Abstract
Background:
Selenium is an essential trace element obtained through diet that plays a critical role in DNA synthesis and protection from oxidative damage. Selenium intake and polymorphisms in selenoproteins have been linked to the risk of certain cancers though data for glioma are sparse.
Methods:
In a case-control study of glioma, we examined the associations of selenium in toenails and genetic variants in the selenoenzyme pathway with the risk of glioma and patient survival. A total of 423 genetic variants in 29 candidate genes in the selenoenzyme pathway were studied in 1,547 glioma cases and 1,014 healthy controls. Genetic associations were also examined in the UK Biobank cohort comprised of 313,868 persons with 322 incident glioma cases. Toenail selenium was measured in a subcohort of 300 glioma cases and 300 age-matched controls from the case-control study.
Results:
None of the 423 variants studied were consistently associated with glioma risk in the case-control and cohort studies. Moreover, toenail selenium in the case-control study had no significant association with glioma risk (p trend = 0.70) or patient survival among 254 patients with high grade tumors (p trend = 0.70).
Conclusion:
The present study offers no support for the hypothesis that selenium plays a role in the onset of glioma or patient outcome.
Keywords: glioma, selenium, single nucleotide polymorphism, case-control study, UK Biobank
Introduction
Gliomas represent the majority of primary intracranial tumors; approximately 80% of malignant brain tumors arise from the glial cells of the brain (1). Mortality from glioma is high with only 5% survival at 5 years in glioblastoma multiforme (GBM), the most aggressive and common subtype of glioma (WHO Grade IV) (1). Gliomas occur more frequently in males, and in Caucasians when compared to other racial groups (2). Genetic susceptibility (3), as well as rare Mendelian disorders including neurofibromatosis, tuberous sclerosis, and Li-Fraumeni syndrome also contributes to disease (4–6). A history of chicken pox, allergies, or atopic disease has a protective association with glioma suggesting that the immune response may play a role in glioma development (7, 8). A prolonged adolescent growth phase is positively associated with glioma risk (9), as is a higher BMI during adolescence (10), and a later age at menarche in women (11, 12). The only known environmental risk factor is ionizing radiation (13). The role of diet in glioma including trace elements such as selenium is poorly studied.
Selenium (Se) is an essential trace element obtained through diet that plays a critical role in reproduction, thyroid hormone metabolism, DNA synthesis, and protection from oxidative damage and infection (14). Se is naturally present in many foods and is also available as a dietary supplement. Concentrations of Se in foods vary with Se in the soil in which food is produced (14). Toenail concentrations of Se offer a useful measure of dietary exposure and reflect long-term dietary intake (15). In the brain, Se and the selenoproteins play a central role in brain function and neuroprotection (16–19). Se stores in the body reflect not only diet but GI absorption and tissue uptake, and vary according to alcohol consumption and cigarette smoking which may deplete Se stores in the body by contributing to oxidative stress (20).
Epidemiologic studies have suggested a protective association between Se and cancer risk though data are inconsistent (21). A Cochrane meta-analysis of prospective observational studies showed lower cancer incidence among patients with higher Se exposure for cancers of the prostate, gastric cardia, bladder, and lung, but not for breast or colon (21). In contrast, randomized clinical trials have not found evidence that Se supplementation is associated with risk reduction for primary liver cancer, non-melanoma skin cancer, basal cell carcinoma, squamous cell carcinoma, prostate cancer, lung, bladder, or colorectal cancer, and with cancer-related mortality; it is speculated that supplementation may not reduce cancer in persons already replete for Se as would apply to the majority of persons in the US (21). Two studies have examined Se levels and brain tumor risk in human populations. One small study (N=22) found no difference in Se levels in cerebrospinal fluid of patients with glioma as compared to patients with benign tumors of the brain (meningioma), or diagnosed with hydrocephalus, arterial malformation, aneurysm, or headaches (22). Another study examined serum levels of Se in patients with malignant brain tumors (n=139) compared to healthy adult individuals (n=294) and found lower Se serum levels in the glioma patients (23); however, as concentrations of Se in blood reflect recent Se intake (24), Se in the serum of cases may have reflected recent changes in diet related to the glioma diagnosis.
Selenium’s actions in the body are exerted through its incorporation as the amino acid selenocysteine into 25 selenoproteins thought to exert most of the functions of Se in the body (14). Nearly all of the well-characterized selenoproteins possess a role in antioxidant function and redox status in the cell (25). A number of other genes exert effects on bioavailability and action of Se in the cell. Among them, SEPSECS is involved in the regulation of selenoprotein synthesis (26). SEPHS1 is one of two selenophosphate synthases in mammals; unlike SEPSH2, a known selenoprotein, the function of SEPHS1 has not been determined though it is hypothesized to play a role in cell differentiation and proliferation (27). Interactions of SPECSECS and SEPHS1 with SEPHS2 play a role in selenocysteine biosynthesis (28). GCLC and GCLM are subunits of GCL (glutamate cysteine ligase) which is the first rate-limiting enzyme of glutathione biosynthesis (29). Single-nucleotide polymorphisms (SNP) in selenium pathway genes have been linked to the risk of colorectal, prostate, lung or breast cancers (30) but are poorly studied in glioma.
The aim of this study was to investigate the association between toenail Se concentrations and genetic variants in selenoproteins and related genes in relation to glioma risk and survival in a US case-control study. Associations with genetic variants were also investigated in the UK Biobank cohort (31).
