Abstract
Leptin receptor (LepR)‐positive cells are key components of the bone marrow hematopoietic microenvironment, and highly enrich skeletal stem and progenitor cells that maintain homeostasis of the adult skeleton. However, the heterogeneity and lineage hierarchy within this population has been elusive. Using genetic lineage tracing and single‐cell RNA sequencing, we found that Lepr‐Cre labels most bone marrow stromal cells and osteogenic lineage cells in adult long bones. Integrated analysis of Lepr‐Cre‐traced cells under homeostatic and stress conditions revealed dynamic changes of the adipogenic, osteogenic, and periosteal lineages. Importantly, we discovered a Notch3+ bone marrow sub‐population that is slow‐cycling and closely associated with the vasculatures, as well as key transcriptional networks promoting osteo‐chondrogenic differentiation. We also identified a Sca‐1+ periosteal sub‐population with high clonogenic activity but limited osteo‐chondrogenic potential. Together, we mapped the transcriptomic landscape of adult LepR+ stem and progenitor cells and uncovered cellular and molecular mechanisms underlying their maintenance and lineage specification.
Keywords: bone marrow stromal cells, LepR+ cells, periosteum, single‐cell RNA‐seq, skeletal stem/progenitor cells
Subject Categories: Development, Methods & Resources, Stem Cells & Regenerative Medicine
Extensive expression profiling highlights unexpected diversity of leptin receptor‐positive cell populations in the long bones with context‐dependent function.
Introduction
Multiple waves of stem and progenitor cells contribute to the development, maintenance, and remodeling of the skeleton (Takashima et al, 2007; Nagoshi et al, 2008; Morikawa et al, 2009; Maes et al, 2010; Park et al, 2012; Liu et al, 2013; Isern et al, 2014; Mizoguchi et al, 2014; Ono et al, 2014; Yang et al, 2014; Zhou et al, 2014a, 2014b). Our previous study showed that LepR marks skeletal stem and progenitor cells (SSPCs) in the adult bone marrow (Zhou et al, 2014a), which largely overlap with Cxcl12‐abundant reticular (CAR) cells (Omatsu et al, 2010) and Nestin‐GFPlow cells (Mendez‐Ferrer et al, 2010; Kunisaki et al, 2013). Genetic lineage tracing by Lepr‐Cre labels bone marrow stromal cells (BMSCs) in early postnatal mice that generate most osteoblasts (OBs) and adipocytes during aging or after irradiation (Zhou et al, 2014a). They also generate chondrocytes after bone fracture or articular injury (Mizoguchi et al, 2014; Zhou et al, 2014a). Conditional deletion of LepR from limb bones led to increased osteogenesis and decreased adipogenesis, suggesting that LepR regulates SSPC maintenance and differentiation (Yue et al, 2016). LepR+ cells localize in the perivascular region of the adult bone marrow, which produces a repertoire of extracellular matrix proteins and osteogenic factors to promote bone formation (Yue et al, 2016). LepR+ cells also secrete high levels of hematopoietic stem cell (HSC) maintenance factors to create the hematopoietic microenvironment (Ding et al, 2012; Greenbaum et al, 2013; Zhou et al, 2014a; Crane et al, 2017), highlighting their pivotal roles in orchestrating hematopoiesis and osteogenesis. In addition to LepR+/CAR/Nestin‐GFPlow cells, Pdgfrα+Sca‐1+ (PαS) cells (Morikawa et al, 2009), NG2+ cells (Kunisaki et al, 2013), and non‐myelinating Schwann cells (Yamazaki et al, 2011) were also proposed as critical components of the HSC niche. However, the heterogeneity of LepR+ cells and the extent to which they overlap with other HSC niche cells need to be addressed at single‐cell resolution.
By high‐throughput single‐cell RNA sequencing (scRNA‐seq), we and others have mapped the mouse bone marrow stroma in an unbiased way (Han et al, 2018; Baryawno et al, 2019; Baccin et al, 2020). However, transcriptomic profiling of unfractionated cells resulted in low resolution of SSPCs due to over‐representation of osteoblasts, chondrocytes, vascular endothelial cells, and fibroblasts (Han et al, 2018; Baryawno et al, 2019). scRNA‐seq analysis of Col2‐Cre‐traced cells in young and aged mice revealed a marrow adipogenic lineage precursor (MALP) population (Zhong et al, 2020), which highly resembles LepR+ cells (Zhou et al, 2014a). However, since Col2‐Cre also labels large numbers of chondrocytes and osteogenic lineage cells, the heterogeneity within MALPs was not resolved (Zhong et al, 2020). Another scRNA‐seq study analyzed Lepr‐Cre‐traced cells in steady state and 5‐FU‐treated mouse bone marrow (Tikhonova et al, 2019). Unfortunately, bone fragments containing large amounts of SSPCs in the endosteum and periosteum were excluded from this study, therefore, limited heterogeneity was observed within Lepr‐Cre‐traced cells (Tikhonova et al, 2019). Given that LepR+ cells are largely quiescent under steady state (Zhou et al, 2014a), integrated analysis of stress conditions that efficiently drive their differentiation along the adipogenic, osteogenic, or chondrogenic lineages could help reveal the intrinsic heterogeneity within LepR+ cells in adult mice.
Apart from the bone marrow and endosteum, SSPCs also exist in the periosteum to promote bone formation and fracture repair (Duchamp de Lageneste et al, 2018). Periosteal stem cells (PSCs) were identified in both long bones and calvaria, which mediate intramembranous ossification under steady state and repair fractures by endochondral ossification (Debnath et al, 2018). Mx1+αSMA+ periosteal SSCs (p‐SSCs) were also shown to regulate fracture healing in a CCR5‐dependent manner (Ortinau et al, 2019). Interestingly, Lepr‐Cre labels a sub‐population of periosteal cells, which are expanded in response to bone fracture (Mizoguchi et al, 2014). However, the cellular heterogeneity and functional divergence within periosteal Lepr‐Cre+ cells remain elusive. In this study, we performed single‐cell transcriptomic profiling and functional analysis of SSPCs in adult long bones by focusing on Lepr‐Cre‐traced cells. Integrated analysis under homeostatic and stress conditions (e.g., aging, rosiglitazone feeding, irradiation, and bone fracture) revealed novel cellular and molecular mechanisms underlying SSPC maintenance and lineage specification.
Results
Mapping Prrx1‐Cre‐ and Lepr‐Cre‐traced cells under homeostatic condition
During long bone development, Prrx1 is highly expressed by limb bud mesenchymal progenitors (Cserjesi et al, 1992). Therefore, genetic lineage tracing by Prrx1‐Cre labels all skeletal lineage cells in long bones, including SSPCs, osteoblasts, osteocytes, chondrocytes, and adipocytes (Logan et al, 2002). To resolve the heterogeneity within SSPCs, we crossed Prrx1‐Cre or Lepr‐Cre mice with a loxp‐STOP‐loxp‐tdTomato reporter line to perform genetic lineage tracing (Prrx1‐Cre; tdTomato and Lepr‐Cre; tdTomato, respectively), and compared the extent to which Prrx1‐Cre+ and Lepr‐Cre+ cells overlap in adult long bones. We also crossed a Col2.3‐GFP reporter line with Prrx1‐Cre; tdTomato mice (Prrx1‐Cre; tdTomato; Col2.3‐GFP) to exclude most OBs traced by Prrx1‐Cre during development. Lived non‐hematopoietic and non‐endothelial tdTomato+ GFP− cells (for Prrx1‐Cre), or tdTomato+ cells (for Lepr‐Cre) were sorted from the bone marrows and bone fragments of 8‐week‐old male mice by flow cytometry (Figs 1A and EV1A), followed by scRNA‐seq on 10X Genomics platform (Fig 1A). After quality control, we obtained 4,170 and 3,050 single cells from Prrx1‐Cre; tdTomato; Col2.3‐GFP and Lepr‐Cre; tdTomato mice, respectively.
Figure 1. Comparison of Prrx1‐Cre‐ and Lepr‐Cre‐traced cells in adult long bones.
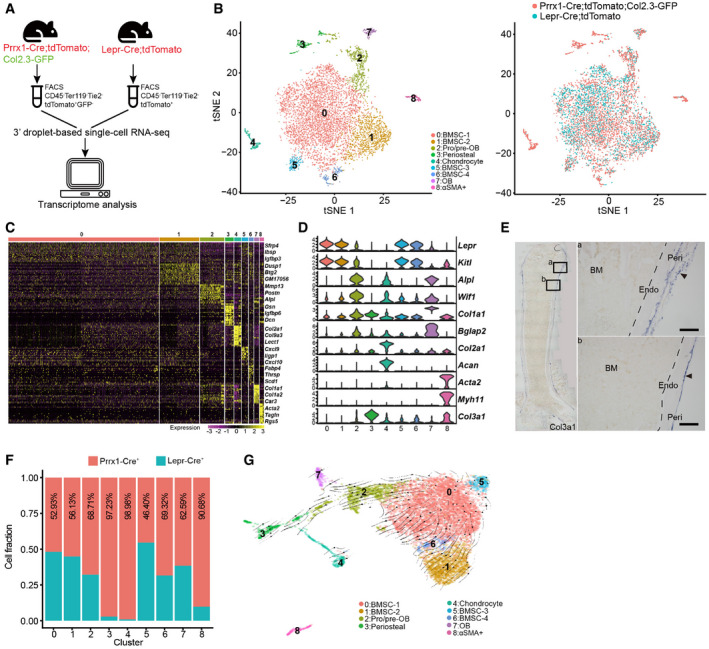
- Schematic overview of the scRNA‐seq workflow.
- t‐SNE plots showing integrated analysis of Prrx1‐Cre‐ (8‐week‐old mice, n = 3 males) and Lepr‐Cre‐traced (8‐week‐old mice, n = 4 males) cells. Cells were colored by clusters (left) or samples (right). Top 20 PCs were chosen for the clustering.
- Heatmap showing the relative expression levels (row‐wide Z score) of the 20 most significant markers for each cluster (rows) across cells in the 9 clusters (columns). Bars on the top were colored as in (B).
- Violin plots showing the expression of feature genes for each cluster.
- In situ hybridization of Col3a1 on femur sections. Col3a1 was expressed in the perichondral (a) and periosteal (b) regions, but not in the bone marrow. B: Bone; BM: Bone marrow; Peri: Periosteum; Endo: Endosteum. Scale bars are 100 µm. Arrowheads indicated the periosteum.
- Relative contribution to each cluster by Prrx1‐Cre‐ and Lepr‐Cre‐traced cells after normalization to total cells (4,170 cells in Prrx1‐Cre, 3,050 cells in Lepr‐Cre).
- UMAP plot showing the inferred trajectories by RNA velocity analysis.
Figure EV1. Further characterizations of Prrx1‐Cre‐ and Lepr‐Cre‐traced cells.
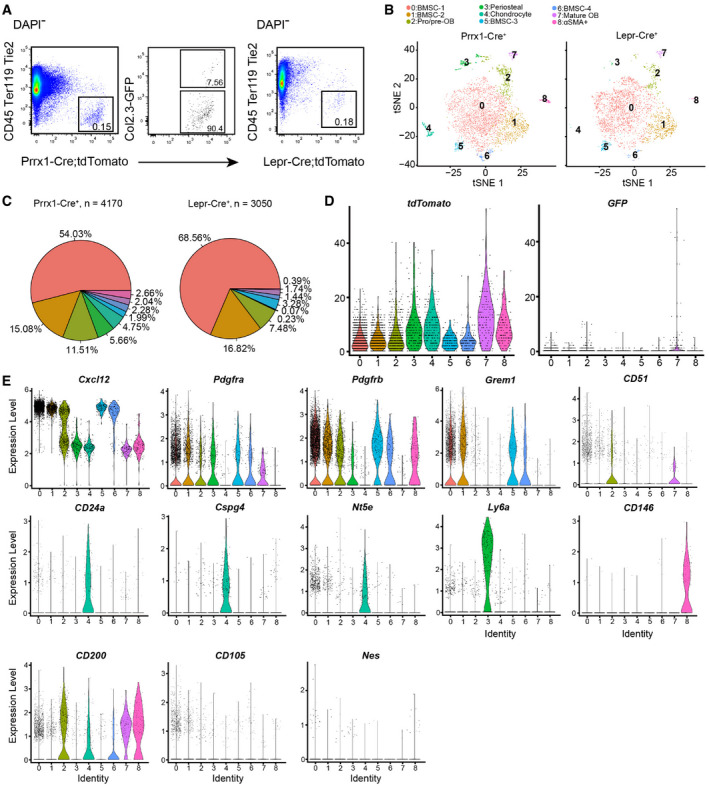
- Flow cytometry gating strategy for Prrx1‐Cre; tdTomato; Col2.3‐GFP and Lepr‐Cre; tdTomato mice.
- t‐SNE plots showing cells sorted from Prrx1‐Cre; tdTomato; Col2.3‐GFP (left) and Lepr‐Cre; tdTomato mice (right). Cells were colored by clustering.
- Pie graphs showing the cell frequency distribution. Colored as in (B).
- Violin plots showing the expression levels of tdTomato (left) and GFP (right) transcripts. Clusters are colored as in (B).
- Violin plots showing the expression levels of skeletal stem and progenitor cell markers in each cluster (colored as in B).
After correcting batch effects, we performed integrated analysis of Prrx1‐Cre‐ and Lepr‐Cre‐traced cells, which revealed nine subsets using non‐linear dimensionality reduction with t‐distributed stochastic neighbor embedding (tSNE) (Figs 1B and EV1B). Specifically, we identified subsets including BMSCs (clusters 0, 1, 5, and 6, expressing Lepr and Kitl) (Ding et al, 2012; Zhou et al, 2014a), pro/pre‐OBs (cluster 2, expressing Alpl and Wif1) (Delgado‐Calle et al, 2011; Baker et al, 2015), OBs (cluster 7, expressing Col1a1 and Bglap2) (Kratochwil et al, 1993; Li et al, 2010), chondrocytes (cluster 4, expressing Col2a1 and Acan) (Doege et al, 1991; Chan et al, 1995), and αSMA+ cells (cluster 8, expressing Acta2 and Myh11; Fig 1C and D). We also identified a subset that highly expressed Col3a1 (Fig 1D). RNA in situ hybridization showed that Col3a1+ cells are mainly localized in the periosteal regions of femur metaphysis and diaphysis (Fig 1E). Interestingly, periosteal cells (cluster 3), chondrocytes (cluster 4), and αSMA+ cells (cluster 8) were mainly derived from Prrx1‐Cre (Figs 1F and EV1C). In contrast, BMSCs (clusters 0, 1, 5, 6) and osteogenic lineage cells (cluster 2, 7) were traced by both Cre lines, suggesting that Lepr‐Cre could reliably label SSPCs and their descendants in adult mice. Consistent with the fact that Col2.3‐GFP labels most but not all OBs in an age‐dependent manner (Kalajzic et al, 2002), we could still observe an OB subset (cluster 7) within Prrx1‐Cre+ cells even after excluding Col2.3‐GFP+ cells (Fig 1B). We confirmed that most cells within this subset expressed tdTomato but not GFP transcripts (Fig EV1D). Pseudotime analysis by RNA velocity (Bergen et al, 2020) showed an osteogenic differentiation trajectory from BMSCs (cluster 0) to pro/pre‐OBs (cluster 2) and OBs (cluster 7; Fig 1G).
