Key Points
Question
How does the association between prior COVID-19 vaccination and symptomatic SARS-CoV-2 infection change with time since vaccination and the SARS-CoV-2 Delta variant?
Findings
In this test-negative, case-control study that included 1 634 271 tests from symptomatic adults, the odds ratio for prior mRNA vaccination and SARS-CoV-2 test positivity was lower before than during Delta variant predominance. The attenuation in effect size related to time since vaccination was greater than the attenuation related to the Delta variant.
Meaning
The findings are consistent with a steady decline in estimated mRNA vaccine effectiveness over time, separate from variant-specific differences in protection.
Abstract
Importance
Monitoring COVID-19 vaccine performance over time since vaccination and against emerging variants informs control measures and vaccine policies.
Objective
To estimate the associations between symptomatic SARS-CoV-2 infection and receipt of BNT162b2, mRNA-1273, and Ad26.COV2.S by day since vaccination before and during Delta variant predominance (pre-Delta period: March 13-May 29, 2021; Delta period: July 18-October 17, 2021).
Design, Setting, and Participants
Test-negative, case-control design with data from 6884 US COVID-19 testing sites in the pharmacy-based Increasing Community Access to Testing platform. This study included 1 634 271 laboratory-based SARS-CoV-2 nucleic acid amplification tests (NAATs) from adults 20 years and older and 180 112 NAATs from adolescents 12 to 19 years old with COVID-19–like illness from March 13 to October 17, 2021.
Exposures
COVID-19 vaccination (1 Ad26.COV2.S dose or 2 mRNA doses) 14 or more days prior.
Main Outcomes and Measures
Association between symptomatic infection and prior vaccination measured using the odds ratio (OR) from spline-based multivariable logistic regression.
Results
The analysis included 390 762 test-positive cases (21.5%) and 1 423 621 test-negative controls (78.5%) (59.9% were 20-44 years old; 9.9% were 12-19 years old; 58.9% were female; 71.8% were White). Among adults 20 years and older, the BNT162b2 mean OR for days 14 to 60 after a second dose (initial OR) was lower during the pre-Delta period (0.10 [95% CI, 0.09-0.11]) than during the Delta period (0.16 [95% CI, 0.16-0.17]) and increased with time since vaccination (per-month change in OR, pre-Delta: 0.04 [95% CI, 0.02-0.05]; Delta: 0.03 [95% CI, 0.02-0.03]). The initial mRNA-1273 OR was 0.05 (95% CI, 0.04-0.05) during the pre-Delta period, 0.10 (95% CI, 0.10-0.11) during the Delta period, and increased with time (per-month change in OR, pre-Delta: 0.02 [95% CI, 0.005-0.03]; Delta: 0.03 [95% CI, 0.03-0.04]). The Ad26.COV2.S initial OR was 0.42 (95% CI, 0.37-0.47) during the pre-Delta period and 0.62 (95% CI, 0.58-0.65) during the Delta period and did not significantly increase with time since vaccination. Among adolescents, the BNT162b2 initial OR during the Delta period was 0.06 (95% CI, 0.05-0.06) among 12- to 15-year-olds, increasing by 0.02 (95% CI, 0.01-0.03) per month, and 0.10 (95% CI, 0.09-0.11) among 16- to 19-year-olds, increasing by 0.04 (95% CI, 0.03-0.06) per month.
Conclusions and Relevance
Among adults, the OR for the association between symptomatic SARS-CoV-2 infection and COVID-19 vaccination (as an estimate of vaccine effectiveness) was higher during Delta variant predominance, suggesting lower protection. For mRNA vaccination, the steady increase in OR by month since vaccination was consistent with attenuation of estimated effectiveness over time; attenuation related to time was greater than that related to variant.
This study estimates the associations between symptomatic SARS-CoV-2 infection and receipt of BNT162b2, mRNA-1273, and Ad26.COV2.S by day since vaccination before and during Delta variant predominance.
Introduction
Randomized clinical trials of COVID-19 vaccines authorized or approved for use in the US reported high efficacy against symptomatic disease in adults: 95% for BNT162b2 (Pfizer-BioNTech),1 94% for mRNA-1273 (Moderna),2 and 67% for Ad26.COV2.S (Janssen/Johnson & Johnson).3 Early observational studies reported similarly high protection.4,5,6 However, subsequent studies indicated that protection from mRNA vaccines (BNT162b2 and mRNA-1273) against infection may decrease with time since vaccination7,8,9,10,11 and appeared to be lower against the SARS-CoV-2 Delta (B.1.617.2) variant,9,11,12 which was the dominant variant in the US from mid-July until late December 2021.13 Because of the timing of COVID-19 vaccine introduction, distinguishing effects of the Delta variant on vaccine effectiveness from waning protection is difficult.
Observational evidence of protection after vaccination among adolescents is also limited. Trial data for BNT162b2 in adolescents 12 to 15 years old showed 100% efficacy against documented infection.14 Emergency Use Authorization (EUA) was issued for this group on May 10, 2021.15
Assessments of the association between COVID-19 vaccination and SARS-CoV-2 infection that separately examine the effects of time since vaccination and of emerging variants can inform decisions regarding booster doses, variant-specific vaccine formulations, and other pandemic mitigation measures. This analysis used data from a national pharmacy-based COVID-19 testing platform to estimate product- and age group–specific associations between vaccination and symptomatic infection by time since vaccination before and during Delta variant predominance.
Methods
The human participants research advisor for the Centers for Disease Control and Prevention’s (CDC) National Center for Immunization and Respiratory Diseases determined this analysis met the requirements for public health surveillance as outlined in 45 CFR §46.102(l)(2). Because data were collected during routine operational procedures, this secondary data analysis did not require informed consent and was conducted consistent with applicable federal law and CDC policy.
Data Source
Data from the Increasing Community Access to Testing 2.0 platform—a Department of Health and Human Services (HHS) partnership facilitating no-cost, drive-through SARS-CoV-2 testing at pharmacies across all 50 states, the District of Columbia, and Puerto Rico—were analyzed.16,17 Testing sites were selected by HHS to prioritize access in racially and ethnically diverse communities and areas with moderate to high social vulnerability.
Individuals registered online for testing and self-reported demographic information; presence of COVID-19–like illness symptoms (eTable 1 in the Supplement); and since March 2021, vaccination status, including product received and number and dates of doses. From March to November 2021, data collection only allowed for self-reporting of 1 Ad26.COV2.S dose or 2 BNT162b2 or mRNA-1273 doses; booster doses were not reported. Vaccination reporting was not mandatory, and information was not verified.
Nasal swabs were tested for SARS-CoV-2 using rapid point-of-care tests or sent to laboratories for nucleic acid amplification testing (NAAT). Data were reported to HHS with an estimated 3-day lag and included test type, specimen collection date, test result, symptom status (asymptomatic, symptomatic [≥1 symptom], not reported), vaccination status, race and ethnicity (required data elements per HHS COVID-19 laboratory reporting requirements18) from fixed categories provided by the pharmacy, sex, age group (prespecified as 12-15, 16-19, 20-44, 45-54, 55-64, ≥65 years), and county and state of residence. Data also included testing site zip code, county, state, and census tract social vulnerability index (SVI).19 No personally identifying information was reported.
Study Design
A retrospective test-negative, case-control analysis20 using laboratory-based NAATs collected through October 17, 2021, from persons reporting 1 or more COVID-19–like symptoms was conducted. The unit of analysis was tests; NAATs with positive results were classified as cases, and NAATs with negative results as controls. NAATs with indeterminate results were excluded.
The start date varied by age group: March 13, 2021, for tests for persons 20 years and older (based on vaccination data availability); April 15, 2021, for persons 16 to 19 years old (before that ages 0-19 years were reported in aggregate); and June 15, 2021, for persons 12 to 15 years old (the earliest date this group could be fully vaccinated based on BNT162b2 EUA date). Tests came from 3 pharmacy chains (A, B, and C); 2 of the chains (A and B) contributed data throughout the entire analysis period while 1 (C) provided eligible tests beginning June 16, 2021.
