Summary
Background
Historically, admission serum albumin concentrations have been considered useful biochemical markers for nutrition assessment. However, there is a lack of randomised trial data investigating whether low albumin concentrations are helpful for identifying patients benefitting from nutritional support.
Methods
This study was a secondary analysis of the EFFORT trial, a Swiss-wide multicentre, randomised controlled trial comparing individualised nutritional support with usual care nutrition in medical inpatients from April 1, 2014, to February 1, 2018. 1389 of 2028 patients at nutritional risk with available albumin concentrations on admission were included. The primary endpoint was all-cause mortality within 30 and 180 days. Patients were stratified into groups of low or normal albumin based on the albumin cut-off of 30 g/L. ClinicalTrials.gov number, NCT02517476.
Findings
1389 patients (mean age, 73.1 (SD 3.5) years; 747 (53.8%) men) were included and 676 (48.7%) had low serum albumin concentrations at admission (<30 g/L). Mortality at 180 days was significantly increased in the low albumin group compared with patients with normal albumin concentrations (219/676 (32.4%) vs. 162/713 (22.7%), fully adjusted HR 1.4, 95%CI 1.11 to 1.77, p = 0.005]. Effects of nutritional support on 30-day mortality were similar for patients with low compared to patients with normal albumin concentrations (HR 0.68, 95%CI 0.44 to 1.05 vs. HR 0.70, 95%CI 0.41 to 1.20), with no evidence for a subgroup effect (p for interaction=0.97).
Interpretation
Based on this secondary analysis of a randomised trial, low admission serum albumin concentrations in hospitalised, non-critically ill, medical patients at nutritional risk had prognostic implications and indicated higher mortality risk but were not helpful in selecting patients for nutritional interventions.
Funding
The Swiss National Science Foundation (SNSF) (PP00P3_150531) and the Research Council of the Kantonsspital Aarau (1410.000.058 and 1410.000.044) provided funding for the EFFORT trial
Keywords: Malnutrition, Inflammation, Serum albumin, Nutritional support, C-reactive protein, Outcomes
Research in context.
Evidence before this study
We searched PubMed and Google Scholar for studies published in English from Nov 1, 1992, to Nov, 1, 2021. We used the search terms “malnutrition” and “albumin”, and “mortality”, or “clinical outcome”, and “nutritional treatment” or “nutritional support”, and found that several studies have examined the cross-sectional relationship between albumin concentrations and mortality and other clinical outcomes. However, there have been no large randomized studies exploring whether low albumin concentrations could be helpful in identifying patients who may benefit from nutritional support.
Added value of this study
This study is, to our knowledge, the first to conclusively assess the ability of albumin concentration to predict the effectiveness of nutritional support and confirms that low admission serum albumin concentrations in hospitalised, non-critically ill, medical patients at nutritional risk predicted higher mortality in the short-term and long-term. Albumin concentrations were, however, not helpful in selecting patients for nutritional interventions.
Implications of all the available evidence
Based on this secondary analysis of a randomised trial, low admission albumin concentrations should not be used to diagnose malnutrition or to help guide nutritional interventions. There is, however, a need for further research investigating biomarkers for better assessing nutritional status and response to nutritional therapy.
Alt-text: Unlabelled box
Introduction
For decades, serum albumin concentration has been used as a surrogate marker to quantify the amount of circulating proteins in the plasma and was, thereby, thought to reflect nutrition status.1,2 For this reason, albumin was historically viewed as a nutrition marker and patients with low albumin concentrations were considered malnourished and in need of nutritional support interventions. However, these considerations were largely based on pathophysiological considerations and more recent data suggested that albumin is also a negative acute phase protein and may reflect inflammation/acute disease severity and not necessarily nutritional status only.3 Still, there is an important lack of data from randomised controlled trials investigating whether low albumin concentrations would be helpful for identification of patients benefitting from nutritional support.2
Malnutrition is common among hospitalised elderly patients and is strongly associated with increased morbidity and mortality.4, 5, 6, 7 In the last few years, several trials and meta-analyses of such trials, have provided evidence that nutritional support reduces risks associated with malnutrition.8,9 The largest trial, the Effect of early nutritional therapy on Frailty, Functional Outcomes, and Recovery of malnourished medical inpatients Trial (EFFORT), included 2028 medical inpatients at nutritional risk and reported significant reductions in severe complications and mortality for patients receiving nutritional support as compared with patients in the control group receiving usual care hospital nutrition.10 While the overall population of medical inpatients did show benefit from nutritional support in this trial, it is possible that some patients experienced more or less benefit from this intervention allowing a more individualised approach to the patient at risk for malnutrition.11,12 In a previous analysis, we found that patients with high inflammation mirrored by high concentrations of C-reactive protein (CRP) at admission showed significantly less benefit from nutrition with significant results in interaction analysis.13 Whether other blood markers – including serum albumin – would also be helpful in identifying patients responding or not responding to nutritional support would be important to further advance nutritional care of the individual patient.
Herein, we performed a secondary analysis of EFFORT with the aim to better define the predictive role of admission albumin concentration in screening and assessment of patients regarding malnutrition. We first investigated the prognostic value of admission serum albumin concentrations and second studied associations of albumin concentrations with effectiveness of nutritional support, overall and within different subgroups.
Methods
Study design and setting
This is a secondary analysis of EFFORT, a multicentre trial that took place in 8 hospitals in Switzerland from April 1st 2014 to February 1st 2018.10 EFFORT was a prospective, non-commercial, randomised-controlled trial comparing the effect of an early individualised nutritional support versus usual care hospital nutrition on medical outcomes in patients at risk of malnutrition. It was approved by the ethics committee of Northwestern Switzerland (EKNZ; 2014_001) and was registered at ClinicalTrials.gov in August 2015 (https://clinicaltrials.gov/ct2/show/NCT02517476). The trial protocol,14 the main results10 as well as results regarding long-term outcomes,15 cost outcomes16 and results of secondary analyses12,13,17, 18, 19, 20, 21, 22 have been published previously. All patients provided written informed consent.
Patient population
EFFORT enrolled consecutive patients at nutritional risk [defined by a Nutritional Risk Screening (NRS 2002) total score ≥3 points23] with an expected hospital stay ≥5 days if they were willing to provide informed consent. The NRS includes the patient's current nutritional status and the severity of the underlining disease.24 Both parts score between 0 and 3 points and an additional point for age above 70 years (maximum of 7 points). Exclusion criteria were initial admission to an intensive care unit or a surgical unit, inability for oral ingestion of food, already established nutritional support at admission, terminal illness, gastric bypass, anorexia nervosa, acute pancreatitis, acute liver failure, cystic fibrosis, stem cell transplantation or contraindications for nutritional support and previous inclusion in the trial. Patients were randomly assigned (1:1) either to the intervention group, receiving individualised nutritional support, or the control group receiving standard hospital food. All patients in the intervention group received individualised nutritional support within 48 h of admission to reach protein and energy goals according to a previously published consensus protocol25 and in accordance with recent international guidelines.26 Individualised energy and protein goals were defined for each individual patient upon hospital admission by a trained registered dietician. We used the weight-adjusted Harris-Benedict equation to estimate energy requirements.27 Daily protein intake goals were set at 1.2–1.5 g/kg body weight per day with lower targets of 0.8 g/kg body weight for patients with renal failure.28 To reach these goals, an individualised nutritional plan was developed by a trained registered dietician for each patient based initially on oral nutrition provided by the hospital kitchen and oral nutritional supplements.29,30 A further increase in nutritional support to enteral tube feeding or parenteral feeding was recommended if at least 75% of energy and protein targets could not be reached through oral feeding within 5 days.