We also systematically analyzed the expression of well‐known SSPC markers in our dataset (Fig EV1E). Cxcl12 (Sugiyama et al, 2006), Pdgfra (Morikawa et al, 2009), Pdgfrb (Koide et al, 2007), and Grem1 (Worthley et al, 2015) were highly expressed in BMSCs (Fig EV1E). CD105 (Chan et al, 2009) is also expressed in BMSCs, although at a lower level (Fig EV1E). Itgav (CD51) (Pinho et al, 2013) was highly expressed in pro/pre‐OBs and OBs, while CD24a (Rodeheffer et al, 2008; Ambrosi et al, 2017), NG2 (Cspg4) (Kunisaki et al, 2013), and CD73 (Nt5e) (Breitbach et al, 2018) were highly expressed in chondrocytes (Fig EV1E). Interestingly, Ly6a (Sca‐1) (Morikawa et al, 2009) was highly expressed in periosteal cells, while the human BMSC marker Mcam (CD146) (Sacchetti et al, 2007) was highly expressed in αSMA+ cells (Fig EV1E). CD200 (Chan et al, 2015) showed a broad distribution among BMSCs, OBs, chondrocytes, and αSMA+ cells (Fig EV1E). In contrast, Nes (Nestin) (Mendez‐Ferrer et al, 2010) was expressed in very few cells among all subsets (Fig EV1E).
Integrated analysis of Lepr‐Cre‐traced cells under homeostatic and stress conditions
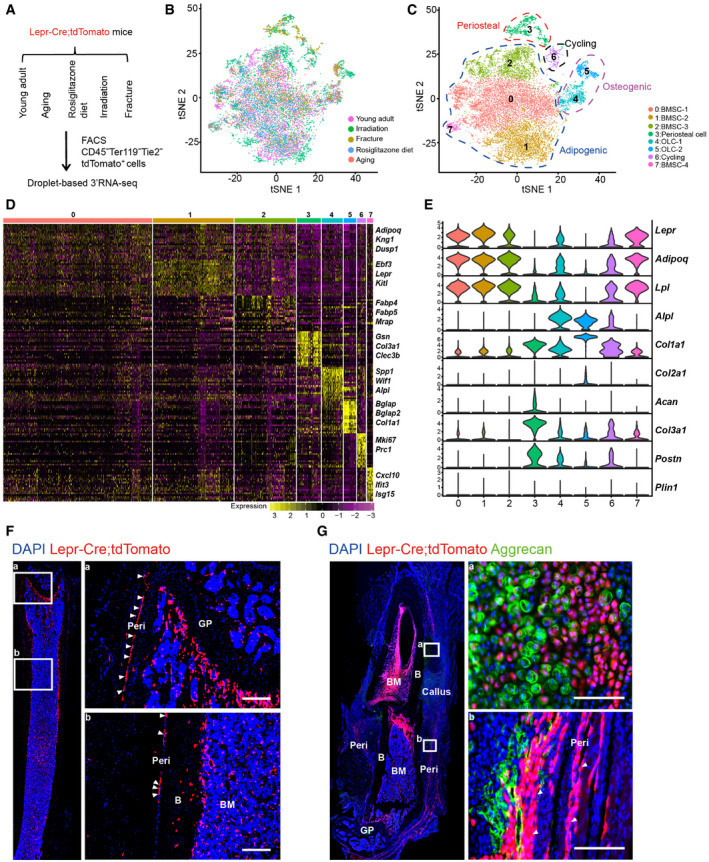
We then focused on Lepr‐Cre; tdTomato mice to analyze the lineage specification of SSPCs under adipogenic or osteo‐chondrogenic stress conditions (Fig 2A). Rosiglitazone is a potent agonist of Pparγ, which has been shown to promote marrow fat accumulation after prolonged feeding (Ackert‐Bicknell et al, 2009; Horowitz et al, 2017; Farrell et al, 2021). Aging also promotes marrow fat accumulation, while irradiation leads to accelerated adipogenic and osteogenic differentiation by LepR+ BMSCs (Zhou et al, 2014a). In contrast, bone fracture drives SSPC differentiation along the osteochondral lineage to promote endochondral ossification and bone remodeling (Worthley et al, 2015). After quality control and filtering, we obtained 5,022 cells from 8‐week‐old mice (young adult, males and females), 2,619 cells from 12‐month‐old mice (aging, females), 2,545 cells from 10‐week‐old mice on rosiglitazone diet for 5 weeks (fed at 5 weeks old, females), 4,514 cells from sub‐lethally irradiated 8‐week‐old mice (irradiated at 7 weeks old, males and females), and 2,524 cells from the fractured femurs of 8‐week‐old mice (unilaterally fractured at 7 weeks old, males and females; Fig 2A).
Figure 2. Integrated analysis of Lepr‐Cre‐traced cells under homeostatic and stress conditions.
-
ASchematic overview of scRNA‐seq strategies in Lepr‐Cre; tdTomato mice. Young adult: 8‐week‐old mice (4 males and 2 females); Aging: 12‐month‐old mice (4 females); Rosiglitazone diet: 10‐week‐old mice (feeding started at 5 weeks old, 2 females); Irradiation: 8‐week‐old mice (sub‐lethally irradiated at 7 weeks old, 2 males and 2 females); Fracture: 8‐week‐old mice (femur fractured at 7 weeks old, 2 males and 2 females).
-
B, Ct‐SNE plots showing 17,224 CD45− Ter119− Tie2− tdTomato+ single cells from all 5 conditions. Cells are colored by conditions (B) or clustering (C). The adipogenic, osteogenic, periosteal, and cycling cells were highlighted by dotted lines in (C). Top 20 PCs were chosen for the clustering.
-
DHeatmap showing the relative expression levels (row‐wide Z score) of the 20 most significant markers for each cluster (rows) across cells in the 8 clusters (columns). Bars on the top were colored as in (C).
-
EViolin plots showing the expression of feature genes for each cluster.
-
FImmunofluorescent images of the distal femur in 8‐week‐old Lepr‐Cre; tdTomato mice. Arrowheads indicated tdTomato+ periosteal cells. Two representative periosteal regions were magnified (a and b). GP: Growth plate. B: Bone. BM: Bone marrow. Peri: Periosteum. Scale bars are 100 µm.
-
GImmunofluorescent images of the distal femur in 8‐week‐old Lepr‐Cre; tdTomato mice 7 days after fracture. Aggrecan (green), tdTomato (red), DAPI (blue). Arrowheads indicated tdTomato+ periosteal cells. Two representative regions were magnified (a and b). Scale bars are 100 µm.
After correcting batch effects, integrated analysis revealed eight subsets based on marker expressions (Figs 2B and C and EV2C), which could be grouped into three lineages: (i) Adipogenic lineage cells (clusters 0, 1, 2, and 7) that expressed Lepr, Adipoq, and Lpl (Kindblom et al, 2005); (ii) Osteogenic lineage cells (OLCs) that expressed Alpl and Col1a1 (clusters 4 and 5); (iii) Periosteal lineage cells that expressed Col3a1 and Postn (Duchamp de Lageneste et al, 2018) (cluster 3), as well as Acan (Fig 2 D and E); and (iv) Cycling cells that expressed Mki67 (Tikhonova et al, 2019) (cluster 6), which were mainly induced by irradiation (Fig EV2C and D). Similar cell clustering was observed after segregating females (expressing Xist, Esr1, or Esr2) and males (expressing Ddx3y, Eif2s3y, Kdm5d, or Uty) cells (Skelly et al, 2018; Fig EV2A and B).
Figure EV2. Further characterizations of Lepr‐Cre‐traced cells under homeostatic and stress conditions.
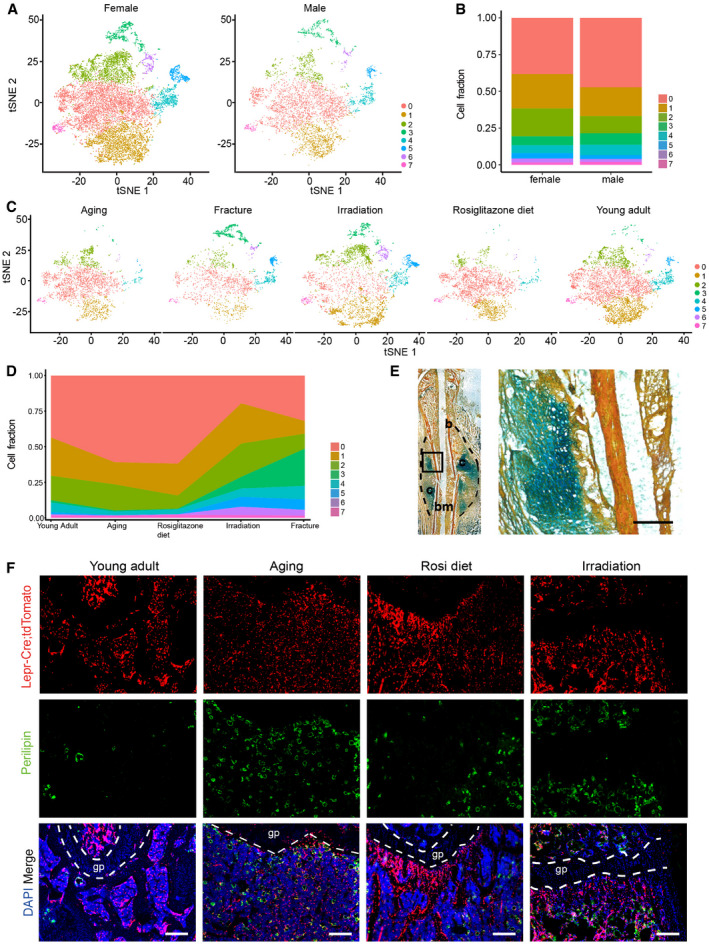
- t‐SNE plots showing segregation of female and male cells. Colored as in Fig 2C.
- Cluster frequencies after segregation of female and male cells.
- t‐SNE plots showing Lepr‐Cre‐traced cells split by conditions. Colored as in Fig 2C.
- Frequencies of different clusters under homeostatic and stress conditions.
- Movat’s pentachrome staining of the bone callus 7 days after femur fracture. Bone (b), cartilage (c), and bone marrow (bm) regions were indicated (left panel). The dashed area indicates the fracture callus. An enlarged view of the boxed cartilaginous area in the left panel is shown on the right. Scale bar is 100 µm.
- Immunofluorescent images of Perilipin (green) in the distal femur metaphysis of Lepr‐Cre; tdTomato mice under homeostatic and adipogenic stress conditions. gp: Growth plate. Scale bars are 100 µm.
Although only a few Lepr‐Cre+ periosteal cells could be detected in young adults (Figs 2F and EV2C and D), they were expanded after bone fracture (Figs 2G and EV2C–E) and irradiation (Fig EV2C and D). As compared to young adults, the frequency of OLCs (clusters 4 and 5) was higher after bone fracture and irradiation, and lower under adipogenic stresses (Fig EV2C and D). The number of bone marrow adipocytes was dramatically increased in aged mice, as well as after rosiglitazone diet feeding and sub‐lethal irradiation (Fig EV2F). Notably, the adipogenic subsets (clusters 0, 1, 2, and 7) highly expressed Adipoq and Lpl (Fig 2E), but not the mature adipocyte marker Perilipin (Plin1) (Fig 2E). This indicated that they represent Lepr‐Cre+ BMSCs with adipogenic potential, but not terminally differentiated bone marrow adipocytes containing large lipid droplets, which could not be sorted by flow cytometrically for scRNA‐seq analysis. As far as we are concerned, these adipogenic lineage cells are similar to previously reported Adipo‐CAR cells (Baccin et al, 2020) and MALPs (Zhong et al, 2020).
Next, we dissected the heterogeneity and lineage hierarchy within each lineage by integrated analysis of the homeostatic and stress conditions.
Heterogeneity analysis within the adipogenic lineage cells
The adipogenic lineage cells were sub‐divided into six subsets (Figs 3 A and EV3A). Cluster 1 expressed the highest level of Lepr, Kitl, and Cxcl12, as well as angiogenesis‐related genes such as Vegfa, Notch3, and Col4a1 (Domenga et al, 2004; Van Agtmael et al, 2010; Chen et al, 2019; Fig 3B). Cell cycle analysis revealed that cluster 1 contains more cells in the G1 phase and less cells in the S phase (Fig 3C). Consistent with this, bromodeoxyuridine (BrdU) incorporation analysis in 8‐week‐old Lepr‐Cre; tdTomato mice showed fewer Lepr‐Cre+Notch3+ cells in the S phase as compared to Lepr‐Cre+Notch3− cells (Fig 3D). Immunostaining on femur sections showed that Lepr‐Cre+Notch3+ BMSCs represented adventitial cells closely associated with the vasculatures (Fig 3E and F), including sinusoids (Fig 3Ea), arterioles (Fig 3Eb), and arteries (Fig 3Ec), while Lepr‐Cre+Notch3− BMSCs represented reticular cells located in between vasculatures (Fig 3Ed).
Figure 3. Identification of a Notch3+ sub‐population within the adipogenic lineage cells.
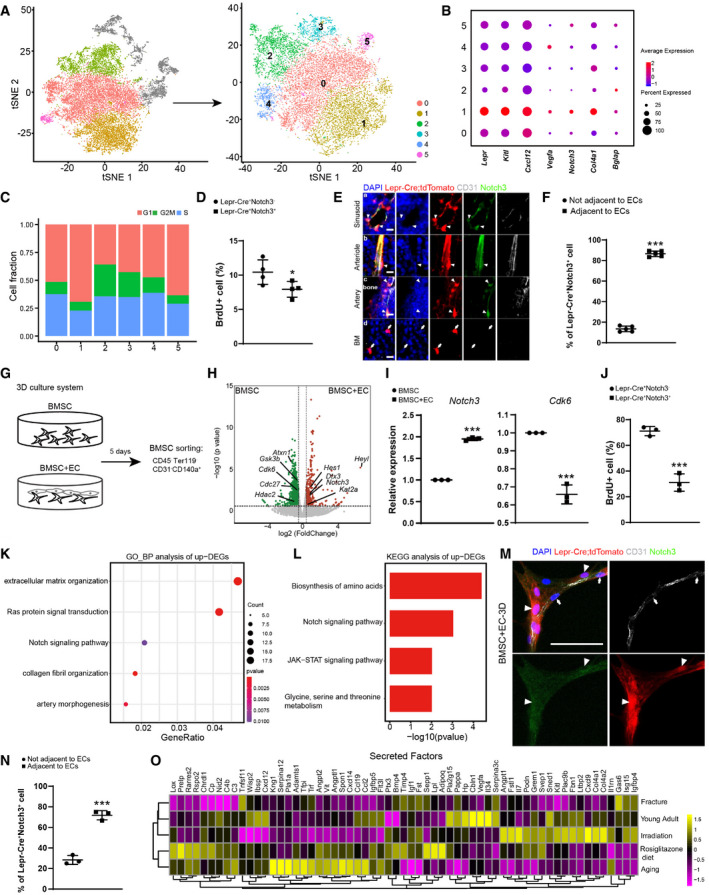
-
At‐SNE plots showing the original (left) and re‐clustered (right) adipogenic lineage cells by integrated analysis of homeostatic and stress conditions. Top 15 PCs were chosen for the clustering.
-
BDot plots showing the gene expression patterns of selected genes in each cluster.
-
CStacked bar charts showing the cell cycle status in all clusters.
-
DFlow cytometry analysis of BrdU incorporation in uncultured Lepr‐Cre+Notch3+/− BMSCs (n = 4 independent experiments). Eight‐week‐old Lepr‐Cre; tdTomato mice were given a single intraperitoneal injection of BrdU (100 mg/kg body mass) and maintained on 0.5 mg/ml of BrdU in the drinking water for 14 days.