Exposure
The exposure of interest was full vaccination with BNT162b2, mRNA-1273, or Ad26.COV2.S. Cases and controls were considered unvaccinated if tests were from persons reporting receipt of no COVID-19 vaccine, and fully vaccinated if tests were from persons reporting receipt of 1 dose of Ad26.COV2.S or 2 doses of mRNA vaccine, with reported date of receipt 14 days or more before testing. Tests from persons reporting receipt of a COVID-19 vaccine but who were not fully vaccinated at time of testing, with missing vaccination status, or with illogical or missing vaccination details were excluded.
Outcome
The primary outcome measure was symptomatic SARS-CoV-2 infection determined by laboratory-based NAAT result. Time periods for the outcome were defined based on percentage of the Delta variant among US sequenced SARS-CoV-2 specimens: pre-Delta (<14%: March 13-May 29, 2021), intermediate (14%-90%: May 30-July 17, 2021), and Delta (>90%: July 18-October 17, 2021).13
Statistical Analysis
Vaccine product–specific association between symptomatic infection and vaccination was estimated by comparing the odds of prior full vaccination (exposed) vs no vaccination (unexposed) in cases vs controls using multivariable logistic regression. The odds ratio (OR) was used to estimate vaccine effectiveness (VE), where VE = (1 − OR) × 100%. ORs by time since vaccination were modeled using a 2-knot quadratic spline for the number of days since full vaccination. ORs were estimated both stratified by age group and for all adults 20 years and older adjusted by age group. The 16- to 19-year age group did not align with mRNA-1273 or Ad26.COV2.S EUA specifications for use among those 18 years and older; therefore, vaccinated individuals were likely 18 to 19 years old, while unvaccinated individuals could have been 16 to 19 years old.21,22 Models were stratified by period (pre-Delta, intermediate, and Delta). Models included race, ethnicity, sex, testing site state, testing site census tract SVI, and test date as covariates to adjust for potential confounding. Unknown race and ethnicity were coded as categories of each variable and missing sex was included with other sex to retain these tests in models. Tests with other missing variables were included in unadjusted estimates but dropped from adjusted models.
To summarize changes in the association between symptomatic infection and full vaccination based on fitted curves, the following were calculated: (1) an initial OR reflecting the mean of the daily OR estimates from days 14 to 60 following Ad26.COV2.S vaccine dose or the second mRNA vaccine dose and (2) the mean of the daily change in OR from day 14 after vaccination to the maximum number of days since vaccination included in the model, expressed per 30-day period (1 month). Although all available data were used, the maximum number of days since vaccination for tests in each model varied by age, vaccine product, and period. This analysis focused on the pre-Delta and Delta periods, because interpretation of the association between vaccination and infection during the intermediate period was complicated by the rapid increase of the Delta variant13 and changing and differential masking guidance for vaccinated and unvaccinated individuals.23,24,25
Because booster doses were not captured in the testing platform but were recommended for certain adults who had received BNT162b2 starting September 24, 2021,26 a sensitivity analysis was conducted restricting the BNT162b2 model for adults 20 years and older during the Delta period to tests performed through September 23, before widespread access to boosters (eFigure 1 in the Supplement). To assess whether the addition of tests from pharmacy chain C starting June 16, 2021, affected pre-Delta/Delta comparisons, a sensitivity analysis was conducted for both periods excluding pharmacy chain C (eFigures 2-4 in the Supplement).
For comparability with other studies, discrete unadjusted and adjusted ORs for periods since final dose, starting at 14 to 30 days after the last dose and in subsequent 30-day increments, stratified by age group, product, and period were calculated (eTables 2-10 in the Supplement). For adolescent age groups in the pre-Delta and intermediate periods, data were too sparse to estimate ORs by day since vaccination, so only discrete estimates were calculated.
Statistical analyses were performed in R (version 4.0.2; R Foundation). The estimated ORs and their corresponding uncertainty are presented with point estimates and 95% CIs. Two-sided P values comparing the magnitude of the association between vaccination and infection across products and study periods for adults 20 years and older were corrected for multiple comparisons using Benjamini-Hochberg false discovery rate, and to account for the possibility of intraclass correlation due to repeat testing by the same individual a conservative P value threshold of less than .001 rather than less than .05 was considered significant.
Results
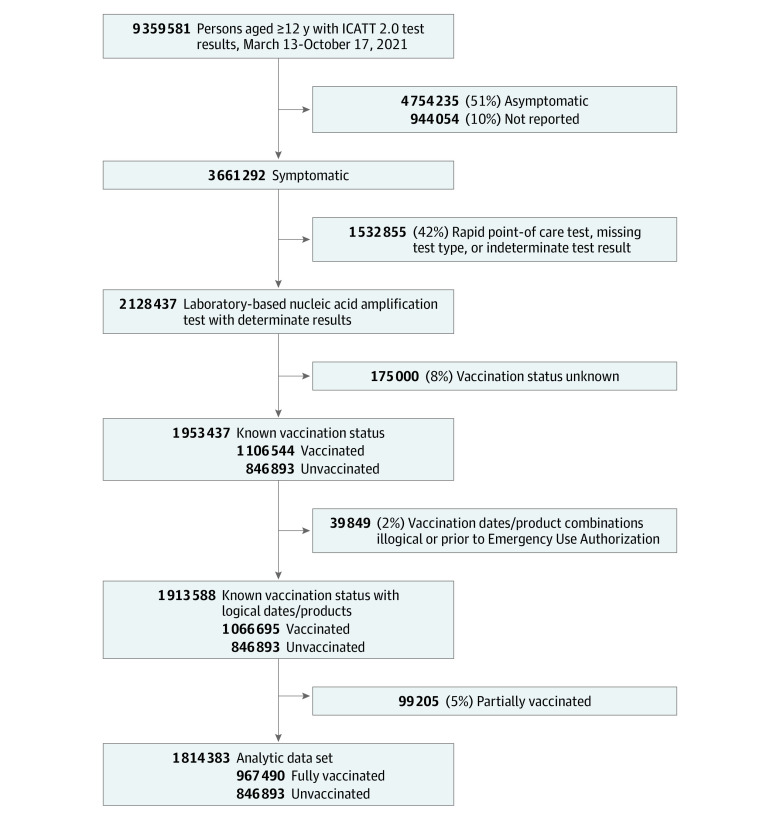
From March 13 to October 17, 2021, 1 814 383 tests from 6884 sites nationwide met inclusion criteria (Figure 1), including 390 762 test-positive cases (21.5%) and 1 423 621 test-negative controls (78.5%) (59.9% aged 20-44 years, 9.9% aged 12-19 years; 58.9% female; 1.0% American Indian/Alaska Native, 6.5% Asian, 12.3% Black/African American, 0.7% Native Hawaiian or Other Pacific Islander, 71.8% White; 20.1% Hispanic/Latino). Records excluded for unknown vaccination status (8%) were similar to those with known status (eTables 11-12 in the Supplement). A total of 560 557 tests (30.9%) were from persons fully vaccinated with BNT162b2, 331 350 (18.3%) with mRNA-1273, and 75 583 (4.2%) with Ad26.COV2.S and 846 893 (46.7%) from unvaccinated persons. Cases were more frequently tests from persons who were male, Black/African American, from the South Atlantic region, and tested at sites with census tract SVI of 0.5 or greater indicating higher social vulnerability (Table).
Figure 1. Inclusion Criteria for a Case-Control Study of Association Between COVID-19 Vaccination and Symptomatic SARS-CoV-2 Infection by Time Since Vaccination and Delta Variant Predominance.
Data were from the Increasing Community Access to Testing (ICATT) 2.0 platform and included persons 12 years and older tested for SARS-CoV-2 in the US during March 13 to October 17, 2021. Data from adolescents 12 to 15 years old were included if their test dates occurred from June 15, 2021 (5 weeks after BNT162b2 Emergency Use Authorization [EUA] for this age group), to October 17, 2021. Data from adolescents 16 to 19 years old were included if their test date occurred from April 15, 2021 (prior to that date ages 0-19 years were reported in aggregate to ICATT), to October 17, 2021. Data were included from 3 pharmacy chains (A [n = 710 758 tests], B [n = 73 913 tests], and C [n = 1 029 712 tests]), with chain C contributing data starting June 16, 2021.