Additionally, medical diagnosis according to International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10) codes, sociodemographic and anthropometric data, baseline muscle strength, and functional status (using the Barthel scale) were assessed in all patients. While EFFORT included a total of 2028 patients, this secondary analysis focuses on 1389 patients (68%) from all participating hospitals. For further subgroup analyses, we excluded another 18 patients due to missing admission CRP concentrations.
Patient groups, research objective and outcomes
The main aims of our study were twofold: first, to investigate role of albumin as a prognostic indicator for mortality, and second as a predictor for the response to nutritional support. For this purpose, we divided the patient population in two groups based on their admission serum albumin concentration choosing a cut-off of 30 g/L.31 Because albumin is highly influenced by inflammation, we further grouped patients according to the level of inflammation based on a CRP cut-off value of 100 mg/L, according to a previous study.13
Endpoints were in line with the original publication, collected through a structured telephone interview at 30 and 180 days after inclusion in the trial.10 The primary endpoint was all-cause mortality within 180 days for prognostic analyses and all-cause mortality within 30 days for the for predictive analysis regarding treatment response. Secondary endpoints included the composite endpoint of adverse outcomes (consisting of all-cause mortality, admission to the intensive care, readmission, major complications, functional decline), major complications (nosocomial infection or abscess requiring antibiotic treatment, major cardiovascular events, acute renal failure), length of hospital stay (LOS), non-elective hospital readmission after 30 and 180 days.
Statistical analysis
Analyses were performed in the intention-to-treat population, including all patients with available serum albumin concentrations on admission. Continuous variables were expressed as mean and standard deviations (SD) for normally distributed data or as median and Interquartile range (IQR) for skewed data, discrete variables as counts and percentages. We compared frequencies using Pearson's χ² test and continuous variables using a two-sample t-statistic or a Wilcoxon rank-sum test. We used Cox regression models for time-to-event data with reporting of hazard ratios (HR), logistic regression for binary outcomes with odds ratios (OR) and linear regression for continuous outcomes with coefficients. We also report corresponding 95% confidence intervals (CI) for all estimates. We fitted different unadjusted and adjusted models including main prognostic indicators (age, sex, main diagnosis, comorbidities, randomisation, centre) and confounders (CRP or NRS). To investigate whether treatment response was different in groups with high or low albumin concentrations, we included interaction terms in the statistical models. We used the KaplanMeier method to visualise the primary outcome data over time by calculating the probability of allcause mortality within 30 days of randomisation. All statistical analyses were performed with STATA 15.1 (Stata Corp, College Station, TX, USA). A P value <0.05 (for a 2-tailed test) was considered to indicate statistical significance.
Role of the funding source
The Swiss National Science Foundation (SNSF) (PP00P3_150531) and the Research Council of the Kantonsspital Aarau (1410.000.058 and 1410.000.044) provided funding for the EFFORT trial. The funders had no role in data collection, analysis, interpretation, writing of the manuscript and the decision to submit.
Results
Patient population
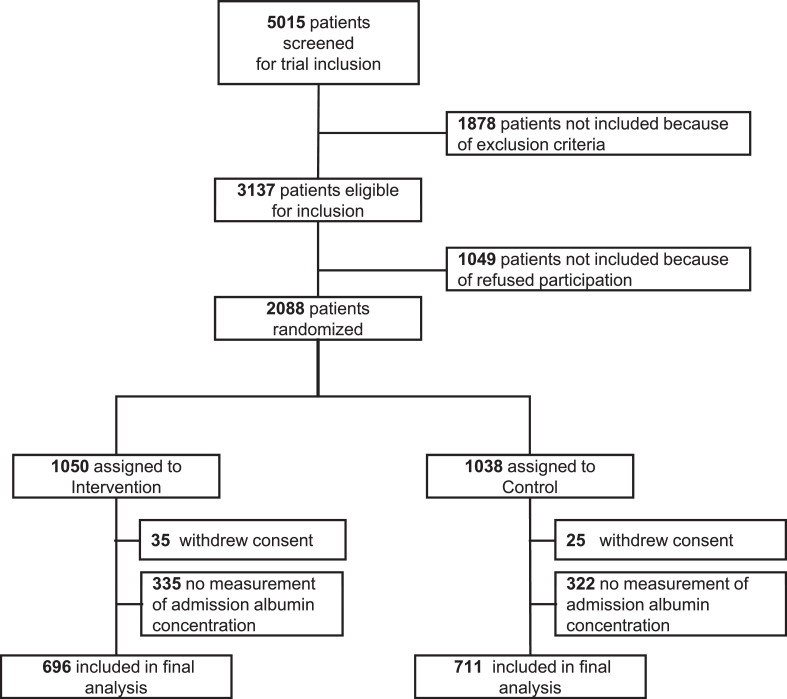
From the 2028 patients enrolled in EFFORT, we had available serum albumin concentrations for 1389 patients (Figure 1), of whom 713 (51.3%) had normal and 676 (48.7%) patients had low albumin concentrations (≥ or <30 g/L). Median age was 73.1 (±13.5) years and 54% were male. All patients were at nutritional risk, with 397 (28.6%), 547 (39.4%) and 445 (32.0%) having NRS-2002 scores of 3, 4 or ≥5 points. Patients had different main admission diagnoses and overall a high burden of comorbidities. Baseline characteristics for the overall population and stratified by albumin concentrations on admission are shown in Table 1. Additional tables of baseline characteristics stratified by CRP concentrations and randomisation can be found in the Supplement 2 (eTables 1 and 2).
Figure 1.
Study profile.
Abbreviations: CRP: C-reactive protein.
Table 1.