Subjects and Methods
Study Populations
Case-Control Study –
The study population included persons enrolled in a case-control study of glioma risk factors (GliomaSE) (11). In brief, cases were aged 18 years or older with a recently diagnosed (within 3 months of recruitment) primary intracranial glioma. Cases were recruited at neurosurgery and neuro-oncology clinics at major medical centers throughout the southeastern United States, which included: Moffitt Cancer Center in Tampa, Florida; Vanderbilt University Medical Center in Nashville, Tennessee; University of Alabama at Birmingham; Emory University in Atlanta, Georgia; and the Kentuckiana Cancer Institute in Louisville, Kentucky. Among eligible glioma patients, 87% were enrolled a median of 1 month following initial diagnosis (IQR: 2 – 7 weeks). Controls were recruited from friends and non-blood related associates of the cases in the study, as well as residents from the same communities as the cases identified in white page listings. Of eligible households contacted, approximately 50% yielded a participant for the study. For cases and controls, a structured interview was administered by a member of the research team to collect information on demographics, medical history, and potential risk factors of glioma. All subjects provided oral DNA as a basis for genetic investigations (32, 33). Subjects were also asked to provide toenail samples for studies of trace elements in glioma risk (11). All subjects were genotyped using the Affymetrix ‘UKBiobank’ array (http://www.ukbiobank.ac.uk/scientists-3/uk-biobank-axiom-array/) that offers genome-wide coverage including the 25 selenoenzymes and other selenium pathway genes of interest. The study was approved by the institutional review board at each participating center.
Cohort Study –
The UKBiobank (UKB) cohort was employed to study associations of genes of interest with glioma incidence. The full UKB cohort consists of 502,619 subjects, ages 40 to 69, recruited from 2006 to 2010 (31). All subjects were genotyped using the Affymetrix ‘UKBiobank’ array with anonymized genotype and descriptive data for each subject downloaded by investigators under an approved protocol (Application #16944). A diagnosis of glioma was determined based on ICD-9 and ICD-10 codes provided by the National Health Service (NHS) Central Registers, with follow up through November 30, 2014 for England and Wales residents and December 31, 2014 for Scotland residents (31). The current study is based on 313,868 UKB nongenetically related cohort members with no history of cancer at baseline (other than non-melanoma skin), generating 322 incident glioma cases. Written consent was obtained at recruitment.
Toenail Selenium Measurement
The substudy of trace metals in glioma has been previously described (11). Toenail samples were analyzed in a sample of 300 cases and 300 age, sex, and state matched controls from the case-control study. Toenail samples were harvested a median of 24 days and a maximum of 88 days following glioma diagnosis (10th-90th percentile range: 10–44 days). Toenail clippings were analyzed by instrumental neutron activation analysis (NAA) at the University of Missouri Reactor. The toenail samples were analyzed in 3 separate batches, each containing 100 cases and 100 matched controls. Matched case-control pairs were handled identically in each analytical run, with laboratory personnel blinded to case-control status. All toenail clippings prior to analysis by sonication in 10% v/v nitric acid and deionized water. To ensure quality control, samples were created in duplicate and analyzed to ensure consistency, as well as with 5 NIST SRM 1577 Bovine liver quality control samples. The Se concentration in the samples ranged from 0.60 μg/g to 4.6 μg/g, and the mean concentration of Se was 0.879 μg/g. The average coefficient of variation (CV) was 3.7% among sample pairs; if a duplicate pair had a CV of greater than 5%, both samples were re-analyzed (27 pairs total). Toenails were not available for Se measurement in the UKB cohort.
Toenail Se Analyses
The association between Se levels in the toenail and glioma risk was estimated with odds ratios (OR) and 95% confidence intervals (CI) using logistic regression. We examined risk associated with increasing quartile of nail Se as defined in the controls, with the lowest quartile considered the referent group. To test for linear trend, an ordinal term reflecting increasing quartile of toenail Se was included in regression models. All regression models included terms for age (5 year age groups), state of residence at diagnosis, sex, and batch. As use of dandruff shampoo can result in abnormally high levels of Se in the nails (34, 35), we excluded subjects with outlying Se concentrations of greater than 2 μg/g (3 cases and 4 controls).
Cox proportional hazards regression was used to estimate hazard ratios (HR) and 95% CIs for the association of nail Se levels with mortality in patients with high grade glioma (HGG), which included grade III and grade IV glioma. Regression models included terms for age, sex, and histology (GBM, high grade astrocytoma, high grade pure/mixed oligodendroglioma), and Karnofsky Performance Score. Associations were considered among patients overall and among the subgroups of patients treated with the Stupp Protocol (36) defining standard of care (SOC). Two HGG cases with outlying Se concentrations were excluded from survival analyses.
Genotyping Association Analyses
The list of all selenoproteins and selenium metabolism genes included in the present analyses (17) are found in a supplemental table. Searching between the GRCh37 gene coordinates, we identified a total of 423 variants in 29 candidate genes tiled on the Affymetrix ‘UKBiobank’ array. Prior to analysis, SNPs with a MAF < 0.05, HWE P-value < 10−5 in controls and a call rate < 95% were removed, as were samples with a call rate < 95%. These quality control measures were applied separately to each study and all analyses were restricted to self-identified Caucasians. Logistic regression under an additive model was used to determine association between genetic variants and glioma risk. To control for multiple testing, Bonferroni correction was independently applied. Validation was sought at P-value < 0.05. Combined p-values between the two studies were calculated based on the unadjusted p-values using the Fisher method (37). Genetic analyses included all histologic subtypes of glioma combined; histologic subgroup (GBM versus nonGBM) specific analyses were conducted in the case-control study, only.
