Abstract
Objective
To determine the association between directly measured physical activity and hospitalisation, intensive care unit (ICU) admission, ventilation and mortality rates in patients with a confirmed diagnosis of COVID-19.
Methods
Directly measured physical activity data from 65 361 adult patients with a COVID-19 diagnosis from 19 March 2020 to 30 June 2021, were grouped by activity level: low (<60 min/week), moderate (60–149 min/week) and high activity (≥150 min/week). The association of physical activity levels and the risk of adverse outcomes was analysed using modified Poisson regression. We accounted for demographics and comorbidities including conditions known to influence COVID-19 outcomes, as well as patient complexity as measured by the Johns Hopkins Adjusted Clinical Group system. The regression approach was further validated with a Bayesian network model built off a directed acyclic graph.
Results
High physical activity was associated with lower rates of hospitalisation (risk ratio, RR 0.66, 95% CI 0.63 to 0.70), ICU admission (RR 0.59, 95% CI 0.52 to 0.66), ventilation (RR 0.55, 95% CI 0.47 to 0.64) and death (RR 0.58, 95% CI 0.50 to 0.68) due to COVID-19 than those who engaged in low physical activity. Moderate physical activity also was associated with lower rates of hospitalisation (RR 0.87, 95% CI 0.82 to 0.91), admission to ICU (RR 0.80, 95% CI 0.71 to 0.89), ventilation (RR 0.73, 95% CI 0.62 to 0.84) and death (RR 0.79, 95% CI 0.69 to 0.91).
Conclusions
Adults with high and moderate physical activity levels had significantly better outcomes than those with low activity when contracting COVID-19. The apparent protective effects of regular physical activity extended to those with concomitant chronic medical conditions.
Keywords: COVID-19, physical activity, exercise
Introduction
The health benefits of regular physical activity have been repeatedly and methodically demonstrated.1–3 Increasingly, and particularly in the context of the COVID-19 pandemic, there is interest in the potential effect of physical activity on the immune system, principally pertaining to shielding against communicable diseases.4 5
The largely immunoprotective effect of aerobic activity is multifaceted. It involves reductions in inflammation, the mobilisation of lymphocytes, alterations in cytokine profiles, enhanced immunosurveillance and the amelioration of psychological stress.6–8
Before the emergence of COVID-19, epidemiological data suggested that physically active people are less likely to report symptoms of upper respiratory illness and that regular physical activity can protect the host from many types of viral infections including influenza, rhinovirus and the reactivation of latent herpes viruses.9 Data support a clear inverse relationship between moderate physical activity and illness risk; regular physical activity has an anti-inflammatory influence mediated through multiple pathways; and regular physical activity improves immune regulation, delaying the onset of age-related dysfunction.10
Recent studies retrospectively evaluating cohorts of COVID-19-positive adults, have described the benefit of regular physical activity in decreasing the incidence of negative outcomes in confirmed cases of COVID-19.11–17 While these studies have made a convincing case for a protective effect of physical activity against severe COVID-19 outcomes, each has acknowledged as a limitation that physical activity levels in study participants had been self-reported. Self-reporting may result in regression dilution and an underestimation or over-estimation of the effect of an intervention.
In this study, we accessed the records of over 65 000 members of a South African private health plan and matched these with physical activity data captured by smart devices, clocked gym attendance and mass event participation via a healthy lifestyle behavioural change programme called Vitality, linked to the insurer. We tested the hypothesis that regular physical activity, even of low to moderate intensity, is associated with reductions in morbidity and mortality from COVID-19 infection, including where the presence of chronic medical conditions may typically worsen the prognosis. The Vitality programme allows for the measurement of activity type, frequency, duration and intensity. Vitality activity points are awarded for both duration and intensity of physical activity (see online supplemental 1) and reflected as equivalent weekly minutes of physical activity in table 1.
Table 1.
Distribution of patients per physical activity group
| Activity group | Patients | Equivalent measure |
| Low | 13 366 (20.4%) | 0–59 min per week |
| Moderate | 22 526 (34.5%) | 60–149 min per week |
| High | 29 469 (45.1%) | 150+ min per week |
bjsports-2021-105159supp001.pdf (103.5KB, pdf)
Methods
Study design
This was a retrospective observational study in which objectively recorded physical activity points data, awarded through the Vitality programme, were extracted for all patients engaged on the Vitality programme in the 2 years preceding the March 2020 lockdown in South Africa.
Setting
This study was conducted with anonymised Discovery Health and Vitality client data. Discovery Health Medical Scheme (DHMS) is the largest open medical plan in South Africa covering approximately 2.8 million beneficiaries. Vitality is a global health promotion and behavioural change programme that encourages and rewards members for engaging in healthy lifestyle choices. Vitality offers members incentives and rewards for taking steps towards a healthier lifestyle.18 Vitality members earn points for engaging in physical activities. A summary of point allocations is listed in online supplemental 1. Points are awarded for one fitness activity a day and recorded via smart devices, clocked gym attendance or recorded mass sports event participation. If a member completes more than one fitness activity in a day, then the higher scoring of the activities is awarded. The programme is voluntary and requires a monthly subscription the equivalent of US$18 for a single person, US$22 for two members of a family and US$26 for three members of a family.
Study cohort
Inclusion criteria consisted of all DHMS members aged 18 years and older with a concluded COVID-19 positive status between 19 March 2020 and 30 June 2021 who engaged in the Vitality health promotion programme. Only Vitality members were included in the study as their physical activity could be independently tracked. The patients were confirmed COVID-19 positive by PCR test. Concluded statuses included patients with a recovery (based on the National Institute for Communicable Diseases (NICD) definition of 14 days since receiving a positive PCR test result in the case of no admission occurring within this time period or 14 days since discharge from hospital for COVID-19 related treatment) or death where a death is associated with a recent COVID-19 diagnosis according to NICD criteria.19
Only patients on the Vitality programme who had at least 3 months in which they had been awarded physical activity points in the 2 years prior to the national lockdown that started in March 2020, were included in the study. This period was selected instead of the period directly prior to a diagnosis of COVID-19 to eliminate any bias that would then exist in the data due to restricted access to exercise facilities during lockdown. Only including members who had at least earned some physical activity points in the period ensured that all in the study were categorised into activity levels. This avoided the distortion that would have arisen by inferring that those members for whom there were no data are not active at all.
Exclusion criteria consisted of all patients with incomplete records, missing critical demographic information, or patients that had not yet reached a conclusion to their illness (ie, were still hospitalised or had not met the NICD criteria as outlined above for their episode). Members not on Vitality or with physical activity points in fewer than 3 months out of the preceding 24 months before the national lockdown were excluded. In addition, all vaccinated patients (either one or two doses) that contracted COVID-19 post their first vaccination date were excluded to remove the effects of the vaccine on COVID-19 outcomes. Patient selection is presented in figure 3. Based on the robust standard errors obtained from the modified Poisson regression model adjusting for other covariates, the sample sizes were calculated to achieve the upper CI of the risk ratio (RR) for the moderate physical activity vs low physical activity to be less than 1 with 90% assurance. The sample sizes required were 22 059, 42 352, 39 804, 63 031 for the admission, intensive care unit (ICU) admission, ventilation and death, respectively. Our final sample size of 65 361 exceeded each of these required sample sizes.
