Abstract
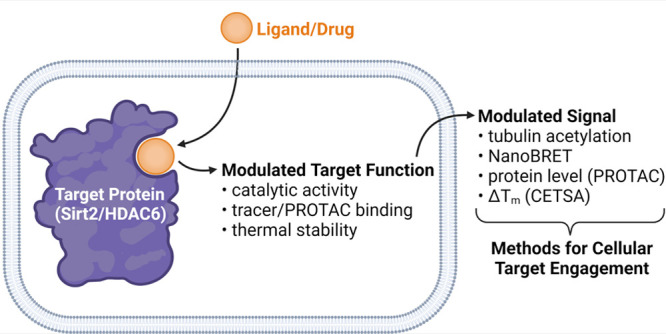
The tubulin deacetylases Sirt2 and HDAC6 have been associated with the development of various diseases. Herein, we discuss recent approaches that enable cellular target engagement studies for these deacetylases and thus play a critical role in the evaluation of small molecule inhibitors of Sirt2 or HDAC6 as potential therapeutic agents.
Keywords: epigenetics, target engagement, tubulin acetylation, NanoBRET, PROTACs
Both histone deacetylase 6 (HDAC6) and Sirtuin 2 (Sirt2) are protein deacylases that cleave off acetyl as well as other acyl groups from the ε-amino group of lysines in their substrate proteins. While HDAC6 is a Zn2+-dependent lysine deacylase and belongs to class IIb of HDACs, Sirt2 features an NAD+-dependent catalytic mechanism and has been classified as a class III HDAC, the so-called Sirtuin family. Despite the fact that both deacylases have been assigned as histone deacetylases (HDACs), they share acetylated α-tubulin (α-tubulin K40ac) as a major substrate and are hence frequently referred to as tubulin deacetylases.1,2 Dysregulation of both Sirt2 and HDAC6 activity has been associated with the pathogenesis of cancer, inflammation, and neurodegeneration, thus making these two enzymes promising targets for pharmaceutical intervention. This has prompted intense efforts in the development of small molecule inhibitors of Sirt2 and HDAC6, which are reviewed elsewhere.3,4 A critical step in preclinical drug discovery is the assessment of the interactions between a drug and its protein target in a physiologically relevant cellular environment.5 This step, also referred to as cellular target engagement, is highly important for successfully delivering compounds with the desired biological and ultimately clinical effects, as the on-target activity of small molecules can be changed significantly when transitioning from a biochemical to a cellular environment. A loss of activity in a cellular environment can be attributed to various factors, including low cell permeability, compound efflux, off-target protein binding, or a change in target protein’s structure/accessibility. Herein, we review different approaches that have recently been applied to study cellular target engagement for Sirt2 and HDAC6, thereby playing a critical role in the evaluation of small molecule inhibitors for these two tubulin deacetylases.
As already mentioned, Sirt2 and HDAC6 are tubulin deacetylases. Therefore, α-tubulin acetylation has widely been applied to confirm cellular inhibition of Sirt2- or HDAC6-mediated deacetylation. The increase in tubulin acetylation is most commonly detected with antibody-based techniques (e.g., Western blot, ELISA, immunofluorescence microscopy). An important benefit of using tubulin acetylation as a readout for cellular Sirt2 or HDAC6 target engagement is that this method does not require any context-specific modification of the targeted proteins. However, α-tubulin acetylation is influenced not only by Sirt2 and HDAC6 activity but also by other factors such as α-tubulin N-acetyltransferase (ATAT1) activity, oxidative stress or high glucose levels. Thus, effects of certain compounds on tubulin acetylation must not necessarily be a consequence of cellular Sirt2 or HDAC6 inhibition. Furthermore, the overall effect of selective Sirt2 inhibition on tubulin acetylation is not highly pronounced and often challenging to demonstrate via Western blot, due to its low dynamic range of detection. This might be one reason, why several recently published studies preferred immunofluorescence microscopy over Western blotting to prove cellular inhibition of Sirt2-mediated α-tubulin deacetylation.6,7 Additionally, antibody-based methods for the detection of α-tubulin acetylation are time and labor intensive because of their heterogeneous assay protocols, thereby limiting the throughput of these methods. Due to the aforementioned drawbacks of using α-tubulin acetylation as a readout for cellular inhibition of Sirt2- or HDAC6-mediated deacetylation, several alternative methods have been recently established to demonstrate cellular target engagement for Sirt2 and HDAC6.