Results
The case-control study population consisted of cases aged 18–87 and controls aged 20–85, with a median age of 54 for both case and control groups (Table 1). The majority of the population was male (61.7%) and predominantly Caucasian (~95%). Glioma cases in the study population had a diagnosis of GBM (194, 64.7%) or a lower grade ‘(nonGBM’) subtype (106, 35.3%).
Table 1.
Descriptive Statistics of study population
| Characteristic | Toenail Samples | Genotype Samples | |||||
|---|---|---|---|---|---|---|---|
| Cases (n=300) | Controls (n=300) | p-value* | Cases (n=1547) | Controls (n=1015) | p-value | ||
| Age | Median | 55 | 55 | Matched | 55 | 56 | 0.002 |
| Range | (18–88) | (20–86) | (18–92) | (18–88) | |||
| Sex, N (%) | Female | 118 (60.7) | 118 (60.7) | Matched | 642 (41.5) | 488 (48.1) | 0.001 |
| Male | 182 (39.3) | 182 (39.3) | 905 (58.5) | 527 (51.9) | |||
| Race, N (%) | Caucasian | 286 (95.3) | 280 (93.3) | 0.29 | 1547 (100) | 1015 (100) | ---- |
| Non-Caucasian | 14 (4.7) | 20 (6.7) | 0 (0) | 0 (0) | |||
| State of Residence, N (%) | Florida | 152 (50.7) | 154 (51.3) | Matched | 683 (44.1) | 347 (34.2) | <0.001 |
| Alabama | 71 (23.7) | 70 (23.3) | 274 (17.7) | 130 (12.8) | |||
| Georgia | 35 (11.7) | 35 (11.7) | 114 (7.4) | 111 (10.9) | |||
| Tennessee | 26 (8.7) | 27 (9.0) | 221 (14.3) | 264 (26.0) | |||
| Other | 16 (5.2) | 14 (4.7) | 255 (14.5) | 163 (16.1) | |||
| Toenail Selenium (μg/g) | Median | 0.87 | 0.87 | 0.28 | ---- | ---- | ---- |
| Range | (0.56–1.88) | (0.60–1.79) | ---- | ---- | |||
| Histology, N (%) | Glioblastoma | 194 (64.7) | ---- | ---- | 941 (60.8) | ---- | ---- |
| Non-GBM | 106 (35.3) | ---- | 606 (39.2) | ---- | |||
t-test conducted for toenail selenium analysis, chi-square test conducted for race
Case-control results for nail Se and glioma risk are shown in Table 2. When considering a referent group for Se concentration of less than 0.742 μg/g, no significant association emerged between Se concentration and glioma risk, after adjustment for age, state of residence, sex, and batch. A nonsignificant inverse association was observed for toenail selenium above 1.037 μg/g (OR=0.70; 95% CI 0.37, 1.32), though without evidence of dose response: the overall trend for Se concentration in toenails versus glioma risk was nonsignificant (OR=0.96; 95% CI 0.80, 1.16). Results were similarly null among males and females, separately (not shown).
Table 2.
Association between toenail selenium concentration and risk of glioma excluding Se levels > 2 (μg/g)
| ALL SAMPLES (N=593) | |||||
|---|---|---|---|---|---|
| Se (μg/g) | Cases N (%) | Controls N (%) | Crude OR (95% CI) | Multivariable OR (95% CI)* | p-value |
| <0.742 | 40 (13) | 39 (13) | Ref | Ref | ---- |
| 0.743–0.890 | 119 (40) | 125 (42) | 0.93 (0.56–1.54) | 0.92 (0.55–1.54) | 0.76 |
| 0.891–1.037 | 104 (35) | 86 (29) | 1.18 (0.70–1.99) | 1.19 (0.70–2.02) | 0.53 |
| 1.038–2 | 34 (11) | 46 (16) | 0.72 (0.39–1.35) | 0.70 (0.37–1.32) | 0.27 |
|
| |||||
| Trend | 0.96 (0.80–1.16) | 0.70 | |||
Unconditional logistic regression model adjusted for age (5 year groups), state of residence (FL, AL, GA, TN, other), sex, and batch.
Associations of nail Se and HGG outcome are shown in Table 3. Adjusting for age, sex, and histology, no statistical significant associations were observed with increasing nail Se concentration and patient survival (HR=1.06; 95% CI 0.89, 1.26 with increasing quartile of Se). Restricting to HGG patients treated with the SOC yielded similarly null results (not shown).
Table 3.
