ABSTRACT
Osmotic stress is a significant physical challenge for free-living cells. Cells from all three domains of life maintain viability during osmotic stress by tightly regulating the major cellular osmolyte potassium (K+) and by import or synthesis of compatible solutes. It has been widely established that in response to high salt stress, many bacteria transiently accumulate high levels of K+, leading to bacteriostasis, with growth resuming only when compatible solutes accumulate and K+ levels are restored to biocompatible levels. Using Bacillus subtilis as a model system, we provide evidence that K+ fluxes perturb Mg2+ homeostasis: import of K+ upon osmotic upshift is correlated with Mg2+ efflux, and Mg2+ reimport is critical for adaptation. The transient growth inhibition resulting from hyperosmotic stress is coincident with loss of Mg2+ and a decrease in protein translation. Conversely, the reimport of Mg2+ is a limiting factor during resumption of growth. Furthermore, we show the essential signaling dinucleotide cyclic di-AMP fluctuates dynamically in coordination with Mg2+ and K+ levels, consistent with the proposal that cyclic di-AMP orchestrates the cellular response to osmotic stress.
KEYWORDS: osmotic up-shock, osmoadaptation, magnesium homeostasis, c-di-AMP, Bacillus subtilis, magnesium, osmotic stress, physiology, potassium transport
INTRODUCTION
It is estimated that half of all enzymes require metals (1), and cells have developed sophisticated mechanisms to regulate the import, intracellular trafficking, and export of metal ions (2). Using Bacillus subtilis as a model system, we have identified the key regulatory proteins that monitor and control the intracellular levels of zinc (Zn2+), manganese (Mn2+), and iron (Fe2+/3+). Ion homeostasis relies on the tight regulation of both import and efflux (2–5), and mutants lacking efflux have an increased sensitivity to metal intoxication (6–10). Previously, we recovered mpfA mutations as suppressors of Mn2+ sensitivity in strains defective for Mn2+ efflux (5, 11). Strains lacking MpfA have an ∼50% increase in intracellular Mg2+ (5), consistent with the assignment of MpfA as a major Mg2+ efflux system (5, 12). Thus, elevated Mg2+ can protect cells against Mn2+ intoxication.
In most cells, Mg2+ import is tightly regulated. In Escherichia coli, Mg2+ import requires the P-type ATPase MgtA. MgtA is under complex regulation, which includes induction through the PhoPQ two-component system and regulation of MgtA activity by the small protein MgtS (13) and allosterically by Mg2+ (14). In B. subtilis, Mg2+ uptake requires MgtE (15), and mgtE expression is controlled at the transcriptional level by a Mg2+-sensing riboswitch (16, 17). MgtE activity was allosterically inhibited by Mg2+ binding to a cytoplasmic cystathionine beta-synthase (CBS) domain (18), likely in combination with ATP (19). Finally, MgtE stability is tightly regulated by the FtsH intramembrane protease and the YqgP adaptor protein (20).
Since uptake is so tightly regulated, the major physiological role of the MpfA Mg2+ efflux protein is not immediately obvious. We hypothesized that Mg2+ efflux is elicited by hyperosmotic shock, which often triggers a large influx of K+. The level of intracellular Mg2+ is second only to that of K+, and a large influx of K+ (to levels that can approach 1 M) could lead to a displacement of Mg2+ from macromolecules and an increase in free Mg2+ levels (21). However, most comprehensive reviews of bacterial osmotic stress responses focus on K+ influx and make little, if any, mention of how K+ might perturb Mg2+ pools (22, 23).
Here, we show that hyperosmotic shock triggers a drastic loss of Mg2+ from cells. Osmoadaptation is then limited by Mg2+ reimport, which in B. subtilis depends on MgtE. In strains deficient in Mg2+ export, elevated Mg2+ is correlated with a reduction in the basal-level expression of MgtE, and this reduced capacity for Mg2+ import contributes to delayed osmoadaptation. We further demonstrate that levels of cyclic di-AMP, a master regulator of osmoadaptation (24), vary, consistent with a role in directly controlling the dynamic and inverse fluctuations of K+ and Mg2+.
RESULTS
Mutants lacking the MpfA Mg2+ efflux pump are impaired in osmoadaptation.
To study the importance of Mg2+ during osmoadaptation, we compared the growth of wild-type B. subtilis strain CU1065 (WT) with an isogenic ΔmpfA deletion mutant (here termed mpfA) missing the primary Mg2+ efflux pump. The subculture of exponentially growing WT cells into a high salt medium elicits osmotic stress and results in a growth lag and reduced growth rate (Fig. 1), as reported previously (25). The mpfA strain grew like the WT in the absence of stress but was significantly delayed in osmoadaptation to both high NaCl and high KCl (Fig. 1).
FIG 1.
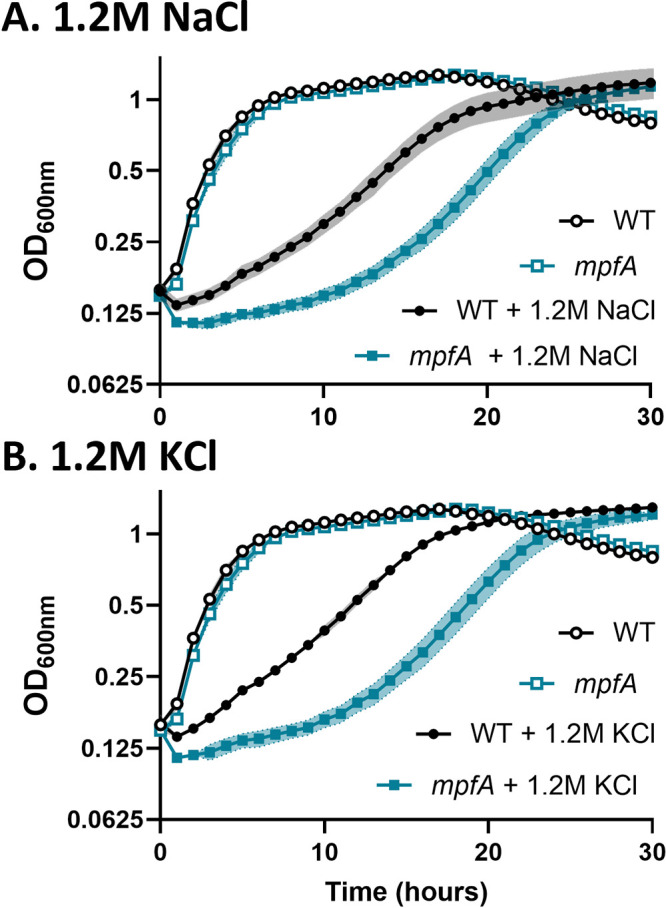
Loss of MpfA impairs osmoadaptation. mpfA mutants exhibit a lag in osmoadaptation relative to wild-type cells when exposed to hyperosmotic stress. In minimal medium at 37°C, mpfA mutants (blue) grow like WT (black) cells, but they lag in osmoadaptation in medium with 1.2 M NaCl (A) or 1.2 M KCl (B). Exponentially growing cells in minimal medium were subcultured (1:4) in triplicate into the indicated medium. Absorbance at 600 nm was recorded every 60 min. The data shown are the averages (symbols) and standard deviations (shading) from at least three biological replicates. Open symbols represent cells grown on minimal medium, and filled symbols represent cells with the indicated addition.
The role of MpfA in osmoadaptation is correlated with K+ import.
Since osmoadaptation triggers a large influx of K+, we hypothesized that MpfA is important for export of displaced Mg2+ ions. To test whether the role of MpfA was related to K+ import, osmoadaptation was investigated in WT and mpfA strains defective in K+ import. In agreement with the established importance of K+ uptake as a first response to osmotic stress, a mutant lacking the high-affinity K+ importer KimA (26) was delayed in osmoadaptation, much like mpfA. In the kimA strain with reduced K+ import, deletion of mpfA did not further slow osmoadaptation (Fig. 2A). This is consistent with the reported role of MpfA in Mg2+ efflux (5) and the hypothesis that osmotically induced K+ import perturbs Mg2+ pools.
FIG 2.
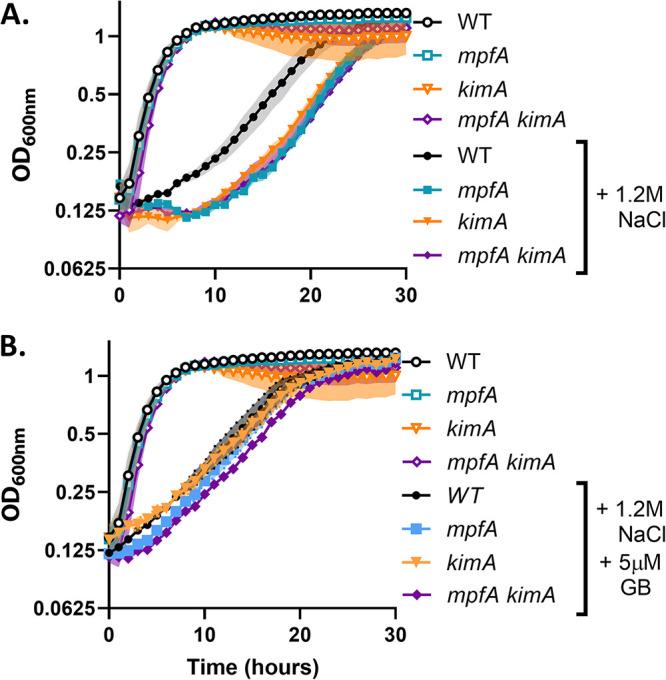
Effects of kimA and mpfA on osmoadaptation are not additive. (A) Mutants defective in one of the osmotically inducible K+ importers, KimA, exhibit a lag in osmoadaptation relative to the WT. This phenotype is not additive with mpfA. (B) Addition of 5 μM glycine betaine (GB) reduces the lag in both mpfA and kimA mutants. This experiment was performed and depicted as indicated in the legend to Fig. 1.
