This cohort study investigates changes in dose-normalized concentrations of the antiseizure medications lamotrigine, levetiracetam, lacosamide, oxcarbazepine, and zonisamide during pregnancy among women with epilepsy.
Key Points
Question
In women with epilepsy, which antiseizure medications undergo concentration declines during pregnancy?
Findings
In this cohort study of 430 participants, women with epilepsy taking lamotrigine, levetiracetam, lacosamide, oxcarbazepine, and zonisamide experienced significant decreases in dose-normalized concentrations during pregnancy. Although dose-normalized concentrations decreased for carbamazepine, they remained stable for unbound carbamazepine and carbamazepine-10,11-epoxide.
Meaning
Results of this study suggest the need for higher doses of several antiseizure medications during pregnancy and support therapeutic drug monitoring beginning early in pregnancy.
Abstract
Importance
During pregnancy in women with epilepsy, lower blood concentrations of antiseizure medications can have adverse clinical consequences.
Objective
To characterize pregnancy-associated concentration changes for several antiseizure medications among women with epilepsy.
Design, Setting, and Participants
Enrollment in this prospective, observational cohort study, Maternal Outcomes and Neurodevelopmental Effects of Antiepileptic Drugs (MONEAD), occurred from December 19, 2012, to February 11, 2016, at 20 US sites. Enrolled cohorts included pregnant women with epilepsy and nonpregnant control participants with epilepsy. Inclusion criteria were women aged 14 to 45 years, an intelligence quotient greater than 70 points, and, for the cohort of pregnant women, a fetal gestational age younger than 20 weeks. A total of 1087 women were assessed for eligibility; 397 were excluded and 230 declined. Data were analyzed from May 1, 2014, to June 30, 2021.
Exposure
Medication plasma concentrations in women taking monotherapy or in combination with noninteracting medications. The cohort of pregnant women was monitored through 9 months post partum, with similar time points for control participants.
Main Outcomes and Measures
Dose-normalized concentrations were calculated as total or unbound plasma medication concentrations divided by total daily dose. Phlebotomy was performed during 4 pregnancy study visits and 3 postpartum visits for the pregnant women and 7 visits over 18 months for control participants. The primary hypothesis was to test pregnancy changes of dose-normalized concentrations from nonpregnant postpartum samples compared with those of control participants.
Results
Of the 351 pregnant women and 109 control participants enrolled in MONEAD, 326 pregnant women (median [range] age, 29 [19-43] years) and 104 control participants (median [range] age, 29 [16-43] years) met eligibility criteria for this analysis. Compared with postpartum values, dose-normalized concentrations during pregnancy were decreased by up to 56.1% for lamotrigine (15.60 μg/L/mg to 6.85 μg/L/mg; P < .001), 36.8% for levetiracetam (11.33 μg/L/mg to 7.16 μg/L/mg; P < .001), 17.3% for carbamazepine (11.56 μg/L/mg to 7.97 μg/L/mg; P = .03), 32.6% for oxcarbazepine (11.55 μg/L/mg to 7.79 μg/L/mg; P < .001), 30.6% for unbound oxcarbazepine (6.15 μg/L/mg to 4.27 μg/L/mg; P < .001), 39.9% for lacosamide (26.14 μg/L/mg to 15.71 μg/L/mg; P < .001), and 29.8% for zonisamide (40.12 μg/L/mg to 28.15 μg/L/mg; P < .001). No significant changes occurred for unbound carbamazepine, carbamazepine-10,11-epoxide, and topiramate, although a decrease was observed for topiramate (29.83 μg/L/mg to 13.77 μg/L/mg; P = .18). Additionally, compared with dose-normalized concentrations from control participants, pregnancy dose-normalized median (SE) concentrations decreased significantly by week of gestational age: carbamazepine, −0.14 (0.06) μg/L/mg (P = .02); carbamazepine unbound, −0.04 (0.01) μg/L/mg (P = .01); lacosamide, −0.23 (0.07) μg/L/mg (P < .001); lamotrigine, −0.20 (0.02) μg/L/mg (P < .001); levetiracetam, −0.06 (0.03) μg/L/mg (P = .01); oxcarbazepine, −0.14 (0.04) μg/L/mg (P < .001); oxcarbazepine unbound, −0.11 (0.03) μg/L/mg (P < .001); and zonisamide, −0.53 (0.14) μg/L/mg (P < .001) except for topiramate (−0.35 [0.20] μg/L/mg per week) and carbamazepine-10,11-epoxide (0.02 [0.01] μg/L/mg).
Conclusions and Relevance
Study results suggest that therapeutic drug monitoring should begin early in pregnancy and that increasing doses of these anticonvulsants may be needed throughout the course of pregnancy.
Introduction
With a point prevalence of 6.85 (95% CI, 5.55-8.47) per 1000, epilepsy is one of the most common disorders requiring daily treatment with potential teratogens during pregnancy.1 Treatment is further complicated by pregnancy-induced pharmacokinetic changes in antiseizure medications (ASMs) (eg, decreased protein binding, altered hepatic metabolism, increased kidney blood flow).2 Increased ASM clearance during pregnancy can decrease concentrations, and ASM gestational concentrations less than 65% of the preconception baseline can significantly increase the risk of seizures.3,4,5,6 Management of epilepsy in pregnancy requires balancing risk of harm from suboptimal therapy and worsening maternal seizures with fetal risk from increased ASM exposure due to unnecessary dose increases.
Increases in ASM clearance, and thus decreases in ASM concentrations, have been observed during pregnancy. Observational studies of lamotrigine during pregnancy report lamotrigine clearance increases as much as 219% above preconception clearance, starting as early as 5 weeks’ gestational age (GA).3,7,8,9,10,11 Clearance changes in total and free lamotrigine are nearly identical.12 Several small studies13,14,15 of levetiracetam also suggest increased clearance during pregnancy, probably owing to increased kidney elimination. Reports of clearance changes for total carbamazepine, unbound carbamazepine, and the metabolite carbamazepine 10,11-epoxide are inconsistent.16,17,18,19,20,21,22 Oxcarbazepine (measured as the monohydroxy derivative) clearance probably increases, but changes in unbound fraction of monohydroxy derivative have not been characterized.4,23,24,25 Increased clearances have been reported for zonisamide and topiramate during pregnancy.4,26,27,28 These relatively small studies of pregnancy-related ASM clearance changes have used variable methods, and to our knowledge, there are no studies that compare clearance or concentration changes in pregnant women with epilepsy with that in a control group of nonpregnant women with epilepsy using the same study protocol.
The American Academy of Neurology practice parameters concluded that clearance changes are largely unknown during pregnancy for most ASMs.6 The Maternal Outcomes and Neurodevelopmental Effects of Antiepileptic Drugs (MONEAD) study provided a unique opportunity to better characterize clearance changes during pregnancy for a variety of ASMs. The MONEAD study compared data from a cohort of pregnant women with that of control participants who were not pregnant at similar sampling time points using a similar protocol. The study findings provided a framework for practitioners to consider when treating women with epilepsy during pregnancy and during the postpartum period. We chose to report the data as dose-normalized concentrations (DNCs) to allow comparisons of concentrations across individuals who were receiving different doses at different times as per their clinical regimen.
Methods
Study Design
The MONEAD study was a National Institutes of Health–funded, 20-site, prospective, observational cohort study with recruitment of pregnant women with epilepsy and a control group of nonpregnant women with epilepsy. Other groups recruited for the MONEAD study were not included in this analysis. Key inclusion criteria for all participants with epilepsy included women aged 14 to 45 years and the ability to maintain a daily medical diary. Exclusion criteria included an intelligence quotient less than 70 points, major medical illness, and progressive cerebral disease. Specific exclusion criteria for the pregnant women included fetal GA older than 20 weeks, exposure to non-ASM teratogens, the detection of major fetal congenital malformations, or a history of genetic disorder in the participant or a primary relative. The study was approved by individual site institutional review boards. Written informed consent was obtained from all participants before study participation. Recruitment occurred from December 19, 2012, through February 11, 2016. Decisions regarding ASM management, including dose adjustments, were made by the treating clinician for both groups. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines.