Baseline characteristics overall and stratified by serum albumin concentration.
| Overall | Albumin ≥30 g/L | Albumin <30 g/L | p-value | |
|---|---|---|---|---|
| n | 1389 | 713 | 676 | |
| Sociodemographics | ||||
| Male sex | 747 (53.8%) | 374 (52.5%) | 373 (55.2%) | 0.31 |
| Age | ||||
| Mean Age (years), mean (SD) | 73.1 (13.5) | 73.6 (13.9) | 72.5 (13.0) | 0.13 |
| Age groups | 0.008 | |||
| < 65 years, n (%) | 220 (15.8%) | 118 (16.5%) | 102 (15.1%) | |
| 65–75 years | 468 (33.7%) | 213 (29.9%) | 255 (37.7%) | |
| > 75 years | 701 (50.5%) | 382 (53.6%) | 319 (47.2%) | |
| Nutritional assessment | ||||
| Mean body-mass index (kg/m2), mean (SD) | 24.6 (5.3) | 24.5 (5.3) | 24.7 (5.2) | 0.47 |
| Mean body weight (kg), median (IQR) | 68.5 (58.4–81.5) | 67.7 (57.7–80.8) | 69.5 (59.1–82.9) | 0.14 |
| NRS 2002 Score n (%) | <0.001 | |||
| 3 points | 397 (28.6%) | 234 (32.8%) | 163 (24.1%) | |
| 4 points | 547 (39.4%) | 282 (39.6%) | 265 (39.2%) | |
| ≥ 5 points | 445 (32.0%) | 197 (27.6%) | 248 (36.7%) | |
| Weight loss, n (%) | 0.23 | |||
| ≤ 5% in 3 months | 679 (48.9%) | 330 (46.3%) | 349 (51.6%) | |
| > 5% in 3 months | 226 (16.3%) | 119 (16.7%) | 107 (15.8%) | |
| > 5% in 2 months | 186 (13.4%) | 103 (14.4%) | 83 (12.3%) | |
| > 5% in 1 month | 298 (21.5%) | 161 (22.6%) | 137 (20.3%) | |
| Loss of appetite, n (%) | 1242 (89.4%) | 624 (87.5%) | 618 (91.4%) | 0.018 |
| Food intake of normal requirement in the past week, n (%) | 0.007 | |||
| > 75% | 130 (9.4%) | 76 (10.7%) | 54 (8.0%) | |
| 50–75% | 430 (31.0%) | 242 (33.9%) | 188 (27.8%) | |
| 25–50% | 581 (41.8%) | 283 (39.7%) | 298 (44.1%) | |
| < 25% | 248 (17.9%) | 112 (15.7%) | 136 (20.1%) | |
| Severity of illness, n (%) | <0.001 | |||
| very mild | 30 (2.2%) | 23 (3.2%) | 7 (1.0%) | |
| mild | 924 (66.5%) | 534 (74.9%) | 390 (57.7%) | |
| moderate | 418 (30.1%) | 148 (20.8%) | 270 (39.9%) | |
| severe | 17 (1.2%) | 8 (1.1%) | 9 (1.3%) | |
| Laboratory measurements | ||||
| Admission albumin concentration (g/L), mean (SD) | 30.2 (6.7) | 35.4 (4.2) | 24.7 (3.8) | <0.001 |
| Admission CRP concentration (mg/L), median (IQR) | 37 (9.4–117) | 17 (5–60) | 80.5 (25–150) | <0.001 |
| Main admission diagnosis n (%) | ||||
| Cardiovascular disease | 154 (11.1%) | 106 (14.9%) | 48 (7.1%) | <0.001 |
| Infection | 390 (28.1%) | 161 (22.6%) | 229 (33.9%) | <0.001 |
| Cancer | 287 (20.7%) | 118 (16.5%) | 169 (25.0%) | <0.001 |
| Pulmonary disease | 84 (6.0%) | 50 (7.0%) | 34 (5.0%) | 0.12 |
| Frailty | 128 (9.2%) | 90 (12.6%) | 38 (5.6%) | <0.001 |
| Other | 249 (17.9%) | 141 (19.8%) | 108 (16.0%) | 0.065 |
| Comorbidities n (%) | ||||
| Coronary heart disease | 391 (28.1%) | 140 (19.6%) | 109 (16.1%) | 0.088 |
| Congestive heart failure | 249 (17.9%) | 381 (53.4%) | 372 (55.0%) | 0.55 |
| Hypertension | 753 (54.2%) | 54 (7.6%) | 51 (7.5%) | 0.98 |
| Cerebrovascular disease | 105 (7.6%) | 69 (9.7%) | 63 (9.3%) | 0.82 |
| Peripheral arterial disease | 132 (9.5%) | 217 (30.4%) | 236 (34.9%) | 0.075 |
| Chronic kidney disease | 453 (32.6%) | 146 (20.5%) | 157 (23.2%) | 0.22 |
| Diabetes | 303 (21.8%) | 109 (15.3%) | 86 (12.7%) | 0.17 |
| COPD | 195 (14.0%) | 37 (5.2%) | 20 (3.0%) | 0.036 |
| Dementia | 57 (4.1%) | 214 (30.0%) | 282 (41.7%) | <0.001 |
| Malignant disease | 496 (35.7%) | 87 (25.0%) | 409 (39.3%) | <0.001 |
Abbreviations: SD: standard deviation, IQR: interquartile range, NRS: nutritional risk screening, CRP: C-reactive protein, COPD: chronic obstructive pulmonary disease.