Cellular thermal shift assays (CETSA) enable the assessment of cellular target engagement by quantifying the changes in the thermal stability of a targeted protein upon ligand binding in intact cells. Similar to the aforementioned methods based on α-tubulin acetylation, CETSA is a label-free technique and is usually combined with an immunosorbent assay (e.g., Western blot) for detecting the amount of stabile protein remaining in solution at a given temperature. In contrast to α-tubulin acetylation, which can be influenced by various factors (see above), ligand-induced shifts of thermal protein stability are a direct and exclusive consequence of ligand-target-binding interactions. Due to its broad applicability, the CETSA technique has revolutionized cell-based target engagement studies and has recently been successfully used for Sirt27 as well as HDAC6.8 However, it should be noted that not all ligand-protein interactions result in a significant change of thermal protein stability, depending on the nature of binding interaction. Thus, negative results from CETSA-based target engagement studies should be verified by an orthogonal method, to rule out false negatives. More recent approaches to increase the throughput of CETSA, so-called high-throughput CETSA (HT-CETSA), use methods other than Western blot (e.g., β-galactosidase and NanoLuciferase reporters, AlphaLISA) for protein detection, but as of now they have not been applied for Sirt2- or HDAC6-based target engagement studies.
The approach of targeted protein degradation, induced by means of so-called proteolysis-targeting chimeras (PROTACs), has recently gained much traction due to a number of key advantages compared to standard inhibition of protein function by small molecules. Two major advantages of PROTACs are their catalytic mode of action and a durable inhibition of protein function as a consequence of irreversible target protein degradation. Besides these pharmacological benefits, which have implications for basic research and clinical applications, PROTACs can also be used as molecular tools to study cellular target engagement. By displacing the PROTAC from its target protein binding site, an unlabeled small molecule competitor can prevent PROTAC-induced protein degradation, which can be assessed via Western blot analysis.9,10 This experiment is commonly performed in the course of PROTAC validation, but it can just as well be used as a method for cellular target engagement. Similar to tubulin acetylation- and CETSA-based approaches for cellular target engagement, the PROTAC-based protocol does not require specific protein modifications. Of course, the availability of PROTACs that are able to induce a significant reduction of target protein levels is required for such an approach. In the case of Sirt29 and HDAC6,10,11 potent and selective degraders have already been reported, thereby laying the key basis for PROTAC-mediated target engagement studies for these two deacylases. Of note, ligands showing noncompetitive binding toward the employed PROTAC cannot be detected with the approach described above. For ligands that show a reduction of PROTAC-mediated protein degradation, an orthogonal target engagement assay should be performed, as an inhibition of the employed E3 ligase or the proteasome might lead to false positive results.
The NanoBRET technique is proximity-based and relies on bioluminescence resonance energy transfer (BRET) from a donor (e.g., Nanoluciferase (Nluc)-labeled fusion protein) to an acceptor (e.g., fluorescently labeled ligand). Thus, the NanoBRET technology requires both a modified ligand and a modified, non-native target protein (Table 1). If used in a displacement setup, the binding of an unlabeled small molecule ligand to the targeted binding site can be detected via the displacement of the fluorescent tracer, thereby resulting in a reduced BRET signal. As with all tracer-dependent assay techniques, there is a potential risk of false negative results for ligands that do not show competitive binding behavior toward the tracer molecule. In contrast to the other methods applied for studying cellular target engagement for Sirt2 or HDAC6 (see above), NanoBRET assays can be performed in a microtiter plate format following a straightforward homogeneous assay protocol, which does not require any antibodies, cell lysis/permeabilization or washing steps. Therefore, the NanoBRET technology allows a quantitative real-time detection of protein–ligand interactions in live cells. Moreover, the assay readout can be performed with a plate reader in a highly accurate and high-throughput manner. Whereas NanoBRET-based target engagement assays for Zn2+-dependent HDACs, including HDAC6, have been available for several years,12 the first method for a member of the NAD+-dependent HDACs, Sirt2, has only very recently been reported.6 Application of the NanoBRET-based Sirt2 target engagement assay enabled the development of small molecule inhibitors with low nanomolar Sirt2 affinities in cells. Moreover, this method was used to prove cellular target engagement for several literature-known Sirt2 inhibitors and provided additional evidence of the low on-target specificity of the broadly used Sirt2 probe Sirtinol.6
Table 1. Comparison of Available Cellular Target Engagement Methods for the Tubulin Deacetylases Sirt2 and HDAC6.