Physical activity points
Three categories of physical activity points (collected for a minimum of 3 months in the 2 years prior to lockdown) were created for this study, namely high activity (those engaging in >150 min of at least moderate-intensity physical activity per week), moderate activity (those engaging in between 60 and 149 min of at least moderate-intensity physical activity per week) and low activity (those engaging in less than 60 min of at least moderate-intensity physical activity per week) (see table 1). Where intensity was not recorded by a wearable device, estimation techniques were used to convert steps to minutes to facilitate the allocation of Vitality points.20 21
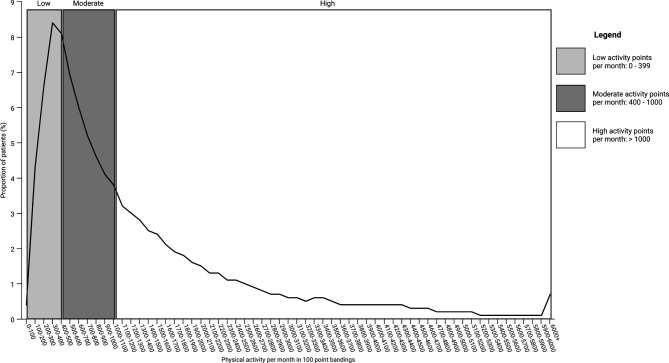
The graph (figure 1) below shows the distribution of patients by average monthly points and illustrates that the highest number (45%) are in the ‘high activity’ group which also showed the largest spread of physical activity engagement.
Figure 1.
Distribution of patients by average monthly points and activity group.
Table 1 shows the number of patients and equivalent measure for patients within each physical activity group:
Data analysis
We conducted modified Poisson regressions to estimate the RRs and their 95% CIs using a robust error variance procedure for the binary outcomes; (1) hospitalisation, (2) admission to ICU, (3) the need for ventilation and (4) death due to COVID-19 in relation to the three physical activity categories, with separate models for each of the outcome variables. Data were analysed using R (V.4.0.4 for Windows). The analysis was adjusted for age and sex and further adjusted for comorbidities and patient complexity as measured by the Johns Hopkins Adjusted Clinical Groups (ACG) Systems software.22 The ACG software assigns a six-level (low to high) simplified morbidity category termed Resource Utilisation Bands (RUB) to each patient. The six RUBs are formed by combining the ACG mutually exclusive cells that measure overall morbidity burden and comorbidities (online supplemental file 2). An extensive list of comorbidities are incorporated in the analysis, including but not limited to, all known comorbidities that influence COVID-19 outcomes (such as hypertension, diabetes, ischaemic heart disease, chronic lung disease, chronic renal failure and HIV). Chronic conditions were those present over the preceding 24 months with information derived from accessible patient billing/coding data. Body mass index (BMI) was available in approximately 50% of the study population. When adding BMI into the model, the overall RR for high activity vs low and similarly moderate activity versus low does not change. This could be because the associated morbidities of a high BMI such as diabetes and hypertension were taken into account. In the case of simultaneous measurements of these variables, it would be difficult to assess the role of BMI.
bjsports-2021-105159supp002.pdf (37.6KB, pdf)
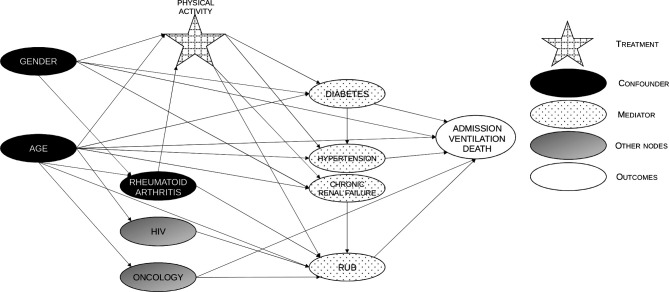
Another approach that was followed was to fit a Bayesian Network to the same dataset to validate the findings and outputs from the modified Poisson regression approach. The starting point was to construct a Directed Acyclic Graph (DAG) (see figure 2) representing the hypothesised causal relationships between the variables in the data. This allows for the explicit modelling of confounding variables—for instance, age may influence the amount of physical activity someone is able to or has the propensity to do, as well as affect the target variable of interest.
Figure 2.
Directed acyclic graph showing the implicitly assumed causal association between physical activity and risk of COVID-19 outcome. Confounders, potential mediators and associations are displayed. RUB, resource utilisation bands.
It also explicitly allows for mediating effects, such as the effect of physical activity, which can have a direct impact on the target outcome of interest while also having a mediated impact through affecting the risk of comorbidities that are relevant to COVID-19.
Variables were identified as confounders when literature supported both a causal link between the variable in question and the exposure (physical activity points), as well as a causal link between the variable and COVID-19 severity. Similarly, variables were identified as mediators in instances where the literature supported a causal relationship between the exposure (physical activity points) and the variable in question, as well as a causal link between the variable and COVID-19 severity.
This approach determines from the data a set of conditional probability distributions of the values that each node can take depending on the values of its parent nodes. While this does not prove causal relationships, by imposing hypothesised causal relationships between the variables, it makes these explicit.
The RRs implied by the Bayesian network model were consistent with those produced by the modified Poisson regression. Where they differ is mainly where the Bayesian network model was better at detecting non-linearities in effects. For example, it finds stronger effects for being over-60, having the higher RUBs or doing more physical activity. With concurrence found between the modified Poisson regression method and the Bayesian Net from the DAG approach, our methodology focuses on the outputs from the modified Poisson regression approach as this is more methodologically familiar and one that delivers well-understood effect measures for statistical significance, with comfort that the effect of confounders has been adjusted for in the study.
Patient and public involvement
Patients and the public were not involved in the design or conduct of this study. All data were anonymised.
