000, Völlm, 2006, Jackson et al., 2006), the shift in the association with OXTRm from the TPJ in childhood to the STG in adults may reflect a fine-tuning of the system across development.
In our exploratory whole brain analysis, we also found that OXTRm is positively correlated with activity in the left IFG in children. While the left IFG is robustly significant in the main effect of Animate > Control condition in both children and adults, the association with OXTRm in the left IFG is a novel finding in childhood that has not previously been shown in adults. Across the lifespan the left IFG is associated with a wide variety of cognitive functions ranging from inhibitory control (Swick et al., 2008) to empathy (Chakrabarti et al., 2006) and is specifically well known to be involved in semantic processing(Binder et al., 2005, Perani, 1999, Noppeney and Price, 2004, Jessen, 2000). It is also proposed to be a region involved in differentiating abstract from concrete concepts (Wang et al., 2010) and interpreting communicative intent (Wang et al., 2006). The left IFG has also been shown to be more active in children and adolescents as compared to adults in nonverbal theory of mind tasks (Kobayashi et al., 2007). There is some evidence to suggest that activity in frontal regions, including the IFG, follows an inverted U-shape from childhood to adulthood as mentalizing becomes more automatic (Wang et al., 2006, Blakemore, 2008). It is possible that the observed association with OXTRm and IFG activity may reflect a mediating factor in a developmental shift in mentalizing abilities during perception of animate motion. Future work should investigate the role of OXTRm on developmental trajectories of mentalizing in a longitudinal sample.
When considering how variability in DNA methylation and social skills might relate to individual differences in neural response during animate motion, we hypothesized that social skills and OXTRm would be negatively correlated with one another and inversely related to TPJ activity. We hypothesized that individuals with higher levels of methylation, and presumably less sensitivity to endogenous oxytocin, recruit brain areas involved in mentalizing to a greater extent possibly due to increased resource intensive processing of social contingencies (Jack et al., 2012). It would follow that those individuals with higher methylation levels would also exhibit lesser social skills and greater social skills would be associated with reduced neural activity. Instead, we find no correlation between OXTRm and parent-reported social skills. Moreover, we find a significant positive association between social skills and left TPJ activity such that individuals with more social skills exhibit greater TPJ activity. Perhaps this is because at these ages, children are developing social skills concurrently with that activity; as the skills mature, greater efficiency may tamp the neural activity. A longitudinal investigation of change in neural activity across childhood may ultimately shed light on this trajectory.
The results of the stepwise multiple regression revealed that incorporating social skills in addition to OXTRm significantly improved the model fit. This model provides evidence that the variance in TPJ activity explained by OXTRm and the variance explained by social skills are unique, with no support for a mediation model. Parent education was also a significant variable but only in the full model including OXTRm and social skills, and importantly we find no evidence of an interaction or mediation for this term either. These data highlight that individual differences in the neural response of social perception is multidimensional and dependent on the environment, biological contributions, and social behaviors, and likely comprised of a combination of these influences.
4.1. Limitations and future directions
The present study focuses on individual differences in the endogenous oxytocin system and neural substrates of social perception and social behavior from a development perspective. While our previous young adult research is informative, we cannot make direct developmental comparisons between children and young adults as these were independent samples. Another important difference is the use of different tissues in children and adults. Working with developmental populations required us to use saliva as a non-invasive peripheral tissue to isolate DNA and derive OXTRm levels while working with young adults allowed for the use of an intravenous blood draw. We have previously shown that blood-derived and brain-derived DNA methylation at our CpG site of interest are correlated in animal models and that both reliably assess ASD phenotype in humans (Gregory, 2009). More recently we have shown that OXTRm derived from saliva and from whole blood and peripheral blood mononuclear cells are highly correlated in a large sample of adults (Krol et al., 2019a) and that methylation does not vary across different cell types in the blood (Puglia et al., 2018). Taken together this suggests that cell type frequency is less likely to impact relationships we identified. Even so, the use of saliva derived DNA methylation levels in developing children warrants consideration (JD et al., 2017, Bakulski et al., 2016). Because the CpG sites we study are not on the Illumina array, testing for cell type differences using saliva cell type reference panels (Middleton, 2021) would require we generate both sets of data to test our sites of interest and control for cell type differences. This limitation should be considered for future studies. These experiments provide compelling evidence for the feasibility of saliva as a non-invasive peripheral marker of OXTRm but do not allow us to make direct comparisons between children and adults regarding methylation values or the association with neural response. Lastly, because methylation levels have been shown to differ as a function of ethnic groups, we restricted our sample to self-identified Caucasian Americans. Future work should explore the impact of epigenetic differences within and between ethnic groups.