-
EImmunofluorescent images of the distal femur in 8‐week‐old Lepr‐Cre; tdTomato mice. Notch3 (green), tdTomato (red), CD31 (gray), DAPI (blue). Sinusoid (a), arteriole (b), artery (c), and avascular bone marrow (BM, d) regions are shown. Arrowheads indicated Notch3+ tdTomato+ adventitial cells closely associated with ECs. Arrows indicated Notch3− tdTomato+ reticular cells in the bone marrow that were not associated with ECs. Scale bars are 10 µm.
-
FLocalization quantification of Lepr‐Cre+Notch3+ cells relative to bone marrow ECs (n = 5 biological replicates from three independent experiments). Cells within 5 µm diameter of ECs were considered adjacent.
-
GGraphical illustration of the 3D co‐culture system.
-
HVolcano plot showing DEGs in the bulk RNA‐seq analysis (n = 3 biological replicates). Green dots represent down‐regulated genes. Red dots represent up‐regulated genes.
-
IqPCR analysis of Notch3 and Cdk6 expression (n = 3 independent experiments).
-
JFlow cytometry analysis of BrdU incorporation in Lepr‐Cre+Notch3+/− BMSCs after 3D co‐cultured with bone marrow ECs (n = 3 independent experiments). BrdU was administered at a final concentration of 10 µM from culture day 1 to day 5.
-
K, LGO terms (K) and KEGG pathways (L) enriched in up‐regulated genes.
-
MRepresentative confocal images of BMSCs 3D co‐cultured with bone marrow ECs. Z‐stack projection is shown. Notch3 (green), tdTomato (red), CD31 (gray), DAPI (blue). Arrowheads indicated Notch3+tdTomato+ adventitial cells. Arrows indicated bone marrow ECs. Scale bar is 50 µm.
-
NLocalization quantification of Lepr‐Cre+Notch3+ cells relative to bone marrow ECs after 3D co‐culture (n = 3 biological replicates from three independent experiments). Cells within 25 µm diameter of ECs were considered adjacent.
-
OHeatmap showing the average expression levels (column‐wide Z score) of secreted factors with clustering.
Data information: All data represented Mean ± SD. The statistical significance of differences was analyzed by two‐tailed unpaired Student’s t‐test. *P < 0.05, ***P < 0.001.
Figure EV3. Further characterizations of adipogenic lineage cells.
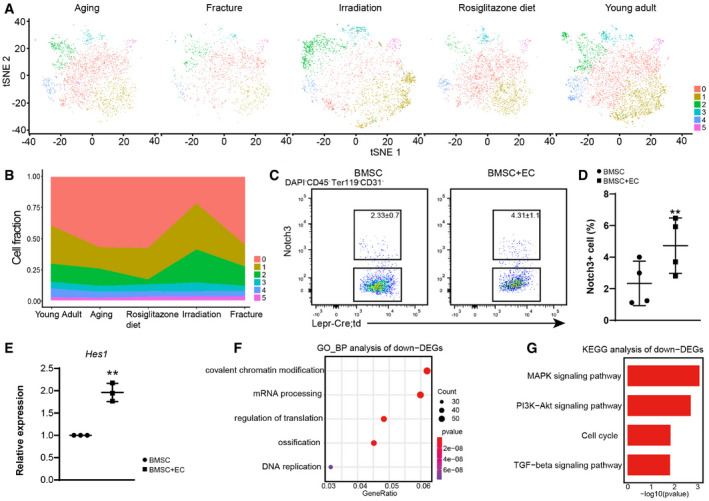
-
At‐SNE plots showing adipogenic sub‐clusters split by conditions. Colored as in Fig 3A.
-
BFrequencies of different adipogenic clusters under homeostatic and stress conditions.
-
C, DFlow cytometry quantification of Lepr‐Cre+Notch3+ BMSCs cultured with or without bone marrow ECs ex vivo (n = 4 independent experiments). The statistical significance of differences was analyzed by two‐tailed paired Student’s t‐test.
-
EqPCR analysis of Hes1 expression (n = 3 independent experiments). The statistical significance of differences was analyzed by two‐tailed unpaired Student’s t‐test.
-
F, GGO terms (F) and KEGG pathways (G) enriched in down‐regulated genes.
Data information: All data represented Mean ± SD. **P < 0.01.
To functionally test whether vasculature association regulates cell cycle and Notch signaling in BMSCs, we performed 3D culture of primary BMSCs with or without bone marrow endothelial cells (ECs) in 4% gelatin methacryloyl (GelMA) hydrogels (Fig 3G). RNA‐seq and qPCR analyses revealed that co‐culture with bone marrow ECs significantly increased Notch3 and Hes1 expression, and significantly decreased Cdk6 expression in BMSCs (Figs 3H and I, and Fig EV3E). Flow cytometry analysis showed significantly increased frequency of Notch3+ cells in Lepr‐Cre+ BMSCs co‐cultured with ECs (Fig EV3C and D). BrdU incorporation analysis showed fewer Lepr‐Cre+Notch3+ cells in S phase as compared to Lepr‐Cre+Notch3− cells during ex vivo culture (Fig 3J). Gene ontology (GO) and KEGG pathway analyses showed significant up‐regulation of genes related to Notch signaling pathway (Fig 3K and L), while genes related to ossification, MAPK signaling, cell cycle, and TGF‐b signaling pathways were significantly down‐regulated (Fig EV3F and G). Consistent with the in vivo data (Fig 3E), immunostaining in the 3D co‐culture system showed Lepr‐Cre+Notch3+ BMSCs closely associated with ECs (Fig 3M and N). Thus, we identified a Notch3+ BMSC sub‐population that corresponds to adventitial cells, which are maintained in a slow cycling state by ECs.
As compared to cluster 1, cluster 0 contains more cells in S phase (Fig 3C), which is sensitive to irradiation (Fig EV3A and B). Cluster 2 enriched ossification genes such as Bglap (Fig 3B), which was expanded after irradiation and fracture (Fig EV3A and B). In contrast, cluster 3 enriched adipocyte marker genes (Dataset EV2), which was expanded by irradiation (Fig EV3A and B). Clusters 4 and 5 enriched immediate early response genes and interferon‐stimulated genes (ISGs; Dataset EV2), respectively, the frequency of which remain largely constant under homeostatic and stress conditions (Fig EV3A and B). We also analyzed the secretory profiles in the adipogenic lineage cells (Fig 3O). As compared to young mice, the HSC niche factors Kitl and Cxcl12 were down‐regulated in aged, rosiglitazone‐fed, and fractured mice (Fig 3O), suggesting a compromised HSC niche. In contrast to Cxcl12, Kitl expression was increased after irradiation (Fig 3O), consistent with a previous study that bone marrow adipogenesis after irradiation could promote hematopoietic regeneration via Kitl secretion (Zhou et al, 2017). Interestingly, the secretory profiles of aged and rosiglitazone‐fed mice clustered together, implicating Pparγ activation as a potential mechanism underlying bone marrow aging (Sadie‐Van Gijsen et al, 2013).
Heterogeneity analysis within the osteogenic lineage cells
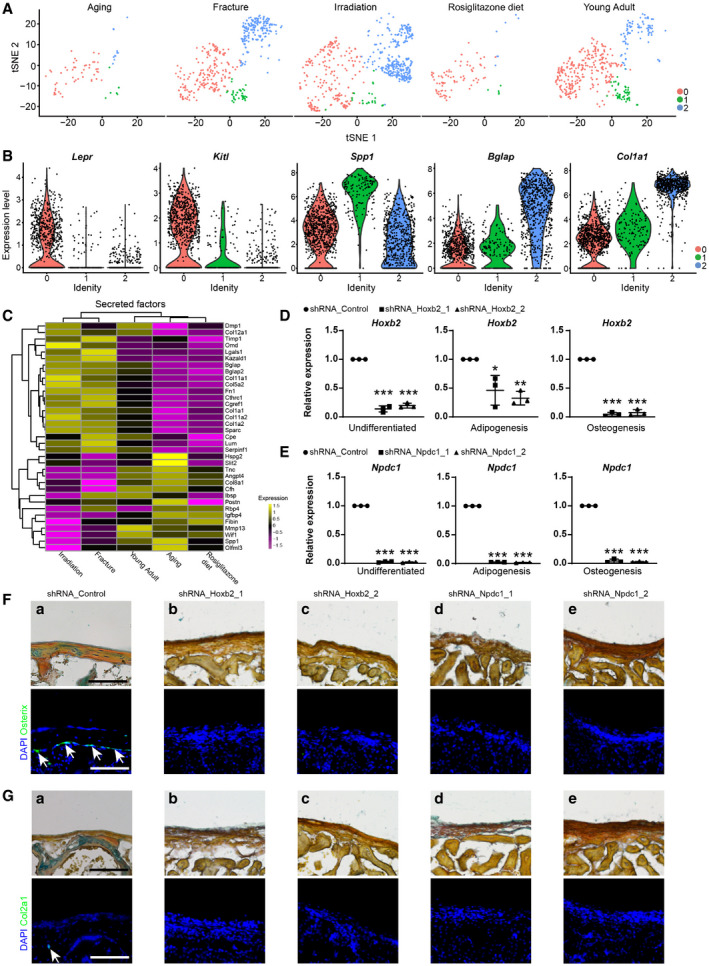
The osteogenic lineage cells were sub‐divided into three subsets (Figs 4A and EV4A), including Pro‐OBs (cluster 0), Pre‐OB (cluster 1), and mature OB (cluster 2). Pseudotime analysis revealed an osteogenic differentiation continuum (Fig 4B). BMSC markers Lepr, Kitl, and Ebf3 (Seike et al, 2018) were dramatically down‐regulated following lineage commitment, while the osteogenic factors, such as Spp1, Postn and Aspn, were gradually up‐regulated (Figs 4C and EV4B). As cells underwent terminal differentiation into mature OBs, they expressed the highest level of mineralization factors such as Col1a1, Bglap, and Bglap2 (Figs 4C and EV4B). As compared to young adult mice, the frequency of mature OBs was lower in aged and rosiglitazone‐fed mice, but much higher after bone fracture and irradiation (Fig 4D). Consistent with this, secreted bone matrix factors such as Col1a1, Col1a2, Sparc, and Bglap were down‐regulated in aged and rosiglitazone‐fed mice (Fig EV4C), and up‐regulated after bone fracture and irradiation (Fig EV4C).
Figure 4. Identification of candidate osteo‐chondrogenic regulators promoting fracture repair.
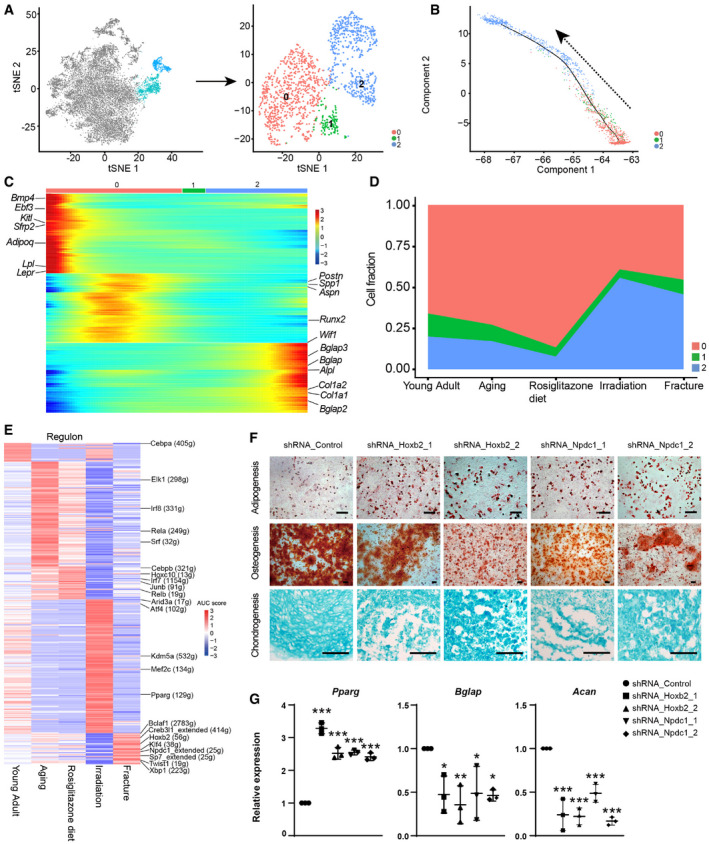
- t‐SNE plots showing the original (left) and re‐clustered (right) osteogenic lineage cells by integrated analysis of homeostatic and stress conditions. Top 10 PCs were chosen for the clustering.
- Pseudotime analysis by Monocle 2. Arrow indicated inferred direction of the differentiation trajectory.
- Heatmap showing pseudotime‐dependent gene expression. Bars on the top were colored as in (A). Representative genes are highlighted on both sides.
- Frequencies of different osteogenic clusters under homeostatic and stress conditions.
- Heatmap showing the AUC score of regulons enriched in each stress. Z‐score (row scaling) was calculated. Representative regulons were highlighted on the right.
- Representative images of adipogenic, osteogenic and chondrogenic differentiation following shRNA knockdown in Lepr‐Cre+ BMSCs. Scale bars are 100 µm.
- qPCR analysis of the expression levels of Pparg, Bglap, and Acan in control or knockdown group (n = 3 independent experiments). The statistical significance of differences was analyzed by one‐way ANOVAs with Tukey’s multiple comparison tests. Data represented Mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001.
Source data are available online for this figure.
Figure EV4. Further characterizations of osteogenic lineage cells.
-
At‐SNE plots showing osteogenic sub‐clusters split by conditions. Colored as in Fig 4A.
-
BViolin plots showing the expression levels of selected marker genes in each sub‐cluster.
-
CHeatmap showing the secreted factors enriched in osteogenic clusters under homeostatic and stress conditions with clustering. Z‐score (row scaling) was calculated.
-
D, EqPCR analysis of Hoxb2 (D) and Npdc1 (E) knockdown efficiency in BMSC before and after adipogenic or osteogenic differentiation (n = 3 independent experiments). The statistical significance of differences was analyzed by two‐tailed unpaired Student’s t test. Data represented Mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001.
-
FRepresentative renal sub‐capsular transplantation images. Movat’s pentachrome (upper panels) and immunostaining of Osterix (Sp7, lower panels) were performed to analyze bone ossicles 4 weeks after transplantation of control (a), Hoxb2‐ (b and c) or Npdc1‐deficient (d and e) BMSCs. Yellow stain: bone matrix and fibrotic tissue; Blue stain: cartilage matrix. Arrows indicated Osterix+ osteolineage cells. DAPI indicated the nucleus. Scale bars are 50 μm.
-
GRepresentative renal sub‐capsular transplantation images. Movat’s pentachrome (upper panels) and immunostaining of Col2a1 (lower panels) were performed to analyze bone ossicles 4 weeks after transplantation of control (a), Hoxb2‐ (b and c) or Npdc1‐deficient (d and e) BMSCs. Yellow stain: bone matrix and fibrotic tissue; Blue stain: cartilage matrix. Arrow indicated Col2a1+ chondrocyte. DAPI indicated the nucleus. Scale bars are 50 μm.
