Abstract
The present study investigated the anti-melanogenic activity of 10 essential oils using the B16F10 cell model. Initially, a wide range of concentrations of these essential oils were screened in order to determine their toxicity levels. The assigned non-toxic concentrations of the tested essential oils were then used to evaluate their effects on melanogenesis. The effects of the essential oils with potent anti-melanogenic activity on cell proliferation, protection against H2O2-induced cell death and the expression of certain melanogenesis-related genes, including MITF, tyrosinase, tyrosinase related protein (TRP)-1 and TRP-2 were also evaluated. The results revealed that the essential oils extracted from Citrus unshiu, Juniperus chinensis L., Zanthoxylum piperitum and Artemisia capillaris (A. capillaris) inhibited melanogenesis. However, among these four extracts, only A. capillaris extract enhanced cell proliferation, exhibited anti-H2O2 activities and decreased the expression level of TRP-1. It was demonstrated that A. capillaris extract inhibited melanin synthesis via the downregulation of the TRP-1 translational level. These essential oil extracts, particularly that of A. capillaris, may thus be used as natural anti-melanogenic agents for therapeutic purposes and in the cosmetic industry for skin whitening effects with beneficial proliferative properties. However, further studies using in vivo models are required to validate these findings and to examine the effects of these extracts on various molecular pathways.
Keywords: Artemisia capillaris, melanogenesis, skin whitening, microphthalmia-associated transcription factor, tyrosinase, tyrosinase related protein 1 and 2
Introduction
Melanogenesis is a crucial physiological process that occurs in melanocytes by which the melanosomes that synthesize and store melanin pigment are loaded with melanin and are translocated into the epidermal keratinocytes (1). Melanin biosynthesis is a tightly regulated process, with different pathways controlled by several enzymes and regulators. Tyrosinase is the main enzyme, initiating and regulating melanogenesis. However, tyrosinase-related protein (TRP)-1 and −2 are eumelanogenic enzymes contributing to the completion of the process and acting as modifiers for pathway velocity. TRP-1 and TRP-2 stabilize tyrosinase activity and TRP-1 possibly maintains melanosome structural integrity. Moreover, several regulators are involved in melanin biosynthesis, such as microphthalmia-associated transcription factor (MITF), which is considered the main transcriptional regulator of melanogenesis, functioning as the ‘central switchboard’ for the routing of various signals involved in the expression of melanogenesis-related genes (2,3).
Melanin pigment plays a crucial role in the protection of epidermal cell DNA from solar ultraviolet radiation damage, and in determining skin, hair and eye color (4,5). Furthermore, it can modulate skin immune responses and serves as a scavenger of reactive oxygen species (ROS), cellular toxins and miscellaneous chemical compounds, preventing further skin damage (6,7). The excessive reduction in melanin production (hypopigmentation) is associated with abnormal melanocyte development and dysfunction (8,9), which subsequently reduces protection from harmful UV radiations present in sunlight. On the other hand, the aberrant excessive production and the accumulation of melanin (hyperpigmentation) can lead to the development of skin disorders, such as melasma, post-inflammatory hyperpigmentation, solar lentigo, ephelides and café-au-lait macules (10). Moreover, the overproduction of melanin is recognized not only as a pathological concern, but also as a cosmetic issue. In this regard, individuals from a number of countries in Asia, Africa, South America and the Middle East have decided to reduce skin pigmentation to obtain a lighter skin tone, as fair skin is considered synonymous with youth, health, wealth and beauty in different cultures (11,12). However, hyperpigmentation may be congenital as a result of skin issues/systemic disease or it may be caused by environmental factors (13).
The active agents used to suppress melanin production and lighten the skin for therapeutic or cosmetic purposes are either natural or synthetic, and may function at various levels during melanogenesis. However, several of these agents have undesired adverse effects, such as irritation, rashes, inflamed skin, itchiness, toxicity and pain (14–17), and some of these agents exhibit relatively poor skin permeability (17,18). Therefore, there is a need for new safe and effective skin depigmenting agents to overcome these issues. The use of natural products, including essential oils as functional ingredients in cosmetics and depigmenting agents, has received increasing attention due to the growing interest of consumers in ingredients from natural sources. Moreover, several of these products have multiple pharmacological activities, including anti-melanogenic activity (19,20).
The aim of the present study was to identify naturally-sourced agents for skin whitening purposes and for use in the cosmetic industry with beneficial proliferative properties. For this purpose, the anti-melanogenic activity of essential oils extracted from 10 medicinal plants was evaluated using the B16F10 melanoma cell line by measuring the melanin content. The effects of essential oils with potent anti-melanogenic activity on cell proliferation, protection against H2O2-induced cell death, and the expression of certain melanogenesis-related proteins, namely MITF, tyrosinase, TRP-1 and TRP-2, were also evaluated.
Materials and methods
Extraction of essential oils
The hydrodistillation method was used to extract the essential oils from 10 medicinal plants using different plant parts (National Institute of Forest Science; Republic of Korea) (Table I). In brief, 1 kg of the plant part was mixed with 10 liters of distilled water and heated at 102°C using a heating mantle (cat. no. MS-DM608; Misung Scientific Co., Ltd.). The volatile steam was then condensed using a Dean-Stark trap (National Institute of Forest Science; Republic of Korea), and the acquired whole essential oil was dehydrated using anhydrous sodium sulfite and stored at 4°C until use.
Table I.
List of the scientific names, common names, and parts used of the investigated medicinal plants.
| No. | Scientific name | Common name | Parts used |
|---|---|---|---|
| 1 | Citrus unshiu | Satsuma orange | Peels |
| 2 | Citrus natsudaidai Hayata | Natsumikan | Peels |
| 3 | Citrus pseudo gulgul | Hill lemon | Peels |
| 4 | Juniperus chinensis L | Chinese juniper | Leaves |
| 5 | Juniperus chinensis var. sargentii | Sargent juniper | Leaves |
| 6 | Zanthoxylum piperitum | Japanese pepper | Fruits |
| 7 | Zanthoxylum schinifolium | Peppertree | Fruits |
| (Siebold & Zucc) | |||
| 8 | Artemisia capillaris | Yin Chen Hao | Grass clumps |
| 9 | Aster glehnii F. Schmidt | Ezo-goma-naa | Grass clumps |
| 10 | Cinnamomum cassia | Chinese cinnamon | Leaves |
Japanese common name.
Cells and cell culture
B16F10 mouse melanoma cells (Korean Cell Line Bank) were cultured in Dulbecco's modified Eagle's medium (DMEM; Welgene Inc.) with or without phenol red supplemented with 10% fetal bovine serum (FBS; Sigma-Aldrich; Merck KGaA) and 1% streptomycin/penicillin (Welgene Inc.) under standard culture conditions for 24 h for recovery. The cultured cells were then treated with the assigned concentrations of the tested essential oils for a further 24 or 72 h (Table II) for further analysis. For cell viability assay and western blot analysis, the B16F10 cells were cultured in DMEM in 24-well plates at a density of 55×104 and 3×105 cells/well, respectively, and treated with the essential oils for 24 h. For the 5-bromo-2-deoxyuridine (BrdU) and melanin quantification assays, the B16F10 cells were cultured in DMEM without phenol red in 6-well plates at a density of 1×105 cells/ml and treated with the essential oils for 24 or 72 h, respectively.