As reported previously, the compatible solute glycine betaine (GB) modestly increases the rate of osmoadaptation. GB import is known to significantly reduce K+ uptake (27). Consistent with reduced K+ import, the kimA and mpfA mutations do not significantly increase the lag time relative to the WT (Fig. 2B). Mutants deficient in the other osmotically inducible K+ transporter, KtrAB, exhibited a shorter lag in osmoadaptation, but, like kimA, the effect of the ktrAB mutation was not additive with mpfA (see Fig. S1A in the supplemental material). Notably, ktrAB kimA double mutants exhibit slower growth in minimal media without osmotic stress and were unable to adapt and grow in the presence of 1.2 M NaCl, with or without MpfA (Fig. S1B). This supports the idea that KimA and KtrAB are partially redundant in their ability to import K+ under osmostress conditions and reinforces the view that this is an essential first response in the absence of compatible solutes (22, 23).
Osmoadaptation requires osmotically induced K+ import. (A) Loss of KtrAB (in strains retaining KimA) has little effect on osmoadaptation in either WT or mpfA strains. (B) Mutant strains lacking both high-affinity K+ importers (kimA ktrAB) have reduced growth rate and fail to adapt to hyperosmotic stress. The experiment was performed and depicted as indicated in the legend to Fig. 1. Download FIG S1, TIF file, 0.1 MB (144.9KB, tif) .
Copyright © 2022 Wendel et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Mg2+ levels fluctuate inversely to K+ levels during osmoadaptation.
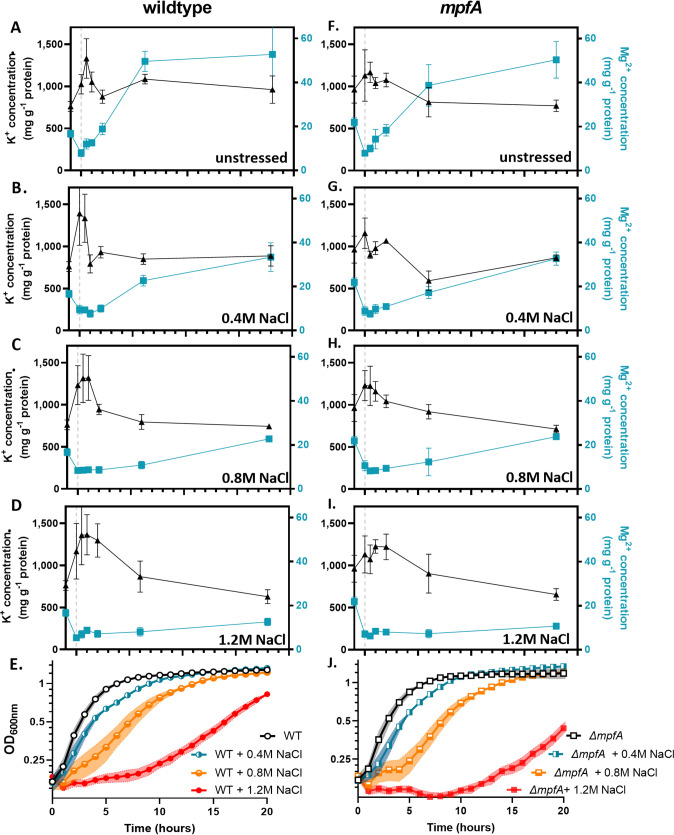
Next, we quantified K+ and Mg2+ fluxes during osmoadaptation by inductively coupled plasma mass spectrometry (ICP-MS). Consistent with previous literature (21), the K+ levels nearly doubled upon exposure to hyperosmotic shock in WT cells (Fig. 3A to D). Surprisingly, even in cells subcultured into fresh medium without added NaCl, K+ levels also transiently increased (Fig. 3A), but the duration of this effect was reduced compared to that of the high salt media. Previous studies have also noted that the phenotypic response to nutrient upshift can mimic that of osmotic upshift, consistent with our observation (28). As the level of osmotic stress increased, so did the duration of the K+ increase (Fig. 3B to D). This spike in K+ was inversely correlated with a precipitous drop in Mg2+. In both WT and mpfA strains, intracellular Mg2+ dropped transiently, but in medium with 1.2 M NaCl this decline was much more persistent. Although we were surprised that the mpfA strain still displayed a rapid loss of Mg2+, this is likely due to the presence of several paralogs (see Discussion).
FIG 3.
Mg2+ and K+ levels are inversely correlated during osmoadaptation. Total cellular K+ and Mg2+ levels were monitored before and after subculture into increasing concentrations of NaCl. (A) WT cells exhibit a rapid spike in K+ and simultaneous drop in Mg2+, recovering to normal K levels after 2 h. (B to D) As the concentration of NaCl increases, the duration of the fluctuations increase. (E) Growth of WT cells correlates with a restoration of Mg2+ levels. (F to I) ΔmpfA mutants exhibit patterns similar to those of WT cells. (J) Similar to WT cells, growth of ΔmpfA mutants appears to correlate with a restoration of Mg levels. The gray dashed line indicates the point of subculture/addition. Samples for analysis by ICP-MS were taken at the indicated time points and processed as described in Materials and Methods. Growth experiments were performed as indicated in the legend to Fig. 1.
We postulate that K+ is displacing Mg2+, leading to an elevation of free Mg2+ pools and triggering efflux. Since ICP-MS measures the total cellular Mg2+ content, we turned to the fluorescent probe Mag-Fura-2 to monitor free (readily chelatable) Mg2+ in the cytosol. Indeed, free Mg2+ levels fall upon osmotic upshift, and this decrease was enhanced when 1.2 M KCl replaced 1.2 M NaCl (Fig. S2). This supports the idea that imported K+ is displacing Mg2+. We conclude that Mg2+ levels fluctuate inversely to K+ during osmoadaptation.
Mg2+ is rapidly lost during hyperosmotic shock. Chelatable (free) Mg2+ levels were monitored using Mag-Fura2 fluorescence. Loss of intracellular, chelatable Mg2+ is greater in cells stressed with 1.2 M KCl than 1.2 M NaCl, consistent with a role for K+ import in displacement of bound Mg2+ in the cytosol. Data shown are averages and standard deviations from three biological replicates. Download FIG S2, TIF file, 0.1 MB (143.3KB, tif) .
Copyright © 2022 Wendel et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Reimport of Mg2+ is critical for adaptation to hyperosmotic stress.
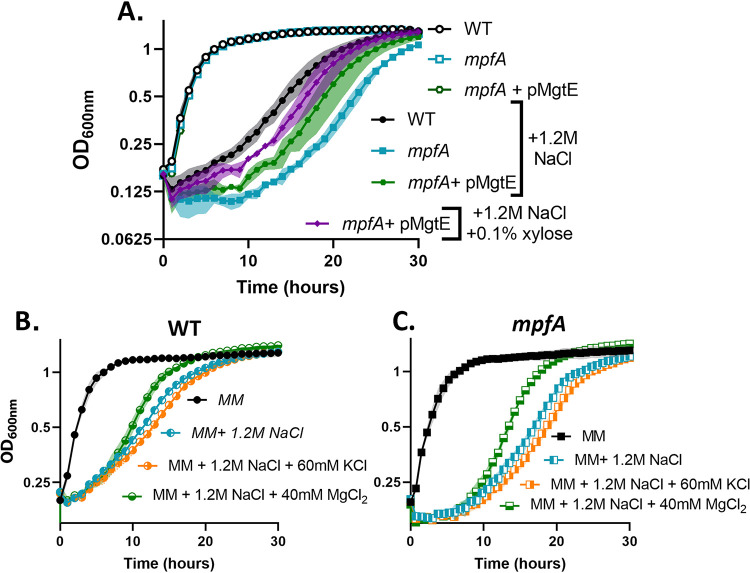
We noted that the time required for osmoadaptation (Fig. 3E) seems to be correlated with the time required for the restoration of intracellular Mg2+ levels (Fig. 3B to D). This led us to hypothesize that reduced growth under hyperosmotic stress may result from Mg2+ limitation rather than a direct effect of high K+. To test this idea, we induced MgtE during osmoadaptation using a xylose-inducible promoter. As predicted, increased expression of mgtE reduced the lag in osmoadaptation relative to the WT (Fig. 4A). Further, addition of 60 mM KCl (120 mosM) to cells already stressed with 1.2 M NaCl slowed osmoadaptation, whereas addition of 40 mM MgCl2 (also 120 mosM) had the opposite effect and actually increased the rate of osmoadaptation (Fig. 4B). A similar effect was observed in mpfA mutants (Fig. 4C). In parallel experiments, we tested the ability of an inducible mpfA construct to increase the rate of osmoadaptation. In contrast to mgtE, induction of mpfA at the time of subculture did not increase the rate of osmoadaptation (Fig. S3). Together, these results support Mg2+ reimport (and not efflux) as the rate-limiting process during adaptation to hyperosmotic stress.
