Abstract
Per- and Polyfluoroalkyl Substances (PFAS) are ubiquitously detected in the environment and some pose significant human and environmental health concerns globally. While some PFAS induce adverse health effects, relatively few toxicological studies adequately address the broad structural diversity of this chemical class. In the current study, we evaluated 58 individual PFAS spanning 14 functional head group classes, and 2 mixtures, at a single, low concentration for developmental toxicity in zebrafish using highly sensitive behavior endpoints. Following developmental exposure to PFAS, zebrafish were assessed for mortality and challenged with an embryonic photomotor response (EPR) assay at 24 hours post fertilization (hpf) and with larval photomotor response (LPR) and larval startle response (LSR) assays at 120 hpf. We found that none of the tested PFAS exposures elicited significant mortality or aberrant EPR; however, exposure to 21 individual PFAS from multiple functional head group classes and 1 mixture induced aberrant larval behavior. We then evaluated developmental toxicity across a concentration range of 0–100 μM for 10 perfluoroalkyl carboxylic acids (PFCAs; 4-carbon perfluorobutanoic acid through the 13-carbon perfluorotridecanoic acid). Exposure to the PFCAs did not cause significant mortality or morphological effects, with the exception of perfluorooctanoic acid (PFOA) and perfluorononanoic acid (PFNA), and did not induce aberrant EPR. All PFCAs, except for longer-chain perfluorododecanoic acid (PFDoA) induced abnormal LPR following exposure to at least one concentration. In this study, we evaluated a broad set of PFAS not previously assessed for in vivo sublethal behavior endpoints and confirmed previous findings that exposure to some PFAS induces abnormal behavior in developing zebrafish. The data from this study will guide the selection of PFAS for which to investigate modes of toxic action.
Keywords: Perfluoroalkyl Substances, Polyfluoroalkyl Substances, PFAS, Developmental Toxicity, Zebrafish, Larval Behavior
Graphical Abstract

Introduction
Per- and Polyfluoroalkyl Substances (PFAS) are a significant global concern due to the highly persistent nature, bioaccumulation, and toxicity of some PFAS.1, 2 As chemicals with at least one aliphatic perfluorocarbon moiety, PFAS comprise a large and structurally diverse class of man-made chemicals for which we still have relatively little toxicological data.1, 3, 4 In a 2018 study, the OECD identified 4,730 PFAS-related CAS numbers.4 Due to their oleophobic and hydrophobic properties, PFAS are used for surface treatments for outdoor gear, carpets, and leather stain blocks; paper food packaging; cosmetics; household cleaning agents; printing inks; cookware; and fire-fighting foams.5 Because of their persistent nature, some PFAS are now ubiquitous in the environment, biota, food, drinking water, and in humans.2, 6, 7 Health effects such as reproductive and developmental outcomes, liver and kidney disease, and immune and thyroid dysfunction have been associated with PFAS exposure. While the breadth of PFAS structures represented in the toxicological literature has expanded somewhat, it is still disproportionately focused on perfluorooctane sulfonic acid (PFOS) and perflorooctanoic acid (PFOA). Given their structural diversity, it is essential to broaden PFAS toxicological investigations across all structural subclasses. A primary goal is to identify structural features that predict hazard.1, 2
Embryonic zebrafish is a near-ideal whole animal system in which to screen for chemical-biological activity. Previous developmental zebrafish studies predominately investigated toxicity from the perfluoroalkyl carboxylic acid (PFCA) and perfluoroalkyl sulfonic acid (PFSA) subclasses.8–12 While these PFAS were generally not teratogenic at low μM concentrations, some did alter larval zebrafish behavior. The PFAS hazard potential was influenced by the functional head group and chain length. PFSAs were typically more toxic than their PFCA counterparts and longer chain length was associated with higher toxicity and bioaccumulation8–12 Few studies have addressed other PFAS subclasses, and additional testing is needed before structure-bioactivity consensus can be established.
In this study, we leveraged a library of 58 individual PFAS spanning 14 structural subclasses and 2 mixtures to screen for bioactivity in developmental zebrafish embryonic and larval behavior assays at single concentrations. We then evaluated the bioactivity of 10 PFCAs across a range of concentrations. This is the most structurally diverse in vivo PFAS bioactivity study to date and is the first to evaluate the effect of developmental PFAS exposure on early embryonic zebrafish behavior. The results offer a better understanding of PFAS structure-bioactivity and priority selection of PFAS for mechanistic investigation.
Experimental Procedures
Chemicals for Toxicological Testing:
A set of 58 individual PFAS encompassing 14 structural subclasses (see Table 1 for a complete list and Table S1 for chemical names, CAS numbers, and molecular weights) was obtained as analytical standards from Wellington Laboratories (Guelph, Canada; https://well-labs.com), all at equal stock concentrations of 50 μg/mL, the highest concentration available. The tested subclasses varied by functional head group, per- or polyfluorinated status, ethers within the structure, chlorination, and the presence of charged atoms (see Table 1 for abbreviated subclass structures and Table S2 for structure visuals). 2 available PFAS mixtures were also obtained for toxicological testing: PFC-MXA (11 PFCAs at a ∑PFAS concentration of 22 μg/mL; Wellington Laboratories) and a Mixture B (46 PFAS originally obtained as analytical standards at a ∑PFAS concentration of 2.3 μg/mL), see Tables S3 and S4 for mixture constituents. PFAS stocks were provided in 100% methanol, stored in glass in the dark at −20 °C, and identified as the highest purity available- guaranteed >98% purity (see Table S5 for purity information). Due to the high level of characterization of these analytical standards, no further validation was conducted prior to toxicological testing. Stock concentrations are provided here in units of mass/volume as originally obtained from the manufacturer; however, throughout the remainder of this study, stock and exposure concentrations will be stated in molar units.