Table II.
The concentrations of the tested essential oils used in the different assays in the present study.
| Tested concentrations (ppm) | |||||
|---|---|---|---|---|---|
|
|
|||||
| No. | Essential oil source | Cell viability assay | Melanin quantification assays | BrdU assay | Western blot analysis |
| 1 | Citrus unshiu | 0.31, 1.25, 5, 20 and 80 | 0.31 and 1.25 | 0.31 and 1.25 | 1.25a |
| 2 | Citrus natsudaidai Hayata | 0.08, 0.31, 1.25, 5 and 20 | 0.08, 0.31, 1.25 and 5 | - | - |
| 3 | Citrus pseudo gulgul | 0.08, 0.31, 1.25, 5 and 20 | 0.08, 0.31 and 1.25 | - | - |
| 4 | Juniperus chinensis L | 0.08, 0.31, 1.25, 5 and 20 | 0.08, 0.31 and 1.25 | 0.08, 0.31 and 1.25 | 1.25a |
| 5 | Juniperus chinensis var. sargentii | 0.31, 1.25, 5, 20 and 80 | 0.31 | - | - |
| 6 | Zanthoxylum piperitum | 0.08, 0.31, 1.25, 5 and 20 | 0.08, 0.31, 1.25 and 5 | 0.08, 0.31, 1.25 and 5 | 5a |
| 7 | Zanthoxylum schinifolium (Siebold & Zucc) | 0.31, 1.25, 5, 20 and 80 | 0.31, 1.25 and 5 | - | - |
| 8 | Artemisia capillaris | 0.08, 0.31, 1.25, 5 and 20 | 0.08, 0.31, 1.25 and 5 | 0.08, 0.31, 1.25 and 5 | 5a |
| 9 | Aster glehnii F. Schmidt | 0.31, 1.25, 5, 20 and 80 | 0.31 and 1.25 | - | - |
| 10 | Cinnamomum cassia | 0.08, 0.31, 1.25, 5 and 20 | 0.08, 0.31, 1.25 and 5 | - | - |
These concentrations were also used to assess the protective effects of essential oils against H2O2-induced cell death.
Cell viability assay and determination of half maximal inhibitory concentration (IC50) values
MTT assay was used to construct a cell viability curve and to determine the IC50 values. The cultured cells were incubated with various concentrations of the essential oils for 24 h. After treatment, the medium containing essential oils was replaced with a solution of 5 mg/ml MTT (Sigma-Aldrich; Merck KGaA) and incubated at 37°C for 2 h. The optical density (OD) was measured at 570 nm using a microplate reader (BioTek Inc.). Cell viability was calculated using the following formula: OD sample/OD control ×100 for each concentration. The cell survival curve and the IC50 value for each treatment were calculated from these values using the SigmaPlot software program (V. 10.0; Systat Software, Inc.).
Measurement of melanin content
For the melanin quantification assay, the cells were pre-incubated with 200 nM α-melanocyte-stimulating hormone (α-MSH; Sigma-Aldrich; Merck KGaA) for 1 h at 37°C before adding the essential oils to promote melanin production. The assigned tested essential oils at non-toxic concentrations (Table II) or anti-melanogenic agent arbutin (250 µM) were added to the culture medium and incubated for a further 72 h at 37°C. α-MSH was used without essential oils as a positive control. DMSO alone was used as a standard control. Following treatment, the extracellular melanin content in 200 µl of culture media was measured using a microplate reader using a microplate reader (Agilent Technologies, Inc.) at 405 nm. To measure the intracellular melanin content, the cells were washed twice with phosphate-buffered saline (PBS) and collected using trypsinization. Centrifugation at 20,000 × g for 15 min at 4°C was performed, and the melanin pellets were dissolved in 1 N NaOH containing 10% DMSO for 1 h at 60°C. The mixed homogenate (100 µl) was placed in a 96-well microplate, and the OD values were measured using a microplate reader (Agilent Technologies, Inc.) at 405 nm. The extracellular and intracellular melanin contents per well were calculated and expressed as a percentage of the control.
Assessment of inhibitory effects of essential oil extracts on tyrosinase activity
Mushroom tyrosinase activity assay was performed according to the manufacturer's recommendations. In a 96-well plates, 20 µl mushroom tyrosinase (2,000 U/ml, Sigma-Aldrich; Merck KGaA), 30 µl essential oil extracts or arbutin (Sigma-Aldrich; Merck KGaA) as a positive control, 210 µl phosphate buffer (0.1 M; pH 6.8) and 40 µl tyrosine (1.5 mM, Sigma-Aldrich; Merck KGaA) were mixed and incubated at 37°C for 20 min. The OD value was then measured using a microplate reader (Agilent Technologies, Inc.) at 490 nm. The tyrosinase activity in the samples were expressed using the following formula: OD sample/OD control ×100.
Measurement of cell proliferation
BrdU assay was carried out using a cell proliferation ELISA BrdU kit (Roche Diagnostics) according to the manufacturer's recommendations. In brief, following the treatment period with the assigned concentrations of the essential oils, 100 µl BrdU solution (100 µM) were added to each well in 1 ml medium, and the plates were then incubated at 37°C for 4 h. Subsequently, the cells were fixed using 1 ml FixDenat (Roche Diagnostics) in each well for 30 min and incubated with the kit-supplied anti-BrdU antibody (1:100; Roche Diagnostics) for 90 min at room temperature. After washing, the cells were incubated with 500 µl substrate for 20 min at room temperature, and 125 µl H2SO4 (1 M) were added. The plates were analyzed at 450 nm using a spectrometer (Agilent Technologies, Inc.).
Assessment of the protective effects of the essential oils against H2O2-induced cell death
B16F10 cells were cultured in DMEM containing the assigned concentrations of the tested essential oils (Table II) for 24 h, as described above. H2O2 (Sigma-Aldrich; Merck KGaA) was then added at a final concentration of 400 µM for 4 h. Cell viability was assessed using MTT assay as aforementioned.