Another important limitation to the present study is the use of a cross-sectional design. Our lab has shown that saliva derived OXTRm is stable in adulthood but dynamic in infancy (Krol et al., 2019b), and in human infants and animal models changes in methylation values at these CpG sites are related to the social environment (Perkeybile, 2019, Krol et al., 2019). It is still unknown if OXTRm at this CpG site is dynamic through childhood and the present study is only informative of OXTRm and neural activity at one time point in development for each individual. We do not observe any chronological age-related associations with variability in the oxytocinergic system itself, or any presence of chronological age confounding the observed associations with neural activity to animate motion. But middle childhood is characterized by sweeping developmental shifts in mentalizing and social interactions and there is robust evidence demonstrating developmental changes in neural processing during middle childhood and adolescence (Blakemore, 2008). There is likely within-individual variability in the trajectories of the establishment and fine tuning of the associations between OXTRm and neural substrates of social perception that cannot be captured without a longitudinal design.
The current study aimed to investigate how individual variability in the oxytocinergic system and neural response to animate social perception inform the development of social skills and behaviors in typically developing children. The results of the multiple regression analysis emphasize that individual variability in social perception is scaffolded on more than DNA methylation alone and indicate the need for future studies to directly probe these complex associations between biological markers, social environment, and social behaviors. The current study assessed children’s social skills only from parent report to obtain a global measure of social skills across several domains of social behavior. Including direct assessments of children’s social behavior may provide more robust information to untangle the correlation between biological and neural associations of variability in social behaviors. Finally, a longitudinal design is needed to investigate these associations and how shifts in the oxytocinergic system, environment, social perception processing, and social skills relate to the formation of social support systems and successful social integration.
4.2. Conclusion
In summary, we find that similar to adults, increased OXTRm is associated with increased activity in the left tempo-parietal area during the perception of animate motion in children. We also find a novel association in left IFG activity and OXTRm in children. When considering other factors that might contribute to individual differences in mentalizing we find that in addition to OXTRm, social skills contribute to individual differences in TPJ activity regardless of chronological age. While we find no evidence of an association between OXTRm and parent-reported social skills, the full model highlights the complexity of social skills, social environment, and biological influence in the development of children’s mentalizing abilities. Nonetheless, these data provide the first evidence in children of the impact of the epigenome on the developing social brain and point to a clear role for the oxytocin system in understanding individual differences in social developmental trajectories.
Funding
This research was supported by the University of Virginia and a UVA Brain Institute Transformative Neuroscience Pilot Grant “Individual variability in the oxytocinergic system and the development of human sociality” (AL, JC, and JM).
CRediT authorship contribution statement
Amalia Skyberg: Conceptualization, Methodology, Software, Validation, Formal analysis, Investigation, Data Curation, Writing – Original Draft, Visualization, Project administration. Stefen Beeler-Duden: Formal analysis, Investigation, Data Curation, Writing – Review & Editing. Alison Goldstein: Investigation, Data Curation, Project administration, Writing – Review & Editing. Christina Gancayco: Software, Data Curation, Writing – Review & Editing. Angeline Lillard: Conceptualization, Resources, Supervision, Funding, Writing – Review & Editing. Jessica Connelly: Conceptualization, Methodology, Resources, Supervision, Funding acquisition, Validation, Writing – Review & Editing. James Morris: Conceptualization, Methodology, Validation, Resources, Supervision, Funding acquisition, Writing – Review & Editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We thank Morgan Lynch, Samuel Wilson, Arpitha Shenoy, Jamie Levin, and Michelle Miller for their assistance with data collection and all the families who participated. We thank Kevin Tarczon and Zoe Bell for their assistance with preparation of the saliva samples. We also thank Drs. Kathleen Krol and Krassimira Botcheva for helpful comments on drafts of this manuscript.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.dcn.2022.101080.