Table 1:
Table of individual PFAS toxicologically assessed at a single concentration.
| Structural Class | Count | Individual PFAS Toxicologically Assessed | Abbreviated Structure |
|---|---|---|---|
| Carboxylic acids | 13 | PFBA, PFPeA, PFHxA, PFHpA, PFOA, PFNA, PFDA, PFUdA, PFDoA, PFTrDA, PFTeDA, PFHxDA, PFODA | CF3[CF2]nCO2− |
| Fluorotelomer carboxylic acids | 3 | FPrPA, FPePA, FHpPA | CF3[CF2]nCH2CH2COO− |
| Unsaturated fluorotelomer carboxylic acids | 2 | FHUEA, FOUEA | CF3[CF2]nCF=CHCOO− |
| Ether/polyether carboxylic acids | 2 | HFPO-DA (GenX) | CF3[CF2]2OCF[CF3]COO− |
| NaDONA | CF3O[CF2]3OCFHCF2COO− | ||
| Sulfonic acids | 10 | PFPrS, PFBS, PFPeS, PFHxS, PFHpS, PFOS, PFNS, PFDS, PFDOS | CF3[CF2]nSO3− |
| 8-Cl PFOS | Cl[CF2]8SO3− | ||
| Sulfonic acids (cyclic) | 1 | PFECHS | [CF2]2CF[CF2CF3][CF2]2CF[SO3−] |
| Fluorotelomer sulfonic acids | 4 | 4:2 FTS, 6:2 FTS, 8:2 FTS, 10:2 FTS | CF3[CF2]n[CH2]2SO3− |
| Sulfonamides | 2 | Methyl FOSA, Ethyl FOSA | CF3[CF2]nSO2N[R]H |
| Sulfonamidoacetic acids | 3 | FOSAA, Methyl FOSAA, Ethyl FOSAA | CF3[CF2]nSO2N[CH2COO−][R] |
| Chlorinated ether sulfonic acids | 2 | 11-Cl PF3OUdS, 9-Cl PF3ONS (minor, major components F-53B) | Cl[CF2]nO[CF2]2SO3− |
| Phosphonic acids | 5 | PFHxPA, PFOPA, PFDPA | CF3[CF2]nPO3− |
| Cl PFHxPA, Cl PFOPA | Cl[CF2]nPO3− | ||
| Phosphate esters (mono-/di-substituted) | 6 | 6:2 PAP, 8:2 PAP, SAMPAP | CF3[CF2]nCH2CH2OP[O][O−]2 |
| 6:2 DiPAP, 8:2 DiPAP, DiSAMPAP | [CF3[CF2]nCH2CH2O]2PO2− | ||
| Phosphinic acids | 2 | 6:6 PFPiA, 8:8 PFPiA | [CF3[CF2]n]2PO2− |
| Zwitterions | 3 | N-AP-FHxSA | CF3[CF2]5SO2N−[CH2]3N+[CH3]2H |
| N-TAMP-FHxSA | CF3[CF2]5SO2N−[CH2]3N+[CH3]3 | ||
| N-CMAMP (6:2 FOSA) | CF3[CF2]5[CH2]2SO2NH[CH2]3N+[CH3]2CH2CO2− |
Individual PFAS are organized by functional head group class with the number of PFAS within each class indicated in the Count column and representative, abbreviated structures in the Abbreviated Structure column. Structures of acid classes are in the deprotonated form to accurately represent predominant structures at environmentally relevant pH. Bolded portions of the abbreviated structures indicate the structural features that deviate between classes. R = Methyl or Ethyl group.
Following initial testing, PFCAs were obtained as commercial reference standards (CRS; PFBA, PFPeA, PFHxA, PFHpA, PFOA, PFNA, PFDA, PFUdA, PFDoA, and PFTrDA; see Table S6 for chemical information). CRS were necessary to achieve stock concentrations high enough to enable an exposure concentration range of 0–100 μM. The 30 mM stocks were made in 100% methanol (Optima® LC/MS grade, Fisher Chemical), except for PFUdA, PFDoA (15 mM), and PFTrDA (7.5 mM) due to solubility (see Section S1 for stock solution preparation information). This same methanol was also used for the carrier solvent control treatment. Stock concentrations were validated using HPLC and triple quadrupole mass spectrometry (LC-MS/MS; see Sections S2–S3 and Table S7 for analytical information). Stock concentration was required to be 70% - 130% of the nominal concentration before acceptance for toxicological use (see Table S8).
Zebrafish Husbandry:
Zebrafish (Danio rerio) of the wild-type Tropical 5D strain were bred and raised at Oregon State University in the Sinnhuber Aquatic Research Laboratory (SARL) according to Institutional Animal Care and Use Committee protocols and procedures described by Barton et al., 2016.13 The specific pathogen-free (SPF) nature of the colony at SARL results in healthier, more productive fish, and less variability in research outcomes, especially for behavior endpoints.13 Brood stock were maintained under a 14:10 h light:dark cycle at 28 ± 1°C and housed at densities of 400 fish/50 gallon tank in recirculating filtered water supplemented with Instant Ocean salts (Spectrum Brands, Blacksburg, VA) and sodium bicarbonate as needed to maintain a pH of 7.4. Spawning was stimulated with insertion of a custom spawning funnel into the tanks the night prior to collections. Embryos were collected, rinsed, staged, and housed in plastic petri dishes at 28°C in embryo medium (EM) consisting of 15 mM NaCl, 0.5 mM KCl, 1 mM MgSO4, 0.15 mM KH2PO4, 0.05 mM Na2HPO4, and 0.7 mM NaHCO3 prior to experimental usage.14, 15
Embryonic Exposures:
Figure 1 shows the experimental workflow and exposure paradigm used to test the PFAS at a single concentration. At 4 hours post-fertilization (hpf), chorions of the embryos were enzymatically removed using pronase and a custom automated dechorionator.16 At 5 hpf, an automated embryo placement system placed one embryo per well into round-bottom 96-well plates (Falcon®, product number: 353227) prefilled with 100 μL EM.16 Following embryo placement, 50 μL of EM was removed from each well.
Figure 1-.