FIG 4.
Magnesium reimport is important for osmoadaptation. (A) Expression of MgtE partially rescues the lag in osmoadaptation in ΔmpfA mutants. (B and C) Supplementation of 60 mM KCl increases the lag in osmoadaptation in both WT and ΔmpfA cells. Supplementation of an equal osmolarity (40 mM) of MgCl2 suppresses the lag in osmoadaptation in both WT and ΔmpfA cells. Growth experiments were performed and depicted as indicated in the legend to Fig. 1.
Restoration of MpfA does not suppress the delay in osmoadaptation. Expression of MpfA does not rescue the lag in osmoadaptation in ΔmpfA mutants. Experiment was performed and depicted as indicated in the legend to Fig. 1. Download FIG S3, TIF file, 0.1 MB (58.5KB, tif) .
Copyright © 2022 Wendel et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Mg2+ deficiency during osmoadaptation impairs translation.
One likely consequence of Mg2+ deficiency is impaired translation (29). Ribosomes contain a significant fraction of the total cellular pool of Mg2+ (30, 31), and translation is a major energy-dependent process in the cell fueled by NTP pools, which function as NTP:Mg2+ salts (29). Furthermore, rpmH mutants deficient in ribosomal assembly due to loss of the large-subunit ribosomal protein L34 are suppressed by supplemental Mg2+ or by mutations in mpfA that increase cytosolic Mg2+ (32). To test whether Mg2+ deficiency during osmoadaptation affects translation, we evaluated the response of the rpmH mutant to salt stress. The rpmH mutant was severely impaired for growth in the presence of 1.2 M NaCl yet was only partially rescued by the addition of the compatible solute glycine betaine (Fig. 5A). Thus, a strain known to have ribosomes sensitive to the depletion of cellular Mg2+ pools (32) is strongly affected in osmoadaptation.
FIG 5.
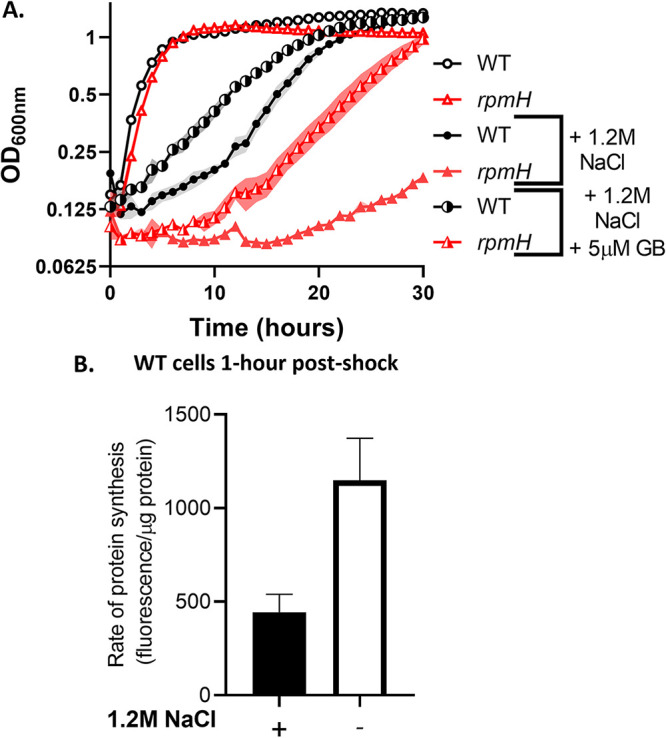
Magnesium limitation during osmoadaptation may contribute to impaired translation. (A) rpmH mutants (defective in ribosomal protein L34) exhibit somewhat reduced growth in minimal media and a severe lag relative to the WT under osmotic stress. This growth defect is partially rescued by 5 μM GB. Cells were grown and depicted as indicated in the legend to Fig. 1. (B) The translation rate in the WT was reduced at 1 h after subculture in the presence of 1.2 M NaCl, as measured by l-azidohomoalanine labeling. Data presented are averages and SD from 3 biological replicates (P < 0.01).
To directly evaluate the effect of osmotic upshift on translation, we labeled cells with the methionine analog l-azidohomoalanine after 1 h of subculture into medium with and without 1.2 M NaCl. In the presence of high salt, nascent translation was significantly reduced (Fig. 5B). These data, together with recent publications highlighting the connection between Mg2+ homeostasis and translation (29, 33, 34), support the idea that a decrease in Mg2+ during osmoadaptation could impair translation and thereby delay the resumption of growth.
c-di-AMP levels fluctuate dynamically during osmoadaptation.
Cyclic di-AMP has been implicated in growth under osmotic stress due to its central role in coordinating K+ homeostasis (26, 35, 36). However, c-di-AMP also binds MgtE (37). To test if c-di-AMP may be regulating Mg2+ homeostasis during osmoadaptation, mutants defective in one of the constitutively expressed diadenylate cyclases, CdaA or DisA, or one of the c-di-AMP-specific phosphodiesterases, GdpP or PgpH, were exposed to osmotic upshock. Interestingly, pgpH mutants exhibited a lag in osmoadaptation similar to mpfA mutants, and the other single mutants also exhibited a lag, but not as much as mpfA (Fig. S4A). A pgpH gdpP double mutant accumulates toxic levels of c-di-AMP, and this strain rapidly picks up suppressors (38, 39). One of our double mutant strains developed suppressor mutations in yfkN, a membrane-bound phosphodiesterase, and ywfM, an unknown putative transporter. This pgpH gdpP double mutant grew more slowly than the WT and was unable to grow under osmotic stress (Fig. S4B). Interestingly, and consistent with the established inhibition of compatible solute import by c-di-AMP (35, 39, 40), the pgpH gdpP double mutant was not rescued by GB (Fig. S4B).
Perturbations in cyclic-di-AMP metabolism impairs osmoadaptation. (A) Single mutants defective in synthesizing (cdaA or disA) or degrading (gdpP or pgpH) c-di-AMP exhibit a lag relative to the WT during osmoadaptation (B). A pgpH gdpP double mutant (with high c-di-AMP) is unable to adapt to hyperosmotic shock, even in the presence of GB. This experiment was performed as described in the legend to Fig. 1. Download FIG S4, TIF file, 0.1 MB (20.1MB, tif) .
Copyright © 2022 Wendel et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Based on the known inhibition of K+ and compatible solute import by c-di-AMP (26, 39) and a proposed role in regulating the activity of MgtE (37), we hypothesized that c-di-AMP levels fluctuate dynamically during osmoadaptation. Specifically, an initial decrease in c-di-AMP might facilitate K+ and compatible solute import, and a subsequent rise in c-di-AMP may be required for K+ efflux, the restoration of Mg2+ import, and resumption of growth. Consistent with this, both c-di-AMP and Mg2+ levels drop following hyperosmotic shock, and both recover in parallel during osmoadaptation (Fig. 6A to C). Thus, fluctuations in c-di-AMP levels are consistent with a direct role in coordinating K+ and Mg2+ fluxes during the response to hyperosmotic stress.
FIG 6.
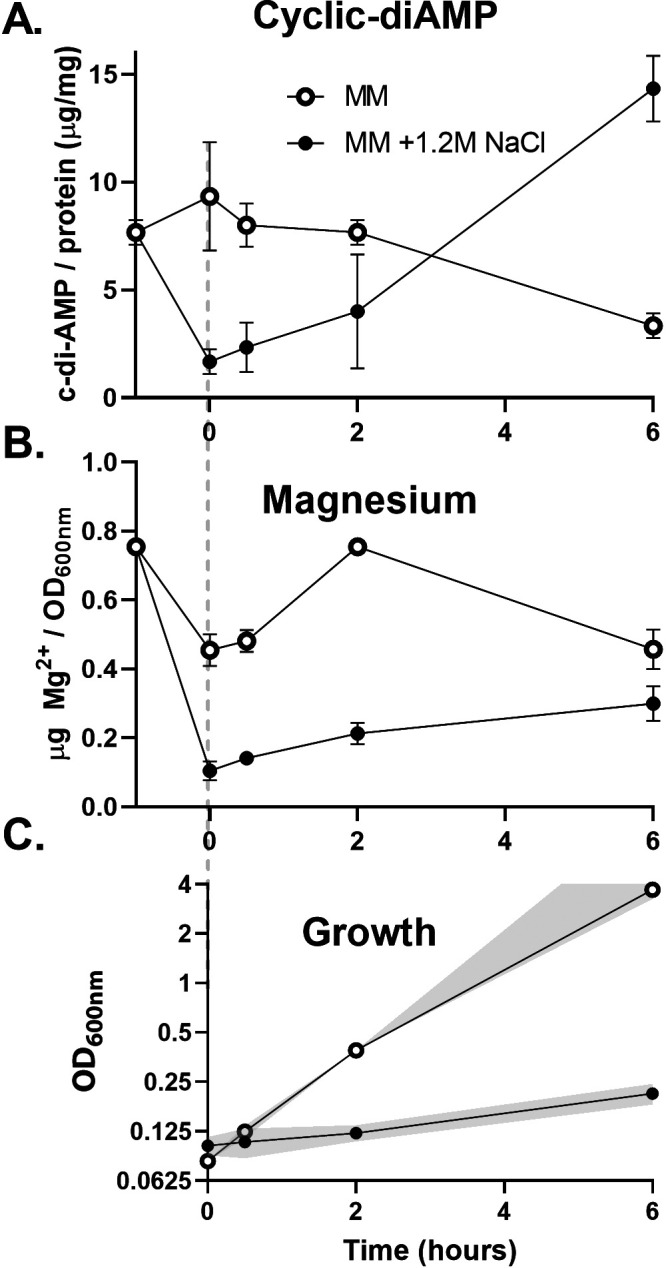
Cyclic-di-AMP levels fluctuate coordinately with Mg2+ levels during osmoadaptation. (A) The levels of c-di-AMP levels drop upon subculture with 1.2 M NaCl but not in the absence of hyperosmotic stress. (B) Mg2+ levels also drop upon subculture with 1.2 M NaCl. (C) This drop in cell-associated Mg is associated with a reduction in growth rate. The gray dashed line indicates the point of subculture/addition. The plots represent the averages and standard deviations from three biological replicates. The experiment was designed and depicted as indicated in the legend to Fig. 1. Mg levels were measured by ICP-OES and c-di-AMP levels by LC-MS. In this experiment, culture density for cultures with an OD600 of >1.0 was determined after 10× dilution and absorbance values calculated accordingly.