Table. Characteristics of Included Cases and Controls Tested for SARS-CoV-2 in Increasing Community Access to Testing (ICATT) 2.0, United States, March 13-October 17, 2021.
| Characteristic | SARS-CoV-2, No. (%) | |
|---|---|---|
| Positive cases | Negative controls | |
| Total | 390 762 | 1 423 621 |
| Age group, y | ||
| 12-15a | 14 988 (3.8) | 56 216 (3.9) |
| 16-19b | 24 434 (6.3) | 84 474 (5.9) |
| 20-44 | 235 430 (60.2) | 851 641 (59.8) |
| 45-54 | 53 868 (13.8) | 186 748 (13.1) |
| 55-64 | 39 401 (10.1) | 149 483 (10.5) |
| ≥65 | 22 641 (5.8) | 95 059 (6.7) |
| Sexc | 390 380 | 1 420 421 |
| Female | 204 129 (52.3) | 862 587 (60.7) |
| Male | 186 044 (47.7) | 556 959 (39.2) |
| Other | 207 (0.1) | 875 (0.1) |
| Raced | 358 985 | 1 321 002 |
| American Indian/Alaska Native | 4082 (1.1) | 13 247 (1.0) |
| Asian | 13 705 (3.8) | 94 690 (7.2) |
| Black or African American | 53 671 (15.0) | 153 806 (11.6) |
| Native Hawaiian or Other Pacific Islander | 2798 (0.8) | 9419 (0.7) |
| White | 258 580 (72.0) | 947 561 (71.7) |
| Other | 26 149 (7.3) | 102 279 (7.7) |
| Ethnicityd | 356 181 | 1 301 519 |
| Hispanic/Latino | 74 971 (21.0) | 257 432 (19.8) |
| Regione | ||
| New England | 10 706 (2.7) | 73 588 (5.2) |
| Mid-Atlantic | 58 293 (14.9) | 251 553 (17.7) |
| South Atlantic | 76 872 (19.7) | 219 527 (15.4) |
| East North Central | 65 966 (16.9) | 227 916 (16.0) |
| East South Central | 20 243 (5.2) | 50 529 (3.5) |
| West North Central | 16 424 (4.2) | 73 745 (5.2) |
| West South Central | 33 408 (8.5) | 85 493 (6.0) |
| Mountain | 26 824 (6.9) | 99 393 (7.0) |
| Pacific | 81 569 (20.9) | 338 474 (23.8) |
| Puerto Rico | 457 (0.1) | 3403 (0.2) |
| Site census tract SVIf | 390 110 | 1 419 346 |
| SVI <0.5 (less socially vulnerable) | 161 022 (41.3) | 649 521 (45.8) |
| SVI, 0.5-1.0 (more socially vulnerable) | 229 088 (58.7) | 769 825 (54.2) |
| Vaccination status | ||
| Unvaccinated | 276 336 (70.7) | 570 557 (40.1) |
| Ad26.COV2.S fully vaccinatedg | 15 587 (4.0) | 59 996 (4.2) |
| mRNA fully vaccinatedg | 98 839 (25.3) | 793 068 (55.7) |
| BNT162b2 fully vaccinatedg | 68 214 (17.5) | 492 343 (34.6) |
| mRNA-1273 fully vaccinatedg | 30 625 (7.8) | 300 725 (21.1) |
| Periodh | ||
| Pre-Delta (March 13–May 29, 2021) | 44 649 (11.4) | 144 619 (10.2) |
| Intermediate (May 30–July 17, 2021) | 17 056 (4.4) | 125 731 (8.8) |
| Delta (July 18–October 17, 2021) | 329 057 (84.2) | 1153 271 (81.0) |
Abbreviation: SVI, social vulnerability index.
Data from adolescents 12 to 15 years old were included if their test dates occurred from June 15, 2021 (5 weeks after BNT162b2 Emergency Use Authorization for this age group), to October 17, 2021.
Data for adolescents 16 to 19 years old were included from April 15, 2021 (prior to that date ages 0-19 years were reported in aggregate to ICATT), to October 17, 2021.
Tests with missing sex (n = 3582) were combined with other sex to retain those tests in regression models.
Race and ethnicity were self-reported from fixed categories provided at test registration. Other race was an available option at test registration at 2 of 3 pharmacy chains. Unknown race (n = 134 396) and ethnicity (n = 156 683) were coded as categorical levels within each variable to retain those tests in regression models.
Regions defined as New England (Connecticut, Maine, Massachusetts, New Hampshire, Rhode Island, and Vermont), Mid-Atlantic (New Jersey, New York, and Pennsylvania), South Atlantic (Delaware, Florida, Georgia, Maryland, North Carolina, South Carolina, Virginia, District of Columbia, and West Virginia), East North Central (Illinois, Indiana, Michigan, Ohio, and Wisconsin), East South Central (Alabama, Kentucky, Mississippi, and Tennessee), West North Central (Iowa, Kansas, Minnesota, Missouri, Nebraska, North Dakota, and South Dakota), West South Central (Arkansas, Louisiana, Oklahoma, and Texas), Mountain (Arizona, Colorado, Idaho, Montana, Nevada, New Mexico, Utah, and Wyoming), Pacific (Alaska, California, Hawaii, Oregon, and Washington), and Puerto Rico.
Testing site census tract SVI was an available variable in ICATT data. SVI is assigned for all US census tracts by Centers for Disease Control and Prevention/Agency for Toxic Substances and Disease Registry based on US Census data.19 Higher SVI indicates greater social vulnerability. Tests with missing SVI (n = 4927) were included in unadjusted analyses but not included in adjusted analyses. All data for Puerto Rico were missing SVI, and therefore records from Puerto Rico were dropped from the adjusted analyses.
Fully vaccinated: 2 doses of an mRNA vaccine or 1 dose of the Ad26.COV2.S vaccine 14 days or more prior to SARS-CoV-2 test date.
Time periods were based on the percentage of the Delta variant among US sequenced SARS-COV-2 specimens: pre-Delta (<14%: March 13-May 29, 2021), intermediate (14%-90%: May 30-July 17, 2021) and Delta (>90%: July 18-October 17, 2021).
Adults 20 Years and Older
Among adults 20 years and older, 499 879 (30.6%) were fully vaccinated with BNT162b2, 324 601 (19.9%) with mRNA-1273, and 73 709 (4.5%) with Ad26.COV2.S and 736 082 (45.0%) were unvaccinated. For BNT162b2 during the pre-Delta period, the initial OR between symptomatic infection and full vaccination (mean daily OR during days 14-60 since second dose) among adults 20 years and older was 0.10 (95% CI, 0.09-0.11) (eTable 13 in the Supplement), and the OR increased by a mean of 0.04 (95% CI, 0.02-0.05) per month during days 14 to 111, reflecting a weakening association over time since vaccination (Figure 2A). During the Delta period (July 18-October 17, 2021), the BNT162b2 initial OR was 0.16 (95% CI, 0.16-0.17), and the OR increased by 0.03 (95% CI, 0.02-0.03) per month from days 14 to 280. During the pre-Delta period, the mRNA-1273 initial OR was 0.05 (95% CI, 0.04-0.05), and the OR increased by 0.02 (95% CI, 0.005-0.03) per month from days 14 to 93 (Figure 2B). During the Delta period, the mRNA-1273 initial OR was 0.10 (95% CI, 0.10-0.11), and the OR increased by 0.03 (95% CI, 0.03-0.04) per month from days 14 to 266. For both mRNA vaccines, models for ages 20 to 44, 45 to 54, and 55 to 64 years yielded similar OR estimates and trends over time since the second dose within each period (Figure 3).
Figure 2. Association of COVID-19 Vaccination and Symptomatic SARS-CoV-2 Infection by Day Since Vaccination Among Adults 20 Years and Older in the Pre-Delta and Delta Periods.