Association between toenail Se concentration and risk of death in high grade tumors (N=254)
| Se (μg/g) | No. Deaths | Crude HR (95% CI) | (n=254) Multivariate HR (95% CI)* | p-value | (n=210) Multivariate HR (95% CI)** | p-value |
|---|---|---|---|---|---|---|
| <0.742 | 24 | Ref | Ref | ---- | Ref | ---- |
| 0.743–0.890 | 70 | 1.22 (0.77, 1.94) | 1.17 (0.73, 1.87) | 0.523 | 0.98 (0.59, 1.62) | 0.92 |
| 0.891–1.037 | 59 | 1.25 (0.78, 2.01) | 1.38 (0.85, 2.25) | 0.20 | 1.18 (0.69, 2.00) | 0.54 |
| >1.037 | 21 | 1.07 (0.59, 1.92) | 1.08 (0.59, 1.98) | 0.81 | 0.99 (0.52, 1.88) | 0.97 |
|
| ||||||
| Trend | 1.06 (0.89, 1.26) | 0.50 | 1.04 (0.86, 1.25) | 0.70 | ||
Model adjusted for age (5 year groups), gender, and histology (GBM, high grade astrocytoma, high grade pure/mixed oligodendroglioma).
Restricted to cases with KPS data. Model adjusted for age (5 year groups), gender, histology (GBM, high grade astrocytoma, high grade pure/mixed oligodendroglioma), and KPS group
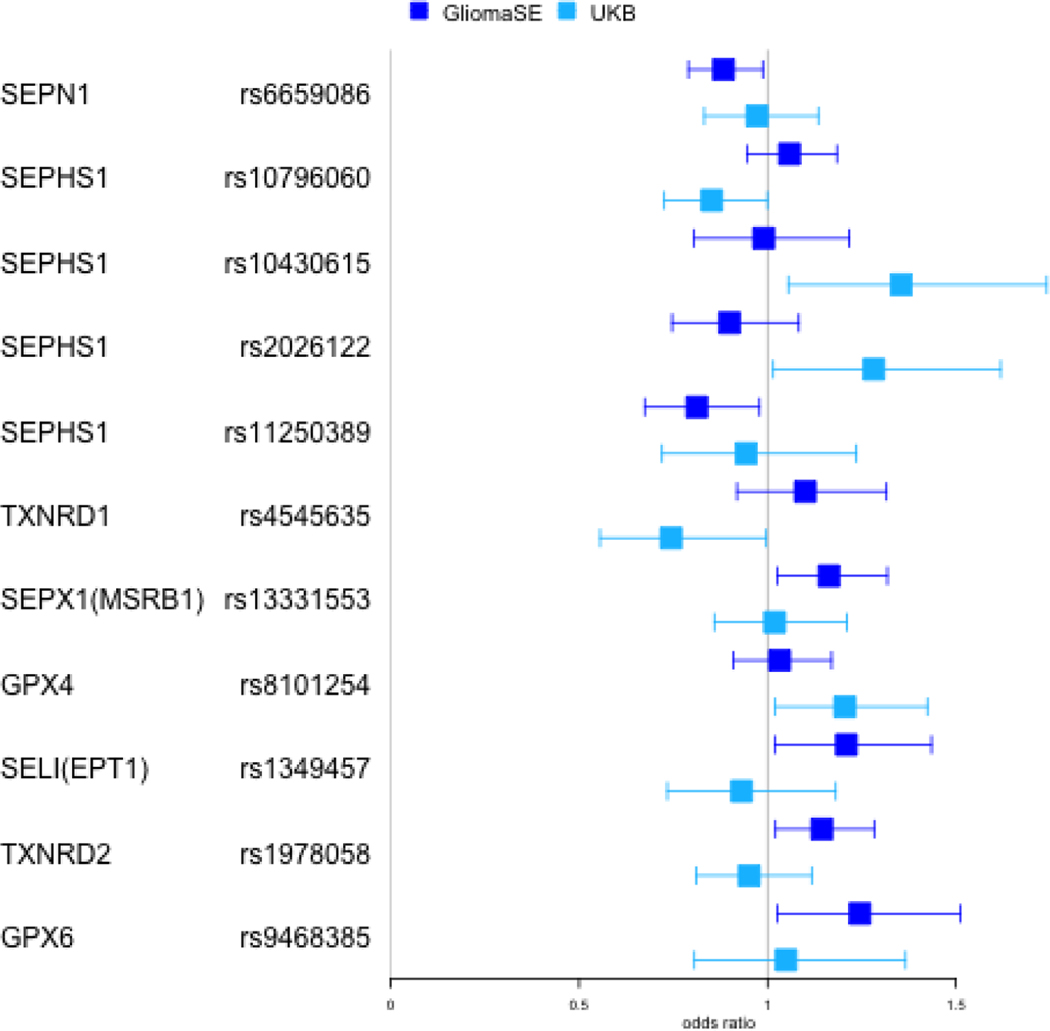
Table 4 summarizes results for genetic association analyses in the case-control study and UKBiobank cohort. Of the initial 423 SNPs in the 29 candidate genes, 198 passed QC filters in both studies. In the case-control study, 6 SNPs in 6 different genes were significantly associated with glioma risk. The minor allele for rs6659086 (SEPN1) and rs11250389 (SEPHS1) had a protective association with glioma (OR=0.88; 95% CI 0.79, 0.99; p=0.03 and OR=0.81; 95% CI 0.67, 0.98; p=0.03, respectively). Conversely an increased risk was observed with each additional copy of the minor allele for rs1349457 (SELI; OR=0.88; 95% CI 1.02, 1.44; p=0.03), rs9468385 (GPX6; OR=1.25; 95% CI 1.03, 1.51; p=0.03), rs13331553 (SEPX1; OR=1.16; 95% CI 1.03, 1.32; p=0.02) and rs1978058 (TXNRD2; OR=1.14; 95% CI 1.02,1.28; p=0.02). None of these variants were validated in analyses based on the UKB cohort. A total of 5 other variants within the UKB cohort were associated with glioma. Among them, rs10430615, rs1079606 and rs2026122 are located in SEPHS1, with the minor allele conferring decreased glioma risk in rs10796060 (OR=0.85; 95% CI 0.73, 1.00; p=0.05) and the minor allele conferring increased risk in rs10430615 and rs2026122 (OR=1.36; 95% CI 1.06, 1.74; p=0.02 and OR=1.28; 95% CI 1.01, 1.62; p=0.04, respectively). A decreased risk of glioma was also associated with the minor allele in rs4545635 (TXNRD1, OR=0.75; 95% CI 0.56, 1.00; p=0.05) and an increased risk was associated with the minor allele in rs8101254 (GPX4, OR=1.20; 95% CI 1.02, 1.42; p=0.03). None of the combined p-values showed significance and no association in either study retained significance after adjusting for multiple testing (not shown). Results by glioma subtype for the case-control study can be found in Supplemental Table 2; no individual variant retained statistical significance after adjustment for multiple testing.