DISCUSSION
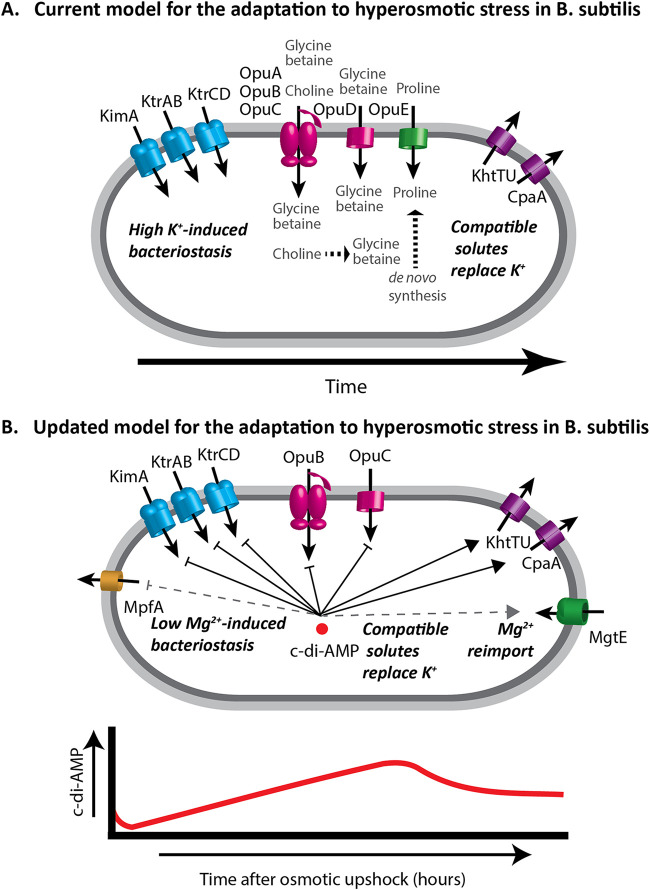
Osmotic upshift can restrict cell growth by dehydration of the cytoplasm. In E. coli, the growth rate is linearly correlated with the amount of free water over a wide range of conditions (41). In the current model for osmoadaptation in B. subtilis (Fig. 7A), dehydration is countered by K+ uptake, mediated by KimA and KtrAB, which can raise the intracellular K+ concentration to levels approaching 1 M (21, 26, 42, 43). This import is transient, as high K+ is proposed to compromise protein function and membrane potential (44). To rebalance the ionic strength of the cytoplasm (45), B. subtilis imports compatible solutes such as glycine betaine (GB) and proline (46, 47). If extracellular osmolyte concentrations are insufficient, then cells defer to the energetically costly de novo synthesis of proline (48). Finally, K+ efflux is proposed to facilitate the resumption of essential cytoplasmic functions and cell growth (49, 50). Here, we amend this model by integration of Mg2+ and c-di-AMP as central players in osmoadaptation (Fig. 7B).
FIG 7.
Osmotic stress response in B. subtilis. (A) In the current model, when cells encounter hyperosmotic stress they rapidly import K+ to stabilize turgor and retain cellular water. Compatible solutes are then accumulated through import or synthesis and K+ is exported. In the presence of compatible solutes K+ import is reduced, and turgor is maintained without gross disruption of ion pools. (B) In our updated model, K+ import is accompanied by Mg2+ loss, and essential cell processes such as translation are thereby inhibited. In the absence of MpfA, Mg2+ homeostasis is perturbed and MgtE levels are reduced, which diminishes the capacity for Mg2+ reimport, thereby delaying osmoadaptation. (Lower panel) Cyclic-di-AMP varies during osmoadaptation and functions to inhibit expression and activity of K+ and compatible solute importers and to activate K+ exporters. This signaling nucleotide may also regulate Mg2+ homeostasis during osmoadaptation.
Mg2+ has not been previously implicated as a major player during osmoadaptation, in part because its concentration (unlike K+) is too low to have a significant role as an osmolyte (44). Indeed, Mg2+ homeostasis is rarely mentioned in discussions of bacterial osmoadaptation (22, 51). However, previous work suggests that osmotic stress and the accompanying rise in K+ levels can perturb intracellular ion pools. For example, in E. coli osmotic stress triggers proton egress and a rise in intracellular pH in E. coli (52), and in osmotically stressed human (HeLa) cells a transient rise in Mg2+ levels was noted (53). A coupling between osmotic stress and Mg2+ pools has also been suggested from system-level modeling of the bacterial metabolome (54).
We hypothesized import of K+ upon osmotic upshift would displace Mg2+ from complexes within the cell, and that this displaced Mg2+ would be lost from the cell through efflux. In support of this idea, mpfA mutants are delayed in osmoadaptation, and this delay appears to be related to K+ influx (Fig. 1 and 2). We further anticipated that mpfA mutant cells would be defective in Mg2+ efflux and perhaps impaired in K+ import. However, that is clearly not the case (Fig. 3), and slower osmoadaptation in the mpfA strain is not correlated with an obvious defect in Mg2+ efflux. Although at first puzzling, we realized that a key difference between the WT and mpfA cells might instead be the rate of Mg2+ reimport (i.e., Fig. 3B versus G). We noted that prior to osmotic upshift, mpfA cells have ∼36% increased intracellular Mg2+ levels (see Table S3 in the supplemental material), consistent with the ∼50% increase noted previously (5). Since MgtE is rate-limiting for the resumption of growth (Fig. 4A) and mgtE transcription is regulated by a Mg2+-sensing riboswitch (16), we hypothesize that mpfA mutants have a reduced capacity for Mg2+ uptake. Indeed, mgtE mRNA levels, monitored by reverse transcription-PCR (RT-PCR), were reduced >2-fold in the mpfA mutant (Fig. S5). This striking decrease, despite a more modest change in Mg2+ levels, might result from cooperativity of Mg2+ binding to the MgtE riboswitch, as proposed previously (55, 56). The persistence of Mg2+ efflux in the mpfA strain is likely due to alternative efflux systems. Indeed, B. subtilis encodes four MpfA paralogs (YrkA, YhdT, YqhB, and YugS), and mutations in any one of these paralogs also lead to a lag in osmoadaptation, similar to the mpfA strain (Fig. S6A), and in each case the observed lag was suppressed by the addition of GB (Fig. S6B), which is known to reduce K+ import (21). We conclude that mutations affecting known (MpfA) or candidate Mg2+ efflux proteins all delay osmoadaptation and that these proteins are partially redundant with respect to Mg2+ egress. Delayed osmoadaptation in these strains highlights the importance of Mg2+ homeostasis and argues for an amendment to our current understanding of bacterial osmostress responses (Fig. 7B).
ΔmpfA mutants have reduced expression of the MgtE Mg2+ importer. MgtE expression is reduced in ΔmpfA mutants relative to the WT as measured using RT-PCR. Gene expression values (2−ΔCT) of mgtE were normalized to gyrA for WT and ΔmpfA strains (P < 0.01). Download FIG S5, TIF file, 0.2 MB (246.9KB, tif) .
Copyright © 2022 Wendel et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Loss of MpfA paralogs delays osmoadaptation. (A) Single mutants lacking any one MpfA paralog demonstrate a lag in osmoadaptation. (B) The growth lag in the mutant strains is suppressed by the presence of a compatible solute (glycine betaine, or GB). Plots are the average (line/point) and SD (shading) from three biological replicates. Open symbols indicate minimal medium with no additives, and solid symbols indicate minimal medium with 1.2 M NaCl or 1.2 M NaCl and 5 μM GB. Download FIG S6, TIF file, 2.1 MB (1.1MB, tif) .
Copyright © 2022 Wendel et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Intracellular metal content of exponentially growing cells. Download Table S3, DOCX file, 0.01 MB (13.4KB, docx) .
