Abstract
Objective
The gut microbiota plays a key role in modulating host immune response. We conducted a prospective, observational study to examine gut microbiota composition in association with immune responses and adverse events in adults who have received the inactivated vaccine (CoronaVac; Sinovac) or the mRNA vaccine (BNT162b2; BioNTech; Comirnaty).
Design
We performed shotgun metagenomic sequencing in stool samples of 138 COVID-19 vaccinees (37 CoronaVac and 101 BNT162b2 vaccinees) collected at baseline and 1 month after second dose of vaccination. Immune markers were measured by SARS-CoV-2 surrogate virus neutralisation test and spike receptor-binding domain IgG ELISA.
Results
We found a significantly lower immune response in recipients of CoronaVac than BNT162b2 vaccines (p<0.05). Bifidobacterium adolescentis was persistently higher in subjects with high neutralising antibodies to CoronaVac vaccine (p=0.023) and their baseline gut microbiome was enriched in pathways related to carbohydrate metabolism (linear discriminant analysis (LDA) scores >2 and p<0.05). Neutralising antibodies in BNT162b2 vaccinees showed a positive correlation with the total abundance of bacteria with flagella and fimbriae including Roseburia faecis (p=0.028). The abundance of Prevotella copri and two Megamonas species were enriched in individuals with fewer adverse events following either of the vaccines indicating that these bacteria may play an anti-inflammatory role in host immune response (LDA scores>3 and p<0.05).
Conclusion
Our study has identified specific gut microbiota markers in association with improved immune response and reduced adverse events following COVID-19 vaccines. Microbiota-targeted interventions have the potential to complement effectiveness of COVID-19 vaccines.
Keywords: immune response, COVID-19, enteric bacterial microflora
Significance of this study.
What is already known on this subject?
Durability of COVID-19 vaccine remains unclear and many countries are offering vaccine booster.
Individuals who received the inactivated vaccine (CoronaVac) had a lower antibody response compared to those who received the mRNA vaccine (BNT162b2).
Increasing evidence suggests that the gut microbiota plays a crucial role in modulating immune responses to various vaccines.
What are the new findings?
We demonstrated for the first time that baseline gut microbiota composition can predict immune response to COVID-19 vaccines and vaccine-related adverse events.
We observed higher abundance of B. adolescentis in CoronaVac high-responders, which is associated with enriched carbodydrate metabolic pathways for immunoprotection.
Body mass index is negatively correlated with neutralising antibody response to CoronaVac and specific baseline bacterial markers are associated with higher immune response among overweight or obese people.
How might it impact on clinical practice in the foreseeable future?
Our data highlight that microbiota-targeted interventions have the potential not only to optimise immune responses to COVID-19 vaccines but also to minimise vaccine-related adverse events.
Introduction
Vaccination elicits protective immune responses against SARS-CoV-2 and provides hope for containing the COVID-19 pandemic. As of 17 January 2022, more than 9.3 billion doses of vaccine have been administrated worldwide1 with substantial efficacy.2–4 Recent observational studies reported a steady decline of antibody levels among vaccinated individuals which implied a growing risk of breakthrough infection over time5 6 but factors influencing immunogenicity and durability of vaccine remains poorly understood. Evidence from clinical or animal studies suggested that the composition and functions of the gut microbiota are crucial in modulating immune responses of vaccination.7–9 Mucosal or systemic microbiota exposure shapes T and B cell repertoires that have an important implication for regulating responses to vaccination.10 11 Whether host microbiota composition influences responses of COVID-19 vaccines in humans has not been determined. We conducted a prospective observational study of adults who have received either the inactivated vaccine (CoronaVac; Sinovac) or the mRNA vaccine (BNT162b2; BioNTech; Comirnaty) to examine gut microbiota determinants of vaccine immune responses and vaccine-related adverse events.
Materials and methods
Study cohorts
Participants were volunteers receiving the mRNA COVID-19 vaccine (BNT162b2; N=101) or the inactivated COVID-19 vaccine (CoronaVac; N=37) recruited for serial blood and stool donations at the Prince of Wales Hospital of the Chinese University of Hong Kong (CUHK), the Queen Mary Hospital of the University of Hong Kong (HKU) or the community between 1 April 2021 and 31 August 2021. Eligible participants were aged 18 or above with no history of SARS-CoV-2 infection receiving either BNT162b2 or CoronaVac vaccine. Exclusion criteria included the presence of clinical signs and symptoms suggestive of acute infection with a positive reverse transcription PCR results for SARS-CoV-2 in saliva, or a positive COVID-19 serology. All participants provided written informed consent and completed both doses of vaccines.
Collection of stool and blood samples
One stool sample in DNA preservative and ~10 mL of blood in anticoagulant were collected from the participants at baseline (within 3 days of the first dose) and 1 month after second dose of vaccination.12 Stool samples were self-collected in DNA preservative tube at home and transferred at room temperature to laboratories within an average of 48 hours and stored at −80°C until DNA extraction. Blood samples were collected at hospital clinics and transported to laboratories for separation of plasma for serological tests.
Collection of demographic and epidemiological data
Standardised questionnaires were used to capture basic demographics and adverse events after both doses of vaccine. Demographics included age, gender, weight, height, comorbidities (hypertension, diabetes mellitus, allergy, diarrhoea, any other comorbidities), medication (antibiotics, hormone, immunomodulator), probiotics, vaccination in the past year, diet, alcohol intake (within 2 weeks prior to the first vaccination) and regular exercise (strenuous/moderate). Overweight or obese (OWOB) was determined according to the Asian-specific cut-off point of body mass index (BMI) ≥23 kg/m2. Adverse events questionnaires are summarised in the online supplemental table S1.
gutjnl-2021-326563supp001.pdf (1.1MB, pdf)
Serological tests
SARS-CoV-2 surrogate virus neutralisation test (sVNT) and spike receptor-binding domain (RBD) IgG ELISA were used to assess antibody levels in plasma collected at baseline and 1 month after second dose of vaccination. sVNT kits were obtained from GenScript, NJ, USA (Catalogue No. L00847-A) and tests were carried out according to manufacturer’s instructions. SARS-CoV-2 spike RBD IgG ELISA was carried out as previously described13 14 (online supplemental methods).
Stool metagenomic sequencing
Faecal DNA was extracted from the pellet using Maxwell RSC PureFood GMO and Authentication Kit (Promega, Madison, Wisconsin, USA). Faecal DNA was subjected to library construction using Nextera DNA Flex Library Preparation kit (Illumina, San Diego, California, USA)15 16 following manufacturer’s instructions (online supplemental methods). Libraries were sequenced on an in-house sequencer Illumina NovaSeq 6000 (250 base pairs paired-end) at the Microbiota I-Centre, Hong Kong, China. Sequence data processing and analysis were fully stated in online supplemental methods.
Statistical analysis
The primary analysis was to compare the relationship between microbiome profile and immune response to COVID-19 vaccines. Detailed statistical analysis can be found in online supplemental methods.