| method |
||||
|---|---|---|---|---|
| α-tubulin acetylation | CETSA | PROTAC | NanoBRET | |
| principle | activity-based | thermal stability | proximity-based | proximity-based |
| secondary detection method | Western blot, fluorescence microscopy | Western blot | Western blot | not required |
| modified ligand (tracer) | not required | not required | required | required |
| modified protein | not required | not required | not required | required |
| assay protocol | heterogeneous | heterogeneous | heterogeneous | homogeneous |
Cellular target engagement studies are of fundamental importance in order to confirm relevant drug targets and to evaluate the cellular on-target activity of biologically active compounds. For the tubulin deacetylases Sirt2 and HDAC6, which are both relevant drug targets, substantial progress in establishing methods to study cellular target engagement has been made in recent years. Currently, researchers can choose between a few techniques to assess cellular target engagement for Sirt2 and HDAC6, including tubulin acetylation, CETSA, as well as PROTAC- and NanoBRET-based approaches. Systematic application of different orthogonal target engagement methods will further aid the development of high-quality probes and drug candidates with a well-validated mechanism of action.
Acknowledgments
M.S. (Li 204/04) is supported by the Verband der Chemischen Industrie (VCI). A.V. and M.J. were supported by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) through SFB992 (Project ID 192904750). We thank M.E. Huber for the preparation of the Table of Contents Graphic.
Glossary
Abbreviations
- ATAT1
α-tubulin N-acetyltransferase
- BRET
bioluminescence resonance energy transfer
- CETSA
cellular thermal shift assays
- ELISA
enzyme-linked immunosorbent assay
- HDAC
histone deacetylase
- NAD
Nicotinamide adenine dinucleotide
- Nluc
nanoluciferase
- PROTAC
proteolysis-targeting chimera
Author Contributions
The manuscript was written through contributions of all authors and all authors have given approval to the final version of the manuscript.
The authors declare no competing financial interest.
Special Issue
Published as part of the ACS Pharmacology & Translational Science special issue “Epigenetics 2022”.
References
- North B. J.; Marshall B. L.; Borra M. T.; Denu J. M.; Verdin E. The human Sir2 ortholog, SIRT2, is an NAD(+)-dependent tubulin deacetylase. Mol. Cell 2003, 11, 437–444. 10.1016/S1097-2765(03)00038-8. [DOI] [PubMed] [Google Scholar]
- Hubbert C.; Guardiola A.; Shao R.; Kawaguchi Y.; Ito A.; Nixon A.; Yoshida M.; Wang X. F.; Yao T. P. HDAC6 is a microtubule-associated deacetylase. Nature 2002, 417, 455–458. 10.1038/417455a. [DOI] [PubMed] [Google Scholar]
- Yang W.; Chen W.; Su H.; Li R.; Song C.; Wang Z.; Yang L. Recent advances in the development of histone deacylase SIRT2 inhibitors. RSC Adv. 2020, 10, 37382–37390. 10.1039/D0RA06316A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X. H.; Qin M.; Wu H. P.; Khamis M. Y.; Li Y. H.; Ma L. Y.; Liu H. M. A Review of Progress in Histone Deacetylase 6 Inhibitors Research: Structural Specificity and Functional Diversity. J. Med. Chem. 2021, 64, 1362–1391. 10.1021/acs.jmedchem.0c01782. [DOI] [PubMed] [Google Scholar]