Association of admission serum albumin concentrations and outcomes
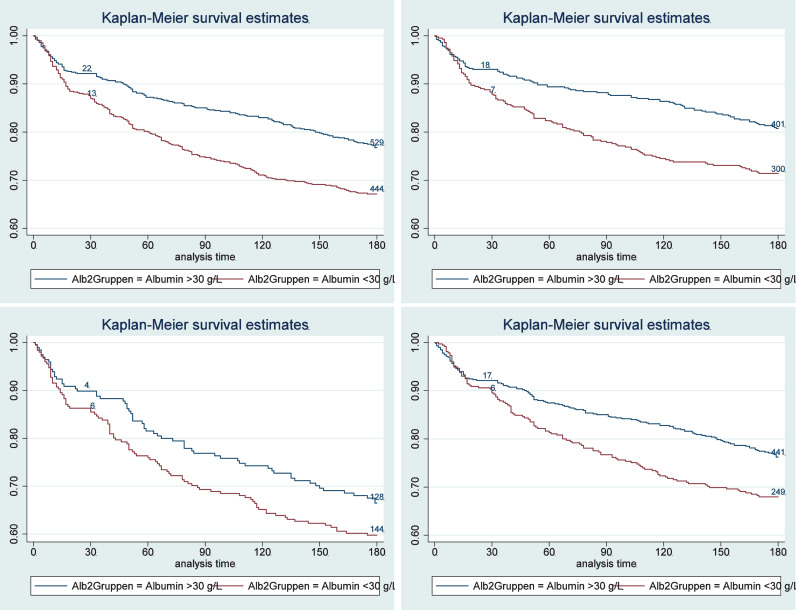
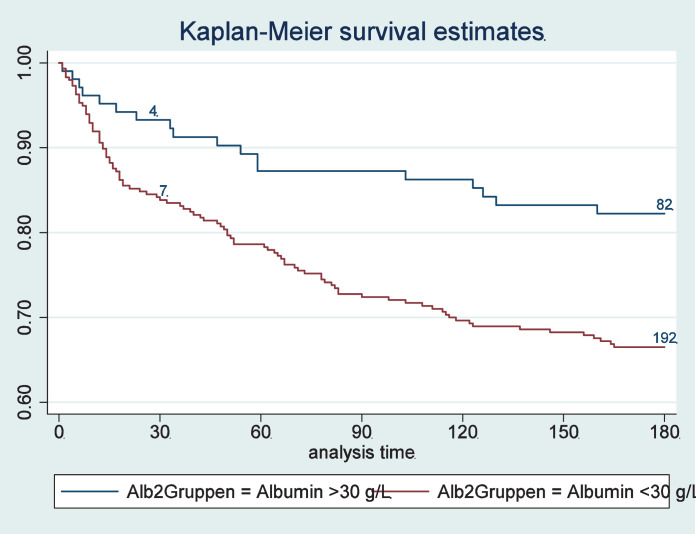
In a first step, we investigated associations of baseline albumin concentration and outcomes at 30 and 180 days (Table 2). Patients with low compared with normal admission albumin concentrations had a significant increase in the risk of mortality within 180 days [32.4% vs. 22.7%, HR 1.53 (95%CI 1.25 to 1.87); p < 0.001]and 30 days [12.6% vs. 7.9%, HR 1.62 (95%CI 1.16 to 2.27), p = 0.005]. Kaplan-Meier survival estimates, shown in Figure 2, confirm these results, showing a higher risk for death in patients with low albumin concentrations at admission. These associations remained significant over 180-days, also in models adjusted for different confounders including Model 3 age, sex, main diagnosis, comorbidities, randomisation, centre, NRS – as well as CRP. For the 30-day outcome, results were no longer significant when adjusting the multivariate analysis for CRP (HR 1.28, 95%CI 0.85 to1.94, p = 0.239). Low admission albumin concentration was also significantly associated with higher risk for adverse outcomes, major complications and LOS, with results remaining significant also in the multivariate models. Figure 2B through E show 180-day mortality stratified according to albumin concentrations within subgroups of patients with high and low nutritional risk and high and low inflammation.
Table 2.
Prognostic value of admission serum albumin concentration.
| Albumin ≥30 g/L | Albumin <30 g/L | Univariate regression analysis (model 1) |
Adjusted regression analysis (Model 2*) |
Adjusted regression analysis (Model 3**) |
||||
|---|---|---|---|---|---|---|---|---|
| n (%) or mean (SD) | n (%) or mean (SD) | OR, HR or Coef (95% CI) | p-value | OR, HR or Coef (95% CI) | p-value | OR, HR or Coef (95% CI) | p-value | |
| Short- and long-term mortality | ||||||||
| All-cause mortality within 30 days | 56/713 (7.9) | 85/676 (12.6) | 1.62 (1.16–2.27) | 0.005 | 1.28 (0.85–1.94) | 0.239 | 1.41 (0.95–2.07) | 0.085 |
| All-cause mortality within 180 days | 162/713 (22.7) | 219/676 (32.4) | 1.53 (1.25–1.87) | <0.001 | 1.4 (1.1–1.79) | 0.007 | 1.4 (1.11–1.77) | 0.005 |
| Secondary short-term outcomes (30 days) | ||||||||
| Adverse outcome | 168/713 (23.6) | 199/676 (29.4) | 1.35 (1.07–1.72) | 0.013 | 1.42 (1.06–1.91) | 0.018 | 1.43 (1.08–1.89) | 0.013 |
| Major complications | 49/713 (6.9) | 61/676 (9) | 1.34 (0.91–1.99) | 0.139 | 1.86 (1.16–2.99) | 0.01 | 1.68 (1.06–2.65) | 0.028 |
| Non-elective hospital readmission | 52/713 (7.3) | 65/676 (9.6) | 1.33 (0.92–1.91) | 0.127 | 1.57 (1.02–2.42) | 0.041 | 1.48 (0.97–2.24) | 0.067 |
| Length of hospital stay | 8.9 (6.1) | 10.5 (7.1) | 1.61 (0.91–2.3) | <0.001 | 2.26 (1.46–3.07) | <0.001 | 2.36 (1.59–3.13) | <0.001 |
| Secondary long-term outcomes (180 days) | ||||||||
| Non-elective hospital readmission | 180/713 (25.2) | 191/676 (28.3) | 1.29 (1.05–1.59) | 0.013 | 1.2 (0.94–1.53) | 0.144 | 1.2 (0.95–1.52) | 0.128 |
*Model 2 adjusted for age, sex, main diagnosis, comorbidities, randomisation, centre, CRP; **Model 3 adjusted for age, sex, main diagnosis, comorbidities, randomisation, centre, NRS. Abbreviations: SD: standard deviation, OR: odd ratio, HR: hazard ratio, Coef: Coefficent, CI: confidence interval, CRP: C-reactive protein, NRS: nutritional risk screening.
Figure 2.
Kaplan-Meier estimates for time to death within 180 days for prognostic value stratified by admission albumin concentrations for the overall population (A); for patients with moderate nutritional risk (B) and high nutritional risk (C); and for patients with low and moderate inflammation (D) and high inflammation (E).
