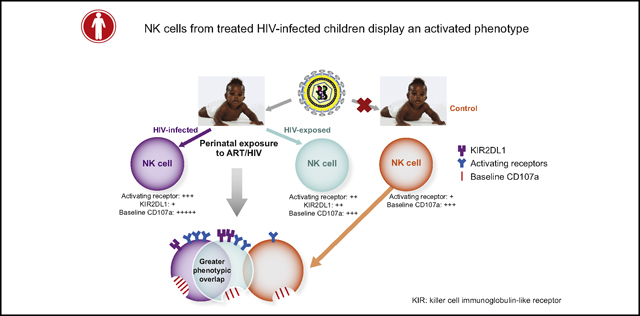
Abstract
Background:
Chronic HIV infection is known to trigger a population redistribution and alteration in the functional capacity of natural killer (NK) cells. Because of improved antiretroviral treatments, there are rising numbers of adolescents and young adults worldwide who are living with HIV infection since birth.
Objective:
We sought to determine how NK-cell phenotypic and functional subsets are altered in treated pediatric patients.
Methods:
NK cells were contrasted among 29 HIV-unexposed and uninfected controls (5–19 years), 23 HIV-exposed but uninfected patients (3–19 years), and 25 HIV-infected patients (3–19 years) using multiparametric flow cytometry.
Results:
Although most NK-cell markers did not differ, activating receptors such as NKp46, DNAX accessory molecule-1, and NKG2C and stimulatory receptors such as CD2 and CD11c were expressed by a higher frequency of NK cells in HIV-infected patients than in controls. Interestingly, there were less differences between HIV-infected and HIV-exposed but uninfected children. There was an inverse relationship between CD4/CD8 T-cell ratio (as a marker of disease progression) and CD11c and NKG2C frequency and CD69 upregulation on stimulation among HIV-infected patients.
Conclusions:
A chronic NK-cell activation phenotype persists in HIV-infected children receiving antiretroviral therapy and is associated with declining CD4/CD8 T-cell ratios. A lower CD4/CD8 T-cell ratio was associated with higher baseline granzyme B (P =.0068; R2 = 0.29) and degranulation potential (P =.022; R2 = 0.22) in stimulated NK cells. Thus, NK cells in HIV-infected children receiving treatment have reduced functional potential and an activated phenotype that distinguishes them from uninfected children.
Keywords: NK cell, treated HIV, flow cytometry, children, vertical exposure
Graphical Abstract
Natural killer (NK) cells are lymphocytes of the innate immune system that kill virally infected and malignant cells. They arise in the bone marrow, undergo central and peripheral development, and ultimately attain functional competency, with the mature subsets in the majority found in the peripheral blood. NK cells are armed with an array of activating, inhibitory, and adhesion surface receptors, which determine the population’s phenotypic diversity and function.1, 2 During viral infections, NK cells can recognize virally infected cells through their activating receptors, or the sensing of decreased MHC-I molecules through their cognate inhibitory receptors.3 They can also be activated by stress-induced ligands that bind to stress-sensing receptors such as NKG2D. Once activated, NK cells mediate antiviral immunity via direct killing of infected cells, production of soluble antiviral mediators such as IFN-γ, and promoting/facilitating broader immunity. Direct killing includes CD16-dependent antibody-dependent cellular cytotoxicity and natural cytotoxicity both via the secretion of preformed lytic granules containing perforin and granzyme. Accordingly, NK cells can contribute to the control of viral infections and evidence exists for their role in the defense against HIV.1, 2
Chronic HIV infection is characterized by phenotypic redistribution of NK-cell subsets that potentially compromises antiviral functions. Defective NK-cell killing has been identified in the context of disease progression and defective antibody-dependent cellular cytotoxicity. Diminished secretion of soluble mediators has also been reported in HIV-infected patients.4 This could be owing to expansion of a dysfunctional CD56− NK-cell subset that has impaired cytotoxicity and cytokine release.5, 6 Abnormal cytotoxicity has additionally been associated with downregulation of activating receptors in viremic patients, 7 but increased NK-cell activity has been identified in some viremic patients.8 Irrespective, NK-cell phenotypic changes have been linked to ongoing viral replication that is partially recovered following viral suppression through antiretroviral therapy (ART).9 However, IFN-γ secretion from NK cells is reduced in aviremic patients and activated NK cells were observed in HIV-infected patients regardless of ART.10, 11 Thus, ART does not fully reconstitute the NK-cell phenotype and function, and exact characteristics of residual NK-cell subset abnormalities in treated patients warrant further investigation.
Although most studies of NK cells in HIV-infected patients have been conducted in adults, few studies in HIV-infected children have identified some limited NK-cell phenotypic changes that persist despite treatment.12, 13 Because we have previously shown that a high-resolution NK-cell phenotype in children differs from that in adults, 14 we pursued this level of detail in treated HIV-infected children. We were especially focused on mother-to-child transmission (MTCT) cases because most children are on ART since birth.15 Moreover, perinatal transmission does not occur in 65% to 85% of infants, even in the absence of ART, leaving children HIV-exposed but uninfected (HEU). HEU infants in the context of MTCT can potentially provide important insights into NK-cell antiviral defense because the HIV-exposed NK cells produce increased chemokine (C-C motif) ligands × to 5, which can suppress viral replication through blocking the cognate chemokine (C-C motif) receptor 5.16 Furthermore, activated and perforin-expressing NK cells decline over the first year of life in HEU infants but not in HIV-unexposed controls, indicating some impact on and functional differences in NK cells from HEU children.17 Therefore, we set out to determine how NK-cell subsets at relatively high resolution might be altered and what distinguishing characteristics, if any, they have in HEU children. We identified differences in NK-cell phenotype and function in both cohorts and could associate the phenotypic variation with markers of disease progression in HIV-infected patients. Given the prevalence of pulmonary complications in HIV-infected patients18 and the role of NK cells in allergic asthma, 19 we also examined NK-cell phenotype on the basis of asthma incidence.
METHODS
Human subjects and sample preparation
A total of 77 subjects (aged 3–19 years) were recruited at Texas Children’s Hospital (Table I). All individuals provided written informed consent approved by the Institutional Review Board of Baylor College of Medicine for the protection of human rights. PBMCs were extracted from whole blood using Ficoll-Paque (ThermoFisher Scientific, Waltham, Mass) density gradient centrifugation and cryopreserved in filter-sterilized heat-inactivated FBS (Atlanta Biologicals, Flowery Branch, Ga) containing 10% dimethyl sulfoxide (ThermoFisher Scientific).
TABLE I.