Western blot analysis
Protein samples from the B16F10 cells treated with the assigned concentrations of the essential oils for 24 h were extracted using Pro-Prep solution (iNtRON Biotechnology Inc.) according to the manufacturer's protocol. The concentration of protein was determined by performing a bicinchoninic acid assay. Subsequently, 5 µg protein were loaded and separated using sodium dodecyl sulfate-polyacrylamide gel electrophoresis on 8–10% gels and transferred onto nitrocellulose membranes (Daeillab Lab Service Co., Ltd.) using the wet transfer system. The membranes were blocked for 2 h with 5% skimmed milk (BD Biosciences) in PBS with 0.05% Tween-20 (PBST) at room temperature. Subsequently, the membranes were incubated with antibodies against MITF (1:300; cat. no. sc-56725; Santa Cruz Biotechnology, Inc.), tyrosinase (1:300; cat. no. sc-20035; Santa Cruz Biotechnology, Inc.), TRP-1 (1:300; cat. no. sc-25543; Santa Cruz Biotechnology, Inc.), TRP-2 (1:300; cat. no. sc-25544; Santa Cruz Biotechnology, Inc.) and β-actin (1:3,000; cat. no. #4967; Cell Signaling Technology, Inc.), which served as an internal control overnight at 4°C, followed by incubation with horseradish peroxidase-conjugated secondary antibodies (1:5,000; cat. nos. ADI-SAB-100 and ADI-SAB-300; Enzo Life Science Inc.) in 5% skimmed milk in PBST for 1 h at room temperature. Luminol reagent (Bio-Rad Laboratories, Inc.) was used to visualize antibody binding. The blots were scanned using Gel Doc 1000, version 1.5 (Bio-Rad Laboratories, Inc.), and band intensities were normalized to β-actin levels.
Statistical analyses
Data are presented as the mean ± standard deviation (SD). Data were analyzed using one-way analysis of variance (ANOVA) with SPSS 10.10 standard version (IBM Corp.). Means obtained from three independent experiments were evaluated using one-way ANOVA and Tukey's post hoc t-test for multiple comparisons. A value of P<0.05 was considered to indicate a statistically significant difference.
Results
Effects of the tested essential oils on cell viability
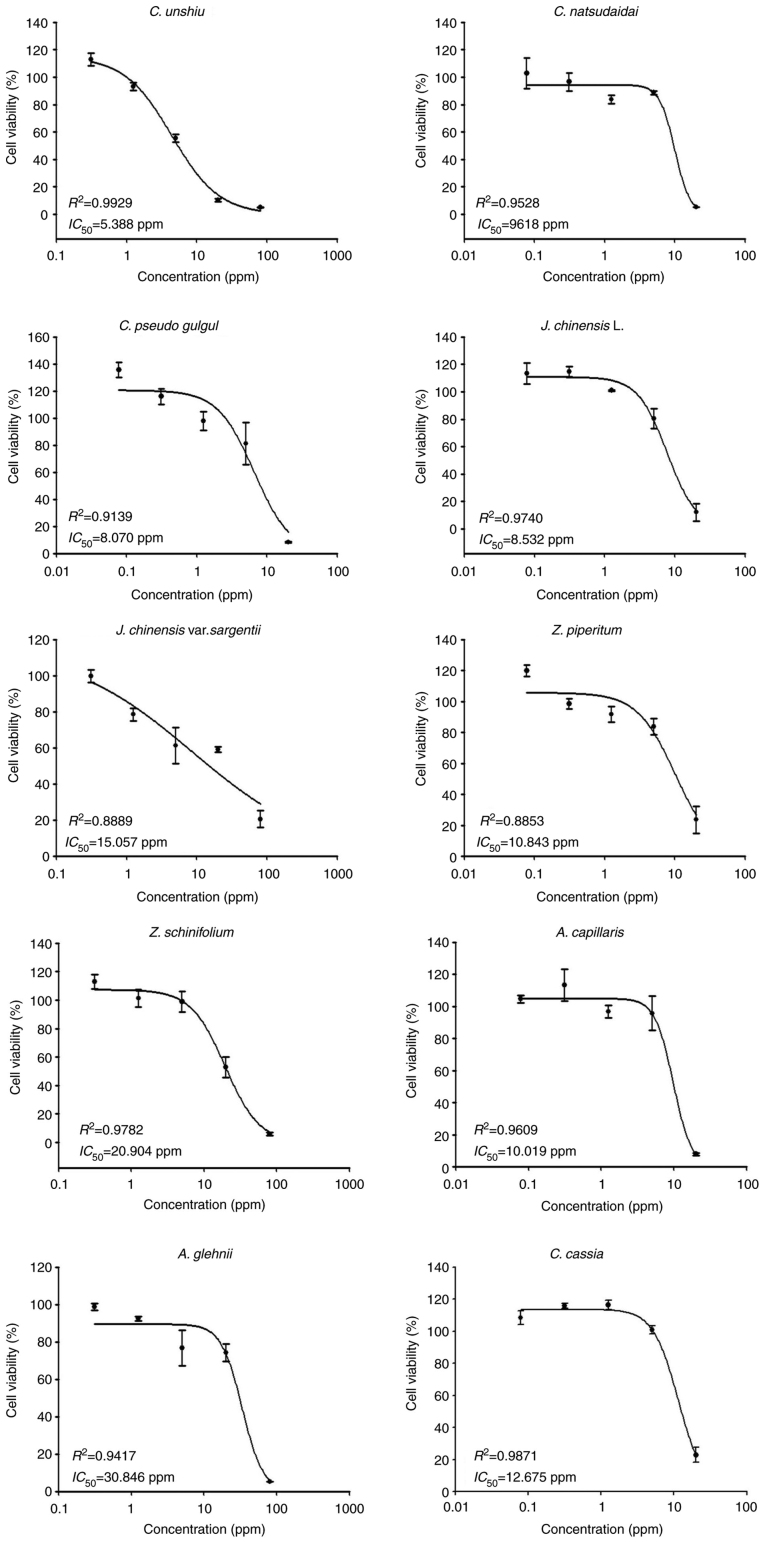
Cell viability assays were conducted using a wide range of concentrations (0.31–80 ppm) for the essential oils extracted from Citrus unshiu (C. unshiu), Juniperus chinensis var. sargentii (J. chinensis var. sargentii), Zanthoxylum schinifolium (Siebold & Zucc) (Z. schinifolium) and Aster glehnii F. Schmidt (A. glehnii), and from 0.08 to 20 ppm for the essential oils extracted from Citrus natsudaidai Hayata (C. natsudaidai), Citrus pseudo gulgul (C. pseudo gulgul), Juniperus chinensis (J. chinensis L.), Zanthoxylum piperitum (Z. piperitum), Artemisia capillaris (A. capillaris) and Cinnamomum cassia (C. cassia) to screen their toxic effects on B16F10 cells. The tested essential oils exhibited variable toxicity levels in the B16F10 cells, as revealed by the cell viability curves and IC50 values (Fig. 1). The essential oils extracted from C. unshiu exhibited the highest toxicity level (IC50, 5.388 ppm), whereas the essential oil extracted from A. glehnii exhibited the lowest toxicity level (IC50, 30.846 ppm) (Fig. 1).
Figure 1.
Cell viability curves and IC50 values of the tested essential oils. IC50, the concentration of the essential oil in the culture media that killed 50% of the B16F10 melanoma cells.