To explore gene regulatory networks (regulons) underlying lineage specification, we applied a single‐cell regulatory network inference and clustering (SCENIC) method to score the activity of regulons by an AUCell algorithm (AUC score), which reflects the co‐expression of transcription factors (TFs) and their downstream target genes in each individual cell (Aibar et al, 2017). In addition to previously reported osteogenic regulons, such as Twist1, Atf4, Klf4, and Mef2c (Yang et al, 2004; Arnold et al, 2007; Miraoui et al, 2010; Kim et al, 2014), SCENIC analysis revealed that Hoxb2 and Npdc1 regulons were up‐regulated after bone fracture (Fig 4E). To functionally test their roles in BMSC regulation, we knocked down Hoxb2 and Npdc1 in BMSCs by shRNA (Fig EV4D and E) and performed in vitro trilineage differentiation. Interestingly, knockdown of Hoxb2 or Npdc1 significantly increased adipogenic differentiation and decreased osteo‐chondrogenic differentiation (Fig 4F), which was confirmed by qPCR analysis of the adipogenic (Pparg), osteogenic (Bglap), and chondrogenic (Acan) markers (Fig 4G). Renal sub‐capsular transplantation experiments showed that knockdown of Hoxb2 or Npdc1 impaired the osteo‐chondrogenic differentiation of BMSCs in vivo (Fig EV4F and G). Thus, these data implicated Hoxb2 and Npdc1 as potential regulators of osteo‐chondrogenic differentiation during fracture repair.
Heterogeneity analysis within the periosteal lineage cells
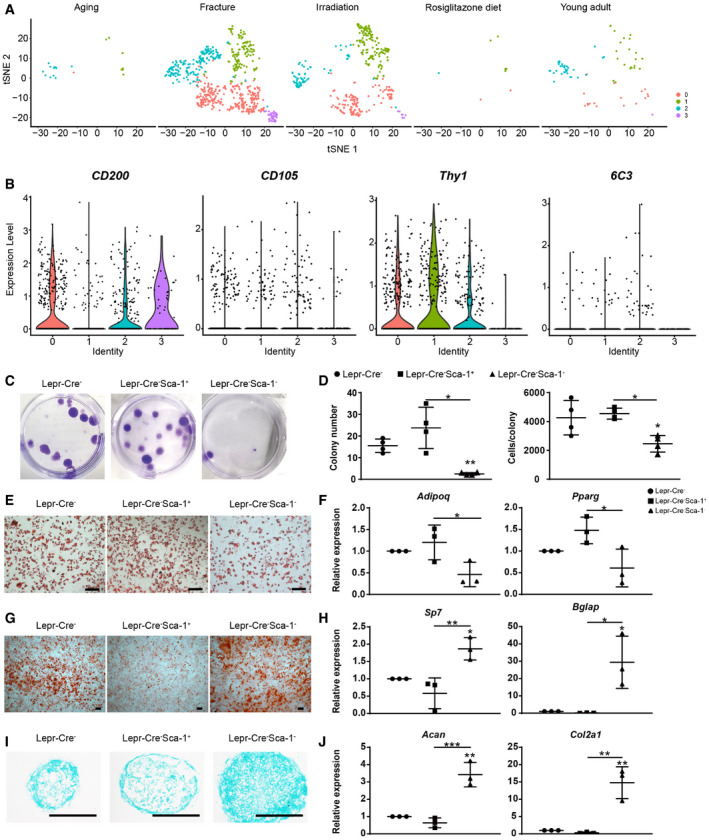
The periosteal lineage cells were expanded after irradiation and fracture (Fig EV5A), which were sub‐divided into four subsets (Fig 5A). Importantly, all subsets expressed high levels of periosteal marker Ctsk (Debnath et al, 2018) but not Lepr (Fig 5B), suggesting that they correspond to CTSK+ periosteal cells (Debnath et al, 2018). Cluster 0 highly expressed tenocyte marker Tnmd (Docheva et al, 2005), tissue remodeling factor Ptx3 (Grcevic et al, 2018), as well as low levels of chondrocyte markers (Fig 5B). Cluster 1 highly expressed Ly6a (Sca‐1) and CD34 (Fig 5B). Cluster 2 highly expressed myofibroblast marker Acta2 (αSMA; Roy et al, 2007; Fig 5B), reminiscent of the αSMA+ p‐SSCs (Ortinau et al, 2019). Cluster 3 highly expressed chondrocyte markers Col2a1, Sox9, and Acan (Fig 5B), as well as PSC markers (CD200+CD105−Thy1−6C3−; Debnath et al, 2018; Fig EV5B).
Figure EV5. Further characterizations of periosteal lineage cells.
-
At‐SNE plots showing periosteal sub‐clusters split by conditions. Colored as in Fig 5A.
-
BViolin plots showing the expression of PSC marker genes in each sub‐cluster.
-
C, DRepresentative CFU‐F colony images with quantifications. Two hundred DAPI− CD45− Ter119− Tie2− tdTomato− (Lepr‐Cre−), DAPI− CD45− Ter119− Tie2− tdTomato− Sca‐1+ (Lepr‐Cre− Sca‐1+) or DAPI− CD45− Ter119− Tie2− tdTomato− Sca‐1− (Lepr‐Cre− Sca‐1−) cells were sorted from the limb bone periosteum of Lepr‐Cre; tdTomato mice and cultured for 8 days before crystal violet staining (C) and quantifications (D). The average number of CFU‐F colonies formed and the average cell number per colony are shown (D) (n = 4 independent experiments).
-
E, FRepresentative oil red staining after 7 days of adipogenic differentiation in Lepr‐Cre+ and Lepr‐Cre− subsets (E) and qPCR analysis of Adipoq and Pparg expression (F) (n = 3 independent experiments). Scale bars are 100 µm.
-
G, HRepresentative alizarin red staining after 14 days of osteogenic differentiation (G) in Lepr‐Cre− subsets and qPCR analysis of Sp7 and Bglap (H) (n = 3 independent experiments). Scale bars are 200 µm.
-
I, JRepresentative alcian blue staining after 21 days of chondrogenic differentiation (I) in Lepr‐Cre− subsets and qPCR analysis of Acan and Col2a1 expression (J) (n = 3 independent experiments). Scale bars are 100 µm.
Data information: The statistical significance of differences was analyzed by one‐way ANOVAs with Tukey’s multiple comparison tests. All data represented Mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001.
Figure 5. Delineating the periosteal lineage cells.
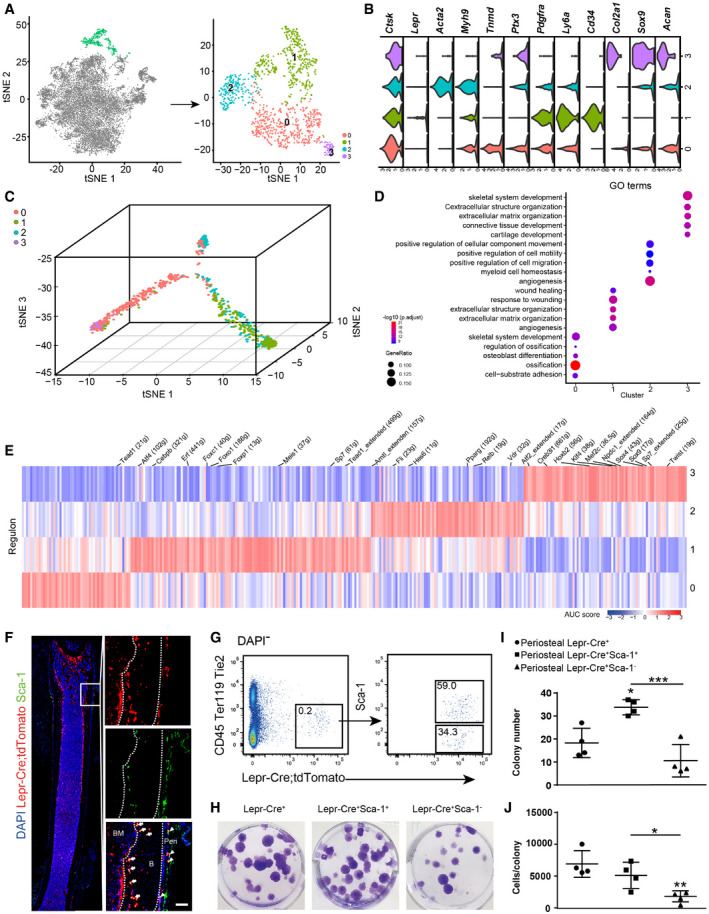
-
At‐SNE plots showing the original (left) and re‐clustered (right) periosteal lineage cells by integrated analysis of homeostatic and stress conditions. Top 10 PCs were chosen for the clustering.
-
BViolin plots showing the expression of feature genes for each cluster.
-
CPseudotime trajectories of four clusters. Cells are colored as in (A).
-
DDot plots showing the top5 GO terms enriched for each cluster.
-
EHeatmap showing the AUC score of regulons enriched in each cluster. Z‐score (column scaling) was calculated. Representative regulons are highlighted on the top.
-
FImmunostaining of Sca‐1 on femur sections of 8‐week‐old Lepr‐Cre; tdTomato mice. Arrows indicate Sca‐1+ tdTomato+ cells inside cortical bones of the femur diaphysis. Arrowheads indicate Sca‐1+ tdTomato+ cells in the femur periosteum. B: Bone. BM: Bone marrow. Peri: Periosteum. Scale bar is 100 µm.
-
GRepresentative flow cytometry plots of long bone periosteum in 8‐week‐old Lepr‐Cre; tdTomato mice. The percentage of DAPI− CD45− Ter119− Tie2− tdTomato+ Sca‐1+/− cells are shown.
-
HRepresentative CFU‐F colony images. Two hundred DAPI− CD45− Ter119− Tie2− tdTomato+ (Lepr‐Cre+), DAPI− CD45− Ter119− Tie2− tdTomato+ Sca‐1+ (Lepr‐Cre+Sca‐1+), or DAPI− CD45− Ter119− Tie2− tdTomato+ Sca‐1− (Lepr‐Cre+ Sca‐1−) cells were sorted from the limb bone periosteum of Lepr‐Cre; tdTomato mice and cultured for 8 days, followed by crystal violet staining.
-
I, JThe average number of CFU‐F colonies formed (I) and the average cell number per colony (J) are shown (n = 4 independent experiments). The statistical significance of differences was assessed using one‐way ANOVAs with Tukey’s multiple comparison tests. All data represented Mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001.
Source data are available online for this figure.
Pseudotime analysis showed that clusters 0 and 3 are closely related, while cluster 2 is intermediate between clusters 0 and 1 (Fig 5C). GO analysis revealed that clusters 0 and 3 enriched genes regulating skeletal development, and that clusters 1 and 2 enriched genes regulating angiogenesis (Fig 5D). Interestingly, SCENIC analysis showed enrichment of both chondrogenic (Sox4 and Sox9) and osteogenic (Twist and Sp7) regulons in cluster 3, while early‐stage adipogenic regulon Cebpb was enriched in cluster 1 (Fig 5E), implicating their distinct differentiation potentials.
Although a similar Sca‐1+ subset (cluster 1 in this study) has been previously identified within CTSK+ periosteal cells (Debnath et al, 2018), its localization, clonogenic and differentiation potentials remain elusive. To test this, we first analyzed the femur sections of 8‐week‐old Lepr‐Cre; tdTomato mice (Fig 5F) and found Lepr‐Cre+ Sca‐1+ cells in the periosteum and cortical bones (Fig 5F). We then sorted Lepr‐Cre+ Sca‐1+ cells from the periosteum of unfractured femurs by flow cytometry (Fig 5G) and compared them with Lepr‐Cre+ and Lepr‐Cre+ Sca‐1− cells by colony‐forming unit‐fibroblast (CFU‐F) culture ex vivo. Lepr‐Cre+ Sca‐1+ cells formed significantly more colonies as compared to Lepr‐Cre+ and Lepr‐Cre+ Sca‐1− cells (Fig 5H and I), while the colonies formed by Lepr‐Cre+ Sca‐1− cells were significantly smaller than Lepr‐Cre+ and Lepr‐Cre+ Sca‐1+ cells (Fig 5J). We also sorted Lepr‐Cre−, Lepr‐Cre− Sca‐1+, and Lepr‐Cre− Sca‐1− cells from the periosteum of unfractured femurs. Lepr‐Cre− Sca‐1− cells formed significantly fewer colonies with significantly smaller colony size as compared to Lepr‐Cre− and Lepr‐Cre− Sca‐1+ cells (Fig EV5C and D). Therefore, these data suggested that Sca‐1 enriched CFU‐F activity in both Lepr‐Cre+ and Lepr‐Cre− fractions of the periosteum.
Next, we tested the tri‐lineage differentiation potential of the periosteal subsets after ex vivo expansion. To our surprise, Lepr‐Cre+ Sca‐1+ cells underwent efficient adipogenic differentiation (Fig 6A and B), but limited osteo‐chondrogenic differentiation (Fig 6C–F). In contrast, Lepr‐Cre+ Sca‐1− cells showed significantly lower adipogenic differentiation (Fig 6A and B), and significantly higher osteo‐chondrogenic differentiation as compared to Lepr‐Cre+ Sca‐1+ cells (Fig 6C–F). Similar results were also obtained in Lepr‐Cre− fraction of the periosteum (Fig EV5E–J). Taken together, we found that Sca‐1+ periosteal cells enrich CFU‐F activity but undergo limited osteo‐chondrogenic differentiation.
Figure 6. Functional analysis of the Sca‐1+ sub‐population in the periosteum.
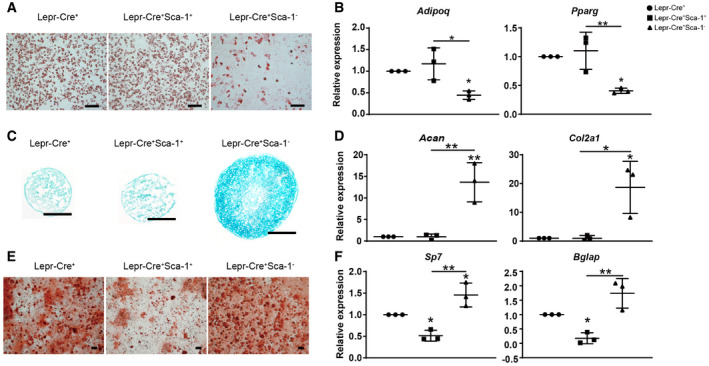
-
A, BRepresentative oil red staining after 7 days of adipogenic differentiation in Lepr‐Cre+ subsets (A) and qPCR analysis of Adipoq and Pparg expression (B) (n = 3 independent experiments). Scale bars are 100 µm.
-
C, DIn vitro chondrogenic differentiation of sorted periosteal cells. Representative alcian blue staining after chondrogenic differentiation for 21 days and cryosection (C). Scale bars are 100 µm. Chondrogenic efficiencies were quantified by qPCR analysis of Acan and Col2a1 expression (D) (n = 3 independent experiments).
-
E, FIn vitro osteogenic differentiation of sorted periosteal cells. Representative alizarin red staining after osteogenic differentiation for 14 days. Scale bars are 200 µm. Osteogenic efficiencies were quantified by qPCR analysis of Sp7 and Bglap expression (F) (n = 3 independent experiments).
Data information: The statistical significance of differences was analyzed by one‐way ANOVAs with Tukey’s multiple comparison tests. All data represented Mean ± SD. *P < 0.05, **P < 0.01.