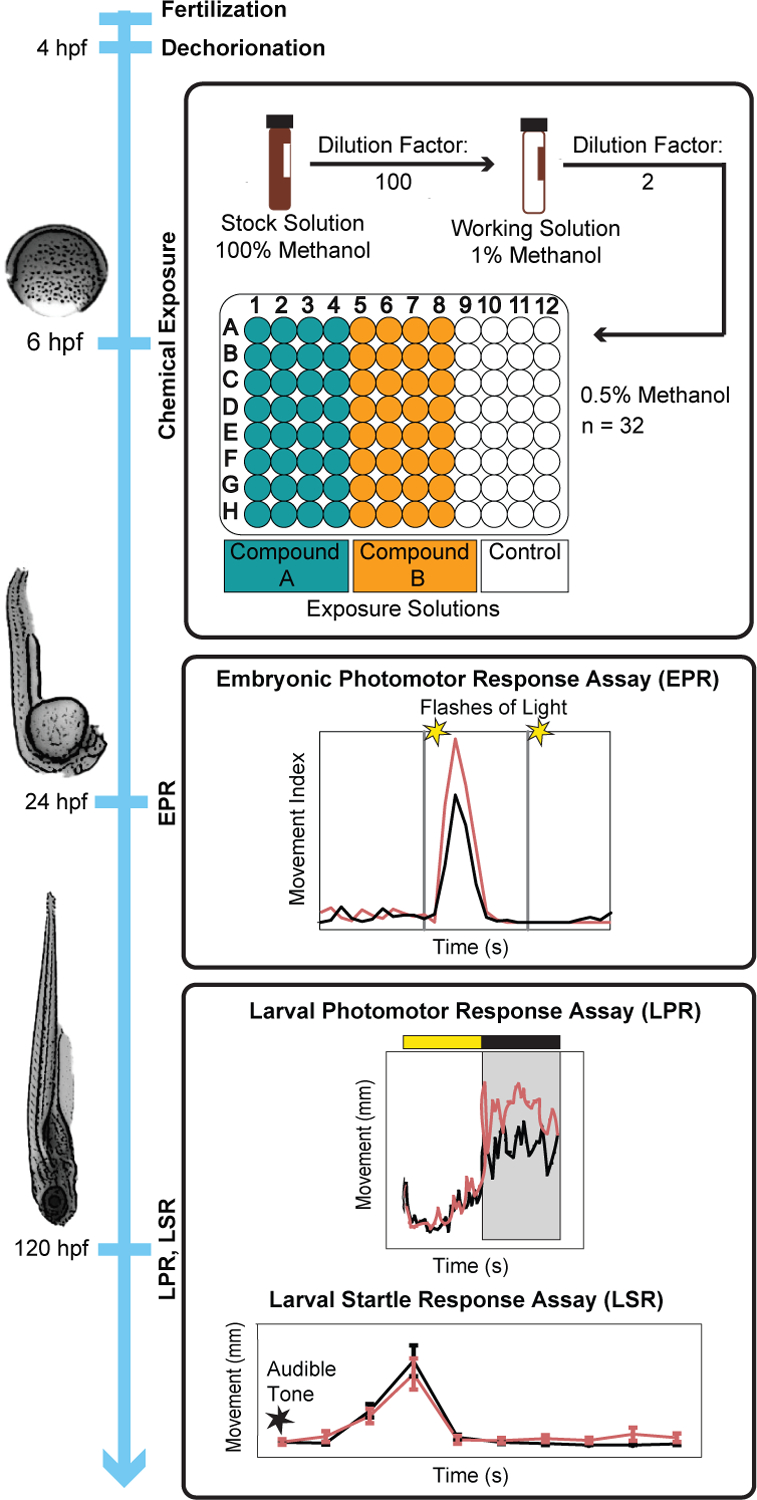
Experimental workflow for single concentration testing. The workflow depicts steps at 4, 6, 24, and 120 hpf. Mortality was assessed at 24 and 120 hpf.
Preliminary screening selected 0.5% methanol as the highest tolerable concentration that did not elicit teratogenic or behavioral effects. Therefore, a total dilution factor of 200 was necessary to achieve 0.5% methanol in the final exposure solutions from stocks in 100% methanol. Embryos were statically exposed starting between 6–8 hpf by adding 50 μL of 1% methanol working solution in EM into each well (already filled with 50 μL EM). Plates were sealed using silicone adhesive film (VWR Cat No. 89134–428) and a heat sealer to minimize evaporation, shaken overnight on an orbital shaker at 235 rpm, maintained at 28°C, and kept in the dark until after the first behavior assay was performed at 24–26 hpf.17
PFAS from analytical stocks, including the two mixtures, were evaluated for bioactivity at a single concentration (see Table 2) due to the high cost and the concentration of stocks (approximately 41–234 μM, see Table S9). To optimize bioactivity detection, the highest achievable concentration, given the required dilution factor of 200, was tested: 0.20–1.17 μM for individual PFAS, 0.27 μM ∑PFAS for PFC-MXA, and 0.023 μM ∑PFAS for Mixture B. The subsequent concentration-response testing with PFCAs followed the same procedure with a few deviations. Nominal exposure concentrations were 0, 1.0, 2.5, 6.5, 16.4, 35.0, 74.8, and 100.0 μM (n = 36 tested with 8 concentrations on all 3 plates), with lower concentrations for PFUdA and PFDoA (0, 1.0, 2.5, 6.5, 16.4, 35.0, 55.0, and 75.0 μM) and PFTrDA (0, 1.0, 2.5, 6.5, 10.0, 16.4, 25.0, and 35.0 μM) due to stock solubility limitations (see Table S10 for purity-adjusted exposure concentrations).
Table 2.
Results of single concentration developmental toxicity testing.