Table 4.
Variants with unadjusted p-value < 0.05 in either study
| Study Cohort | UKB Cohort | Combined | ||||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| Gene | Variant | OR (95% CI) | p-value | OR (95% CI) | p-value | p-value |
| SEPN1 | rs6659086 | 0.88 (0.79–0.99) | 0.03 | 0.97 (0.83–1.14) | 0.72 | 0.10 |
| SELI(EPT1) | rs1349457 | 1.12 (1.02–1.44) | 0.03 | 0.93 (0.73–1.18) | 0.56 | 0.09 |
| GPX6 | rs9468385 | 1.25 (1.03–1.51) | 0.03 | 1.05 (0.81–1.37) | 0.72 | 0.10 |
| SEPHS1 | rs11250389 | 0.81 (0.67–0.98) | 0.03 | 0.94 (0.72–1.24) | 0.67 | 0.10 |
| rs10430615 | 0.99 (0.81–1.22) | 0.92 | 1.36 (1.06–1.74) | 0.02 | 0.09 | |
| rs10796060 | 1.06 (0.95–1.18) | 0.33 | 0.85 (0.73–1.00) | 0.05 | 0.08 | |
| rs2026122 | 0.90 (0.75–1.08) | 0.26 | 1.28 (1.01–1.62) | 0.04 | 0.06 | |
| TXNRD1 | rs4545635 | 1.10 (0.92–1.32) | 0.30 | 0.75 (0.56–1.00) | 0.05 | 0.08 |
| SEPX1(MSRB1) | rs13331553 | 1.16 (1.03–1.32) | 0.02 | 1.02 (0.86–1.21) | 0.83 | 0.08 |
| GPX4 | rs8101254 | 1.03 (0.91–1.17) | 0.63 | 1.20 (1.02–1.42) | 0.03 | 0.09 |
| TXNRD2 | rs1978058 | 1.14 (1.02–1.28) | 0.02 | 0.95 (0.81–1.12) | 0.54 | 0.06 |
Discussion
In the present study, Se in toenails was not associated with risk or survival in glioma. Several germline variants in pathways involved in selenoprotein metabolism were associated with risk of glioma in either the case-control or cohort study. However, none were individually significant after adjustment for FDR or in meta-analyses combining the two studies. Based on these results, neither toenail Se nor functional SNPs among genes involved in selenoprotein metabolism play a significant role in the risk of glioma onset or patient survival.
While other studies have examined the association of other trace elements and glioma (12, 38), this study is novel in its consideration of the role of toenail Se levels, as well as the variants involved in selenoprotein metabolism in association with glioma risk and survival. Se has been implicated as having a protective role in colorectal and prostate cancer (39, 40), and low Se levels have been associated with neurodegenerative disease (41, 42) as well as tumor progression (43). An in vitro study of Se in human brain tumor cell lines showed growth inhibition and apoptosis in glioma cells treated with Se (44), suggesting that higher levels of pre-diagnostic Se might have a protective role in gliomagenesis or reduce aggressiveness of tumors. However, the present data offered no support for a salutary influence of selenium on either outcome in glioma.
The present study offered no support for a role of selenium pathway germline variation in glioma. Several of the members of the selenoprotein family harbor functional variants that affect Se bioavailability and have been associated with susceptibility to specific cancers (45, 46). Among them, SNPs in selenoprotein P thioredoxin reductases 15kDa, GPx-1, GPx-3, GPx-4, and selenoprotein S were evaluated in the present study with overall null results (45, 46). Several variants in the selenoenzyme pathway linked to colon or rectal cancer in one study (47) were associated with overall risk of glioma (rs6659086) or with nonGBM (rs9637365) in the case-control study; however, they were not validated in the cohort study analysis.
Rajaraman et al examined the relationship between the innate immune system and glioma (48), and reported two statistically significant SNPs (at p<0.01) in SELP. Of these, one SNP (rs3917727) was in linkage disequilibrium (LD) (LD=0.71) with a SNP (rs3917687) associated with nonGBM glioma in the case-control study (OR=0.86; 95% CI 0.74, 0.99; p=0.03). However, the association was not observed in the UKB cohort and may be due to chance.