Effectiveness of nutritional support on outcomes depending on admission albumin concentration
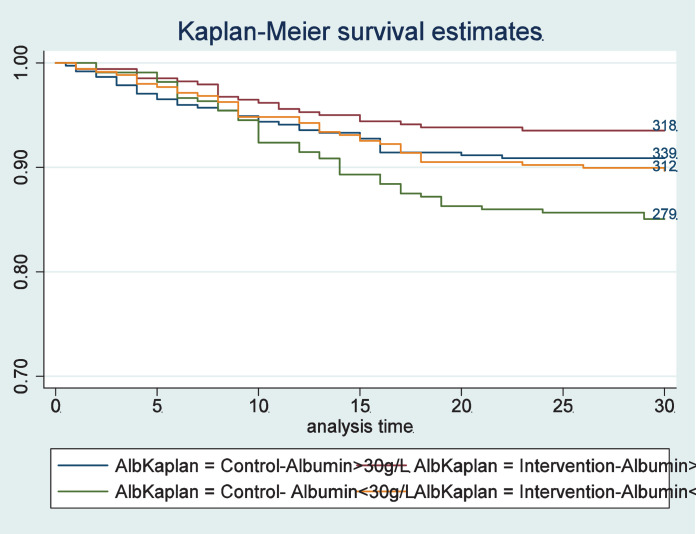
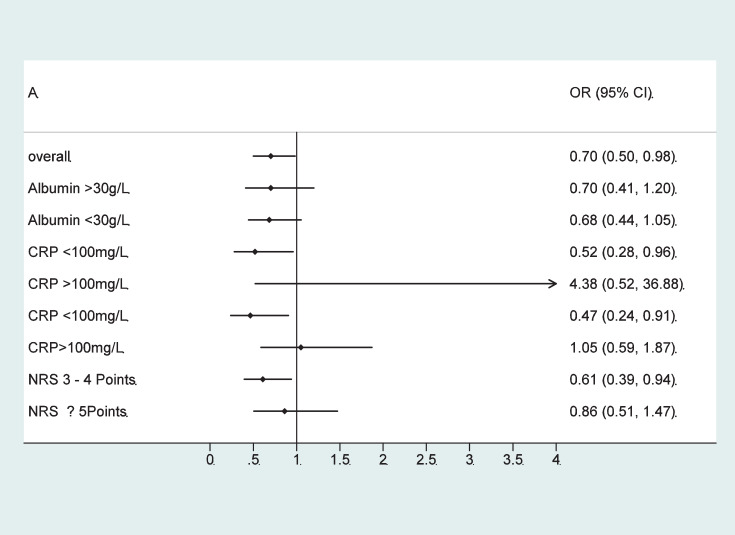
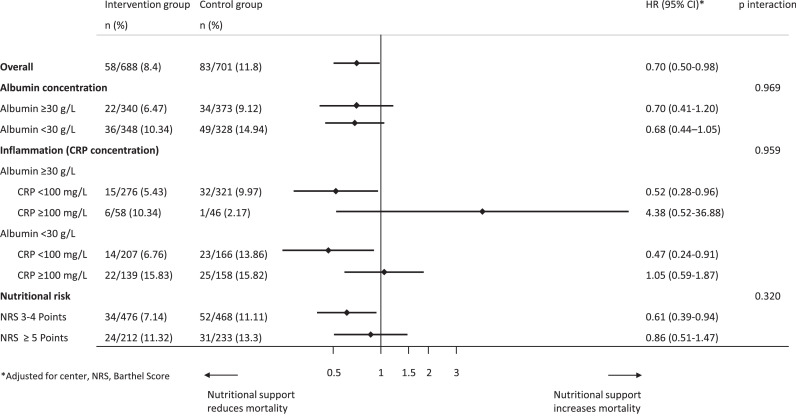
In a second step, we compared effectiveness of individualised nutritional support in patients with normal or low admission albumin concentrations (Table 3). There was no significant difference in the effect of the nutritional support intervention on mortality in both groups (HR 0.68, 95%CI 0.44 to 1.05, p = 0.084 vs 0.70 95%CI 0.41 to 1.20, p = 0.196) with no evidence for interaction (p = 0.969). Figure 3 shows effects of nutritional support in patients stratified according to admission albumin concentration. When additionally stratifying patients according to their baseline CRP concentration, we found more effect of nutritional support in patients with low and moderate CRP concentrations (<100 mg/L) and no effects in the high CRP group (CRP ≥100 mg/L), but again no differences in response according to admission albumin concentrations. Results are also displayed in a forest plot (Figure 4). Results remained similar for secondary endpoints including adverse outcome, LOS as well as 180-day mortality with no evidence for effect modification according to admission albumin concentrations except for a borderline result regarding 180 day mortality.
Table 3.
Predictive value of serum albumin concentration regarding effectiveness of nutritional support.
| Albumin ≥30 g/L |
Albumin <30 g/L |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Intervention group | Control group | Regression analysis* | Intervention group | Control group | Regression analysis* | Interaction terms | |||
| n (%) or mean (SD) | n (%) or mean (SD) | OR, HR or Coef (95% CI) | p-value | n (%) or mean (SD) | n (%) or mean (SD) | OR, HR or Coef (95% CI) | p-value | p interaction* | |
| All-cause mortality within 30 days | |||||||||
| All patients | 22/340 (6.5) | 34/373 (9.1) | 0.70 (0.41–1.2) | 0.196 | 36/348 (10.3) | 49/328 (14.9) | 0.68 (0.44–1.05) | 0.084 | 0.969 |
| CRP <100 mg/l | 15/276 (5.4) | 32/321 (10) | 0.52 (0.28–0.96) | 0.036 | 14/207 (6.8) | 23/166 (13.9) | 0.47 (0.24–0.91) | 0.024 | 0.801 |
| CRP ≥100 mg/l | 6/58 (10.3) | 1/46 (2.2) | 4.38 (0.52–36.88) | 0.174 | 22/139 (15.8) | 25/158 (15.8) | 1.05 (0.24–1.87) | 0.868 | 0.165 |
| Adverse outcome | |||||||||
| All patients | 80/340 (23.5) | 88/373 (23.6) | 1.02 (0.72–1.44) | 0.914 | 91/348 (26.2) | 108/328 (32.9) | 0.72 (0.52–1) | 0.052 | 0.171 |
| CRP <100 mg/l | 66/276 (23.9) | 81/321 (25.2) | 0.95 (0.65–1.38) | 0.792 | 49/207 (23.7) | 51/166 (30.7) | 0.69 (0.43–1.09) | 0.115 | 0.29 |
| CRP ≥100 mg/l | 11/58 (19) | 6/46 (13) | 1.57 (0.52–4.76) | 0.426 | 42/139 (30.2) | 55/158 (34.8) | 0.79 (0.48–1.29) | 0.34 | 0.287 |
| Length of hospital stay | |||||||||
| All patients | 8.8 (6.6) | 9 (5.7) | -0.26 (-1.14–0.61) | 0.554 | 10.57471 (7.3) | 10.5 (6.8) | 0.09 (-0.94–1.12) | 0.865 | 0.599 |
| CRP <100 mg/l | 8.8 (6.4) | 9 (5.8) | -0.15 (-1.11–0.8) | 0.752 | 10.4 (7.1) | 10 (5.8) | 0.42 (-0.89–1.73) | 0.529 | 0.473 |
| CRP ≥100 mg/l | 8.7 (7.4) | 9.4 (4.6) | -0.58 (-3.04–1.88) | 0.641 | 10.8 (7.6) | 11 (7.7) | -0.31 (-2.01–1.4) | 0.722 | 0.747 |
| All-cause mortality within 180 days | |||||||||
| All patients | 70/340 (20.6) | 92/373 (24.7) | 0.82 (0.6–1.13) | 0.223 | 106/348 (30.5) | 113/328 (34.5) | 0.87 (0.67–1.14) | 0.308 | 0.797 |
| CRP <100 mg/l | 54/276 (19.6) | 85/321 (26.5) | 0.71 (0.51–1) | 0.051 | 65/207 (31.4) | 53/166 (31.9) | 0.96 (0.67–1.38) | 0.815 | 0.266 |
| CRP ≥100 mg/l | 14/58 (24.1) | 4/46 (8.7) | 2.76 (0.19–17.31) | 0.077 | 40/139 (28.8) | 58/158 (36.7) | 0.8 (0.53–1.2) | 0.281 | 0.022 |
*Adjusted for centre, NRS, Barthel Score; Abbreviations: SD: standard deviation, OR: odd ratio, HR: hazard ratio, Coef: Coefficent, CI: confidence interval, CRP: C-reactive protein, NRS: nutritional risk screening.