Appendix A. Supplementary material
Supplementary material
.
Data statement
This data will be made available by request on OSF.
References
- Anagnostou E. Intranasal oxytocin in the treatment of autism spectrum disorders: a review of literature and early safety and efficacy data in youth. Brain Res. 2014 doi: 10.1016/j.brainres.2014.01.049. [DOI] [PubMed] [Google Scholar]
- Andersson J.L.R., Jenkinson M., Smith S. 2007. Non-linear Registration Aka Spatial Normalisation. FMRIB Technical Report TRO7JA2. [Google Scholar]
- Bakulski K.M., Halladay A., Hu V.W., Mill J., Fallin M.D. Epigenetic research in neuropsychiatric disorders: the “tissue issue”. Curr. Behav. Neurosci. Rep. 2016;3:264. doi: 10.1007/s40473-016-0083-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee R., Watling D., Caputi M. Peer relations and the understanding of faux pas: longitudinal evidence for bidirectional associations. Child Dev. 2011 doi: 10.1111/j.1467-8624.2011.01669.x. [DOI] [PubMed] [Google Scholar]
- Beckmann C.F., Jenkinson M., Smith S.M. General multilevel linear modeling for group analysis in FMRI. Neuroimage. 2003 doi: 10.1016/S1053-8119(03)00435-X. [DOI] [PubMed] [Google Scholar]
- Bigelow B.J. Children’s friendship expectations: a cognitive-developmental study. Child Dev. 1977 doi: 10.2307/1128905. [DOI] [Google Scholar]
- Binder J.R., Westbury C.F., McKiernan K.A., Possing E.T., Medler D.A. Distinct brain systems for processing concrete and abstract concepts. J. Cogn. Neurosci. 2005 doi: 10.1162/0898929054021102. [DOI] [PubMed] [Google Scholar]
- Blakemore S.J. The social brain in adolescence. Nat. Rev. Neurosci. 2008 doi: 10.1038/nrn2353. [DOI] [PubMed] [Google Scholar]
- Carlson S.M., Moses L.J., Breton C. How specific is the relation between executive function and theory of mind? Contributions of inhibitory control and working memory. Infant Child Dev. 2002 doi: 10.1002/icd.298. [DOI] [Google Scholar]
- Casey B.J., et al. The Adolescent Brain Cognitive Development (ABCD) study: imaging acquisition across 21 sites. Dev. Cogn. Neurosci. 2018 doi: 10.1016/j.dcn.2018.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelli F., Happé F., Frith U., Frith C. Movement and mind: a functional imaging study of perception and interpretation of complex intentional movement patterns. Neuroimage. 2000 doi: 10.1006/nimg.2000.0612. [DOI] [PubMed] [Google Scholar]
- Chakrabarti B., Bullmore E., Baron-Cohen S. Empathizing with basic emotions: common and discrete neural substrates. Soc. Neurosci. 2006 doi: 10.1080/17470910601041317. [DOI] [PubMed] [Google Scholar]
- Cox R.W. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Comput. Biomed. Res. 1996 doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Crawley M.J. The R Book. 2007. The R Book. [DOI] [Google Scholar]
- Dadds M.R., et al. Methylation of the oxytocin receptor gene and oxytocin blood levels in the development of psychopathy. Dev. Psychopathol. 2014 doi: 10.1017/S0954579413000497. [DOI] [PubMed] [Google Scholar]
- Del Giudice M. Middle childhood: an evolutionary-developmental synthesis. Child Dev. Perspect. 2014 doi: 10.1111/cdep.12084. [DOI] [PubMed] [Google Scholar]
- Demirci E., Ozmen S., Kilic E., Oztop D.B. The relationship between aggression, empathy skills and serum oxytocin levels in male children and adolescents with attention deficit and hyperactivity disorder. Behav. Pharmacol. 2016 doi: 10.1097/FBP.0000000000000234. [DOI] [PubMed] [Google Scholar]
- Devine R.T., White N., Ensor R., Hughes C. Theory of mind in middle childhood: longitudinal associations with executive function and social competence. Dev. Psychol. 2016 doi: 10.1037/dev0000105. [DOI] [PubMed] [Google Scholar]