Effect of essential oils on melanin content
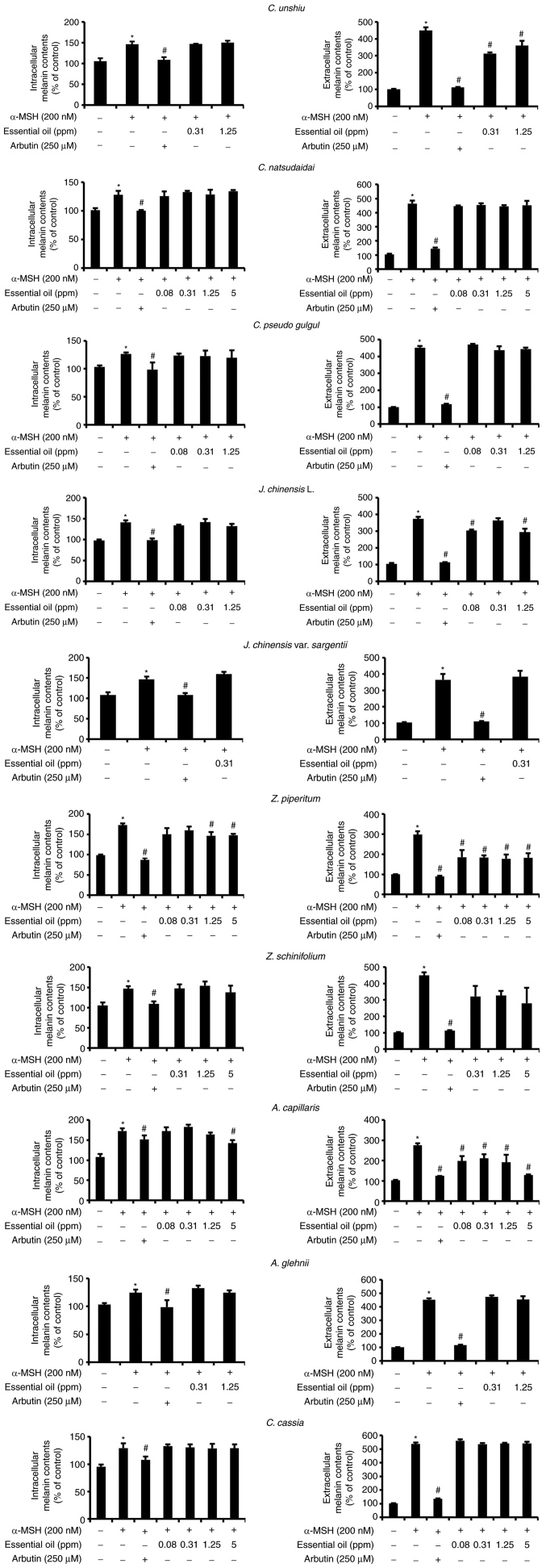
A set of non-toxic concentrations of the tested essential oils was used to investigate their anti-melanogenic activity in B16F10 cells. The extracellular and intracellular melanin contents were quantified after culturing the cells with the assigned treatments for 72 h (Fig. 2). The extracellular and intracellular melanin contents were significantly higher (P<0.05) in the positive control group (α-MSH) than in the other treatment groups. However, arbutin treatment significantly decreased the extracellular and intracellular melanin content compared to that in the positive control group (P<0.05). Of note, the essential oils extracted from C. unshiu, J. chinensis L., Z. piperitum and A. capillaris significantly decreased the extracellular melanin contents at all concentrations tested compared to the positive control group (P<0.05), excluding the concentration of 0.31 ppm for J. chinensis L. The highest concentrations of these essential oils decreased the extracellular melanin content by 20, 38.8, 21 and 53.5% compared with the positive control group, respectively. However, only the elevated concentrations of Z. piperitum and A. capillaris significantly decreased the intracellular melanin content (P<0.05) compared to that in the positive control group by 14.4 and 17.5%, respectively (Figs. 2 and 3). Of note, none of these essential oils significantly altered the tyrosinase activity (Fig. S1).
Figure 2.
Intracellular and extracellular melanin contents measured following the indicated treatments of the B16F10 melanoma cells. Each value for melanin contents was calculated relative to that of the control group. Values represent the mean ± SD of three independent experiments. *P<0.05, significant difference between the control and α-MSH group; #P<0.05, significant difference compared to the group treated with α-MSH only. α-MSH, α-melanocyte-stimulating hormone.
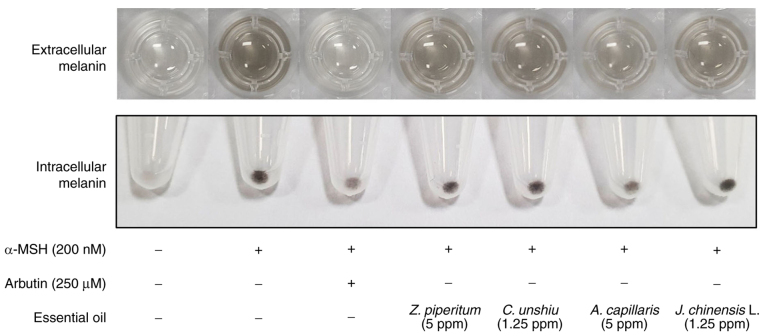
Figure 3.
Effects of C. unshiu, J. chinensis L., Z. piperitum and A. capillaris extracts on α-MSH-induced melanogenesis. B16F10 Cells were incubated with the assigned concentrations of the essential oils extracts or arbutin (250 µM) in the presence of α-MSH (200 nM) for 72 h, collected in a microfuge tube, and photographed. Experiments were performed three times with similar results, and a typical image is presented. α-MSH, α-melanocyte-stimulating hormone.
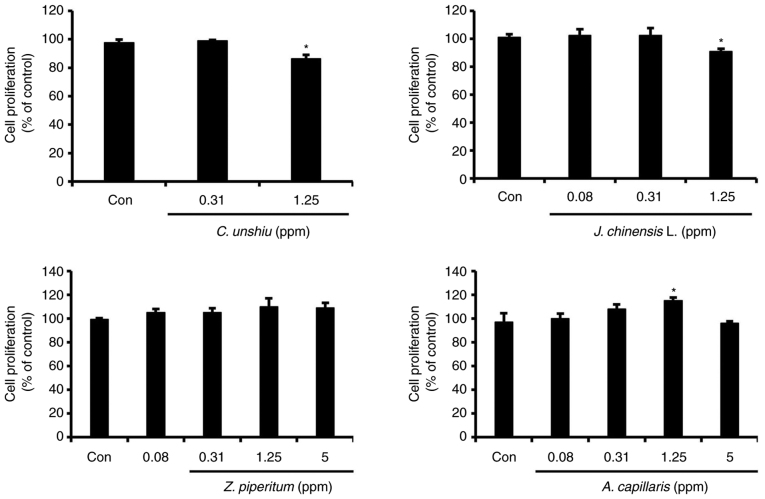
Effect of essential oils on B16F10 cell proliferation
Among the 10 essential oils examined in the present study, only four essential oils with anti-melanogenic activity (C. unshiu, J. chinensis L., Z. piperitum, and A. capillaris) were selected to investigate their effects on cell proliferation using BrdU assay. In general, the essential oils extracted from Z. piperitum and A. capillaris enhanced cell proliferation, although only A. capillaris extract at a concentration of 1.25 ppm significantly (P<0.05) increased cell proliferation by 18.7% compared to that in the control group. However, the highest concentrations tested for the C. unshiu and J. chinensis L. extracts significantly decreased cell proliferation by 11.5 and 10.1%, respectively (P<0.05; Fig. 4).