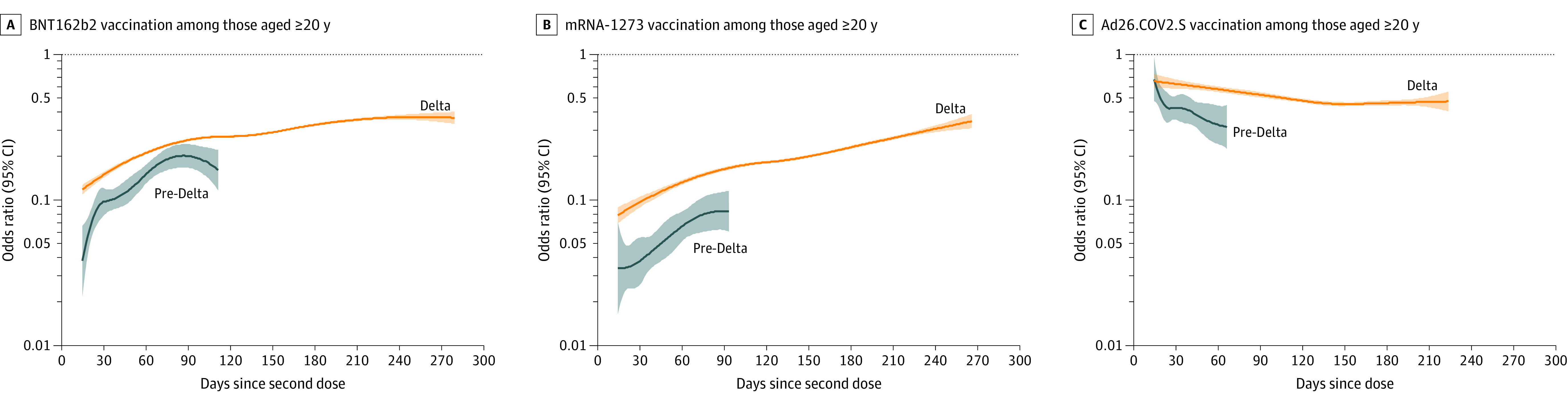
Panels display odds ratios (ORs), plotted on a logarithmic scale, for prior COVID-19 vaccination (by vaccine product) and SARS-CoV-2 test positivity by day since vaccination (starting at day 14 since second mRNA dose or Ad26.COV2.S dose) in the pre-Delta (March 13-May 29, 2021; shown in blue) and Delta (July 18-October 17; shown in orange) periods with 95% CIs (shaded areas). ORs were adjusted for age group, race, ethnicity, sex, testing site state, testing site census tract social vulnerability index, and calendar date as a continuous variable. Tests with missing social vulnerability index were excluded from adjusted analyses. The presented (fitted) curves were truncated on the day after which 10 or fewer cases remained for each product- and period-specific model, beyond which CIs widened. ORs (95% CIs) for day 14, mean of the daily OR estimates from days 14 to 60 (initial OR), and end day for each period are shown in eTable 13 in the Supplement.
Figure 3. Association of COVID-19 Vaccination and Symptomatic SARS-CoV-2 Infection by Day Since Vaccination by Adult Age Group and Vaccine Product in the Pre-Delta and Delta Periods.
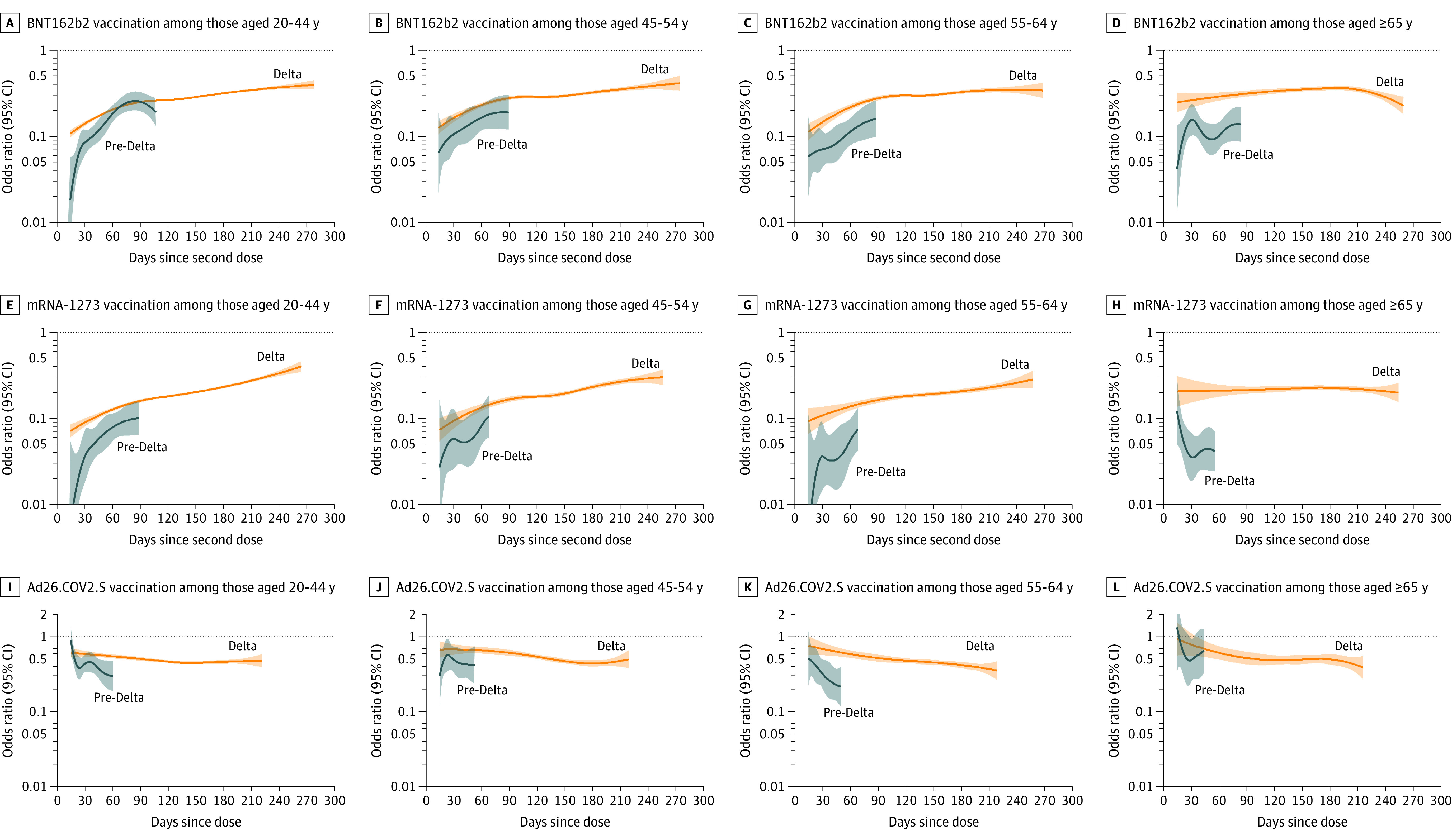
Panels display odds ratios (ORs), plotted on a logarithmic scale, for prior COVID-19 vaccination (by age group and vaccine product) and SARS-CoV-2 test positivity by day since vaccination (starting at day 14 since second mRNA dose or Ad26.COV2.S dose) in the pre-Delta (March 13-May 29, 2021; shown in blue) and Delta (July 18-October 17, 2021; shown in orange) periods with 95% CIs (shaded areas). ORs were adjusted for race, ethnicity, sex, testing site state, testing site census tract social vulnerability index, and calendar date as a continuous variable. Tests with missing social vulnerability index were excluded from adjusted analyses. The presented (fitted) curves were truncated on the day after which 10 or fewer cases remained for each product-, age group–, and period-specific model, beyond which CIs widened. For Ad26.COV2.S, the y-axis was truncated at 2.0, and data points above 2.0 are not shown. ORs (95% CIs) for day 14, mean of the daily OR estimates from days 14 to 60 (initial OR), and end day for each period are shown in eTable 13 in the Supplement.
Among adults 65 years and older during the pre-Delta period, the initial OR between symptomatic infection and full vaccination for BNT162b2 was 0.11 (95% CI, 0.08-0.14) (eTable 13 in the Supplement) and the OR increased by 0.04 (95% CI, 0.01-0.08) per month from days 14 to 83 after the second dose (Figure 3D). During the Delta period, the initial BNT162b2 OR was 0.27 (95% CI, 0.22-0.31) and did not significantly increase (per-month change in OR, 0.00 [95% CI, –0.01 to 0.01] from days 14-260). For mRNA-1273, the initial OR was 0.05 (95% CI, 0.03-0.06) during the pre-Delta period and 0.21 (95% CI, 0.15-0.26) during the Delta period and did not significantly increase in either period (per-month change in OR, pre-Delta: −0.06 [95% CI, –0.14 to 0.02] from days 14-55; Delta: 0.00 [95% CI, –0.01 to 0.01] from days 14-254) (Figure 3H).