Copyright © 2022 Wendel et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Mg2+ homeostasis is tightly regulated by both uptake and export. B. subtilis MgtE is an essential transporter required for regulated, high-affinity import (15). Expression of mgtE is transcriptionally regulated by a Mg2+-sensitive riboswitch, and MgtE activity is feedback inhibited by Mg2+ (16, 18, 57, 58). Despite the long history of work on Mg2+ homeostasis (59), Mg2+ efflux pumps were only recently identified (5, 12). MpfA was discovered in genetic screens for suppressors of ribosome assembly defects and metal intoxication (5, 33, 60, 61). A Listeria monocytogenes homolog, lmo233, was reported to be important for growth in high salt prior to recognition of its role in Mg2+ homeostasis (62). MpfA is now appreciated as a Mg2+ efflux pump in both B. subtilis and S. aureus (5, 63). Interestingly, B. subtilis encodes four MpfA paralogs (5), all with salt-responsive transcriptional regulation (64).
Given the central role of Mg2+ in cell physiology and the large-scale perturbation of the cellular metallome by K+ import during hyperosmotic stress, we monitored changes in Mg2+ during a time course of osmoadaptation. We demonstrate that osmotically induced changes in K+ and Mg2+ levels are inversely correlated. The depletion of free Mg2+ early during osmoadaptation is expected to impair energy-requiring processes in the cell by virtue of the role of free Mg2+ as a cofactor for NTPs. Translation is the single most energy-intensive process in the cell, and Mg2+-limited cells may become growth-limited due to defects in translation (29). Indeed, an rpmH strain in which ribosomes have an elevated requirement for Mg2+ is impaired in osmoadaptation (Fig. 5A). We further show that translation is reduced after osmotic upshift (Fig. 5B). Thus, osmotic upshift reduces translation, and a mutation that renders translation more sensitive to a reduction in Mg2+ levels slows osmoadaptation. Conversely, conditions that increase reimport of Mg2+ increase the rate of osmoadaptation (excess Mg2+, overexpression of MgtE). These results support a model in which hyperosmotic stress triggers Mg2+ depletion as the proximate cause of bacteriostasis, and Mg2+uptake is then limiting for recovery (Fig. 7B).
Recently, the dinucleotide second messenger c-di-AMP has been implicated in the control of K+ and compatible solute transport, suggesting that it acts as a central regulator of osmoadaptation (24). Increased c-di-AMP levels inhibit both transcription and activity of the osmotically induced K+ transporters KimA and KtrAB and activate the K+ exporters CpaA and KhtU (36, 37, 65). Furthermore, increased c-di-AMP inhibits compatible solute uptake by the Opu-family proteins (35, 46). c-di-AMP often binds to proteins that have a CBS (cystathionine-beta-synthase) or an RCK_C (regulator of conductance of K+) domain (24). MgtE contains a CBS domain, as do MpfA and its paralogs. c-di-AMP binds to MgtE (37), which supports a role in controlling both Mg2+ as well as K+ homeostasis. Indeed, upon osmotic upshift c-di-AMP levels are rapidly reduced (Fig. 6A), which, based on our current understanding of c-di-AMP regulation, would facilitate K+ and/or compatible solute import (24, 65). During osmoadaptation, c-di-AMP levels rise again, which would reduce K+ and compatible solute import, activate K+ efflux by KhtTU and CpaA (27, 48), and may serve to activate MgtE-dependent Mg2+ reimport, which then allows a resumption of growth (37). Whether or not c-di-AMP also regulates the activity of MpfA and its paralogs remains to be determined (Fig. 7B). Thus, c-di-AMP likely choreographs these responses by regulating both transcription and activity of the transporters for K+, Mg2+, and compatible solutes throughout osmoadaptation (Fig. 7B).
MATERIALS AND METHODS
Bacterial strains and growth conditions.
All strains used in the study are derivatives of B. subtilis strain CU1065 (WT), are listed in Table S1 in the supplemental material, and were verified using primers listed in Table S1. Gene replacement cassettes were obtained through the Bacillus Genetic Stock Center from the BKE collection (66). Cells were grown in liquid LB, on solid LB agar plates with appropriate antibiotic selection, or in minimal media adapted by Chen et al. from Belitsky minimal medium with vigorous shaking at 37°C (67, 68). Briefly, the minimal media consisted of 15 mM (NH4)2SO4, 1.6 mM MgSO4, 4.5 mM potassium glutamate, 40 mM morpholinepropanesulfonic acid (MOPS), pH 7.4, 5 mM KPO4, pH 7, 49 mM tryptophan, 2% glucose. The antibiotics (concentrations) used are the following: ampicillin (amp; 100 μg mL−1), chloramphenicol (cm; 10 μg mL−1), kanamycin (kan; 15 μg mL−1), neomycin (neo; 8 μg mL−1), and macrolide lincosamide-streptogramin B (MLS; 1 μg mL−1 erythromycin and 25 μg mL−1 lincomycin). For the minimal media used for c-di-AMP null strains, the MgSO4 concentration was raised to 20 mM, the potassium glutamate was omitted, and KH2PO4 was replaced with NaH2PO4, as in reference 37.
Strains and plasmids used in this study. Download Table S1, DOCX file, 0.02 MB (17.1KB, docx) .
Copyright © 2022 Wendel et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Growth curves.
Cells were grown overnight in LB medium, subcultured at a 1:100 ratio into minimal medium, and grown to exponential phase (optical density at 600 nm [OD600], ∼0.4). Cells were subsequently subcultured 1:4 into a prewarmed Bioscreen plate with the indicated conditions. Cell growth (OD600) was monitored every 15 min for 30 h using a Bioscreen growth analyzer (Growth Curves USA, Piscataway, NJ) at 37°C with continuous shaking. In Fig. 6, cultures were measured by hand in a spectrophotometer, with densities at an OD600 of >1 diluted and values calculated accordingly. Data shown are averages and standard deviations or representative plots from at least three biological replicates.
RNA extraction and qPCR.
Gene expression for mgtE was determined by real-time PCR using primers mentioned in Table S2. RNA was purified from 1.5 mL of exponentially growing cells (OD600 of ∼0.4) in minimal media using an RNeasy kit from Qiagen per the manufacturer’s instructions. Two micrograms of RNA was used to prepare 20 μL of cDNA to achieve a final concentration of 100 ng/μL using a high-capacity cDNA reverse transcription kit from Applied Biosystems. The gene expression levels were measured using 100 ng of cDNA using 0.5 μM gene-specific primers and 1× SYBR green (Bio-Rad) in a Quantstudio 7 Pro. Gene expression values (2−ΔCT) were plotted after normalization with gyrA. A Student’s t test was performed to determine statistical significance.
Primer oligonucleotides. Download Table S2, DOCX file, 0.01 MB (15.8KB, docx) .
Copyright © 2022 Wendel et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Quantification of intracellular metal content by ICP-MS.
Cells were grown in LB medium overnight and subcultured at a 1:100 ratio into fresh minimal medium to an OD600 of ∼0.4. Cells were then subcultured 1:4 into fresh prewarmed minimal medium with or without the indicated osmotic stressor. Cells were harvested at the indicated time points, and levels of intracellular metals (K, Mg, Fe, Mn, Zn, and Co) were monitored at each time point by inductively coupled plasma mass spectrometry (ICP-MS). All samples were washed once with Chelex-treated phosphate-buffered saline (PBS) buffer. Cell pellets were resuspended in 400 μl of buffer 2 (1× Chelex-treated PBS buffer, 75 mM NaN3, 1% Triton X-100) and incubated at 37°C for 90 min to lyse the cells. Lysed samples were spun down by centrifugation, and the total protein content was quantified using a Bradford assay. The samples then were mixed with 600 μl buffer 4 (5% HNO3, 0.1% [vol/vol] Triton X-100) and heated in a 95°C sand bath for 30 min. The debris was removed by centrifugation, and the total metal ions in the diluted samples were analyzed by a Perkin-Elmer Elan DRC II ICP-MS. Gallium was used as an internal standard. The total cellular ion levels are expressed as total molar content (means ± standard errors; n = 3). An average cell volume of 0.9 μm3 and average cell protein content of 0.121 pg was used to determine molarity from the ICP-MS unit in micrograms per gram of protein (69, 70).
Quantification of intracellular metal content by ICP-OES.
B. subtilis cells were harvested by centrifugation (3 min, 4°C, 8,500 × g). Cell pellets were washed twice with Na-PBS buffer and transferred onto ash-free filter discs (pore size, 0.45 mm; diameter, 47 mm). The cells were dried overnight at room temperature, followed by 3 h at 70°C. The dried filter discs were cut into small pieces and reduced into a fluid state through pressure and 2 mL of 65% HNO3 for 7 h at 185°C in Teflon beakers (25 mL) (PDS-6 pressure digestion system; Loftfield). After the digestion process, the fluid content in the beakers was transferred into an Erlenmeyer flask and diluted with demineralized water to a volume of 50 mL. The total potassium and magnesium content of the bacterial cells in this solution was determined by ICP-OES analysis (Optima 5300 DV; PerkinElmer). This common type of emission spectroscopy technique uses the ICP to produce element atoms and ions that emit electromagnetic radiation at wavelengths of specific characteristics of a particular chemical element. The intensity of light emission at 766.49 nm and 285.21 nm indicates the potassium and magnesium concentration, respectively. The plasma is built by argon gas ionized in an intense electromagnetic field at a temperature of about 7,000 to 10,000°C, generated as the result of the collisions between the neutral argon atoms and the charged particles (71).
Quantification of free magnesium by Mag-Fura 2.