Study cohort
| Age (y) | Ethnicity | Sex | Cohort |
|---|---|---|---|
| 3 | Hispanic | F | HIV+ |
| 4 | Unknown | F | HIV+ |
| 5 | Black | F | HIV+ |
| 5 | Black | F | HIV+ |
| 7 | Black | F | HIV+ |
| 8 | Black | F | HIV+ |
| 9 | Hispanic | F | HIV+ |
| 11 | Black | M | HIV+ |
| 12 | Black | F | HIV+ |
| 12 | Hispanic | F | HIV+ |
| 14 | Black | F | HIV+ |
| 15 | Hispanic | F | HIV+ |
| 15 | Black | M | HIV+ |
| 16 | Black | F | HIV+ |
| 16 | Black | F | HIV+ |
| 16 | Black | F | HIV+ |
| 17 | Hispanic | M | HIV+ |
| 17 | Black | M | HIV+ |
| 18 | Black | F | HIV+ |
| 18 | Black | M | HIV+ |
| 18 | Black | F | HIV+ |
| 19 | White (non-Hispanic) | M | HIV+ |
| 19 | Black | F | HIV+ |
| 19 | Black | M | HIV+ |
| 19 | Black | M | HIV+ |
| 3 | Hispanic | M | HEU |
| 5 | Black | F | HEU |
| 5 | Black | M | HEU |
| 6 | Black | F | HEU |
| 7 | Black | F | HEU |
| 7 | Black | F | HEU |
| 8 | Black | M | HEU |
| 9 | Black | M | HEU |
| 9 | White | M | HEU |
| 10 | Black | M | HEU |
| 11 | White (non-Hispanic) | M | HEU |
| 11 | Black | F | HEU |
| 13 | Black | M | HEU |
| 14 | Black | F | HEU |
| 14 | Black | M | HEU |
| 15 | Black | M | HEU |
| 15 | Black | M | HEU |
| 15 | Hispanic | M | HEU |
| 16 | Black | F | HEU |
| 17 | Black | F | HEU |
| 18 | Black | M | HEU |
| 18 | Black | F | HEU |
| 19 | Black | F | HEU |
| 5 | Hispanic | M | Control |
| 6 | Hispanic | M | Control |
| 9 | Hispanic | M | Control |
| 9 | Hispanic | F | Control |
| 9 | Black | M | Control |
| 10 | Black | M | Control |
| 11 | Black | F | Control |
| 11 | Hispanic | M | Control |
| 12 | Hispanic | M | Control |
| 12 | Black | M | Control |
| 12 | Black | M | Control |
| 13 | Hispanic | M | Control |
| 14 | Hispanic | F | Control |
| 14 | Hispanic | M | Control |
| 15 | Hispanic | F | Control |
| 15 | Hispanic | F | Control |
| 15 | Black | F | Control |
| 16 | Unknown | M | Control |
| 16 | Hispanic | F | Control |
| 16 | Hispanic | M | Control |
| 16 | Hispanic | F | Control |
| 16 | Black | F | Control |
| 17 | Black | F | Control |
| 18 | Black | F | Control |
| 18 | Black | F | Control |
| 18 | Black | F | Control |
| 18 | Asian | M | Control |
| 18 | Hispanic | F | Control |
| 19 | Black | M | Control |
| 19 | White (non-Hispanic) | F | Control |
F, Female; M, male.
HIV-infected patients were recruited from Retrovirology clinic and controls from the Allergy and Immunology clinic.14 HEU subjects were recruited from those enrolled in the Pediatric HIV/AIDS Cohort Study.18 Inclusion criteria was MTCT for HIV-infected and perinatal exposure for HEU children.
Antibodies
All commercial antibodies were obtained from sources as previously outlined.14
Cell staining, acquisition, and data analysis
Cryopreserved PBMCs were thawed and stained as previously described.14 Briefly, cells were suspended at a concentration of approximately 4 × 106 cells/mL. For surface staining (panels 2–4) (see Table E1 in this article’s Online Repository at http://www.jacionline.org), cells were stained in PBS (ThermoFisher Scientific) 2% FBS buffer with a cocktail of antibodies for 15 to 20 minutes in the dark. For panels 1 and 5, cells were stimulated with phorbol 12-myristate 13-acetate (10 ng/mL; Sigma-Aldrich, St Louis, Mo) and ionomycin (1 μg/mL; Sigma-Aldrich) and incubated for 4 hours. Cells were washed and fixed with 1× PBS containing 1% paraformaldehyde (Sigma-Aldrich) at the end of staining.
A modified LSR Fortessa (BD Biosciences, San Jose, Calif) was used to acquire approximately 2000 NK cells for each donor. Data analysis was conducted using Kaluza (Version 1.3; Beckman Coulter, Brea, Calif) with the gating strategy illustrated earlier.14
Statistics
Comparisons across 3 groups were made using the Kruskal-Wallis test. If the overall test for differences was significant at the 0.05 level, then all pairwise comparisons were assessed using Wilcoxon rank-sum test with Bonferroni adjustment for multiple comparisons (STATA Version 14; Stata Corp, College Station, Tex). Two-γroup comparisons were assessed using the Wilcoxon rank-sum test. Staining, acquisition, and analysis were repeated for 3 controls, and interex perimental variability for controls was determined using Bland-Altman analysis (Prism, Version 6, GraphPad Software, Inc, San Diego, Calif). Variability was defined as the mean difference in percentages for each marker in panels 2 to 4. Combinatorial subsets were identified at a minimum threshold of 3%. The mean values were compared between HIV-infected and HEU cohorts using a general linear mixed model (SAS, Version 9.4; SAS Institute, Cary, NC).14
RESULTS
NK cells in treated HIV-infected pediatric patients display an activated phenotype
Chronic HIV infection triggers an abnormal redistribution of NK-cell subsets, which does not fully recover post-ART. The mature CD56+CD16+ NK-cell subset is selectively depleted in HIV-infected adolescents with low CD4+ T-cell counts, 20 and HIV-infected patients have lower levels of NK-cell degranulation despite more than 3 years of ART.13 Therefore, we sought to evaluate whether functional and phenotypic NK-cell defects persist in HIV-infected children on long-term therapy using high-resolution multiparametric flow cytometry. Our cohort included 25 MTCT perinatally HIV-infected children (3–20 years), 23 perinatally HEU children (3–20 years), and 29 demographic-matched control subjects (5–20 years). Because NK-cell development and effector functions are dependent on a range of activating, adhesion, and inhibitory receptors, progressively acquired or lost through different stages of maturation, we used = previously designed panels that encompass the spectrum of NK-cell biology and function (Table E1).14
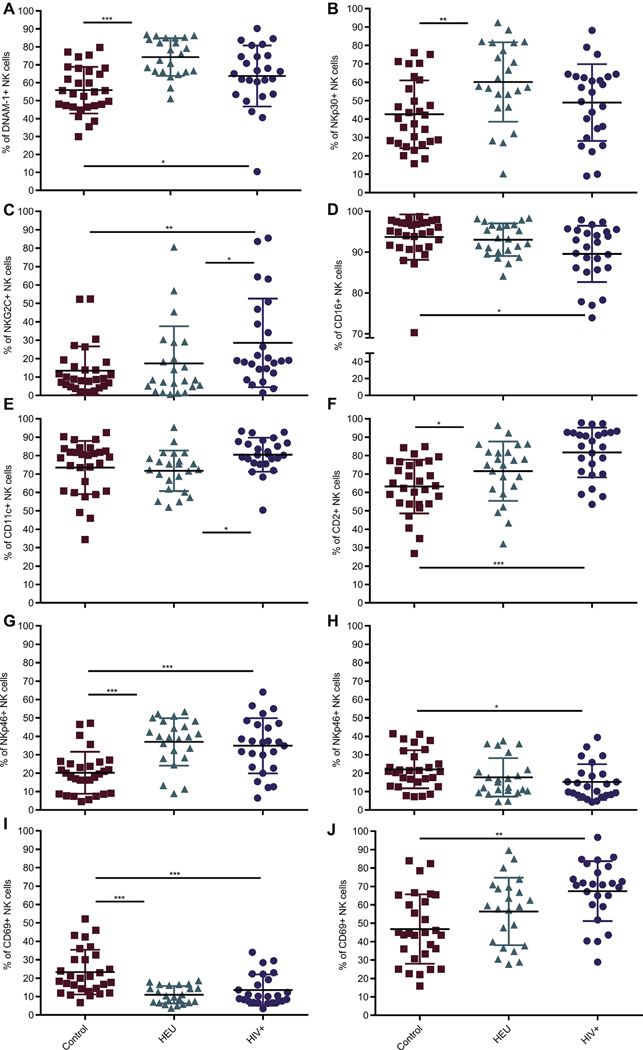