Results
SARS-CoV-2 vaccine cohort
Between 1 April 2021 and 31 August 2021, we recruited 138 adults who have received two doses of either the inactivated vaccines (CoronaVac; n=37) or the mRNA vaccine (BNT162b2; n=101) from CUHK and HKU (figure 1A). The participants ranged in age from 18 to 67 years (median=47 years, IQR 31.2–55.0) and 32.6% were male. 38.4% was classified as OWOB (ie, BMI ≥23) (table 1). Compared with BNT162b2 vaccinees, CoronaVac vaccinees were older in age (55.0 (CoronaVac) vs 42.0 (BNT162b2); p=0.003) and a higher proportion had hypertension (18.9% (CoronaVac) vs 6.9% (BNT162b2), p=0.055). Plasma SARS-CoV-2 sVNT and spike RBD IgG ELISA before vaccination were negative in all participants. At 1 month after completion of two doses of vaccines, CoronaVac vaccinees had a significantly lower immune response against SARS-CoV-2 compared with BNT162b2 vaccinees (sVNT: 57.6% vs 95.2%, p<0.001; anti-RBD: 1725.0 vs 8696.0, p<0.001) (table 1 and online supplemental figure 1A, B) based on adjusted linear regression and propensity score matching analysis matched for age and comorbidities (p<0.001, (online supplemental tables S2, S3). Moreover, sVNT were negatively correlated with BMI in the CoronaVac group (BMI; Spearman’s r=−0.385, p=0.018, (online supplemental table S4), and it was significant in both males and females (r=−0.817, p=0.007 and r=−0.403, p=0.033, respectively).
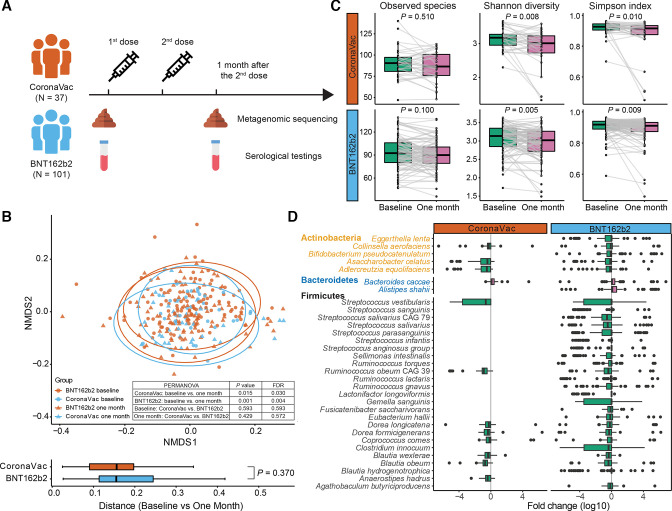
Figure 1.
Study design and changes in beta diversity, alpha diversity and bacterial species from baseline to 1 month after second dose of vaccination. (A) Study design. (B) Beta diversity was significantly different between baseline and 1 month after completion of vaccination (CoronaVac baseline, n=37; BNT162b2 baseline, n=101; CoronaVac 1 month, n=36; BNT162b2 1 month, n=98). P values were given by PERMANOVA and Wilcoxon rank-sum test (two sided), and adjusted for FDR, respectively. (C) Alpha diversity decreased significantly from baseline to 1 month after completion of vaccination for CoronaVac (n=36) and BNT162b2 (n=98). P values were given by paired Wilcoxon rank-sum test (two sided). (D) Differentially abundant species between baseline and 1 month after completion of vaccination for CoronaVac (n=36) and BNT162b2 (n=98). Differentially abundant species were detected using paired Wilcoxon rank-sum test (FDR corrected p<0.05). Elements on boxplots: centre line, median; box limits, upper and lower quartiles; whiskers, 1.5×IQR; points, outliers. FDR, false discovery rate; NMDS, non-metric multi-dimensional scaling; PERMANOVA, permutational multivariate analysis of variance.
Table 1.
Baseline characteristics of study population
| Variable | Overall (N=138) | BNT162b2 (N=101) | CoronaVac (N=37) | P value |
| Characteristics | ||||
| Age, years, (median (IQR)) | 47 (31.2–55.0) | 42 (29.0–53.0) | 55 (44.0–57.0) | 0.003 |
| Female* | 93 (67.9) | 65 (65.0) | 28 (75.7) | 0.304 |
| BMI, kg/m2, (median (IQR)) | 21.8 (20.2–24.5) | 21.8 (20.1–24.6) | 22.2 (20.4–23.7) | 0.946 |
| Overweight or obese† | 53 (38.7) | 38 (38.0) | 15 (40.5) | 0.844 |
| Obese† | 27 (19.7) | 22 (22.0) | 5 (13.5) | 0.338 |
| Presence of comorbidity | ||||
| Hypertension | 14 (10.1) | 7 (6.9) | 7 (18.9) | 0.055 |
| Diabetes mellitus | 4 (2.9) | 3 (3.0) | 1 (2.7) | 1.000 |
| Allergy ever | 49 (35.5) | 40 (39.6) | 9 (24.3) | 0.111 |
| Diarrhoea (past 3 months to current) | 55 (40.4) | 42 (42.0) | 13 (36.1) | 0.560 |
| Other comorbidities‡ | 15 (10.9) | 13 (12.9) | 2 (5.4) | 0.354 |
| Current medication | ||||
| Antibiotic intake (past 3 months and/or currently) | 6 (4.3) | 6 (5.9) | 0 (0.0) | 0.192 |
| Hormone therapy | 4 (2.9) | 4 (4.0) | 0 (0.0) | 0.574 |
| Immunomodulator | 3 (2.2) | 3 (3.0) | 0 (0.0) | 0.564 |
| Probiotics | 18 (13.1) | 12 (12.0) | 6 (16.2) | 0.572 |
| Vaccination in the past year | 53 (38.7) | 38 (38.0) | 15 (40.5) | 0.844 |
| Dietary habit | ||||
| Vegetarian | 1 (1.0) | 1 (1.0) | 0 (0.0) | 1.000 |
| Diet change during vaccination | 0 (0.0) | 0 (0.0) | 0 (0.0) | – |
| Alcohol intake (within 2 weeks prior to first vaccine dose) | 31 (22.5) | 25 (24.8) | 6 (16.2) | 0.361 |
| Exercise | ||||
| Regular exercise (strenuous/moderate) | 86 (62.3) | 62 (61.4) | 24 (64.9) | 0.843 |
| SARS-CoV-2 antibody response | ||||
| AUC of spike RBD IgG level (median (IQR))§ | 7889.5 (3110.8–9588.5) | 8696.0 (7628.0–11048.0) | 1725.0 (1418.0–2459.0) | <0.001 |
| sVNT (>60%) | 116 (84.1) | 100 (99.0) | 16 (43.2) | <0.001 |
| sVNT (inhibition %) (median (IQR)) | 93.9 (79.7–95.9) | 95.2 (92.1–96.4) | 57.6 (42.1–69.3) | <0.001 |
| Any adverse events¶ | ||||
| After the first dose | 116 (84.7) | 93 (93.0) | 23 (62.2) | <0.001 |
| After the second dose | 120 (87.6) | 95 (95.0) | 25 (67.6) | <0.001 |
Categorical data are presented as number (percentage) and continuous data as median (IQR). Within-group valid percentages are shown.
*One participant requested concealment of gender.
†BMI between 23.0 and 25.0 kg/m2 is classified as overweight and BMI above 25.0 kg/m2 is classified as obese.
‡Any other comorbidities: asthma, depression, eczema, high cholesterol, systemic lupus erythematosus, attention deficit hyperactivity disorder.