| Structural Class | PFAS | CFC | Conc (μM) | Mort 24 | Mort 120 | EPR | LPR | LSR | n (LPR/LSR) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B | E | R | Light | Dark | AUC | Peak | Controls | Treated | |||||||
| Carboxylic acids | PFBA | 3 | 1.17 | HYPER | 31 | 32 | |||||||||
| PFPeA | 4 | 0.95 | HYPO | 31 | 29 | ||||||||||
| PFHxA | 5 | 0.80 | HYPER | 30 | 32 | ||||||||||
| PFHpA | 6 | 0.69 | 30 | 32 | |||||||||||
| PFOA | 7 | 0.60 | 31/32 | 32 | |||||||||||
| PFNA | 8 | 0.54 | HYPO | 31/32 | 32 | ||||||||||
| PFDA | 19 | 0.49 | 31 | 30/32 | |||||||||||
| PFUdA | 10 | 0.44 | 31 | 30 | |||||||||||
| PFDoA | 11 | 0.41 | HYPER | 32 | 31 | ||||||||||
| PFTrDA | 12 | 0.38 | 32 | 31 | |||||||||||
| PFTeDA | 13 | 0.35 | HYPO | 32 | 31 | ||||||||||
| PFHxDA | 15 | 0.31 | 32 | 29 | |||||||||||
| PFODA | 17 | 0.27 | HYPER | 32 | 32 | ||||||||||
| Fluorotelomer carboxylic acids | FPrPA | 3 | 1.03 | 28 | 30 | ||||||||||
| FPePA | 5 | 0.73 | 31 | 29 | |||||||||||
| FHpPA | 7 | 0.57 | HYPO | 31 | 30 | ||||||||||
| Unsaturated fluorotelomer carboxylic acids | FHUEA | 6 | 0.70 | 30 | 29 | ||||||||||
| FOUEA | 8 | 0.55 | 30 | 29 | |||||||||||
| Ether/polyether carboxylic acids | HFPO-DA | 3,2 | 0.76 | HYPO | 32 | 31 | |||||||||
| NaDONA | 1,3,2 | 0.62 | 32 | 30 | |||||||||||
| Sulfonic acids | PFPrS | 3 | 0.92 | HYPO | 28 | 29 | |||||||||
| PFBS | 4 | 0.74 | HYPER | 27 | 28 | ||||||||||
| PFPeS | 5 | 0.67 | HYPER | 27 | 27 | ||||||||||
| PFHxS | 6 | 0.57 | 28 | 27 | |||||||||||
| PFHpS | 7 | 0.53 | HYPO | 28 | 26 | ||||||||||
| PFOS | 8 | 0.46 | HYPO | 29 | 29 | ||||||||||
| 8-Cl PFOS | 8 | 0.46 | HYPO | 30 | 29 | ||||||||||
| PFNS | 9 | 0.44 | 24 | 26 | |||||||||||
| PFDS | 10 | 0.40 | 30 | 31 | |||||||||||
| PFDoS | 11 | 0.35 | 31 | 30 | |||||||||||
| Sulfonic acids (cyclic) | PFECHS | 8 | 0.50 | HYPO | 29 | 31 | |||||||||
| Fluorotelomer sulfonic acids | 4:2 FTS | 4 | 0.71 | 31 | 30 | ||||||||||
| 6:2 FTS | 6 | 0.56 | 31 | 32 | |||||||||||
| 8:2 FTS | 8 | 0.45 | 28 | 29 | |||||||||||
| 10:2 FTS | 10 | 0.38 | 31 | 30 | |||||||||||
| Sulfonamides | N-Methyl FOSA | 8 | 0.49 | 30 | 31 | ||||||||||
| N-Ethyl FOSA | 8 | 0.47 | 30 | 30 | |||||||||||
| Sulfonamidoacetic acids | FOSAA | 8 | 0.45 | 27 | 28 | ||||||||||
| N-Methyl FOSAA | 8 | 0.44 | 27 | 27 | |||||||||||
| N-Ethyl FOSAA | 8 | 0.43 | 27 | 25 | |||||||||||
| Chlorinated ether sulfonic acids | 9-Cl PF3ONS | 6,2 | 0.44 | 27 | 27 | ||||||||||
| 11-Cl PF3OUdS | 8,2 | 0.37 | 29 | 32 | |||||||||||
| Phosphonic acids | PFHxPA | 6 | 0.62 | HYPO | 32 | 30 | |||||||||
| Cl PFHxPA * | 6 | 0.40 | 28 | 28 | |||||||||||
| PFOPA | 8 | 0.50 | 30 | 31 | |||||||||||
| Cl PFOPA | 8 | 0.48 | HYPER | 25 | 25 | ||||||||||
| PFDPA | 10 | 0.42 | 32 | 31 | |||||||||||
| Phosphate ethers (mono-/di-substituted) | 6:2 PAP | 6 | 0.51 | 29 | 28 | ||||||||||
| 8:2 PAP | 8 | 0.43 | 29 | 23 | |||||||||||
| SAMPAP | 8 | 0.36 | 30 | 30 | |||||||||||
| 6:2 DiPAP | 6,6 | 0.31 | 28 | 29 | |||||||||||
| 8:2 DiPAP | 8,8 | 0.25 | HYPER | 28 | 31 | ||||||||||
| DiSAMPAP | 8,8 | 0.20 | HYPER | 28 | 26 | ||||||||||
| Phosphinic acids | 6:6 PFPiA | 6,6 | 0.35 | HYPO | 27 | 25 | |||||||||
| 8:8 PFPiA | 8,8 | 0.27 | 27 | 26 | |||||||||||
| Zwitterions | N-CMAMP | 6 | 0.44 | 24 | 24 | ||||||||||
| N-AP-FHxSA | 6 | 0.52 | 26 | 28 | |||||||||||
| N-TAMP-FHxSA | 6 | 0.50 | 26 | 31 | |||||||||||
| Mixtures | PFC-MXA | - | 0.27** | 28 | 27 | ||||||||||
| Mixture B | - | 0.023** | HYPER | 28 | 25 | ||||||||||
CFC = Continuously fluorinated carbons; Conc = Concentration; Mort = Mortality (at 24 and 120 hpf, respectively); EPR = Embryonic Photomotor Response: B = Background, E = Excitatory, R = Refractory period; LPR = Larval Photomotor Response (light and dark periods, respectively); LSR = Larval Startle Response: AUC = Area Under the Curve analysis, Peak = peak analysis; n (LPR/LSR) = Sample sizes of the control and treated groups; Results: HYPER = hyperactive (yellow), HYPO = hypoactive (blue) compared to controls. Grey coloring indicates no significant effect.
Tested at half the target concentration due to dilution error (0.4 μM).
Concentration describes ∑PFAS in mixture.
Mortality Assessment and Behavior Assays:
At 24 hpf, having maintained the animals in darkness overnight, embryo photomotor response (EPR) behavior was assessed. As described by Reif et al., 2016, the EPR assay entailed 30 s of darkness with IR light (background period), a 1 s pulse of intense visible light (13,500 lux) followed by 9 s of darkness (excitation period), and another pulse of visible light followed by 10 s of darkness (refractory period). The total movement, characterized by spontaneous tail contractions, was recorded using a custom-built Photomotor Response Analysis Tool. Before analysis, data from dead embryos were removed. Statistical significance was calculated for each period using a Kolmogorov-Smirnov (K-S) test (Bonferroni-corrected p-value threshold = 0.05) for chemically treated embryos compared to controls.18 Following the EPR assay, a 24 hpf visual mortality assessment was conducted and any dead embryos were automatically excluded from the subsequent EPR analysis.