Cells were treated with modifications as described in reference 72. Briefly, overnight cultures of cells were diluted into minimal media to an OD600 of 0.2 in the presence of the acetoxymethyl ester form of Mag-Fura 2. Cells were loaded with AM-Mag-Fura-2 at a final concentration of 5 μM with 15 μM pluronic F-127 as a cell permeant. After a 75-min loading incubation at 37°C with shaking, cells were washed 2× with prewarmed minimal medium, and 100 μL of cell suspension was added to a 96-well plate. After a 30-min incubation at 37°C with shaking, fluorescent signals in samples were measured in a Synergy H1 reader (BioTek) at 37°C for the bound (340-nm excitation and 509-nm emission) and unbound (380-nm excitation and 509-nm emission) form of Mag-Fura 2 at the minimum interval. Additives dissolved in 1× minimal medium were used as indicated. The ratio of bound to unbound fluorescence signal was plotted.
AHA labeling of nascent proteins.
Strains were grown overnight in rich medium. The following day, cells were subcultured (1:100) into fresh MM to an OD600 of 0.4. Cells were again subcultured (1:4) in MM with and without 1.2 M NaCl. At the indicated time points, cultures were labeled with 400 μM l-azidohomoalanine (AHA) (Click Chemistry Tools) for 30 min. Cultures were treated with 100 μg/mL−1 chloramphenicol at the end of the labeling and collected by centrifugation at 4°C. Cells were washed 3× with ice-cold PBS and stored at −80°C. Cell pellets were thawed and resuspended in a lysis buffer consisting of 1 mg/mL−1 lysozyme, 50 mM Tris-HCl, pH 8.0, 0.5% SDS. Cells were lysed by sonication, and insoluble debris was removed by centrifugation (10 min, 10,000× rpm, 4°C). Covalent attachment of fluorescent tetramethylrhodamine (TAMRA)-alkyne (Thermo Fisher Scientific) to AHA-containing proteins was carried out using a Click-iT protein reaction buffer kit (Thermo Fisher Scientific) according to the manufacturer's instructions. Protein concentrations were determined by Bradford assay. Fluorescent signals in samples were measured in a Synergy H1 reader (BioTek, VT) with 545-nm excitation and 580-nm emission wavelengths. The fluorescence signal was normalized by the protein content of the sample to determine the translation rate. A Student’s t test was performed to determine statistical significance.
Analysis of cyclic-di-AMP pools.
The concentration of c-di-AMP in B. subtilis cells was determined by a liquid chromatography–tandem mass spectrometry method, as described previously (38). The cells were harvested by centrifugation (4°C, 8,500 × g), shock-frozen in liquid nitrogen, and stored at −80°C. This sample was used for c-di-AMP extraction (38). The chromatographic separation was performed on a Series 200 HPLC (high-performance liquid chromatography) system (PerkinElmer Life Sciences) or an LC-10AD HPLC system (Shimadzu), as described previously (73). Detection of c-di-AMP was performed on an API 3000 or API 4000 triple quadrupole mass spectrometer equipped with an electrospray ionization source (AB Sciex) using selected reaction monitoring (SRM) analysis in positive ionization mode. The SRM transitions labeled as “quantifier” were used to quantify the compound of interest, whereas “identifier” SRM transitions were monitored as confirmatory signals. The quantifier SRM transitions were most intense and used for quantification.
ACKNOWLEDGMENTS
This work was supported by a grant from the National Institutes of Health (R35GM122461) to J.D.H.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
This article is a direct contribution from John D. Helmann, a Fellow of the American Academy of Microbiology, who arranged for and secured reviews by Erhard Bremer, University of Marburg, and Reinhard Krämer, University of Koln.
Contributor Information
John D. Helmann, Email: jdh9@cornell.edu.
Mark S. Turner, University of Queensland
REFERENCES
- 1.Osman D, Martini MA, Foster AW, Chen J, Scott AJP, Morton RJ, Steed JW, Lurie-Luke E, Huggins TG, Lawrence AD, Deery E, Warren MJ, Chivers PT, Robinson NJ. 2019. Bacterial sensors define intracellular free energies for correct enzyme metalation. Nat Chem Biol 15:241–249. doi: 10.1038/s41589-018-0211-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chandrangsu P, Rensing C, Helmann JD. 2017. Metal homeostasis and resistance in bacteria. Nat Rev Microbiol 15:338–350. doi: 10.1038/nrmicro.2017.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Imlay JA. 2014. The mismetallation of enzymes during oxidative stress. J Biol Chem 289:28121–28128. doi: 10.1074/jbc.R114.588814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chandrangsu P, Helmann JD. 2016. Intracellular Zn(II) intoxication leads to dysregulation of the PerR regulon resulting in heme toxicity in Bacillus subtilis. PLoS Genet 12:e1006515. doi: 10.1371/journal.pgen.1006515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pi H, Wendel BM, Helmann JD. 2020. Dysregulation of magnesium transport protects Bacillus subtilis against manganese and cobalt intoxication. J Bacteriol 202:e00711-19. doi: 10.1128/JB.00711-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Que Q, Helmann JD. 2000. Manganese homeostasis in Bacillus subtilis is regulated by MntR, a bifunctional regulator related to the diphtheria toxin repressor family of proteins. Mol Microbiol 35:1454–1468. doi: 10.1046/j.1365-2958.2000.01811.x. [DOI] [PubMed] [Google Scholar]
- 7.Guan G, Pinochet-Barros A, Gaballa A, Patel SJ, Argüello JM, Helmann JD. 2015. PfeT, a P1B4 -type ATPase, effluxes ferrous iron and protects Bacillus subtilis against iron intoxication. Mol Microbiol 98:787–803. doi: 10.1111/mmi.13158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pi H, Helmann JD. 2017. Sequential induction of Fur-regulated genes in response to iron limitation in Bacillus subtilis. Proc Natl Acad Sci USA 114:12785–12790. doi: 10.1073/pnas.1713008114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pinochet-Barros A, Helmann JD. 2020. Bacillus subtilis Fur is a transcriptional activator for the PerR-repressed pfeT gene, encoding an iron efflux pump. J Bacteriol 202:e00697-19. doi: 10.1128/JB.00697-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paruthiyil S, Pinochet-Barros A, Huang X, Helmann JD. 2020. Bacillus subtilis TerC family proteins help prevent manganese intoxication. J Bacteriol 202:e00624-19. doi: 10.1128/JB.00624-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sachla AJ, Luo Y, Helmann JD. 2021. Manganese impairs the QoxABCD terminal oxidase leading to respiration-associated toxicity. Mol Microbiol 116:729–742. doi: 10.1111/mmi.14767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Armitano J, Redder P, Guimarães VA, Linder P. 2016. An essential factor for high Mg2+ tolerance of Staphylococcus aureus. Front Microbiol 7:1888. doi: 10.3389/fmicb.2016.01888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang H, Yin X, Wu Orr M, Dambach M, Curtis R, Storz G. 2017. Increasing intracellular magnesium levels with the 31-amino acid MgtS protein. Proc Natl Acad Sci USA 114:5689–5694. doi: 10.1073/pnas.1703415114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Subramani S, Perdreau-Dahl H, Morth JP. 2016. The magnesium transporter A is activated by cardiolipin and is highly sensitive to free magnesium in vitro. Elife 5:e11407. doi: 10.7554/eLife.11407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wakeman CA, Goodson JR, Zacharia VM, Winkler WC. 2014. Assessment of the requirements for magnesium transporters in Bacillus subtilis. J Bacteriol 196:1206–1214. doi: 10.1128/JB.01238-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dann CE, Wakeman CA, Sieling CL, Baker SC, Irnov I, Winkler WC. 2007. Structure and mechanism of a metal-sensing regulatory RNA. Cell 130:878–892. doi: 10.1016/j.cell.2007.06.051. [DOI] [PubMed] [Google Scholar]
- 17.Helmann JD. 2007. Measuring metals with RNA. Mol Cell 27:859–860. doi: 10.1016/j.molcel.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hattori M, Iwase N, Furuya N, Tanaka Y, Tsukazaki T, Ishitani R, Maguire ME, Ito K, Maturana A, Nureki O. 2009. Mg2+‐dependent gating of bacterial MgtE channel underlies Mg2+ homeostasis. EMBO J 28:3602–3612. doi: 10.1038/emboj.2009.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tomita A, Zhang M, Jin F, Zhuang W, Takeda H, Maruyama T, Osawa M, Hashimoto K, Kawasaki H, Ito K, Dohmae N, Ishitani R, Shimada I, Yan Z, Hattori M, Nureki O. 2017. ATP-dependent modulation of MgtE in Mg2+ homeostasis. Nat Commun 8:148. doi: 10.1038/s41467-017-00082-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Began J, Cordier B, Březinová J, Delisle J, Hexnerová R, Srb P, Rampírová P, Kožíšek M, Baudet M, Couté Y, Galinier A, Veverka V, Doan T, Strisovsky K. 2020. Rhomboid intramembrane protease YqgP licenses bacterial membrane protein quality control as adaptor of FtsH AAA protease. EMBO J 39:e102935. doi: 10.15252/embj.2019102935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Whatmore AM, Reed RH. 1990. Determination of turgor pressure in Bacillus subtilis: a possible role for K+ in turgor regulation. J Gen Microbiol 136:2521–2526. doi: 10.1099/00221287-136-12-2521. [DOI] [PubMed] [Google Scholar]