Figure 4.
Effects of the essential oils on B16F10 melanoma cell proliferation. *P<0.05, significant difference compared to the control.
Assessment of the protective effects of the essential oils against H2O2-induced cell death
The protective effects of the essential oils extracted from C. unshiu, J. chinensis L., Z. piperitum, and A. capillaris against H2O2-induced cell death were assessed using MTT assay. The most effective concentration of the essential oils for an anti-melanogenic effect was used for MTT assay. As shown in Fig. 5, only the essential oil extracted from A. capillaris attenuated the effects of H2O2 on cell death induction and significantly increased cell viability in the presence of H2O2 in the culture media compared to the other essential oils (P<0.05).
Figure 5.
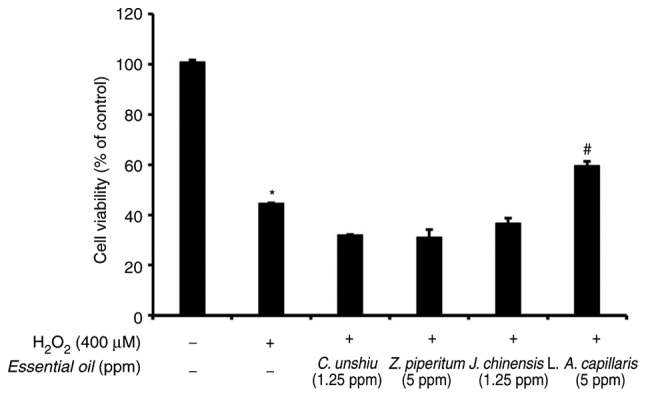
Protective effects of the essential oils against the H2O2-induced death of B16F10 cells as revealed using MTT assay. *P<0.05, significant difference between the control and the group treated with H2O2 only; #P<0.05, significant difference compared to the group treated with H2O2 only.
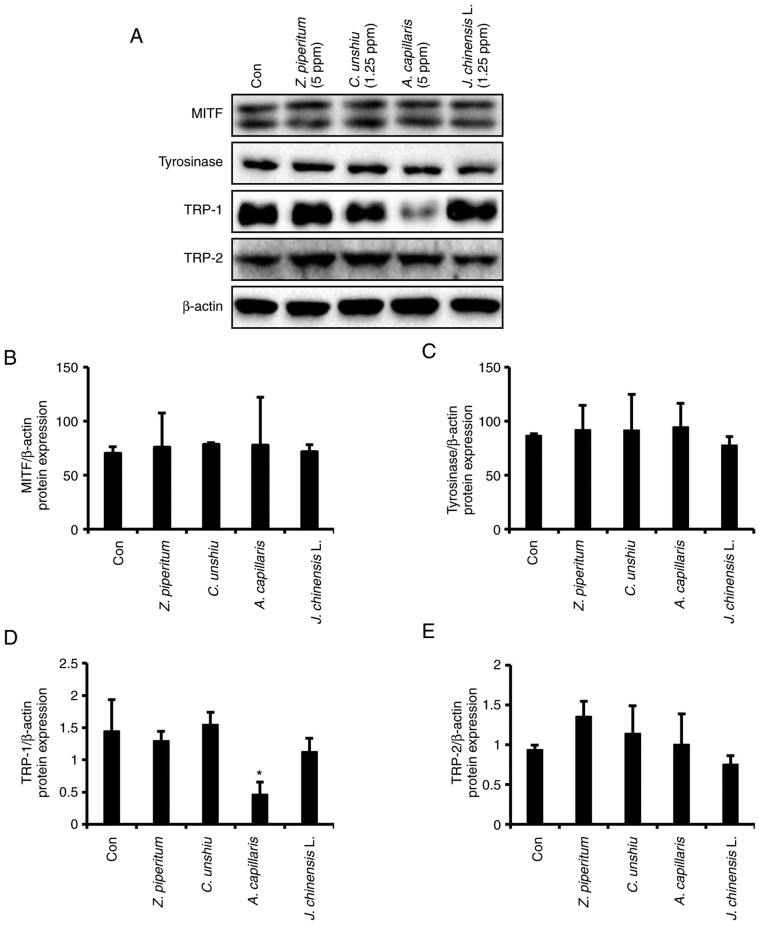
Effects of the essential oils on the translational levels of melanogenesis-related genes
The effects of the four essential oils extracted from C. unshiu, J. chinensis L., Z. piperitum, and A. capillaris on the MITF, tyrosinase, TRP-1 and TRP-2 translational levels in B16F10 cells were examined using western blot analysis. The most effective concentration of the essential oils for an anti-melanogenic effect was used in western blot analysis. The essential oil extracted from A. capillaris at a concentration of 5 ppm significantly decreased the TRP-1 protein level by ~68% compared to that in the control group (P<0.05). However, the other essential oils did not induce any significant changes in the protein levels of tyrosinase, TRP-1 and TRP-2 (Figs. 6 and S2). Among the proteins related to melanogenesis, only MITF exhibited double bands. The upper band has been assigned as a shift of the lower band due to phosphorylation (21). However, none of the essential oils altered the expression levels of MITF (Figs. 6 and S2).
Figure 6.
Translational level of melanogenesis-related genes in B16F10 cells treated with different essential oils. (A) Translational levels of melanogenesis-related genes examine using western blot analysis. The expression levels of (B) MITF, (C) tyrosinase, (D) TRP-1, and (E) TRP-2are represented as schematic graphs and normalized to β-actin levels. Data are expressed as the mean ± SD. *P<0.05, significant difference compared to the control. MITF, microphthalmia-associated transcription factor; TRP, tyrosinase related protein.
Discussion
Essential oils extracted from 10 medicinal plants were assessed in the present study to determine their anti-melanogenic activities using B16F10 cell line model. These plants represent various groups of medicinal plants that are widely distributed in a number of Asian countries and have a long history of use in folk medicine to treat various diseases. Moreover, over the past few decades, increased attention has been directed towards the use of functional components from these plants in biomedical applications to treat various diseases, such as cancer (22–25), allergies (26), dermatopathology (27,28) and other diseases (29–32). However, little is known about their effects and functions as natural anti-melanogenic agents.