For Ad26.COV2.S, the initial OR between symptomatic infection and full vaccination during the pre-Delta period among adults 20 years and older was 0.42 (95% CI, 0.37-0.47) and the OR decreased nonlinearly from days 14 to 66 (Figure 2C; eTable 13 in the Supplement). During the Delta period, the initial OR was 0.62 (95% CI, 0.58-0.65) and the OR decreased nonlinearly from days 14 to 224 after vaccination.
Comparisons of vaccine products between periods for adults 20 years and older demonstrated that initial ORs were significantly lower during the pre-Delta vs Delta period for all products (P < .001; eTable 14 in the Supplement). Comparisons between products within the same time period demonstrated that initial ORs were significantly lower in both the pre-Delta and Delta periods for mRNA-1273 vs BNT162b2 (P < .001), for BNT162b2 vs Ad26.COV2.S (P < .001), and for mRNA-1273 vs Ad26.COV2.S (P < .001; eTable 14 in the Supplement).
Sensitivity analyses excluding pharmacy chain C and restricting the BNT162b2 model for adults 20 years and older during the Delta period to tests performed through September 23, 2021, yielded similar magnitudes of association and patterns over time (eFigures 1-4 in the Supplement). Estimates of ORs during the intermediate period for all products are shown in eFigures 5-6 in the Supplement.
Adolescents 12 to 19 Years Old
Among 180 112 adolescents 12 to 19 years old, 60 678 (33.7%) were fully vaccinated with BNT162b2, 6749 (3.7%) with mRNA-1273, and 1874 (1.0%) with Ad26.COV2.S and 110 811 (61.5%) were unvaccinated (eTable 15 in the Supplement). During the Delta period among adolescents 12 to 15 years old, the BNT162b2 initial OR between symptomatic infection and full vaccination was 0.06 (95% CI, 0.05-0.06), and the OR increased by 0.02 (95% CI, 0.01-0.03) per month from days 14 to 127 (Figure 4; eTable 13 in the Supplement). During the Delta period among adolescents 16 to 19 years old, the BNT162b2 initial OR was 0.10 (95% CI, 0.09-0.11), and the OR increased by 0.04 (95% CI, 0.03-0.06) per month from days 14 to 231. The mRNA-1273 initial OR among adolescents 16 to 19 years old was 0.06 (95% CI, 0.04-0.08), and the OR increased by 0.03 (95% CI, 0.02-0.05) per month from days 14 to 203. The initial Ad26.COV2.S OR among adolescents 16 to 19 years old was 0.46 (95% CI, 0.30-0.62) and was stable over time since vaccination.
Figure 4. Association of COVID-19 Vaccination and Symptomatic SARS-CoV-2 Infection by Day Since Vaccination by Adolescent Age Group and Vaccine Product in the Delta Period.
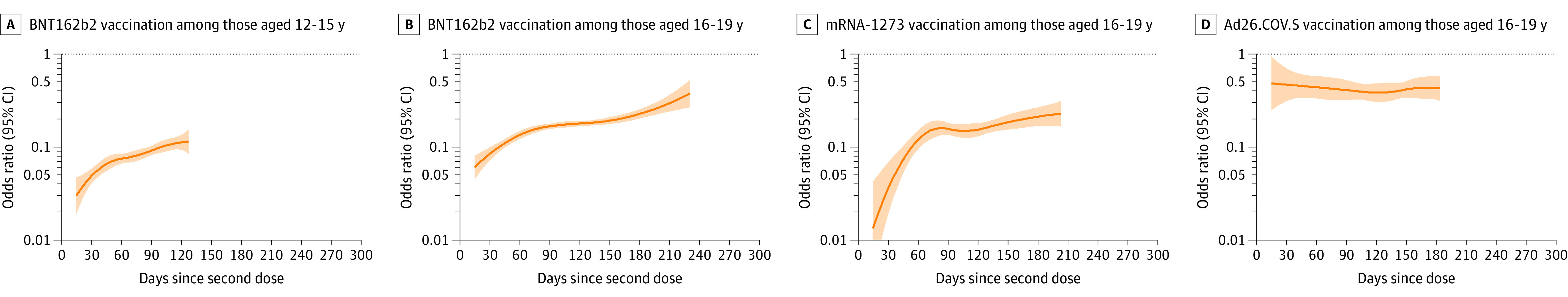
Panels display odds ratios (ORs), plotted on a logarithmic scale, for prior COVID-19 vaccination (by adolescent age group and vaccine product) and SARS-CoV-2 test positivity by day since vaccination (starting at day 14 since second mRNA dose or Ad26.COV2.S dose) in the Delta period (July 18-October 17, 2021) with 95% CIs. ORs were adjusted for race, ethnicity, sex, testing site state, testing site census tract social vulnerability index, and calendar date as a continuous variable. Tests with missing social vulnerability index were excluded from adjusted analyses. The presented (fitted) curves were truncated on the day after which 10 or fewer cases remained for each product- and age group–specific model, beyond which CIs widened. ORs (95% CIs) for day 14, mean of the daily OR estimates from days 14 to 60 (initial OR), and end day for each period are shown in eTable 13 in the Supplement. mRNA-1273 and Ad26.COV2.S COVID-19 vaccines were only authorized for those 18 years and older during these time periods.
Discussion
In this analysis of SARS-CoV-2 tests performed at sites across the US from March 13 to October 17, 2021, among adults the OR for the association between symptomatic SARS-CoV-2 infection and vaccination (as an estimate of vaccine effectiveness) was higher during Delta variant predominance, suggesting lower protection. For mRNA vaccination, the steady increase in OR by month since vaccination was consistent with attenuation of effectiveness over time. The magnitude of the association between mRNA vaccination and symptomatic SARS-CoV-2 infection was less affected by the emergence of the Delta variant than by time since second dose of vaccine. Among adults, the ORs for mRNA vaccination increased by 0.02 to 0.04 per month, corresponding to a reduction in vaccine effectiveness of approximately 30% over 8 to 9 months. In adolescents 12 to 19 years old, the association between infection and mRNA vaccination similarly attenuated with time since vaccination. Booster doses are now recommended in the US for persons 12 years and older26 and may help optimize protection.
Other observational studies have reported declining protection from mRNA vaccines against infection7,8,9,11,27,28,29 and declining protection in the Delta period compared with the pre-Delta period,9,11,12,27,30,31,32,33 but few could assess these effects separately because Delta emerged several months after initial vaccine introduction.9,11 In this analysis, the pattern of attenuation related to the time since vaccination for mRNA vaccines was consistent before and after the emergence of Delta as the predominant variant, although the initial ORs were higher during the Delta than pre-Delta periods. More recent data from this same testing platform suggest that 2 doses of mRNA vaccine offer less protection against infection with the Omicron variant than against infection with the Delta variant, but the pattern of attenuation over time since vaccination was similar for both variants.17 Together these data suggest that while the starting point for protection among recent recipients of 2 doses of mRNA vaccine can differ across variants, declines in effectiveness over time may be more predictable. It will be important to evaluate whether protection from booster doses wanes in a similar fashion as that of 2 doses.
In this analysis, the magnitude of the association between vaccination and infection was greater for mRNA-1273 than for BNT162b2. Early observational data found nearly identical estimates for the association between mRNA-1273 and BNT162b2 vaccination and infection,4,5 but emerging evidence during the Delta period suggests that mRNA-1273 vaccination may be more protective against both infection8,29,33 and hospitalization34 than BNT162b2 vaccination. Important differences between these vaccines include the mRNA dose (mRNA-1273, 100 μg; BNT162b2, 30 μg) and dosing interval (mRNA-1273, 28 days; BNT162b2, 21 days).1,2 Further investigation of the reasons for differing protection is needed to guide product-specific recommendations.