At 120 hpf, larval behavior was evaluated with a larval photomotor response (LPR) assay using ZebraBoxes (ViewPoint Behavior Technologies).19, 20 The software tracked each larvae’s movement and integrated over 6 s time bins. The assay was 24 min and consisted of 4 cycles of a 3 min light period (900 lux) and 3 min dark period (IR). Only the last light/dark cycle (6 min) was used as data, the preceding 3 cycles were treated as acclimation to reduce noisy variation in the data. Statistical analysis followed the workflow described by Zhang et al., 2017. Briefly, using a custom R script, dead fish were removed from the analysis and at least 70% of the original exposure group was required to be phenotypically normal to undergo statistical analysis. Total distance moved was plotted, differential entropy was modeled, and statistical significance was evaluated based on the area under the curve ratios between treatments and control groups using a K-S test.21 At 30 s after the LPR assay, also using the ZebraBox platform, larval startle response (LSR) behavior was evaluated in a 1.5 min assay where an audible 100 dB, 600 Hz tone sounded for 900 ms from speakers physically coupled to the assay plate stage. The stimulus imparted a vibration frequency of 3 mm/s to the 96-well plate, measured at the central wells. Larval motion was tracked prior to the sounding of the tone and for 9 s following. LSR statistical analysis was conducted similar to LPR. A visual mortality/morphology assessment followed the 120 hpf behavioral assays where dead animals were flagged for removal from LPR and LSR analyses.
Morphology Assessment:
In addition to mortality, EPR, and LPR assessments, for the initial solvent screening and the PFCA CRS, 11 morphological endpoints were assessed as described by Truong et al., 2011. These include developmental progression and normal spontaneous movement at 24 hpf and 9 endpoints at 120 hpf (see the raw data guide supporting material).22
Results and Discussion:
0.5% methanol vehicle did not cause significant developmental effects, but isopropanol did
To utilize PFAS in their original solvent and avoid solvent exchange and ensuing losses, it was first necessary to evaluate the developmental toxicity of methanol (0.5, 0.75, and 1%). None of the tested methanol concentrations elicited morphological effects. Only fish exposed to 1% methanol exhibited significant hyperactivity in the dark and light phases of the LPR assay (see Figure S1). Fish exposed to 0.5% and 0.75% methanol were not statistically different from controls. Exposure to 0.5% methanol resulted in behavior more similar to controls and was therefore selected as the carrier solvent concentration for exposure solutions, establishing the required dilution factor of 200.
Several PFAS analytical standards were only available in isopropanol. Significant morphological and behavioral effects were observed in zebrafish exposed to 0.75 and 1% isopropanol. Exposure to 0.5% isopropanol was associated with apparent depressed activity and produced significantly abnormal LSR. Thus, PFAS available as isopropanol solutions were not assessed, i.e., FOSA was not evaluated.
Some PFAS tested at single concentrations impacted larval behavior
None of the structurally diverse PFAS or mixtures tested at single concentrations induced significant EPR effects. This is important as abnormal EPR is often predictive of teratogenic outcomes resulting from diverse modes of action.18 This is the largest study of PFAS bioactivity utilizing behavior endpoints in a whole animal system and the first study to assess the effects of PFAS on embryonic zebrafish behavior as early as 24 hpf. At least 70% of the compounds tested in the present study had not been previously assessed for behavioral effects in zebrafish based on a search of PubMed using the terms PFAS, zebrafish, behavior, and perfluoro and limited to the last 12 years as zebrafish developmental behavior was not widely reported prior to 2009.
Bioactivity spanned multiple subclasses beyond PFCAs and PFSAs. Exposure to 17 of the 58 PFAS, representing 7 of the 14 tested subclasses, produced aberrant LPR, while exposure to 4 PFAS from 2 subclasses induced aberrant LSR (see Table 2 for all results). The carboxylic acid, sulfonic acid, phosphate ether, and phosphonic acid subclasses, the most highly represented in the library containing 13, 10, 6, and 5 individual PFAS, respectively, all had at least 2 PFAS that induced larval behavior effects. PFCAs and PFSAs associated with abnormal larval behavior had 3–17 and 3–8 continuously fluorinated carbons (CFCs), respectively. No PFAS from the unsaturated fluorotelomer carboxylic acid, fluorotelomer sulfonic acid, sulfonamide, sulfonamidoacetic acid, chlorinated ether sulfonic acid, and zwitterionic subclasses induced abnormal behavior. The PFC-MXA mixture did not induce abnormal behavior, perhaps a result of the slightly lower ∑PFAS exposure concentration (0.27 μM) compared to nearly all the other PFCAs; however, Mixture B elicited abnormal LPR effects at an even lower ∑PFAS concentration of 0.023 μM. The behavior effects caused by Mixture B exposure suggest that individual mixture components may have contributed unequally to the mixture toxicity, and additional testing is needed to better understand PFAS mixture effects.
Previous zebrafish studies primarily measured abnormal behavior in larval zebrafish after developmental exposure to PFCAs and PFSAs. PFSAs were generally reported as more toxic than other subclasses and toxicity to increase with chain length.8, 9, 12 PFOS, the most commonly studied PFSA, induced abnormal behavior at concentrations of 0.02,10 0.6 (measured),8 1,23 4.3,9 and 5.46 μM,24 aligning with our findings of abnormal behavior at 0.46 μM. While Ulhaq et al. and the present study observed hypoactivity and others reported hyperactivity,8, 10, 23, 24 there is widespread agreement that PFOS impacts zebrafish development, manifested as abnormal behavior. Gaballah et al. reported the following trend in PFSA developmental neurotoxicity: PFOS>PFHpS>PFPeS>PFHxS, and that PFBS had no observed effect.8 We also identified PFHxS among the least bioactive PFSAs with no larval behavior effects at 0.57 μM, but we did observe behavior effects following PFBS exposure. Concordant PFSA behavior effects between past and the present study validate important new information about less-studied PFAS subclasses.
A few zebrafish studies have investigated the behavioral effects of less-studied PFAS subclasses. Both Menger et al. and the present study found that 6:2 FTS, a fluorotelomer sulfonic acid, did not induce abnormal behavior. Among ether/polyether carboxylic acids, our findings concurred with those of Gaballah et al. that developmental exposure to ADONA (4.4–80.0 μM)8 did not result in abnormal behavior. In contrast, we observed abnormal behavior following exposure to HFPO-DA (GenX), whereas Gaballah et al. did not. Additionally, Kim et al. reported abnormal locomotor behavior in zebrafish larvae exposed to 8:8 PFPiA, a phosphinic acid, as low as 0.343 μM.25 While we did not observe any effect at 0.27 μM 8:8 PFPiA, we did observe abnormal behavior after exposure to 6:6 PFPiA at 0.35 μM.