Melanogenesis is a complex and multistep process that results in melanin formation. Therefore, the measurement of the melanin content is a direct strategy which can be used to assess the melanogenic activity. It has been reported that in an in vitro culture system, melanin is synthesized intracellularly and is then transported into the surrounding culture medium (33,34). In the present study, the extracellular and intracellular melanin contents were determined simultaneously to measure the total amount of melanin synthesized under various treatment conditions in B16F10 cells. The anti-melanogenic effects of the essential oils were further examined on the human melanoma cell line, A375SM; however, when treated with α-MSH as a positive control, the cells did not produce a sufficient amount of melanin to evaluate the anti-melanogenic effects (data not shown). Among the essential oil extracts examined, only four essential oils from C. unshiu, J. chinensis L., Z. piperitum and A. capillaris successfully decreased melanogenesis compared to the other extracts, which reflects their potency as anti-melanogenic agents. In agreement with these findings, previous studies have reported the inhibitory effects of some fractions extracted from these four plants on melanin formation using various cell models (31,35–37). Notably, the non-toxic concentrations of all essential oils used in the present study, which inhibited melanin formation, were very low compared to the fraction concentrations used in a previous study, which reached up to 100 µg/ml (36). Although the whole extracts of the essential oils exerted beneficial effects on melanogenesis, active substances were not evaluated in the present study. Therefore, further studies on the active compounds for each essential oil are warranted. However, using the whole essential oils has an advantage over purified components as they have multi-pharmacological activities, and the different components may exert a synergistic or potentiating effects and be important for the bioactivity of the essential oils (38,39). Moreover, unlike previous studies (35–37), the present study used the hydrodistillation method for essential oil extraction, avoiding the hazards of organic solvents and emulsion formation using other methods (40). To v further alidate efficiency of the extracts, their effects on cell proliferation were assessed using BrdU assay, which established the positive effects of two extracts, Z. piperium and A. capillaris, at specific concentrations on cell proliferation. Additionally, as the skin, more than other tissues, is exposed to numerous external stresses generating several types of ROS, such as H2O2 that is also produced as a response to a multitude of very complex cellular events causing various deleterious effects and apoptosis in keratinocytes (41,42), the protective effects of A. capillaris against H2O2-induced cell death were also examined. Of note, A. capillaris was also able to significantly reduce H2O2-induced cell death, suggesting that it is an anti-melanogenic agent with proliferative and antioxidant properties. Hong et al (43) reported that ethyl acetate fraction from A. capillaris exerted significant ROS scavenging and protective effects against oxidative DNA.
The anti-melanogenic effects of the extracted essential oils on the B16F10 cell line were also investigated at the molecular level by assessing the MITF, tyrosinase, TRP-1, and TRP-2 protein expression levels using western blot analysis. Although the four essential oils tested successfully decreased the synthesized melanin content, they did not affect the expression levels of MITF, tyrosinase and TRP-2 proteins, or even tyrosinase activity, and only the essential oil extracted from A. capillaris significantly decreased the expression level of TRP-1 compared to that in the control group. Although tyrosinase is the key enzyme in melanogenesis, TRP-1 is considered an eumelanogenic enzymes with a vital role in the completion of melanogenesis. TRP-1 is a protein producing eumelanin in the last stage of the melanogenesis. Eumelanin is the most common type of melanin comprising cross-linked 5,6-dihydroxyindole (DHI) and 5,6-dihydroxyindole-2-carboxylic acid (DHICA). TRP-1 induces the oxidative conversion of DHICA to indole-5,6-quinone-2-carboxylic acid, which is a structural unit of eumelanin (2,3). Therefore, the overexpression of TRP-1 causes skin color to darken (44). Eberle et al (45) examined the expression levels of tyrosinase family genes in melanoma and normal melanocyte human cell lines and found that the expression levels of tyrosinase and TRP-2 are regulated differently than TRP-1. Accordingly, in the present study, A. capillaris at a concentration of the 5 ppm did not affect the MITF, tyrosinase and TRP-2 expression levels or tyrosinase activity, whereas it significantly decreased TRP-1 expression compared to that in the control group. It appears that A. capillaris inhibits melanogenesis via a tyrosinase-independent pathway. Therefore, any materials or components that suppress TRP-1 expression may affect melanogenesis by reducing the oxidation of DHICA to a carboxylated indole-quinone. The findings of the present study suggest that the low expression of TRP-1 can reduce melanin synthesis. The present study initially examined various concentrations of A. capillaris (0.08, 0.13, 1.25 and 5 ppm) on cell viability and melanin synthesis. While the concentrations <5 ppm (0.08, 0.13 and 1.25 ppm) did not affect cytotoxicity, these concentrations did not reduce intracellular melanin contents. The concentration of 5 ppm exerted the optimal effect on melanin synthesis, and significantly decreased the extracellular and intracellular melanin contents by 53.5 and 17.5%, respectively compared to the control group. Therefore, this concentration was used to examine the effect of A. capillaris against the protein expression levels of TRP-1. Although the C. unshiu, J. chinensis L. and Z. piperitum extracts decreased the melanin content, none of them altered the translational level of the proteins involved in melanin synthesis. These extracts appear to exert their effects on melanogenesis via mechanisms different from those of A. capillaris. As melanogenesis is a tightly regulated process that includes various enzymes and other factors controlling different pathways, materials that exert an inhibitory effect on any of these factors are anticipated to inhibit melanogenesis.
In conclusion, the essential oils extracted from C. unshiu, J. chinensis L., Z. piperitum and A. capillaris using the hydrodistillation method inhibited melanin synthesis. A. capillaris extract was the most potent inhibitor of melanin synthesis, with good potential to enhance cell viability and anti-H2O2 activity. A. capillaris extract inhibited melanin synthesis by downregulating the TRP-1 expression level. The present study did not perform animal experiments to reveal the effects of the essential oils. Further animal studies are thus required to address the systemic effects of the essential oils. However, in general, animal experiments to evaluate the functional effects of certain materials on the skin are prohibited for animal protection. Even though the present study did not determine the effects of the extracts in in vivo conditions, these four essential oil extracts, particularly A. capillaris, may be considered as natural anti-melanogenic agents with beneficial proliferative properties for the treatment of skin pigmentary disorders and for skin whitening in the cosmetic industry. However, future studies using in vivo models are required for further validation and to investigate the effects of these extracts at the molecular level through various mechanisms and pathways.
Supplementary Material
Acknowledgements
Not applicable.