Pregnant women had 3 visits during gestation, 1 visit at delivery, and 1 visit at 1.5 to 3, 6, and 9 months post partum each. GA was calculated based on the last menstrual period confirmed by first-trimester ultrasound. Second-trimester visits occurred at 21 to 27 weeks’ GA, and third-trimester visits occurred at 30 to 36 weeks’ GA. When the first visit occurred within the second trimester, the next visit was separated by 4 or more weeks. Women participants self-identified their race (American Indian or Alaska Native, Asian, Black or African American, Native Hawaiian or Pacific Islander, White, multiracial, other/unknown) and ethnicity (Hispanic, non-Hispanic) based on options defined by the protocol, and they were asked to choose other if they did not identify with one of the specified race categories. Race and ethnicity information was required by the funding body. The control cohort of nonpregnant women were recruited to resemble the cohort of pregnant women with epilepsy regarding race and ethnicity, seizure type, seizure frequency, ASM monotherapy vs polytherapy, and specific ASMs. The control cohort had 7 study visits over a time frame and time interval similar to that of the pregnant cohort.
Pharmacokinetic Sampling and Bioanalysis
At each study visit, convenience blood samples were collected (≥3 days after last dose change to reflect steady state). At the first visit, detailed demographic characteristics; ASMs; concomitant medications; and family, medical, and epilepsy history were obtained. Participants used an electronic daily diary application29 developed for this study, which included details of the ASM regimen with dosing, including dates of any changes and missed doses. At each subsequent study visit, demographic and medical history were updated, and research staff reviewed electronic daily diary data to verify accuracy. ASM logs documented start and stop dates of all ASM doses.
ASM plasma concentrations were measured via validated liquid chromatography–mass spectrometry assays at the University of Minnesota in the MONEAD Pharmacokinetics Core Laboratory.30 The ASMs measured and quantification ranges were lamotrigine, 0.3 to 25 μg/mL; levetiracetam, 0.1 to 80 μg/mL; total and unbound carbamazepine, 0.3 to 25 μg/mL; carbamazepine-10,11-epoxide, 0.3 to 25 μg/mL; total and unbound monohydroxy derivative of oxcarbazepine, 0.5 to 40 μg/mLeach; topiramate, 0.4 to 30 μg/mL; zonisamide, 0.6 to 50 μg/mL; and lacosamide, 0.1 to 80 μg/mL.
Statistical Analysis
Women taking ASM monotherapy or noninteracting polytherapy with any blood samples were considered for this analysis. To account for varied and changing doses, we used DNCs in all analyses: DNC = ASM plasma concentration (μg/mL) / ASM total daily dose (mg). It was assumed that owing to the ASM concentrations reflecting steady state, any variability introduced by not accounting for the timing of blood samples would be small. Most samples (approximately 60%) were collected 3 to 7 hours after the dose was taken. Within the cohort of pregnant women, to characterize changes in DNC during pregnancy compared with those during the nonpregnant period, we used the last postpartum dose and concentration measurement available as the nonpregnant comparison. Postpartum values used as baselines were measured a median (range) of 38 (14-48) weeks after delivery. Samples during delivery hospitalization were included in the third trimester. A repeated-measures analysis of variance was used, and each visit was listed as a categorical variable. For multiple comparisons, the Tukey post hoc test was used with a 2-sided significance level of P < .05.
To characterize differences in DNC between the cohort of pregnant women and the control participants, we first created 2 subsets of data: (1) pregnancy-only data, which included all pregnancy visits from the cohort of pregnant women and visits 1 to 4 from the control participants and (2) postpartum-only data, which included all postpartum visits from the cohort of pregnant women and visits 5 to 7 from the control participants. It should be noted that although the data are named as pregnancy only and post partum only, the control participants were never pregnant. Visits 1 to 4 and 5 to 7 were in the same time sequence as the pregnancy and postpartum visits, respectively, from the cohort of pregnant women and included in the data as control or reference values. The last postpartum value was used as the nonpregnant baseline. We used linear mixed-effect models treating time as a continuous variable, the cohort as a categorical variable, and an interaction term on both data sets to characterize changes in ASM DNCs. The models were parametrized as shown in
| Yij = (β0 + bi) + (β1 + ci) × Timeij + β2 × Cohorti + β3 × Timeij × Cohorti, |
where Yij was the ith individual DNC at time j, β0 was the intercept with participant-specific random-effect intercept bi, β1 was the coefficient for continuous variable time (GA or postpartum weeks for the pregnant cohort and time since enrollment for the control cohort) with participant-specific random-effect slope ci, β2 was the coefficient for the categorical covariate cohort (0 input for controls, 1 input for pregnant women), β3 was the coefficient for the time and cohort interaction, and bi and ci were normally distributed random effects with a mean of 0 and variability of σ2b and σ2c, respectively. For a typical individual, bi and ci were set at the population mean of 0. Given the way the model was coded, β2 and β3 were estimated only for the cohort of pregnant women.
Thus, for a typical pregnant woman at 30 weeks’ GA, the model was written as
| Y30 weeks (pregnant) = (β0 + β2) + (β1 + β3) × 30, |
whereas for a typical woman in the control group at 30 study weeks, the model was written as
| Y30 weeks (control) = β0 + β1 × 30. |
The equations show that the common intercept β0 and the common slope β1 were informed by data from both the cohort of pregnant women and the cohort of control participants (eFigure 1 in Supplement 1). The additional intercept β2 and additional slope β3 were only informed by data from the cohort of pregnant women. Significant P values for β2 would indicate that generally (holding other variables constant), a typical pregnant woman’s DNC during the nonpregnant state (post partum) significantly differed from that of a woman from the control cohort. On the other hand, significant P values for β3 (holding other variables constant) indicate that generally, women in the pregnant cohort experience significant changes in DNC with time (ie, GA) compared with women in the control cohort.
For all ASMs, we compared model performance between a participant-specific intercept vs participant-specific slope and intercept using a likelihood ratio test. In addition, for lamotrigine and levetiracetam analyses, the use of estrogen-based hormonal medication (estrogen only and combined estrogen and progestins) was also considered as a covariate. For the remaining ASMs, less than 10% of women were taking hormonal medications; hence, this covariate was not tested.
All analyses were performed in R, version 3.6.1 (R Foundation for Statistical Computing) using the packages nlme, version 3.1-141, and multcomp, version 1.4-10. Data were analyzed from May 1, 2014, to June 30, 2021.
Results
Participants
Of 1087 women with epilepsy who were assessed for eligibility, 351 pregnant women and 109 control participants were enrolled in the MONEAD study; 397 were ineligible (350 pregnant women, 47 control participants), and 230 were eligible but declined participation (152 pregnant women, 78 control participants). Of the women enrolled in MONEAD, 326 pregnant women (median [range] age, 29 [19-43] years; race: 10 [3.1%] Asian, 20 [6.1%] Black or African American, 1 [0.3%] Native Hawaiian or Pacific Islander, 278 [85.3%] White, 11 [3.4%] multiracial, 6 [1.2%] other/unknown; ethnicity: 65 [19.9%] Hispanic, 261 [80.1%] non-Hispanic) and 104 control participants (median [range] age, 29 [16-43] years; race: 4 [3.8%] Asian, 4 [3.8%] Black or African American, 0 Native Hawaiian or Pacific Islander, 95 [91.3%] White, 0 multiracial, 1 [1.0%] other/unknown; ethnicity: 15 [14.4%] Hispanic, 89 [85.6%] non-Hispanic) met eligibility criteria for analysis. Eligible women were taking either ASM monotherapy or polytherapy with noninteracting ASMs (eFigures 2 and 3 in Supplement 1). Ninety-four pregnant women (28.8%) and 27 control women (26.0%) contributed to analysis of ASMs in polytherapy. Of the 6 ASMs, lamotrigine and levetiracetam had the highest sample size for both cohorts. Demographic characteristics of the pregnant and control women were similar. The demographic, pertinent medical, and ASM dosing data used in this analysis by specific ASM are presented in Table 1. We observed that most ASMs had higher total daily doses during pregnancy than during the postpartum period and when compared with the control cohort (eg, median [range] daily dose of lamotrigine: second trimester, 500 [50-190] mg; post partum, 400 [50-100] mg; control, 400 [100-800] mg).