Results
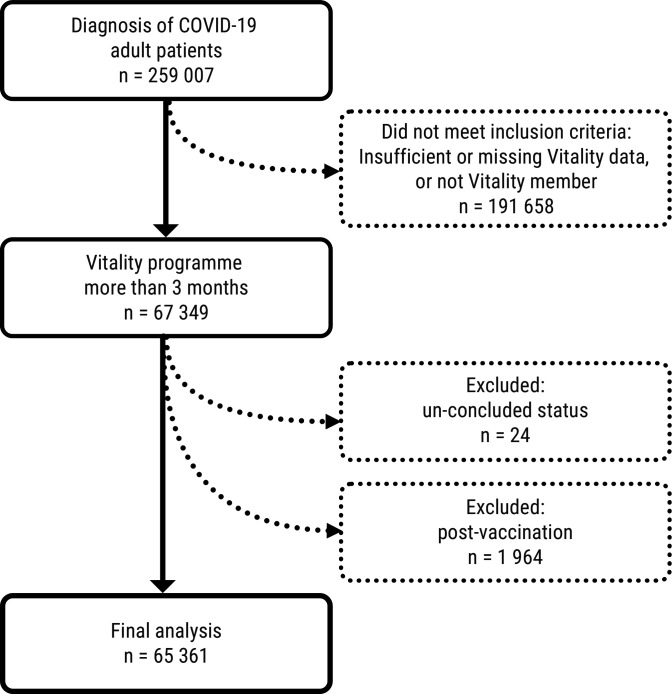
There were 259 007 patients on the DHMS with a confirmed diagnosis of COVID-19 at the start of our study. Only those members of the Vitality health promotion programme (n=67 349) were included in the study as their physical activity could be independently tracked. Those whose health statuses were not concluded (n=24) or who had been vaccinated against COVID-19 (n=1 964) were excluded. The final number included in our analysis was 65 361 (see figure 3).
Figure 3.
CONSORT diagram of patient selection. CONSORT, Consolidated Standards of Reporting Trials.
Demographics
The population had a median age of 41 years (SD 12.1) and males represented 51.8% of the cohort. The distribution of patients across the different included model factors are shown in table 2. The selected age groupings correspond with vaccine rollout age groupings in South Africa. A sensitivity analysis has been performed, which included age as a continuous variable in the modified Poisson regression instead of the age bands, to ascertain the reliability of the RRs for each of the reported COVID-19 outcomes. This analysis revealed stability and no major change in the RRs reported for the different levels of physical activity and hence can conclude that the age band categorisation does not lead to any residual confounding for the reported RRs.
Table 2.
Study patient characteristics
| Characteristics | Low activity | Moderate activity | High activity | Total |
| (N=13 366) | (N=22 526) | (N=29 469) | (N=65 361) | |
| Age band—no (%) | ||||
| 18–34 | 3822 (28.6) | 7149 (31.7) | 8823 (29.9) | 19 794 (30.3) |
| 35–49 | 5975 (44.7) | 9273 (41.2) | 13 341 (45.3) | 28 589 (43.7) |
| 50–59 | 2517 (18.8) | 4013 (17.8) | 5114 (17.4) | 11 644 (17.8) |
| 60+ | 1052 (7.9) | 2091 (9.3) | 2191 (7.4) | 5334 (8.2) |
| Sex—no (%) | ||||
| Female | 7658 (57.3) | 11 276 (50.1) | 12 564 (42.6) | 31 498 (48.2) |
| Male | 5708 (42.7) | 11 250 (49.9) | 16 905 (57.4) | 33 863 (51.8) |
| RUB—no (%) | ||||
| 0—Non-users | 835 (6.2) | 1549 (6.9) | 2322 (7.9) | 4706 (7.2) |
| 1—Healthy users | 1169 (8.7) | 2134 (9.5) | 3215 (10.9) | 6518 (10) |
| 2—Low morbidity | 2161 (16.2) | 3811 (16.9) | 5104 (17.3) | 11 076 (16.9) |
| 3—Moderate | 7176 (53.7) | 12 010 (53.3) | 15 651 (53.1) | 34 837 (53.3) |
| 4—High | 1492 (11.2) | 2280 (10.1) | 2534 (8.6) | 6306 (9.6) |
| 5—Very high | 533 (4) | 742 (3.3) | 643 (2.2) | 1918 (2.9) |
| Chronic conditions—no (%) | ||||
| Oncology | 308 (2.30) | 533 (2.37) | 616 (2.09) | 1457 (2.23) |
| Essential hypertension | 2115 (15.82) | 3254 (14.45) | 3284 (11.14) | 8653 (13.24) |
| Hypercholesterolaemia | 1440 (10.77) | 2322 (10.31) | 2426 (8.23) | 6188 (9.47) |
| Asthma | 809 (6.05) | 1299 (5.77) | 1827 (6.2) | 3935 (6.02) |
| Diabetes mellitus | 918 (6.87) | 1183 (5.25) | 931 (3.16) | 3032 (4.64) |
| Hypothyroidism | 432 (3.23) | 636 (2.82) | 746 (2.53) | 1814 (2.78) |
| HIV infection | 274 (2.05) | 427 (1.90) | 297 (1.01) | 998 (1.53) |
| Epilepsy | 165 (1.23) | 235 (1.04) | 291 (0.99) | 691 (1.06) |
| Rheumatoid arthritis | 151 (1.13) | 211 (0.94) | 211 (0.72) | 573 (0.88) |
| Conduction disorder | 104 (0.78) | 199 (0.88) | 207 (0.70) | 510 (0.78) |
| Congestive cardiac failure | 78 (0.58) | 100 (0.44) | 68 (0.23) | 246 (0.38) |
| Ulcerative colitis | 50 (0.37) | 81 (0.36) | 91 (0.31) | 222 (0.34) |
| Systemic lupus erythematosus | 57 (0.43) | 55 (0.24) | 53 (0.18) | 165 (0.25) |
| Chronic renal failure | 43 (0.32) | 57 (0.25) | 50 (0.17) | 150 (0.23) |
| Crohn’s disease | 25 (0.19) | 40 (0.18) | 55 (0.19) | 120 (0.18) |
| Cardiomyopathy | 25 (0.19) | 41 (0.18) | 39 (0.13) | 105 (0.16) |
| Multiple sclerosis | 15 (0.11) | 31 (0.14) | 34 (0.12) | 80 (0.12) |
| Bronchiectasis | 4 (0.03) | 10 (0.04) | 16 (0.05) | 30 (0.05) |
| Angina syndrome | 4 (0.03) | 5 (0.02) | 13 (0.04) | 22 (0.03) |
RUB, resource utilisation bands.
Poor outcomes (hospitalisations, ICU admissions, ventilation and deaths)
Outcomes and adjusted analyses
Among the study population, 11.1% were hospitalised, 2.4% were admitted to the ICU, 1.3% were ventilated and 1.6% died. Adjusting for demographic and clinical factors, tables 3–6 summarise the associations for each feature included in the model with each COVID-19 outcome in terms of the RRs estimated from modified Poisson regression models.
Table 3.