A previous GWAS found a significant association between genetic variants in the selenoprotein TXNRD1 and toenail Se levels (49). This association could potentially explain the significant risk association observed in the UK Biobank with rs4545635 which is in LD (LD=0.61) with the GWAS SNP associated with toenail selenium (rs7975161). However, this SNP was not associated with glioma risk in the case-control study analysis. Furthermore, among 480 persons with genotyping data that were included in the toenail risk analyses, we found no association between toenail Se and the GWAS linked SNP (rs4545635) nor any of the SNPs associated with glioma risk in either study (Table 4), overall, or among the 192 controls only, after adjustment for FDR (not shown).
Selenoprotein P (SePP) is the major plasma selenoprotein with both transport and antioxidant functions (17). Two functional SNPs in SePP, rs7579 and rs3877899, have been shown to influence levels of SePP isoforms in plasma as well as risk of colorectal cancer (50). However, in the present study, neither rs3877899 nor a SNP in high LD with rs7579 (rs7717985; LD = 0.94) was associated with glioma risk in either the case-control or cohort study analysis.
The present study had a number of strengths and limitations. The case-control study was based on subjects residing in the southeastern US including the state of FL where soil Se is relatively low compared to other regions of the US (51) thus potentially increasing range of toenail Se in participants and our ability to detect associations contrasting extreme levels of exposure. The study of genetic association in selenoenzyme pathway variants was based on a large case-control study for a rare tumor and we were able to attempt to validate associations in an independent prospective cohort study in which genotyping of subjects was performed based on the identical array. Glioma cases were rapidly ascertained and enrolled, generally within 2 months of diagnosis and, as toenail Se reflects dietary intake 6–12 months in the past (34), Se measures in most cases would have predated changes in diet that occurred from the disease or its treatment. Within the 300 cases studied, we found no correlation between toenail Se concentrations and the number of days between diagnosis and toenail collection (Pearson r = 0.03; p=0.65), which ranged from 0 to 88 days; hence, any delay to nail collection in cases would not have affected results. On the other hand, if there is a long latency to glioma diagnosis, Se measured in toenail samples may not have reflected exposure during an etiologically relevant timeframe in glioma. In addition, the sample size for toenail Se analyses was relatively limited (300 cases and 300 controls) and we lacked power to evaluate associations by glioma subtype or according to exposures like smoking or obesity associated with oxidative stress that may increase Se requirements (52–54). Se levels vary by demographic and lifestyle factors (53) and low response rates in controls could potentially have biased selenium associations. Finally, previous studies show that selenium is efficiently retained in the brain even in conditions of Se deficiency (19); hence, it possible that only severe Se depletion not typically encountered in the US has an impact on brain tumor development.
In summary, the present results do not support a role for Se levels in glioma risk or patient outcome. None of the genetic variants were associated with overall glioma risk in both studies; however, several of the variants had suggestive findings. Prospective studies would be of value for definitive study of dietary Se in relation to glioma risk.
Supplementary Material
Figure 1:
Odds ratios for genetic variants with unadjusted p value < 0.05
Odds ratios and 95% confidence intervals in both GliomaSE and UKB for the 11 variants with an unadjusted p-value < 0.05 in either study. Effect estimates for GliomaSE are in dark blue and light blue for UKB.
Acknowledgement
The authors would like to thank the participants and their families, as well as the clinicians and research staff from participating medical centers, for their contributions.
Funding
The research was supported by the National Institutes of Health [grant number R01 CA116174 and R03 CA171612]. The work is based in part on the UK Biobank Resource under application number 16944.
References:
- 1.Ostrom QT, Bauchet L, Davis FG, Deltour I, Fisher JL, Langer CE, et al. The epidemiology of glioma in adults: a “state of the science” review. Neuro Oncol. 2014;16:896–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kohler BA, Ward E, McCarthy BJ, Schymura MJ, Ries LA, Eheman C, et al. Annual report to the nation on the status of cancer, 1975–2007, featuring tumors of the brain and other nervous system. J Natl Cancer Inst. 2011;103:714–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Melin BS, Barnholtz-Sloan JS, Wrensch MR, Johansen C, Il’yasova D, Kinnersley B, et al. Genome-wide association study of glioma subtypes identifies specific differences in genetic susceptibility to glioblastoma and non-glioblastoma tumors. Nat Genet. 2017;49:789–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Farrell CJ, Plotkin SR. Genetic causes of brain tumors: neurofibromatosis, tuberous sclerosis, von Hippel-Lindau, and other syndromes. Neurol Clin. 2007;25:925–46, viii. [DOI] [PubMed] [Google Scholar]