Table 1. Demographic Characteristics of the Cohort of Pregnant Women and Control Participants With Antiseizure Medication Concentrations Available, Stratified by Medication.
| Cohort variable | Median (range) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Carbamazepine | Lacosamide | Lamotrigine | Levetiracetam | Oxcarbazepine | Topiramate | Zonisamide | ||||||||
| Pregnant | Control | Pregnant | Control | Pregnant | Control | Pregnant | Control | Pregnant | Control | Pregnant | Control | Pregnant | Control | |
| Sample size, No. | 18 | 4 | 16 | 11 | 162 | 49 | 151 | 46 | 20 | 8 | 15 | 6 | 22 | 9 |
| Plasma samples collected, No. | 101 | 28 | 67 | 58 | 891 | 277 | 821 | 261 | 111 | 37 | 53 | 26 | 112 | 52 |
| Age, y | 30.0 (28.0-31.0) | 33.5 (28.0-42.0) | 31.0 (30.0-35.0) | 27.0 (18.0-38.0) | 31.0 (20.0-43.0) | 29.0 (16.0-40.0) | 31.0 (20.0-42.0) | 28.0 (20.0-44.0) | 29.0 (19.0-32.0) | 27.0 (22.0-28.0) | 28.0 (26.0-34.0) | 25.0 (22.0-28.0) | 29.0 (23.0-33.0) | 33.0 (24.0-43.0) |
| Race, No. (%) | ||||||||||||||
| Asian | 3 (16.7) | 0 | 1 (6.3) | 1 (9.1) | 4 (2.5) | 1 (2.0) | 2 (1.3) | 2 (4.3) | 0 | 1 (12.5) | 1 (6.7) | 0 | 0 | 0 |
| Black or African American | 1 (5.6) | 0 | 3 (18.8) | 10 (90.9) | 10 (6.2) | 2 (4.1) | 9 (6.0) | 2 (4.3) | 2 (10.0) | 0 | 0 | 0 | 0 | 0 |
| Native Hawaiian or Pacific Islander | 0 | 0 | 0 | 0 | 0 | 0 | 1 (0.7) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| White | 11 (61.1) | 4 (100) | 12 (75.0) | 0 | 141 (87.0) | 45 (91.8) | 130 (86.1) | 42 (91.3) | 18 (90.0) | 7 (87.5) | 13 (86.7) | 6 (100) | 21 (95.5) | 9 (100) |
| Multiracial | 1 (5.6) | 0 | 0 | 0 | 5 (3.1) | 0 | 6 (4.0) | 0 | 0 | 0 | 0 | 0 | 1 (4.5) | 0 |
| Other/uknown | 2 (11.1) | 0 | 0 | 0 | 2 (1.2) | 1 (2.0) | 3 (2.0) | 0 | 0 | 0 | 1 (6.7) | 0 | 0 | 0 |
| Ethnicity, No. (%) | ||||||||||||||
| Hispanic | 5 (27.8) | 0 | 2 (12.5) | 0 | 25 (15.4) | 9 (18.4) | 44 (29.1) | 5 (10.9) | 4 (20.0) | 1 (12.5) | 4 (26.7) | 1 (16.7) | 4 (18.2) | 4 (44.4) |
| Non-Hispanic | 13 (72.2) | 4 (100) | 14 (87.5) | 11 (100) | 137 (84.6) | 40 (81.6) | 107 (7.09) | 41 (89.1) | 16 (80.0) | 7 (87.5) | 11 (73.3) | 5 (83.3) | 18 (81.8) | 5 (55.6) |
| Gestational age, wk | 29 (7-41) | NA | 28.5 (10-39) | NA | 26 (5-42) | NA | 27 (6-41) | NA | 26 (6-41) | NA | 23 (7-41) | NA | 28 (7-41) | NA |
| Postpartum period, wk | 26 (6-47) | NA | 26 (7-43) | NA | 25 (4-48) | NA | 25 (4-47) | NA | 26 (4-41) | NA | 26 (11-41) | NA | 25 (6-42) | NA |
| Weight, kg | ||||||||||||||
| First trimester | 59.1 (52.7-85.8) | NA | 73.9 (65.1-76.8) | NA | 65.5 (47.0-122.5) | NA | 66.0 (47.3-122.5) | NA | 73.6 (58.0-105.5) | NA | 70.6 (51.8-110.0) | NA | 68.6 (53.6-122.5) | NA |
| Second trimester | 69.4 (50.7-112.7) | NA | 74.2 (54.7-89.5) | NA | 73.9 (47.5-127.0) | NA | 71.8 (43.8-162.7) | NA | 74.1 (46.8-120.7) | NA | 78.3 (56.7-123.6) | NA | 66.9 (52.3-105.6) | NA |
| Third trimester | 76.8 (53.6-112.7) | NA | 75.7 (62.6-98.0) | NA | 76.5 (52.2-127.2) | NA | 76.20 (51.3-161.7) | NA | 77.3 (52.3-122.7) | NA | 76.0 (57.9-98.3) | NA | 68.0 (57.5-113.6) | NA |
| Post partum | 70.7 (50.0-115.2) | 62.7 (48.4-90.4) | 75.6 (55.5-117.5) | 60.9 (53.0-111.6) | 70.5 (41.2-138.3) | 68.6 (51.2-111.8) | 69.1 (44.5-138.3) | 62.4 (53.6-110.0) | 67.3 (42.7-117.4) | 58.8 (51.5-77.2) | 76.0 (54.9-133.0) | 51.1(49.8-110.0) | 64.3 (49.4-136.9) | 68.0 (55.0-81.7) |
| Hormonal medication, No. | ||||||||||||||
| Progestin only | 0 | 0 | 2 | 2 | 20 | 4 | 18 | 2 | 0 | 1 | 1 | 0 | 0 | 1 |
| Estrogen only | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Combination | 0 | 1 | 0 | 2 | 9 | 13 | 8 | 13 | 1 | 0 | 0 | 1 | 0 | 3 |
| None | 17 | 3 | 14 | 7 | 133 | 32 | 125 | 31 | 19 | 7 | 14 | 5 | 22 | 5 |
| Total daily dose, mg | ||||||||||||||
| First trimester | 500 (400-1000) | NA | 375 (200-500) | NA | 400 (100-900) | NA | 2000 (200-4500) | NA | 1200 (600-2400) | NA | 450 (200-450) | NA | 400 (200-800) | NA |
| Second trimester | 800 (400-1400) | NA | 400 (200-700) | NA | 500 (50-1900) | NA | 2000 (200-7000) | NA | 1050 (600-2100) | NA | 300 (50-500) | NA | 400 (100-600) | NA |
| Third trimester | 800 (400-1600) | NA | 400 (200-700) | NA | 600 (50-2100) | NA | 2000 (500-7000) | NA | 1500 (600-3300) | NA | 200 (150-300) | NA | 400 (100-600) | NA |
| Post partum | 800 (300-1600) | 550 (100-1400) | 350 (50-600) | 400 (50-700) | 400 (50-1000) | 400 (100-800) | 2000 (375-6000) | 2000 (250-5000) | 1200 (450-2400) | 1050 (300-2100) | 200 (50-400) | 200 (100-400) | 350 (100-500) | 300 (100-400) |
Abbreviation: NA, not applicable.
DNCs During Pregnancy
Using repeated-measures analysis of variance for the cohort of pregnant women only, statistically significant differences in DNCs during pregnancy compared with postpartum values were present for all ASM compounds except topiramate, carbamazepine-10,11-epoxide, and unbound carbamazepine. The Tukey multiple comparisons test showed that for lamotrigine, DNCs were significantly lower in all 3 trimesters of pregnancy compared with DNCs during the postpartum period (median [SE] DNC, first trimester: −5.47 [0.58] μg/L/mg; second trimester: −7.63 [0.41] μg/L/mg; third trimester: −7.67 [0.42] μg/L/mg; P < .001). Additionally, lamotrigine DNCs were significantly lower in the second trimester (median [SE] DNC, −2.16 [0.57] μg/L/mg; P < .001) and the third trimester (median [SE] DNC, −2.19 [0.57] μg/L/mg; P < .001) compared with the first trimester (Figure 1). Overall, DNCs during pregnancy had decreased by up to 56.1% for lamotrigine (15.60 μg/L/mg to 6.85 μg/L/mg; P < .001).