Risk ratios, 95% CIs and p values for all the features from the modified Poisson regression and Bayesian network models for admissions
| Modified poisson | Bayesian network | |||||||
| Admission risk ratio | Admission lower CI | Admission upper CI | Admission p value | Admission risk ratio | Admission lower CI | Admission upper CI | Admission p value | |
| Moderate activity versus low activity | 0.87 | 0.82 | 0.91 | <0.001 | 0.83 | 0.79 | 0.88 | <0.001 |
| High activity versus low activity | 0.66 | 0.63 | 0.70 | <0.001 | 0.59 | 0.55 | 0.62 | <0.001 |
| 35–49 vs below 35 | 2.39 | 2.21 | 2.58 | <0.001 | 2.43 | 2.25 | 2.61 | <0.001 |
| 50–59 vs below 35 | 3.29 | 3.03 | 3.57 | <0.001 | 3.90 | 3.61 | 4.21 | <0.001 |
| 60+vs below 35 | 4.04 | 3.69 | 4.42 | <0.001 | 6.26 | 5.78 | 6.77 | <0.001 |
| Males versus females | 1.87 | 1.79 | 1.97 | <0.001 | 1.83 | 1.75 | 1.92 | <0.001 |
| RUB 1 vs RUB 0 | 3.96 | 2.93 | 5.33 | <0.001 | 3.35 | 2.52 | 4.46 | <0.001 |
| RUB 2 vs RUB 0 | 6.56 | 4.95 | 8.71 | <0.001 | 6.52 | 4.99 | 8.52 | <0.001 |
| RUB 3 vs RUB 0 | 8.51 | 6.45 | 11.23 | <0.001 | 8.42 | 6.48 | 10.94 | <0.001 |
| RUB 4 vs RUB 0 | 19.04 | 14.39 | 25.18 | <0.001 | 20.17 | 15.50 | 26.25 | <0.001 |
| RUB 5 vs RUB 0 | 30.21 | 22.81 | 40.01 | <0.001 | 35.78 | 27.45 | 46.64 | <0.001 |
| Oncology | 1.29 | 1.17 | 1.42 | <0.001 | 1.17 | 1.02 | 1.33 | 0.026 |
| Hypercholesterolaemia | 0.90 | 0.85 | 0.96 | <0.001 | ||||
| Angina syndrome | 1.29 | 0.82 | 2.03 | 0.138 | ||||
| Congestive cardiac failure | 1.07 | 0.92 | 1.26 | 0.198 | ||||
| Cardiomyopathy | 0.89 | 0.66 | 1.20 | 0.222 | ||||
| Essential hypertension | 1.08 | 1.03 | 1.14 | 0.002 | 1.22 | 1.15 | 1.29 | <0.001 |
| Diabetes mellitus | 1.43 | 1.34 | 1.52 | <0.001 | 1.51 | 1.39 | 1.65 | <0.001 |
| Hypothyroidism | 1.02 | 0.91 | 1.13 | 0.380 | ||||
| Crohns disease | 0.77 | 0.49 | 1.23 | 0.139 | ||||
| Ulcerative colitis | 0.68 | 0.49 | 0.95 | 0.012 | ||||
| HIV | 1.39 | 1.23 | 1.56 | <0.001 | 1.15 | 0.98 | 1.35 | 0.096 |
| Systemic lupus erythematosus | 1.27 | 0.93 | 1.72 | 0.069 | ||||
| Rheumatoid arthritis | 1.19 | 1.04 | 1.37 | 0.005 | 1.62 | 1.36 | 1.93 | <0.001 |
| Chronic renal failure | 1.19 | 1.01 | 1.43 | 0.031 | 2.13 | 1.61 | 2.85 | <0.001 |
| Epilepsy | 0.93 | 0.78 | 1.10 | 0.184 | ||||
| Asthma | 1.08 | 1.01 | 1.16 | 0.016 | ||||
| Conduction disorder | 0.69 | 0.60 | 0.81 | <0.001 | ||||
| Multiple sclerosis | 1.05 | 0.69 | 1.58 | 0.413 | ||||
| Bronchiectasis | 1.05 | 0.67 | 1.64 | 0.414 | ||||
RUB, resource utilisation bands.
Table 4.
Risk ratios, 95% CIs and p values for all the features from the modified Poisson regression and Bayesian network models for ICU admissions
| Modified poisson | Bayesian network | |||||||
| ICU risk ratio | ICU lower CI | ICU upper CI | ICU p value | ICU risk ratio | ICU lower CI | ICU upper CI | ICU p value | |
| Moderate activity versus low activity | 0.80 | 0.71 | 0.89 | <0.001 | 0.70 | 0.62 | 0.79 | <0.001 |
| High activity versus low activity | 0.59 | 0.52 | 0.66 | <0.001 | 0.44 | 0.39 | 0.49 | <0.001 |
| 35–49 vs below 35 | 3.61 | 2.85 | 4.57 | <0.001 | 3.40 | 2.79 | 4.14 | <0.001 |
| 50–59 vs below 35 | 5.05 | 3.96 | 6.45 | <0.001 | 6.37 | 5.20 | 7.80 | <0.001 |
| 60+ vs below 35 | 6.03 | 4.67 | 7.79 | <0.001 | 12.80 | 10.45 | 15.70 | <0.001 |
| Males versus females | 2.75 | 2.44 | 3.10 | <0.001 | 2.73 | 2.44 | 3.06 | <0.001 |
| RUB 1 vs RUB 0 | 1.41 | 0.74 | 2.70 | 0.147 | 1.01 | 0.55 | 1.86 | 0.97 |
| RUB 2 vs RUB 0 | 2.50 | 1.43 | 4.39 | <0.001 | 2.10 | 1.26 | 3.50 | 0.005 |
| RUB 3 vs RUB 0 | 3.98 | 2.34 | 6.76 | <0.001 | 3.52 | 2.19 | 5.67 | <0.001 |
| RUB 4 vs RUB 0 | 19.12 | 11.22 | 32.59 | <0.001 | 18.68 | 11.61 | 30.05 | <0.001 |
| RUB 5 vs RUB 0 | 52.69 | 30.90 | 89.86 | <0.001 | 52.59 | 32.69 | 84.61 | <0.001 |
| Oncology | 1.56 | 1.28 | 1.90 | <0.001 | 1.35 | 1.01 | 1.79 | 0.040 |
| Hypercholesterolaemia | 0.80 | 0.71 | 0.91 | <0.001 | ||||
| Angina syndrome | 0.41 | 0.06 | 2.82 | 0.183 | ||||
| Congestive cardiac failure | 0.90 | 0.64 | 1.25 | 0.243 | ||||
| Cardiomyopathy | 0.94 | 0.54 | 1.64 | 0.428 | ||||
| Essential hypertension | 1.31 | 1.17 | 1.47 | <0.001 | 1.29 | 1.13 | 1.48 | <0.001 |
| Diabetes mellitus | 1.39 | 1.22 | 1.60 | <0.001 | 1.81 | 1.51 | 2.16 | <0.001 |
| Hypothyroidism | 0.85 | 0.65 | 1.11 | 0.117 | ||||
| Crohns disease | 0.56 | 0.21 | 1.53 | 0.123 | ||||
| Ulcerative colitis | 0.70 | 0.40 | 1.24 | 0.111 | ||||
| HIV | 1.22 | 0.92 | 1.61 | 0.091 | 1.21 | 0.85 | 1.74 | 0.294 |
| Systemic lupus erythematosus | 0.65 | 0.26 | 1.64 | 0.183 | ||||
| Rheumatoid arthritis | 1.48 | 1.12 | 1.94 | 0.003 | 2.32 | 1.65 | 3.26 | <0.001 |
| Chronic renal failure | 0.83 | 0.57 | 1.21 | 0.139 | 3.51 | 2.07 | 5.97 | <0.001 |
| Epilepsy | 0.75 | 0.51 | 1.10 | 0.063 | ||||
| Asthma | 1.01 | 0.86 | 1.19 | 0.470 | ||||
| Conduction disorder | 0.76 | 0.57 | 1.01 | 0.060 | ||||
| Multiple sclerosis | 0.64 | 0.24 | 1.70 | 0.188 | ||||
| Bronchiectasis | 1.22 | 0.52 | 2.83 | 0.318 | ||||
ICU, intensive care unit; RUB, resource utilisation bands.