Funding Statement
The present study was performed with the support of the National Institute of Forest Science (Project no. FP0702-2016-03-2020) and was partially supported by the BK21 FOUR Program (grant no. F20YY8109033) through the National Research Foundation of Korea (NRF), funded by the Ministry of Education, Korea.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
MJK, EAM and BSA designed the experiments and wrote the manuscript. MJK, DSK, MJP and BJA performed the experiments and analyzed the data. EAM and BSA confirmed the authenticity of all the raw data. EAM, EBJ and BSA analyzed the data and revised the manuscript. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Tayarani-Najaran Z, Akaberi M, Vatani M, Emami SA. Evaluation of antioxidant and anti-melanogenic activities of different extracts from aerial parts of Nepeta binaludensis Jamzad in murine melanoma B16F10 cells. Iran J Basic Med Sci. 2016;19:662–669. [PMC free article] [PubMed] [Google Scholar]
- 2.Slominski A, Tobin DJ, Shibahara S, Wortsman J. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol Rev. 2004;84:1155–1228. doi: 10.1152/physrev.00044.2003. [DOI] [PubMed] [Google Scholar]
- 3.Slominski A, Zmijewski MA, Pawelek J. L-tyrosine and L-dihydroxyphenylalanine as hormone-like regulators of melanocyte functions. Pigment Cell Melanoma Res. 2012;25:14–27. doi: 10.1111/j.1755-148X.2011.00898.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang L, Oh JY, Kim YS, Lee HG, Lee JS, Jeon YJ. Anti-photoaging and anti-melanogenesis effects of fucoidan isolated from Hizikia fusiforme and its underlying mechanisms. Mar Drugs. 2020;18:427. doi: 10.3390/md18080427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rok J, Otręba M, Buszman E, Wrześniok D. Melanin-from melanocyte to keratinocyte, that is how melanin is transported within the skin. Ann Acad Med Siles. 2012;66:60–66. [Google Scholar]
- 6.Slominski A, Wortsman J, Luger T, Paus R, Solomon S. Corticotropin releasing hormone and proopiomelanocortin involvement in the cutaneous response to stress. Physiol Rev. 2000;80:979–1020. doi: 10.1152/physrev.2000.80.3.979. [DOI] [PubMed] [Google Scholar]
- 7.Slominski AT, Zmijewski MA, Skobowiat C, Zbytek B, Slominski RM, Steketee JD. Sensing the environment: Regulation of local and global homeostasis by the skin's neuroendocrine system. Adv Anat Embryol Cell Biol. 2012;212:v. doi: 10.1007/978-3-642-19683-6_1. vii, 1, 115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Otręba M, Buszman E, Miliński M, Wrześniok D. Hypomelanoses transmitted from generation to generation. Postepy Hig Med Dosw (Online) 2014;68:1081–1090. doi: 10.5604/17322693.1119791. (In Polish) [DOI] [PubMed] [Google Scholar]
- 9.Otręba M, Miliński M, Buszman E, Wrześniok D, Beberok A. Hereditary hypomelanocytoses: the role of PAX3, SOX10, MITF, SNAI2, KIT, EDN3 and EDNRB genes. Postepy Hig Med Dosw (Online) 2013;67:1109–1118. doi: 10.5604/17322693.1077722. (In Polish) [DOI] [PubMed] [Google Scholar]
- 10.Goswami P, Sharma HK. Skin hyperpigmentation disorders and use of herbal extracts: A review. Curr Trends Pharm Res. 2020;7:81–104. [Google Scholar]
- 11.Burger P, Landreau A, Azoulay S, Michel T, Fernandez X. Skin whitening cosmetics: Feedback and challenges in the development of natural skin lighteners. Cosmetics. 2016;3:36. doi: 10.3390/cosmetics3040036. [DOI] [Google Scholar]
- 12.Pollock S, Taylor S, Oyerinde O, Nurmohamed S, Dlova N, Sarkar R, Galadari H, Manela-Azulay M, Chung HS, Handog E, Kourosh AS. The dark side of skin lightening: An international collaboration and review of a public health issue affecting dermatology. Int J Womens Dermatol. 2020;7:158–164. doi: 10.1016/j.ijwd.2020.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cestari TF, Dantas LP, Boza JC. Acquired hyperpigmentations. An Bras Dermatol. 2014;89:11–25. doi: 10.1590/abd1806-4841.20142353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blaut M, Braune A, Wunderlich S, Sauer P, Schneider H, Glatt H. Mutagenicity of arbutin in mammalian cells after activation by human intestinal bacteria. Food Chem Toxicol. 2006;44:1940–1947. doi: 10.1016/j.fct.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 15.Hwang KS, Yang JY, Lee JY, Lee YR, Kim SS, Kim GR, Chae JS, Ahn JH, Shin DS, Choi TY, Bae MA. A novel anti-melanogenic agent, KDZ-001, inhibits tyrosinase enzymatic activity. J Dermatol Sci. 2018;89:165–171. doi: 10.1016/j.jdermsci.2017.11.004. [DOI] [PubMed] [Google Scholar]
- 16.Saeedi M, Eslamifarb M, Khezri K. Kojic acid applications in cosmetic and pharmaceutical preparations. Biomed Pharmacother. 2019;110:582–593. doi: 10.1016/j.biopha.2018.12.006. [DOI] [PubMed] [Google Scholar]
- 17.Lee R, Ko HJ, Kim K, Sohn Y, Min SY, Kim JA, Na D, Yeon JH. Anti-melanogenic effects of extracellular vesicles derived from plant leaves and stems in mouse melanoma cells and human healthy skin. J Extracell Vesicles. 2019;9:1703480. doi: 10.1080/20013078.2019.1703480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fong P, Tong HH. In silico prediction of the cosmetic whitening effects of naturally occurring lead compounds. Nat Prod Commun. 2012;7:1287–1294. [PubMed] [Google Scholar]
- 19.Huang HC, Wang HF, Yih KH, Chang LZ, Chang TM. The dual antimelanogenic and antioxidant activities of the essential oil extracted from the leaves of acorus macrospadiceus (Yamamoto) F. N. Wei et Y.K. Li. Evid Based Complement Alternat Med. 2012;2012:781280. doi: 10.1155/2012/781280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang HC, Ho YC, Lim JM, Chang TY, Ho CL, Chang TM. Investigation of the anti-melanogenic and antioxidant characteristics of eucalyptus camaldulensis flower essential oil and determination of its chemical composition. Int J Mol Sci. 2015;16:10470–10490. doi: 10.3390/ijms160510470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu M, Hemesath TJ, Takemoto CM, Horstmann MA, Wells AG, Price ER, Fisher DZ, Fisher DE. c-Kit triggers dual phosphorylations, which couple activation and degradation of the essential melanocyte factor Mi. Genes Dev. 2000;14:301–12. doi: 10.1101/gad.14.3.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Herdwiani W, Soemardji AA, Elfahmi Tan MI. Review of cinnamon as a potent anticancer drug. Asian J Pharma Clin Res. 2016;9:8–13. [Google Scholar]
