Abstract
Background
High prevalence, severity, and formidable morbidity have marked the recent emergence of the novel coronavirus disease (COVID-19) pandemic. The significant association with the pre-existing co-morbid conditions has increased the disease burden of this global health emergency, pushing the patients, healthcare workers and facilities to the verge of complete disruption.
Methods
Meta-analysis of pooled data was undertaken to assess the cumulative risk assessment of multiple co-morbid conditions associated with severe COVID-19. PubMed, Scopus, and Google Scholar were searched from January 1st to June 27th 2020 to generate a well-ordered, analytical, and critical review. The exercise began with keying in requisite keywords, followed by inclusion and exclusion criteria, data extraction, and quality evaluation. The final statistical meta-analysis of the risk factors of critical/severe and non-critical COVID-19 infection was carried out on Microsoft Excel (Ver. 2013), MedCalc (Ver.19.3), and RevMan software (Ver.5.3).
Results
We investigated 19 eligible studies, comprising 12037 COVID-19 disease patients, representing the People’s Republic of China (PRC), USA, and Europe. 18.2% (n = 2200) of total patients had critical/severe COVID-19 disease. The pooled analysis showed a significant association of COVID-19 disease severity risk with cardiovascular disease (RR: 3.11, p < 0.001), followed by diabetes (RR: 2.06, p < 0.001), hypertension (RR: 1.54, p < 0.001), and smoking (RR: 1.52, p < 006).
Conclusion
The review involved a sample size of 12037 COVID-19 patients across a wide geographical distribution. The reviewed reports have focussed on the association of individual risk assessment of co-morbid conditions with the heightened risk of COVID-19 disease. The present meta-analysis of cumulative risk assessment of co-morbidity from cardiovascular disease, diabetes, hypertension, and smoking signals a novel interpretation of inherent risk factors exacerbating COVID-19 disease severity. Consequently, there exists a definite window of opportunity for increasing survival of COVID-19 patients (with high risk and co-morbid conditions) by timely identification and implementation of appropriately suitable treatment modalities.
Keywords: COVID-19, Co-morbidity, risk factor, SARS-CoV-2, smoking, acute cardiac injury, hypertension
1. INTRODUCTION
The emergence of novel coronavirus (nCoV), or severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), and novel coronavirus associated diseases (COVID-19) has resulted in a global medical emergency, affecting the human population in 219 countries and territories [1, 2]. It has created an unprecedented health challenge for mankind in general and medical sciences in particular. Globally, as of 25th July 2021 (05:00 GMT), the total number of confirmed cases has crossed 194 million, with over 4.1 million deaths [3].
Co-morbidities, such as obesity, diabetes, cardiovascular disease, etc., have been recognised as life-threatening risk factors in various serious diseases, such as cancer and multi-organ failure; they are also considered as potent indicators of poor prognostic outcomes [4, 5]. Survival and severity of COVID-19 disease in patients with co-morbid conditions have been one of the most persistent challenges for healthcare professionals [6]. As of now, there is no universally defined standard treatment protocol available to cure COVID-19 and/or vaccine to reduce the spread of the infection. Timely identification and management of high-risk co- morbid condition patients and subsequent treatment intervention are mandatory to reduce fatality.
COVID-19 patients with co-morbid systemic diseases like diabetes, hypertension, cardiovascular conditions, chronic liver disease, and cancer have an incremental risk of serious clinical complications (acute respiratory distress syndrome (ARDS) & respiratory failure, sepsis, acute cardiac injury (ACI), heart failure, and acute kidney injury) [7, 8].
Cardiovascular disease, cerebrovascular disease, chronic obstructive pulmonary disease (C OPD), hypertension, diabetes, age over 65 years, and smoking have been often described as independent risk factors for poor clinical outcomes in COVID-19 patients [9, 10]. Meta-analysis studies have shown that individual co-morbid risk factors (diabetes, IL-6 levels, pregnancy, arterial hypertension) are associated with severity and non-severity of COVID-19 disease [11-13], but their sample size and single-factor analysis may be a limitation for strong evidence.
Previous pooled meta-analysis studies have indicated male gender, diabetes, hypertension, cardiovascular disease, COPD, and malignancies as significant risk factors for severity and adverse clinical outcome of COVID-19 disease [5, 8, 11, 12, 14-16]. However, cumulative risk assessment of multiple co-morbid conditions with severity and mortality of COVID-19 disease has not been reported in previous meta-analysis studies. Smoking has been reported in multiple epidemiological studies as one of the most significant risk factors for diabetes, lung diseases, cardiovascular disease, cancer, etc. In addition, co-morbidities, such as diabetes, are reported to predispose individuals to enhanced risk of infections, cardiovascular diseases, etc.
Therefore, in our meta-analysis, we aimed to pool and analyse two or more co-morbid factors, such as smoking, diabetes, hypertension and cardiovascular disease. In addition, an appraisal of their cumulative risk associated with clinical outcomes in terms of severity, morbidity and mortality of hospitalized COVID-19 patients has been reviewed.
2. METHODS
2.1. Search Approach and Literature Selection Criteria
Relevant e-literatures were retrieved from the PubMed, Scopus, and Google Scholar search engines published during 1st January to 27th June 2020 using the keywords coronavirus or COVID-19, Severe Acute Respiratory Syndrome Coronavirus 2 or SARS-CoV-2 or SARS2, recovered or death mortality, or smoking effect and Acute Respiratory Distress Syndrome (ARDS) or ICU. No language limitations were made compulsory on the search. The systematic review and meta-analysis in this work was carried out in accordance with PRISMA and MOOSE guidelines, and the supplementary checklist has been furnished [16, 17].
2.2. Inclusion and Exclusion Criteria
The titles and abstracts of potentially significant studies retrieved by the electronic search were critically reviewed. Subsequently, full-text articles were accessed for comprehensive assessment, and the relevant studies generated were included in the systematic review and meta-analysis. The enclosure criteria were as following: all case studies of COVID-19, all confirmed positive cases with laboratory-identified SARS-CoV-2 infection by real-time reverse-transcriptase polymerase chain reaction (RT-PCR), demographic data (sex, age, smoking habit, study period, location, deaths, and recovery of patients), clinical symptoms (fever, cough, shortness of breath, muscle ache, confusion, headache sore throat, rhinorrhea, chest pain, diarrhoea, nausea, vomiting, hypertension, diabetes, cardiovascular disease, chronic liver disease and cancer) and treatments (antibiotics, antiviral treatment, corticosteroids, intravenous immunoglobin, high-flow nasal cannula, oxygen therapy, non-invasive mechanical ventilation, invasive mechanical ventilation, ECMO and adrenal replacement therapy). All the data thus engendered were segregated as severe and non-severe cases. The exclusion categories were the absence of laboratory and radiological characteristics, treatment results, medical relevance, and case reviews.
2.3. Data Extraction and Quality Evaluation
Three authors independently screened and evaluated all the retrieved data, including their titles and abstracts. All relevant information on baseline details, such as clinical manifestations, risk factors, and co-morbid conditions, were maintained using Microsoft Office Excel (Ver. 2013) tool. Data presented in this study was considered as basic data on gender, age, diagnosis, and treatment strategies. Data extraction and quality evaluation of the presence of diabetes, hypertension, cardiovascular diseases, liver disease, cancer, and smoking habit in tandem with COVID-19 disease severity, complications, and mortality, were in line with the objectives of the review.
2.4. Statistical Analysis
All meta-analysis was performed using Microsoft Excel (Ver. 2013), MedCalc (Ver.19.3), and RevMan software (Ver.5.3). For dichotomized data, risk ratio (RR) and 95% confidence interval (CI) were applied, while heterogeneity and the random-effect model were used for analysing continuous data and considerable heterogeneity, respectively [14]. The degree of disease severity was categorized as low (25%), moderate (50%), and high (75%) by using Chi-square and I2 tests [11]. In addition, funnel plots were applied to check the probable bias in published data [18].