Figure 1. Lamotrigine and Levetiracetam Dose-Normalized Concentrations During Pregnancy and the Postpartum Period.
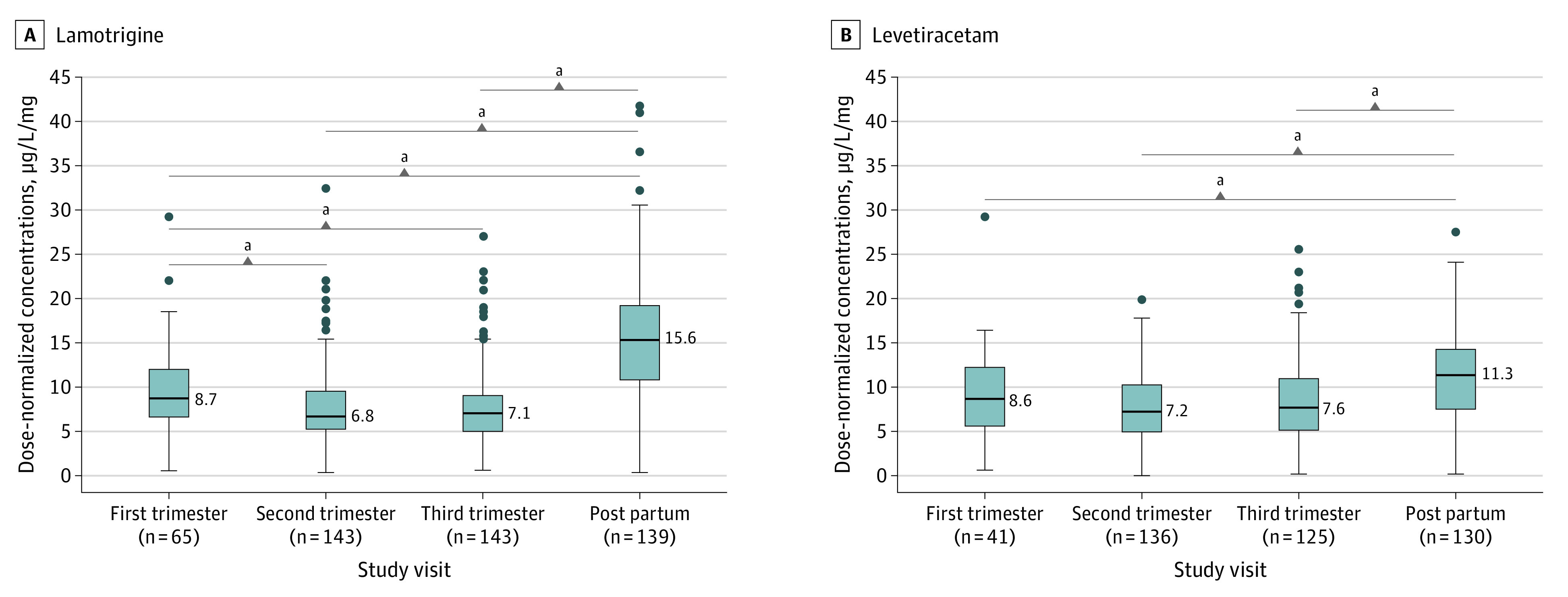
Boxplots showing lower dose-normalized concentrations during pregnancy and the postpartum period for lamotrigine (A) and levetiracetam (B). The midline of the box plots indicates the median, and the box indicates the 25th and 75th percentiles. Whiskers represent 1.5 times the IQR. Circles represent outliers. The postpartum period and the first trimester were used as comparators.
aP < .001.
For levetiracetam, lacosamide, carbamazepine, total and unbound oxcarbazepine, and zonisamide, DNCs were significantly lower in pregnancy than in the postpartum period (median [SE] DNC, levetiracetam: −3.55 [0.43] μg/L/mg; P < .001; lacosamide: −9.21 [1.78] μg/L/mg; P < .001; carbamazepine: −2.44 [0.84] μg/L/mg; P = .03; total oxcarbazepine: −4.44 [0.83] μg/L/mg; P < .001; unbound oxcarbazepine: −2.17 [0.55] μg/L/mg; P < .001; zonisamide: −15.04 [2.23] μg/L/mg; P < .001), but no significant differences were found between trimesters (Figures 1, 2, and 3 and eFigure 4 in Supplement 1). DNCs had decreased by up to 36.8% for levetiracetam (11.33 μg/L/mg to 7.16 μg/L/mg; P < .001), 17.3% for carbamazepine (11.56 μg/L/mg to 7.97 μg/L/mg; P = .03), 32.6% for oxcarbazepine (11.55 μg/L/mg to 7.79 μg/L/mg; P < .001), 30.6% for unbound oxcarbazepine (6.15 μg/L/mg to 4.27 μg/L/mg; P < .001), 39.9% for lacosamide (26.14 μg/L/mg to 15.71 μg/L/mg; P < .001), and 29.8% for zonisamide (40.12 μg/L/mg to 28.15 μg/L/mg; P < .001). DNCs for topiramate, carbamazepine-10,11-epoxide, and unbound carbamazepine had no significant differences either within pregnancy (first vs second and third trimester) or during pregnancy compared with the postpartum period (Figure 2 and eFigure 4 in Supplement 1).
Figure 2. Zonisamide, Lacosamide, and Topiramate Dose-Normalized Concentrations During Pregnancy and the Postpartum Period.
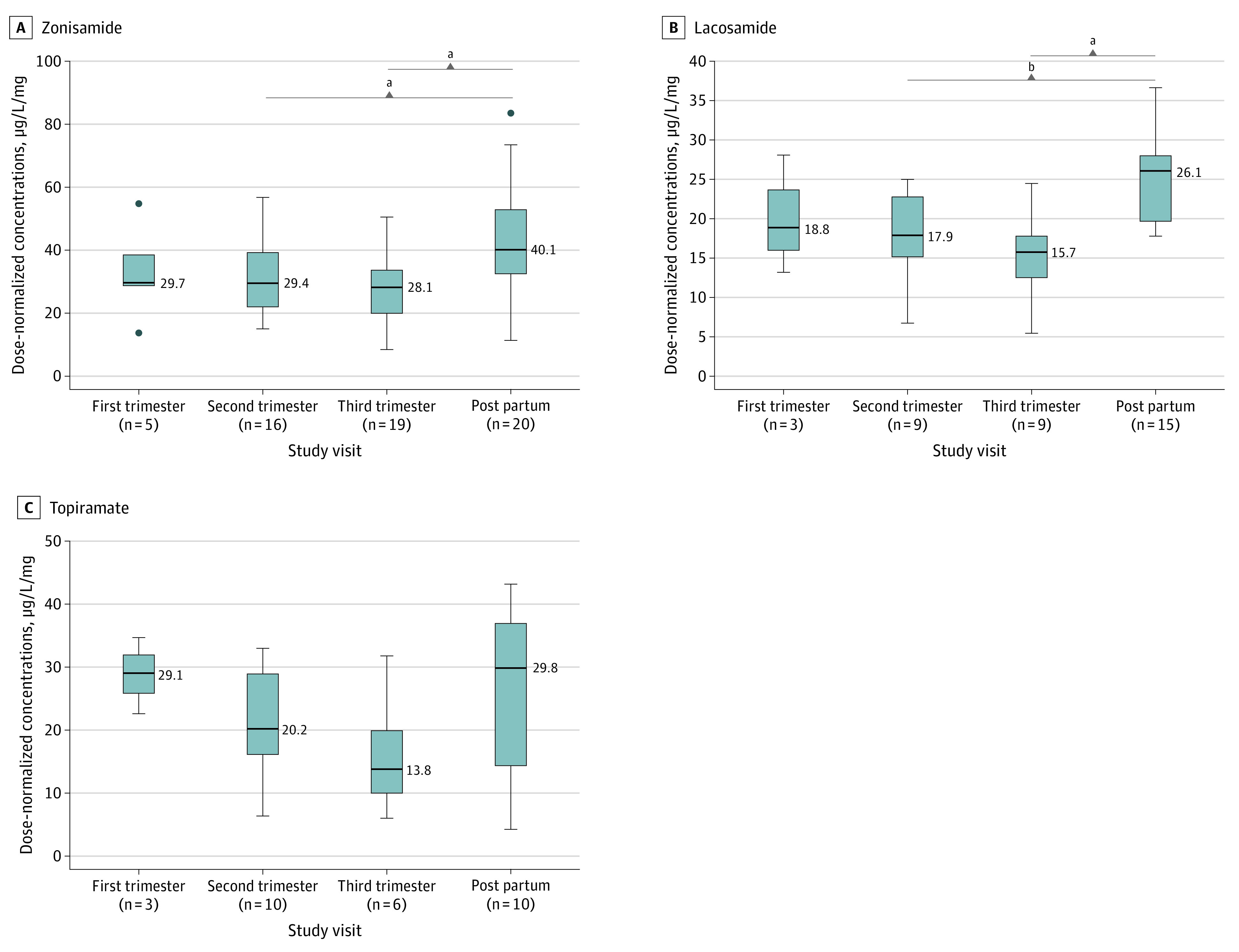
Boxplots showing the median lower dose-normalized concentrations during pregnancy and the postpartum period for zonisamide (A), lacosamide (B), and topiramate (C). The midline of the box plots indicates the median, and the box indicates the 25th and 75th percentiles. Whiskers represent 1.5 times the IQR. Circles represent outliers. The postpartum period was used as a comparator.