Different toxicological outcomes between studies may result from variations in exposure paradigm and study design. Among the published studies, exposure initiation ranged from 3–6 hpf and continued until 5 days post-fertilization (dpf) either statically, as in the present study,9–11 or with daily renewals,8 all in 96-well plate format except for Jantzen et al. and Annunziato et al. who exposed in glass vials.10, 11 Zebrafish embryos were enzymatically dechorionated prior to exposure in our study, whereas other studies conducted exposures with the chorions intact.8–11, 25 Several studies mixed PFAS directly in water without use of a carrier solvent,9–11 while others used DMSO8, 24 or methanol.25 The time point at which swimming behavior was assessed also varied from 5 dpf as in the present study and Kim et al.,25 to 6 dpf,8, 9 and 14 dpf.10, 11 Variation between studies is inevitable because the field has yet to converge on a standard praxis for developmental zebrafish assessment. However, we believe that the present study highlights the value of examining as many structures as possible (here 58) under rigorously consistent experimental conditions within an SPF facility that enables the collection of consistent, high-quality behavior data.
We screened PFAS at the single highest concentration possible given available stock concentrations and the limitation of 0.5% methanol in the exposure solutions. These individual PFAS nominal concentrations of 0.20–1.17 μM still represent a high exposure concentration relative to environmentally measured levels. PFCAs and PFSAs, particularly short chain constituents, are detected worldwide at nM levels.26, 27 Some PFAS are more abundant. PFOA has been detected in water bodies around the Bohai Sea, China, at a maximum measured concentration of approximately 0.1 μM,28 closer to the 0.60 μM at which PFOA was tested in the present study. The Mixture B ∑PFAS concentration tested in this study (0.023 μM) is even closer to typical environmentally detected concentrations. Additionally, with half-lives of some PFAS on the order of years in humans, there is significant potential for bioaccumulation and persistence.29
We discovered that approximately 36% of the individual PFAS tested and Mixture B elicited larval behavior effects after developmental exposure, but none caused significant mortality or aberrant embryonic behavior. Given this and the sensitivity of behavior assays, PFAS as a class appear to have relatively low bioactivity at the tested concentrations. The present study is a significant expansion of toxicological data from numerous PFAS subclasses and supports the emerging consensus that structural subclass, and specifically functional head group, influences PFAS toxicity. However, our findings on the impact of CFC chain length are less conclusive. A more in-depth structure-bioactivity analysis utilizing chemoinformatic and computational modeling might elucidate other driving chemical features.
Only PFOA and PFNA induced morphological effects when tested at various concentrations
This section and the following will report nominal exposure concentrations not adjusted for manufacturer-reported purity; however, adjusted concentrations are provided in Table S10. PFOA and PFNA were the only PFCAs to induce morphological effects. The lowest concentrations at which they elicited any mortality or morphological effects were 16.4 and 74.8 μM, respectively (see Figure S3 for PFCA morphology results).
Nearly all PFCAs elicited larval behavior effects when tested at various concentrations
None of the PFCAs tested induced abnormal EPR. Apart from PFDoA, all PFCA exposures produced abnormal LPR effects during the dark (most active) period of the assay for at least 1 nominal exposure concentration (see Figure S4 for all PFCA LPR results). Exposure to all PFBA concentrations induced hyperactivity in the LPR assay. Exposure to PFPeA caused hyperactivity at 16.4, 35.0, and 100.0 μM, trending towards hypoactivity at 74.8 μM. PFHxA elicited hypoactivity at 2.5, 6.5, 35.0, and 74.8 μM, and PFHpA caused abnormal behavior at 2.5 and 6.5 μM (hyperactivity) and 100.0 μM (hypoactivity). PFOA induced hyperactivity at 1 μM, however, the incidence of morphological effects was too high by 6.5 μM to enable LPR analysis; this was also true for PFNA at 100.0 μM. Exposure to PFNA, PFUdA, and PFTrDA induced hypoactivity only at 35.0, 55.0, and 10.0 μM, respectively. PFDA elicited hyperactivity at 2.5 and 35.0 μM and hypoactivity at 100.0 μM, while PFDoA did not induce any abnormal LPR behavior.
LPR effects in the less active light period of the assay were also observed. Similar to the dark period, in the light PFBA also induced hyperactivity at 2.5, 6.5, 35.0, 74.8, and 100.0 μM. Exposure to PFPeA and PFHxA resulted in hyperactivity at 6.5, 16.4, and 35.0 μM, and at 2.5 and 16.4 μM, respectively. No aberrant behavior during the LPR light period was observed following PFHpA, PFOA (as stated above, PFOA concentrations of 6.5 and greater were not useable for LPR), or PFDoA exposure. Exposure to PFNA induced hyperactivity at 1.0, and 74.8 μM and hypoactivity at 2.5 and 35.0 μM. Hyperactivity resulted from exposure to PFDA and PFTrDA at 2.5 and 10.0 μM, respectively. Hypoactivity was induced by PFDA, PFUdA, and PFTrDA at 16.4, 1 and 2.5, and 35.0 μM, respectively.
Comparing PFCA larval behavior effects across concentrations to those observed in the single concentration testing, we noted concordances and differences. PFBA in both tests impacted the light phase of LPR with hyperactivity as low as 2.5 μM and at 1.17 μM, respectively. Across the tested concentration range, PFPeA exposure caused hyperactivity as low as 6.5 μM, while the single 0.95 μM induced hypoactivity. PFHpA and PFOA did not induce abnormal LPR light phase effects in either test; however, the concentration-response testing revealed LPR dark period effects at 2.5 and 1.0 μM, respectively. PFHxA, PFNA, PFDA, PFUdA, and PFTrDA elicited abnormal LPR effects at 2.5, 1.0, 2.5, 1.0, and 10.0 μM, respectively, when tested across concentrations, but the corresponding single concentration test did not induce abnormal LPR, likely the result of lower concentrations (0.80, 0.54, 0.49, 0.44, and 0.38 μM, respectively). Interestingly, PFDoA exposure did not cause aberrant LPR during concentration-response testing, but did cause hyperactivity at the lower, single concentration of 0.41 μM. Concentration-response testing enabled detection of abnormal larval behavior, specifically in the dark phase of the LPR assay.