For Ad26.COV2.S, the initial OR was higher than that of both mRNA vaccines, consistent with lower vaccine effectiveness, but there was no attenuation over time. Additionally, the initial OR was higher during the Delta than pre-Delta period, suggesting reduced protection against the Delta variant. Although in this analysis fewer tests were from persons who received Ad26.COV2.S compared with the mRNA vaccines, these results add to the limited number of observational Ad26.COV2.S studies. These studies have reported effectiveness for Ad26.COV2.S against infection (with or without symptoms) ranging from 50% to 87%,6,8,29,31 effectiveness against symptomatic infection lower than that of mRNA vaccines,8,29,31 and stable protection over time.29,31 Booster doses are now recommended for all Ad26.COV2.S recipients 2 months or more after vaccination.26 Additional evaluations are needed to estimate effectiveness of a 2-dose Ad26.COV2.S schedule as well as protection against newly emerging variants such as Omicron.
This analysis also adds to limited observational evidence on COVID-19 vaccines in adolescents.9,35,36,37 In this analysis, the ORs since the second mRNA dose remained 0.11 or less for adolescents 12 to 15 years old over a period of 4 months and 0.38 or less for adolescents 16 to 19 years old over a period of 7.5 months, corresponding to an estimated mRNA vaccine effectiveness of 89% or more and 62% or more, respectively. This supports continued efforts to increase vaccine coverage of both the primary series and booster doses in adolescents.
Among adults 65 years and older, the association between mRNA vaccination and symptomatic infection was stable over time since vaccination during the Delta period. This finding contrasts with other studies reporting waning protection from BNT162b2 among older adults.9,31 During the Delta period, the observed BNT162b2 OR decreased for older adults at about 6 months after the second dose, which might be attributable to booster doses (not captured in these data) recommended on September 23, 2021, for older adults more than 6 months after their primary series.26 However, the sensitivity analysis that only included tests from before the authorization of booster doses yielded similar results. The results for adults 65 years and older should be interpreted with caution due to low numbers of recently vaccinated older adults during Delta predominance and because older adults tested at drive-through sites may differ from the general older adult population.
Limitations
This study had additional limitations. First, vaccination status and symptoms were based on self-report, potentially leading to misclassification. Second, exclusion of tests from persons with missing vaccination status (8%) could introduce bias due to differences in behavior and COVID-19 risk. Third, this analysis could not account for some potentially important confounders including underlying conditions, prior COVID-19 disease, and mitigation behaviors such as masking, which likely changed with changing public health guidance for vaccinated individuals.23,24 Fourth, the likelihood of testing might change over time or with vaccination status; however, as long as it did not differ systematically between individuals with COVID-19 and non–COVID-19 acute respiratory infections, these changes would not cause bias.38 Fifth, because these data do not include identifiers, test was used as the unit of analysis and individuals may have been included more than once. However, a more restrictive significance threshold of P less than .001 was used to account for the possibility of intraindividual correlation in significance tests.
Sixth, within the 16- to 19-year age group, those vaccinated with mRNA-1273 or Ad26.COV2.S were likely 18 to 19 years old, while unvaccinated persons may have been 16 to 19 years old, due to differences between prespecified age groups in this data set and EUA age cutoffs.21,22 Seventh, vaccination eligibility varied by jurisdiction; some tests in March and April 2021 may have been from those not yet eligible for vaccination. Eighth, these data do not include sequencing results, so time was used as a proxy for Delta infection, similar to other analyses.27,28,34 Ninth, the inclusion of pharmacy chain C’s tests starting June 16, 2021 (57% of tests), may have affected the comparability of pre-Delta and Delta results. However, the sensitivity analysis conducted excluding pharmacy chain C showed similar results. Tenth, during the time of this study, the Increasing Community Access to Testing platform did not allow for reporting of more than 2 vaccine doses; some individuals may have received additional or booster doses that were not recorded.
Conclusions
Among adults, the OR for the association between symptomatic SARS-CoV-2 infection and COVID-19 vaccination (as an estimate of vaccine effectiveness) was higher during Delta variant predominance, suggesting lower protection. For mRNA vaccination, the steady increase in OR by month since vaccination was consistent with attenuation of estimated effectiveness over time; attenuation related to time was greater than that related to variant.
eTable 1. COVID-like Illness Symptoms Collected by Self-report on Questionnaire Administered at Online Test Registration by Pharmacy Chain
eTable 2. Unadjusted and Adjusted BNT162b2 Odds Ratios (OR) With 95% Confidence Intervals (CI) by Time Since Second Dose and Age Group, During the Pre-Delta Period (March 13-May 29, 2021)
eTable 3. Unadjusted and Adjusted BNT162b2 Odds Ratios (OR) With 95% Confidence Intervals (CI) by Time Since Second Dose and Age Group, During the Intermediate Period (May 30-July 17, 2021)
eTable 4. Unadjusted and Adjusted BNT162b2 Odds Ratios (OR) With 95% Confidence Intervals (CI) by Time Since Second Dose and Age Group, During the Delta Period (July 18-October 17, 2021)
eTable 5. Unadjusted and Adjusted mRNA-1273 Odds Ratios (OR) With 95% Confidence Intervals (CI) by Time Since Second Dose and Age Group, During the Pre-Delta Period (March 13-May 29, 2021)
eTable 6. Unadjusted and Adjusted mRNA-1273 Odds Ratios (OR) With 95% Confidence Intervals (CI) by Time Since Second Dose and Age Group, During the Intermediate Period (May 30-July 17, 2021)
eTable 7. Unadjusted and Adjusted mRNA-1273 Odds Ratios (OR) With 95% Confidence Intervals (CI) by Time Since Second Dose and Age Group, During the Delta Period (July 18-October 17, 2021)
eTable 8. Unadjusted and Adjusted Ad26.COV2.S Odds Ratios (OR) With 95% Confidence Intervals (CI) by Time Since Dose and Age Group, During the Pre-Delta Period (March 13-May 29, 2021)
eTable 9. Unadjusted and Adjusted Ad26.COV2.S Odds Ratios (OR) With 95% Confidence Intervals (CI) by Time Since Dose and Age Group, During the Intermediate Period (May 30-July 17, 2021)
eTable 10. Unadjusted and Adjusted Ad26.COV2.S Odds Ratios (OR) With 95% Confidence Intervals (CI) by Time Since Dose and Age Group, During the Delta Period (July 18-October 17, 2021)
eTable 11. Comparison of Characteristics of Symptomatic Persons Ages ≥20 Years Tested for SARS-CoV-2 in Increasing Community Access to Testing (ICATT) 2.0 With Laboratory-Based Nucleic Acid Amplification Test (NAAT) With Known and Unknown Vaccination Status—United States, March 13-October 17, 2021
eTable 12. Comparison of Characteristics of Symptomatic Persons Ages 12-19 Years Tested for SARS-CoV-2 in Increasing Community Access to Testing (ICATT) 2.0 With Laboratory-Based Nucleic Acid Amplification Test (NAAT) With Known and Unknown Vaccination Status—United States, March 13-October 17, 2021
eTable 13. Odds Ratios (OR) a (95% Confidence Intervals [CI]) for Day 14, Mean of Daily OR Estimates From Days 14-60 (initial OR), and End Day for Figures 2, 3, and 4.