aP < .001.
bP < .05.
Figure 3. Total and Unbound Oxcarbazepine Dose-Normalized Concentrations During Pregnancy and the Postpartum Period.
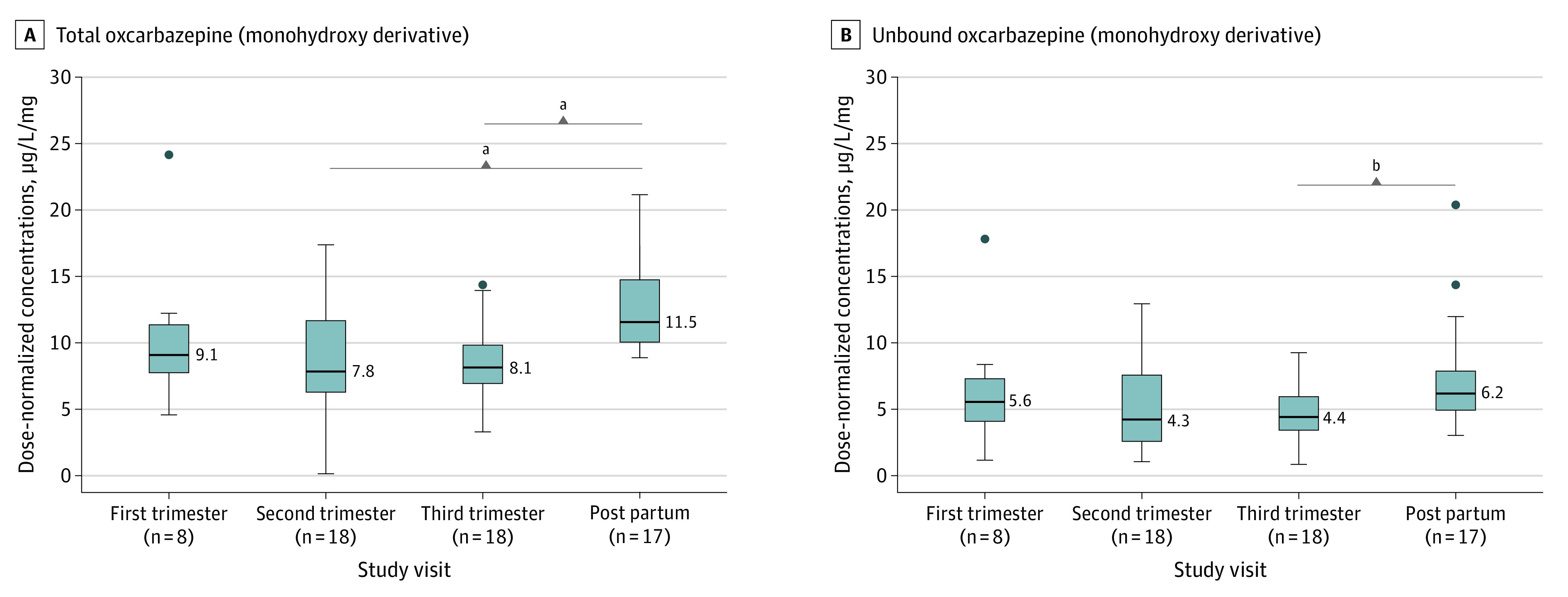
Boxplots showing statistically significant median lower dose-normalized concentrations during pregnancy and the postpartum period for total (A) and unbound (B) oxcarbazepine (measured as total and unbound monohydroxy derivative). The midline of the box plots indicates the median, and the box indicates the 25th and 75th percentiles. Whiskers represent 1.5 times the IQR. Circles represent outliers. The postpartum period was used as a comparator.
aP < .001.
bP < .01.
Linear Mixed-Effect Models
For all linear mixed-effect models, a participant-specific intercept and slope was used. A representative figure for lamotrigine is displayed in eFigure 1 in Supplement 1. Using data from both cohorts during visits 5 to 7, it was identified that for all ASMs, there were no significant differences in DNCs between the cohorts, and the DNCs did not change with postpartum weeks. The only exceptions were for unbound carbamazepine and lacosamide, as the cohort of pregnant women had higher DNCs during postpartum visits 5 to 7 than the control cohort (median [SE], unbound carbamazepine: 0.05 [0.02] μg/L/mg per week; P = .02; lacosamide: 0.32 [0.11] μg/L/mg per week; P = .01). The results of the postpartum linear mixed models are presented in the eTable in Supplement 1.
For these models using data from visits 1 to 4 in both cohorts, we identified that for topiramate and carbamazepine-10,11-epoxide, there were no differences in DNCs between the cohorts (pregnant and post partum), and the DNCs did not change with GA. However, the DNCs decreased significantly with GA in the cohort of pregnant women compared with the control cohort for lamotrigine (median [SE] DNC, −0.20 [0.02] μg/L/mg per week; P < .001), levetiracetam (−0.06 [0.03] μg/L/mg per week; P = .01), lacosamide (−0.23 [0.07] μg/L/mg per week; P < .001), total oxcarbazepine (−0.14 [0.04] μg/L/mg per week; P < .001), unbound oxcarbazepine (−0.11 [0.03] μg/L/mg per week; P < .001), zonisamide (−0.53 [0.14] μg/L/mg per week; P < .001), total carbamazepine (−0.14 [0.06] μg/L/mg per week; P = .02), and unbound carbamazepine (−0.04 [0.01] μg/L/mg per week; P = .01).
For lamotrigine, use of estrogen-based medication (eg, contraceptives) was found to significantly decrease DNCs (median [SE] DNC, −2.57 [1.15] μg/L/mg; P = .03) compared with women taking either progestin only or no hormonal medications. No change was noted in the dosages of any hormonal medications for levetiracetam. Levetiracetam (median [SE] DNC, −0.06 [0.03] μg/L/mg per week; P = .01) and unbound carbamazepine (−0.04 [0.01] μg/L/mg per week; P = .01) resulted in a significant decrease in DNCs with GA in pregnant women compared with control women; however, the cohort of pregnant women also had significantly lower baseline DNCs than the control cohort (median [SE] DNC, levetiracetam:−2.37 [0.7] μg/L/mg; P < .001; unbound carbamazepine: −1.76 [0.7] μg/L/mg; P = .03). The results of the pregnancy linear mixed models are presented in Table 2.