Discussion
By comparing Prrx1‐Cre‐ and Lepr‐Cre‐traced cells under homeostatic condition, we found that SSPC and osteogenic subsets were labeled by both Cre lines, thereby justifying the use of Lepr‐Cre to study SSCPs maintenance and lineage specification in adult long bones. The Prrx1‐Cre‐biased subsets, including periosteal cells, chondrocytes, and αSMA+ cells, could be derived from perichondrial cells during embryonic skeletal development (Logan et al, 2002). In contrast, Lepr‐Cre is not detected in long bones until perinatal stage (Zhou et al, 2014a), which could explain their distinct recombination patterns. Cautions must be taken that Lepr‐Cre is a germline Cre that is not organ‐ or lineage specific. Since we could not temporally control the expression of Cre recombinase, Lepr‐Cre might be ectopically induced upon certain stress conditions. Thus, careful analysis of Lepr expression is needed when inferring lineage relationships among different cell types traced by Lepr‐Cre. Notably, a recent lineage tracing study by LepR‐CreER reveals the transition of postnatal skeletal progenitor cells from chondrocytes to LepR+ BMSCs (Shu et al, 2021). Future studies are needed to test the cellular heterogeneity within Lepr‐CreER‐traced cells in the adult long bones.
Integrated analysis of Prrx1‐Cre‐ and Lepr‐Cre‐traced cells allowed us to systemically examine the distribution of known SSPC markers, as well as the extent to which different HSC niche cells overlap (Fig EV1E). Lepr‐Cre+ BMSCs expressed high levels of Pdgfra, Pdgfrb, Kitl, Cxcl12, and Grem1, which confirmed that they overlap with CAR cells (Omatsu et al, 2010) and Grem1+ cells (Worthley et al, 2015). Previous studies showed that Lepr‐Cre+ BMSCs largely overlap with HSC niche‐forming Nestin‐GFP+ cells, especially Nestin‐GFPlow cells (Mendez‐Ferrer et al, 2010; Kunisaki et al, 2013; Zhou et al, 2014a). Interestingly, Nestin and CD105 were expressed in a smaller fraction of Lepr‐Cre+ BMSCs, which could be caused by sparse sampling by scRNA‐seq, or by the heterogeneity within Lepr‐Cre+ BMSCs. Therefore, cautions must be taken when interpreting the expressional data reported in this study. We were not able to determine the relationship between Lepr‐Cre+ BMSCs and non‐myelinating Schwann cells that were also implicated as critical HSC niche (Yamazaki et al, 2011), since these rare glial cells are derived from neural crest cells that could not be traced by Prrx1‐Cre or Lepr‐Cre.
Integrated analysis of Lepr‐Cre‐traced cells under homeostatic and stress conditions revealed adipogenic, osteogenic, and periosteal lineage cells. The adipogenic lineage cells are mainly composed of Lepr‐Cre+ BMSCs highly expressing HSC niche factors such as Kitl and Cxcl12, which were significantly down‐regulated in aged, rosiglitazone‐fed, and fractured mice, suggesting loss of the ability to create HSC niche. Importantly, we identified a slow cycling Notch3+ sub‐population within Lepr‐Cre+ BMSCs, which correspond to adventitial cells closely associated with the bone marrow vasculatures, reminiscent of the quiescent Cxcl12‐CreER+ BMSCs in the perivascular region (Matsushita et al, 2020). Interestingly, Notch signaling has been previously shown to promote SSPC maintenance by suppressing osteogenic differentiation (Engin et al, 2008; Hilton et al, 2008). Conditional deletion of the Notch ligand Dll4 in bone marrow ECs leads to myeloid skewing of hematopoietic progenitors (Tikhonova et al, 2019), significantly shorter long bones, and loss of trabeculae (Ramasamy et al, 2014). Together with our findings, these data indicate that bone marrow ECs create a vascular niche for SSPCs via the Dll‐Notch axis, and play critical roles in orchestrating osteogenesis and hematopoiesis. Notably, the Notch3+ sub‐population expresses a panel of angiogenic factors such as Vegfa and Col4a1, highlighting the intimate crosstalk between adventitial cells and bone marrow ECs. Future studies are needed to test whether different types of bone marrow ECs, including sinusoidal, arteriolar, and arterial ECs, constitute distinct niches for SSPC to regulate their self‐renewal and lineage commitment.
Lepr‐Cre+ cells have been extensively studied in the bone marrow, while their periosteal functions remain largely unknown. Although Lepr‐Cre labeled a small fraction of periosteal cells under steady state, they were expanded after bone fracture and irradiation, suggesting potential roles in skeletal repair in response to physical insults. Notably, since Lepr‐Cre labels cells in the bone marrow, endosteal, and periosteal compartments, we could not rule out the possibility that bone marrow and endosteal Lepr‐Cre+ cells also give rise to chondrocytes after bone fracture (Fig 2G). In this study, we focused on the Sca‐1+ sub‐population in both Lepr‐Cre+ and Lepr‐Cre− fractions of the periosteum, which enriches CFU‐F activity and exhibits adipogenic progenitor characteristics. This contrasts sharply with bone marrow PαS cells that exhibit trilineage differentiation potential (Morikawa et al, 2009), suggesting functional divergence of Sca‐1+ cells isolated from different skeletal compartments. On the contrary, Sca‐1− periosteal cells seem to enrich SSPCs including PSCs (Debnath et al, 2018; cluster 3 in Fig EV5B, CD200+CD105−Thy1−6C3−) and αSMA+ p‐SSCs (Ortinau et al, 2019; cluster 2 in Fig 5B, expressing Acta2). Consistent with our findings (Fig 6), Ambrosi et al found that CD45−CD31−Sca1+ cells enrich adipogenic progenitors, while CD45−CD31−Sca1− cells enrich osteo‐chondrogenic progenitors (Ambrosi et al, 2017). Given that crushed bones containing both bone marrow and bone fragments were analyzed (Ambrosi et al, 2017), and that Ly6a (Sca‐1) is expressed at a much higher level in periosteal/fibroblast populations as compared to BMSCs (Fig EV1E; Baryawno et al, 2019; Sivaraj et al, 2021), the CD45−CD31−Sca1+ cells studied by Ambrosi et al might overlap, as least in part, with the Sca‐1+ periosteal cells that we analyzed.
Materials and Methods
Reagents and Tools table
| Reagent/Resource | Reference or Source | Identifier or Catalog Number |
|---|---|---|
| Experimental Models | ||
| Prrx1‐Cre | Logan et al (2002) | JAX: 005584 |
| Lepr‐Cre | DeFalco et al (2001) | JAX: 008320 |
| Loxp‐STOP‐loxp‐tdTomato | Madisen et al (2010) | JAX: 007914 |
| Col2.3‐GFP | Kalajzic et al (2002) | JAX: 013134 |
|
Recombinant DNA | ||
| pLKO.1 ‐ TRC Cloning Vector | Addgene | Cat# 10878 |
| pLKO.1_Hoxb2_1 | This study | |
| pLKO.1_Hoxb2_2 | This study | |
| pLKO.1_Npdc1_1 | This study | |
| pLKO.1_Npdc1_2 | This study | |
| Antibodies | ||
| Anti‐CD45‐APC (Clone 30‐F11) | eBioscience | Cat# 17‐0451‐83 |
| Anti‐Ter119‐APC (Clone TER‐119) | eBioscience | Cat# 17‐5921‐82 |
| Anti‐Tie2‐APC (Clone TEK4) | Biolegend | Cat# 124010 |
| Anti‐Pdgfra‐Biotin Antibody (Clone APA5) | eBioscience | Cat# 13‐1401‐82 |
| Anti‐Notch 3 Armenian Hamster Monoclonal Antibody (Clone HMN3‐133) | Biolegend | Cat# 130512 |
| Anti‐Ly‐6A/E (Sca‐1)‐PerCP/Cy5.5 (Clone E13‐161.7) | Biolegend | Cat# 122524 |
| FITC anti‐BrdU Antibody (Clone 3D4) | Biolegend | Cat# 364104 |
| Anti‐CD31‐biotin (Clone MEC13.3) | Biolegend | Cat# 102504 |
| Mouse/Rat CD31/PECAM‐1 Antibody | R&D system | Cat# AF3628‐SP |
| Anti‐Ly‐6A/E‐Alexa Fluor 647 (Clone E13‐16.7) | Biolegend | Cat# 122518 |
| Rabbit anti‐Aggrecan Antibody | Millipore | Cat# AB1031 |
| Rabbit anti‐Perilipin Antibody | Sigma | Cat# P1873 |
| Donkey anti‐rabbit‐Alexa Fluor 488 | Invitrogen | Cat# A21206 |
| Donkey anti‐goat‐Alexa Fluor 488 | Invitrogen | Cat# A‐11055 |
| Donkey anti‐rabbit‐Alexa Fluor 647 | Invitrogen | Cat# A‐31573 |
|
Oligonucleotides and other sequence‐based reagents | ||
| Acan (NM_007424.2) | This study | qPCR primers |
| F: TGA AGC AGA AGG TCT GGA CA | ||
| R: CCA GAA GGA ATC CCA CTA ACA | ||
| Col2a1 (NM_031163.3) | This study | qPCR primers |
| F: GTC CCC CTG GCC TTA GTG | ||
| R: CCA CCA GCC TTC TCG TCA | ||
| Adipoq (NM_001310597.1) | This study | qPCR primers |
| F: TGT TCC TCT TAA TCC TGC CCA | ||
| R: CCA ACC TGC ACA AGT TCC CTT | ||
| Pparg (NM_011146.3) | This study | qPCR primers |
| F: ACC ACT CGC ATT CCT TTG AC | ||
| R: TGG GTC AGC TCT TGT GAA TG | ||
| Sp7 (NM_001348205.1) | This study | qPCR primers |
| F: CAA GAG TGA GCT GGC CTG A | ||
| R: TGG AGC CAT AGT GAG CTT CTT | ||
| Bglap (NM_007541.3) | This study | qPCR primers |
| F: TGA GGA CCA TCT TTC TGC TCA | ||
| R: TGG ACA TGA AGG CTT TGT CA | ||
| Hoxb2 (NM_134032.2) | This study | qPCR primers |
| F: CGA GGT CGG ATC ACC ATC | ||
| R: GGT ACT TAT TGA AGT GGA ACT CCT TCT | ||
| Npdc1 (NM_008721.4) | This study | qPCR primers |
| F: GTG CCA AGG AAA CA CTA TCTT | ||
| R: CTC CTT CAG TGC CAG TTC CT | ||
| ShRNA_Hoxb2_1 | This study | shRNA‐sequence |
| CCGGGTCTGGTCCTCCTTGGCTGTTCTCGAGAACAGCCAAGGAGGACCAGACTTTTTG | ||
| ShRNA_Hoxb2_2 | This study | shRNA‐sequence |
| AATTCAAAAAGCCTTCTCCACTCGTGGATACCTCGAGGTATCCACGAGTGGAGAAGGC | ||
| ShRNA_Npdc1_1 | This study | shRNA‐sequence |
| AATTCAAAAAGCTTTCTGCTTAGCAAGCTCACTCGAGTGAGCTTGCTAAGCAGAAAGC | ||
| ShRNA_Npdc1_2 | This study | shRNA‐sequence |
| AATTCAAAAAGGCATAAGGAACCTCCAAAGGCTCGAGCCTTTGGAGGTTCCTTATGCC | ||
| Chemicals, Enzymes and other reagents | ||
| Anti‐Biotin Microbeads | Miltenyi Biotec | Cat# 130‐090‐485 |
| LS Columns | Miltenyi Biotec | Cat# 130‐042‐401 |
| Type I Collagenase | Worthington | Cat# LS004197 |
| Dispase II | Sigma‐Aldrich | Cat# 04942078001 |
| DNase I | Sigma‐Aldrich | Cat# D4527‐200KU |
| GelMA | Engineering for Life | EFL‐GM‐90 |
| Sodium pyruvate solution | Gibco | Cat# S8636 |
| Bovine serum albumin (BSA) | Sigma‐Aldrich | Cat# A9647 |
| Fetal bovine serum (FBS) | HyClone | Cat# SH30070.03 |
| MEM alpha medium | Thermo Fisher Scientific | Cat# 12561‐056 |
| Penicillin‐Streptomycin Solution | Thermo Fisher Scientific | Cat# 15140‐122 |
| LM‐DMEM | Corning | Cat# 10‐014‐CV |
| DMEM with 4.5 g/l glucose l‐glutamine & sodium pyruvate | Corning | Car# 10‐013‐CVR |
| L‐Ascorbic acid 2‐phosphate sesquimagnesium salt hydrate, ≥95% | Sigma‐Aldrich | Cat# A8960‐5G |
| L‐proline | Sigma‐Aldrich | Cat# P0380 |
| Recombinant human TGF‐b3 | PeproTech | Cat# 100‐36E |
| Indomethacin | Sigma‐Aldrich | Cat# I7378 |
| Alizarin Red S | Sigma‐Aldrich | Cat# A5533 |
| Oil Red O solution | Sigma‐Aldrich | Cat# O1391 |
| Alcian blue 8GX | Sangon Biotech | Cat# A600298‐0001 |
| Crystal Violet | Solarbio | Cat# C8470 |
| 5X All‐In‐One MasterMix Kit | ABM | Cat# G492 |
| APC BrdU Flow Kit | BD Bioscience | Cat# 552598 |
| 5‐Bromo‐2′‐deoxyuridine ≥ 99% (HPLC) | Sigma‐Aldrich | Cat# B5002‐5G |
| RQ1 RNase‐Free Dnase, | Promega, | Cat# M6101 |
| Matrigel | Corning | Cat# 354234 |
| Chromium Single Cell 3’ Library and Gel Bead Kit V2 | 10× Genomics | Cat# PN‐120237 |
| Chromium Next GEM Single Cell 3’ GEM, Library & Gel Bead Kit V3.1 | 10× Genomics | Cat# 1000128 |
| iTaq Universal SYBR Green Supermix | BioRad | Cat# 172‐5125 |
| Software | ||
| Zen 2.3 software | Zeiss | |
| FlowJo V10.1 | FlowJo LLC | https://www.flowjo.com/ solutions/flowjo |
| GraphPad Prism 7.0 | GraphPad | https://www.graphpad.com/ |
| Image J: Fiji | Image J | https://imagej.nih.gov/ij/ |
| Snapgene V2.3.2 | Snapgene | https://www.snapgene.com |
| Cell Ranger v3.0.1 | 10× Genomics | https://support.10xgenomics.com |
| Python 3 | N/A | www.python.org |
| R 3.5.0 | R‐Project | https://cran.r‐project.org/ mirrors.html |
| Seurat v2.3.4 | Satija et al (2015) | https://satijalab.org/seurat/ |
| Scanpy v1.4 | Wolf et al (2018) | https://github.com/theislab/scanp |
| SCENIC | Aibar et al (2017) | https://github.com/aertslab/SCENIC |
| jvenn | Bardou et al (2014) | http://jvenn.toulouse.inra.fr/app/example.html |
| ClusterProfiler v3.2.14 | Yu et al (2012) | https://guangchuangyu.github.io/software/clusterProfiler/ |
| TACS | Kernfeld et al (2018) | N/A |
| CellphoneDB v2.0.0 | Vieira Braga et al (2019) | www.cellphonedb.org |
| Cytoscape v3.6 | Otasek et al (2019) | www.cytoscape.org |
| GSEA v2.2.4 |
Subramanian et al (2005), Mootha et al (2003) |
|
Methods and Protocols
Mice
All mice were maintained in C57BL/6 background, including Lepr‐Cre (DeFalco et al, 2001), Prrx1‐Cre (Logan et al, 2002), loxp‐STOP‐loxp‐tdTomato (Madisen et al, 2010), and Col2.3‐GFP mice (Kalajzic et al, 2002). For scRNA‐seq experiments in Prrx1‐Cre; tdTomato; Col2.3‐GFP mice, 8‐week‐old mice were analyzed (three males). For scRNA‐seq experiments in Lepr‐Cre; tdTomato mice, 8‐week‐old mice (“Young adult,” four males and two females), 12‐month‐old mice (“Aging,” four females), 10‐week‐old mice on 20 g/kg rosiglitazone‐containing chow for 5 weeks (“Rosiglitazone diet,” feeding started at 5 weeks old, two females), 8‐week‐old sub‐lethally irradiated (5 Gy) mice (“Irradiation,” two males and two females), and 8‐week‐old fractured mice (“Fracture,” two males and two females) were analyzed. All mice were kept in an SPF facility operated in a 12‐h light/dark cycle. Animal procedures were approved by the Tongji University Animal Care and Use Committee. No statistical methods were used to estimate sample size. Mice were randomly chosen for each sample, and blinding of the investigator was not considered in this study.