Figure 3.
Kaplan-Meier estimate for time to death within 30 days stratified by randomization into the intervention group and control group; and stratified by admission albumin concentrations.
Figure 4.
Effect of nutritional support on 30-day mortality overall, stratified by serum albumin concentration, inflammation level (CRP) and nutritional risk (NRS).
Abbreviations: HR: hazard ratio, CI: confidence interval, CRP: C-reactive protein, NRS: nutritional risk screening.
Sensitivity analysis
In a final sensitivity analysis, we also investigated whether results would change according to different albumin and CRP cut-off concentrations (i.e., albumin cut-off of 35 g/L and CRP cut-off of 10 mg/L) (data not shown). However, results remained robust regarding the prognostic models as well as regarding the predictive value for effectiveness of nutritional support.
Discussion
In this secondary analysis of a prospective randomised controlled trial comparing the effect of individualised nutritional support versus standard hospital food in a large population of mainly polymorbid, medical inpatients at nutritional risk, we investigated the prognostic and predictive value of admission serum albumin concentrations. First, our results indicate that admission albumin is a strong and independent prognostic indicator regarding longer-term mortality with patients showing low albumin concentrations having a roughly 40% increase in mortality risk in statistical models adjusted for important confounders. Second, our results confirm that the response to nutritional support was independent of admission albumin concentration and clinicians should, thus, not view albumin as a nutritional marker for nutritional interventions.
Our results regarding the prognostic role of albumin are largely concordant with previous studies demonstrating that albumin concentration is a strong prognostic indictor.3,32, 33, 34, 35, 36 Importantly, we were also able to adjust our analysis for important confounders including age, sex, main diagnosis, comorbidities, and nutritional risk as assessed by NRS. In addition, we adjusted the model for the degree of inflammation – reflected by admission CRP concentrations – which directly influences albumin concentrations. These results suggest that albumin concentration could be used as an indicator for poor outcome across medical inpatient populations and, thereby, be helpful in comparing populations for study purposes, in selecting patients at high risk for specific treatments or to individualise care according to expected mortality risks of patients. Our data do not indicate whether albumin concentration is part of the causal pathway or a surrogate marker for disease severity leading to adverse outcome and death. Also, whether monitoring increase in albumin concentration over time would be helpful in determining recovery of patients should be investigated in future studies.
This report is, to our knowledge, the first to conclusively assess the ability of albumin concentration to predict effectiveness of nutritional support. Regarding our primary endpoint 30-day mortality, there was no significant interaction between albumin concentration and response to nutritional support. When stratified according to inflammation, a stronger treatment response was found in patients with low to moderate inflammation compared to high inflammation (CRP ≥100 g/L) confirming previous reports.13,37 Still, treatment response did not differ according to albumin concentrations within different groups of patients stratified by inflammation level. Thus, albumin concentrations did not provide additional information regarding benefit of treatment and should not be viewed as a nutritional marker. These results are in line with a recent consensus paper and a meta-analysis concluding that albumin concentrations should be used in the evaluation of severity of disease but not to assess nutritional status or diagnose malnutrition.2,32,38 This may be explained by serum albumin concentrations being affected by a variety of factors, mostly reflecting acute disease or inflammation but not reflecting plasma proteins or nutritional status.39 The visceral protein albumin is a negative acute-phase protein and albumin concentrations are inversely correlated to CRP concentrations.2 Normalisation of albumin concentrations may, therefore, not depend on nutritional support or albumin treatment, but rather on the resolution of disease and inflammation.40
Importantly, hypoalbuminaemia and an elevated CRP largely identify the same underlying metabolic response to injury and inflammation, but CRP is more sensitive and specific largely related to is short half-life of about 24–48 h and broad range.2,40 Albumin concentrations in response to inflammation have a long half-life of several weeks and may not be restored to normal until the stress response remits, and then only after several weeks. Furthermore, the serum albumin concentration is dramatically altered by a number of other variables not affecting CRP to the same degree, such as the state of hydration and underlying liver and renal function.40,41 It will be interesting in future studies to look at the value of prealbumin (transthyretin) concentrations, which have a much shorter half-life, to potentially guide nutritional support interventions.42
Our report has strengths and limitations. To our knowledge, this is the first secondary analysis based on a randomised controlled clinical trial to investigate whether hypoalbuminemia is associated with effectiveness of nutritional support. Still, we focussed only on albumin concentrations and other biomarkers such as prealbumin could have shown different results. We excluded patients with no albumin measurements which may introduce selection bias. We also only focused on medical inpatients at nutritional risk and had some exclusion criteria in the original trial including surgical and critically ill patients limiting the generalisability of results. Also, it is likely that patients with the greatest inflammatory response (corresponding to high CRP concentrations and low albumin concentrations) may have been more difficult to feed due to the difficulty of feeding in the more acutely ill. This may explain some of the differences in improvements in outcomes in response to nutritional support seen patients with high vs. low levels of inflammation. Because this was a secondary analysis, our results are hypothesis generating rather than definite and require validation in an independent sample.
Based on this secondary analysis of a randomised trial, low admission albumin concentrations in hospitalised, non-critically ill, medical patients at nutritional risk had prognostic implications and indicated higher mortality risk, but were not helpful in selecting patients for nutritional support interventions.
Declaration of interests
Dr. Schuetz reports grants from Neste Health Science, grants from Abbott Nutrition (outside the submitted work). Dr. Stanga reports grants and personal fees from Neste Health Science, grants from Abbott Nutrition, personal fees from Fresenius Kabi (outside the submitted work). All other authors report no conflicts of interest.
Acknowledgments
Contributors
Drs. Bretscher, Boesiger Schuetz, Kaegi-Braun, Boesiger, Hersberger, Mueller, Stanga, Rodondi, Aujesky, Rutishauser, Thomann, Henzen, Brändle, Sigrist, Bilz, Pavlicek, Hoess, Gomes, Tribolet, Evans, Donzé and Lobo were involved in concept and design of this analysis. Drs. Bretscher, Boesiger Schuetz, Kaegi-Braun, Boesiger, Hersberger, Mueller, Stanga, Rodondi, Aujesky, Rutishauser, Thomann, Henzen, Brändle, Sigrist, Bilz, Pavlicek, Hoess, Gomes, Tribolet, Evans, Donzé and Lobo helped in the acquisition of data, analysis and/or interpretation of data. Drs. Bretscher, Schuetz and Kaegi-Braun performed the statistical analysis and drafted the manuscript. All authors provided critical revision of the manuscript. Drs. Schuetz, Stanga and Mueller were responsible for obtaining funding for the study. All authors confirm that they had full access to all the data in the study and accept responsibility for the decision to submit for publication.