These panels were used to distinguish the frequency of NK-cell markers among our 3 cohorts. Panels 2 to 4 addressed baseline expression of surface markers, whereas panels 1 and = characterize both baseline characteristics and changes after stimulation with phorbol 12-myristate 13-acetate and ionomycin. In all graphs, % of NK cells positive for a particular marker refers to the percentage of total NK cells expressing that marker. Although the overall NK-cell frequencies did not differ between our study groups (data not shown), certain activating, adhesion, and stimulatory receptors were elevated in HIV-infected children (Fig 1). Baseline frequency of DNAX accessory molecule-1 (DNAM-1), CD2, and NKp46 was elevated in both HIV-infected and HEU subjects compared with controls (Fig 1, A, F, and G). In contrast, NKG2C was specifically increased in HIV-infected individuals (Fig 1, C). Although this has been repeatedly demonstrated in HIV-infected adults, 21, 22 it is less established in perinatally infected children. NK-cell frequency of CD11c and ability to upregulate activation marker CD69 poststimulation were also higher in HIV-infected children (Fig 1, E and J). However, baseline frequency of CD16, CD69 (Fig 1, D and I), and CD11b, 2B4, and CD18 (data not shown) and stimulated expression of NKp46 (Fig 1, H) were reduced in HIV-infected patients, whereas that of NKp30 was unaffected (Fig 1, B). Although the HIV-infected and HEU cohorts were processed and acquired in a paired manner, the demographic-matched control subjects were from our previous study and used here for comparison.14 To evaluate any impact this might have had, Bland-Altman analysis was performed to estimate agreement between the older set of experiments for × random controls (of 29 total patients) and the experiments with the same parameters used for HIV-infected and HEU children. The maximum difference in frequency for any 1 fluorochrome between the 2 sets of experiments was less than 10% (see Table E2, A, in this article’s Online Repository at www.jacionline.org). The difference for each fluorochrome was accordingly applied to the frequency values of all 29 controls for each marker such that the values would be skewed toward those of HIV-infected patients (Table E2, B). Of all the activating receptors increased in HIV-infected patients, only DNAM-1 was no longer significant on repeating the statistical analysis. Given the validity identified through these analyses, we have used the historical age-matched control cohort14 for comparison to the HIV-infected and HEU cohorts for all frequency comparisons. This agreement study was also repeated for median fluorescent intensity (MFI). Here, the variation identified was more than 10%, and thus MFI was contrasted in further analyses only between HIV-infected and HEU children and revealed no significant differences for any marker (data not shown).
FIG 1.
NK-cell baseline frequency of most activating receptors in treated HIV-infected patients is elevated. NK cells were defined as CD3−CD45highCD56+ using flow cytometry and gated above fluorescence minus one (FMO) controls for all markers. A-F, Frequency of NK cells expressing DNAM-1, NKp30, NKG2C, CD16, CD11c, and CD2 without stimulation. NKp46 and CD69 incubated in absence of stimulation (G and I) or after 4-hour stimulation with phorbol 12-myristate 13-acetate/ionomycin (H and J). All markers indicate surface expression. Each data point represents a donor; control (n = 29) (red); HEU (n = 23) (green); HIV-infected (n = 25) (purple). All data shown are mean ± S.D. *P < .05, **P < .01, ***P < .001 among 3 cohorts by Kruskal-Wallis and post hoc comparison by Wilcoxon rank-sum test using Bonferroni adjustment.
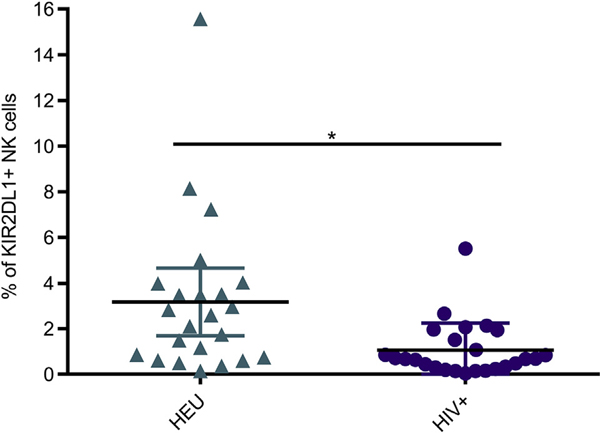
Because disparity in frequencies of the individual receptors among our cohorts was identified, we additionally contrasted the simultaneous expression of these markers between HIV-infected and HEU children. Treeplot analysis was used to generate the frequencies of all 256 combinations of 8 markers in each panel. Although most combinatorial subsets did not differ, the killer cell immunoglobulin-like receptor (KIR) 2DL1+ subset that lacked all other inhibitory receptors such as CD158b, CD158e, NKG2A, and killer cell lectin-like receptor subfamily G member 1 and activating receptors such as KIR2DS4 and NKG2C was significantly lower in HIV-infected patients (Fig 2). Interestingly, NK cells of the only patient with a viral load of more than × logs (see Table E3 in this article’s Online Repository at www.jacionline.org) in our cohort had the lowest KIR2DL11 subset frequency (0.05%). Few studies have indicated that HIV-infected patients with KIR2DL1+ NK cells who also express the cognate HLA-C2 ligand have enhanced NK-cell activity, while the presence of KIR/HLA discordance provides a protective advantage against HIV.23–25 Of the 10 HIV-infected patients we performed HLA typing on, 9 were mismatched to KIR2DL1 (data not shown). Therefore, one explanation could be that this mismatch did not protect against HIV due to low KIR2DL1 expression. That said, our cohort is underpowered for such considerations because these hypotheses are resolvable only in larger population studies. Furthermore, conclusions cannot be drawn because we do not have the HLA haplotype of all patients. Thus, HIV-infected patients not only have elevated expression of individual activating receptors but also decreased expression of a combinatorial inhibitory subset. Although the wide age range of our patients may confound these results, we do not believe this to be a major limitation because we have included age-matched controls. Moreover, our previous study noted far fewer differences among NK cells of healthy children than between children and adults.14
FIG 2.