3. RESULTS
3.1. Characteristics and Quality Parameters of Searched Literature
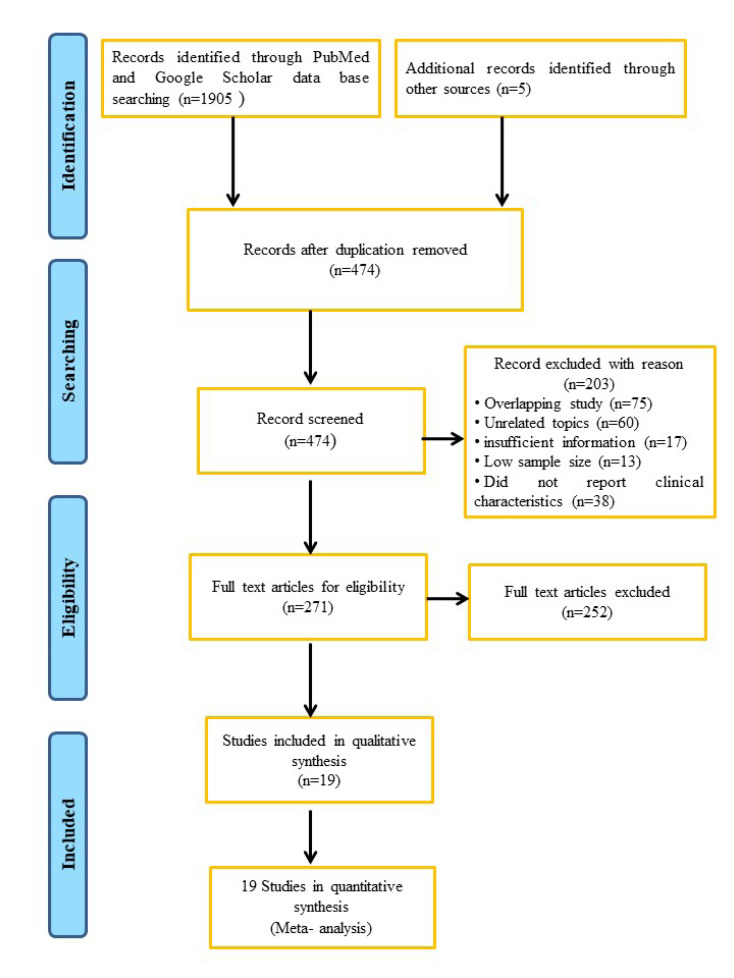
A preliminary search for relevant e-literature in various databases resulted in 1910 articles involving a study on SARS-Cov-2 disease. A total of 474 duplicate articles were removed by carefully checking the titles and abstracts and using the quotation director's ability to create partitions by recognizing duplications. For individual citations of the same paper, the citations were screened and de-copied. Post appraisal of same creator, title, distribution date, volume, issue, and test measure, the citations were recorded on a Microsoft Excel spreadsheet. Another 203 articles were excluded for overlapping study (n=75), unrelated topics (n=60), insufficient information (n=17), low sample size (n=13) and lack of clinical characteristics (n=38). In addition, the lack of information on co-morbid systemic disease leads to the exclusion of another 271 articles. Thus, in the qualitative and quantitative synthesis of the current COVID-19 meta-analysis reports, only nineteen (19) retrospective studies fitted the inclusion criteria (Fig. 1). These retrospective studies involved a sample size of 12037 COVID-19 patients drawn from a wide range of geographical locations.
Fig. (1).
Flow chart of the study selection. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
3.2. Demographic Characteristics
The demographic characteristics of the COVID-19 patients (n=12037) included in the current study are presented in Table 1. Meta-analysis of gender information, based on 17 retrospective literatures, shows 55.68% male (n=2581) and 42.76% females (n=1982) in COVID-19 patients. The sex ratio (male to female) in severe (2.66) and non-severe (1.21) COVID-19 patient groups had no statistical significance. The prevalence significance exhibited for males [Prevalence (%): 56.71, 95% (CI): (53.20-60.19), p<0.0001, I2: 80.54%] was higher as compared to females (Supplementary Fig. 2 (526.2KB, pdf) ). The majority of the COVID-19 patients were from China, followed by the USA and France. The median age ranged from 23 to 91 years, and the mean age was ~60 years.
Table 1.
Main Characteristics of the included patients in meta-analysis.
| Authors | Period of Recruitment | Country | Number of Cases | Deaths (%) | Recovered (%) |
Male/Female
(%) |
Age (Year)
P-Value |
Mean (Year)
(Median) |
|---|---|---|---|---|---|---|---|---|
| Guan W J. et al., 2020 [1] | 11-12-2019 to 29-12-2019 | China | 1099 | 15 (1.4) |
9 (0.8) |
640/459 (58.2/41.7) |
<65/≥65 0.9 |
47.0 (35.0-58.0) |
| Tao Guoet al., 2020 [19] | 23-01-2020 to 23-02-2020 | China | 187 | 43 (23.0) |
144 (77.0) |
91/96 (48.7/51.3) |
<.001 | 58.5 (14.7) |
| Zhang X et al., 2020 [20] | 17-01-2020 to 8-02-2020 | China | 645 | NA | NA | 295/278 (51.5/48.5) |
<0.001 | 46.7 |
| Zhang JJ et al., 2020 [21] | 16-01-2020 - 03-02-2020 | China | 140 | NA | NA | 71/69 (50.7/49.3) |
<50/≥5 <0.01 |
57.0 (25.0-87.0) |
| Chen T et al., 2020 [22] | 13-01-2020 to 12-02-2020 | China | 274 | 113 (41.2) |
161 (58.8) |
171/103 (62.4/37.6) |
<60/≥60 <0.001 |
62.0 (44.0-70.0) |
| Simonnet A et al., 2020 [23] | 27-02-2020 to 05-04-2020 | France | 124 | NA | NA | 90/34 (72.6/27.4) |
0.87 | 60.0 (51-70) |
| Goyal P et al., 2020 [24] | 03-03-2020 to 27-03-2020 | USA | 393 | 40 (10.2) |
260 (66.2) |
238/155 (60.6/39.4) |
<60/≥60 <.001 |
62.2 (48.6-73.7) |
| Mo P et al., 2020 [25] | 01-01-2020 to 05-02-2020 | China | 155 | 22 (14.2) |
NA | 86/69 (55.5/44.5) |
<0.001 | 54.0 (42.0-66.0) |
| Hu L et al., 2020 [26] | 08-01-2020 to 20-02-2020 | China | 323 | NA | NA | 166/157 (51.4/48.6) |
<65/≥65 <0.0001 |
61.0 (23.0-91.0) |
| Wan S et al., 2020 [27] | 23-01-2020 to 08-02-2020 | China | 135 | 1 (0.7) |
15 (11.1) |
72/63 (53.3/46.7) |
<65/≥65 <.0001 |
47.0 (36.0-55.0) |
| Liu Wet al., 2020 [28] | 30-12-2019 to 15-01-2020 | China | 78 | 2 (2.6) |
67 (77.0) |
39/39 (50.0/50.0) |
<60/≥60 0.001 |
38.0 (33.0-57.0) |
| Dreher M et al., 2020 [29] | February to March 2020 | China | 50 | 7 (14.0) |
35 (70.0) |
33/17 (66.0/34.0) |
NA | 65.0 (58.0-76.0) |
| Huang C et al., 2020 [30] | 31-12-2019 to 01-01-2020 | China | 41 | 6 (14.6) |
28 (68.3) |
30/11 (73.0/27.0) |
0·60 | 49.0 (41.0-58.0) |
| Peng YD et al., 2020 [31] | 20 -01-2020 to15-02-2020 | China | 112 | 17 (15.2) | 95 (84.8) |
53/59 (47.3/52.7) |
0.61 | 57.5 (54.0- 63.0) |
| Zhou F et al., 2020 [32] | 29-12-2019 to 31-01-2020 | China | 191 | 54 (28.3) |
137 (71.7) |
119/72 (62.0/38.0) |
<0·0001 | 56.0 (46.0-67.0) |
| Fleming K et al., 2020 [33] | 12-02-2020 to 28-03-2020 | USA | 7,162 | 184 (2.6) |
NA | NA | NA | NA |
| O'Reilly GM et al., 2020 [34] | 1-14 April 2020 | Australia | 240 | NA | NA | NA | NA | 60.0 (21.0) |
| Shi Y et al., 2020 [35] | Until 17 Feb, 2020 | China | 487 | NA | NA | 259/228 (53.2/46.8) |
<0.001 | NA |
| Wu C et al., 2020 [36] | 25-12-2019 to 26-01 2020 | China | 201 | 44 (21.9) | 40 (19.9) |
128/73 (63.7/36.3) |
<.001 | 51.0 (43.0-60.0) |
3.3. Clinical Characteristics of COVID-19 Disease
The clinicopathological characteristics of COVID-19 patients are tabulated in Supplementary Table 1 (526.2KB, pdf) . The most reported symptoms among COVID-19 patients were fever (47.57%), cough (44.67%), shortness of breath (6.51%), headache (5.60%), diarrhoea (5.89%), nausea and vomiting (4.80%). We categorized COVID-19 cases into two sub-groups viz. critical/severe (18.27%, n=2200) and non-critical/non-severe (81.73%, n=9837) groups. Our pooled analysis showed that patients with critical/severe condition had lower prevalence rate compared to non-critical/severe patients [Prevalence (%): 32.96%, 95% (CI): 19.90-47.52] (Supplementary Fig. 1 (526.2KB, pdf) ). The random effect model showed significant heterogeneity for critical/severe condition COVID-19 patients [I2: 99.46%, p<0.001] (Supplementary Fig. 1 (526.2KB, pdf) ). In the studies selected, most of the COVID-19 disease patients, particularly in the critical/severe category, were clinically managed with antibiotics (30.65%), antiviral (41.34%), corticosteroids (22.86%), intravenous immunoglobin (4.46%), oxygen (30.41%) and non-invasive mechanical ventilation (16.15%) treatment. The modalities are summarized in Supplementary Table 1 (526.2KB, pdf) .