- 22.Bremer E, Krämer R. 2019. Responses of microorganisms to osmotic stress. Annu Rev Microbiol 73:313–334. doi: 10.1146/annurev-micro-020518-115504. [DOI] [PubMed] [Google Scholar]
- 23.Wood JM. 2011. Bacterial osmoregulation: a paradigm for the study of cellular homeostasis. Annu Rev Microbiol 65:215–238. doi: 10.1146/annurev-micro-090110-102815. [DOI] [PubMed] [Google Scholar]
- 24.Stülke J, Krüger L. 2020. Cyclic di-AMP signaling in bacteria. Annu Rev Microbiol 74:159–179. doi: 10.1146/annurev-micro-020518-115943. [DOI] [PubMed] [Google Scholar]
- 25.Sherman JM, Holm GE, Albus WR. 1922. Salt effects in bacterial growth. J Bacteriol 7:583–588. doi: 10.1128/jb.7.6.583-588.1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gundlach J, Herzberg C, Kaever V, Gunka K, Hoffmann T, Weiß M, Gibhardt J, Thürmer A, Hertel D, Daniel R, Bremer E, Commichau FM, Stülke J. 2017. Control of potassium homeostasis is an essential function of the second messenger cyclic di-AMP in Bacillus subtilis. Sci Signal 10:eaal3011. doi: 10.1126/scisignal.aal3011. [DOI] [PubMed] [Google Scholar]
- 27.Whatmore AM, Chudek JA, Reed RH. 1990. The effects of osmotic upshock on the intracellular solute pools of Bacillus subtilis. J Gen Microbiol 136:2527–2535. doi: 10.1099/00221287-136-12-2527. [DOI] [PubMed] [Google Scholar]
- 28.Oldewurtel ER, Kitahara Y, van Teeffelen S. 2021. Robust surface-to-mass coupling and turgor-dependent cell width determine bacterial dry-mass density. Proc Natl Acad Sci USA 118:e2021416118. doi: 10.1073/pnas.2021416118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pontes MH, Yeom J, Groisman EA. 2016. Reducing ribosome biosynthesis promotes translation during low Mg2+ stress. Mol Cell 64:480–492. doi: 10.1016/j.molcel.2016.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McCarthy BJ. 1962. The effects of magnesium starvation on the ribosome content of Escherichia coli. Biochim Biophys Acta 55:880–889. doi: 10.1016/0926-6550(62)90345-6. [DOI] [Google Scholar]
- 31.Goldberg A. 1966. Magnesium binding by Escherichia coli ribosomes. J Mol Biol 15:663–673. doi: 10.1016/s0022-2836(66)80134-1. [DOI] [PubMed] [Google Scholar]
- 32.Akanuma G, Kobayashi A, Suzuki S, Kawamura F, Shiwa Y, Watanabe S, Yoshikawa H, Hanai R, Ishizuka M. 2014. Defect in the formation of 70S ribosomes caused by lack of ribosomal protein L34 can be suppressed by magnesium. J Bacteriol 196:3820–3830. doi: 10.1128/JB.01896-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Akanuma G, Yamazaki K, Yagishi Y, Iizuka Y, Ishizuka M, Kawamura F, Kato-Yamada Y. 2018. Magnesium suppresses defects in the formation of 70S ribosomes as well as in sporulation caused by lack of several individual ribosomal proteins. J Bacteriol 200:e00212-18. doi: 10.1128/JB.00212-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee D-YD, Galera-Laporta L, Bialecka-Fornal M, Moon EC, Shen Z, Briggs SP, Garcia-Ojalvo J, Süel GM. 2019. Magnesium flux modulates ribosomes to increase bacterial survival. Cell 177:352–360. doi: 10.1016/j.cell.2019.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schuster CF, Bellows LE, Tosi T, Campeotto I, Corrigan RM, Freemont P, Gründling A. 2016. The second messenger c-di-AMP inhibits the osmolyte uptake system OpuC in Staphylococcus aureus. Sci Signal 9:ra81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cereija TB, Guerra JPL, Jorge JMP, Morais-Cabral JH. 2021. c-di-AMP, a likely master regulator of bacterial K+ homeostasis machinery, activates a K+ exporter. Proc Natl Acad Sci USA 118:e2020653118. doi: 10.1073/pnas.2020653118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gundlach J, Krüger L, Herzberg C, Turdiev A, Poehlein A, Tascón I, Weiss M, Hertel D, Daniel R, Hänelt I, Lee VT, Stülke J. 2019. Sustained sensing in potassium homeostasis: cyclic di-AMP controls potassium uptake by KimA at the levels of expression and activity. J Biol Chem 294:9605–9614. doi: 10.1074/jbc.RA119.008774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gundlach J, Mehne FMP, Herzberg C, Kampf J, Valerius O, Kaever V, Stülke J. 2015. An essential poison: synthesis and degradation of cyclic Di-AMP in Bacillus subtilis. J Bacteriol 197:3265–3274. doi: 10.1128/JB.00564-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pham HT, Nhiep NTH, Vu TNM, Huynh TN, Zhu Y, Huynh ALD, Chakrabortti A, Marcellin E, Lo R, Howard CB, Bansal N, Woodward JJ, Liang Z-X, Turner MS. 2018. Enhanced uptake of potassium or glycine betaine or export of cyclic-di-AMP restores osmoresistance in a high cyclic-di-AMP Lactococcus lactis mutant. PLoS Genet 14:e1007574. doi: 10.1371/journal.pgen.1007574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huynh TN, Choi PH, Sureka K, Ledvina HE, Campillo J, Tong L, Woodward JJ. 2016. Cyclic di-AMP targets the cystathionine beta-synthase domain of the osmolyte transporter OpuC. Mol Microbiol 102:233–243. doi: 10.1111/mmi.13456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Record MT, Jr, Courtenay ES, Cayley S, Guttman HJ. 1998. Biophysical compensation mechanisms buffering E. coli protein–nucleic acid interactions against changing environments. Trends Biochem Sci 23:190–194. doi: 10.1016/S0968-0004(98)01207-9. [DOI] [PubMed] [Google Scholar]
- 42.Holtmann G, Bakker EP, Uozumi N, Bremer E. 2003. KtrAB and KtrCD: two K+ uptake systems in Bacillus subtilis and their role in adaptation to hypertonicity. J Bacteriol 185:1289–1298. doi: 10.1128/JB.185.4.1289-1298.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vieira-Pires RS, Szollosi A, Morais-Cabral JH. 2013. The structure of the KtrAB potassium transporter. Nature 496:323–328. doi: 10.1038/nature12055. [DOI] [PubMed] [Google Scholar]
- 44.Record MT, Jr, Courtenay ES, Cayley DS, Guttman HJ. 1998. Responses of E. coli to osmotic stress: large changes in amounts of cytoplasmic solutes and water. Trends Biochem Sci 23:143–148. doi: 10.1016/S0968-0004(98)01196-7. [DOI] [PubMed] [Google Scholar]
- 45.Csonka LN. 1989. Physiological and genetic responses of bacteria to osmotic stress. Microbiol Rev 53:121–147. doi: 10.1128/mr.53.1.121-147.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hoffmann T, Wensing A, Brosius M, Steil L, Völker U, Bremer E. 2013. Osmotic control of opuA expression in Bacillus subtilis and its modulation in response to intracellular glycine betaine and proline pools. J Bacteriol 195:510–522. doi: 10.1128/JB.01505-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zaprasis A, Bleisteiner M, Kerres A, Hoffmann T, Bremer E. 2015. Uptake of amino acids and their metabolic conversion into the compatible solute proline confers osmoprotection to Bacillus subtilis. Appl Environ Microbiol 81:250–259. doi: 10.1128/AEM.02797-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hoffmann T, von Blohn C, Stanek A, Moses S, Barzantny H, Bremer E. 2012. Synthesis, release, and recapture of compatible solute proline by osmotically stressed Bacillus subtilis cells. Appl Environ Microbiol 78:5753–5762. doi: 10.1128/AEM.01040-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fujisawa M, Kusumoto A, Wada Y, Tsuchiya T, Ito M. 2005. NhaK, a novel monovalent cation/H+ antiporter of Bacillus subtilis. Arch Microbiol 183:411–420. doi: 10.1007/s00203-005-0011-6. [DOI] [PubMed] [Google Scholar]
- 50.Lundberg ME, Becker EC, Choe S. 2013. MstX and a putative potassium channel facilitate biofilm formation in Bacillus subtilis. PLoS One 8:e60993. doi: 10.1371/journal.pone.0060993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wood JM. 2015. Bacterial responses to osmotic challenges. J Gen Physiol 145:381–388. doi: 10.1085/jgp.201411296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McLaggan D, Naprstek J, Buurman ET, Epstein W. 1994. Interdependence of K+ and glutamate accumulation during osmotic adaptation of Escherichia coli. J Biol Chem 269:1911–1917. doi: 10.1016/S0021-9258(17)42113-2. [DOI] [PubMed] [Google Scholar]
- 53.Koldenkova VP, Matsuda T, Nagai T. 2015. MagIC, a genetically encoded fluorescent indicator for monitoring cellular Mg2+ using a non-Förster resonance energy transfer ratiometric imaging approach. J Biomed Opt 20:101203. doi: 10.1117/1.JBO.20.10.101203. [DOI] [PubMed] [Google Scholar]