Immunofluorescent staining
Freshly dissected bones were fixed in 4% paraformaldehyde overnight at 4°C, decalcified in 10% EDTA for 3 days, and sectioned at 10 µm using the CryoJane Tape‐Transfer system (Leica). Sections were blocked in PBS with 10% horse serum and 0.1% Triton X‐100 at room temperature for 1 h, and stained overnight at 4°C with rabbit anti‐Aggrecan (Millipore, AB1031 1:200), rabbit anti‐Perilipin (Sigma, 1:1,000) rabbit‐anti‐Col2a1 (Boster, BA0533, 1:200), rabbit‐anti‐Sp7 (Abcam, ab209484, 1:200), or goat anti‐CD31 (R&D systems, AF3628‐SP, 1:200) primary antibodies. Sections were washed three times (10 min/each) at room temperature, and stained with anti‐Ly‐6A/E‐Alexa Fluor 647 (Biolegend, clone: E13‐161.7, 1:50), anti‐Notch3‐Alexa Fluor 647 (Biolegend, clone: HMN3‐133, 1:100), donkey anti‐rabbit‐Alexa Fluor 488, anti‐goat‐Alexa Fluor 488, or donkey anti‐rabbit‐Alexa Fluor 647 antibodies (Invitrogen, 1:500) at room temperature for 1 h. Slides were mounted with Anti‐fade Prolong Gold with DAPI (Invitrogen). Images were acquired with an Olympus IX73 fluorescent microscope. For immunostaining of the 3D coculture system, the GelMA hydrogels were treated as described above at 4°C during the whole process. Blocking, primary, and secondary antibody application were each performed overnight, and the washing time was extended to 30 min/each. Images were acquired with Zeiss LSM8800 confocal microscope and z‐stack images were analyzed using Zen 2.3 software.
Bone fracture
After anesthesia, an incision was made on the skin of the right leg to expose the femur through the muscles. A stainless‐steel wire was inserted into the intramedullary canal through the knee, and an incision was made by the cranial drill in the mid‐diaphysis to introduce a fracture. The muscle and skin were then closed by sutures and wound clips. Mice were closely monitored until they resumed full activity. Ketoprofen was given for analgesia before and after surgery.
Enzymatic dissociation and flow cytometry
Enzymatic digestion of the bone and bone marrow cells were performed as previously described (Suire et al, 2012; Yue et al, 2016). Briefly, intact marrow plugs were flushed from the limb bones and subjected to two rounds of enzymatic digestion at 37°C for 15 min each. The remaining bone fragments were crushed and digested in the same ways as the marrow plugs. The digestion buffer contained 3 mg/ml type I collagenase (Worthington), 4 mg/ml Dispase (Sigma), and 1 U/ml DNase I (Ambion) in HBSS with calcium and magnesium. The cells were resuspended in staining medium (HBSS + 2% fetal bovine serum) with 2 mM EDTA to stop the digestion. The bone and marrow fractions were combined and filtered through 70‐µm cell strainers before sorting. Fractured femurs were dissected and crushed for enzymatic digestion without flushing the marrows. To isolate periosteal cells for flow cytometry and functional analyses, long bones with periosteum were digested twice at 37°C for 15 min each. The loosened periosteum was scraped off with a razor blade and digested twice at 37°C for 15 min each. For flow cytometry, digested cells were stained with anti‐CD45‐APC (eBioscience, clone: 30‐F11, 1:200), anti‐Ter119‐APC (eBioscience, clone TER‐119, 1:200), and anti‐Tie2‐APC (Biolegend, clone: TEK4, 1:200) antibodies in staining buffer for 30 min on ice, washed and resuspended in 1 ml staining buffer with 1 µg/ml DAPI (Invitrogen). Cell sorting was performed on FACSAriaII flow cytometer (BD Biosciences) with a 100‐µm nozzle, and sorted cells were collected in PBS supplemented with 0.08% UltraPure BSA in 96‐well clear round bottom ultra‐low attachment microplates (Corning 7007). When analyzing Lepr‐Cre+Sca‐1+/− cells, anti‐Ly‐6A/E (Sca‐1)‐PerCP/Cy5.5 (Biolegend, clone: E13‐161.7, 1:100) was used in combination with the above antibodies.
CFU‐F culture and in vitro differentiation
To form CFU‐F colonies, flow cytometrically sorted cells were plated in six‐well plates (200 cells/well) with BMSC medium containing α‐MEM (Gibco), 20% fetal bovine serum (FBS, Gibco 10270‐106, Lot #: 42F6480K, selected to support CFU‐F growth), 10 µM ROCK inhibitor, 1 mM sodium pyruvate, and 1% penicillin/streptomycin. Cultures were maintained at 37°C with 5% O2 and 5% CO2 to create a low oxygen environment that promoted cell survival and proliferation (Morrison et al, 2000). The culture medium was changed every 4 days. CFU‐F colonies were counted 8 days after plating by crystal violet staining (1% in formalin solution). Adipogenic and osteogenic differentiation was assessed by replating primary CFU‐F colonies into 24‐well plates (4–6 × 104 cells/cm2). The adipogenic differentiation medium contained DMEM (Gibco), 10% fetal bovine serum, 0.5 μM isobutylmethylxanthine, 60 μM indomethacin, 1% penicillin/ streptomycin, 5 μg/ml insulin, and 1 μM dexamethasone. The medium was changed every 3–4 days, and the differentiation efficiency was determined by oil red O staining on day 7. The osteogenic differentiation medium contained α‐MEM (Gibco), 10% fetal bovine serum (Gibco 10270‐106, Lot #: 42F6480K, selected to support CFU‐F growth), 1 mM sodium pyruvate, 1% penicillin/streptomycin, 50 µg/ml L‐ascorbic acid, 10 mM β‐glycerophosphate, and 100 nM dexamethasone. The medium was changed every 3–4 days, and the differentiation efficiency was determined by alizarin red staining on day 14. The chondrogenic medium contained 10 ng/ml recombinant transforming growth factor‐b3 (Peprotech), 100 nM dexamethasone, 50 µg/ml ascorbic acid 2‐phosphate, 1 mM sodium pyruvate, 40 µg/ml proline, 1% penicillin/streptomycin, and 1X ITS cell culture supplement (Cyagen) containing 6.25 μg/ml bovine insulin, 6.25 μg/ml transferrin, 6.25 μg/ml selenous acid, 5.33 μg/ml linoleic acid, and 1.25 mg/ml BSA in high glucose DMEM. To form cell pellets, 2.5 × 105 cells were centrifuged in 15‐ml polypropylene tubes. The medium was changed every 2 days in the first week, and then once a week. The differentiation efficiency was determined by cryosection of the cell pellets and alcian blue staining on day 21.
BrdU incorporation assay
For cell cycle analysis in vivo, 8‐week‐old Lepr‐Cre; tdTomato mice were given a single intraperitoneal injection of BrdU (100 mg/kg body mass) and maintained on 0.5 mg/ml of BrdU in the drinking water for 14 days. For cell cycle analysis during ex vivo 3D co‐culture, BrdU was administered at a final concentration of 10 µM from day 1 to day 5. The frequency of BrdU+ cells was analyzed by flow cytometry using the BrdU Flow Kit (BD Biosciences) and FITC anti‐BrdU Antibody (Biolegend, clone: 3D4, 1:100).
Ex vivo 3D co‐culture
Primary BMSCs and bone marrow ECs were isolated from 8‐week‐old wild‐type (for RNA‐seq) or Lepr‐Cre; tdTomato (for immunostaining) mice. Bone marrow plugs were flushed out of the limb bones and digested as described above. For 2D expansion of BMSCs, whole bone marrow cells were cultured at a density of 8 × 106 per 10‐cm dish with BMSC medium for 8 days. For 2D expansion of bone marrow ECs, CD31+ cells were immunomagnetically enriched from digested whole bone marrow cells using anti‐CD31‐biotin (Biolegend, clone: MEC13.3, 1:200) antibody, anti‐Biotin MicroBeads (Miltenyi Biotech, 130‐090‐485), and MACS LS column (Miltenyi Biotech, 130‐042‐401). The enriched CD31+ cells were cultured at a density of 2 × 107 cells per 10 cm fibronectin (Sigma, F0895) pre‐coated dish with EGM2 medium (Lonza, CC‐3202) for 8 days. 2D expanded BMSCs and bone marrow ECs were then purified by flow cytometry using anti‐CD45‐APC, anti‐Ter119‐APC, anti‐CD140a‐biotin (eBioscience, clone: APA5, 1:200), and anti‐CD31‐biotin antibodies. Ten thousand BMSCs and bone marrow ECs were mixed at 1:1 ratio in 50 μl GelMA (4%, Engineering for Life, GM‐60) in a 24‐well culture plate, and cross‐linked with a 405‐nm light source for 25 s to form hydrogel. 3D hydrogels were cultured in 500 μl EGM2 medium (changed once after 2–3 days) and maintained at 37°C with 5% O2 and 5% CO2 for 5 days.
Lentiviral transfection
Short hairpin RNAs (shRNA) targeting Hoxb2 and Npdc1 were cloned into pLKO.1 vector. HEK293T cells (2 × 105) were seeded in a six‐well plate and transfected with pLKO.1‐Hoxb2‐shRNA, pLKO.1‐Npdc1‐shRNA, or pLKO.1 vector (control) together with packaging vectors (pAX2 and VSV‐G) by PEI (Polysciences, 23966‐1) for 8 h. The lentivirus‐containing culture medium was harvested at 48 and 72 h post‐transfection and stored at −80°C. For lentiviral transfection of BMSCs, 2 × 104 Lepr‐Cre+ BMSCs were seeded in a six‐well plate and transfected with the lentivirus‐containing medium (1:10 volume ratio). Cells were expanded after 48 h for in vitro differentiation and qPCR analyses.
Renal sub‐capsular transplantation
Briefly, 2 × 05 cells were resuspended in 5 μl Matrigel (Corning, 354234) on ice and then aspirated into a micropipette (Drummond Scientific, 5‐000‐2010). The cell mixture was transplanted underneath the renal capsule of 7–8‐week‐old wild‐type mice. Four weeks after transplantation, the kidneys were surgically removed, fixed in 4% paraformaldehyde for 6 h at 4°C, decalcified in 10% EDTA for 2 days, and then dehydrated in 30% sucrose at room temperature. Cryosections were cut at 10 µm and subjected to immunofluorescent staining, followed by Movat’s Pentachrome staining (ScyTek, MPS‐1). Images were acquired using Olympus IX73 microscope.
RNA extraction and qPCR
Total RNAs were extracted by RNAiso Plus (Takara) and reverse transcribed into cDNA using 5X All‐In‐One MasterMix kit (ABM). qPCR was performed using iTaq Universal SYBR Green Supermix (BioRad) on a CFX96 real‐time system (BioRad).
scRNA‐seq library preparation and sequencing
Flow cytometry‐sorted cells were examined under a microscope, counted with a hemocytometer, and then subjected to 3’ scRNA‐seq library construction on a Chromium Controller (10× Genomics). All libraries were constructed with Single Cell 3’ v2 kit according to the manufacturer’s instructions except for the irradiation sample of female mice (Single Cell 3’ v3 kit). Our target output was 4,000 cells for each sample. Reverse transcription, cDNA amplification, and library constructions were performed on T100 Touch Thermal cycler (Bio‐Rad). Amplified cDNA and libraries were evaluated on an Agilent Tape‐Station system using D5000 and D1000 screentapes (Agilent Technologies) and diluted to 10 ng/µl. Pooled samples were sequenced on a HiSeq X Ten system (Illumina) as paired‐end 150‐bp reads, to ~90% saturation level for all samples.
Pre‐processing of scRNA‐seq data
Paired‐end sequencing reads were processed using the Cell Ranger pipelines (version 3.0, 10× Genomics) for sample demultiplexing, read mapping (mm10/GRCm38), barcode processing, and single‐cell transcript counting. After removing poorly mapped reads and PCR duplicates, reads with valid cell barcodes and unique molecular identifiers (UMIs) were used to generate the gene‐barcode matrix. To remove low‐quality and contaminating hematopoietic cells, we excluded the following cells: (i) cells with fewer than 500 detected genes (each gene must have at least one UMI aligned); (ii) cells with more than 15% mitochondrial transcripts (UMIs); (iii) cells with more than 2% hemoglobin transcripts (UMIs) including Hbb‐bt, Hbb‐bs, Hbb‐bh1, Hbb‐bh2, Hbb‐y; (iv) contaminating endothelial cells. After filtering, gene expression in each cell was calculated as transcripts per 10k transcripts (TP10K), representing the fraction of each gene’s UMI count with respect to total UMIs in the cell and multiplied by 10,000. Only genes expressed in at least three cells were retained. The resulting digital expression matrix was log‐transformed before downstream analyses. Detailed sample and single‐cell information was listed in Dataset EV1.
Dimensionality reduction, clustering, and visualization
We performed dimensionality reduction and clustering using the Seurat package (v3.0.1) (Macosko et al, 2015). Briefly, we first selected a subset of highly variable genes based on dispersion of binned variance to mean expression ratios using the FindVariableFeatures function (with the MeanVarPlot method, 0.1 < mean < 8, dispersion > 1) followed by filtering of dissociation artifact genes (van den Brink et al, 2017) and mitochondrial genes. We then performed principal component analysis (PCA) and reduced the data to the top PCs (number of top PCs was chosen based on the “knee” point at the cumulative curve of standard deviations of each PCs). The PCA‐reduced data were then used to compute a shared nearest neighbor graph, and were further subjected to graph‐based clustering with the Louvain Method (Vincent D Blondel, 2008). The clusters were visualized on a two‐dimensional t‐distributed stochastic neighbor embedding (t‐SNE) map (van der Maaten & Hinton, 2008), implemented by the Seurat software with perplexity = 30. Cell clustering is not driven by the cell cycle state except for cluster 6 in Fig 2C.
Batch effect correction
Batch effect correction was based on the assumption that different samples share similar subsets. Successfully batch correction was determined by overlapping of the same subsets from different samples. To correct batch effects, we used the Seurat alignment method for data integration. Briefly, we identified highly variable genes for each dataset as described above, and took the union of these genes to run a canonical correlation analysis to determine the common sources of variation among datasets by FindIntegrationAnchors function with the dims = 30. We then used IntegrateData function to align the sub‐spaces on the basis of the first 30 canonical correlation vectors, which generated a new dimensional reduction that was used for further analysis (Tikhonova et al, 2019). We integrated Prrx1‐Cre‐ and Lepr‐Cre‐traced samples and clustered 7,220 cells into 9 subsets (Fig 1B). The 17,224 cells from Lepr‐Cre‐traced samples were clustered into 8 subsets (Fig 2B).