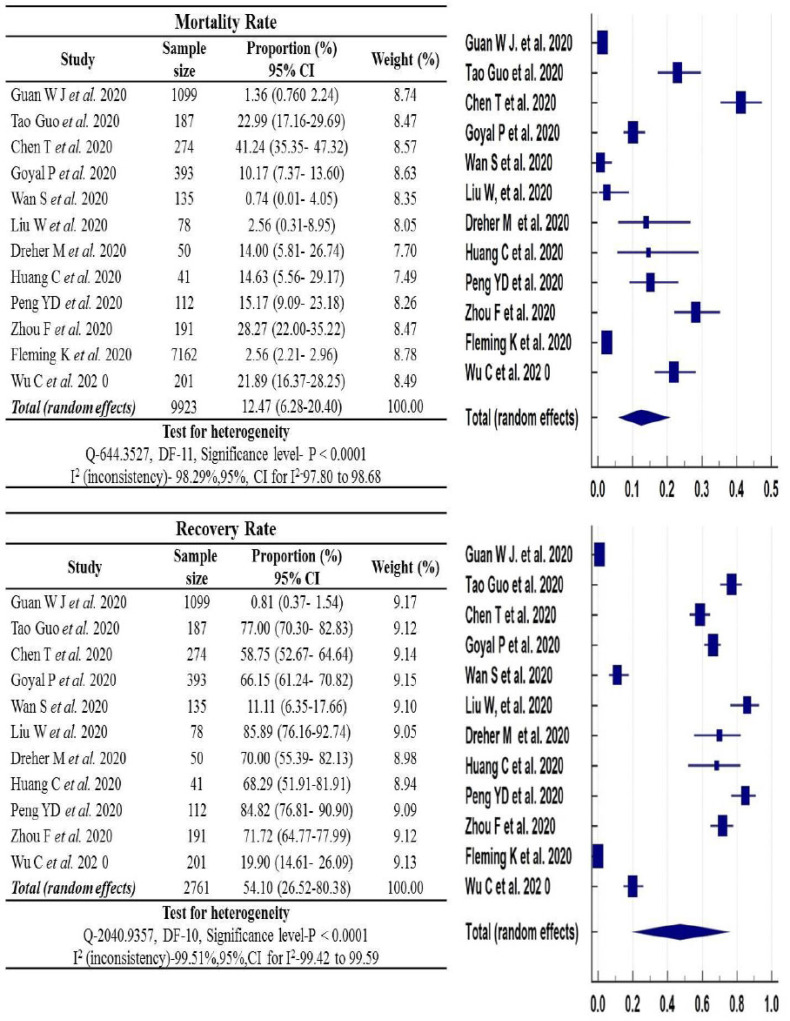
3.4. Recovery and Mortality Rate of COVID-19 Disease
Among COVID-19 patients under critical/severe category, the recovery and mortality rate showed statistically significant association. Random-effects meta-analysis of selected studies, among critical/severe category COVID-19 patients, has shown higher recovery rate [Prevalence (%): 54.10, 95% (CI): (26.52-80.38), p<0.0001, I2: 99.51%, n=2761] compared to the mortality rate [Prevalence (%):12.47, 95% (CI): (6.28-20.40), p<0.0001, I2:98.29%, n=9923] (Fig. 2).
Fig. (2).
Pooled prevalence of mortality and recovery rate in critical/severe patients. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
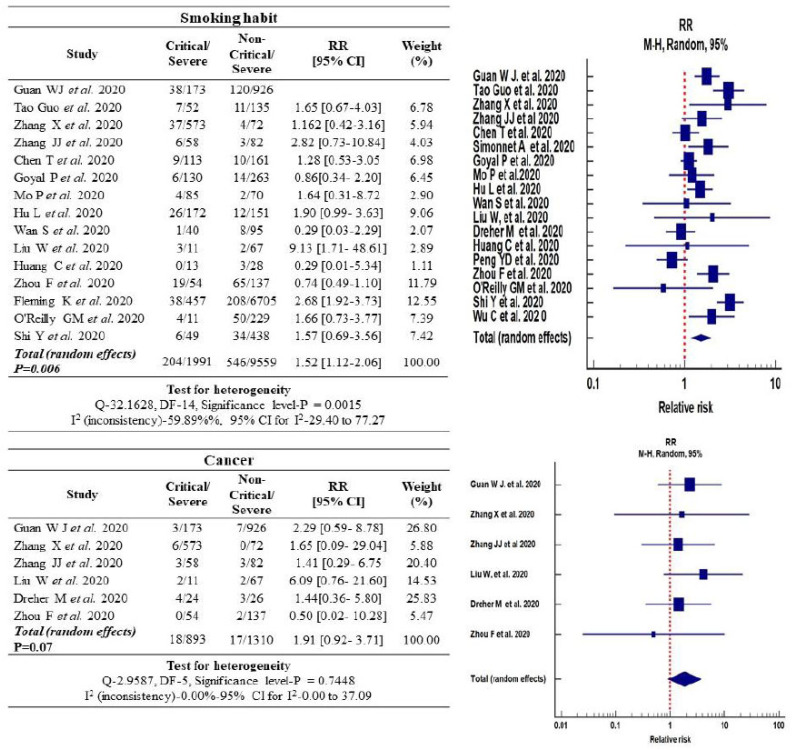
3.5. Association of Smoking with the Severity of COVID-19 Disease
The exposure to smoking (including current and ex-smokers) among severe and non-severe COVID-19 category of patients was 10.24% (204/1991) and 5.71% (546/9559), respectively. The relative risk (RR) estimation of COVID-19 disease among patients with smoking history exhibited a statistically significant association with severity of COVID-19 disease [RR: 1.52, 95% (CI): (1.12-2.06), p<0.006]. Funnel plot evaluation of publication bias among the pooled and selected studies indicated significant heterogeneity [I2: 59.89%, p<0.001] (Fig. 3).
Fig. (3).
Forest plot of the relative risk in smoking habits and cancer associated with COVID-19 infected cases. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
3.6. COVID-19 Disease Risk Association with Cancer Co-morbidities
A total of 19 retrospective studies were considered, based on relative risk induced by co-morbidities (n=2200) in severe COVID-19 disease cases [1, 19-36]. The resultant associations of co-morbidities and COVID-19 severity are presented in Figs. (3-5). Cancer increased the relative risk of COVID-19, which was 1.9-fold higher for the critical patient group [95% (CI): 0.92-3.71, p=0.07] and the heterogenicity between the critical and non-critical group was I2: 0.00%, p=0.74. However, no significance was observed in the case of cancer alone (Fig. 3).