- 5.Jemal A, Ward EM, Johnson CJ, Cronin KA, Ma J, Ryerson B, et al. Annual Report to the Nation on the Status of Cancer, 1975–2014, Featuring Survival. J Natl Cancer Inst. 2017;109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rice T, Lachance DH, Molinaro AM, Eckel-Passow JE, Walsh KM, Barnholtz-Sloan J, et al. Understanding inherited genetic risk of adult glioma - a review. Neurooncol Pract. 2016;3:10–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amirian ES, Scheurer ME, Zhou R, Wrensch MR, Armstrong GN, Lachance D, et al. History of chickenpox in glioma risk: a report from the glioma international case-control study (GICC). Cancer Med. 2016;5:1352–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amirian ES, Zhou R, Wrensch MR, Olson SH, Scheurer ME, Il’yasova D, et al. Approaching a Scientific Consensus on the Association between Allergies and Glioma Risk: A Report from the Glioma International Case-Control Study. Cancer Epidemiol Biomarkers Prev. 2016;25:282–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Little RB, Nabors LB, Olson JJ, Thompson ZJ, Rozmeski CM, LaRocca RV, et al. Older age at the completion of linear growth is associated with an increased risk of adult glioma. Cancer Causes Control. 2017;28:709–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Little RB, Madden MH, Thompson RC, Olson JJ, Larocca RV, Pan E, et al. Anthropometric factors in relation to risk of glioma. Cancer Causes Control. 2013;24:1025–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anic GM, Madden MH, Thompson RC, Nabors LB, Olson JJ, Larocca RV, et al. Toenail iron, genetic determinants of iron status, and the risk of glioma. Cancer Causes Control. 2013;24:2051–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krishnamachari B, Il’yasova D, Scheurer ME, Bondy ML, Wrensch M, Davis FG. A pooled multisite analysis of the effects of female reproductive hormones on glioma risk. Cancer Causes Control. 2014;25:1007–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Braganza MZ, Kitahara CM, Berrington de Gonzalez A, Inskip PD, Johnson KJ, Rajaraman P. Ionizing radiation and the risk of brain and central nervous system tumors: a systematic review. Neuro Oncol. 2012;14:1316–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Winkel LH, Johnson CA, Lenz M, Grundl T, Leupin OX, Amini M, et al. Environmental selenium research: from microscopic processes to global understanding. Environ Sci Technol. 2012;46:571–9. [DOI] [PubMed] [Google Scholar]
- 15.Garland M, Morris JS, Rosner BA, Stampfer MJ, Spate VL, Baskett CJ, et al. Toenail trace element levels as biomarkers: reproducibility over a 6-year period. Cancer Epidemiol Biomarkers Prev. 1993;2:493–7. [PubMed] [Google Scholar]
- 16.Brauer AU, Savaskan NE. Molecular actions of selenium in the brain: neuroprotective mechanisms of an essential trace element. Rev Neurosci. 2004;15:19–32. [DOI] [PubMed] [Google Scholar]
- 17.Moghadaszadeh B, Beggs AH. Selenoproteins and their impact on human health through diverse physiological pathways. Physiology (Bethesda). 2006;21:307–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Savaskan NE, Ufer C, Kuhn H, Borchert A. Molecular biology of glutathione peroxidase 4: from genomic structure to developmental expression and neural function. Biol Chem. 2007;388:1007–17. [DOI] [PubMed] [Google Scholar]
- 19.Schweizer U, Schomburg L, Savaskan NE. The neurobiology of selenium: lessons from transgenic mice. J Nutr. 2004;134:707–10. [DOI] [PubMed] [Google Scholar]
- 20.Park K, Rimm E, Siscovick D, Spiegelman D, Morris JS, Mozaffarian D. Demographic and lifestyle factors and selenium levels in men and women in the U.S. Nutr Res Pract. 2011;5:357–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vinceti M, Dennert G, Crespi CM, Zwahlen M, Brinkman M, Zeegers MP, et al. Selenium for preventing cancer. Cochrane Database Syst Rev. 2014:CD005195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.El-Yazigi A, Al-Saleh I, Al-Mefty O. Concentrations of Ag, Al, Au, Bi, Cd, Cu, Pb, Sb, and Se in cerebrospinal fluid of patients with cerebral neoplasms. Clin Chem. 1984;30:1358–60. [PubMed] [Google Scholar]
- 23.Philipov P, Tzatchev K. Selenium concentrations in serum of patients with cerebral and extracerebral tumors. Zentralbl Neurochir. 1988;49:344–7. [PubMed] [Google Scholar]
- 24.Sunde RA, Ross A, Caballero B, Cousins R, Tucker K, Ziegler T. Modern Nutrition in Health and Disease. 11th ed: Lippincott Williams & Wilkins; 2012. [Google Scholar]
- 25.Johansson L, Gafvelin G, Arner ES. Selenocysteine in proteins-properties and biotechnological use. Biochim Biophys Acta. 2005;1726:1–13. [DOI] [PubMed] [Google Scholar]
- 26.Li JL, Li HX, Gao XJ, Zhang JL, Li S, Xu SW, et al. Priority in selenium homeostasis involves regulation of SepSecS transcription in the chicken brain. PLoS One. 2012;7:e35761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tobe R, Carlson BA, Huh JH, Castro NP, Xu XM, Tsuji PA, et al. Selenophosphate synthetase 1 is an essential protein with roles in regulation of redox homoeostasis in mammals. Biochem J. 2016;473:2141–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oudouhou F, Casu B, Dopgwa Puemi AS, Sygusch J, Baron C. Analysis of Novel Interactions between Components of the Selenocysteine Biosynthesis Pathway, SEPHS1, SEPHS2, SEPSECS, and SECp43. Biochemistry. 2017;56:2261–70. [DOI] [PubMed] [Google Scholar]