Marker gene identification
To identify marker genes that define individual clusters, we performed pairwise differential expression analyses using the FindAllMarkers function in Seurat with the MAST method (Finak et al, 2015) for statistical tests. Differentially expressed autosomal genes that were detected in at least 20% cells within the cluster and with a log2 fold change > 0.2 compared with all other clusters were considered to be marker genes. Detailed DEG information for all figures were listed in Dataset EV2.
Reconstructing cell development trajectories
To infer the developmental progression of cells in different clusters and to order them in pseudotime, we used the non‐linear reconstruction algorithm DDRTree in the Monocle 2 package (v2.10.0) (Qiu et al, 2017). The differentially expressed genes of different clusters were used as the ordering genes for the trajectory reconstruction.
Single‐cell gene regulatory network inference using SCENIC
We performed SCENIC analysis to predict single‐cell gene regulatory networks by following the standard workflow (Aibar et al, 2017). The raw count expression matrix was loaded onto GENIE3 for building the initial co‐expression gene regulatory networks (GRN). The regulon data were then analyzed based on TF motifs from mm10‐tss‐centered‐10 kb (for mouse) database by RcisTarget (Imrichova et al, 2015). The regulon activity scores in single cells were calculated using AUCell (Holland et al, 2020). Detailed regulon information for all figures was listed in source data for Figs 4 and 5.
RNA velocity
The spliced and unspliced reads were quantified by velocyto (version 0.17.11) with mouse genome reference. The velocities of each gene were calculated following the proposed workflow of scVelo (version 0.2.2; Bergen et al, 2020) with the default parameter. The trajectories were visualized on Uniform Manifold Approximation and Projection (UMAP) (Becht et al, 2018; Fig 1G).
GO and KEGG pathway analysis
The DEGs in each cluster were used to perform GO and KEGG pathway enrichment analysis by clusterProfiler package (Yu et al, 2012). The significantly expressed genes of each cluster were used as input and ontology was set to BP (biological process). The enriched GO terms and pathways were filtered by setting P‐value cutoff to 0.05. Simplify function was performed to select the most significantly enriched terms.
Bulk RNA‐seq of 3D‐cultured BMSCs
Ten thousand lived BMSCs 3D cultured alone or together with bone marrow ECs in 4% GelMA hydrogels were sorted by flow cytometry (CD45−Ter119−CD31−CD140a+). Total RNAs were extracted, digested with DNase I, and purified using RNAclean Kit (TIANGEN, DP412). RNA‐seq libraries were constructed by a modified Smart‐seq2 protocol (Zhou et al, 2016) and sequenced using Nova‐seq (PE150). Adaptor sequences were removed using CutAdapt and trimmed reads shorter than 30 bp were discarded. Trimmed data were aligned to mouse reference genome mm10 using STAR (Dobin et al, 2013; unique mapping rate > 80%). HTseq‐count (Anders et al, 2015) was used to count the number of reads mapped to each gene. Counts of genes were normalized and differentially expressed genes (DEGs, P < 0.05, fold‐change > 1.35) were determined by the DESeq2 package (Love et al, 2014). GO and KEGG enrichment of DEGs was performed using clusterProfiler package (Yu et al, 2012). Top 2% GO terms and top 20 KEGG pathways with P < 0.05 were chosen. DEGs, GO terms, and KEGG pathways were visualized using ggplot2. Normalized gene counts for all samples were listed in Dataset EV3.
Statistical analysis
The statistical significance of differences was analyzed by two‐tailed unpaired Student’s t‐tests, or one‐way ANOVAs with Tukey’s multiple comparison tests. Detailed statistical methods were specified in the figure legends. All data represented Mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001.
Author contributions
CM performed most mouse experiments. JG and LS performed most bioinformatic analyses. HW performed the bone fracture surgeries. JQ helped with the scRNA‐seq experiments. XZ, DC, and Yiying Z. performed 3D co‐culture experiment and bulk RNA‐seq analysis. Yuxi S. performed renal sub‐capsular transplantation. CZ, YX, and Yong Z. helped with bioinformatic analysis. Yao S. and LS critically read the manuscript. RY designed and interpreted all experiments and wrote the manuscript.
Supporting information
Expanded View Figures PDF
Dataset EV1
Dataset EV2
Dataset EV3
Source Data for Figure 4
Source Data for Figure 5
Acknowledgments
This work was supported by grants from the National Key R&D Program on Stem Cell and Translational Research (2017YFA0106400, 2017YFC1001500, 2021YFA1100900), National Natural Science Foundation of China (91749124, 81772389, 82070108, 31871478, 32022023), Zhejiang Provincial Natural Science Foundation of China (LR18C060001), Fundamental Research Funds for the Central Universities (22120190149, 22120200411, kx0200020173386), and Peak Disciplines (Type IV) of Institutions of Higher Learning in Shanghai.
Conflict of interest
The authors declare that they have no conflict of interest.
The EMBO Journal (2022) 41: e108415.
See also: J Sun & MB Greenblatt (February 2022)
Contributor Information
Li Shen, Email: li_shen@zju.edu.cn.
Rui Yue, Email: ryue@tongji.edu.cn.
Data availability
The scRNA‐seq and bulk RNA‐seq data reported in this study have been deposited to Gene Expression Omnibus (GEO) with the accession number GSE138689 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE138689). R scripts for data analysis are available upon request.
References
- Ackert‐Bicknell CL, Shockley KR, Horton LG, Lecka‐Czernik B, Churchill GA, Rosen CJ (2009) Strain‐specific effects of rosiglitazone on bone mass, body composition, and serum insulin‐like growth factor‐I. Endocrinology 150: 1330–1340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Agtmael T, Bailey MA, Schlotzer‐Schrehardt U, Craigie E, Jackson IJ, Brownstein DG, Megson IL, Mullins JJ (2010) Col4a1 mutation in mice causes defects in vascular function and low blood pressure associated with reduced red blood cell volume. Hum Mol Genet 19: 1119–1128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aibar S, González‐Blas CB, Moerman T, Huynh‐Thu VA, Imrichova H, Hulselmans G, Rambow F, Marine J‐C, Geurts P, Aerts J et al (2017) SCENIC: single‐cell regulatory network inference and clustering. Nat Methods 14: 1083–1086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrosi TH, Scialdone A, Graja A, Gohlke S, Jank A‐M, Bocian C, Woelk L, Fan H, Logan DW, Schürmann A et al (2017) Adipocyte accumulation in the bone marrow during obesity and aging impairs stem cell‐based hematopoietic and bone regeneration. Cell Stem Cell 20: 771–784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anders S, Pyl PT, Huber W (2015) HTSeq–a Python framework to work with high‐throughput sequencing data. Bioinformatics 31: 166–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold MA, Kim Y, Czubryt MP, Phan D, McAnally J, Qi X, Shelton JM, Richardson JA, Bassel‐Duby R, Olson EN (2007) MEF2C transcription factor controls chondrocyte hypertrophy and bone development. Dev Cell 12: 377–389 [DOI] [PubMed] [Google Scholar]
- Baccin C, Al‐Sabah J, Velten L, Helbling PM, Grunschlager F, Hernandez‐Malmierca P, Nombela‐Arrieta C, Steinmetz LM, Trumpp A, Haas S (2020) Combined single‐cell and spatial transcriptomics reveal the molecular, cellular and spatial bone marrow niche organization. Nat Cell Biol 22: 38–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker EK, Taylor S, Gupte A, Chalk AM, Bhattacharya S, Green AC, Martin TJ, Strbenac D, Robinson MD, Purton LE et al (2015) Wnt inhibitory factor 1 (WIF1) is a marker of osteoblastic differentiation stage and is not silenced by DNA methylation in osteosarcoma. Bone 73: 223–232 [DOI] [PubMed] [Google Scholar]
- Bardou P, Mariette J, Escudié F, Djemiel C, Klopp C (2014) jvenn: an interactive Venn diagram viewer. BMC Bioinform 15: 293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baryawno N, Przybylski D, Kowalczyk MS, Kfoury Y, Severe N, Gustafsson K, Kokkaliaris KD, Mercier F, Tabaka M, Hofree M et al (2019) A Cellular Taxonomy of the Bone Marrow Stroma in Homeostasis and Leukemia. Cell 177: 1915–1932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becht E, McInnes L, Healy J, Dutertre CA, Kwok IWH, Ng LG, Ginhoux F, Newell EW (2018) Dimensionality reduction for visualizing single‐cell data using UMAP. Nat Biotechnol 37: 38–44 [DOI] [PubMed] [Google Scholar]
- Bergen V, Lange M, Peidli S, Wolf FA, Theis FJ (2020) Generalizing RNA velocity to transient cell states through dynamical modeling. Nat Biotechnol 38: 1408–1414 [DOI] [PubMed] [Google Scholar]
- Vincent D Blondel J‐LG, Renaud Lambiotte and Etienne Lefebvre (2008) Fast unfolding of communities in large networks. J Stat Mech Theory Exp 10: arXiv:0803.0476 [Google Scholar]
- Breitbach M, Kimura K, Luis TC, Fuegemann CJ, Woll PS, Hesse M, Facchini R, Rieck S, Jobin K, Reinhardt J et al (2018) In vivo labeling by CD73 marks multipotent stromal cells and highlights endothelial heterogeneity in the bone marrow niche. Cell Stem Cell 22: 262–276 [DOI] [PubMed] [Google Scholar]
- van den Brink SC, Sage F, Vertesy A, Spanjaard B, Peterson‐Maduro J, Baron CS, Robin C, van Oudenaarden A (2017) Single‐cell sequencing reveals dissociation‐induced gene expression in tissue subpopulations. Nat Methods 14: 935–936 [DOI] [PubMed] [Google Scholar]
- Chan CK, Chen CC, Luppen CA, Kim JB, DeBoer AT, Wei K, Helms JA, Kuo CJ, Kraft DL, Weissman IL (2009) Endochondral ossification is required for haematopoietic stem‐cell niche formation. Nature 457: 490–494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan C, Seo E, Chen J, Lo D, McArdle A, Sinha R, Tevlin R, Seita J, Vincent‐Tompkins J, Wearda T et al (2015) Identification and specification of the mouse skeletal stem cell. Cell 160: 285–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan D, Cole WG, Chow CW, Mundlos S, Bateman JF (1995) A COL2A1 mutation in achondrogenesis type II results in the replacement of type II collagen by type I and III collagens in cartilage. J Biol Chem 270: 1747–1753 [PubMed] [Google Scholar]
- Chen Q, Liu Y, Jeong HW, Stehling M, Dinh VV, Zhou B, Adams RH (2019) Apelin(+) endothelial niche cells control hematopoiesis and mediate vascular regeneration after myeloablative injury. Cell Stem Cell 25: 768–783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane GM, Jeffery E, Morrison SJ (2017) Adult haematopoietic stem cell niches. Nat Rev Immunol 17: 573–590 [DOI] [PubMed] [Google Scholar]
- Cserjesi P, Lilly B, Bryson L, Wang Y, Sassoon DA, Olson EN (1992) MHox: a mesodermally restricted homeodomain protein that binds an essential site in the muscle creatine kinase enhancer. Development 115: 1087–1101 [DOI] [PubMed] [Google Scholar]
- Debnath S, Yallowitz AR, McCormick J, Lalani S, Zhang T, Xu R, Li NA, Liu Y, Yang YS, Eiseman M et al (2018) Discovery of a periosteal stem cell mediating intramembranous bone formation. Nature 562: 133–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFalco J, Tomishima M, Liu H, Zhao C, Cai X, Marth JD, Enquist L, Friedman JM (2001) Virus‐assisted mapping of neural inputs to a feeding center in the hypothalamus. Science 291: 2608–2613 [DOI] [PubMed] [Google Scholar]
- Delgado‐Calle J, Sanudo C, Sanchez‐Verde L, Garcia‐Renedo RJ, Arozamena J, Riancho JA (2011) Epigenetic regulation of alkaline phosphatase in human cells of the osteoblastic lineage. Bone 49: 830–838 [DOI] [PubMed] [Google Scholar]
- Ding L, Saunders TL, Enikolopov G, Morrison SJ (2012) Endothelial and perivascular cells maintain haematopoietic stem cells. Nature 481: 457–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, Batut P, Chaisson M, Gingeras TR (2013) STAR: ultrafast universal RNA‐seq aligner. Bioinformatics 29: 15–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docheva D, Hunziker EB, Fassler R, Brandau O (2005) Tenomodulin is necessary for tenocyte proliferation and tendon maturation. Mol Cell Biol 25: 699–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doege KJ, Sasaki M, Kimura T, Yamada Y (1991) Complete coding sequence and deduced primary structure of the human cartilage large aggregating proteoglycan, aggrecan. Human‐specific repeats, and additional alternatively spliced forms. J Biol Chem 266: 894–902 [PubMed] [Google Scholar]
- Domenga V, Fardoux P, Lacombe P, Monet M, Maciazek J, Krebs LT, Klonjkowski B, Berrou E, Mericskay M, Li Z et al (2004) Notch3 is required for arterial identity and maturation of vascular smooth muscle cells. Genes Dev 18: 2730–2735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchamp de Lageneste O, Julien A, Abou‐Khalil R, Frangi G, Carvalho C, Cagnard N, Cordier C, Conway SJ, Colnot C (2018) Periosteum contains skeletal stem cells with high bone regenerative potential controlled by Periostin. Nat Commun 9: 773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engin F, Yao Z, Yang T, Zhou G, Bertin T, Jiang MM, Chen Y, Wang L, Zheng H, Sutton RE et al (2008) Dimorphic effects of Notch signaling in bone homeostasis. Nat Med 14: 299–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell M, Fairfield H, Costa S, D'Amico A, Falank C, Brooks DJ, Reagan MR (2021) Sclerostin‐neutralizing antibody treatment rescues negative effects of rosiglitazone on mouse bone parameters. J Bone Miner Res 36: 158–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finak G, McDavid A, Yajima M, Deng J, Gersuk V, Shalek AK, Slichter CK, Miller HW, McElrath MJ, Prlic M et al (2015) MAST: a flexible statistical framework for assessing transcriptional changes and characterizing heterogeneity in single‐cell RNA sequencing data. Genome Biol 16: 278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grčević D, Sironi M, Valentino S, Deban L, Cvija H, Inforzato A, Kovačić N, Katavić V, Kelava T, Kalajzić I et al (2018) The long pentraxin 3 plays a role in bone turnover and repair. Front Immunol 9: 417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenbaum A, Hsu YM, Day RB, Schuettpelz LG, Christopher MJ, Borgerding JN, Nagasawa T, Link DC (2013) CXCL12 in early mesenchymal progenitors is required for haematopoietic stem‐cell maintenance. Nature 495: 227–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X, Wang R, Zhou Y, Fei L, Sun H, Lai S, Saadatpour A, Zhou Z, Chen H, Ye F et al (2018) Mapping the mouse cell atlas by Microwell‐seq. Cell 172: 1091–1107 [DOI] [PubMed] [Google Scholar]