Data sharing statement
We intend to make data collected for the study, including deidentified individual participant data and a data dictionary defining each field in the set, available to others; Also, related documents will be available including the trial protocol and the statistical analysis plan. These data will be available with the publication of our main manuscript and all secondary projects as outlined in our trial protocol upon receipt of a letter of intention detailing the study hypothesis and statistical analysis plan. The steering committee of this trial will discuss all requests and decide based on the scientific rigour of the proposal whether data sharing is appropriate. All applicants are asked to sign a data access agreement. Please send any request to the principal investigator of this trial (Philipp.Schuetz@unibas.ch).
Acknowledgments
We thank all the contributors to the Effect of early nutritional therapy on Frailty, Functional Outcomes, and Recovery of malnourished medical inpatients Trial (EFFORT) for their support.
Funding
The Swiss National Science Foundation (SNSF) (PP00P3_150531) and the Research Council of the Kantonsspital Aarau (1410.000.058 and 1410.000.044) provided funding for the EFFORT trial.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2022.101301.
Supplementary materials
References
- 1.Blackburn G.L., Bistrian B.R., Maini B.S., Schlamm H.T., Smith M.F. Nutritional and metabolic assessment of the hospitalized patient. JPEN J Parenter Enter Nutr. 1977;1(1):11–21. doi: 10.1177/014860717700100101. [DOI] [PubMed] [Google Scholar]
- 2.Evans D.C., Corkins M.R., Malone A., et al. The use of visceral proteins as nutrition markers: an ASPEN position paper. Nutr Clin Pract. 2021;36(1):22–28. doi: 10.1002/ncp.10588. [DOI] [PubMed] [Google Scholar]
- 3.Eckart A., Struja T., Kutz A., et al. Relationship of nutritional status, inflammation, and serum albumin levels during acute illness: a prospective study. Am J Med. 2020;133(6):713–722. doi: 10.1016/j.amjmed.2019.10.031. e7. [DOI] [PubMed] [Google Scholar]
- 4.Schuetz P., Seres D., Lobo D.N., Gomes F., Kaegi-Braun N., Stanga Z. Management of disease-related malnutrition for patients being treated in hospital. Lancet. 2021;398(10314):1927–1938. doi: 10.1016/S0140-6736(21)01451-3. [DOI] [PubMed] [Google Scholar]
- 5.Felder S., Braun N., Stanga Z., et al. Unraveling the link between malnutrition and adverse clinical outcomes: association of acute and chronic malnutrition measures with blood biomarkers from different pathophysiological states. Ann Nutr Metab. 2016;68(3):164–172. doi: 10.1159/000444096. [DOI] [PubMed] [Google Scholar]
- 6.Konturek P.C., Herrmann H.J., Schink K., Neurath M.F., Zopf Y. Malnutrition in hospitals: it was, is now, and must not remain a problem! Med Sci Monit. 2015;21:2969–2975. doi: 10.12659/MSM.894238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Corkins M.R., Guenter P., DiMaria-Ghalili R.A., et al. Malnutrition diagnoses in hospitalized patients: United States, 2010. JPEN J Parenter Enter Nutr. 2014;38(2):186–195. doi: 10.1177/0148607113512154. [DOI] [PubMed] [Google Scholar]
- 8.Gomes F., Baumgartner A., Bounoure L., et al. Association of nutritional support with clinical outcomes among medical inpatients who are malnourished or at nutritional risk: an updated systematic review and meta-analysis. JAMA Netw Open. 2019;2(11) doi: 10.1001/jamanetworkopen.2019.15138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaegi-Braun N., Faessli M., Kilchoer F., et al. Nutritional trials using high protein strategies and long duration of support show strongest clinical effects on mortality.: results of an updated systematic review and meta-analysis. Clin Nutr ESPEN. 2021;45:45–54. doi: 10.1016/j.clnesp.2021.08.003. [DOI] [PubMed] [Google Scholar]
- 10.Schuetz P., Fehr R., Baechli V., et al. Individualised nutritional support in medical inpatients at nutritional risk: a randomised clinical trial. Lancet. 2019;393(10188):2312–2321. doi: 10.1016/S0140-6736(18)32776-4. [DOI] [PubMed] [Google Scholar]
- 11.Kaegi-Braun N., Baumgartner A., Gomes F., Stanga Z., Deutz N.E., Schuetz P. Evidence-based medical nutrition - a difficult journey, but worth the effort! Clin Nutr. 2020;39(10):3014–3018. doi: 10.1016/j.clnu.2020.01.023. [DOI] [PubMed] [Google Scholar]
- 12.Kaegi-Braun N., Tribolet P., Baumgartner A., et al. Value of handgrip strength to predict clinical outcomes and therapeutic response in malnourished medical inpatients: secondary analysis of a randomized controlled trial. Am J Clin Nutr. 2021;114(2):731–740. doi: 10.1093/ajcn/nqab042. [DOI] [PubMed] [Google Scholar]
- 13.Merker M., Felder M., Gueissaz L., et al. Association of baseline inflammation with effectiveness of nutritional support among patients with disease-related malnutrition: a secondary analysis of a randomized clinical trial. JAMA Netw Open. 2020;3(3) doi: 10.1001/jamanetworkopen.2020.0663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schuetz P., Fehr R., Baechli V., et al. Design and rationale of the effect of early nutritional therapy on frailty, functional outcomes and recovery of malnourished medical inpatients trial (EFFORT): a pragmatic, multicenter, randomized-controlled trial. Int J Clin Trials. 2018;5(3):142–150. [Google Scholar]
- 15.Kaegi-Braun N., Tribolet P., Gomes F., et al. Six-month outcomes after individualized nutritional support during the hospital stay in medical patients at nutritional risk: secondary analysis of a prospective randomized trial. Clin Nutr. 2021;40(3):812–819. doi: 10.1016/j.clnu.2020.08.019. [DOI] [PubMed] [Google Scholar]
- 16.Schuetz P., Sulo S., Walzer S., et al. Economic evaluation of individualized nutritional support in medical inpatients: secondary analysis of the EFFORT trial. Clin Nutr. 2020;39(11):3361–3368. doi: 10.1016/j.clnu.2020.02.023. [DOI] [PubMed] [Google Scholar]
- 17.Efthymiou A., Hersberger L., Reber E., et al. Nutritional risk is a predictor for long-term mortality: 5-Year follow-up of the EFFORT trial. Clin Nutr. 2021;40(4):1546–1554. doi: 10.1016/j.clnu.2021.02.032. [DOI] [PubMed] [Google Scholar]