eTable 14. Significance Testing for Comparisons of Mean of Daily Odds Ratios (OR) Estimates From Days 14-60 After Vaccination (Initial OR) Between Vaccine Products (BNT162b2, mRNA-1273, and Ad26.COV2.S) and Time Periods (pre-Delta vs. Delta) for Adults Ages ≥20 Years
eTable 15. Characteristics of Included Adolescent Cases and Controls Ages 12-19 Years Tested for SARS-CoV-2 in Increasing Community Access to Testing (ICATT) 2.0 — United States, April 15-October 17, 2021 for Ages 12-15 years and June 15-October 17, 2021 for Ages 16-19 Years
eFigure 1. Sensitivity Analysis Restricting to Tests Performed Through September 23, 2021: Association of BNT162b2 Vaccination and Symptomatic Infection by Day Since Vaccination by Age Group
eFigure 2. Sensitivity Analysis Excluding Pharmacy Chain C: Association of COVID-19 Vaccination and Symptomatic Infection by Day Since Vaccination Among Adults Ages ≥20 Years
eFigure 3. Sensitivity Analysis Excluding Pharmacy Chain C: Association of COVID-19 Vaccination and Symptomatic Infection by Day Since Vaccination by Adult Age Group and Vaccine Product
eFigure 4. Sensitivity Analysis Excluding Pharmacy Chain C: Association of COVID-19 Vaccination and Symptomatic Infection by Day Since Vaccination Among Ages 12-15 Years and 16-19 Years
eFigure 5. Association of COVID-19 Vaccination and Symptomatic Infection by Day Since Vaccination Among Adults Ages ≥20 Years During the Intermediate Period (May 30-July 17, 2021)
eFigure 6. Association of COVID-19 Vaccination and Symptomatic Infection by Day Since Vaccination by Adult Age Group and Vaccine Product During the Intermediate Period (May 30-July 17, 2021)
References
- 1.Polack FP, Thomas SJ, Kitchin N, et al. ; C4591001 Clinical Trial Group . Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med. 2020;383(27):2603-2615. doi: 10.1056/NEJMoa2034577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baden LR, El Sahly HM, Essink B, et al. ; COVE Study Group . Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403-416. doi: 10.1056/NEJMoa2035389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sadoff J, Gray G, Vandebosch A, et al. ; ENSEMBLE Study Group . Safety and efficacy of single-dose Ad26.COV2.S vaccine against COVID-19. N Engl J Med. 2021;384(23):2187-2201. doi: 10.1056/NEJMoa2101544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pilishvili T, Gierke R, Fleming-Dutra KE, et al. ; Vaccine Effectiveness Among Healthcare Personnel Study Team . Effectiveness of mRNA COVID-19 vaccine among US health care personnel. N Engl J Med. 2021;385(25):e90. doi: 10.1056/NEJMoa2106599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thompson MG, Burgess JL, Naleway AL, et al. Prevention and attenuation of COVID-19 with the BNT162b2 and mRNA-1273 vaccines. N Engl J Med. 2021;385(4):320-329. doi: 10.1056/NEJMoa2107058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Corchado-Garcia J, Zemmour D, Hughes T, et al. Analysis of the effectiveness of the Ad26.COV2.S adenoviral vector vaccine for preventing COVID-19. JAMA Netw Open. 2021;4(11):e2132540-e2132540. doi: 10.1001/jamanetworkopen.2021.32540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chemaitelly H, Tang P, Hasan MR, et al. Waning of BNT162b2 vaccine protection against SARS-CoV-2 infection in Qatar. N Engl J Med. 2021;385(24):e83. doi: 10.1056/NEJMoa2114114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martínez-Baz I, Trobajo-Sanmartín C, Miqueleiz A, et al. ; Working Group for the Study of COVID-19 in Navarre; Investigators, other members of the Working Group for the Study of COVID-19 in Navarre . Product-specific COVID-19 vaccine effectiveness against secondary infection in close contacts, Navarre, Spain, April to August 2021. Euro Surveill. 2021;26(39):2100894. doi: 10.2807/1560-7917.ES.2021.26.39.2100894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tartof SY, Slezak JM, Fischer H, et al. Effectiveness of mRNA BNT162b2 COVID-19 vaccine up to 6 months in a large integrated health system in the USA: a retrospective cohort study. Lancet. 2021;398(10309):1407-1416. doi: 10.1016/S0140-6736(21)02183-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thomas SJ, Moreira ED Jr, Kitchin N, et al. ; C4591001 Clinical Trial Group . Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine through 6 months. N Engl J Med. 2021;385(19):1761-1773. doi: 10.1056/NEJMoa2110345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bruxvoort KJ, Sy LS, Qian L, et al. Effectiveness of mRNA-1273 against Delta, Mu, and other emerging variants of SARS-CoV-2: test negative case-control study. BMJ. 2021;375:e068848. doi: 10.1136/bmj-2021-068848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lopez Bernal J, Andrews N, Gower C, et al. Effectiveness of COVID-19 vaccines against the B.1.617.2 (Delta) variant. N Engl J Med. 2021;385(7):585-594. doi: 10.1056/NEJMoa2108891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention . COVID data tracker: variant proportions. Accessed September 7, 2021. https://covid.cdc.gov/covid-data-tracker/#variant-proportions
- 14.Frenck RW Jr, Klein NP, Kitchin N, et al. ; C4591001 Clinical Trial Group . Safety, immunogenicity, and efficacy of the BNT162b2 COVID-19 vaccine in adolescents. N Engl J Med. 2021;385(3):239-250. doi: 10.1056/NEJMoa2107456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Food and Drug Administration . Pfizer-BioNTech COVID-19 vaccine EUA letter of authorization. Accessed October 4, 2021. https://www.fda.gov/media/144412/download
- 16.Miller MF, Shi M, Motsinger-Reif A, Weinberg CR, Miller JD, Nichols E. Community-based testing sites for SARS-CoV-2: United States, March 2020-November 2021. MMWR Morb Mortal Wkly Rep. 2021;70(49):1706-1711. doi: 10.15585/mmwr.mm7049a3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Accorsi EK, Britton A, Fleming-Dutra KE, et al. Association between 3 doses of mRNA COVID-19 vaccine and symptomatic infection caused by the SARS-CoV-2 Omicron and Delta variants. JAMA. Published January 21, 2022. doi: 10.1001/jama.2022.0470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Centers for Disease Control and Prevention . 07/31/2020: Lab advisory: update on COVID-19 laboratory reporting requirements. Accessed January 11, 2022. https://www.cdc.gov/csels/dls/locs/2020/update-on-covid-19-reporting-requirements.html
- 19.Agency for Toxic Substances and Disease Registry . CDC/ATSDR Social Vulnerability Index. Accessed September 24, 2021. https://www.atsdr.cdc.gov/placeandhealth/svi/index.html
- 20.Chua H, Feng S, Lewnard JA, et al. The use of test-negative controls to monitor vaccine effectiveness: a systematic review of methodology. Epidemiology. 2020;31(1):43-64. doi: 10.1097/EDE.0000000000001116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.US Food and Drug Administration . Moderna COVID-19 vaccine letter of authorization. Accessed October 4, 2021. https://www.fda.gov/media/144636/download
- 22.US Food and Drug Administration . Janssen COVID-19 vaccine EUA letter of authorization. Accessed October 4, 2021. https://www.fda.gov/media/146303/download
- 23.Centers for Disease Control and Prevention . Interim public health recommendations for fully vaccinated people. Updates as of May 28, 2021. Accessed September 24, 2021. https://web.archive.org/web/20210530040439/https://www.cdc.gov/coronavirus/2019-ncov/vaccines/fully-vaccinated-guidance.html
- 24.Centers for Disease Control and Prevention . Interim public health recommendations for fully vaccinated people. Updated July 27, 2021. Accessed January 10, 2022. https://web.archive.org/web/20210727235224/https:/www.cdc.gov/coronavirus/2019-ncov/vaccines/fully-vaccinated-guidance.html
- 25.Centers for Disease Control and Prevention . US state and territorial public mask mandates from April 10, 2020, through August 15, 2021, by county by day. Accessed October 4, 2021. https://data.cdc.gov/Policy-Surveillance/U-S-State-and-Territorial-Public-Mask-Mandates-Fro/62d6-pm5i