§Plasma IgG antibody binding to SARS-Cov-2 RBD was reported as area under the curve.
¶Any adverse events: injection site pain/burn, fatigue, fever, injection site swelling/pruritus/erythema/induration, myalgia, drowsiness, headache, chills, dizziness, arthralgia, loss of appetite, abdominal pain, rhinorrhea, sore throat, diarrhoea, pruritus, coughing, constipation, abdominal distension, nausea, flushing, hypersensitivity, muscle spasms, nasal congestion, oedema, vomiting, tremor, eyelid oedema, nosebleeds, hyposmia, ocular congestion, low back pain, increase of appetite, muscle pain, rib pain, eyes pain, palpitations.
AUC, area under the curve; BMI, body mass index; DM, diabetes mellitus; RBD, receptor-binding domain; sVNT, surrogate virus neutralisation test.
Gut microbiota composition in CoronaVac and BNT162b2 vaccinees
We performed shotgun metagenomic analysis on stool samples to determine whether baseline gut microbiome composition was associated with immune response to COVID-19 vaccines. In total, 272 stool samples were sequenced to generate an average of 7.7 Gb (33.7M reads) per sample. We observed a significant change in the gut microbiome composition including shifts in beta diversity (figure 1B) and a decrease in alpha diversity (figure 1C) at 1 month after the second dose of vaccination compared with baseline samples in both vaccine groups. These changes were not significantly different between the two vaccine groups. Baseline gut microbiome was significantly associated with several comorbidities, antibiotic use within 3 months prior to vaccination, regular exercise and recent symptoms of diarrhoea (online supplemental table S5). At the species level, only the abundance of Bacteroides caccae was found to be increased in CoronaVac vaccinees whereas BNT162b2 vaccinees had increased abundances of both B. caccae and Alistipes shahii, 1 month after two doses of vaccination. On the other hand, a relative decline in abundances of common bacterial species including Adlercreutzia equolifaciens, Asaccharobacter celatus, Blautia obeum, Blautia wexlerae, Dorea formicigenerans, Dorea longicatena, Coprococcus comes, Streptococcus vestibularis, Collinsella aerofaciens, and Ruminococcus obeum CAG 39 (figure 1D) were observed in both vaccine groups. A significant decline in Actinobacteria and Firmicutes abundances could be explained by altered physiological functions and drastic inflammation during vaccine regimen.17 Importantly, none of the participants reported significant dietary changes during the study period. Among 72 randomly selected participants, no significant changes in detailed dietary intake were recorded at baseline and 1 month after second dose of vaccination (p>0.05; online supplemental table S6).
Baseline gut microbiome composition predicts immune response at one month after COVID-19 vaccine
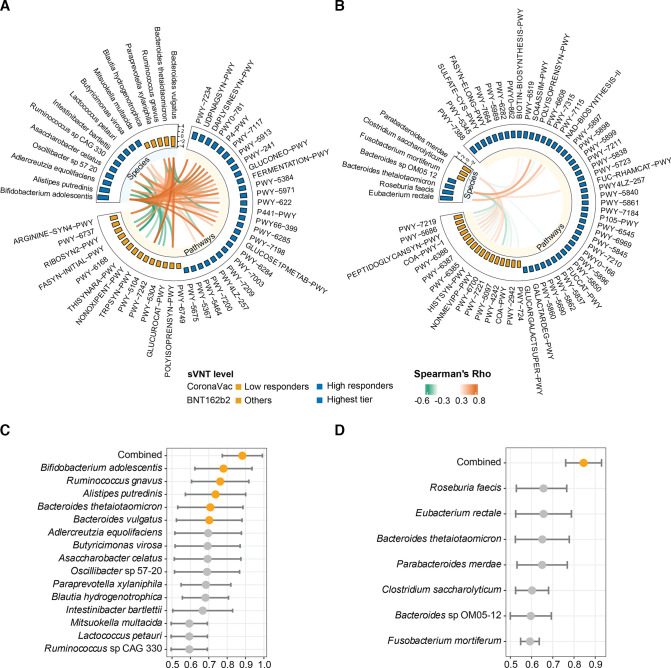
Consistent with previous findings,18 19 our study showed a high correlation between neutralising antibody by sVNT and anti-spike RBD IgG measured by ELISA (Spearman’s r=0.85, p<0.001 in CoronaVac; r=0.48, p<0.001 in BNT162b2, (online supplemental figure S1C, D), thus, we focused our analysis using results of sVNT. Khoury et al reported that 50% protection from neutralisation was related to antibody levels that were 20% of convalescent antibody titers.20 People with a sVNT lower than 50% may prone to re-infection. Since there was waning of antibody from peak titres observed at 1 month after second dose of vaccination, we set our target titre achieved at 1 month after second dose of vaccination to be twice the 50% protection titre which corresponded to sVNT inhibition of 60%.19 Among CoronaVac vaccinees, 21 of 37 (56.8%) who showed sVNT lower than 60% (low-responders) had a distinct baseline gut microbiome from those with sVNT higher than 60% (high responders). We observed that certain baseline gut microbiota species were associated with antibody response to COVID-19 vaccines. In particular, a total of 15 bacterial species in the baseline gut microbiome were identified, of which Bifidobacterium adolescentis was enriched in high-responders while Bacteroides vulgatus, Bacteroides thetaiotaomicron and Ruminococcus gnavus were more abundant in low-responders (figure 2A). B. adolescentis which was present in 64.9% of subjects showed a significant correlation with sVNT% in the CoronaVac group (table 2). At 1 month after second dose of vaccination, seven species including B. adolescentis, A. equolifaciens and A. celatus were more abundant whereas B. vulgatus remained less abundant in high responders (online supplemental figure S2A). Using mixed effect modeling,21 we showed that B. adolescentis was persistently higher while B. vulgatus was persistently lower from baseline to 1 month after second dose in high-responders (online supplemental table S7). We further interrogated functional pathways (online supplemental table S8) in the baseline gut microbiome and found that CoronaVac vaccinees with sVNT >60% had higher abundances of pathways related to carbohydrate metabolism and most of these pathways were positively correlated with abundance of B. adolescentis (figure 2A). In contrast, low responders had a relatively higher abundance of L-ornithine22 biosynthesis II pathway which was positively correlated with abundances of B. vulgatus and B. thetaiotaomicron at baseline (figure 2A).
Figure 2.
Baseline gut bacterial species and functions associated with high and low responders to vaccines at 1 month after second dose of vaccination. (A) Baseline bacterial species and pathways associated with high responders among CoronaVac vaccinees (n=37) (sVNT of 10-fold diluted plasma >60%). Differential baseline gut bacterial species and pathways were detected by LEfSe. Pairwise correlations between selected bacterial species and pathways markers with FDR corrected p<0.05 were shown. (B) Baseline bacterial species and pathways for highest-tier responders among BNT162b2 vaccinees (n=101) (the first quartile (Q1) of sVNT of 200-fold diluted plasma). sVNT-10: sVNT level of 10-fold diluted plasma; sVNT-200: sVNT level of 200-fold diluted plasma. Differential baseline gut bacterial species and pathways were detected by LEfSe. Pairwise correlations between selected bacterial species and pathways markers with FDR corrected p<0.05 were shown. Full names of differentially abundant pathways between high/low responders in (A, B) are described in online supplemental table S7C), AUROC (95% CI) values of models based on individual biomarkers and a combined model based on all biomarkers for high responders (n=16) vs low responders (n=21) among CoronaVac vaccinees. (D) AUROC (95% CI) values of models based on individual biomarkers and a combined model based on all biomarkers for the highest-tier responders (n=25) vs others (n=76) among BNT162b2 vaccines. each AUROC was presented as an orange dot with a bar showing the 95% CI. AUROC, area under the receiver operating characteristic curve; FDR, false discovery rate; LEfSe, linear discriminant analysis effect size; sVNT, surrogate virus neutralisation test.