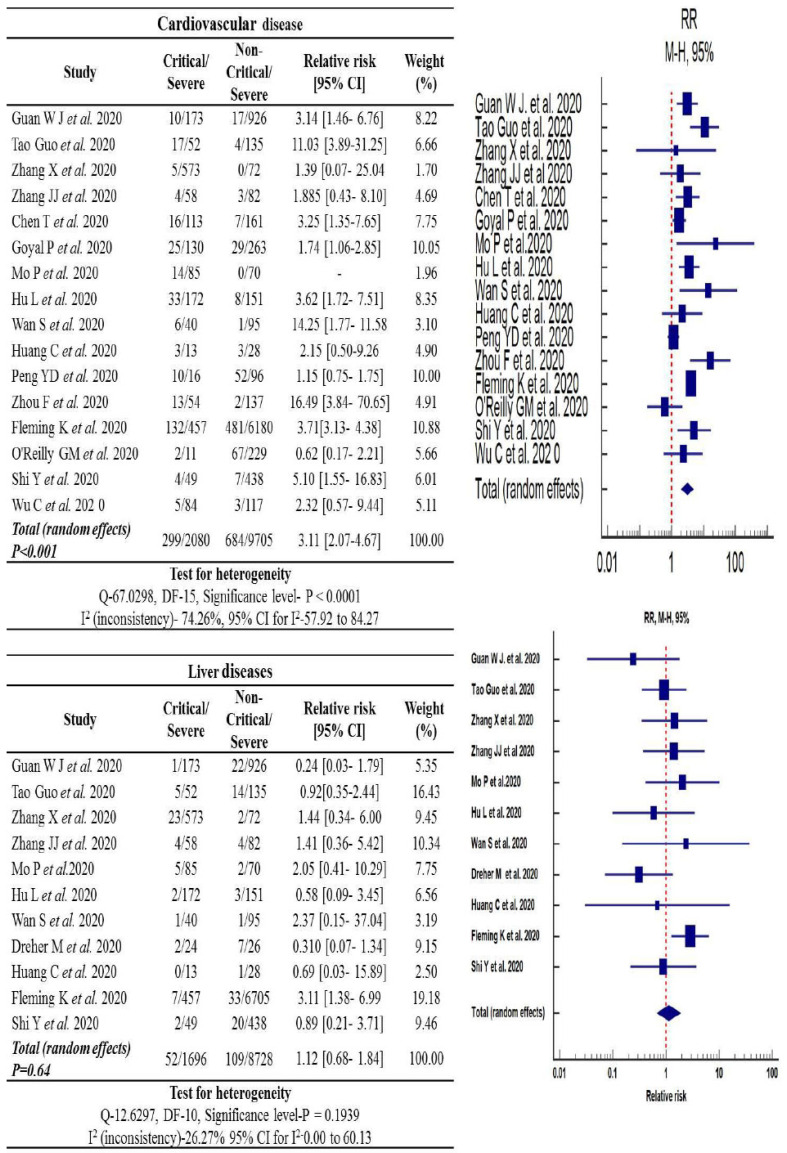
Fig. (5).
Forest plot of the relative risk in cardiovascular and liver diseases associated with COVID-19 infected cases. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
3.7. Prevalence of Hypertension
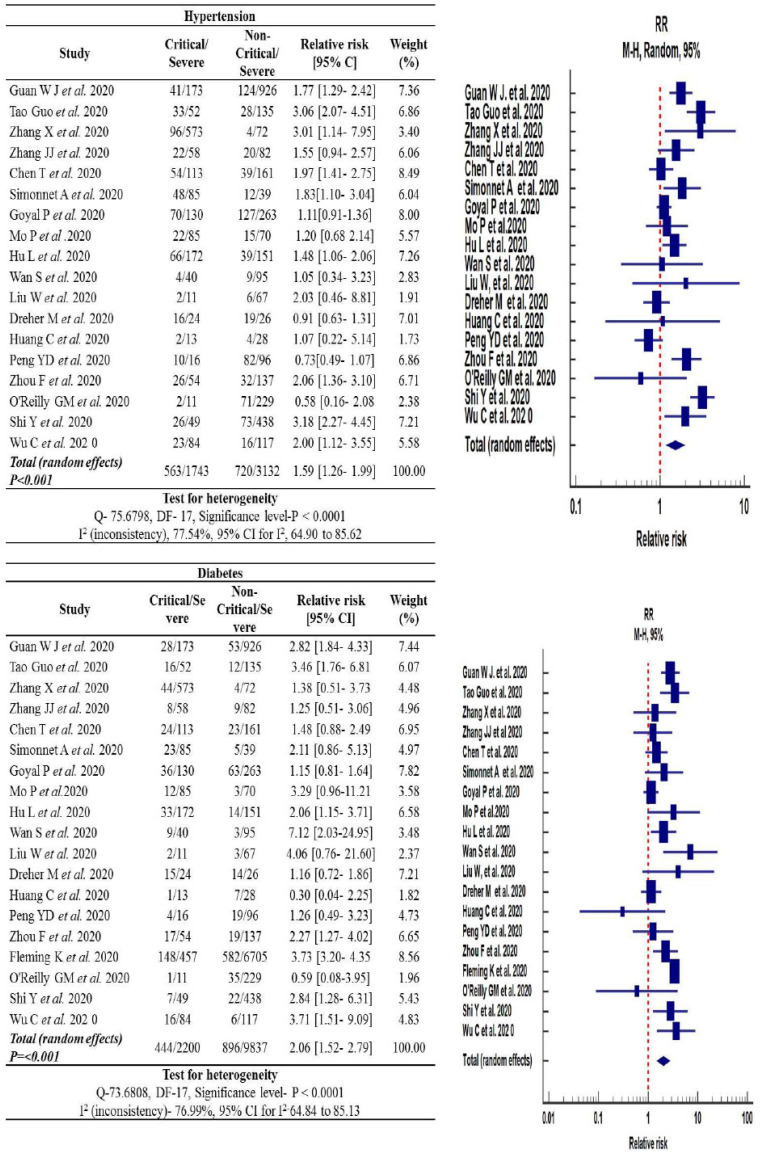
The pooled analysis of the prevalence of hypertension among critical (32.3%) and non-critical (22.9%) COVID-19 patients displayed a significant increase in the risk of severity of COVID-19 disease in the critical group [RR: 1.59, 95% (CI): 1.26-1.99, p<0.0001]. Cochran Q statistics showed significantly higher heterogeneity (75.67%) among studies [I2: 75.54%, p<0.0001], as shown in Fig. (4).
Fig. (4).
Forest plot of the relative risk in hypertension and diabetes associated with COVID-19 infected cases. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
3.8. Prevalence of Diabetes Mellitus
Diabetes was almost two times higher (20.1%; 444/2200) in critical COVID-19 patients as compared to non-critical COVID-19 patients (9.1%; 896/9837). The relative risk of COVID-19 disease severity was found to be statistically significant for the critical group versus the non-critical group [RR: 2.06, 95% (CI):1.52-2.79, p<0.001]. The heterogenicity observed among the COVID-19 patients with diabetes patients was significantly high [I2: 76.99%, p<0.0001] (Fig. 4).
3.9. Prevalence of Cardiovascular Diseases
Cardiovascular diseases were higher (14.3%; 299/2080) in the critical COVID-19 patient's group compared to the non-critical patients (7.0%; 684/9705) group. The random-effect model was employed on 16 a pooled analysis of cardiovascular diseases in COVID-19 patients. The cardiovascular diseases increased the relative risk of severity of COVID-19 disease between critical and non-critical COVID-19 patients by 3.11-fold [95% (CI): 2.07-4.67, p<0.001]. The heterogenicity between the critical and non-critical group of COVID-19 patients was also found to be statistically significant [I2: 74.26%, p<0.0001] (Fig. 5).
3.10. Prevalence of Chronic Liver Disease
According to our statistical analysis, the incidence rate of chronic liver disease in hospitalized COVID-19 patients was 1.12-fold higher for the critical patient group [95% (CI): 0.68-1.84, p=0.64] and the heterogenicity was I2: 26.27%, p=0.193. To estimate the pooled prevalence, a fixed model was used, and the results are shown in Fig. (5).
Statistical significance was not observed for chronic liver disease in COVID-19 patients in the critical group when compared to the non-critical group.