- 23.Kuo ZK, Lin MW, Lu IH, Yao HJ, Wu HC, Wang CC, Lin SH, Wu SY, Tong TS, Cheng YC, et al. Antiangiogenic and antihepatocellular carcinoma activities of the Juniperus chinensis extract. BMC Complement Altern Med. 2016;16:277. doi: 10.1186/s12906-016-1250-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chung KS, Cheon SY, Roh SS, Lee M, An HJ. Chemopreventive effect of Aster glehni on inflammation-induced colorectal carcinogenesis in mice. Nutrients. 2018;10:202. doi: 10.3390/nu10020202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim J, Jung KH, Yan HH, Cheon MJ, Kang S, Jin X, Park S, Oh MS, Hong SS. Artemisia capillaris leaves inhibit cell proliferation and induce apoptosis in hepatocellular carcinoma. BMC Complement Altern Med. 2018;18:147. doi: 10.1186/s12906-018-2217-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kono R, Nomura S, Okuno Y, Kagiya T, Nakamura M, Utsunomiya H, Ueno M. Two Japanese pepper (Zanthoxylum piperitum) fruit-derived compounds attenuate IgE-mediated allergic response in vitro and in vivo via inhibition of mast cell degranulation. Eur J Pharmacol. 2020;885:173435. doi: 10.1016/j.ejphar.2020.173435. [DOI] [PubMed] [Google Scholar]
- 27.Kim SS, Baik JS, Oh TH, Yoon WJ, Lee NH, Hyun CG. Biological activities of Korean Citrus obovoides and Citrus natsudaidai essential oils against acne-inducing bacteria. Biosci Biotechnol Biochem. 2008;72:2507–2513. doi: 10.1271/bbb.70388. [DOI] [PubMed] [Google Scholar]
- 28.Kang GJ, Han SC, Yi EJ, Kang HK, Yoo ES. The inhibitory effect of premature Citrus unshiu extract on atopic dermatitis in vitro and in vivo. Toxicol Res. 2011;27:173–180. doi: 10.5487/TR.2011.27.3.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Choi SY, Ko HC, Ko SY, Hwang JH, Park JG, Kang SH, Han SH, Yun SH, Kim SJ. Correlation between flavonoid content and the NO production inhibitory activity of peel extracts from various citrus fruits. Biol Pharm Bull. 2007;30:772–778. doi: 10.1248/bpb.30.772. [DOI] [PubMed] [Google Scholar]
- 30.Jin KS, Lee JY, Hyun SK, Kim BW, Kwon HJ. Juniperus chinensis and the functional compounds, cedrol and widdrol, ameliorate α-melanocyte stimulating hormone-induced melanin formation in B16F10 Cells. Food Sci Biotechnol. 2015;24:611–618. doi: 10.1007/s10068-015-0080-5. [DOI] [Google Scholar]
- 31.Jin S, Yun HJ, Jeong HY, Oh YN, Park HJ, Yun SG, Kim BW, Kwon HJ. Widdrol, a sesquiterpene isolated from Juniperus chinensis, inhibits angiogenesis by targeting vascular endothelial growth factor receptor 2 signaling. Oncol Rep. 2015;34:1178–1184. doi: 10.3892/or.2015.4075. [DOI] [PubMed] [Google Scholar]
- 32.Lee SW, Lim JM, Mohan H, Seralathan KK, Park YJ, Lee JH, Oh BT. Enhanced bioactivity of Zanthoxylum schinifolium fermented extract: Anti-inflammatory, anti-bacterial, and anti-melanogenic activity. J Biosci Bioeng. 2020;129:638–645. doi: 10.1016/j.jbiosc.2019.12.003. [DOI] [PubMed] [Google Scholar]
- 33.Laskin JD, Piccinini L, Engelhardt DL, Weinstein IB. Control of melanin synthesis and secretion by B16/C3 melanoma cells. J Cell Physiol. 1982;113:481–486. doi: 10.1002/jcp.1041130318. [DOI] [PubMed] [Google Scholar]
- 34.Bhatnagar V, Srirangam A, Abburi R. In vitro modulation of proliferation and melanization of melanoma cells by citrate. Mol Cell Biochem. 1998;187:57–65. doi: 10.1023/A:1006870621424. [DOI] [PubMed] [Google Scholar]
- 35.Jeong CH, Shim KH. Tyrosinase inhibitor isolated from the leaves of Zanthoxylum piperitum. Biosci Biotechnol Biochem. 2004;68:1984–1987. doi: 10.1271/bbb.68.1984. [DOI] [PubMed] [Google Scholar]
- 36.Saba E, Oh MJ, Lee YY, Kwak D, Kim S, Rhee MH. Artemisia capillaris thunb. Inhibits melanin synthesis activity via ERK-dependent MITF pathway in B16/F10 melanoma cells. Korean J Vet Res. 2018;58:1–7. doi: 10.14405/kjvr.2018.58.1.1. [DOI] [Google Scholar]
- 37.Kim JK, Park NH, Hwang JS. Skin lightening effect of the dietary intake of citrus peel extract against UV-induced pigmentation. Nat Prod Commun. 2019;14:1934578X19859979. [Google Scholar]
- 38.Burt S. Essential oils: Their antibacterial properties and potential applications in foods-a review. Int J food Microbiol. 2004;94:223–253. doi: 10.1016/j.ijfoodmicro.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 39.Popa M, Măruțescu L, Oprea E, Bleotu C, Kamerzan C, Chifiriuc MC, Grădișteanu Pircalabioru G. In vitro evaluation of the antimicrobial and immunomodulatory activity of culinary herb essential oils as potential perioceutics. Antibiotics (Basel) 2020;9:428. doi: 10.3390/antibiotics9070428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dangkulwanich M, Charaslertrangsi T. Hydrodistillation and antimicrobial properties of lemongrass oil (Cymbopogon citratus, Stapf): An undergraduate laboratory exercise bridging chemistry and microbiology. J Food Sci Educ. 2020;19:41–48. doi: 10.1111/1541-4329.12178. [DOI] [Google Scholar]
- 41.Baldea I, Mocan T, Cosgarea R. The role of ultraviolet radiation and tyrosine stimulated melanogenesis in the induction of oxidative stress alterations in fair skin melanocytes. Exp Oncol. 2009;31:200–208. [PubMed] [Google Scholar]
- 42.Kim ES, Park SJ, Goh MJ, Na YJ, Jo DS, Jo YK, Shin JH, Choi ES, Lee HK, Kim JY, et al. Mitochondrial dynamics regulate melanogenesis through proteasomal degradation of MITF via ROS-ERK activation. Pigment Cell Melanoma Res. 2014;27:1051–1062. doi: 10.1111/pcmr.12298. [DOI] [PubMed] [Google Scholar]
- 43.Hong JH, Lee JW, Park JH, Lee IS. Antioxidative and cytoprotective effects of Artemisia capillaris fractions. Biofactors. 2007;31:43–53. doi: 10.1002/biof.5520310105. [DOI] [PubMed] [Google Scholar]
- 44.Kim ZH, Hwang JW, Lee JH, Kim H, Lim DS, Kang S, Lee HS, Choi YS. Whitening effect of storage protein 2 from silkworm hemolymph. Adv Biosci Biotechnol. 2014;5:758–767. doi: 10.4236/abb.2014.59089. [DOI] [Google Scholar]
- 45.Eberle J, Garbe C, Wang N, Orfanos CE. Incomplete expression of the tyrosinase gene family (tyrosinase, TRP-1, and TRP-2) in human malignant melanoma cells in vitro. Pigment Cell Res. 1995;8:307–313. doi: 10.1111/j.1600-0749.1995.tb00679.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.