Table 2.
Correlations between relative abundance of selected differential bacterial species at baseline and 1-month sVNT%
| Bacterial species | Prevalence (%) | Spearman correlation | |||
| Crude | Adjusted for age | ||||
| r | P value | r | P value | ||
| CoronaVac | |||||
| Bifidobacterium adolescentis | 64.9 | 0.354 | 0.032 | 0.329 | 0.050 |
| Alistipes putredinis | 78.4 | 0.380 | 0.020 | 0.294 | 0.082 |
| Adlercreutzia equolifaciens | 81.1 | 0.202 | 0.230 | 0.154 | 0.368 |
| Oscillibacter sp 57 20 | 73 | 0.261 | 0.118 | 0.207 | 0.225 |
| Asaccharobacter celatus | 78.4 | 0.204 | 0.227 | 0.175 | 0.308 |
| Ruminococcus sp CAG 330 | 8.1 | 0.300 | 0.071 | 0.257 | 0.130 |
| Intestinibacter bartlettii | 37.8 | 0.276 | 0.099 | 0.228 | 0.181 |
| Lactococcus petauri | 8.1 | 0.211 | 0.211 | 0.212 | 0.214 |
| Mitsuokella multacida | 8.1 | 0.253 | 0.131 | 0.147 | 0.393 |
| Butyricimonas virosa | 59.5 | 0.136 | 0.423 | 0.046 | 0.791 |
| Blautia hydrogenotrophica | 27 | −0.399 | 0.014 | −0.388 | 0.019 |
| Paraprevotella xylaniphila | 32.4 | −0.310 | 0.062 | −0.273 | 0.107 |
| Ruminococcus gnavus | 59.5 | −0.281 | 0.092 | −0.198 | 0.246 |
| Bacteroides thetaiotaomicron | 100 | −0.074 | 0.662 | −0.015 | 0.931 |
| Bacteroides vulgatus | 100 | −0.147 | 0.385 | −0.127 | 0.461 |
| BNT162b2 | |||||
| Eubacterium rectale | 71.3 | 0.227 | 0.023 | 0.223 | 0.026 |
| Roseburia faecis | 76.2 | 0.214 | 0.031 | 0.215 | 0.031 |
| Bacteroides thetaiotaomicron | 95 | 0.191 | 0.056 | 0.204 | 0.042 |
| Bacteroides sp OM05 12 | 13.9 | 0.101 | 0.317 | 0.088 | 0.383 |
| Fusobacterium mortiferum | 13.9 | −0.167 | 0.096 | −0.161 | 0.108 |
| Clostridium saccharolyticum | 25.7 | −0.097 | 0.335 | −0.085 | 0.403 |
| Parabacteroides merdae | 70.3 | −0.276 | 0.005 | −0.273 | 0.006 |
Partial Spearman correlation was used to adjust for age.
sVNT, surrogate virus neutralisation test.
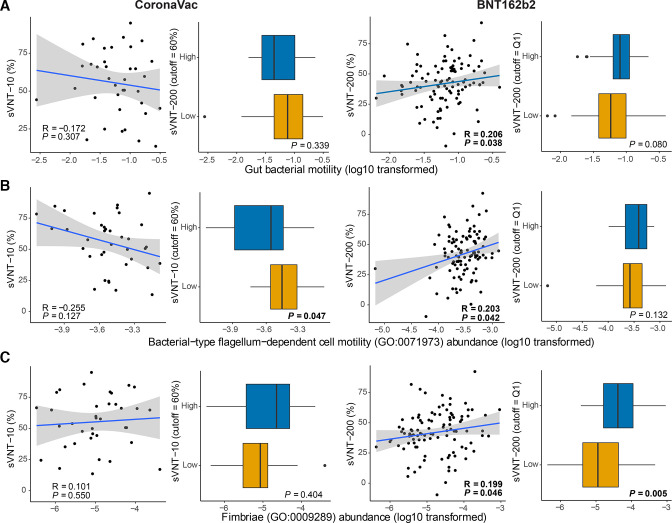
The sVNT kit has a ceiling of detection limit using the standard dilution.23 Studies showed that most people who received the BNT162b2 vaccine reached this detection limit 1 month after two doses of vaccination.24 Only one participant who received BNT162b2 vaccine had very low sVNT inhibition (29.3%) (online supplemental figure S1A). The participant was overweight, had a history of kidney transplant and was on corticosteroids and antihypertensive therapy. Similar to CoronaVac low responders, the gut microbiota of BNT162b2 low responders had a persistently low level of Actinobacteria particularly B. adolescentis (online supplemental figure S3). To further differentiate response among the participants, we performed sVNT using plasma samples after 200-fold of dilution to differentiate neutralising antibody level from samples of BNT162b2 (online supplemental figure S1B). We then defined the quartiles from the sVNT results of BNT162b2 cohort. Four specific bacteria in the baseline gut microbiome including Eubacterium rectale, Roseburia faecis and two Bacteroides species, B. thetaiotaomicron and Bacteroides sp OM05-12 were significantly increased in the highest-tier responders with top 25% of sVNT level (figure 2B). Abundance of these species except Bacteroides sp OM05-12 also significantly correlated with the sVNT% (table 2). Interestingly, a higher relative abundance of bacteria with flagella in the baseline gut microbiome was associated with a higher antibody response to BNT162b2 vaccine. R. faecis is one of the major contributors to gut bacterial motility, according to both bacterial phenotype databases25 26 (online supplemental methods) and Gene Ontology annotation (GO:0071973, (online supplemental figures 4,5), which was positively correlated with sVNT levels in BNT162b2 vaccinees (figure 3A, B). Moreover, R. faecis and E. rectale which were likely to express fimbriae (according to GO:0009289, (online supplemental figure S6) also positively correlated with sVNT levels in BNT162b2 vaccinees (figure 3C). Among these bacterial biomarkers, two Bacteroides species remained persistently enriched at 1 month after BNT162b2 vaccination in highest-tier responders (online supplemental figure S2B). Enriched pathways for biosynthesis of several menaquinols were found in highest-tier responders’ samples collected before but not after vaccination. There was decreased abundance of pathways for adenosine27 ribonucleotide biosynthesis and peptidoglycan biosynthesis (figure 2B) in the baseline gut microbiome.
Figure 3.