Table 5.
Risk ratios, 95% CIs and p values for all the features from the modified Poisson regression and Bayesian network models for ventilation admissions
| Modified poisson | Bayesian network | |||||||
| Ventilation risk ratio | Ventilation lower CI | Ventilation upper CI | Ventilation p value | Ventilation Risk ratio | Ventilation lower CI | Ventilation upper CI | Ventilation p value | |
| Moderate activity versus low activity | 0.73 | 0.62 | 0.84 | <0.001 | 0.63 | 0.54 | 0.74 | <0.001 |
| High activity versus low activity | 0.55 | 0.47 | 0.64 | <0.001 | 0.39 | 0.33 | 0.46 | <0.001 |
| 35–49 vs below 35 | 3.51 | 2.54 | 4.84 | <0.001 | 3.48 | 2.64 | 4.58 | <0.001 |
| 50–59 vs below 35 | 4.90 | 3.51 | 6.84 | <0.001 | 7.10 | 5.37 | 9.39 | <0.001 |
| 60+ vs below 35 | 5.53 | 3.90 | 7.85 | <0.001 | 13.67 | 10.31 | 18.12 | <0.001 |
| Males versus females | 2.53 | 2.15 | 2.96 | <0.001 | 2.47 | 2.12 | 2.87 | <0.001 |
| RUB 1 vs RUB 0 | 2.03 | 0.80 | 5.18 | 0.069 | 2.26 | 0.82 | 6.26 | 0.159 |
| RUB 2 vs RUB 0 | 2.46 | 1.04 | 5.81 | 0.020 | 3.11 | 1.21 | 8.00 | 0.019 |
| RUB 3 vs RUB 0 | 4.18 | 1.86 | 9.40 | <0.001 | 5.62 | 2.29 | 13.78 | <0.001 |
| RUB 4 vs RUB 0 | 26.83 | 11.92 | 60.37 | <0.001 | 38.33 | 15.66 | 93.77 | <0.001 |
| RUB 5 vs RUB 0 | 87.82 | 38.98 | 197.84 | <0.001 | 131.74 | 53.91 | 321.95 | <0.001 |
| Oncology | 2.31 | 1.70 | 3.15 | <0.001 | 0.93 | 0.58 | 1.48 | 0.758 |
| Hypercholesterolaemia | 0.76 | 0.64 | 0.90 | <0.001 | ||||
| Angina syndrome | 0.77 | 0.11 | 5.20 | 0.398 | ||||
| Congestive cardiac failure | 0.78 | 0.48 | 1.28 | 0.156 | ||||
| Cardiomyopathy | 0.79 | 0.32 | 1.95 | 0.320 | ||||
| Essential hypertension | 1.44 | 1.24 | 1.67 | <0.001 | 1.31 | 1.09 | 1.57 | 0.003 |
| Diabetes mellitus | 1.26 | 1.04 | 1.53 | 0.008 | 1.48 | 1.14 | 1.93 | 0.003 |
| Hypothyroidism | 0.90 | 0.64 | 1.27 | 0.282 | ||||
| Crohns disease | 0.66 | 0.20 | 2.16 | 0.241 | ||||
| Ulcerative colitis | 0.43 | 0.14 | 1.31 | 0.065 | ||||
| HIV | 0.76 | 0.46 | 1.25 | 0.139 | 1.26 | 0.78 | 2.04 | 0.343 |
| Systemic lupus erythematosus | 0.73 | 0.26 | 2.05 | 0.281 | ||||
| Rheumatoid arthritis | 1.40 | 1.01 | 2.01 | 0.033 | 2.60 | 1.67 | 4.04 | <0.001 |
| Chronic renal failure | 0.64 | 0.36 | 1.14 | 0.053 | 4.25 | 2.19 | 8.25 | <0.001 |
| Epilepsy | 0.85 | 0.53 | 1.37 | 0.211 | ||||
| Asthma | 1.03 | 0.84 | 1.27 | 0.422 | ||||
| Conduction disorder | 0.61 | 0.40 | 0.95 | 0.013 | ||||
| Multiple sclerosis | 0.70 | 0.19 | 2.59 | 0.298 | ||||
| Bronchiectasis | 0.66 | 0.12 | 3.54 | 0.324 | ||||
RUB, resource utilisation band.
Table 6.