Inhibitory receptor KIR2DL1 is found on a lower frequency of NK cells in treated HIV-infected patients. Frequency of NK cells with positive expression of KIR2DL1 but lacking CD158b, CD158e, KIR2DS4, CD94, NKG2A, NKG2C, and KLRG1. KLRG1, Killer cell lectin-like receptor subfamily G member 1. Each data point represents a donor; HEU (n = 23) (green); HIV-infected (n = 25) (purple). Mean ± SD. *P < .05 using a general linear mixed model and adjusted for multiple comparisons using Holm’s stepdown Bonferroni method.
Reciprocal relationship between NK-cell activating receptors and CD4/CD8 T-cell ratio
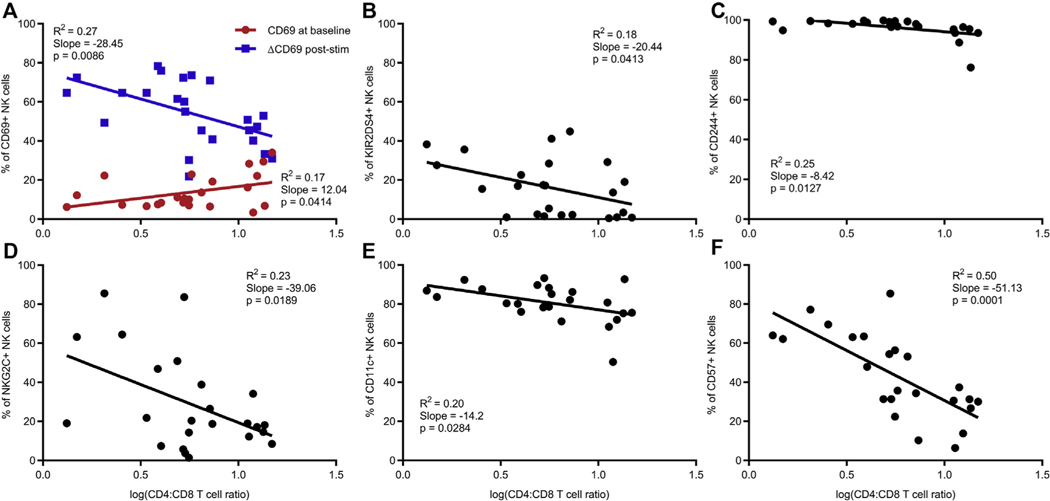
HIV infection is characterized by chronic immune activation that endures despite suppression of viral replication with persistent evidence of T-cell activation.26 CD4/CD8 T-cell ratio is used as a reliable biomarker of disease progression in HIV infection because it is more stable than CD4+ T-cell counts and normalizes gradually during ART.27, 28 CD4/CD8 ratio is also posited to be an indicator of T-cell activation, senescence, and inflammation. Because our data allude to an NK-cell activation phenotype in HIV-infected patients, we hypothesized that declining CD4/CD8 ratio associates with the frequency of activating receptors on NK cells. Indeed, CD69 upregulation by NK cells poststimulation negatively associatedwithCD4/CD8ratio (Fig 3, A). The same correlation was observed on a per-cell basis as a change in CD69 MFI negatively associated with both CD4/CD8 ratio and frequency of CD4+ T cells (see Fig E1, A and B, in this article’s Online Repository at www.jacionline.org). Activating and stimulatory receptors such as KIR2DS4, CD244, NKG2C, and CD11c also had a reciprocal relationship with CD4/CD8 ratio (Fig 3, B-E). Only NKG2C was significant when the same markers were tested against CD4+ T-cell counts, underscoring the value of using CD4/CD8 ratios (see Fig E2, D, in this article’s Online Repository at www.jacionline.org). CD69, NKG2C, and CD11c were particularly relevant because they were significantly higher in HIV-infected patients than in controls (Fig 1). Moreover, CD94, which associates with NKG2C, also had a reverse association with both CD4+ T-cell frequency and count (Fig E1, C and D). Thus, NK-cell activating receptors are more highly expressed in HIV-infected individuals having greater disease severity.
FIG 3.
Frequency of NK-cell activating and stimulatory receptors and CD57 negatively associates with CD4/CD8 T-cell ratio. A, Relationship between CD4/CD8 T-cell ratio and frequency of NK cells expressing CD69 in the absence of stimulation (red) or increase in CD69 after stimulation with phorbol 12-myristate 13-acetate/ionomycin (blue). B-F, Relationship between CD4/CD8 T-cell ratio and percentage of NK cells expressing KIR2DS4, CD244, NKG2C, CD11c, and CD57. CD4/CD8 ratios were log transformed after a constant value of 1 was added to each ratio. All markers indicate surface expression. Each data point represents a donor; HIV-infected (n = 24). P values were identified by linear regression with the coefficient of determination (R2) and slope defined.