- 26.Centers for Disease Control and Prevention . Interim clinical considerations for use of COVID-19 vaccines currently approved or authorized in the United States. Accessed November 5, 2021. https://www.cdc.gov/vaccines/covid-19/clinical-considerations/covid-19-vaccines-us.html
- 27.Keehner J, Horton LE, Binkin NJ, et al. ; SEARCH Alliance . Resurgence of SARS-CoV-2 infection in a highly vaccinated health system workforce. N Engl J Med. 2021;385(14):1330-1332. doi: 10.1056/NEJMc2112981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pouwels KB, Pritchard E, Matthews PC, et al. Effect of Delta variant on viral burden and vaccine effectiveness against new SARS-CoV-2 infections in the UK. Nat Med. 2021;27(12):2127-2135. doi: 10.1038/s41591-021-01548-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tabak YP, Sun X, Brennan TA, Chaguturu SK. Incidence and estimated vaccine effectiveness against symptomatic SARS-CoV-2 infection among persons tested in US retail locations, May 1 to August 7, 2021. JAMA Netw Open. 2021;4(12):e2143346-e2143346. doi: 10.1001/jamanetworkopen.2021.43346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fowlkes A, Gaglani M, Groover K, Thiese MS, Tyner H, Ellingson K; HEROES-RECOVER Cohorts . Effectiveness of COVID-19 vaccines in preventing SARS-CoV-2 infection among frontline workers before and during B.1.617.2 (Delta) variant predominance: eight US locations, December 2020-August 2021. MMWR Morb Mortal Wkly Rep. 2021;70(34):1167-1169. doi: 10.15585/mmwr.mm7034e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rosenberg ES, Dorabawila V, Easton D, et al. COVID-19 vaccine effectiveness in New York State. N Engl J Med. 2022;386(2):116-127. doi: 10.1056/NEJMoa2116063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sheikh A, McMenamin J, Taylor B, Robertson C; Public Health Scotland and the EAVE II Collaborators . SARS-CoV-2 Delta VOC in Scotland: demographics, risk of hospital admission, and vaccine effectiveness. Lancet. 2021;397(10293):2461-2462. doi: 10.1016/S0140-6736(21)01358-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tang P, Hasan MR, Chemaitelly H, et al. BNT162b2 and mRNA-1273 COVID-19 vaccine effectiveness against the SARS-CoV-2 Delta variant in Qatar. Nat Med. 2021;27(12):2136-2143. doi: 10.1038/s41591-021-01583-4 [DOI] [PubMed] [Google Scholar]
- 34.Grannis SJ, Rowley EA, Ong TC, et al. ; VISION Network . Interim estimates of COVID-19 vaccine effectiveness against COVID-19-associated emergency department or urgent care clinic encounters and hospitalizations among adults during SARS-CoV-2 B.1.617.2 (Delta) variant predominance: nine states, June-August 2021. MMWR Morb Mortal Wkly Rep. 2021;70(37):1291-1293. doi: 10.15585/mmwr.mm7037e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Glatman-Freedman A, Hershkovitz Y, Kaufman Z, Dichtiar R, Keinan-Boker L, Bromberg M. Effectiveness of BNT162b2 vaccine in adolescents during outbreak of SARS-CoV-2 Delta variant infection, Israel, 2021. Emerg Infect Dis. 2021;27(11):2919-2922. doi: 10.3201/eid2711.211886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reis BY, Barda N, Leshchinsky M, et al. Effectiveness of BNT162b2 vaccine against Delta variant in adolescents. N Engl J Med. 2021;385(22):2101-2103. doi: 10.1056/NEJMc2114290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lutrick K, Rivers P, Yoo YM, et al. Interim estimate of vaccine effectiveness of BNT162b2 (Pfizer-BioNTech) vaccine in preventing SARS-CoV-2 infection among adolescents aged 12-17 years: Arizona, July-December 2021. MMWR Morb Mortal Wkly Rep. 2021;70(5152):1761-1765. doi: 10.15585/mmwr.mm705152a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jackson ML, Phillips CH, Benoit J, et al. The impact of selection bias on vaccine effectiveness estimates from test-negative studies. Vaccine. 2018;36(5):751-757. doi: 10.1016/j.vaccine.2017.12.022 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. COVID-like Illness Symptoms Collected by Self-report on Questionnaire Administered at Online Test Registration by Pharmacy Chain
eTable 2. Unadjusted and Adjusted BNT162b2 Odds Ratios (OR) With 95% Confidence Intervals (CI) by Time Since Second Dose and Age Group, During the Pre-Delta Period (March 13-May 29, 2021)
eTable 3. Unadjusted and Adjusted BNT162b2 Odds Ratios (OR) With 95% Confidence Intervals (CI) by Time Since Second Dose and Age Group, During the Intermediate Period (May 30-July 17, 2021)
eTable 4. Unadjusted and Adjusted BNT162b2 Odds Ratios (OR) With 95% Confidence Intervals (CI) by Time Since Second Dose and Age Group, During the Delta Period (July 18-October 17, 2021)
eTable 5. Unadjusted and Adjusted mRNA-1273 Odds Ratios (OR) With 95% Confidence Intervals (CI) by Time Since Second Dose and Age Group, During the Pre-Delta Period (March 13-May 29, 2021)
eTable 6. Unadjusted and Adjusted mRNA-1273 Odds Ratios (OR) With 95% Confidence Intervals (CI) by Time Since Second Dose and Age Group, During the Intermediate Period (May 30-July 17, 2021)
eTable 7. Unadjusted and Adjusted mRNA-1273 Odds Ratios (OR) With 95% Confidence Intervals (CI) by Time Since Second Dose and Age Group, During the Delta Period (July 18-October 17, 2021)
eTable 8. Unadjusted and Adjusted Ad26.COV2.S Odds Ratios (OR) With 95% Confidence Intervals (CI) by Time Since Dose and Age Group, During the Pre-Delta Period (March 13-May 29, 2021)
eTable 9. Unadjusted and Adjusted Ad26.COV2.S Odds Ratios (OR) With 95% Confidence Intervals (CI) by Time Since Dose and Age Group, During the Intermediate Period (May 30-July 17, 2021)
eTable 10. Unadjusted and Adjusted Ad26.COV2.S Odds Ratios (OR) With 95% Confidence Intervals (CI) by Time Since Dose and Age Group, During the Delta Period (July 18-October 17, 2021)
eTable 11. Comparison of Characteristics of Symptomatic Persons Ages ≥20 Years Tested for SARS-CoV-2 in Increasing Community Access to Testing (ICATT) 2.0 With Laboratory-Based Nucleic Acid Amplification Test (NAAT) With Known and Unknown Vaccination Status—United States, March 13-October 17, 2021
eTable 12. Comparison of Characteristics of Symptomatic Persons Ages 12-19 Years Tested for SARS-CoV-2 in Increasing Community Access to Testing (ICATT) 2.0 With Laboratory-Based Nucleic Acid Amplification Test (NAAT) With Known and Unknown Vaccination Status—United States, March 13-October 17, 2021
eTable 13. Odds Ratios (OR) a (95% Confidence Intervals [CI]) for Day 14, Mean of Daily OR Estimates From Days 14-60 (initial OR), and End Day for Figures 2, 3, and 4.
eTable 14. Significance Testing for Comparisons of Mean of Daily Odds Ratios (OR) Estimates From Days 14-60 After Vaccination (Initial OR) Between Vaccine Products (BNT162b2, mRNA-1273, and Ad26.COV2.S) and Time Periods (pre-Delta vs. Delta) for Adults Ages ≥20 Years
eTable 15. Characteristics of Included Adolescent Cases and Controls Ages 12-19 Years Tested for SARS-CoV-2 in Increasing Community Access to Testing (ICATT) 2.0 — United States, April 15-October 17, 2021 for Ages 12-15 years and June 15-October 17, 2021 for Ages 16-19 Years
eFigure 1. Sensitivity Analysis Restricting to Tests Performed Through September 23, 2021: Association of BNT162b2 Vaccination and Symptomatic Infection by Day Since Vaccination by Age Group
eFigure 2. Sensitivity Analysis Excluding Pharmacy Chain C: Association of COVID-19 Vaccination and Symptomatic Infection by Day Since Vaccination Among Adults Ages ≥20 Years
eFigure 3. Sensitivity Analysis Excluding Pharmacy Chain C: Association of COVID-19 Vaccination and Symptomatic Infection by Day Since Vaccination by Adult Age Group and Vaccine Product
eFigure 4. Sensitivity Analysis Excluding Pharmacy Chain C: Association of COVID-19 Vaccination and Symptomatic Infection by Day Since Vaccination Among Ages 12-15 Years and 16-19 Years
eFigure 5. Association of COVID-19 Vaccination and Symptomatic Infection by Day Since Vaccination Among Adults Ages ≥20 Years During the Intermediate Period (May 30-July 17, 2021)
eFigure 6. Association of COVID-19 Vaccination and Symptomatic Infection by Day Since Vaccination by Adult Age Group and Vaccine Product During the Intermediate Period (May 30-July 17, 2021)