Association of baseline gut bacterial motility and fimbrial gene abundance with neutralising antibody response to CoronaVac and BNT162b2 vaccines at 1 month after second dose of vaccination. (A) Association of baseline gut bacterial motility (based on bacterial relative abundance and bacterial motility phenotype, the Methods section) with neutralising antibody response at 1 month after second dose of vaccination. (B) Association of flagellum-dependent cell motility (GO:0071973) of baseline gut microbiome with neutralising antibody response at 1 month after second dose of vaccination. (C) Association of fimbrial gene abundance (GO:0009289) of baseline gut microbiome with neutralising antibody response at 1 month after second dose of vaccination. CoronaVac (n=37): high-responders, n=16; low responders, n=21. BNT162b2 (n=101) highest tier, n=25; others, n=76. sVNT-10: sVNT level of 10-fold diluted plasma; sVNT-200: sVNT level of 200-fold diluted plasma. Correlation between motility/fimbrial gene abundance and sVNT data was examined using Spearman’s correlation test. Regression lines with 95% CI (grey area) were shown on scatter plots. Comparison between high versus low responder groups/highest tier versus others was made using Wilcoxon’s rank-sum test (two-sided). Elements on boxplots: centre line, median; box limits, upper and lower quartiles; whiskers, 1.5×IQR; points, outliers. sVNT, surrogate virus neutralisation test.
We further tested predictive power of the abovementioned baseline gut bacterial species markers based on area under the receiver operating characteristic curve (AUROC) to each type of vaccine. Predictive power of B. adolescentis alone (AUROC (95% CI): 0.780 (0.624 to 0.935) was higher than other bacterial species in predicting high responders versus low responders to CoronaVac (figure 2C) but this was not significantly different from the AUROC of combined bacterial species markers, 0.882 (0.773 to 0.992). For BNT162b2, the best predictive power was observed in the model using a combination of seven bacterial species, AUROC (95% CI): 0.845 (0.761 to 0.930) (figure 2D).
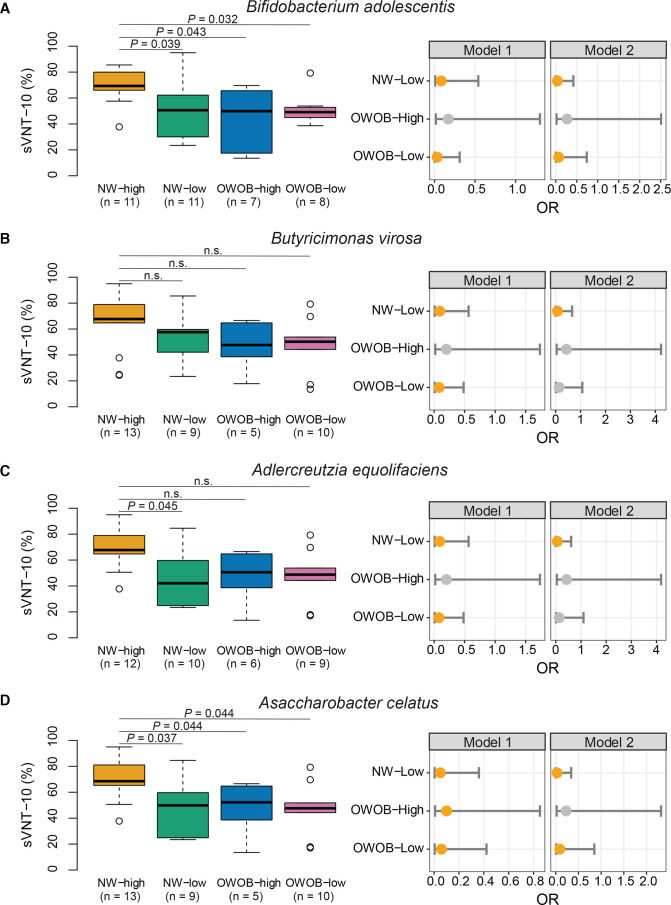
Effect of beneficial bacteria on immune response to inactivated vaccine is modified by BMI
Gut microbiome is known to be influenced by host physiological status and lifestyle factors. Reciprocally, gut microbiome orchestrates host immune system and modulates responses to vaccines.7 We found that sVNT levels were correlated with BMI (online supplemental table S4 and figure 4) and abundance of certain bacteria in the CoronaVac group. This observation prompted us to further investigate the potential role of weight as an effect modifier of bacteria-immune response relationship. Based on comparison between strata of weight status and abundance of bacterial species markers of the baseline gut microbiome, associations of the four bacterial species with immune response were significantly influenced by body weight. Positive associations between the four bacterial biomarkers with immune response were compromised in OWOB people. These species included two short-chain fatty acid (SCFA) producers, B. adolescentis and Butyricimonas virosa, and A. equolifaciens and A. celatus (figure 4). However, compared with normal weight people with high abundances of B. adolescentis and A. celatus, the risk of being low responders was not significant for OWOB people if they had a high abundance of the same bacterial species (model 2: adjusted OR 0.27, 95% CI 0.02 to 2.51 and OR 0.43, 95% CI 0.04 to 4.23, respectively). These results suggest that the beneficial effect of these bacteria on the immune responses to CoronaVac vaccine was attenuated in OWOB people. Therefore, we further identified specific bacterial species in the high BMI population. LEfSe analysis showed enrichment of three bacterial species including Ruminococcs torques, Eubacterium ventriosum and Streptococcus salivarius in CoronaVac high responders who were OWOB (online supplemental figure S7).
Figure 4.
Weight status modifies the assocaitions between baseline gut bacterial species and immune response in CoronaVac vaccinees at 1 month after second dose of vaccination. Immune response and ORs to be high responders separated by baseline bacterial abundance within weight strata (A) by Bifidobacterium adolescentis abundance. (B) By Butyricimonas virosa abundance (C) by Adlercreutzia equolifaciens abundance. (D) by Asaccharobacter celatus abundance. sVNT-10: sVNT of 10-fold diluted plasma. Sample size per group was indicated on the figure. Comparisons between subgroups were done using Dunn’s test (one sided) with FDR correction. Model 1: crude model. Model 2: adjusted for age. Reference group: NW with high bacterial abundance. Elements on boxplots: centre line, median; box limits, upper and lower quartiles; whiskers, 1.5×IQR; points, outliers. Each OR was presented as an orange dot with a bar showing the 95% CI. NW, normal weight; FDR, false discovery rate; OWOB, overweight or obese; sVNT, surrogate virus neutralisation test.
Gut microbiome composition is associated with vaccine-related adverse events
None of the participants had serious adverse events that led to hospitalisation. Consistent with the previous report,28 a greater proportion of BNT162b2 vaccinees reported adverse events than CoronaVac vaccines. Compared with CoronaVac vaccinees, more BNT162b2 vaccinees developed injection site pain, fatigue, fever, myalgia, drowsiness, headache and chills (table 1 and online supplemental table S1). We hypothesised that gut microbiome composition may associate with adverse events caused by vaccination. Among BNT162b2 vaccinees, participants who reported any adverse effect after the first dose of vaccination had a significant decrease in observed bacterial species richness (p=0.011) (online supplemental figure S8). To assess whether specific baseline bacterial species was associated with vaccine-related adverse events, we applied partitioning around medoids clustering,29 which optimally clustered the gut microbiome composition of CoronaVac vaccinees into two distinct groups (online supplemental figure 9A–C) with varying proportions of adverse events after both doses of vaccine (online supplemental table S9). Consistent with previous studies including Asian populations,30–32 two distinct gut microbiota clusters can be distinguished primarily by levels of Bacteroides and Prevotella. The cluster associated with fewer adverse events after CoronaVac vaccination had a higher abundance of Prevotella copri and two Megamonas species (M. funiformis and M. hypermegale) in their baseline gut microbiome (online supplemental figure S9D). Similarly, baseline gut microbiota cluster enriched by P. copri and two Megamonas species was associated with fewer adverse events in BNT162b2 vaccinees (online supplemental figure s9E–H), indicating that these species may play an anti-inflammatory role in both vaccine groups. Interestingly, symptoms of fatigue after the first dose of vaccination were associated with a higher sVNT inhibition in BNT162b2 vaccinees but lower inhibition in CoronaVac vaccinees (online supplemental tables S10, S11).