CD57 is a terminal maturation marker on NK cells that can be acquired after cytokine stimulation in vitro.29 Its expression has been reported to increase with aging as well as in chronic infections such as human cytomegalovirus (CMV) and HIV.30 In our cohort, the CD4/CD8 ratio was linked to the variation in CD57 (R2 = 0.5) and had the strongest inverse relationship of all markers tested (Fig 3, F). This pattern was repeated with both frequency and CD4+ T-cell count as well as between CD57 MFI and CD4/CD8 ratio (see Fig E3 in this article’s Online Repository at www.jacionline.org). Although CD57 frequency did not differ among the 3 cohorts we studied (data not shown), high CD57 expression on NK cells was associated with disease severity. This increased NK-cell maturation could be due to the proinflammatory cytokine environment in active HIV disease or human CMV co-infection, both of which can also mediate chronic immune activation in HIV-infected individuals.26
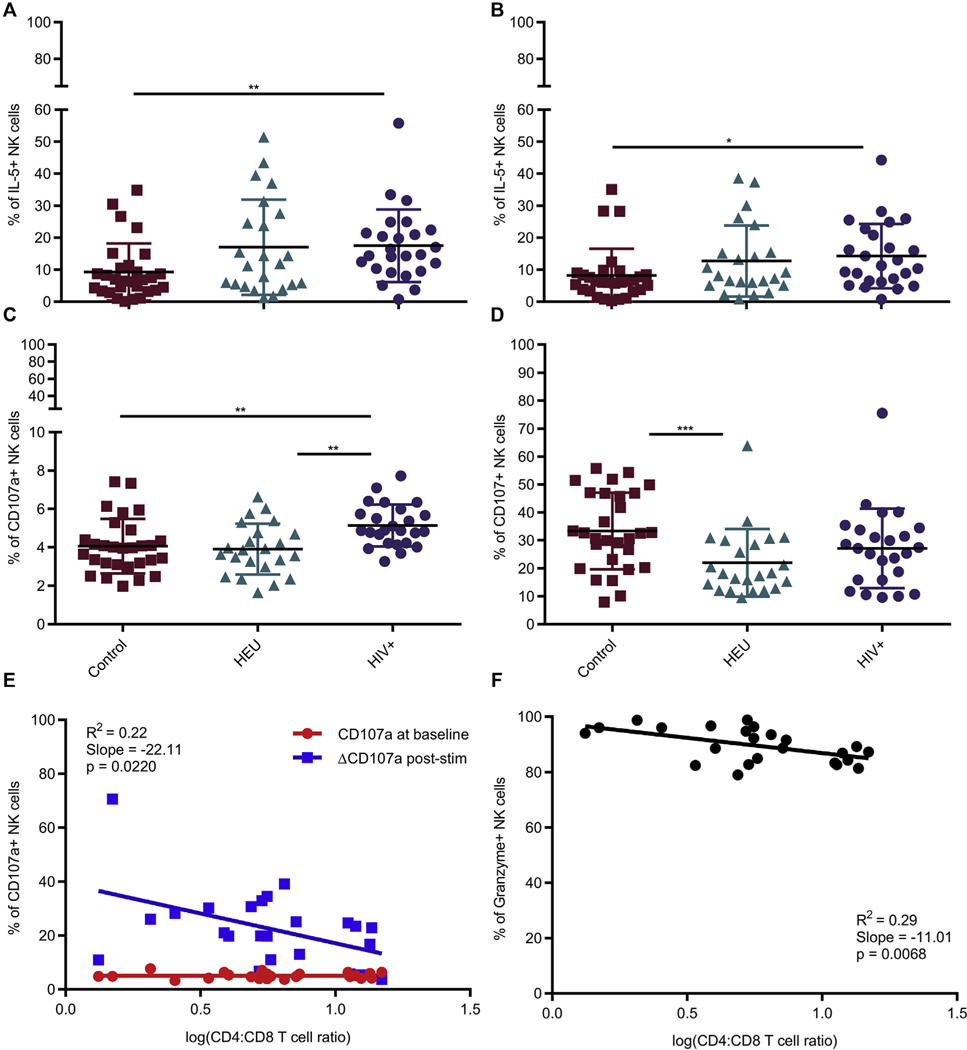
In some studies, impaired NK-cell activity and degranulation potential during HIV infection is not recovered post-ART.13, 20 In our cohorts, NK cells in HIV-infected children had significantly higher baseline IL-5 and CD107a expression (Fig 4, A and C). However, the increased CD107a expression was not retained after stimulation (Fig 4, D). IL-5 presumably did not increase on stimulation (Fig 4, B) due to lack of IL-2 in addition to PMA/ionomycin stimulation as previously shown.31 On repeating analysis, the increased baseline CD107a expression in HIV-infected individuals was still statistically significant from that in control donors but not IL-5 (Table E2, B). Interestingly, the stimulated but not baseline levels of CD107a and granzyme B were inversely associated with CD4/CD8 ratio and CD4+ T-cell counts (Fig 4, E and F; see Fig E4, A, in this article’s Online Repository at www.jacionline.org). Similar trends existed between increase in CD107a MFI after stimulation and CD4/CD8 ratio as well as granzyme and CD4+ T-cell frequency but not count (Fig E4, B; see Fig E5 in this article’s Online Repository at www.jacionline.org). Thus, the HIV-infected children with the lowest CD4/CD8 ratio had the highest baseline granzyme levels and exhibited greatest degranulation potential on stimulation, implying that chronic activation owing to disease severity was promoting NK-cell activation state.
FIG 4.
Unstimulated NK cells in treated HIV-infected patients have higher degranulation potential and type 2 (NK2) cytokine bias. IL-5 and CD107a incubated in absence of stimulation (A and C) or stimulated with phorbol 12-myristate 13-acetate/ionomycin (B and D). Control (n = 29) (red); HEU (n = 23) (green); HIV-infected (n = 25) (purple). All data shown are mean ± SD. *P < .05, **P < .01, ***P < .001 among 3 cohorts by Kruskal-Wallis and post hoc comparison by Wilcoxon rank-sum test using Bonferroni adjustment. E, Relationship between CD4/CD8 T-cell ratio and frequency of NK cells expressing CD107a in the absence of stimulation (red) or increase in CD107a after stimulation (blue). F, Relationship between CD4/CD8 T-cell ratio and percentage of NK cells expressing granzyme in the absence of stimulation. CD107a indicates surface expression while granzyme and IL-5 were detected intracellularly as described in the Methods section. Each data point represents a donor; HIV-infected (n = 24). P values were identified by linear regression with the coefficient of determination (R2) and slope defined.
We also evaluated viremia as an association for the activated NK-cell phenotype observed in HIV-infected patients. Of the 25 patients in our cohort, 12 had detectable viral loads but only 1 had a viral load of more than 3 logs (Table E3). One patient had less than 200 CD4+ T cells and clinically defined to have AIDS but was not excluded from the analysis. On comparing the NK-cell phenotype, the frequency of CD18 and granzyme B at baseline and MFI of CD11a were significantly higher in patients with detectable viral loads (see Fig E6 in this article’s Online Repository at www.jacionline.org). Because our cohort had diverse ART regimens, we also evaluated phenotypic differences by treatment. NK cells in patients who received a combination of integrase and nucleoside reverse transcriptase inhibitors had the highest CD11b MFI (data not shown). Viremia and medication regimen therefore might provide some influence on the activated NK-cell phenotype found in HIV-infected children.