- 54.Akbari A, Yurkovich JT, Zielinski DC, Palsson BO. 2021. The quantitative metabolome is shaped by abiotic constraints. Nat Commun 12:3178. doi: 10.1038/s41467-021-23214-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wakeman CA, Ramesh A, Winkler WC. 2009. Multiple metal-binding cores are required for metalloregulation by M-box riboswitch RNAs. J Mol Biol 392:723–735. doi: 10.1016/j.jmb.2009.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ramesh A, Wakeman CA, Winkler WC. 2011. Insights into metalloregulation by M-box riboswitch RNAs via structural analysis of manganese-bound complexes. J Mol Biol 407:556–570. doi: 10.1016/j.jmb.2011.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Smith RL, Thompson LJ, Maguire ME. 1995. Cloning and characterization of MgtE, a putative new class of Mg2+ transporter from Bacillus firmus OF4. J Bacteriol 177:1233–1238. doi: 10.1128/jb.177.5.1233-1238.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ramesh A, Winkler WC. 2010. Magnesium-sensing riboswitches in bacteria. RNA Biol 7:77–83. doi: 10.4161/rna.7.1.10490. [DOI] [PubMed] [Google Scholar]
- 59.Papp-Wallace KM, Maguire ME. 2008. Magnesium transport and magnesium homeostasis. EcoSal Plus doi: 10.1128/ecosalplus.5.4.4.2. [DOI] [PubMed] [Google Scholar]
- 60.Formstone A, Errington J. 2005. A magnesium-dependent mreB null mutant: implications for the role of mreB in Bacillus subtilis. Mol Microbiol 55:1646–1657. doi: 10.1111/j.1365-2958.2005.04506.x. [DOI] [PubMed] [Google Scholar]
- 61.Dajkovic A, Tesson B, Chauhan S, Courtin P, Keary R, Flores P, Marlière C, Filipe SR, Chapot‐Chartier M, Carballido‐Lopez R. 2017. Hydrolysis of peptidoglycan is modulated by amidation of meso‐diaminopimelic acid and Mg2+ in Bacillus subtilis. Mol Microbiol 104:972–988. doi: 10.1111/mmi.13673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gardan R, Cossart P, Labadie J, The European Listeria Genome Consortium. 2003. Identification of Listeria monocytogenes genes involved in salt and alkaline-pH tolerance. Appl Environ Microbiol 69:3137–3143. doi: 10.1128/AEM.69.6.3137-3143.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Trachsel E, Redder P, Linder P, Armitano J. 2019. Genetic screens reveal novel major and minor players in magnesium homeostasis of Staphylococcus aureus. PLoS Genet 15:e1008336. doi: 10.1371/journal.pgen.1008336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nicolas P, Mäder U, Dervyn E, Rochat T, Leduc A, Pigeonneau N, Bidnenko E, Marchadier E, Hoebeke M, Aymerich S, Becher D, Bisicchia P, Botella E, Delumeau O, Doherty G, Denham EL, Fogg MJ, Fromion V, Goelzer A, Hansen A, Härtig E, Harwood CR, Homuth G, Jarmer H, Jules M, Klipp E, Chat LL, Lecointe F, Lewis P, Liebermeister W, March A, Mars RAT, Nannapaneni P, Noone D, Pohl S, Rinn B, Rügheimer F, Sappa PK, Samson F, Schaffer M, Schwikowski B, Steil L, Stülke J, Wiegert T, Devine KM, Wilkinson AJ, van Dijl JM, Hecker M, Völker U, Bessières P, Noirot P. 2012. Condition-dependent transcriptome reveals high-level regulatory architecture in Bacillus subtilis. Science 335:1103–1106. doi: 10.1126/science.1206848. [DOI] [PubMed] [Google Scholar]
- 65.Ren A, Patel DJ. 2014. c-di-AMP binds the ydaO riboswitch in two pseudo-symmetry-related pockets. Nat Chem Biol 10:780–786. doi: 10.1038/nchembio.1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Koo B-M, Kritikos G, Farelli JD, Todor H, Tong K, Kimsey H, Wapinski I, Galardini M, Cabal A, Peters JM, Hachmann A-B, Rudner DZ, Allen KN, Typas A, Gross CA. 2017. Construction and analysis of two genome-scale deletion libraries for Bacillus subtilis. Cell Syst 4:291–305. doi: 10.1016/j.cels.2016.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen L, James LP, Helmann JD. 1993. Metalloregulation in Bacillus subtilis: isolation and characterization of two genes differentially repressed by metal ions. J Bacteriol 175:5428–5437. doi: 10.1128/jb.175.17.5428-5437.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Neidhardt FC, Bloch PL, Smith DF. 1974. Culture medium for enterobacteria. J Bacteriol 119:736–747. doi: 10.1128/jb.119.3.736-747.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jeong JW, Snay J, Ataai MM. 1990. A mathematical model for examining growth and sporulation processes of Bacillus subtilis. Biotechnol Bioeng 35:160–184. doi: 10.1002/bit.260350208. [DOI] [PubMed] [Google Scholar]
- 70.Dauner M, Storni T, Sauer U. 2001. Bacillus subtilis metabolism and energetics in carbon-limited and excess-carbon chemostat culture. J Bacteriol 183:7308–7317. doi: 10.1128/JB.183.24.7308-7317.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Aceto M, Abollino O, Bruzzoniti MC, Mentasti E, Sarzanini C, Malandrino M. 2002. Determination of metals in wine with atomic spectroscopy (flame-AAS, GF-AAS and ICP-AES); a review. Food Addit Contam 19:126–133. doi: 10.1080/02652030110071336. [DOI] [PubMed] [Google Scholar]
- 72.Froschauer EM, Kolisek M, Dieterich F, Schweigel M, Schweyen RJ. 2004. Fluorescence measurements of free [Mg2+] by use of mag-fura 2 in Salmonella enterica. FEMS Microbiol Lett 237:49–55. doi: 10.1111/j.1574-6968.2004.tb09677.x. [DOI] [PubMed] [Google Scholar]
- 73.Mehne FMP, Gunka K, Eilers H, Herzberg C, Kaever V, Stülke J. 2013. Cyclic di-AMP homeostasis in Bacillus subtilis: both lack and high level accumulation of the nucleotide are detrimental for cell growth. J Biol Chem 288:2004–2017. doi: 10.1074/jbc.M112.395491. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Osmoadaptation requires osmotically induced K+ import. (A) Loss of KtrAB (in strains retaining KimA) has little effect on osmoadaptation in either WT or mpfA strains. (B) Mutant strains lacking both high-affinity K+ importers (kimA ktrAB) have reduced growth rate and fail to adapt to hyperosmotic stress. The experiment was performed and depicted as indicated in the legend to Fig. 1. Download FIG S1, TIF file, 0.1 MB (144.9KB, tif) .
Copyright © 2022 Wendel et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Mg2+ is rapidly lost during hyperosmotic shock. Chelatable (free) Mg2+ levels were monitored using Mag-Fura2 fluorescence. Loss of intracellular, chelatable Mg2+ is greater in cells stressed with 1.2 M KCl than 1.2 M NaCl, consistent with a role for K+ import in displacement of bound Mg2+ in the cytosol. Data shown are averages and standard deviations from three biological replicates. Download FIG S2, TIF file, 0.1 MB (143.3KB, tif) .
Copyright © 2022 Wendel et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Restoration of MpfA does not suppress the delay in osmoadaptation. Expression of MpfA does not rescue the lag in osmoadaptation in ΔmpfA mutants. Experiment was performed and depicted as indicated in the legend to Fig. 1. Download FIG S3, TIF file, 0.1 MB (58.5KB, tif) .
Copyright © 2022 Wendel et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Perturbations in cyclic-di-AMP metabolism impairs osmoadaptation. (A) Single mutants defective in synthesizing (cdaA or disA) or degrading (gdpP or pgpH) c-di-AMP exhibit a lag relative to the WT during osmoadaptation (B). A pgpH gdpP double mutant (with high c-di-AMP) is unable to adapt to hyperosmotic shock, even in the presence of GB. This experiment was performed as described in the legend to Fig. 1. Download FIG S4, TIF file, 0.1 MB (20.1MB, tif) .
Copyright © 2022 Wendel et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
ΔmpfA mutants have reduced expression of the MgtE Mg2+ importer. MgtE expression is reduced in ΔmpfA mutants relative to the WT as measured using RT-PCR. Gene expression values (2−ΔCT) of mgtE were normalized to gyrA for WT and ΔmpfA strains (P < 0.01). Download FIG S5, TIF file, 0.2 MB (246.9KB, tif) .
Copyright © 2022 Wendel et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Loss of MpfA paralogs delays osmoadaptation. (A) Single mutants lacking any one MpfA paralog demonstrate a lag in osmoadaptation. (B) The growth lag in the mutant strains is suppressed by the presence of a compatible solute (glycine betaine, or GB). Plots are the average (line/point) and SD (shading) from three biological replicates. Open symbols indicate minimal medium with no additives, and solid symbols indicate minimal medium with 1.2 M NaCl or 1.2 M NaCl and 5 μM GB. Download FIG S6, TIF file, 2.1 MB (1.1MB, tif) .
Copyright © 2022 Wendel et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Intracellular metal content of exponentially growing cells. Download Table S3, DOCX file, 0.01 MB (13.4KB, docx) .
Copyright © 2022 Wendel et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Strains and plasmids used in this study. Download Table S1, DOCX file, 0.02 MB (17.1KB, docx) .
Copyright © 2022 Wendel et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Primer oligonucleotides. Download Table S2, DOCX file, 0.01 MB (15.8KB, docx) .
Copyright © 2022 Wendel et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.