Discussion
To our knowledge, this is the first human study to show that baseline gut microbiota composition reflects immunogenicity and adverse events of COVID-19 vaccines. We found that differential baseline bacterial species were associated with higher vaccine response. Specifically, the presence of an immunomodulatory bacteria, B. adolescentis, was associated with higher neutralising antibodies to CoronaVac suggesting that this bacteria may serve as an adjuvant to potentially overcome waning immunity of inactivated vaccine. Interestingly, abundance of P. copri and two Megamonas species were found to be more enriched in the baseline gut microbiome of participants with fewer adverse events after inactivated and mRNA vaccines.
Data from clinical studies8 and animal models33 34 suggest that gut microbiota composition plays a crucial role in modulating immune responses to vaccines but mechanisms by which the gut microbiota modulate immune responses to different vaccines in different populations are poorly understood. One potential mechanism is via the provision of natural adjuvants that enhances responses to vaccination.7 Commonly used vaccine adjuvants can directly or indirectly activate antigen-presenting cells such as dendritic cells via pattern recognition receptors (PRRs) like TLRs or NOD-like receptors.35 Flagellin and peptidoglycan produced by the gut microbiota can act as natural adjuvants to vaccines and can be sensed by PRRs.7 For example, TLR5-mediated sensing of flagellin has been shown to be required for optimal antibody response to influenza vaccine.34 Moreover, adhesin portion of bacterial fimbriae can induce innate immune system via TLR4,36 which is one of the immune activator proteins that has been proposed as an effective adjuvant for mRNA vaccines.37 Consistently, a higher relative abundance of bacteria with flagella and fimbriae (E. rectale and R. faecis) was associated with a higher antibody response to mRNA vaccine. Microbiota-derived SCFAs enhance B cell metabolism and gene expression to support optimal homeostatic and pathogen-specific antibody responses.38 E. rectale and R. faecis which produce butyrate may in part account for the elevated immunogenicity in highest-tier BNT162b2 responders. These bacterial species may play a beneficial role in vaccine immunogenicity serving as adjuvants through immunomodulatory TLR agonists. With waning antibody levels,39 whether microbiota-derived flagella/fimbirae or SCFAs can contribute to sustaining long-term COVID-19 immunisation efficacy deserves further investigation.
Consistent with previous reports supporting the immunomodulatory properties of B. adolescentis,40 E. retale, and R. faecis,41 we observed enriched B. adolescentis in CoronaVac high-responders and increased abundances of E. retale, R. faecis, B. theaiotaomicron and Bacteroides. sp OM05-12 in BNT162b2 highest-tier responders. Moreover, reduced abundance of B. adolescentis was identified in a single BNT162b2 vaccinee with low level of sVNT. Studies in infants have shown that the abundance of Bifidobacteria was associated with CD4+ T cell responses and increased antibody responses to several vaccines.42 43 A recent study also reported that vaccine-induced T cell responses showed broad cross-reactivity against SARS-CoV-2 variants.44 Thus, gut microbiota-associated T cell responses would benefit not only vaccine immunogenicity but also cross-protection against multiple variants. Apart from higher abundance of B. adolescentis, we also observed enriched carbohydrate metabolic pathways in CoronaVac high-responders. Carbohydrates play a crucial role in appropriate stimulation of the immune response,45 hence association of B. adolescentis with higher antibody response could be explained by carbohydrate-driven immunopotentiation effects. These data indicate that vaccinees with a higher abundance of these beneficial bacteria may have an optimal immune response and potentially stronger protection.
Obesity is often associated with an adverse impact on the immune system. A recent study reported an inverse correlation between titre of antibody against SARS-CoV-2 spike protein and BMI in men who received BNT162b2 vaccine.46 Herein, we observed that immune response based on percent inhibition in sVNT correlated with BMI and the abundance of certain bacteria (B. adolescentsi, B. virosa, A. equolifaciens and A. celatus) in CoronaVac vaccinees. These results suggest that beneficial effects of these bacteria on immune response to CoronaVac vaccine was modified by body weight. We identified baseline gut microbiota species (R. torques, E. ventriosum and S. salivarius) that were associated with high-responders.
Gut microbiota cluster with a higher abundance of P. copri and Megamonas species was associated with less adverse events to both types of vaccines likely mediated through their anti-inflammatory functions. A higher prevalence of P. copri has been reported in non-westernised populations.47 P. copri also enhanced farnesoid X receptor signalling48 49 via modulating bile acid metabolism. Among the Megamonas species, M. funiformis could ferment glucose into acetate and propionate50 51 which are beneficial for immune homeostasis whereas M. hypermegale can regulate the balance between regulatory T cell and type 17 helper T cells (Th17).52
Although BNT162b2 vaccine induced over 90% neutralising antibody response, waning of pike-antibody levels has been reported in infection-naïve individuals over a period of 3–10 weeks after second vaccine dose.53 Both Spike-antibody and neutralising antibody levels at 1 month after the second dose of mRNA vaccine also positively correlated with vaccine efficacy.54 Longitudinal assessment of the gut microbiota profile and antibody response beyond 1 month after the second dose of vaccines will further delineate how gut microbiota influences immunogenicity and long term durability of vaccine response.
In a prospective study, we found that baseline gut microbiota was significantly associated with immunogenicity and adverse events of COVID-19 vaccines. These novel findings have potential in facilitating microbiota-targeted interventions to optimise vaccine immune response and enhance durability of protection.
Acknowledgments
We thank all study participants for providing specimens and devoting time to our research; the CUHK and HKU Hospital nursing staff and Clinical Research Support Office. We would like to thank Chun Pan Cheung, Ananya Prasad, Crystal YC Wong, Uuriinsaran Purevsuren, Yao Huang, Chengyu Liu and Yao Zeng, Effie YT Lau, and Alan LC Chu for sample collection; Arthur Chung, Wenye Xu, Shilan Wang and Hui Zhan for metagenomic sequencing; Yuk Lam Chan, Alice HY Chu, Hogan KF Wai, Hilda On and Suet Y Ng for subjects recruitment and sample collection; Winnie Lin for dietary analysis; and Dr. Annie Chiu for blood collection. The recombinant RBD protein was kindly provided as gifts by Prof Ian A Wilson and Dr Meng Yuan from Scripps Research Institute. The computations were performed using research computing facilities offered by Information Technology Services, the University of Hong Kong. Chris KP Mok is the visiting scientist of Lee Kong Chian School of Medicine, Nanyang Technological University, Singapore. Hein M Tun is an Adjunct Professor at School of Public Health, Nanjing Medical University, China.
Footnotes
Twitter: @Siew_C_Ng, @YePeng21, @linzhang8385, @hk_kennethwong, @FrancisKLChan, @thetunlab
SCN, YP and LZ contributed equally.