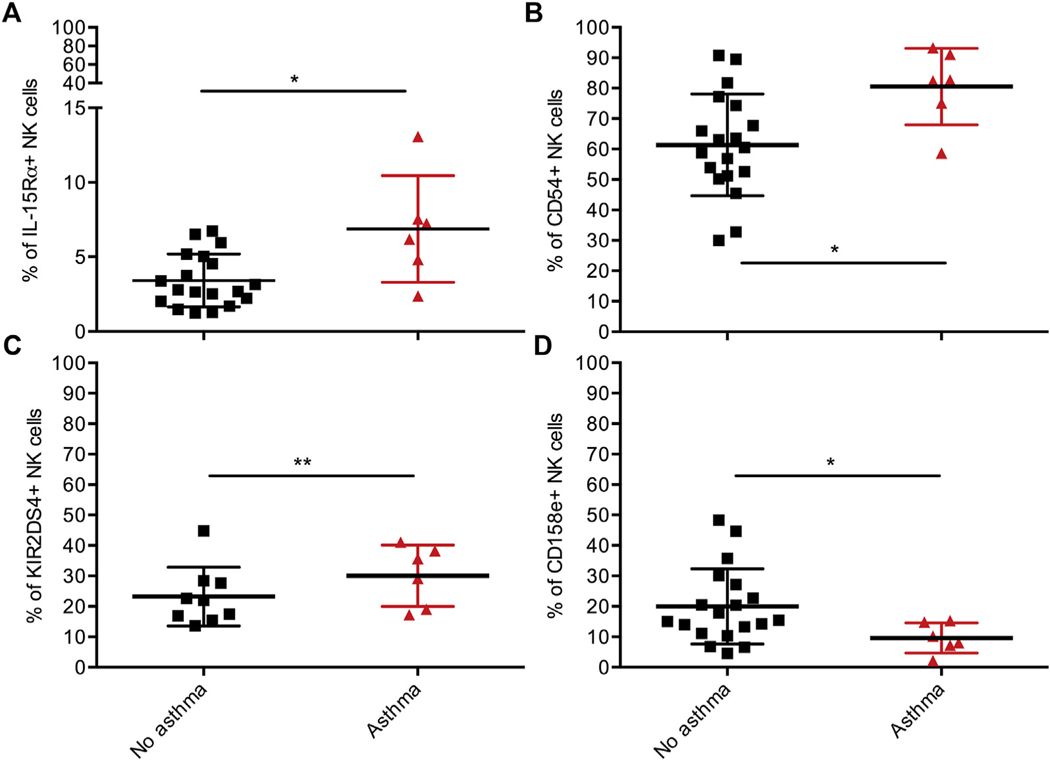
We also considered asthma as an inflammatory disease in our patients that could potentially affect NK-cell phenotype because an increased asthma burden has been documented in HIV-infected patients since the onset of the ART era.32 NK cells in the 6 HIV-infected patients with asthma had higher frequency of IL-15 receptor-α chain, KIR2DS4 and CD54, but lower CD158e (Fig 5). Thus, the incidence of asthma may contribute to but does not account for the activation phenotype in HIV-infected patients.
FIG 5.
NK cells in HIV-infected patients with asthma have increased frequency of IL-15 receptor-α chain, ICAM-1, and KIR2DS4 but reduced inhibitory receptor KIR3DL1. A-D, Frequency of NK cells expressing IL-15Rα, CD54, KIR2DS4, and CD158e among HIV-infected patients. No asthma (n = 19) (black); asthma (n = 6) (red). KIR2DS4, No asthma (n = 9). All data shown are mean ± SD. *P < .05, **P < .01 by Wilcoxon rank-sum test.
NK-cell subsets of HIV-exposed and HIV-infected patients are phenotypically similar
It is increasingly appreciated that HEU individuals have specific characteristics. For example, an increased incidence of respiratory infections in HEU children has been reported in South Africa and India.33 Immunologically, HEU children have a more mature and activated phenotype compared with HIV-unexposed children. These studies suggest that exposure to HIV and/or ART drives phenotypic changes that persist beyond infancy. To examine this, we compared the NK-cell phenotype in HEU children with that in both HIV-infected patients and controls. Although several activating receptors were upregulated in HIV-infected patients, some such as NKp46 and DNAM-1 were also increased in HEU children (Fig 1, A and G). Moreover, NKp30 and inhibitory receptor killer cell lectin-like receptor subfamily G member 1 differed only between HEU subjects and controls, suggesting a potential correlate for HIV infection resistance within the NK-cell population of children (Fig 1, B; see Fig E7, A, in this article’s Online Repository at www.jacionline.org). Thus, HEU children have elevated activating receptors but fewer than those in HIV-infected patients.
Interestingly, CD122 was highest in HEU children (Fig E7, B). NK cells of HEU adults have been previously reported to have higher CD57 compared with those of HIV-infected patients.34 Although the results were not significant, the frequency of CD57 alone (data not shown) and simultaneous expression of CD57, CD122, CD11b, and CD16 trended toward being higher in HEU children (Fig E7, C).
On tabulating the top combinatorial subsets by panel, only the developmental subset differed between HIV-infected and HEU children due to lower CD57 while the remaining subsets were overlapping between the 2 cohorts (see Table E4 in this article’s Online Repository at www.jacionline.org). Thus, higher expression of CD122 and developmentally mature NK-cell subset convey a more mature phenotype in HEU children compared with HIV-infected patients.
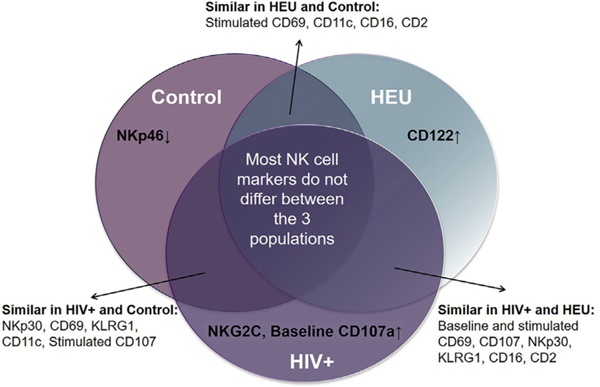
All NK-cell markers that differed among our 3 cohorts are summarized in Fig 6. The baseline frequency of NKp46 was selectively reduced in controls in contrast with elevated NKG2C and baseline degranulation in HIV-infected children. Several markers significantly differed only between 2 cohorts. For example, NKp30 was significantly different between HEU children and controls. However, the HIV-infected cohort was not significantly different from either the HEU cohort or the control cohort. Altogether, HIV-infected and HEU children had the most similarities in NK-cell marker frequency followed by HIV-infected and controls. Given the degree of overlap in top NK-cell subsets between HIV-infected and HEU children (Table E4), overall, they appear to be more phenotypically similar than HEU children and controls.
FIG 6.
NK-cell markers distinguishing HIV-infected, HEU, and HIV-unexposed children. Frequency of NK-cell markers that were statistically significant between one cohort and both other cohorts are indicated as being increased (↑) or decreased (↓). All other markers that did not differ between a pair of cohorts were considered similar as illustrated.
DISCUSSION
Although MTCT rates are 1% to 2% in developed countries, pediatric HIV infection remains a significant problem in low-income countries.15 Given the likely importance of NK cells in HIV pathogenesis, maintaining NK-cell integrity contributes to controlling disease progression. However, reports about restoration of NK-cell subsets post-ART have been inconsistent and studies were limited in the NK-cell markers examined.9, 13, 35 Therefore, we comprehensively characterized NK-cell phenotype in both HEU and HIV-infected children to better understand NK-cell alterations. We identified a predominant activation phenotype in the NK cells of HIV-infected pediatric patients as well as several activating receptors that were inversely associated with CD4/CD8 T-cell ratio.