- 18.Baumgartner A., Pachnis D., Parra L., et al. The impact of nutritional support on malnourished inpatients with aging-related vulnerability. Nutrition. 2021;89 doi: 10.1016/j.nut.2021.111279. [DOI] [PubMed] [Google Scholar]
- 19.Baumgartner A., Hasenboehler F., Cantone J., et al. Effect of nutritional support in patients with lower respiratory tract infection: secondary analysis of a randomized clinical trial. Clin Nutr. 2021;40(4):1843–1850. doi: 10.1016/j.clnu.2020.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bargetzi A., Emmenegger N., Wildisen S., et al. Admission kidney function is a strong predictor for the response to nutritional support in patients at nutritional risk. Clin Nutr. 2021;40(5):2762–2771. doi: 10.1016/j.clnu.2021.03.013. [DOI] [PubMed] [Google Scholar]
- 21.Bargetzi L., Brack C., Herrmann J., et al. Nutritional support during the hospital stay reduces mortality in patients with different types of cancers: secondary analysis of a prospective randomized trial. Ann Oncol. 2021;32(8):1025–1033. doi: 10.1016/j.annonc.2021.05.793. [DOI] [PubMed] [Google Scholar]
- 22.Hersberger L., Dietz A., Burgler H., et al. Individualized nutritional support for hospitalized patients with chronic heart failure. J Am Coll Cardiol. 2021;77(18):2307–2319. doi: 10.1016/j.jacc.2021.03.232. [DOI] [PubMed] [Google Scholar]
- 23.Hersberger L., Bargetzi L., Bargetzi A., et al. Nutritional risk screening (NRS 2002) is a strong and modifiable predictor risk score for short-term and long-term clinical outcomes: secondary analysis of a prospective randomised trial. Clin Nutr. 2020;39(9):2720–2729. doi: 10.1016/j.clnu.2019.11.041. [DOI] [PubMed] [Google Scholar]
- 24.Kondrup J., Rasmussen H.H., Hamberg O., Stanga Z. Ad Hoc EWG. Nutritional risk screening (NRS 2002): a new method based on an analysis of controlled clinical trials. Clin Nutr. 2003;22(3):321–336. doi: 10.1016/s0261-5614(02)00214-5. [DOI] [PubMed] [Google Scholar]
- 25.Bounoure L., Gomes F., Stanga Z., et al. Detection and treatment of medical inpatients with or at-risk of malnutrition: suggested procedures based on validated guidelines. Nutrition. 2016;32(7–8):790–798. doi: 10.1016/j.nut.2016.01.019. [DOI] [PubMed] [Google Scholar]
- 26.Gomes F., Schuetz P., Bounoure L., et al. ESPEN guidelines on nutritional support for polymorbid internal medicine patients. Clin Nutr. 2018;37(1):336–353. doi: 10.1016/j.clnu.2017.06.025. [DOI] [PubMed] [Google Scholar]
- 27.MacDonald A., Hildebrandt L. Comparison of formulaic equations to determine energy expenditure in the critically ill patient. Nutrition. 2003;19(3):233–239. doi: 10.1016/s0899-9007(02)01033-x. [DOI] [PubMed] [Google Scholar]
- 28.Genton L., Pichard C. Protein catabolism and requirements in severe illness. Int J Vitam Nutr Res. 2011;81(2–3):143–152. doi: 10.1024/0300-9831/a000058. [DOI] [PubMed] [Google Scholar]
- 29.Potter J.M., Roberts M.A., McColl J.H., Reilly J.J. Protein energy supplements in unwell elderly patients–a randomized controlled trial. JPEN J Parenter Enter Nutr. 2001;25(6):323–329. doi: 10.1177/0148607101025006323. [DOI] [PubMed] [Google Scholar]
- 30.Milne A.C., Potter J., Vivanti A., Avenell A. Protein and energy supplementation in elderly people at risk from malnutrition. Cochrane Database Syst Rev. 2009;(2) doi: 10.1002/14651858.CD003288.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weimann A., Braga M., Carli F., et al. ESPEN guideline: clinical nutrition in surgery. Clin Nutr. 2017;36(3):623–650. doi: 10.1016/j.clnu.2017.02.013. [DOI] [PubMed] [Google Scholar]
- 32.Zhang Z., Pereira S.L., Luo M., Matheson E.M. Evaluation of blood biomarkers associated with risk of malnutrition in older adults: a systematic review and meta-analysis. Nutrients. 2017;9(8):829. doi: 10.3390/nu9080829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fulks M., Stout R.L., Dolan V.F. Albumin and all-cause mortality risk in insurance applicants. J Insur Med. 2010;42(1):11–17. [PubMed] [Google Scholar]
- 34.Hannan J.L., Radwany S.M., Albanese T. In-hospital mortality in patients older than 60 years with very low albumin levels. J Pain Symptom Manag. 2012;43(3):631–637. doi: 10.1016/j.jpainsymman.2011.04.009. [DOI] [PubMed] [Google Scholar]
- 35.Jellinge M.E., Henriksen D.P., Hallas P., Brabrand M. Hypoalbuminemia is a strong predictor of 30-day all-cause mortality in acutely admitted medical patients: a prospective, observational, cohort study. PLoS One. 2014;9(8) doi: 10.1371/journal.pone.0105983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vincent J.L., Dubois M.J., Navickis R.J., Wilkes M.M. Hypoalbuminemia in acute illness: is there a rationale for intervention? A meta-analysis of cohort studies and controlled trials. Ann Surg. 2003;237(3):319–334. doi: 10.1097/01.SLA.0000055547.93484.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Casaer M.P., Van den Berghe G. Nutrition in the acute phase of critical illness. N Engl J Med. 2014;370(13):1227–1236. doi: 10.1056/NEJMra1304623. [DOI] [PubMed] [Google Scholar]
- 38.Cederholm T., Barazzoni R., Austin P., et al. ESPEN guidelines on definitions and terminology of clinical nutrition. Clin Nutr. 2017;36(1):49–64. doi: 10.1016/j.clnu.2016.09.004. [DOI] [PubMed] [Google Scholar]
- 39.Covinsky K.E., Covinsky M.H., Palmer R.M., Sehgal A.R. Serum albumin concentration and clinical assessments of nutritional status in hospitalized older people: different sides of different coins? J Am Geriatr Soc. 2002;50(4):631–637. doi: 10.1046/j.1532-5415.2002.50156.x. [DOI] [PubMed] [Google Scholar]
- 40.Soeters P.B., Wolfe R.R., Shenkin A. Hypoalbuminemia: pathogenesis and clinical significance. JPEN J Parenter Enter Nutr. 2019;43(2):181–193. doi: 10.1002/jpen.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Erstad B.L. Albumin disposition in critically Ill patients. J Clin Pharm Ther. 2018;43(5):746–751. doi: 10.1111/jcpt.12742. [DOI] [PubMed] [Google Scholar]
- 42.Keller U. Nutritional laboratory markers in malnutrition. J Clin Med. 2019;8(6):775. doi: 10.3390/jcm8060775. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.