Contributors: SCN, LZ, CKPM, FKLC and HMT conceived and designed the study. CKPM and CC carried out serology testing and analysis. AYL, SZ, YP, SY and DLSC recruited participants, JYLC executed clinical protocols. HMT, YP, SZ and JZ performed bioinformatic and statistical analyses. SCN, YP, LZ, CKPM, FKLC and HMT wrote the manuscript with input from all co-authors. HMT acts as the guarantor for this study and publication.
Funding: The project was supported by the Health and Medical Research Fund (HMRF) Commissioned Research Grant (COVID193002) (FKLC); Enhanced start-up research grant of HKU and RGC Research Impact Fund (R7033-18) (HMT) and the National Research Foundation of Korea (NRF) grant funded through the Korea government (NRF-2018M3A9H4055203) (KPM).
Competing interests: The Chinese University of Hong Kong and The University of Hong Kong have filed a provisional patent application in connection with this work on which SCN, FKLC and HMT are inventors (US patent application no. 63/273,088). FKLC and SCN are the scientific co-founders and sit in the board of Directors of GenieBiome Ltd.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available in a public, open access repository. Quality-controlled and human DNA-removed sequence data are deposited in the European Nucleotide Archive under BioProject PRJEB48269. Additional datasets generated and/or analysed in this study are available from the corresponding author on reasonable request.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
The study was approved by The Joint Chinese University of Hong Kong – New Territories East Cluster Clinical Research Ethics Committee (The Joint CUHK-NTEC CREC) (2021.260) and The Institutional Review Board of the University of Hong Kong/Hospital Authority Hong Kong West Cluster (HKU/HA HKW) (UW 21–203). The study was conducted in accordance with the Declaration of Helsinki (1975) and Good Clinical Practice.
References
- 1. World Health Organization. Available: https://www.who.int/emergencies/diseases/novel-coronavirus-2019 [Accessed 17 Jan 2022].
- 2. Voysey M, Clemens SAC, Madhi SA, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021;397:99–111. 10.1016/S0140-6736(20)32661-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jara A, Undurraga EA, González C, et al. Effectiveness of an inactivated SARS-CoV-2 vaccine in Chile. N Engl J Med 2021;385:875–84. 10.1056/NEJMoa2107715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Haas EJ, Angulo FJ, McLaughlin JM, et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet 2021;397:1819–29. 10.1016/S0140-6736(21)00947-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Naaber P, Tserel L, Kangro K, et al. Dynamics of antibody response to BNT162b2 vaccine after six months: a longitudinal prospective study. Lancet Reg Health Eur 2021;10:100208. 10.1016/j.lanepe.2021.100208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Skowronski DM, De Serres G, Thomas SJ. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med 2021;384:1576–8. 10.1056/NEJMc2036242 [DOI] [PubMed] [Google Scholar]
- 7. Lynn DJ, Benson SC, Lynn MA, et al. Modulation of immune responses to vaccination by the microbiota: implications and potential mechanisms. Nat Rev Immunol 2022;22:33–46. 10.1038/s41577-021-00554-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hagan T, Cortese M, Rouphael N, et al. Antibiotics-driven gut microbiome perturbation alters immunity to vaccines in humans. Cell 2019;178:e1313:1313–28. 10.1016/j.cell.2019.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. de Jong SE, Olin A, Pulendran B. The impact of the microbiome on immunity to vaccination in humans. Cell Host Microbe 2020;28:169–79. 10.1016/j.chom.2020.06.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. New JS, Dizon BLP, Fucile CF, et al. Neonatal exposure to commensal-bacteria-derived antigens directs polysaccharide-specific B-1 B cell repertoire development. Immunity 2020;53:e176:172–86. 10.1016/j.immuni.2020.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bi Q, Wu Y, Mei S, et al. Epidemiology and transmission of COVID-19 in 391 cases and 1286 of their close contacts in Shenzhen, China: a retrospective cohort study. Lancet Infect Dis 2020;20:911–9. 10.1016/S1473-3099(20)30287-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Song SJ, Amir A, Metcalf JL, et al. Preservation methods differ in fecal microbiome stability, affecting suitability for field studies. mSystems 2016;1:e00021–16. 10.1128/mSystems.00021-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Perera RA, Mok CK, Tsang OTY, et al. Serological assays for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), March 2020. Euro Surveill 2020;25:200042. 10.2807/1560-7917.ES.2020.25.16.2000421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Perera RAPM, Ko R, Tsang OTY, et al. Evaluation of a SARS-CoV-2 surrogate virus neutralization test for detection of antibody in human, canine, cat, and hamster sera. J Clin Microbiol 2021;59:e02504–20. 10.1128/JCM.02504-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zuo T, Zhang F, Lui GCY, et al. Alterations in gut microbiota of patients with COVID-19 during time of hospitalization. Gastroenterology 2020;159:944–55. 10.1053/j.gastro.2020.05.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yeoh YK, Zuo T, Lui GC-Y, et al. Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID-19. Gut 2021;70:698–706. 10.1136/gutjnl-2020-323020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ciabattini A, Olivieri R, Lazzeri E, et al. Role of the microbiota in the modulation of vaccine immune responses. Front Microbiol 2019;10:1305. 10.3389/fmicb.2019.01305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tan CW, Chia WN, Qin X, et al. A SARS-CoV-2 surrogate virus neutralization test based on antibody-mediated blockage of ACE2-spike protein-protein interaction. Nat Biotechnol 2020;38:1073–8. 10.1038/s41587-020-0631-z [DOI] [PubMed] [Google Scholar]
- 19. Lau EH, Hui DS, Tsang OT, et al. Long-term persistence of SARS-CoV-2 neutralizing antibody responses after infection and estimates of the duration of protection. EClinicalMedicine 2021;41:101174. 10.1016/j.eclinm.2021.101174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Khoury DS, Cromer D, Reynaldi A, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med 2021;27:1205–11. 10.1038/s41591-021-01377-8 [DOI] [PubMed] [Google Scholar]
- 21. Zhang X, Yi N. NBZIMM: negative binomial and zero-inflated mixed models, with application to microbiome/metagenomics data analysis. BMC Bioinformatics 2020;21:488. 10.1186/s12859-020-03803-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dröge W, Männel D, Falk W, et al. Suppression of cytotoxic T lymphocyte activation by L-ornithine. J Immunol 1985;134:3379–83. [PubMed] [Google Scholar]
- 23. Mok CKP, Cohen CA, Cheng SMS, et al. Comparison of the immunogenicity of BNT162b2 and CoronaVac COVID ‐19 vaccines in Hong Kong. Respirology. 10.1111/resp.14191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Turner JS, O'Halloran JA, Kalaidina E, et al. SARS-CoV-2 mRNA vaccines induce persistent human germinal centre responses. Nature 2021;596:109–13. 10.1038/s41586-021-03738-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mukhejee S, Stamatis D, Bertsch J. Data from: genomes online database (gold) v.8: overview and updates. The genomes online database. Available: https://gold.jgi.doe.gov/downloads [Accessed 22 Sep 2021]. [DOI] [PMC free article] [PubMed]