- Feeser M. Oxytocin improves mentalizing - pronounced effects for individuals with attenuated ability to empathize. Psychoneuroendocrinology. 2015 doi: 10.1016/j.psyneuen.2014.12.015. [DOI] [PubMed] [Google Scholar]
- Feldman R., Gordon I., Influs M., Gutbir T., Ebstein R.P. Parental oxytocin and early caregiving jointly shape children’s oxytocin response and social reciprocity. Neuropsychopharmacology. 2013 doi: 10.1038/npp.2013.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher H.L. Reading the mind in cartoons and stories: an fMRI study of ‘theory of mind’ in verbal and nonverbal tasks. Neuropsychologia. 2000 doi: 10.1016/S0028-3932(99)00053-6. [DOI] [PubMed] [Google Scholar]
- Gordon I. Oxytocin enhances brain function in children with autism. Proc. Natl. Acad. Sci. USA. 2013 doi: 10.1073/pnas.1312857110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon I., et al. Intranasal oxytocin enhances connectivity in the neural circuitry supporting social motivation and social perception in children with autism. Sci. Rep. 2016 doi: 10.1038/srep35054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory S.G., et al. Genomic and epigenetic evidence for oxytocin receptor deficiency in autism. BMC Med. 2009 doi: 10.1186/1741-7015-7-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gresham, A.F., Elliott, S.N. Social Skills Improvement System (SSIS) Rating Scales. Bloom. MN Pearson Assessments (2008). doi:10.1007/s00586-009-0944-6.
- Guastella A.J. Intranasal oxytocin improves emotion recognition for youth with autism spectrum disorders. Biol. Psychiatry. 2010 doi: 10.1016/j.biopsych.2009.09.020. [DOI] [PubMed] [Google Scholar]
- Gweon H., Dodell-Feder D., Bedny M., Saxe R. Theory of mind performance in children correlates with functional specialization of a brain region for thinking about thoughts. Child Dev. 2012 doi: 10.1111/j.1467-8624.2012.01829.x. [DOI] [PubMed] [Google Scholar]
- Hughes C., Ensor R. Executive function and theory of mind in 2 year olds: a family affair? Dev. Neuropsychol. 2005 doi: 10.1207/s15326942dn2802_5. [DOI] [PubMed] [Google Scholar]
- Hughes C., Ensor R. Executive function and theory of mind: predictive relations from ages 2 to 4. Dev. Psychol. 2007 doi: 10.1037/0012-1649.43.6.1447. [DOI] [PubMed] [Google Scholar]
- Jack A., Connelly J.J., Morris J.P. DNA methylation of the oxytocin receptor gene predicts neural response to ambiguous social stimuli. Front. Hum. Neurosci. 2012;6 doi: 10.3389/fnhum.2012.00280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson P.L., Meltzoff A.N., Decety J. Neural circuits involved in imitation and perspective-taking. Neuroimage. 2006 doi: 10.1016/j.neuroimage.2005.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holbrook J.D., Huang R.C., Bartan S.J., Saffery R., Lillycrop K.A. Is cellular heterogeneity merely a confounder to be removed from epigenome-wide association studies? Epigenomics. 2017;9:1143–1150. doi: 10.2217/epi-2017-0032. [DOI] [PubMed] [Google Scholar]
- Jenkinson M., Bannister P., Brady M., Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002 doi: 10.1016/S1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Jessen F. The concreteness effect: evidence for dual coding and context availability. Brain Lang. 2000 doi: 10.1006/brln.2000.2340. [DOI] [PubMed] [Google Scholar]