Table 2. Linear Mixed-Effect Model Parameter Estimates for Antiseizure Medication Dose-Normalized Concentrations in the Cohort of Pregnant Women and Control Participants Using Data From Pregnancy Visits.
| Antiseizure medication | Intercepts, μg/L/mg | Slopes, 1/wk | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| β0: Common baselinea | β2: Additional baseline for pregnantb | Estrogen-based hormonal medication | β1: Common slopec | β3: Additional slope for pregnantd | ||||||
| Estimate (SE) | P value | Estimate (SE) | P value | Estimate (SE) | P value | Estimate (SE) | P value | Estimate (SE) | P value | |
| Carbamazepine | 13.32 (2.46) | <.001 | −3.17 (2.67) | .25 | NA | NA | 0.09 (0.05) | .11 | −0.14 (0.06) | .02 |
| Carbamazepine- 10,11-epoxide | 1.73 (0.38) | <.001 | 0.042 (0.42) | .92 | NA | NA | 0.01 (0.01) | .19 | 0.02 (0.01) | .82 |
| Carbamazepine unbound | 2.98 (0.63) | <.001 | −1.76 (0.72) | .02 | NA | NA | 0.03 (0.01) | .02 | −0.04 (0.01) | .01 |
| Lacosamide | 23.49 (1.76) | <.001 | 1.22 (2.39) | .62 | NA | NA | −0.05 (0.05) | .30 | −0.23 (0.07) | <.001 |
| Lamotrigine | 15.04 (0.92) | <.001 | −1.14 (1.03) | .27 | −2.57 (1.15) | .03 | 0.01 (0.02) | .81 | −0.20 (0.02) | <.001 |
| Levetiracetam | 12.51 (0.59) | <.001 | −2.37 (0.69) | <.001 | 0.23 (1.45) | .32 | −0.01 (0.02) | .68 | −0.06 (0.03) | .01 |
| Oxcarbazepine | 14.12 (1.80) | <.001 | −1.97 (2.15) | .37 | NA | NA | 0.01 (0.04) | .74 | −0.14 (0.04) | <.001 |
| Oxcarbazepine unbound | 6.60 (1.44) | <.001 | 0.44 (1.71) | .80 | NA | NA | 0.04 (0.03) | .18 | −0.11 (0.03) | <.001 |
| Topiramate | 28.42 (4.37) | <.001 | −1.51 (5.39) | .78 | NA | NA | 0.07 (0.16) | .65 | −0.35 (0.20) | .09 |
| Zonisamide | 46.31 (5.15) | <.001 | −4.69 (6.22) | .46 | NA | NA | 0.09 (0.11) | .42 | −0.53 (0.14) | <.001 |
Abbreviation: NA, not applicable.
Dose-normalized concentration estimate during the nonpregnant state in both the cohort of pregnant women and control participants.
A significant P value for β2 indicates that the cohort of pregnant women has differences in dose-normalized concentration estimates during the nonpregnant state compared with the cohort of control participants.
Change in dose-normalized concentrations with respect to time in both the cohort of pregnant women and the control participants.
A significant P value for β3 indicates that the cohort of pregnant women has a significant change in dose-normalized concentrations during pregnancy compared with the cohort of control participants.
Discussion
The MONEAD study was, to our knowledge, the largest cohort study with regular ASM blood concentration measurements during pregnancy in women with epilepsy and the only study with a control group of nonpregnant women with epilepsy. In this analysis, we found that the DNCs decreased during pregnancy for lamotrigine, levetiracetam, lacosamide, oxcarbazepine, and zonisamide. Additionally, the DNCs for lamotrigine was lower in the second and third trimester than in the first trimester, which was consistent with a prior study.3 The observation that total daily doses of most ASMs were higher during pregnancy than during the daily doses in the control group during the relevant study visits 1 to 4 was consistent with our prior published analysis of seizure control from this same cohort.31 We reported that the percentages of women with worsening seizure frequency did not differ between pregnant women with epilepsy and the control group. However, the group of pregnant women had more dosage changes (specifically, dose increases).
In the MONEAD study, most women were taking lamotrigine and/or levetiracetam.32 This was similar to ASM pregnancy registries, most likely owing to the fact that these 2 ASMs have the most data demonstrating low rates of major congenital malformations.12,33,34 Additionally, there are ample data for lamotrigine and some data for levetiracetam supporting favorable neurodevelopmental outcomes in children after prenatal exposure, which was further supported by the MONEAD study.35,36,37,38,39 Therefore, in many parts of the world, lamotrigine and levetiracetam are the most frequently prescribed ASM for women with epilepsy during pregnancy.32,40 Our findings for lamotrigine and levetiracetam showed that the DNC changes for these medications occurred early in pregnancy (ie, first trimester), which suggests that it is not sufficient to wait until later in pregnancy to consider therapeutic drug monitoring and dose changes. Both kidney elimination and hepatic elimination via glucuronidation begin very early during gestation. The enhanced glucuronidation is likely related to estrogen upregulation of the primary metabolizing enzyme uridine 5'-diphospho-glucuronosyltransferases 1A4 (UGT1A4).9,10,41 The interindividual variability supported the current recommendations to perform therapeutic monitoring with monthly ASM blood concentrations and to make dosage adjustments as necessary to prevent seizure worsening.6,42
This study also added information about DNC alterations during pregnancy for other ASMs for which there are, to our knowledge, relatively minimal to no prior published data (ie, lacosamide, oxcarbazepine, zonisamide).4 The lower DNC of oxcarbazepine in the second and third trimester is similar to that from a prior study.4 The clinical relevance is further supported in this study with lower DNC of unbound oxcarbazepine, albeit only in the third trimester of the cohort of 20 pregnant women taking oxcarbazepine. This study, to our knowledge, was the first published study of lacosamide DNCs during pregnancy and the postpartum period; study results showed significantly lower DNCs of lacosamide during the second and third trimester. There were a small number of samples for lacosamide (n = 3) and zonisamide (n = 5) in the first trimester. Given that both drugs are primarily eliminated via the kidneys, larger sample sizes may demonstrate significant changes in DNC in the first trimester similar to levetiracetam.
Our finding of no DNC alterations detected for unbound carbamazepine or the metabolite carbamazepine-10,11-epoxide was similar to that in a prior study with 17 pregnancies20; although we did find a lower DNC in the third trimester for total carbamazepine, the unbound concentrations are thought to be more clinically relevant. Owing to the presence of interparticipant variability, therapeutic drug monitoring may be beneficial in individual cases; however, drug monitoring may not be as essential with carbamazepine as with some other ASMs. Although we did not find differences in DNC for topiramate, the markedly lower median value in the third trimester compared with the nonpregnant postpartum period (13.77 μg/L/mg vs 29.83 μg/L/mg; P = .18) suggests potential clinical relevance and should be examined further. Participants taking topiramate represented the lowest sample size (Table 1), and the 25th and 75th percentiles were large. These factors likely contributed to the different finding from a prior study, which demonstrated increased clearance of topiramate in the second and third trimesters.4
Although analysis of variance and linear mixed-effect models agreed for all ASMs, there were some unexpected observations. In the linear mixed-effect models for levetiracetam and unbound carbamazepine, the cohort of pregnant women had lower baseline DNCs compared with those of the control participants, but this baseline was informed by postpartum values imputed for the baseline in the cohort of pregnant women. One reason for this observation could be differences in sampling times after dose administration between the groups; however, we observed no such differences for either ASM. Additionally, a similar difference in levetiracetam DNC between cohorts was not observed in the postpartum-only linear mixed-effect model. We believe that these differences for both ASMs were not clinically relevant for several reasons. First, the difference could have been introduced by use of the last visit postpartum data in the cohort of pregnant women into a time-invariant baseline. Second, sparsity of early pregnancy samples (defined as <10 weeks’ GA) could artificially lower the intercept to better fit the steeper slope. Even if not a data artifact, the outcome is minimal: for a 500-mg levetiracetam dose and 250-mg carbamazepine dose, the model predicted concentrations would be 1.15 μg/mL (levetiracetam) and 0.44 μg/mL (unbound carbamazepine) lower for the cohort of pregnant women at baseline than in the cohort of control participants. Both concentrations fell within the range of unexplained model variabilities (eg, assay variabilities, model misspecifications) and would not be clinically relevant.