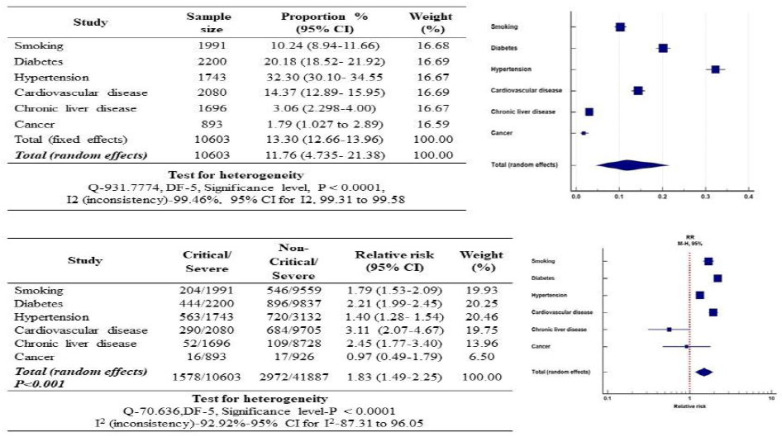
3.11. Relationship of the Smoking Habit, Diabetes, Hypertension, Cardiovascular Disease, Chronic Liver Disease and Cancer with COVID-19 Disease Severity
The complex association of smoking habits and co-morbidities (diabetes, hypertension, cardiovascular disease, chronic liver disease, and cancer) with the severity of COVID-19 disease among critical and non-critical COVID-19 patients group cases are presented in Fig. (6). It is evident that diabetes significantly increased the relative risk of COVID-19 disease severity by 2.06-fold, followed by cardiovascular disease (3.11-fold) and smoking habit (1.52-fold). Smoking habit and other co-morbidities in combination significantly increased the relative risk of COVID-19 disease severity by 1.83-fold [95% (CI): 1.49-2.25, p<0.001]. The heterogenicity between the critical and non-critical group of COVID-19 patients was also found to be statistically significant [I2: 99.92%, p<0.0001] (Fig. 6). Significant heterogeneity was also observed among all analysed co-morbidities except for cancer and chronic liver disease. The relative risk of smoking and other co-morbidities in COVID-19 disease severity was expressed in the form of a funnel plot (Fig. 7).
Fig. (6).
Forest plot of the relative risk in comorbidities associated with COVID-19 infected cases. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
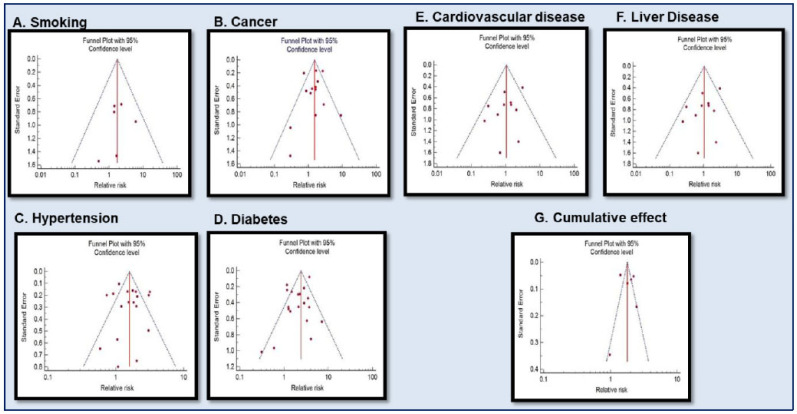
Fig. (7).
Funnel plot for Meta-analysis of the relative risk of underlying disease in COVID-19 infected cases, [A] Smoking, [B] Cancer, [C] Hypertension, [D] Diabetes, [E] Cardiovascular disease, [F] Liver disease [G] Cumulative effect. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
4. DISCUSSION
In this systematic review and meta-analysis, a total of 19 clinical retrospective studies represented the data culled from 12037 COVID-19 patients. There exists a definite window of opportunity for enhanced COVID-19 patients’ survival, in spite of high risk and co-morbid conditions, by timely identification and implementation of appropriately suitable treatment modalities.
Although COVID-19 patients mostly belonged to the 45-60-year age group [ 13, 15, 18] in a few studies, our meta-analysis indicated that a majority of severe COVID-19 patients were from the higher age bracket of 50-60 years. The distinct age-related variation (older adults versus younger children) in the severity of COVID-19 symptoms has recently been attributed to a potential mechanistic pathway [37]. Novel Coronavirus, the causative agent of COVID-19, interacts with angiotensin-converting enzyme 2 (ACE2), a cellular binding site expressed in the heart, kidney, and pulmonary alveolar type II cells [38]. ACE2 is a possible facilitator for SARS-CoV2 spike protein binding on lung cell surface receptors [39]. The expression of TMPRSS2, another cellular protein in the lung tissue, was assessed using mouse models. Very low levels were seen in younger mouse, against the significantly higher levels in the older mouse. The expression of TMPRSS2 was also reported to be significantly higher in the lung tissues of older COVID-19 patients [37]. Therefore, it has been hypothesized that higher TMPRSS2 expression in older individuals may be associated with severity and COVID-19 symptoms and suggests potential therapeutic application in devising a new treatment strategy for COVID-19 [37].
Both male and female sexes have different energy consumption and energy requirement, which depends upon the interaction between sex hormones and environmental factors [40]. Females have a better capacity of producing antibodies that provide effective resistance to COVID-19 infection; consequently, females may be at a lower risk of COVID-19 infection [40]. According to another theory, women may be less vulnerable to viral infection than men, possibly because of the protection of X chromosome and sex hormones, which play a cardinal role in innate and adaptive immunity [41]. Whereas in men, increased risk of COVID-19 infection may also be associated with poor lifestyle habits, such as smoking and underlying diseases [42]. In our meta-analysis and other COVID-19 studies, it was found that the majority of critical or fatal COVID-19 patients were male [23, 30]. The results of our demographic study showed a higher prevalence of COVID-19 disease in the male versus the female population, which is consistent with analytical results from earlier meta-analysis reports and related research [14, 23, 30, 36]. Our meta-analysis shows a significantly higher prevalence rate (54%) of pooled severity compared to pooled mortality (12.44%) in COVID-19 patients (Fig. 2). Similar observations were also reported in a previous meta-analysis study [15]. Early detection of severe and critical COVID-19 symptoms in diseased cases may hold better promise and significance in improving the therapeutic intervention efficacy and subsequent reduction in mortality [43, 44]. However, our prognosis of pooled analysis between critical/severe COVID-19 patients and non-critical/ severe patients indicated a significantly higher mortality risk prevalence (32%) for critical/severe COVID-19 patients (Supplementary Fig. 1 (526.2KB, pdf) ). However, previous studies observed no statistical significance among the severe condition of COVID-19 patients with the prognostic outcome [8]. Application of a more comprehensive evaluation of larger COVID-19 patient samples in conjunction with accurate and suitable statistical methodologies may lead to a better understanding of COVID-19 prognosis influencing factors.
The antagonistic effect of smoking on pulmonary immune function advanced the progression of severe COVID-19 disease [45]. Smoking is thus a significant risk factor for the emergence of acute respiratory distress syndrome (ARDS) and the associated economic burden of patient care in severe cases of COVID-19 in a dose-dependent manner [46]. Hypothetically, smoking might impact the consequences of COVID-19 patients directly by inducing inflammation and damaging endothelial function in the cardiopulmonary systems [46, 47]. The fact that smoking increases the expression of ACE2 in the secretory cells of the respiratory tract corroborated the enhanced access into respiratory epithelial cells by SARS-CoV-2 interaction with ACE2 (angiotensin-converting enzyme 2) receptors [48, 49]. Consequently, the high expression of ACE2 in smokers might play an important role in inducing direct damage to the epithelial cell or through downstream inflammatory cascade events [46]. Our previous report substantiates the cumulative risk of COVID-19 disease severity due to smoking, hypertension, and cardiovascular diseases [13]. In our present investigations, smokers had a 1.51-fold greater risk of COVID-19 disease compared to non-smokers. Our current findings are not only consistent with previous meta-analysis findings [50] but suggest that current and previous smoking habits may have an association with COVID-19 disease severity risk prevalence. Smoking has been correlated with adverse COVID-19 disease progression; this is due to the deleterious impact of tobacco use on lung health and its underlying association with a plethora of respiratory ailments [51].
It has been hypothesized, but not verified that the pre-existing use of type 1 receptor blockers (ARBs) for angiotensin II will upregulate membrane-bound ACE2, thus raising human susceptibility to virus entry [52]. It is likewise conceivable that people with pre-existing chronic conditions, such as high blood pressure and coronary heart disease (receiving ARBs) may be more susceptible to SARS- CoV-2 severity, including mortality [38].
ACE2 has a diversity of physiological roles that revolve around its trivalent function [39]. ACE2 is extensively expressed in the kidneys, cardiovascular system, lungs, gut, central nervous system, and adipose tissue. ACE2 may be a critical link between immunity, inflammation, cardiovascular disease [53].