- Stefaniak J.; Huber K. V. M. Importance of Quantifying Drug-Target Engagement in Cells. ACS Med. Chem. Lett. 2020, 11, 403–406. 10.1021/acsmedchemlett.9b00570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogelmann A.; Schiedel M.; Wössner N.; Merz A.; Herp D.; Hammelmann S.; Colcerasa A.; Komaniecki G.; Hong J. Y.; Sum M.; Metzger E.; Neuwirt E.; Zhang L.; Einsle O.; Groß O.; Schüle R.; Lin H.; Sippl W.; Jung M.. Development of a NanoBRET Assay to Validate Dual Inhibitors of Sirt2-Mediated Lysine Deacetylation and Defatty-Acylation That Block Prostate Cancer Cell Migration. ChemRxiv, December 16, 2021, ver. 1. 10.26434/chemrxiv-2021-1r1dn. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen A. L.; Rajabi N.; Kudo N.; Lundo K.; Moreno-Yruela C.; Baek M.; Fontenas M.; Lucidi A.; Madsen A. S.; Yoshida M.; Olsen C. A. Mechanism-based inhibitors of SIRT2: structure-activity relationship, X-ray structures, target engagement, regulation of alpha-tubulin acetylation and inhibition of breast cancer cell migration. RSC Chem. Biol. 2021, 2, 612–626. 10.1039/D0CB00036A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakur A.; Tawa G. J.; Henderson M. J.; Danchik C.; Liu S.; Shah P.; Wang A. Q.; Dunn G.; Kabir M.; Padilha E. C.; Xu X.; Simeonov A.; Kharbanda S.; Stone R.; Grewal G. Design, Synthesis, and Biological Evaluation of Quinazolin-4-one-Based Hydroxamic Acids as Dual PI3K/HDAC Inhibitors. J. Med. Chem. 2020, 63, 4256–4292. 10.1021/acs.jmedchem.0c00193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiedel M.; Herp D.; Hammelmann S.; Swyter S.; Lehotzky A.; Robaa D.; Olah J.; Ovadi J.; Sippl W.; Jung M. Chemically induced degradation of sirtuin 2 (Sirt2) by a proteolysis targeting chimera (PROTAC) based on sirtuin rearranging ligands (SirReals). J. Med. Chem. 2018, 61, 482–491. 10.1021/acs.jmedchem.6b01872. [DOI] [PubMed] [Google Scholar]
- Yang K.; Wu H.; Zhang Z.; Leisten E. D.; Nie X.; Liu B.; Wen Z.; Zhang J.; Cunningham M. D.; Tang W. Development of Selective Histone Deacetylase 6 (HDAC6) Degraders Recruiting Von Hippel-Lindau (VHL) E3 Ubiquitin Ligase. ACS Med. Chem. Lett. 2020, 11, 575–581. 10.1021/acsmedchemlett.0c00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinatra L.; Bandolik J. J.; Roatsch M.; Sonnichsen M.; Schoeder C. T.; Hamacher A.; Scholer A.; Borkhardt A.; Meiler J.; Bhatia S.; Kassack M. U.; Hansen F. K. Hydroxamic Acids Immobilized on Resins (HAIRs): Synthesis of Dual-Targeting HDAC Inhibitors and HDAC Degraders (PROTACs). Angew. Chem., Int. Ed. Engl. 2020, 59, 22494–22499. 10.1002/anie.202006725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolbinger F. R.; Koeneke E.; Ridinger J.; Heimburg T.; Muller M.; Bayer T.; Sippl W.; Jung M.; Gunkel N.; Miller A. K.; Westermann F.; Witt O.; Oehme I. The HDAC6/8/10 inhibitor TH34 induces DNA damage-mediated cell death in human high-grade neuroblastoma cell lines. Arch. Toxicol. 2018, 92, 2649–2664. 10.1007/s00204-018-2234-8. [DOI] [PMC free article] [PubMed] [Google Scholar]