Concordant with the hyperactivity we observed following exposure to 1.0 μM PFOA, Jantzen et al. found that developmental exposure to PFOA at 0.2 and 2.0 μM caused an increase in distance traveled and that 2.0 μM induced an increase in swimming velocity.10 Menger et al. also reported significantly increased swimming distance during the dark phase of their larval zebrafish assay following PFOA exposure, but at a higher concentration of 12 μM.9 In contrast, Gaballah et al. did not find any effects following PFOA exposure up to 80 μM.8 In the present study, PFPeA induced hyperactivity at as low as 6.5 μM in the light phase and 16.4 μM in the dark phases, and PFHxA caused hyperactivity in the light phase and hypoactivity in the dark phase at as low as 2.5 μM. Gaballah et al. also reported that PFHxA caused hyperactivity in larval zebrafish at as low as 25 μM in the light and 14 μM in the dark, while Menger et al. reported no significant larval behavior effects up to 84 μM PFPeA and 69 μM PFHxA.8, 9 Similarly, we report hyperactivity caused by PFHpA as low as 2.5 μM in the dark phase, whereas Menger et al. only found hyperactivity 89 μM.9 We found that PFNA induced hyperactivity at 1.0 μM in the light, hypoactivity at 2.5 and 35.0 μM, and hyperactivity at 74.8 μM. Other studies have reported that exposure to PFNA caused hyperactivity (0.2 μM) but decreased swimming velocity (0.02, 0.2, 2.0 μM),10 or that PFNA only induced hypoactivity at 48 μM in the light phase.9 It is clear that many studies indicate PFCAs induce behavior effects in larval zebrafish, and a significant strength of our study was comparing the behavioral toxicity of PFCAs to other subclasses in the same controlled system.
EPR, LPR, and LSR are sensitive indicators of bioactivity
EPR, LPR, and LSR can only be measured below the threshold for teratogenicity, making them, by definition, highly sensitive endpoints of bioactivity. Abnormal LPR or LSR outcomes do not identify a specific affected pathway, nor do they mean that a similar behavior effect should be anticipated in humans. However, an abnormal LPR or LSR does indicate that, within this critical developmental window, some PFAS interact with biological targets sufficiently to perturb normal development. Therefore, those PFAS structures associated with developmental bioactivity in a rapid model like larval zebrafish behavior can be quickly prioritized for more comprehensive hazard assessment and investigation of toxic mechanisms.
Future considerations
Bioactivity observed in any test system is the result of the combination of chemical uptake, metabolism, and intrinsic chemical biological activity. Some PFAS bioaccumulate more than others and this can affect toxicity.8, 9, 30 Understanding the toxicokinetics of PFAS from broad subclasses is essential to understand the health risks that PFAS may pose. While we did not assess PFAS toxicokinetics in this study, our highly controlled system and exposure paradigm enabled comparisons between compounds that represented much of PFAS structural diversity. Further investigation of PFAS mixture toxicity will also be essential to understanding PFAS hazard potential. The present dataset will inform the targeted selection of PFAS for which to investigate toxicokinetics and elucidate modes of toxic action. The field is getting closer to a better understanding of which PFAS are most bioactive, the structural drivers of bioactivity, and their biological targets. This information will be key to informing regulation and manufacturer selection of PFAS that are safest for consumer use versus those with a clear safety liability.
Conclusion
PFAS of a variety of structural subclasses and fluorinated carbon chain lengths are bioactive in developmental zebrafish, primarily eliciting larval behavior effects. Our dataset spans a PFAS structural breadth previously unassessed in a single in vivo system and will further inform PFAS structure-bioactivity relationships and selection of PFAS for future investigations of toxicity.
Supplementary Material
Funding Information
This research was funded by the U.S. Environmental Protection Agency grant #83948101, and the National Institute of Health Sciences grant #P30ES030287 and T32 Integrated Regional Training Program in Environmental Health Sciences grant #ES007060.
Footnotes
Supporting Information
Includes additional information on PFAS chemicals, subclass structures, stock solution preparation and analytical validation, methanol and isopropanol developmental toxicity, and PFCA behavioral effects. Raw toxicological data and a raw data guide also provided.
References
- 1.Wang ZY; DeWitt JC; Higgins CP; Cousins IT, A Never-Ending Story of Per- and Polyfluoroalkyl Substances (PFASs)? Environmental Science & Technology 2017, 51 (5), 2508–2518. [DOI] [PubMed] [Google Scholar]
- 2.Fenton SE; Ducatman A; Boobis A; DeWitt JC; Lau C; Ng C; Smith JS; Roberts SM, Per- and Polyfluoroalkyl Substance Toxicity and Human Health Review: Current State of Knowledge and Strategies for Informing Future Research. Environ Toxicol Chem 2021, 40 (3), 606–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buck RC; Franklin J; Berger U; Conder JM; Cousins IT; de Voogt P; Jensen AA; Kannan K; Mabury SA; van Leeuwen SP, Perfluoroalkyl and polyfluoroalkyl substances in the environment: terminology, classification, and origins. Integr Environ Assess Manag 2011, 7 (4), 513–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.OECD Toward a new comprehensive global database of per- and polyfluoroalkyl substances (PFASs): Summary report on updating the OECD 2007 list of per- and polyfluoroalkyl substances (PFASs); 2018.