The diabetic individual has a higher risk of respiratory infections due to a compromised immune system, especially innate immunity [54]. Besides, diabetes is characterized by exaggerated proinflammatory cytokine response, particularly interleukin (IL)-1, IL-6, and tumor necrosis factor (TNF)-α in the absence of appropriate immune stimulation. This may be further complicated in response to a cytokine stimulus observed in patients with COVID-19 disease due to acute respiratory distress syndrome (ARDS) [55]. A thorough review of the recent meta-analysis showed that diabetes plays a significant role in the severity of COVID-19 disease and associated mortality [7, 14]. Our meta-analysis has emphatically shown the associated risk posed by diabetes for mortality and severity of COVID-19 disease [56].
Our meta-analysis findings are consistent with the previously published study, that hypertension posed a higher risk for COVID-19 disease severity, particularly among critical cases. According to another pooled meta-analysis study, hypertension is associated with a 2.54-fold increased risk for critical/severe COVID-19 disease, particularly among older age groups [57]. Acute cardiac injury is frequently reported in patients with COVID-19 [58]. COVID-19 may perhaps attack cardiomyocytes through different pathways [59]. Higher-level expression of ACE2 directly in the myocardium may lead to the release of cytokine and chemokine signals in COVID-19, which invariably resulted in myocardial inflammation in severe cases [59]. Our meta-analysis showed a 3.11-fold higher risk of severity of COVID-19 in patients with cardiovascular disease.
Liver disease of advanced stage and post-liver transplantation patients are more susceptible to COVID-19 infection [60]. However, in the present meta-analysis, no significant association between chronic liver disease and mortality in severe COVID-19 disease was evident. Our analysis has also not found a significant association between the severity of COVID-19 disease and cancer. However, cancer patients have a higher risk of COVID-19 disease severity compared to those without cancer (39% vs. 8%; hazard ratio (39% vs. 8%, HR: 5.34; 95% (CI): 1.80-16.18, p<0.0026) [61]. It is hypothesized that cancer-induced inflammation and cytokine-associated lung injury may have a role in COVID-19 disease, but it may not be a universal mechanism in all types of cancer patients. The individualistic variation in the genotype of inflammation and cytokine-associated genes may also have a role in cancer-induced lung injury and subsequent COVID-19 disease severity [61].
Our pooled meta-analysis data underscores the significant association of extant co-morbidity (predominantly smoking, diabetes, hypertension, and cardiovascular disease) with severity and rapid deterioration of COVID-19 disease-related symptoms. Though many reports cite the co- morbidity arising out of individual systemic diseases [7, 8, 13], there is a definite paucity on the combined presence of multiple co-morbid conditions with severity and mortality of COVID-19 disease. Diabetes mellitus in the presence of hypertension in males is known to synergistically amplify the severity of coronary artery disease (CAD) [62]. To the best of our knowledge, our pooled meta-analysis is the first report showing the synergistic association of the smoking habit, diabetes, hypertension, and cardiovascular diseases as significant co-morbidity factors for COVID-19 disease severity.
CONCLUSION
We conclude that the cumulative action of multiple co- morbidities involving smoking, diabetes, hypertension, and cardiovascular diseases may reinforce COVID-19 disease severity and mortality. The findings of our meta-analysis provide unambiguous insight into cumulative co-morbid risk factors and their intense adverse impact on COVID-19 disease. We would also like to stress the need to develop personalized, effective therapeutic regimens for better management of disease severity and treatment outcome in individuals with co-morbid health risks.
ACKNOWLEDGEMENTS
The authors are grateful to the Royal Global University, Guwahati, Assam for their support.
AUTHORS’ CONTRIBUTIONS
RK conceived and designed the manuscript; RK, MMP, AH, and PC contributed to materials/analysis,; RK, AH, MMP, DB, BG, AKR and AKB wrote the paper,; all authors reviewed and approved the manuscript.
CONSENT FOR PUBLICATION
Not applicable.
STANDARDS OF REPORTING
The systematic review and meta-analysis in this work was carried out in accordance with PRISMA and MOOSE guidelines.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
SUPPLEMENTARY MATERIAL
Supplementary material is available on the publisher’s website along with the published article.
Table S1 (526.2KB, pdf) . Descriptive clinical symptoms and treatments of patients.
Fig. (S1 (526.2KB, pdf) ). The pooled prevalence of critical/severe patients in COVID-19.
Fig. (S2 (526.2KB, pdf) ). Pooled prevalence of gender in COVID-19.
Fig. (S3 (526.2KB, pdf) ). Checklist: PRISMA 2009 checklist.
REFERENCES
- 1.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X., Liu L., Shan H., Lei C.L., Hui D.S.C., Du B., Li L.J., Zeng G., Yuen K.Y., Chen R.C., Tang C.L., Wang T., Chen P.Y., Xiang J., Li S.Y., Wang J.L., Liang Z.J., Peng Y.X., Wei L., Liu Y., Hu Y.H., Peng P., Wang J.M., Liu J.Y., Chen Z., Li G., Zheng Z.J., Qiu S.Q., Luo J., Ye C.J., Zhu S.Y., Zhong N.S. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Worldometer. Coronavirus Update (Live): Cases and Deaths from COVID-19 Virus Pandemic. Worldometers. Available from: https://www.worldometers.info/coronavirus/%0Ahttps://www.worldometers.info/coronavirus/ (Accessed November 18, 2020).
- 3.WHO Coronavirus (COVID-19) Dashboard Update. 2021. Available from: https://covid19.who.int/ (Accessed July 21, 2020).
- 4.Etrakis D., Margină D., Tsarouhas K., Tekos F., Stan M., Nikitovic D., Kouretas D., Spandidos DA., Tsatsakis A. Obesity - a risk factor for increased COVID-19 prevalence, severity, and lethality. Mol. Med. Rep. 2020;22(1):9–19. doi: 10.3892/mmr.2020.11127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou Y., Yang Q., Chi J., Dong B., Lv W., Shen L., Wang Y. Comorbidities and the risk of severe or fatal outcomes associated with coronavirus disease 2019: A systematic review and meta-analysis. Int. J. Infect. Dis. 2020;99:47–56. doi: 10.1016/j.ijid.2020.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Callender L.A., Curran M., Bates S.M., Mairesse M., Weigandt J., Betts C.J. The impact of pre-existing comorbidities and therapeutic interventions on COVID-19. Front. Immunol. 1991;2020(11):11. doi: 10.3389/fimmu.2020.01991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang I., Lim M.A., Pranata R. Diabetes mellitus is associated with increased mortality and severity of disease in COVID-19 pneumonia - A systematic review, meta-analysis, and meta-regression. Diabetes Metab. Syndr. 2020;14(4):395–403. doi: 10.1016/j.dsx.2020.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hu Y., Sun J., Dai Z., Deng H., Li X., Huang Q., Wu Y., Sun L., Xu Y. Prevalence and severity of corona virus disease 2019 (COVID-19): A systematic review and meta-analysis. J. Clin. Virol. 2020;127:104371. doi: 10.1016/j.jcv.2020.104371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dariya B., Nagaraju G.P. Understanding novel COVID-19: Its impact on organ failure and risk assessment for diabetic and cancer patients. Cytokine Growth Factor Rev. 2020;53:43–52. doi: 10.1016/j.cytogfr.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McKeigue P.M., Weir A., Bishop J., McGurnaghan S.J., Kennedy S., McAllister D., Robertson C., Wood R., Lone N., Murray J., Caparrotta T.M., Smith-Palmer A., Goldberg D., McMenamin J., Ramsay C., Hutchinson S., Colhoun H.M. Rapid epidemiological analysis of comorbidities and treatments as risk factors for COVID-19 in scotland (react-scot): A population-based case-control study. PLoS Med. 2020;17(10):e1003374. doi: 10.1371/journal.pmed.1003374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coomes E.A., Haghbayan H. Interleukin-6 in Covid-19: A systematic review and meta-analysis. Rev. Med. Virol. 