- 29.Lu SC. Glutathione synthesis. Biochim Biophys Acta. 2013;1830:3143–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meplan C, Hesketh J. Selenium and cancer: a story that should not be forgotten-insights from genomics. Cancer Treat Res. 2014;159:145–66. [DOI] [PubMed] [Google Scholar]
- 31.Collins R. What makes UK Biobank special? Lancet. 2012;379:1173–4. [DOI] [PubMed] [Google Scholar]
- 32.Egan KM, Nabors LB, Olson JJ, Monteiro AN, Browning JE, Madden MH, et al. Rare TP53 genetic variant associated with glioma risk and outcome. J Med Genet. 2012;49:420–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Egan KM, Thompson RC, Nabors LB, Olson JJ, Brat DJ, Larocca RV, et al. Cancer susceptibility variants and the risk of adult glioma in a US case-control study. J Neurooncol. 2011;104:535–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Longnecker MP, Stampfer MJ, Morris JS, Spate V, Baskett C, Mason M, et al. A 1-y trial of the effect of high-selenium bread on selenium concentrations in blood and toenails. Am J Clin Nutr. 1993;57:408–13. [DOI] [PubMed] [Google Scholar]
- 35.Longnecker MP, Stram DO, Taylor PR, Levander OA, Howe M, Veillon C, et al. Use of selenium concentration in whole blood, serum, toenails, or urine as a surrogate measure of selenium intake. Epidemiology. 1996;7:384–90. [DOI] [PubMed] [Google Scholar]
- 36.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–96. [DOI] [PubMed] [Google Scholar]
- 37.R.R. Combining the results of independent studies. Psychological Bulletin. 1978;85:185–93. [Google Scholar]
- 38.Arslan M, Demir H, Arslan H, Gokalp AS, Demir C. Trace elements, heavy metals and other biochemical parameters in malignant glioma patients. Asian Pac J Cancer Prev. 2011;12:447–51. [PubMed] [Google Scholar]
- 39.Li H, Stampfer MJ, Giovannucci EL, Morris JS, Willett WC, Gaziano JM, et al. A prospective study of plasma selenium levels and prostate cancer risk. J Natl Cancer Inst. 2004;96:696–703. [DOI] [PubMed] [Google Scholar]
- 40.Hughes DJ, Fedirko V, Jenab M, Schomburg L, Meplan C, Freisling H, et al. Selenium status is associated with colorectal cancer risk in the European prospective investigation of cancer and nutrition cohort. Int J Cancer. 2015;136:1149–61. [DOI] [PubMed] [Google Scholar]
- 41.Rayman MP. Selenium and human health. Lancet. 2012;379:1256–68. [DOI] [PubMed] [Google Scholar]
- 42.Rita Cardoso B, Silva Bandeira V, Jacob-Filho W, Franciscato Cozzolino SM. Selenium status in elderly: relation to cognitive decline. J Trace Elem Med Biol. 2014;28:422–6. [DOI] [PubMed] [Google Scholar]
- 43.Yakubov E, Buchfelder M, Eyupoglu IY, Savaskan NE. Selenium action in neuro-oncology. Biol Trace Elem Res. 2014;161:246–54. [DOI] [PubMed] [Google Scholar]
- 44.Sundaram N, Pahwa AK, Ard MD, Lin N, Perkins E, Bowles AP Jr. Selenium causes growth inhibition and apoptosis in human brain tumor cell lines. J Neurooncol. 2000;46:125–33. [DOI] [PubMed] [Google Scholar]
- 45.Mathers JC, Meplan C, Hesketh JE. Polymorphisms affecting trace element bioavailability. Int J Vitam Nutr Res. 2010;80:314–8. [DOI] [PubMed] [Google Scholar]
- 46.Hesketh J, Meplan C. Transcriptomics and functional genetic polymorphisms as biomarkers of micronutrient function: focus on selenium as an exemplar. Proc Nutr Soc. 2011:1–9. [DOI] [PubMed] [Google Scholar]
- 47.Slattery ML, Lundgreen A, Welbourn B, Corcoran C, Wolff RK. Genetic variation in selenoprotein genes, lifestyle, and risk of colon and rectal cancer. PLoS One. 2012;7:e37312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rajaraman P, Brenner AV, Butler MA, Wang SS, Pfeiffer RM, Ruder AM, et al. Common variation in genes related to innate immunity and risk of adult glioma. Cancer Epidemiol Biomarkers Prev. 2009;18:1651–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cornelis MC, Fornage M, Foy M, Xun P, Gladyshev VN, Morris S, et al. Genome-wide association study of selenium concentrations. Hum Mol Genet. 2015;24:1469–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Meplan C, Nicol F, Burtle BT, Crosley LK, Arthur JR, Mathers JC, et al. Relative abundance of selenoprotein P isoforms in human plasma depends on genotype, se intake, and cancer status. Antioxid Redox Signal. 2009;11:2631–40. [DOI] [PubMed] [Google Scholar]
- 51.Gustavsson N. Geochemical landscapes of the conterminous United States : new map presentations for 22 elements. Denver, CO: U.S. Dept. of the Interior U.S. Geological Survey, Informations Services distributor; 2001. [Google Scholar]
- 52.Beane Freeman LE, Karagas MR, Baris D, Schwenn M, Johnson AT, Colt JS, et al. Is the inverse association between selenium and bladder cancer due to confounding by smoking? Am J Epidemiol. 2015;181:488–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jain RB, Choi YS. Normal reference ranges for and variability in the levels of blood manganese and selenium by gender, age, and race/ethnicity for general U.S. population. J Trace Elem Med Biol. 2015;30:142–52. [DOI] [PubMed] [Google Scholar]
- 54.Kim Y, Wei J, Citronberg J, Hartman T, Fedirko V, Goodman M. Relation of Vitamin E and Selenium Exposure to Prostate Cancer Risk by Smoking Status: A Review and Meta-Analysis. Anticancer Res. 2015;35:4983–96. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.