- Hilton MJ, Tu X, Wu X, Bai S, Zhao H, Kobayashi T, Kronenberg HM, Teitelbaum SL, Ross FP, Kopan R et al (2008) Notch signaling maintains bone marrow mesenchymal progenitors by suppressing osteoblast differentiation. Nat Med 14: 306–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland CH, Tanevski J, Perales‐Patón J, Gleixner J, Kumar MP, Mereu E, Joughin BA, Stegle O, Lauffenburger DA, Heyn H et al (2020) Robustness and applicability of transcription factor and pathway analysis tools on single‐cell RNA‐seq data. Genome Biol 21: 36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz MC, Berry R, Holtrup B, Sebo Z, Nelson T, Fretz JA, Lindskog D, Kaplan JL, Ables G, Rodeheffer MS et al (2017) Bone marrow adipocytes. Adipocyte 6: 193–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imrichova H, Hulselmans G, Atak ZK, Potier D, Aerts S (2015) i‐cisTarget 2015 update: generalized cis‐regulatory enrichment analysis in human, mouse and fly. Nucleic Acids Res 43: W57–W64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isern J, Garcia‐Garcia A, Martin AM, Arranz L, Martin‐Perez D, Torroja C, Sanchez‐Cabo F, Mendez‐Ferrer S (2014) The neural crest is a source of mesenchymal stem cells with specialized hematopoietic stem cell niche function. Elife 3: e03696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalajzic I, Kalajzic Z, Kaliterna M, Gronowicz G, Clark SH, Lichtler AC, Rowe D (2002) Use of type I collagen green fluorescent protein transgenes to identify subpopulations of cells at different stages of the osteoblast lineage. J Bone Miner Res 17: 15–25 [DOI] [PubMed] [Google Scholar]
- Kernfeld EM, Genga RMJ, Neherin K, Magaletta ME, Xu P, Maehr R (2018) A single‐cell transcriptomic atlas of thymus organogenesis resolves cell types and developmental maturation. Immunity 48: 1258–1270.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Kim K, Youn BU, Lee J, Kim I, Shin HI, Akiyama H, Choi Y, Kim N (2014) Kruppel‐like factor 4 attenuates osteoblast formation, function, and cross talk with osteoclasts. J Cell Biol 204: 1063–1074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindblom JM, Gevers EF, Skrtic SM, Lindberg MK, Gothe S, Tornell J, Vennstrom B, Ohlsson C (2005) Increased adipogenesis in bone marrow but decreased bone mineral density in mice devoid of thyroid hormone receptors. Bone 36: 607–616 [DOI] [PubMed] [Google Scholar]
- Koide Y, Morikawa S, Mabuchi YO, Muguruma Y, Hiratsu E, Hasegawa K, Kobayashi M, Ando K, Kinjo K, Okano H et al (2007) Two distinct stem cell lineages in murine bone marrow. Stem Cells 25: 1213–1221 [DOI] [PubMed] [Google Scholar]
- Kratochwil K, Ghaffari‐Tabrizi N, Holzinger I, Harbers K (1993) Restricted expression of Mov13 mutant alpha 1(I) collagen gene in osteoblasts and its consequences for bone development. Dev Dyn 198: 273–283 [DOI] [PubMed] [Google Scholar]
- Kunisaki Y, Bruns I, Scheiermann C, Ahmed J, Pinho S, Zhang D, Mizoguchi T, Wei Q, Lucas D, Ito K et al (2013) Arteriolar niches maintain haematopoietic stem cell quiescence. Nature 502: 637–643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Ge C, Franceschi RT (2010) Differentiation‐dependent association of phosphorylated extracellular signal‐regulated kinase with the chromatin of osteoblast‐related genes. J Bone Miner Res 25: 154–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Strecker S, Wang L, Kronenberg MS, Wang W, Rowe DW, Maye P (2013) Osterix‐cre labeled progenitor cells contribute to the formation and maintenance of the bone marrow stroma. PLoS One 8: e71318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan M, Martin JF, Nagy A, Lobe C, Olson EN, Tabin CJ (2002) Expression of Cre recombinase in the developing mouse limb bud driven by a Prxl enhancer. Genesis 33: 77–80 [DOI] [PubMed] [Google Scholar]
- Love MI, Huber W, Anders S (2014) Moderated estimation of fold change and dispersion for RNA‐seq data with DESeq2. Genome Biol 15: 550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Maaten L, Hinton G (2008) Visualizing data using t‐SNE. J Mach Learn Res 9: 2579–2605 [Google Scholar]
- Macosko E, Basu A, Satija R, Nemesh J, Shekhar K, Goldman M, Tirosh I, Bialas A, Kamitaki N, Martersteck E et al (2015) Highly parallel genome‐wide expression profiling of individual cells using nanoliter droplets. Cell 161: 1202–1214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, Ng LL, Palmiter RD, Hawrylycz MJ, Jones AR et al (2010) A robust and high‐throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci 13: 133–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes C, Kobayashi T, Selig MK, Torrekens S, Roth SI, Mackem S, Carmeliet G, Kronenberg HM (2010) Osteoblast precursors, but not mature osteoblasts, move into developing and fractured bones along with invading blood vessels. Dev Cell 19: 329–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushita Y, Nagata M, Kozloff KM, Welch JD, Mizuhashi K, Tokavanich N, Hallett SA, Link DC, Nagasawa T, Ono W et al (2020) A Wnt‐mediated transformation of the bone marrow stromal cell identity orchestrates skeletal regeneration. Nat Commun 11: 332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez‐Ferrer S, Michurina TV, Ferraro F, Mazloom AR, Macarthur BD, Lira SA, Scadden DT, Ma'ayan A, Enikolopov GN, Frenette PS (2010) Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature 466: 829–834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miraoui H, Severe N, Vaudin P, Pages JC, Marie PJ (2010) Molecular silencing of Twist1 enhances osteogenic differentiation of murine mesenchymal stem cells: implication of FGFR2 signaling. J Cell Biochem 110: 1147–1154 [DOI] [PubMed] [Google Scholar]
- Mizoguchi T, Pinho S, Ahmed J, Kunisaki Y, Hanoun M, Mendelson A, Ono N, Kronenberg HM, Frenette PS (2014) Osterix marks distinct waves of primitive and definitive stromal progenitors during bone marrow development. Dev Cell 29: 340–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mootha VK, Lindgren CM, Eriksson KF, Subramanian A, Sihag S, Lehar J, Puigserver P, Carlsson E, Ridderstråle M, Laurila E et al (2003) PGC‐1α‐responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet 34: 267–273 [DOI] [PubMed] [Google Scholar]
- Morikawa S, Mabuchi YO, Kubota Y, Nagai Y, Niibe K, Hiratsu E, Suzuki S, Miyauchi‐Hara C, Nagoshi N, Sunabori T et al (2009) Prospective identification, isolation, and systemic transplantation of multipotent mesenchymal stem cells in murine bone marrow. J Exp Med 206: 2483–2496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison SJ, Perez SE, Qiao Z, Verdi JM, Hicks C, Weinmaster G, Anderson DJ (2000) Transient Notch activation initiates an irreversible switch from neurogenesis to gliogenesis by neural crest stem cells. Cell 101: 499–510 [DOI] [PubMed] [Google Scholar]
- Nagoshi N, Shibata S, Kubota Y, Nakamura M, Nagai Y, Satoh E, Morikawa S, Okada Y, Mabuchi YO, Katoh H et al (2008) Ontogeny and multipotency of neural crest‐derived stem cells in mouse bone marrow, dorsal root ganglia, and whisker pad. Cell Stem Cell 2: 392–403 [DOI] [PubMed] [Google Scholar]
- Omatsu Y, Sugiyama T, Kohara H, Kondoh G, Fujii N, Kohno K, Nagasawa T (2010) The essential functions of adipo‐osteogenic progenitors as the hematopoietic stem and progenitor cell niche. Immunity 33: 387–399 [DOI] [PubMed] [Google Scholar]
- Ono N, Ono W, Nagasawa T, Kronenberg HM (2014) A subset of chondrogenic cells provides early mesenchymal progenitors in growing bones. Nat Cell Biol 16: 1157–1167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortinau LC, Wang H, Lei K, Deveza L, Jeong Y, Hara Y, Grafe I, Rosenfeld SB, Lee D, Lee B et al (2019) Identification of functionally distinct Mx1+alphaSMA+ periosteal skeletal stem cells. Cell Stem Cell 25: 784–796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otasek D, Morris JH, Bouças J, Pico AR, Demchak B (2019) Cytoscape automation: empowering workflow‐based network analysis. Genome Biol 20: 185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park D, Spencer JA, Koh BI, Kobayashi T, Fujisaki J, Clemens TL, Lin CP, Kronenberg HM, Scadden DT (2012) Endogenous bone marrow MSCs are dynamic, fate‐restricted participants in bone maintenance and regeneration. Cell Stem Cell 10: 259–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinho S, Lacombe J, Hanoun M, Mizoguchi T, Bruns I, Kunisaki Y, Frenette PS (2013) PDGFRalpha and CD51 mark human nestin+ sphere‐forming mesenchymal stem cells capable of hematopoietic progenitor cell expansion. J Exp Med 210: 1351–1367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu X, Mao Q, Tang Y, Wang L, Chawla R, Pliner HA, Trapnell C (2017) Reversed graph embedding resolves complex single‐cell trajectories. Nat Methods 14: 979–982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramasamy SK, Kusumbe AP, Wang L, Adams RH (2014) Endothelial Notch activity promotes angiogenesis and osteogenesis in bone. Nature 507: 376–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodeheffer MS, Birsoy K, Friedman JM (2008) Identification of white adipocyte progenitor cells in vivo. Cell 135: 240–249 [DOI] [PubMed] [Google Scholar]
- Roy S, Khanna S, Rink T, Radtke J, Williams WT, Biswas S, Schnitt R, Strauch AR, Sen CK (2007) P21waf1/cip1/sdi1 as a central regulator of inducible smooth muscle actin expression and differentiation of cardiac fibroblasts to myofibroblasts. Mol Biol Cell 18: 4837–4846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacchetti B, Funari A, Michienzi S, Di Cesare S, Piersanti S, Saggio I, Tagliafico E, Ferrari S, Robey PG, Riminucci M et al (2007) Self‐renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell 131: 324–336 [DOI] [PubMed] [Google Scholar]
- Sadie‐Van Gijsen H, Hough FS, Ferris WF (2013) Determinants of bone marrow adiposity: the modulation of peroxisome proliferator‐activated receptor‐gamma2 activity as a central mechanism. Bone 56: 255–265 [DOI] [PubMed] [Google Scholar]
- Satija R, Farrell JA, Gennert D, Schier AF, Regev A (2015) Spatial reconstruction of single‐cell gene expression data. Nat Biotechnol 33: 495–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seike M, Omatsu Y, Watanabe H, Kondoh G, Nagasawa T (2018) Stem cell niche‐specific Ebf3 maintains the bone marrow cavity. Genes Dev 32: 359–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu HS, Liu YL, Tang XT, Zhang XS, Zhou B, Zou W, Zhou BO (2021) Tracing the skeletal progenitor transition during postnatal bone formation. Cell Stem Cell 28: 2122–2136 [DOI] [PubMed] [Google Scholar]
- Sivaraj KK, Jeong HW, Dharmalingam B, Zeuschner D, Adams S, Potente M, Adams RH (2021) Regional specialization and fate specification of bone stromal cells in skeletal development. Cell Rep 36: 109352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skelly DA, Squiers GT, McLellan MA, Bolisetty MT, Robson P, Rosenthal NA, Pinto AR (2018) Single‐cell transcriptional profiling reveals cellular diversity and intercommunication in the mouse heart. Cell Rep 22: 600–610 [DOI] [PubMed] [Google Scholar]
- Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES et al (2005) Gene set enrichment analysis: a knowledge‐based approach for interpreting genome‐wide expression profiles. Proc Natl Acad Sci 102: 15545–15550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama T, Kohara H, Noda M, Nagasawa T (2006) Maintenance of the hematopoietic stem cell pool by CXCL12‐CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity 25: 977–988 [DOI] [PubMed] [Google Scholar]
- Suire C, Brouard N, Hirschi K, Simmons PJ (2012) Isolation of the stromal‐vascular fraction of mouse bone marrow markedly enhances the yield of clonogenic stromal progenitors. Blood 119: e86–e95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takashima Y, Era T, Nakao K, Kondo S, Kasuga M, Smith AG, Nishikawa S (2007) Neuroepithelial cells supply an initial transient wave of MSC differentiation. Cell 129: 1377–1388 [DOI] [PubMed] [Google Scholar]
- Tikhonova AN, Dolgalev I, Hu H, Sivaraj KK, Hoxha E, Cuesta‐Domínguez Á, Pinho S, Akhmetzyanova I, Gao J, Witkowski M et al (2019) The bone marrow microenvironment at single‐cell resolution. Nature 569: 222–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira Braga FA, Kar G, Berg M, Carpaij OA, Polanski K, Simon LM, Brouwer S, Gomes T, Hesse L, Jiang J et al (2019) A cellular census of human lungs identifies novel cell states in health and in asthma. Nat Med 25: 1153–1163 [DOI] [PubMed] [Google Scholar]
- Wolf FA, Angerer P, Theis FJ (2018) SCANPY: large‐scale single‐cell gene expression data analysis. Genome Biol 19: 15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worthley D, Churchill M, Compton J, Tailor Y, Rao M, Si Y, Levin D, Schwartz M, Uygur A, Hayakawa Y et al (2015) Gremlin 1 identifies a skeletal stem cell with bone, cartilage, and reticular stromal potential. Cell 160: 269–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki S, Ema H, Karlsson G, Yamaguchi T, Miyoshi H, Shioda S, Taketo MM, Karlsson S, Iwama A, Nakauchi H (2011) Nonmyelinating Schwann cells maintain hematopoietic stem cell hibernation in the bone marrow niche. Cell 147: 1146–1158 [DOI] [PubMed] [Google Scholar]
- Yang L, Tsang KY, Tang HC, Chan D, Cheah KS (2014) Hypertrophic chondrocytes can become osteoblasts and osteocytes in endochondral bone formation. Proc Natl Acad Sci USA 111: 12097–12102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Matsuda K, Bialek P, Jacquot S, Masuoka HC, Schinke T, Li L, Brancorsini S, Sassone‐Corsi P, Townes TM et al (2004) ATF4 is a substrate of RSK2 and an essential regulator of osteoblast biology; implication for Coffin‐Lowry Syndrome. Cell 117: 387–398 [DOI] [PubMed] [Google Scholar]
- Yu G, Wang LG, Han Y, He QY (2012) clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS 16: 284–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue R, Zhou BO, Shimada IS, Zhao Z, Morrison SJ (2016) Leptin receptor promotes adipogenesis and reduces osteogenesis by regulating mesenchymal stromal cells in adult bone marrow. Cell Stem Cell 18: 782–796 [DOI] [PubMed] [Google Scholar]
- Zhong L, Yao L, Tower RJ, Wei Y, Miao Z, Park J, Shrestha R, Wang L, Yu W, Holdreith N et al (2020) Single cell transcriptomics identifies a unique adipose lineage cell population that regulates bone marrow environment. Elife 9: e54695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou BO, Yu H, Yue R, Zhao Z, Rios JJ, Naveiras O, Morrison SJ (2017) Bone marrow adipocytes promote the regeneration of stem cells and haematopoiesis by secreting SCF. Nat Cell Biol 19: 891–903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou BO, Yue R, Murphy MM, Peyer JG, Morrison SJ (2014a) Leptin‐receptor‐expressing mesenchymal stromal cells represent the main source of bone formed by adult bone marrow. Cell Stem Cell 15: 154–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F, Li X, Wang W, Zhu P, Zhou J, He W, Ding M, Xiong F, Zheng X, Li Z et al (2016) Tracing haematopoietic stem cell formation at single‐cell resolution. Nature 533: 487–492 [DOI] [PubMed] [Google Scholar]
- Zhou X, von der Mark K, Henry S, Norton W, Adams H, de Crombrugghe B (2014b) Chondrocytes transdifferentiate into osteoblasts in endochondral bone during development, postnatal growth and fracture healing in mice. PLoS Genet 10: e1004820 [DOI] [PMC free article] [PubMed] [Google Scholar]