2020;30(6):1–9. doi: 10.1002/rmv.2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Di Mascio D., Khalil A., Saccone G., Rizzo G., Buca D., Liberati M., Vecchiet J., Nappi L., Scambia G., Berghella V., D’Antonio F. Outcome of coronavirus spectrum infections (SARS, MERS, COVID-19) during pregnancy: A systematic review and meta-analysis. Am. J. Obstet. Gynecol. MFM. 2020;2(2):100107. doi: 10.1016/j.ajogmf.2020.100107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zheng Z., Peng F., Xu B., Zhao J., Liu H., Peng J., Li Q., Jiang C., Zhou Y., Liu S., Ye C., Zhang P., Xing Y., Guo H., Tang W. Risk factors of critical & mortal COVID-19 cases: A systematic literature review and meta-analysis. J. Infect. 2020;81(2):e16–e25. doi: 10.1016/j.jinf.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kumar A., Arora A., Sharma P., Anikhindi S.A., Bansal N., Singla V., Khare S., Srivastava A. Is diabetes mellitus associated with mortality and severity of COVID-19? A meta-analysis. Diabetes Metab. Syndr. 2020;14(4):535–545. doi: 10.1016/j.dsx.2020.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fu L., Wang B., Yuan T., Chen X., Ao Y., Fitzpatrick T., Li P., Zhou Y., Lin Y.F., Duan Q., Luo G., Fan S., Lu Y., Feng A., Zhan Y., Liang B., Cai W., Zhang L., Du X., Li L., Shu Y., Zou H. Clinical characteristics of coronavirus disease 2019 (COVID-19) in China: A systematic review and meta-analysis. J. Infect. 2020;80(6):656–665. doi: 10.1016/j.jinf.2020.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang B, Li R, Lu Z, Huang Y. Does comorbidity increase the risk of patients with COVID-19: evidence from meta-analysis. Aging (Albany NY) 2020;12(7):6049–6057. doi: 10.18632/aging.103000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liberati A., Altman D.G., Tetzlaff J., Mulrow C., Gøtzsche P.C., Ioannidis J.P., Clarke M., Devereaux P.J., Kleijnen J., Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6(7):e1000100. doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang Z., Liu J., Zhou Y., Zhao X., Zhao Q., Liu J. The effect of corticosteroid treatment on patints with coronavirus infection: A systematic review and meta-analysis. J. Infect. 2020;81(1):13–20. doi: 10.1016/j.jinf.2020.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guo T., Fan Y., Chen M., Wu X., Zhang L., He T., Wang H., Wan J., Wang X., Lu Z. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19). JAMA Cardiol. 2020;5(7):811–818. doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang X., Cai H., Hu J., Lian J., Gu J., Zhang S., Ye C., Lu Y., Jin C., Yu G., Jia H., Zhang Y., Sheng J., Li L., Yang Y. Epidemiological, clinical characteristics of cases of SARS-CoV-2 infection with abnormal imaging findings. Int. J. Infect. Dis. 2020;94:81–87. doi: 10.1016/j.ijid.2020.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang J.J., Dong X., Cao Y.Y., Yuan Y.D., Yang Y.B., Yan Y.Q., Akdis C.A., Gao Y.D. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. 2020;75(7):1730–1741. doi: 10.1111/all.14238. [DOI] [PubMed] [Google Scholar]
- 22.Chen T., Wu D., Chen H., Yan W., Yang D., Chen G., Ma K., Xu D., Yu H., Wang H., Wang T., Guo W., Chen J., Ding C., Zhang X., Huang J., Han M., Li S., Luo X., Zhao J., Ning Q. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Simonnet A., Chetboun M., Poissy J., Raverdy V., Noulette J., Duhamel A., Labreuche J., Mathieu D., Pattou F., Jourdain M. High prevalence of obesity in severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) requiring invasive mechanical ventilation. Obesity (Silver Spring) 2020;28(7):1195–1199. doi: 10.1002/oby.22831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goyal P., Choi J.J., Pinheiro L.C., Schenck E.J., Chen R., Jabri A., Satlin M.J., Campion T.R., Jr, Nahid M., Ringel J.B., Hoffman K.L., Alshak M.N., Li H.A., Wehmeyer G.T., Rajan M., Reshetnyak E., Hupert N., Horn E.M., Martinez F.J., Gulick R.M., Safford M.M. Clinical characteristics of Covid-19 in New York city. N. Engl. J. Med. 2020;382(24):2372–2374. doi: 10.1056/NEJMc2010419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mo P, Xing Y, Xiao Y, Deng L, Zhao Q, Wang H, Xiong Y, Cheng Z, Gao S, Liang K, Luo M, Chen T, Song S, Ma Z, Chen X, Zheng R, Cao Q, Wang F, Zhang Y. Clinical characteristics of refractory COVID-19 pneumonia in Wuhan, China. Clin. Infect. Dis. 2020:ciaa270. doi: 10.1093/cid/ciaa270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hu L, Chen S, Fu Y, Gao Z, Long H, Wang JM, Ren HW, Zuo Y, Li H, Wang J, Xu QB, Yu WX, Liu J, Shao C, Hao JJ, Wang CZ, Ma Y, Wang Z, Yanagihara R, Deng Y. Risk factors associated with clinical outcomes in 323 COVID-19 hospitalized patients in Wuhan, China. Clin. Infect. Dis. 2020:ciaa539. doi: 10.1093/cid/ciaa539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wan S., Xiang Y., Fang W., Zheng Y., Li B., Hu Y., Lang C., Huang D., Sun Q., Xiong Y., Huang X., Lv J., Luo Y., Shen L., Yang H., Huang G., Yang R. Clinical features and treatment of COVID-19 patients in northeast Chongqing. J. Med. Virol. 2020;92(7):797–806. doi: 10.1002/jmv.25783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu W, Tao ZW, Wang L, Yuan ML, Liu K, Zhou L, Wei S, Deng Y, Liu J, Liu HG, Yang M, Hu Y. Analysis of factors associated with disease outcomes in hospitalized patients with 2019 novel coronavirus disease. Chin. Med. J. (Engl) 2020;133(9):1032–1038. doi: 10.1097/CM9.0000000000000775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dreher M., Kersten A., Bickenbach J., Balfanz P., Hartmann B., Cornelissen C., Daher A., Stöhr R., Kleines M., Lemmen S.W., Brokmann J.C., Müller T., Müller-Wieland D., Marx G., Marx N. The characteristics of 50 hospitalized COVID-19 patients with and without ards. Dtsch. Arztebl. Int. 2020;117(16):271–278. doi: 10.3238/arztebl.2020.0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peng YD, Meng K, Guan HQ, Leng L, Zhu RR, Wang BY, He MA, Cheng LX, Huang K, Zeng QT. Clinical characteristics and outcomes of 112 cardiovascular disease patients infected by 2019-nCoV. Zhonghua Xin Xue Guan Bing Za Zhi. 2020;48(6):450–455. doi: 10.3760/cma.j.cn112148-20200220-00105. [DOI] [PubMed] [Google Scholar]
- 32.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang Y., Song B., Gu X., Guan L., Wei Y., Li H., Wu X., Xu J., Tu S., Zhang Y., Chen H., Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chow N., Fleming-Dutra K., Gierke R. Preliminary estimates of the prevalence of selected underlying health conditions among patients with coronavirus disease 2019-United States, February 12- March 28, 2020. MMWR Morb. Mortal. Wkly. Rep. 2020;69(13):382–386. doi: 10.15585/mmwr.mm6913e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O’Reilly G.M., Mitchell R.D., Rajiv P., Wu J., Brennecke H., Brichko L., Noonan M.P., Hiller R., Mitra B., Luckhoff C., Paton A., Smit V., Santamaria M.J., Cameron P.A. Epidemiology and clinical features of emergency department patients with suspected COVID-19: Initial results from the COVID-19 Emergency Department Quality Improvement Project (COVED-1). Emerg. Med. Australas. 2020;32(4):638–645. doi: 10.1111/1742-6723.13540. [DOI] [PubMed] [Google Scholar]