Chronic immune activation in HIV-infection is linked to shorter survival.26 Studies in treated adults have shown that persistent NK-cell activation is indicated by high CD38 and low FcR0γ.11, 36 To our knowledge, the work presented here is the first to demonstrate elevated NK-cell activating and stimulatory receptors in pediatric patients. Potential mechanisms behind immune activation include reactivation of infections such as CMV and hepatitis C because CMV intervention has been shown to reduce T-cell activation in HIV-infected patients.26, 37 Inflammation marked by IFN-I production could also activate NK cells.38 Similarly, destruction of mucosal membrane could release microbial products that activate innate immunity promoting inflammation, further driving systemic activation.26 Finally, although low-level viremia has been associated with chronic T-cell activation, a similar link has not been established with regard to NK cells but could explain some of the phenotype in our patient cohort (most of which are aviremic).
Most of the activating receptors were elevated in relation to healthy controls in our studies and not HEU. Thus, our data revealed greater similarities between HIV-infected and HEU subjects than with controls (Fig 6; Table E4). HEU infants mount immune responses that potentially protect them from HIV infection.33 Accordingly, higher KIR2DL1 and CD122 subsets in HEU individuals may suggest correlates of protection (Fig 2; Fig E7). However, a more activated and memory phenotype associated with increased mortality and morbidity has also been reported in HEU infants compared with controls.33 This might explain the phenotypic similarities between HEU and HIV-infected children. Exposure to HIV or viral particles in utero potentially creates an immune imprint that persists beyond infancy, which could in turn account for the increased disease burden in HEU adolescents.33 The contributions of ART and HIV exposure, however, are difficult to disentangle. Although we used Bland-Altman analysis to evaluate the usability of significant results from previous data, it may be inadequate for estimating the values of all controls, which needs to be explicitly stated as a limitation of our study.
Understanding NK-cell phenotype and function in the context of HIV is likely to be key to optimizing strategies for disease treatment. Although it is unclear whether an NK-cell activation phenotype leads to HIV persistence in treated patients, we identified an association between disease progression and several NK-cell activating receptors. CD4/CD8 ratio has been previously implicated in T-cell activation in vertical transmission but has not yet been shown in NK cells.39 Collectively, we established that most NK-cell markers do not differ among our cohorts. This is unsurprising because most HIV-infected children had low viral loads and were on ART for several years. One caveat is that these results may be confounded by lack of adherence to medication regimens and co-infection status. Because ours is a small cross-sectional study, prospective multicenter longitudinal studies are needed to assess long-term variation in NK-cell phenotype. At a minimum however, there are important signatures within the NK-cell populations of children that may provide insights into the underlying antiviral immune response and/or resistance to infection and disease progression.
Supplementary Material
Key messages.
NK cells persist in an activated state in perinatally HIV-infected children regardless of age.
Similarities in NK-cell phenotype between HIV-exposed and HIV-infected children suggest an impact on NK cells through HIV exposure.
Acknowledgments
We acknowledge all nurses involved in recruiting patients, especially Norma Cooper and Jane Head, as well as everyone else in the Center for Human Immunobiology and the Retrovirology clinic for their support. This work is dedicated to the memory of Dr William T. Shearer. He received the review of this manuscript and participated with great joy in the revisions. It was of great solace to us all knowing that he was able to spend his last days working on his passion, which was always his wish.
This work was supported by the National Institutes of Health (grant no. R01AI120989 to J.S.O.).
Abbreviations used
- ART
Antiretroviral therapy
- CMV
Cytomegalovirus
- DNAM-1
DNAX accessory molecule-1
- HEU
HIV-exposed but uninfected
- KIR
Killer cell immunoglobulin-like receptor
- MFI
Median fluorescent intensity
- MTCT
Mother-to-child transmission
- NK
Natural killer
Footnotes
Disclosure of potential conflict of interest: The authors declare that they have no relevant conflicts of interest.
REFERENCES
- 1.Lanier LL. NK cell recognition. Annu Rev Immunol 2005;23:225–74. [DOI] [PubMed] [Google Scholar]
- 2.Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat Immunol 2008;9:503–10. [DOI] [PubMed] [Google Scholar]
- 3.Iannello A, Debbeche O, Samarani S, Ahmad A. Antiviral NK cell responses in HIV infection, I: NK cell receptor genes as determinants of HIV resistance and progression to AIDS. J Leukoc Biol 2008;84:1–26. [DOI] [PubMed] [Google Scholar]
- 4.Fauci AS, Mavilio D, Kottilil S. NK cells in HIV infection: paradigm for protection or targets for ambush. Nat Rev Immunol 2005;5:835–43. [DOI] [PubMed] [Google Scholar]
- 5.Bjorkstrom NK, Ljunggren HG, Sandberg JK. CD56 negative NK cells: origin, function, and role in chronic viral disease. Trends Immunol 2010;31:401–6. [DOI] [PubMed] [Google Scholar]
- 6.Mavilio D, Lombardo G, Benjamin J, Kim D, Follman D, Marcenaro E, et al. Characterization of CD562/CD161 natural killer (NK) cells: a highly dysfunctional NK subset expanded in HIV-infected viremic individuals. Proc Natl Acad Sci U S A 2005;102:2886–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mavilio D, Benjamin J, Daucher M, Lombardo G, Kottilil S, Planta MA, et al. Natural killer cells in HIV-1 infection: dichotomous effects of viremia on inhibitory and activating receptors and their functional correlates. Proc Natl Acad Sci U S A 2003;100:15011–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alter G, Malenfant JM, Delabre RM, Burgett NC, Yu XG, Lichterfeld M, et al. Increased natural killer cell activity in viremic HIV-1 infection. J Immunol 2004;173:5305–11. [DOI] [PubMed] [Google Scholar]
- 9.Sondergaard SR, Aladdin H, Ullum H, Gerstoft J, Skinhoj P, Pedersen BK. Immune function and phenotype before and after highly active antiretroviral therapy. J Acquir Immune Defic Syndr 1999;21:376–83. [PubMed] [Google Scholar]
- 10.Azzoni L, Papasavvas E, Chehimi J, Kostman JR, Mounzer K, Ondercin J, et al. Sustained impairment of IFN-gamma secretion in suppressed HIV-infected patients despite mature NK cell recovery: evidence for a defective reconstitution of innate immunity. J Immunol 2002;168:5764–70. [DOI] [PubMed] [Google Scholar]