Our protocol specified that phlebotomy should be performed as convenience samples when the enrolled women presented for study visits, which were often coordinated with obstetric and neurologic clinic visits. This decision was made during the study design, as we felt that it would not be ethical to have women hold doses to obtain trough samples, especially in the setting of increased clearances during pregnancy. However, our future analyses will include formal pharmacokinetic modeling with consideration of ASM formulations (immediate vs extended release) and amount of time between when the last dose was taken and the sample was drawn. We will also examine data for 2 subpopulations with different rates of gestational-induced lamotrigine clearance changes, as we described in prior clinical data from a single clinical site.43 It is hypothesized that group differences are due to differences in upregulation of UGT1A4 or the estrogen promoter region.43 We will also examine how changes in ASM concentrations affect seizure frequency in pregnant and control women.
Strengths and Limitations
This study, to our knowledge, was one of the largest studies to contain pharmacokinetic information of ASMs in both pregnant and control women. Limitations of our study included the relatively low number of women taking ASMs other than lamotrigine and levetiracetam; the resultant limited number of samples in the first trimester may have affected the characterization of actual changes in concentrations throughout the study. Future pharmacogenomic studies may also account for some of the interindividual variability. Additionally, it should be noted that the study was not powered to analyze changes in DNCs in the cohort of pregnant women and the control participants.
Conclusions
Results of this cohort study suggest the need for higher doses of several ASMs during pregnancy and support therapeutic drug monitoring beginning early in pregnancy. The goal of the study was to provide a personalized treatment approach for women with epilepsy during pregnancy to include ASM dosage changes to prevent seizure worsening and to provide the optimal balance of maternal and child outcomes.
eFigure 1. Representative Figure for the Linear Mixed-Effect Analysis for Lamotrigine
eFigure 2. Number of Pregnant Women With Epilepsy Included in the Analysis
eFigure 3. Number of Nonpregnant Women With Epilepsy Included in the Analysis
eFigure 4. Total and Unbound Carbamazepine and Carbamazepine-10,11-Epoxide Dose-Normalized Concentrations During Pregnancy Compared With Postpartum
eTable. Linear Mixed-Effect Model Parameter Estimates for Antiseizure Medication Dose-Normalized Concentrations in Pregnant Women With Epilepsy and Control Nonpregnant Women With Epilepsy Cohorts Using Data From Postpartum Visits
MONEAD Nonauthor Collaborators
References
- 1.Fiest KM, Sauro KM, Wiebe S, et al. Prevalence and incidence of epilepsy: a systematic review and meta-analysis of international studies. Neurology. 2017;88(3):296-303. doi: 10.1212/WNL.0000000000003509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson GD. Pregnancy-induced changes in pharmacokinetics: a mechanistic-based approach. Clin Pharmacokinet. 2005;44(10):989-1008. doi: 10.2165/00003088-200544100-00001 [DOI] [PubMed] [Google Scholar]
- 3.Pennell PB, Peng L, Newport DJ, et al. Lamotrigine in pregnancy: clearance, therapeutic drug monitoring, and seizure frequency. Neurology. 2008;70(22, pt 2):2130-2136. doi: 10.1212/01.wnl.0000289511.20864.2a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Voinescu PE, Park S, Chen LQ, et al. Antiepileptic drug clearances during pregnancy and clinical implications for women with epilepsy. Neurology. 2018;91(13):e1228-e1236. doi: 10.1212/WNL.0000000000006240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reisinger TL, Newman M, Loring DW, Pennell PB, Meador KJ. Antiepileptic drug clearance and seizure frequency during pregnancy in women with epilepsy. Epilepsy Behav. 2013;29(1):13-18. doi: 10.1016/j.yebeh.2013.06.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harden CL, Pennell PB, Koppel BS, et al. ; American Academy of Neurology; American Epilepsy Society . Management issues for women with epilepsy--focus on pregnancy (an evidence-based review): III. vitamin K, folic acid, blood levels, and breast-feeding: report of the Quality Standards Subcommittee and Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology and the American Epilepsy Society. Epilepsia. 2009;50(5):1247-1255. doi: 10.1111/j.1528-1167.2009.02130.x [DOI] [PubMed] [Google Scholar]
- 7.Giri N, Agarwal S, Shaik N, Pan G, Chen Y, Elmquist WF. Substrate-dependent breast cancer resistance protein (Bcrp1/Abcg2)-mediated interactions: consideration of multiple binding sites in in vitro assay design. Drug Metab Dispos. 2009;37(3):560-570. doi: 10.1124/dmd.108.022046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reimers A, Helde G, Bråthen G, Brodtkorb E. Lamotrigine and its N2-glucuronide during pregnancy: the significance of renal clearance and estradiol. Epilepsy Res. 2011;94(3):198-205. doi: 10.1016/j.eplepsyres.2011.02.002 [DOI] [PubMed] [Google Scholar]
- 9.Ohman I, Beck O, Vitols S, Tomson T. Plasma concentrations of lamotrigine and its 2-N-glucuronide metabolite during pregnancy in women with epilepsy. Epilepsia. 2008;49(6):1075-1080. doi: 10.1111/j.1528-1167.2007.01471.x [DOI] [PubMed] [Google Scholar]
- 10.Karanam A, Pennell PB, French JA, et al. Lamotrigine clearance increases by 5 weeks gestational age: relationship to estradiol concentrations and gestational age. Ann Neurol. 2018;84(4):556-563. doi: 10.1002/ana.25321 [DOI] [PubMed] [Google Scholar]
- 11.Tran TA, Leppik IE, Blesi K, Sathanandan ST, Remmel R. Lamotrigine clearance during pregnancy. Neurology. 2002;59(2):251-255. doi: 10.1212/WNL.59.2.251 [DOI] [PubMed] [Google Scholar]
- 12.Battino D, Tomson T, Bonizzoni E, et al. ; EURAP Study Group . Seizure control and treatment changes in pregnancy: observations from the EURAP epilepsy pregnancy registry. Epilepsia. 2013;54(9):1621-1627. doi: 10.1111/epi.12302 [DOI] [PubMed] [Google Scholar]
- 13.Berlin M, Barchel D, Gandelman-Marton R, et al. Therapeutic levetiracetam monitoring during pregnancy: “mind the gap”. Ther Adv Chronic Dis. 2019;10:2040622319851652. doi: 10.1177/2040622319851652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Westin AA, Reimers A, Helde G, Nakken KO, Brodtkorb E. Serum concentration/dose ratio of levetiracetam before, during and after pregnancy. Seizure. 2008;17(2):192-198. doi: 10.1016/j.seizure.2007.11.027 [DOI] [PubMed] [Google Scholar]
- 15.Tomson T, Palm R, Källén K, et al. Pharmacokinetics of levetiracetam during pregnancy, delivery, in the neonatal period, and lactation. Epilepsia. 2007;48(6):1111-1116. doi: 10.1111/j.1528-1167.2007.01032.x [DOI] [PubMed] [Google Scholar]
- 16.Kuhnz W, Jäger-Roman E, Rating D, et al. Carbamazepine and carbamazepine-10,11- epoxide during pregnancy and postnatal period in epileptic mother and their nursed infants: pharmacokinetics and clinical effects. Pediatr Pharmacol (New York). 1983;3(3-4):199-208. [PubMed] [Google Scholar]
- 17.Tomson T, Lindbom U, Ekqvist B, Sundqvist A. Disposition of carbamazepine and phenytoin in pregnancy. Epilepsia. 1994;35(1):131-135. doi: 10.1111/j.1528-1157.1994.tb02922.x [DOI] [PubMed] [Google Scholar]