- Kobayashi C., Glover G.H., Temple E. Children’s and adults’ neural bases of verbal and nonverbal ‘theory of mind’. Neuropsychologia. 2007 doi: 10.1016/j.neuropsychologia.2006.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krol K.M., Puglia M.H., Morris J.P., Connelly J.J., Grossmann T. Epigenetic modification of the oxytocin receptor gene is associated with emotion processing in the infant brain. Dev. Cogn. Neurosci. 2019 doi: 10.1016/j.dcn.2019.100648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krol K.M., Moulder R.G., Lillard T.S., Grossmann T., Connelly J.J. Epigenetic dynamics in infancy and the impact of maternal engagement. Sci. Adv. 2019 doi: 10.1126/sciadv.aay0680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusui C. DNA methylation of the human oxytocin receptor gene promoter regulates tissue-specific gene suppression. Biochem. Biophys. Res. Commun. 2001 doi: 10.1006/bbrc.2001.6024. [DOI] [PubMed] [Google Scholar]
- Lecce S., Caputi M., Hughes C. Does sensitivity to criticism mediate the relationship between theory of mind and academic achievementŽ. J. Exp. Child Psychol. 2011 doi: 10.1016/j.jecp.2011.04.011. [DOI] [PubMed] [Google Scholar]
- Lecce S., Bianco F., Devine R.T., Hughes C. Relations between theory of mind and executive function in middle childhood: a short-term longitudinal study. J. Exp. Child Psychol. 2017 doi: 10.1016/j.jecp.2017.06.011. [DOI] [PubMed] [Google Scholar]
- McCullough M.E., Churchland P.S., Mendez A.J. Problems with measuring peripheral oxytocin: can the data on oxytocin and human behavior be trusted? Neurosci. Biobehav. Rev. 2013 doi: 10.1016/j.neubiorev.2013.04.018. [DOI] [PubMed] [Google Scholar]
- Middleton, L.Y. M. et al. Saliva cell type DNA methylation reference panel for epidemiological studies in children. https://doi.org/10.1080/15592294.2021.1890874 (2021). doi:10.1080/15592294.2021.1890874. [DOI] [PMC free article] [PubMed]
- Miller S.A. Children’s understanding of second-order mental states. Psychol. Bull. 2009 doi: 10.1037/a0016854. [DOI] [PubMed] [Google Scholar]
- Miller S.E., Reavis R.E., Avila B.N. Associations between theory of mind, executive function, and friendship quality in middle childhood. Merrill. Palmer Q. 2018 doi: 10.13110/merrpalmquar1982.64.3.0397. [DOI] [Google Scholar]
- Mukerji C.E., Lincoln S.H., Dodell-Feder D., Nelson C.A., Hooker C.I. Neural correlates of theory-of-mind are associated with variation in children’s everyday social cognition. Soc. Cogn. Affect. Neurosci. 2019 doi: 10.1093/scan/nsz040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noppeney U., Price C.J. Retrieval of abstract semantics. Neuroimage. 2004 doi: 10.1016/j.neuroimage.2003.12.010. [DOI] [PubMed] [Google Scholar]
- Parker K.J. Plasma oxytocin concentrations and OXTR polymorphisms predict social impairments in children with and without autism spectrum disorder. Proc. Natl. Acad. Sci. USA. 2014 doi: 10.1073/pnas.1402236111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peirce J. PsychoPy - Psychology software for Python. Integr. Vlsi J. 2009 [Google Scholar]
- Perani D. The neural correlates of verb and noun processing a PET study. Brain. 1999 doi: 10.1093/brain/122.12.2337. [DOI] [PubMed] [Google Scholar]
- Perkeybile A.M. Early nurture epigenetically tunes the oxytocin receptor. Psychoneuroendocrinology. 2019 doi: 10.1016/j.psyneuen.2018.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puglia M.H., Connelly J.J., Morris J.P. Epigenetic regulation of the oxytocin receptor is associated with neural response during selective social attention. Transl. Psychiatry. 2018 doi: 10.1038/s41398-018-0159-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qualtrics. Qualtrics XM // The Leading Experience Management Software. Qualtrics, 2019.