- 11.Lichtfuss GF, Cheng WJ, Farsakoglu Y, Paukovics G, Rajasuriar R, Velayudham P, et al. Virologically suppressed HIV patients show activation of NK cells and persistent innate immune activation. J Immunol 2012;189:1491–9. [DOI] [PubMed] [Google Scholar]
- 12.Azzoni L, Rutstein RM, Chehimi J, Farabaugh MA, Nowmos A, Montaner LJ. Dendritic and natural killer cell subsets associated with stable or declining CD41 cell counts in treated HIV-1-infected children. J Infect Dis 2005;191: 1451–9. [DOI] [PubMed] [Google Scholar]
- 13.Ballan WM, Vu BAN, Long BR, Loo CP, Michaelsson J, Barbour JD, et al. Natural killer cells in perinatally HIV-1-infected children exhibit less degranulation compared to HIV-1-exposed uninfected children and their expression of KIR2DL3, NKG2C, and NKp46 correlates with disease severity. J Immunol 2007;179: 3362–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mahapatra S, Mace EM, Minard CG, Forbes LR, Vargas-Hernandez A, Duryea TK, et al. High-resolution phenotyping identifies NK cell subsets that distinguish healthy children from adults. PLoS One 2017;12:e0181134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tiemessen CT, Kuhn L. Immune pathogenesis of pediatric HIV-1 infection. Curr HIV/AIDS Rep 2006;3:13–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bernstein HB, Kinter AL, Jackson R, Fauci AS. Neonatal natural killer cells produce chemokines and suppress HIV replication in vitro. AIDS Res Hum Retroviruses 2004;20:1189–95. [DOI] [PubMed] [Google Scholar]
- 17.Slyker JA, Lohman-Payne B, John-Stewart GC, Dong T, Mbori-Ngacha D, Tapia K, et al. The impact of HIV-1 infection and exposure on natural killer (NK) cell phenotype in Kenyan infants during the first year of life. Front Immunol 2012;3: 399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shearer WT, Jacobson DL, Yu W, Siberry GK, Purswani M, Siminski S, et al. Long-term pulmonary complications in perinatally HIV-infected youth. J Allergy Clin Immunol 2017;140:1101–11.e1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mathias CB. Natural killer cells in the development of asthma. Curr Allergy Asthma Rep 2015;15:500. [DOI] [PubMed] [Google Scholar]
- 20.Douglas SD, Durako SJ, Tustin NB, Houser J, Muenz L, Starr SE, et al. Natural killer cell enumeration and function in HIV-infected and high-risk uninfected adolescents. AIDS Res Hum Retroviruses 2001;17:543–52. [DOI] [PubMed] [Google Scholar]
- 21.Brunetta E, Fogli M, Varchetta S, Bozzo L, Hudspeth KL, Marcenaro E, et al. Chronic HIV-1 viremia reverses NKG2A/NKG2C ratio on natural killer cells in patients with human cytomegalovirus co-infection. AIDS 2010;24:27–34. [DOI] [PubMed] [Google Scholar]
- 22.Guma M, Cabrera C, Erkizia I, Bofill M, Clotet B, Ruiz L, et al. Human cytomegalovirus infection is associated with increased proportions of NK cells that express the CD94/NKG2C receptor in aviremic HIV-1–positive patients. J Infect Dis 2006; 194:38–41. [DOI] [PubMed] [Google Scholar]
- 23.K€orner C, Granoff ME, Amero MA, Sirignano MN, Vaidya SA, Jost S, et al. Increased frequency and function of KIR2DL1–3(+) NK cells in primary HIV-1 infection are determined by HLA-C group haplotypes. Eur J Immunol 2014;44: 2938–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jennes W, Verheyden S, Demanet C, Adje-Toure CA, Vuylsteke B, Nkengasong JN, et al. Cutting edge: resistance to HIV-1 infection among African female sex workers is associated with inhibitory KIR in the absence of their HLA ligands. J Immunol 2006;177:6588–92. [DOI] [PubMed] [Google Scholar]
- 25.Jennes W, Verheyden S, Mertens JW, Camara M, Seydi M, Dieye TN, et al. Inhibitory KIR/HLA incompatibility between sexual partners confers protection against HIV-1 transmission. Blood 2013;121:1157–64. [DOI] [PubMed] [Google Scholar]
- 26.Paiardini M, M€uller-Trutwin M. HIV-associated chronic immune activation. Immunol Rev 2013;254:78–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Serrano-Villar S, Deeks SG. CD4/CD8 ratio: an emerging biomarker for HIV. Lancet HIV 2015;2:e76–7. [DOI] [PubMed] [Google Scholar]
- 28.Lu W, Mehraj V, Vyboh K, Cao W, Li T, Routy J-P. CD4:CD8 ratio as a frontier marker for clinical outcome, immune dysfunction and viral reservoir size in virologically suppressed HIV-positive patients. J Int AIDS Soc 2015;18:20052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lopez-Verges S, Milush JM, Pandey S, York VA, Arakawa-Hoyt J, Pircher H, et al. CD57 defines a functionally distinct population of mature NK cells in the human CD56(dim)CD16(+) NK-cell subset. Blood 2010;116:3865–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nielsen CM, White MJ, Goodier MR, Riley EM. Functional significance of CD57 expression on human NK cells and relevance to disease. Front Immunol 2013;4: 422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perussia B. The cytokine profile of resting and activated NK cells. Methods 1996;9: 370–8. [DOI] [PubMed] [Google Scholar]
- 32.Foster SB, McIntosh K, Thompson B, Lu M, Yin W, Rich KC, et al. Increased incidence of asthma in HIV-infected children treated with highly active antiretroviral therapy in the National Institutes of Health Women and Infants Transmission Study. J Allergy Clin Immunol 2008;122:159–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Afran L, Garcia Knight M, Nduati E, Urban BC, Heyderman RS, Rowland-Jones SL. HIV-exposed uninfected children: a growing population with a vulnerable immune system? Clin Exp Immunol 2014;176:11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lima JF, Oliveira LM, Pereira NZ, Mitsunari GE, Duarte AJ, Sato MN. Distinct natural killer cells in HIV-exposed seronegative subjects with effector cytotoxic CD56(dim) and CD56(bright) cells and memory-like CD57(+)NKG2C(+) CD56(dim) cells. J Acquir Immune Defic Syndr 2014;67:463–71. [DOI] [PubMed] [Google Scholar]
- 35.Parato KG, Kumar A, Badley AD, Sanchez-Dardon JL, Chambers KA, Young CD, et al. Normalization of natural killer cell function and phenotype with effective anti-HIV therapy and the role of IL-10. AIDS 2002;16:1251–6. [DOI] [PubMed] [Google Scholar]
- 36.Leeansyah E, Zhou J, Paukovics G, Lewin SR, Crowe SM, Jaworowski A. Decreased NK cell FcRγ in HIV-1 infected individuals receiving combination antiretroviral therapy: a cross sectional study. PLoS One 2010;5:e9643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hunt PW, Martin JN, Sinclair E, Epling L, Teague J, Jacobson MA, et al. Valgan-ciclovir reduces T cell activation in HIV-infected individuals with incomplete CD4+ T cell recovery on antiretroviral therapy. J Infect Dis 2011;203:1474–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Welsh RM. Natural killer cells and interferon. Crit Rev Immunol 1984;5:55–93. [PubMed] [Google Scholar]
- 39.Sainz T, Serrano-Villar S, Diaz L, Gonzalez Tome MI, Gurbindo MD, de Jose MI, et al. The CD4/CD8 ratio as a marker T-cell activation, senescence and activation/exhaustion in treated HIV-infected children and young adults. AIDS 2013;27:1513–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.