Risk ratios, 95% CIs and p values for all the features from the modified Poisson regression and Bayesian network models for death
| Modified poisson | Bayesian network | |||||||
| Death risk ratio | Death lower CI | Death upper CI | Death p value | Death risk ratio | Death lower CI | Death upper CI | Death p value | |
| Moderate activity versus low activity | 0.79 | 0.69 | 0.91 | <0.001 | 0.69 | 0.60 | 0.80 | <0.001 |
| High activity versus low activity | 0.58 | 0.50 | 0.68 | <0.001 | 0.43 | 0.37 | 0.50 | <0.001 |
| 35–49 vs below 35 | 4.34 | 3.03 | 6.22 | <0.001 | 4.32 | 3.19 | 5.86 | <0.001 |
| 50–59 vs below 35 | 7.58 | 5.25 | 10.94 | <0.001 | 10.38 | 7.66 | 14.06 | <0.001 |
| 60+ vs below 35 | 11.37 | 7.80 | 16.56 | <0.001 | 27.45 | 20.35 | 37.03 | <0.001 |
| Males versus females | 2.52 | 2.16 | 2.93 | <0.001 | 2.46 | 2.14 | 2.82 | <0.001 |
| RUB 1 vs RUB 0 | 3.47 | 1.18 | 10.22 | 0.012 | 2.48 | 0.98 | 6.26 | 0.054 |
| RUB 2 vs RUB 0 | 5.38 | 1.95 | 14.83 | <0.001 | 4.50 | 1.92 | 10.54 | <0.001 |
| RUB 3 vs RUB 0 | 7.48 | 2.79 | 20.08 | <0.001 | 6.61 | 2.90 | 15.04 | <0.001 |
| RUB 4 vs RUB 0 | 31.69 | 11.76 | 85.40 | <0.001 | 28.57 | 12.53 | 65.16 | <0.001 |
| RUB 5 vs RUB 0 | 98.43 | 36.51 | 265.32 | <0.001 | 107.35 | 47.18 | 244.30 | <0.001 |
| Oncology | 1.03 | 0.85 | 1.25 | 0.393 | 2.33 | 1.77 | 3.06 | <0.001 |
| Hypercholesterolaemia | 0.80 | 0.69 | 0.94 | 0.002 | ||||
| Angina syndrome | 1.09 | 0.27 | 4.48 | 0.454 | ||||
| Congestive cardiac failure | 1.18 | 0.84 | 1.65 | 0.183 | ||||
| Cardiomyopathy | 0.75 | 0.38 | 1.47 | 0.199 | ||||
| Essential hypertension | 1.27 | 1.11 | 1.46 | <0.001 | 1.37 | 1.16 | 1.61 | <0.001 |
| Diabetes mellitus | 1.61 | 1.37 | 1.89 | <0.001 | 3.00 | 2.49 | 3.61 | <0.001 |
| Hypothyroidism | 0.90 | 0.67 | 1.22 | 0.246 | ||||
| Crohns disease | 0.30 | 0.04 | 2.13 | 0.110 | ||||
| Ulcerative colitis | 0.67 | 0.32 | 1.39 | 0.138 | ||||
| HIV | 1.67 | 1.19 | 2.34 | 0.002 | 1.04 | 0.64 | 1.69 | 0.866 |
| Systemic lupus erythematosus | 1.68 | 0.82 | 3.43 | 0.080 | ||||
| Rheumatoid arthritis | 1.74 | 1.29 | 2.35 | <0.001 | 2.11 | 1.35 | 3.29 | 0.001 |
| Chronic renal failure | 1.21 | 0.82 | 1.79 | 0.164 | 2.04 | 0.85 | 4.9 | 0.113 |
| Epilepsy | 1.02 | 0.70 | 1.49 | 0.416 | ||||
| Asthma | 0.89 | 0.72 | 1.10 | 0.169 | ||||
| Conduction disorder | 0.81 | 0.59 | 1.11 | 0.109 | ||||
| Multiple sclerosis | 0.59 | 0.20 | 1.72 | 0.164 | ||||
| Bronchiectasis | 1.75 | 0.77 | 4.00 | 0.097 | ||||
RUB, resource utilisation band.
After accounting for demographic factors and other risk factors, patients in the high activity band have a 34% lower risk of admission, a 41% lower risk of ICU admission, a 45% lower risk of requiring ventilation and a 42% lower risk of death, compared with those with low levels of activity. Males are at greater risk for a severe COVID-19 outcome. As patient complexity increases, the risk of a severe COVID-19 outcome significantly increases with RUB 5 (Very High Morbidity) patients having a 30 times higher risk of admission, a 53 times higher risk of ICU admission, an 88 times higher risk of requiring ventilation and a 98 times higher risk of dying compared with an RUB 0 (non-healthcare user) patient. Patients with chronic conditions are also at greater risk of severe COVID-19 outcomes as can be seen in tables 3–6.
A summary of the goodness-of-fit/performance metrics for all models are presented in online supplemental 3.
bjsports-2021-105159supp003.pdf (29.6KB, pdf)
Discussion
Main findings
To our knowledge, this is the first study to examine adverse outcomes in a large cohort of COVID-19 positive patients using directly measured physical activity data. It is also the first such study outside of the western world or Asia, in a region in which a number of SARS-CoV-2 strains including the Beta variant were prevalent over the study period.23 Direct measures of physical activity allow for less regression dilution and more accuracy than previous studies that used self-reported activity levels and confirm the potentially protective effect of regular physical activity against poor outcomes in COVID-19. Moderate to high levels of regular activity show a distinct beneficial association compared with a low level of physical activity. Even levels of physical activity below recommended guidelines of at least 150 min of moderate physical activity per week may have significant benefits in preventing hospitalisation, ICU admission, ventilation and death as demonstrated by the associations and reductions in risk.
Those who were in the high activity group were much less likely than those in the low activity group to be hospitalised, admitted to the ICU, be ventilated or die from COVID-19. Even those who were in the moderate activity group had a significantly lower risk of poor outcomes compared with those in the low activity group, suggesting that regular physical activity, even below recommended weekly guidelines for health, could have substantial benefits.
The findings of this study confirm those from studies in similar large COVID-19 cohorts examining the association between regular physical activity and poor disease outcomes but with the added input of directly measured and recorded physical activity levels in the months preceding lockdown.11 12 The directly recorded measures strengthen the findings and may also explain the differences between our RRs and those of previous studies.
While our findings were comparable to two similar studies of large cohorts,11 12 specific differences to previous findings, some explained by the direct measures versus self-reported physical activity, include:
The demonstration of a more significant protective effect even of moderate levels of physical activity (60–149 mins per week).
A greater protective effect in essential hypertension, diabetes mellitus and chronic renal failure.
A potential protective benefit in HIV positive patients (not previously evaluated).
A potential protective effect in rheumatoid arthritis (not previously evaluated).
In addition, during the 16 months of the study, this COVID-19 positive cohort was specifically exposed to the infectious Beta variant found to have emerged in South Africa in May 2020, as well as a number of South African-specific lineages,24 25 before the Delta variant became prevalent globally and in South Africa.23 This suggests a potential protective effect of physical activity against a spectrum of strains and is significant in the light of the most recent ‘Omicron’ variant also first described in South Africa.23
Restrictions on physical activity
Concerningly, and in contrast to the association of regular physical activity and more positive outcomes in COVID-19 from these and other data, there is emerging evidence of significant decreases in physical activity levels associated with COVID-19 lockdowns becoming more permanently entrenched.26
The mortality rate from this pandemic is significant with approximately 5.5 million global deaths being recorded.27 Patients requiring medical attention have also chosen not to attend medical facilities for fear of infection with COVID-19. Alterations in patient behaviour and a reprioritising of elective care have led to further deaths.28 In the light of strong evidence for the immune benefits of physical activity, it is concerning that physical activity levels have decreased significantly during the pandemic.29 In this context, it seems apparent that we should encourage lifestyle choices and behaviours that mitigate the effects of COVID-19.
Physical activity guidelines
Various international bodies have recommended similar minimum daily physical activity levels.30 31 Guidelines are at least 150 min (2 hours and 30 min) to 300 min (5 hours) a week of moderate-intensity or 75 min (1 hour and 15 min) to 150 min (2 hours and 30 min) a week of vigorous-intensity aerobic physical activity, or an equivalent combination of moderate-intensity and vigorous-intensity aerobic activity.