- 26. Guittar J, Shade A, Litchman E. Data from: trait-based community assembly and succession of the infant gut microbiome. Figshare. Available: https://figshare.com/articles/dataset/International_Journal_of_Systematic_and_Evolutionary_Microbiology_IJSEM_phenotypic_database/4272392 [Accessed 07 Dec 2016.]. [DOI] [PMC free article] [PubMed]
- 27. Galván-Peña S, Leon J, Chowdhary K, et al. Profound Treg perturbations correlate with COVID-19 severity. Proc Natl Acad Sci U S A 2021;118:37. 10.1073/pnas.2111315118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lim WW, Mak L, Leung GM, et al. Comparative immunogenicity of mRNA and inactivated vaccines against COVID-19. Lancet Microbe 2021;2:e423. 10.1016/S2666-5247(21)00177-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tun HM, Peng Y, Chen B, et al. Ethnicity associations with food sensitization are mediated by gut microbiota development in the first year of life. Gastroenterology 2021;161:94–106. 10.1053/j.gastro.2021.03.016 [DOI] [PubMed] [Google Scholar]
- 30. Levy R, Magis AT, Earls JC, et al. Longitudinal analysis reveals transition barriers between dominant ecological states in the gut microbiome. Proc Natl Acad Sci U S A 2020;117:13839–45. 10.1073/pnas.1922498117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wan Y, Wang F, Yuan J, et al. Effects of dietary fat on gut microbiota and faecal metabolites, and their relationship with cardiometabolic risk factors: a 6-month randomised controlled-feeding trial. Gut 2019;68:1417–29. 10.1136/gutjnl-2018-317609 [DOI] [PubMed] [Google Scholar]
- 32. Wu GD, Chen J, Hoffmann C, et al. Linking long-term dietary patterns with gut microbial enterotypes. Science 2011;334:105–8. 10.1126/science.1208344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lynn MA, Tumes DJ, Choo JM, et al. Early-Life Antibiotic-Driven dysbiosis leads to dysregulated vaccine immune responses in mice. Cell Host Microbe 2018;23:e655:653–60. 10.1016/j.chom.2018.04.009 [DOI] [PubMed] [Google Scholar]
- 34. Oh JZ, Ravindran R, Chassaing B, et al. TLR5-mediated sensing of gut microbiota is necessary for antibody responses to seasonal influenza vaccination. Immunity 2014;41:478–92. 10.1016/j.immuni.2014.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pulendran B, S Arunachalam P, O'Hagan DT. Emerging concepts in the science of vaccine adjuvants. Nat Rev Drug Discov 2021;20:454–75. 10.1038/s41573-021-00163-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ashkar AA, Mossman KL, Coombes BK, et al. FimH adhesin of type 1 fimbriae is a potent inducer of innate antimicrobial responses which requires TLR4 and type 1 interferon signalling. PLoS Pathog 2008;4:e1000233. 10.1371/journal.ppat.1000233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pardi N, Hogan MJ, Porter FW, et al. mRNA vaccines - a new era in vaccinology. Nat Rev Drug Discov 2018;17:261–79. 10.1038/nrd.2017.243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kim M, Qie Y, Park J, et al. Gut microbial metabolites fuel host antibody responses. Cell Host Microbe 2016;20:202–14. 10.1016/j.chom.2016.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tartof SY, Slezak JM, Fischer H, et al. Effectiveness of mRNA BNT162b2 COVID-19 vaccine up to 6 months in a large integrated health system in the USA: a retrospective cohort study. Lancet 2021;398:1407–16. 10.1016/S0140-6736(21)02183-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Huda MN, Lewis Z, Kalanetra KM, et al. Stool microbiota and vaccine responses of infants. Pediatrics 2014;134:e362–72. 10.1542/peds.2013-3937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Stražar M, Mourits VP, Koeken VACM, et al. The influence of the gut microbiome on BCG-induced trained immunity. Genome Biol 2021;22:275. 10.1186/s13059-021-02482-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhao T, Li J, Fu Y, et al. Influence of gut microbiota on mucosal IgA antibody response to the polio vaccine. NPJ Vaccines 2020;5:47. 10.1038/s41541-020-0194-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Huda MN, Ahmad SM, Alam MJ, et al. Bifidobacterium Abundance in Early Infancy and Vaccine Response at 2 Years of Age. Pediatrics 2019;143:e20181489. 10.1542/peds.2018-1489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Geers D, Shamier MC, Bogers S, et al. SARS-CoV-2 variants of concern partially escape humoral but not T cell responses in COVID-19 convalescent donors and vaccine recipients. Sci Immunol 2021;6:59. 10.1126/sciimmunol.abj1750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Pifferi C, Fuentes R, Fernández-Tejada A, Tejada AF. Natural and synthetic carbohydrate-based vaccine adjuvants and their mechanisms of action. Nat Rev Chem 2021;5:197–216. 10.1038/s41570-020-00244-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Shohei Y, Tetsuya M, Akihito T. Sex–associated differences between body mass index and SARS-CoV-2 antibody titers following the BNT162b2 vaccine among 2,435 healthcare workers in Japan. medRxiv 2021. [Google Scholar]
- 47. Tett A, Huang KD, Asnicar F, et al. The prevotella copri complex comprises four distinct clades underrepresented in westernized populations. Cell Host Microbe 2019;26:e667:666–79. 10.1016/j.chom.2019.08.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Péan N, Le Lay A, Brial F, et al. Dominant gut Prevotella copri in gastrectomised non-obese diabetic Goto-Kakizaki rats improves glucose homeostasis through enhanced FXR signalling. Diabetologia 2020;63:1223–35. 10.1007/s00125-020-05122-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hollman DAA, Milona A, van Erpecum KJ, et al. Anti-Inflammatory and metabolic actions of FXR: insights into molecular mechanisms. Biochim Biophys Acta 2012;1821:1443–52. 10.1016/j.bbalip.2012.07.004 [DOI] [PubMed] [Google Scholar]
- 50. Zhang X, Shen D, Fang Z, et al. Human gut microbiota changes reveal the progression of glucose intolerance. PLoS One 2013;8:e71108. 10.1371/journal.pone.0071108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sakon H, Nagai F, Morotomi M, et al. Sutterella parvirubra sp. nov. and Megamonas funiformis sp. nov., isolated from human faeces. Int J Syst Evol Microbiol 2008;58:970–5. 10.1099/ijs.0.65456-0 [DOI] [PubMed] [Google Scholar]
- 52. Shimizu J, Kubota T, Takada E, et al. Relative abundance of Megamonas hypermegale and Butyrivibrio species decreased in the intestine and its possible association with the T cell aberration by metabolite alteration in patients with Behcet's disease (210 characters). Clin Rheumatol 2019;38:1437–45. 10.1007/s10067-018-04419-8 [DOI] [PubMed] [Google Scholar]
- 53. Shrotri M, Navaratnam AMD, Nguyen V, et al. Spike-antibody waning after second dose of BNT162b2 or ChAdOx1. Lancet 2021;398:385–7. 10.1016/S0140-6736(21)01642-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Gilbert PB, Montefiori DC, McDermott AB. Immune correlates analysis of the mRNA-1273 COVID-19 vaccine efficacy clinical trial. Science 2022;375:eab3435. 10.1126/science.abm3425 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
gutjnl-2021-326563supp001.pdf (1.1MB, pdf)
Data Availability Statement
Data are available in a public, open access repository. Quality-controlled and human DNA-removed sequence data are deposited in the European Nucleotide Archive under BioProject PRJEB48269. Additional datasets generated and/or analysed in this study are available from the corresponding author on reasonable request.