- 18.Tomson T, Lindbom U, Ekqvist B, Sundqvist A. Epilepsy and pregnancy: a prospective study of seizure control in relation to free and total plasma concentrations of carbamazepine and phenytoin. Epilepsia. 1994;35(1):122-130. doi: 10.1111/j.1528-1157.1994.tb02921.x [DOI] [PubMed] [Google Scholar]
- 19.Battino D, Binelli S, Bossi L, et al. Plasma concentrations of carbamazepine and carbamazepine 10,11-epoxide during pregnancy and after delivery. Clin Pharmacokinet. 1985;10(3):279-284. doi: 10.2165/00003088-198510030-00007 [DOI] [PubMed] [Google Scholar]
- 20.Johnson EL, Stowe ZN, Ritchie JC, et al. Carbamazepine clearance and seizure stability during pregnancy. Epilepsy Behav. 2014;33:49-53. doi: 10.1016/j.yebeh.2014.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yerby MS, Friel PN, Miller DQ. Carbamazepine protein binding and disposition in pregnancy. Ther Drug Monit. 1985;7(3):269-273. doi: 10.1097/00007691-198507030-00005 [DOI] [PubMed] [Google Scholar]
- 22.Bernus I, Hooper WD, Dickinson RG, Eadie MJ. Metabolism of carbamazepine and co-administered anticonvulsants during pregnancy. Epilepsy Res. 1995;21(1):65-75. doi: 10.1016/0920-1211(95)00012-Y [DOI] [PubMed] [Google Scholar]
- 23.Christensen J, Sabers A, Sidenius P. Oxcarbazepine concentrations during pregnancy: a retrospective study in patients with epilepsy. Neurology. 2006;67(8):1497-1499. doi: 10.1212/01.wnl.0000240047.11166.0e [DOI] [PubMed] [Google Scholar]
- 24.Wegner I, Edelbroek P, de Haan GJ, Lindhout D, Sander JW. Drug monitoring of lamotrigine and oxcarbazepine combination during pregnancy. Epilepsia. 2010;51(12):2500-2502. doi: 10.1111/j.1528-1167.2010.02771.x [DOI] [PubMed] [Google Scholar]
- 25.Mazzucchelli I, Onat FY, Ozkara C, et al. Changes in the disposition of oxcarbazepine and its metabolites during pregnancy and the puerperium. Epilepsia. 2006;47(3):504-509. doi: 10.1111/j.1528-1167.2006.00459.x [DOI] [PubMed] [Google Scholar]
- 26.Westin AA, Nakken KO, Johannessen SI, Reimers A, Lillestølen KM, Brodtkorb E. Serum concentration/dose ratio of topiramate during pregnancy. Epilepsia. 2009;50(3):480-485. doi: 10.1111/j.1528-1167.2008.01776.x [DOI] [PubMed] [Google Scholar]
- 27.Ohman I, Sabers A, de Flon P, Luef G, Tomson T. Pharmacokinetics of topiramate during pregnancy. Epilepsy Res. 2009;87(2-3):124-129. doi: 10.1016/j.eplepsyres.2009.08.004 [DOI] [PubMed] [Google Scholar]
- 28.Reimers A, Helde G, Becser Andersen N, et al. Zonisamide serum concentrations during pregnancy. Epilepsy Res. 2018;144:25-29. doi: 10.1016/j.eplepsyres.2018.05.002 [DOI] [PubMed] [Google Scholar]
- 29.Irody. Irody home page. Accessed January 15, 2012. http://www.irody.com
- 30.Subramanian M, Birnbaum AK, Remmel RP. High-speed simultaneous determination of nine antiepileptic drugs using liquid chromatography-mass spectrometry. Ther Drug Monit. 2008;30(3):347-356. doi: 10.1097/FTD.0b013e3181678ecb [DOI] [PubMed] [Google Scholar]
- 31.Pennell PB, French JA, May RC, et al. ; MONEAD Study Group . Changes in seizure frequency and antiepileptic therapy during pregnancy. N Engl J Med. 2020;383(26):2547-2556. doi: 10.1056/NEJMoa2008663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meador KJ, Pennell PB, May RC, et al. ; MONEAD Investigator Group . Changes in antiepileptic drug-prescribing patterns in pregnant women with epilepsy. Epilepsy Behav. 2018;84:10-14. doi: 10.1016/j.yebeh.2018.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tomson T, Battino D, Bonizzoni E, et al. ; EURAP Study Group . Comparative risk of major congenital malformations with eight different antiepileptic drugs: a prospective cohort study of the EURAP registry. Lancet Neurol. 2018;17(6):530-538. doi: 10.1016/S1474-4422(18)30107-8 [DOI] [PubMed] [Google Scholar]
- 34.Hernández-Díaz S, Smith CR, Shen A, et al. ; North American AED Pregnancy Registry; North American AED Pregnancy Registry . Comparative safety of antiepileptic drugs during pregnancy. Neurology. 2012;78(21):1692-1699. doi: 10.1212/WNL.0b013e3182574f39 [DOI] [PubMed] [Google Scholar]
- 35.Meador KJ, Baker GA, Browning N, et al. ; NEAD Study Group . Fetal antiepileptic drug exposure and cognitive outcomes at age 6 years (NEAD study): a prospective observational study. Lancet Neurol. 2013;12(3):244-252. doi: 10.1016/S1474-4422(12)70323-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bromley RL, Baker GA. Fetal antiepileptic drug exposure and cognitive outcomes. Seizure. 2017;44:225-231. doi: 10.1016/j.seizure.2016.10.006 [DOI] [PubMed] [Google Scholar]
- 37.Bromley RL, Calderbank R, Cheyne CP, et al. ; UK Epilepsy and Pregnancy Register . Cognition in school-age children exposed to levetiracetam, topiramate, or sodium valproate. Neurology. 2016;87(18):1943-1953. doi: 10.1212/WNL.0000000000003157 [DOI] [PubMed] [Google Scholar]
- 38.Shallcross R, Bromley RL, Cheyne CP, et al. ; Liverpool and Manchester Neurodevelopment Group; UK Epilepsy and Pregnancy Register . In utero exposure to levetiracetam vs valproate: development and language at 3 years of age. Neurology. 2014;82(3):213-221. doi: 10.1212/WNL.0000000000000030 [DOI] [PubMed] [Google Scholar]
- 39.Meador KJ, Cohen MJ, Loring DW, et al. ; Maternal Outcomes and Neurodevelopmental Effects of Antiepileptic Drugs Investigator Group . Two-year-old cognitive outcomes in children of pregnant women with epilepsy in the maternal outcomes and neurodevelopmental effects of antiepileptic drugs study. JAMA Neurol. 2021;78(8):927-936. doi: 10.1001/jamaneurol.2021.1583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tomson T, Battino D, Bonizzoni E, et al. ; EURAP Study Group . Declining malformation rates with changed antiepileptic drug prescribing: an observational study. Neurology. 2019;93(9):e831-e840. doi: 10.1212/WNL.0000000000008001 [DOI] [PubMed] [Google Scholar]
- 41.Chen H, Yang K, Choi S, Fischer JH, Jeong H. Up-regulation of UDP-glucuronosyltransferase (UGT) 1A4 by 17beta-estradiol: a potential mechanism of increased lamotrigine elimination in pregnancy. Drug Metab Dispos. 2009;37(9):1841-1847. doi: 10.1124/dmd.109.026609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tomson T, Battino D, Bromley R, et al. Executive summary: management of epilepsy in pregnancy: a report from the International League Against Epilepsy Task Force on Women and Pregnancy. Epilepsia. 2019;60(12):2343-2345. doi: 10.1111/epi.16395 [DOI] [PubMed] [Google Scholar]
- 43.Polepally AR, Pennell PB, Brundage RC, et al. Model-based lamotrigine clearance changes during pregnancy: clinical implication. Ann Clin Transl Neurol. 2014;1(2):99-106. doi: 10.1002/acn3.29 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Representative Figure for the Linear Mixed-Effect Analysis for Lamotrigine
eFigure 2. Number of Pregnant Women With Epilepsy Included in the Analysis
eFigure 3. Number of Nonpregnant Women With Epilepsy Included in the Analysis
eFigure 4. Total and Unbound Carbamazepine and Carbamazepine-10,11-Epoxide Dose-Normalized Concentrations During Pregnancy Compared With Postpartum
eTable. Linear Mixed-Effect Model Parameter Estimates for Antiseizure Medication Dose-Normalized Concentrations in Pregnant Women With Epilepsy and Control Nonpregnant Women With Epilepsy Cohorts Using Data From Postpartum Visits
MONEAD Nonauthor Collaborators