- 35.Shi Y, Yu X, Zhao H, Wang H, Zhao R, Sheng J. Host susceptibility to severe COVID-19 and establishment of a host risk score: findings of 487 cases outside Wuhan. Crit Care. 2020;24(1):108. doi: 10.1186/s13054-020-2833-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu C., Chen X., Cai Y., Xia J., Zhou X., Xu S., Huang H., Zhang L., Zhou X., Du C., Zhang Y., Song J., Wang S., Chao Y., Yang Z., Xu J., Zhou X., Chen D., Xiong W., Xu L., Zhou F., Jiang J., Bai C., Zheng J., Song Y. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern. Med. 2020;180(7):934–943. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schuler B.A., Habermann A.C., Plosa E.J., Taylor C.J., Jetter C., Negretti N.M., Kapp M.E., Benjamin J.T., Gulleman P., Nichols D.S., Braunstein L.Z., Hackett A., Koval M., Guttentag S.H., Blackwell T.S., Webber S.A., Banovich N.E., Kropski J.A., Sucre J.M.S. Age-determined expression of priming protease TMPRSS2 and localization of SARS-CoV-2 in lung epithelium. J. Clin. Invest. 2021;131(1):140766. doi: 10.1172/JCI140766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ssentongo P., Ssentongo A.E., Heilbrunn E.S., Ba D.M., Chinchilli V.M. Association of cardiovascular disease and 10 other pre-existing comorbidities with COVID-19 mortality: A systematic review and meta-analysis. PLoS One. 2020;15(8):e0238215. doi: 10.1371/journal.pone.0238215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gheblawi M., Wang K., Viveiros A., Nguyen Q., Zhong J.C., Turner A.J., Raizada M.K., Grant M.B., Oudit G.Y. Angiotensin-converting enzyme 2: SARS-CoV-2 receptor and regulator of the renin-angiotensin system: celebrating the 20th anniversary of the discovery of ACE2. Circ. Res. 2020;126(10):1456–1474. doi: 10.1161/CIRCRESAHA.120.317015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Taneja V. Sex hormones determine immune response. Front. Immunol. 2018;9:1931. doi: 10.3389/fimmu.2018.01931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gal-Oz S.T., Maier B., Yoshida H., Seddu K., Elbaz N., Czysz C., Zuk O., Stranger B.E., Ner-Gaon H., Shay T. ImmGen report: sexual dimorphism in the immune system transcriptome. Nat. Commun. 2019;10(1):4295. doi: 10.1038/s41467-019-12348-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pelman R.S., Elterman D.S. Lifestyle and disease, male health and risks. Rev. Med. Clin. Las Condes. 2014;25(1):25–29. doi: 10.1016/S0716-8640(14)70006-9. [DOI] [Google Scholar]
- 43.Ye Q., Wang B., Mao J. The pathogenesis and treatment of the ‘Cytokine Storm’ in COVID-19. J. Infect. 2020;80(6):607–613. doi: 10.1016/j.jinf.2020.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Arabi Y.M., Shalhoub S., Mandourah Y., Al-Hameed F., Al-Omari A., Al Qasim E., Jose J., Alraddadi B., Almotairi A., Al Khatib K., Abdulmomen A., Qushmaq I., Sindi A.A., Mady A., Solaiman O., Al-Raddadi R., Maghrabi K., Ragab A., Al Mekhlafi G.A., Balkhy H.H., Al Harthy A., Kharaba A., Gramish J.A., Al-Aithan A.M., Al-Dawood A., Merson L., Hayden F.G., Fowler R. Ribavirin and interferon therapy for critically ill patients with middle east respiratory syndrome: A multicenter observational study. Clin. Infect. Dis. 2020;70(9):1837–1844. doi: 10.1093/cid/ciz544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Willemse B.W., Postma D.S., Timens W., ten Hacken N.H. The impact of smoking cessation on respiratory symptoms, lung function, airway hyperresponsiveness and inflammation. Eur. Respir. J. 2004;23(3):464–476. doi: 10.1183/09031936.04.00012704. [DOI] [PubMed] [Google Scholar]
- 46.Salah H.M., Sharma T., Mehta J. Smoking doubles the mortality risk in COVID-19: A meta-analysis of recent reports and potential mechanisms. Cureus. 2020;12(10):e10837. doi: 10.7759/cureus.10837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rossato M., Russo L., Mazzocut S., Di Vincenzo A., Fioretto P., Vettor R. Current smoking is not associated with COVID-19. Eur. Respir. J. 2020;55(6):2001290. doi: 10.1183/13993003.01290-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W., Si H.R., Zhu Y., Li B., Huang C.L., Chen H.D., Chen J., Luo Y., Guo H., Jiang R.D., Liu M.Q., Chen Y., Shen X.R., Wang X., Zheng X.S., Zhao K., Chen Q.J., Deng F., Liu L.L., Yan B., Zhan F.X., Wang Y.Y., Xiao G.F., Shi Z.L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Qiu F., Liang C.L., Liu H., Zeng Y.Q., Hou S., Huang S., Lai X., Dai Z. Impacts of cigarette smoking on immune responsiveness: Up and down or upside down? Oncotarget. 2017;8(1):268–284. doi: 10.18632/oncotarget.13613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Patanavanich R., Glantz S.A. Smoking is associated with COVID-19 progression: A meta-analysis. Nicotine Tob. Res. 2020;22(9):1653–1656. doi: 10.1093/ntr/ntaa082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vardavas C.I., Nikitara K. COVID-19 and smoking: A systematic review of the evidence. Tob. Induc. Dis. 2020;18:20. doi: 10.18332/tid/119324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Danser A.H.J., Epstein M., Batlle D. Renin-angiotensin system blockers and the COVID-19 pandemic: At present there is no evidence to abandon renin-angiotensin system blockers. Hypertension. 2020;75(6):1382–1385. doi: 10.1161/HYPERTENSIONAHA.120.15082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sharma R.K., Stevens B.R., Obukhov A.G., Grant M.B., Oudit G.Y., Li Q., Richards E.M., Pepine C.J., Raizada M.K. ACE2 (angiotensin-converting enzyme 2) in cardiopulmonary diseases: Ramifications for the control of SARS-CoV-2. Hypertension. 2020;76(3):651–661. doi: 10.1161/HYPERTENSIONAHA.120.15595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ayelign B., Negash M., Genetu M., Wondmagegn T., Shibabaw T. Immunological impacts of diabetes on the susceptibility of Mycobacterium tuberculosis. J. Immunol. Res. 2019;2019:6196532. doi: 10.1155/2019/6196532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pal R., Bhansali A. COVID-19, diabetes mellitus and ACE2: The conundrum. Diabetes Res. Clin. Pract. 2020;162:108132. doi: 10.1016/j.diabres.2020.108132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jafar N., Edriss H., Nugent K. The effect of short-term hyperglycemia on the innate immune system. Am. J. Med. Sci. 2016;351(2):201–211. doi: 10.1016/j.amjms.2015.11.011. [DOI] [PubMed] [Google Scholar]
- 57.Roncon L., Zuin M., Zuliani G., Rigatelli G. Patients with arterial hypertension and COVID-19 are at higher risk of ICU admission. Br. J. Anaesth. 2020;125(2):e254–e255. doi: 10.1016/j.bja.2020.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lippi G., Lavie C.J., Sanchis-Gomar F. Cardiac troponin I in patients with coronavirus disease 2019 (COVID-19): Evidence from a meta-analysis. Prog. Cardiovasc. Dis. 2020;63(3):390–391. doi: 10.1016/j.pcad.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li X., Guan B., Su T., Liu W., Chen M., Bin Waleed K., Guan X., Gary T., Zhu Z. Impact of cardiovascular disease and cardiac injury on in-hospital mortality in patients with COVID-19: A systematic review and meta-analysis. Heart. 2020;106(15):1142–1147. doi: 10.1136/heartjnl-2020-317062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Boettler T., Newsome P.N., Mondelli M.U., Maticic M., Cordero E., Cornberg M., Berg T. Care of patients with liver disease during the COVID-19 pandemic: EASL-ESCMID position paper. JHEP Rep. 2020;2(3):100113. doi: 10.1016/j.jhepr.2020.100113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Desai A., Sachdeva S., Parekh T., Desai R. COVID-19 and cancer: Lessons from a pooled meta-analysis. JCO Glob Oncol. 2020;6:557–559. doi: 10.1200/GO.20.00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Masoudkabir F., Poorhosseini H., Vasheghani-Farahani A., Hakki E., Roayaei P., Kassaian S.E. Synergistic effect of hypertension with diabetes mellitus and gender on severity of coronary atherosclerosis: Findings from Tehran Heart Center registry. ARYA Atheroscler. 2015;11(6):317–322. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material is available on the publisher’s website along with the published article.
Table S1 (526.2KB, pdf) . Descriptive clinical symptoms and treatments of patients.
Fig. (S1 (526.2KB, pdf) ). The pooled prevalence of critical/severe patients in COVID-19.
Fig. (S2 (526.2KB, pdf) ). Pooled prevalence of gender in COVID-19.
Fig. (S3 (526.2KB, pdf) ). Checklist: PRISMA 2009 checklist.