- R Development Core Team R. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; 2011. [DOI] [Google Scholar]
- Saxe R.R., Whitfield-Gabrieli S., Scholz J., Pelphrey K.A. Brain regions for perceiving and reasoning about other people in school-aged children. Child Dev. 2009 doi: 10.1111/j.1467-8624.2009.01325.x. [DOI] [PubMed] [Google Scholar]
- Skuse, D. [Ed], Bruce, H. [Ed] & Dowdney, L. [Ed]. Child psychology and psychiatry: Frameworks for clinical training and practice. Child Psychol. psychiatry Fram. Clin. Train. Pract. (2017). doi:http://dx.doi.org/10.1002/9781119170235.
- Smith S.M., et al. Advances in functional and structural MR image analysis and implementation as FSL. in. NeuroImage. 2004 doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Sommer M., et al. Modulation of the cortical false belief network during development. Brain Res. 2010 doi: 10.1016/j.brainres.2010.07.057. [DOI] [PubMed] [Google Scholar]
- Swick D., Ashley V., Turken A.U. Left inferior frontal gyrus is critical for response inhibition. BMC Neurosci. 2008 doi: 10.1186/1471-2202-9-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager T.D. NeuroSynth: a new platform for large-scale automated synthesis of human functional neuroimaging data. Front. Neuroinform. 2011 doi: 10.3389/conf.fninf.2011.08.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker-Drob E.M., Briley D.A. Continuity of genetic and environmental influences on cognition across the life span: A meta-analysis of longitudinal twin and adoption studies. Psychol. Bull. 2014 doi: 10.1037/a0035893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Völlm B.A., et al. Neuronal correlates of theory of mind and empathy: a functional magnetic resonance imaging study in a nonverbal task. Neuroimage. 2006 doi: 10.1016/j.neuroimage.2005.07.022. [DOI] [PubMed] [Google Scholar]
- Wang A.T., Lee S.S., Sigman M., Dapretto M. Developmental changes in the neural basis of interpreting communicative intent. Soc. Cogn. Affect. Neurosci. 2006 doi: 10.1093/scan/nsl018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Conder J.A., Blitzer D.N., Shinkareva S.V. Neural representation of abstract and concrete concepts: a meta-analysis of neuroimaging studies. Hum. Brain Mapp. 2010 doi: 10.1002/hbm.20950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolrich M.W., Ripley B.D., Brady M., Smith S.M. Temporal autocorrelation in univariate linear modeling of FMRI data. Neuroimage. 2001 doi: 10.1006/nimg.2001.0931. [DOI] [PubMed] [Google Scholar]
- Woolrich M.W., Behrens T.E.J., Beckmann C.F., Jenkinson M., Smith S.M. Multilevel linear modelling for FMRI group analysis using Bayesian inference. Neuroimage. 2004 doi: 10.1016/j.neuroimage.2003.12.023. [DOI] [PubMed] [Google Scholar]
- Worsley, K.J. Statistical analysis of activation images. in Functional Magnetic Resonance Imaging: An Introduction to Methods (2012). doi:10.1093/acprof:oso/9780192630711.003.0014.
- Zhang, F.F. et al. Significant differences in global genomic DNA methylation by gender and race/ethnicity in peripheral blood. https://doi.org/10.4161. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Data Availability Statement
This data will be made available by request on OSF.