Of our cohort, 45.1% achieved those minimum requirements. For this subgroup the significant benefits of physical activity as a strong shield against adverse outcomes of COVID-19 from our data are clear. But even those who were physically active for less than the recommended weekly amount, benefitted. As such, everyone should be encouraged to be active, even if limited by time or access. Public health messaging should include the immune benefits of physical activity, and indeed the negative effects of sedentary behaviour.
We also acknowledge that Vitality points recorded leisure time physical activity and that other activities such as occupational and daily living activities may also contribute to better health.
Vaccination
In this study, we chose to exclude patients who had been vaccinated against COVID-19 (either partially or fully vaccinated as seen in figure 3) so as not to bias the results. With vaccination becoming more widespread and accessible, the outcomes of severe COVID-19 are expected to change. Encouragingly, investigations into the effects of physical activity on vaccine effectiveness, suggest improved effectiveness among those who are consistently physically active.32
Strengths and limitations
The study’s main strengths are the large number of COVID-19 patients with directly measured recording of physical activity data (as opposed to self-reporting), for at least 3 months in a 24-month period immediately before lockdown, a location that exposes the cohort to different virus strains compared with previous studies, and the accurate measure of severe outcomes. Moreover, this study included analysis of the effects of physical activity on COVID-19 positive patients with HIV and rheumatoid arthritis, which has not been previously evaluated.
Limitations include the cohort all being members of a medical insurance plan implying some selection bias based on affordability, reflecting a certain socioeconomic status that facilitates more leisure time physical activity,33 so the findings may not be entirely generalisable to a broader population. The cohort also lacks data on sociodemographic criteria such as education, income, marital status and race as well as potentially influential behavioural risk factors such as smoking and diet. Measurement bias in exposure, unmeasured confounders and time-varying confounding affected by prior exposure for example, BMI, diabetes and hypertension are also acknowledged as potentially limiting.34 For workouts in which intensity was not collected, physical activity points had to be converted to intensity minutes which required estimation. Finally, we acknowledge the possible effect of sparse data bias in both Poisson regression and Bayesian network models in some groups where the outcome (eg, death) is uncommon.35
Conclusion
The 45% of this cohort who engaged in higher levels of physical activity had a significantly better chance of avoiding negative outcomes (admission, ICU admission, ventilation and death) compared with those with low physical activity levels. Even those with moderate levels of physical activity, well below recommended guidelines, were associated with more positive outcomes. While older age, male sex, having a diagnosis of hypertension or type 2 diabetes increases the likelihood of poor COVID-19 outcomes, exercising at high levels may have an even more significant effect than in healthy individuals.
More education about the potential immune benefits of regular physical activity in the context of communicable diseases is needed. Physical activity of even low intensity is beneficial with engagement in physical activity of at least 150 min per week of moderate to vigorous physical activity providing optimal protection. More accurate measures of physical activity are of benefit in clinical practice and the effects of regular physical activity have a positive influence on public health services as well as the individual. As a means of improving outcomes and decreasing the burden on healthcare systems, regular physical activity in both healthy individuals and those with chronic medical conditions should be encouraged at all times and facilitated, not restricted, during a pandemic. In the context of a persistent pandemic and the emergence of new variants globally, evidence of a protective effect against more severe outcomes in communicable disease such as COVID-19 should lead to enhanced efforts at promoting physical activity at recommended levels as an adjunct to vaccination and other preventive measures, especially for those at high risk. Even small steps afford a strong shield.
Key messages.
What is already known on this topic?
Patient-reported physical activity data from the northern hemisphere suggest that regular physical activity has a protective effect against adverse outcomes from COVID-19.
What this study adds?
This is the first study (1) using objectively measured physical activity data to demonstrate a positive association between physical exercise and protection against adverse outcomes from COVID-19 (2) the data are from an African country where different SARS-CoV-2 variants were prevalent (3) demonstrates a protective effect of physical activity even at less than recommended levels (4) emphasises the benefits of physical activity across a range of chronic medical conditions.
How this might influence research, policy or practice
The study reinforces the need to facilitate physical activity during pandemics as part of government policy and promote its beneficial effects in medical practice across all patient cohorts.
Footnotes
Twitter: @janesthornton, @jonpatricios
Contributors: LS and DP conceived the format of the paper. LS led the actuarial and statistical analysis of the cohort data with oversight from Y-HC, SS and JST. RB and RTS conducted the literature review and formulated the tables. JP coordinated the interinstitutional collaboration, drafted the initial manuscript and led the subsequent iterations. SR and SH developed the Bayesian Network models on the data. SR, SH, LS, RB and RTS formulated the tables and graphs. All authors reviewed and edited each iteration of the manuscript and approved the final version for submission. All authors also accept responsibility for the content. LS as lead author has access to all the data while JP acts as guarantor.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: LS, DP, SR and SH are employed by Discovery Health; JP and JST are editors of BJSM.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
No data are available. Not applicable.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
Permission for use of DHMS anonymised medical and physical activity data was obtained from the Research Governance Committee of Discovery Health. Ethical approval was obtained from the Human Research Ethics Committee of the University of the Witwatersrand, Johannesburg (clearance certificate number M210725.)