- 5.KEMI Occurence and Use of Highly Fluorinated Substances and Alternatives; 361 164; Stockholm, 2015. [Google Scholar]
- 6.Sunderland EM; Hu XDC; Dassuncao C; Tokranov AK; Wagner CC; Allen JG, A review of the pathways of human exposure to poly- and perfluoroalkyl substances (PFASs) and present understanding of health effects. J Expo Sci Env Epid 2019, 29 (2), 131–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mamsen LS; Bjorvang RD; Mucs D; Vinnars MT; Papadogiannakis N; Lindh CH; Andersen CY; Damdimopoulou P, Concentrations of perfluoroalkyl substances (PFASs) in human embryonic and fetal organs from first, second, and third trimester pregnancies. Environment International 2019, 124, 482–492. [DOI] [PubMed] [Google Scholar]
- 8.Gaballah S; Swank A; Sobus JR; Howey XM; Schmid J; Catron T; McCord J; Hines E; Strynar M; Tal T, Evaluation of Developmental Toxicity, Developmental Neurotoxicity, and Tissue Dose in Zebrafish Exposed to GenX and Other PFAS. Environ Health Perspect 2020, 128 (4), 47005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Menger F; Pohl J; Ahrens L; Carlsson G; Orn S, Behavioural effects and bioconcentration of per- and polyfluoroalkyl substances (PFASs) in zebrafish (Danio rerio) embryos. Chemosphere 2019, 245, 125573. [DOI] [PubMed] [Google Scholar]
- 10.Jantzen CE; Annunziato KA; Bugel SM; Cooper KR, PFOS PFNA, and PFOA sub-lethal exposure to embryonic zebrafish have different toxicity profiles in terms of morphometrics, behavior and gene expression. Aquatic Toxicology 2016, 175, 160–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Annunziato KM; Jantzen CE; Gronske MC; Cooper KR, Subtle morphometric, behavioral and gene expression effects in larval zebrafish exposed to PFHxA, PFHxS and 6:2 FTOH. Aquatic Toxicology 2019, 208, 126–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ulhaq M; Orn S; Carlsson G; Morrison DA; Norrgren L, Locomotor behavior in zebrafish (Danio rerio) larvae exposed to perfluoroalkyl acids. Aquatic Toxicology 2013, 144, 332–340. [DOI] [PubMed] [Google Scholar]
- 13.Barton CL; Johnson EW; Tanguay RL, Facility Design and Health Management Program at the Sinnhuber Aquatic Research Laboratory. Zebrafish 2016, 13 Suppl 1, S39–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kimmel CB; Ballard WW; Kimmel SR; Ullmann B; Schilling TF, Stages of embryonic development of the zebrafish. Developmental dynamics : an official publication of the American Association of Anatomists 1995, 203 (3), 253–310. [DOI] [PubMed] [Google Scholar]
- 15.Westerfield M, The zebrafish book. A guide for the laboratory use of zebrafish (Danio rerio). 4 ed.; University of Oregon Press, Eugene: 2000. [Google Scholar]
- 16.Mandrell D; Truong L; Jephson C; Sarker MR; Moore A; Lang C; Simonich MT; Tanguay RL, Automated zebrafish chorion removal and single embryo placement: optimizing throughput of zebrafish developmental toxicity screens. J Lab Autom 2012, 17 (1), 66–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Truong L; Bugel SM; Chlebowski A; Usenko CY; Simonich MT; Simonich SL; Tanguay RL, Optimizing multi-dimensional high throughput screening using zebrafish. Reprod Toxicol 2016, 65, 139–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reif DM; Truong L; Mandrell D; Marvel S; Zhang G; Tanguay RL, High-throughput characterization of chemical-associated embryonic behavioral changes predicts teratogenic outcomes. Arch Toxicol 2016, 90 (6), 1459–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saili KS; Corvi MM; Weber DN; Patel AU; Das SR; Przybyla J; Anderson KA; Tanguay RL, Neurodevelopmental low-dose bisphenol A exposure leads to early life-stage hyperactivity and learning deficits in adult zebrafish. Toxicology 2012, 291 (1–3), 83–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Truong L; Saili KS; Miller JM; Hutchison JE; Tanguay RL, Persistent adult zebrafish behavioral deficits results from acute embryonic exposure to gold nanoparticles. Comp Biochem Physiol C Toxicol Pharmacol 2012, 155 (2), 269–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang G; Truong L; Tanguay RL; Reif DM, A New Statistical Approach to Characterize Chemical-Elicited Behavioral Effects in High-Throughput Studies Using Zebrafish. PLoS One 2017, 12 (1), e0169408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Truong L; Harper SL; Tanguay RL, Evaluation of embryotoxicity using the zebrafish model. Methods Mol Biol 2011, 691, 271–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang HH; Huang CJ; Wang LJ; Ye XW; Bai CL; Simonich MT; Tanguay RL; Dong QX, Toxicity, uptake kinetics and behavior assessment in zebrafish embryos following exposure to perfluorooctanesulphonicacid (PFOS). Aquatic Toxicology 2010, 98 (2), 139–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khezri A; Fraser TW; Nourizadeh-Lillabadi R; Kamstra JH; Berg V; Zimmer KE; Ropstad E, A Mixture of Persistent Organic Pollutants and Perfluorooctanesulfonic Acid Induces Similar Behavioural Responses, but Different Gene Expression Profiles in Zebrafish Larvae. Int J Mol Sci 2017, 18 (2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim S; Stroski KM; Killeen G; Smitherman C; Simcik MF; Brooks BW, 8:8 Perfluoroalkyl phosphinic acid affects neurobehavioral development, thyroid disruption, and DNA methylation in developing zebrafish. Sci Total Environ 2020, 736, 139600. [DOI] [PubMed] [Google Scholar]
- 26.Cai Y; Wang X; Wu Y; Zhao S; Li Y; Ma L; Chen C; Huang J; Yu G, Temporal trends and transport of perfluoroalkyl substances (PFASs) in a subtropical estuary: Jiulong River Estuary, Fujian, China. Sci Total Environ 2018, 639, 263–270. [DOI] [PubMed] [Google Scholar]
- 27.Nakayama SF; Yoshikane M; Onoda Y; Nishihama Y; Iwai-Shimada M; Takagi M; Kobayashi Y; Isobe T, Worldwide trends in tracing poly- and perfluoroalkyl substances (PFAS) in the environment. TrAC Trends in Analytical Chemistry 2019, 121, 115410. [Google Scholar]
- 28.Chen H; Han J; Zhang C; Cheng J; Sun R; Wang X; Han G; Yang W; He X, Occurrence and seasonal variations of per- and polyfluoroalkyl substances (PFASs) including fluorinated alternatives in rivers, drain outlets and the receiving Bohai Sea of China. Environ Pollut 2017, 231 (Pt 2), 1223–1231. [DOI] [PubMed] [Google Scholar]
- 29.Li Y; Fletcher T; Mucs D; Scott K; Lindh CH; Tallving P; Jakobsson K, Half-lives of PFOS, PFHxS and PFOA after end of exposure to contaminated drinking water. Occupational and environmental medicine 2018, 75 (1), 46–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vogs C; Johanson G; Naslund M; Wulff S; Sjodin M; Hellstrandh M; Lindberg J; Wincent E, Toxicokinetics of Perfluorinated Alkyl Acids Influences Their Toxic Potency in the Zebrafish Embryo (Danio rerio). Environ Sci Technol 2019, 53 (7), 3898–3907. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