References
- 1. Myers J. Exercise and cardiovascular health. Circulation 2003;107:e2–5. 10.1161/01.CIR.0000048890.59383.8D [DOI] [PubMed] [Google Scholar]
- 2. Joyner MJ, Green DJ. Exercise protects the cardiovascular system: effects beyond traditional risk factors. J Physiol 2009;587:5551–8. 10.1113/jphysiol.2009.179432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pinckard K, Baskin KK, Stanford KI. Effects of exercise to improve cardiovascular health. Front Cardiovasc Med 2019;6:69. 10.3389/fcvm.2019.00069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Woods JA, Hutchinson NT, Powers SK, et al. The COVID-19 pandemic and physical activity. Sports Med Health Sci 2020;2:55–64. 10.1016/j.smhs.2020.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cho D-H, Lee SJ, Jae SY, et al. Physical activity and the risk of COVID-19 infection and mortality: a nationwide population-based case-control study. J Clin Med 2021;10:1539. 10.3390/jcm10071539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Simpson RJ, Kunz H, Agha N. Exercise and the regulation of immune functions. In: Bouchard C, ed. Progress in molecular biology and translational science. 135. Burlington: Academic Press, 2015: 355–80. [DOI] [PubMed] [Google Scholar]
- 7. Simpson RJ, Katsanis E. The immunological case for staying active during the COVID-19 pandemic. Brain Behav Immun 2020;87:6–7. 10.1016/j.bbi.2020.04.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zbinden-Foncea H, Francaux M, Deldicque L, et al. Does high cardiorespiratory fitness confer some protection against proinflammatory responses after infection by SARS-CoV-2? Obesity 2020;28:1378–81. 10.1002/oby.22849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Simpson RJ, Hussain M, Baker F, et al. Cardiorespiratory fitness is associated with better control of latent herpesvirus infections in a large ethnically diverse community sample: evidence from the Texas City stress and health study. Brain Behav Immun 2017;66:e35. 10.1016/j.bbi.2017.07.128 [DOI] [Google Scholar]
- 10. Nieman DC, Wentz LM. The compelling link between physical activity and the body’s defense system. J Sport Health Sci 2019;8:201–17. 10.1016/j.jshs.2018.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sallis R, Young DR, Tartof SY, et al. Physical inactivity is associated with a higher risk for severe COVID-19 outcomes: a study in 48 440 adult patients. Br J Sports Med 2021;55:1099–105. 10.1136/bjsports-2021-104080 [DOI] [PubMed] [Google Scholar]
- 12. Lee SW, Lee J, Moon SY, et al. Physical activity and the risk of SARS-CoV-2 infection, severe COVID-19 illness and COVID-19 related mortality in South Korea: a nationwide cohort study. Br J Sports Med 2021. 10.1136/bjsports-2021-104203. [Epub ahead of print: 22 Jul 2021]. [DOI] [PubMed] [Google Scholar]
- 13. Tavakol Z, Ghannadi S, Tabesh MR, et al. Relationship between physical activity, healthy lifestyle and COVID-19 disease severity; a cross-sectional study. Z Gesundh Wiss 2021:1–9. 10.1007/s10389-020-01468-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Brandenburg JP, Lesser IA, Thomson CJ, et al. Does higher self-reported cardiorespiratory fitness reduce the odds of hospitalization from COVID-19? J Phys Act Health 2021;18:782–8. 10.1123/jpah.2020-0817 [DOI] [PubMed] [Google Scholar]
- 15. Cunningham GB. Physical activity and its relationship with COVID-19 cases and deaths: analysis of U.S. counties. J Sport Health Sci 2021;10:570–6. 10.1016/j.jshs.2021.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Christensen RAG, Arneja J, St Cyr K, et al. The association of estimated cardiorespiratory fitness with COVID-19 incidence and mortality: a cohort study. PLoS One 2021;16:e0250508. 10.1371/journal.pone.0250508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. de Souza FR, Motta-Santos D, Dos Santos Soares D, et al. Association of physical activity levels and the prevalence of COVID-19-associated hospitalization. J Sci Med Sport 2021;24:913–8. 10.1016/j.jsams.2021.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Patel DN, Nossel C, Patricios J, et al. Bright spots, physical activity investments that work: vitality active Rewards-a smartphone APP that incentivises programme members to be physically active. Br J Sports Med 2018;52:1494–6. 10.1136/bjsports-2018-099271 [DOI] [PubMed] [Google Scholar]
- 19. National Institute for Communicable Diseases . Available: https://www.nicd.ac.za/wp-content/uploads/2020/05/COVID_Recovered-Definition.pdf [Accessed 10 Oct 2021].
- 20. Hoeger WWK, Bond L, Ransdell L, et al. One-mile step count at walking and running speeds. ACSMs Health Fit J 2008;12:14–19. 10.1249/01.FIT.0000298459.30006.8d [DOI] [Google Scholar]
- 21. Pedometer Conversion Chart . Gundersen health system. Available: https://www.gundersenhealth.org/health-wellness/move/physical-activity/minutes-in-motion/pedometer-conversion-chart/ [Accessed 13 Jan 2022].
- 22. Johns Hopkins ACG® system. Available: https://www.hopkinsacg.org [Accessed 10 October 2021].
- 23. Nextstrain . Genomic epidemiology of novel coronavirus - Global subsampling. Available: https://nextstrain.org/ncov/gisaid/global?c=clade_membership&dmax [Accessed 23 October 2021].
- 24. World Health Organization . Currently designated variants of concern. Available: https://www.who.int/en/activities/tracking-SARS-CoV-2-variants/ [Accessed 23 October 2021].
- 25. Gimati YMT, Alrasheed AA, Bashir AM. Effect of a COVID-19 on social, psychological, economic and health conditions in Libya. J App Sci Eng Tech Ed 2021;3:160–70. [Google Scholar]
- 26. Dunton GF, Do B, Wang SD. Early effects of the COVID-19 pandemic on physical activity and sedentary behavior in children living in the U.S. BMC Public Health 2020;20:1351. 10.1186/s12889-020-09429-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Johns Hopkins University of Medicine . Coronavirus Resource Center. Available: https://coronavirus.jhu.edu/map.html [Accessed 8 Jan 2022].
- 28. Department of Health and Social Care, Office for National Statistics, Government Actuary’s Department and Home Office . Direct and indirect impacts of COVID-19 on excess deaths and morbidity: Executive summary, 2020. Available: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/918738/S0650_Direct_and_Indirect_Impacts_of_COVID-19_on_Excess_Deaths_and_Morbidity.pdf [Accessed 26 Aug 2021].
- 29. Tison GH, Avram R, Kuhar P, et al. Worldwide effect of COVID-19 on physical activity: a descriptive study. Ann Intern Med 2020;173:767–70. 10.7326/M20-2665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. World Health Organization . WHO guidelines on physical activity and sedentary behaviour. Available: https://www.who.int/publications/i/item/9789240015128 [Accessed 10 Oct 2021].
- 31. US Department of Health and Human Services . Physical activity guidelines for Americans. 2nd edn, 2021. https://health.gov/sites/default/files/2019-09/Physical_Activity_Guidelines_2nd_edition.pdf [Google Scholar]
- 32. Ledo A, Schub D, Ziller C, et al. Elite athletes on regular training show more pronounced induction of vaccine-specific T-cells and antibodies after tetravalent influenza vaccination than controls. Brain Behav Immun 2020;83:135–45. 10.1016/j.bbi.2019.09.024 [DOI] [PubMed] [Google Scholar]
- 33. Stalsberg R, Pedersen AV. Are differences in physical activity across socioeconomic groups associated with choice of physical activity variables to report? Int J Environ Res Public Health 2018;15:922. 10.3390/ijerph15050922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mansournia MA, Etminan M, Danaei G, et al. Handling time varying confounding in observational research. BMJ 2017;359:j4587. 10.1136/bmj.j4587 [DOI] [PubMed] [Google Scholar]
- 35. Greenland S, Mansournia MA, Altman DG. Sparse data bias: a problem hiding in plain sight. BMJ 2016;352:i1981. 10.1136/bmj.i1981 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bjsports-2021-105159supp001.pdf (103.5KB, pdf)
bjsports-2021-105159supp002.pdf (37.6KB, pdf)
bjsports-2021-105159supp003.pdf (29.6KB, pdf)
Data Availability Statement
No data are available. Not applicable.