Abstract
We investigated the prevalence of spirometric restriction in liver transplantation (LT) candidates and the clinical impacts of restriction. We performed a cross-sectional study within the Pulmonary Vascular Complications of Liver Disease 2 (PVCLD2) study, a multicenter prospective cohort study of patients being evaluated for LT. Patients with obstructive lung disease or missing spirometry or chest imaging were excluded. Patients with and without restriction, defined as a forced vital capacity (FVC) <70% predicted, were compared. Restriction prevalence was 18.4% (63/343). Higher Model for End-Stage Liver Disease–sodium score (odds ratio [OR], 1.06; 95% confidence interval [CI], 1.02–1.11; P = 0.007), the presence of pleural effusions (OR, 3.59; 95% CI, 1.96–6.58; P < 0.001), and a history of ascites (OR, 2.59; 95% CI, 1.26–5.33; P = 0.01) were associated with the presence of restriction, though one-third with restriction had neither pleural effusions nor ascites. In multivariate analysis, restriction was significantly and independently associated with lower 6-minute walk distances (least squares mean, 342.0 [95% CI, 316.6–367.4] m versus 395.7 [95% CI, 381.2–410.2] m; P < 0.001), dyspnea (OR, 2.69; 95% CI, 1.46–4.95; P = 0.002), and lower physical component summary Short Form 36 scores indicating worse quality of life (least squares mean, 34.1 [95% CI, 31.5–36.7] versus 38.2 [95% CI, 36.6–39.7]; P = 0.004). Lower FVC percent predicted was associated with an increased risk of death (hazard ratio, 1.16; 95% CI, 1.04–1.27 per 10-point decrease in FVC percent predicted; P = 0.01). Restriction and abnormal lung function are common in LT candidates; can be present in the absence of an obvious cause, such as pleural effusions or ascites; and is associated with worse exercise capacity, quality of life, and survival.
Dyspnea, reduced exercise capacity, and impaired quality of life are common in patients with cirrhosis and are often attributed to the underlying liver disease or its complications.(1–3) Spirometry is often performed during liver transplantation (LT) evaluation, but clinical implications of abnormal lung function are not well known. In a single-center retrospective study, restrictive ventilatory defects were present in 27% of patients undergoing LT and were associated with increased posttransplant morbidity.(4) Restrictive defects may be related to diffuse parenchymal lung disease (eg, idiopathic pulmonary fibrosis), pleural effusions, extrapulmonary causes (such as ascites and obesity), or respiratory muscle weakness. Although restriction is common in patients with advanced liver disease, its impact on exercise capacity, symptoms, and quality of life are not well understood.
We sought to define the prevalence of spirometric restriction in a prospective multicenter study of LT candidates, to determine the relative contribution of ascites and pleural effusions to restriction, and to assess the clinical impact of restriction on exercise capacity, symptoms, quality of life, and survival. We hypothesized that spirometric restriction would be associated with worse exercise capacity and quality of life and increased dyspnea independent of liver disease severity.
Patients and Methods
STUDY SAMPLE
The Pulmonary Vascular Complications of Liver Disease 2 (PVCLD2) study is a multicenter, prospective cohort study of adult patients with portal hypertension undergoing evaluation for LT or with prevalent portopulmonary hypertension and has been previously described.(5) The inclusion criteria for cohort assembly were the presence of portal hypertension with or without intrinsic liver disease and undergoing an initial evaluation for LT. Patients with active infection, recent gastrointestinal bleeding (<2 weeks from date of evaluation), or a history of prior liver or lung transplantation were excluded. The study sample for this analysis was drawn from 425 patients undergoing initial LT evaluation enrolled at the University of Pennsylvania, Mayo Clinic, and University of Texas at Houston between 2013 and 2017. For this analysis, we excluded patients who did not have spirometry performed, those who had obstructive ventilatory defects (forced expiratory volume in 1 second [FEV1]/forced vital capacity [FVC] <0.7 with FEV1 <80% predicted) and patients who did not have chest imaging (chest radiograph or chest computed tomography [CT]) available for review. Patients with obstructive disease were excluded due to the possibility of air trapping leading to reductions in FVC.
STUDY PROCEDURES
Patients being seen in LT evaluation clinics were screened for eligibility at each study site. Informed consent was obtained from eligible patients, who were then scheduled for research assessment, which included a medical history analysis and physical examination (including assessment of dyspnea), completion of the Medical Outcomes Study Short Form 36 (SF-36), anthropometrics, pulse oximetry, 6-minute walk distance (6MWD) testing, including the modified Borg dyspnea scale to assess breathlessness, arterial blood gas (ABG) sampling, spirometry, and contrast-enhanced transthoracic echocardiogram.(6,7)
Clinical data were collected from the medical record and formal patient interviews. Clinical laboratory results obtained closest to the date of the study visit were recorded. Model for End-Stage Liver Disease–sodium (MELD-Na) scores were calculated using the following equations that include creatinine, bilirubin, international normalized ratio (INR), and serum sodium (Na): Model for End-Stage Liver Disease (MELD) score = 10 × ([0.957 × ln(creatinine)] + [0.378 × ln(bilirubin)] + [1.12 × ln(INR)]) + 6.43 and MELD-Na score = MELD score − serum Na − (0.025 × MELD score × [140 − serum Na]) + 140.
Spirometry was performed according to American Thoracic Society (ATS)–European Respiratory Society (ERS) recommendations. A minimum of 3 efforts with no acceptability errors and at least 2 with repeatability per ATS-ERS standards (FVC within 150 mL of largest, FEV1 within 150 mL of largest, and peak flow within 15% of largest) were required.(8) Testing was continued until the above criteria were met, a total of 8 tests were performed, or the patient was unable to continue testing.
We compared patients with and without restriction, defined as an FVC <70% predicted. Sex-, age-, and race-specific prediction equations were used to determine percent predicted volumes based on spirometric reference values derived from the National Health and Nutrition Survey (NHANES) III for the primary analysis with a multiplication factor of 0.88 for Asians.(9) We also performed a sensitivity analysis where restriction was defined using the lower limit of normal (LLN) as calculated from NHANES prediction equations with a similar adjustment of 0.88 for Asians.(9)
Chest and abdominal imaging, ie, CT or chest radiographs, was performed for clinical indications at the individual study sites within 1 year of study enrollment and was interpreted locally. The presence of pleural effusions, interstitial lung disease, pulmonary artery enlargement, cardiomegaly, and ascites were assessed.
Vital status and outcomes (hospitalization or LT) were assessed at follow-up telephone interviews every 6 months. LT was treated as a competing risk in the survival analysis. Patients were censored at death, last follow-up, or May 25, 2017, whichever occurred first.
STATISTICAL ANALYSIS
Categorical variables were summarized by frequencies and proportions, and continuous variables were summarized by means and standard deviations (SDs) for normally distributed data and by median (interquartile range [IQR]) for nonparametric data. Categorical variables were compared using a χ2 test or Fisher’s exact test. Continuous variables were compared using a Student t test or Wilcoxon rank sum test as appropriate. Multivariate logistic and linear regression and generalized additive models were used to assess independent predictors of restriction and to assess the relationship between restriction as well as FVC percent predicted as a continuous variable with symptoms, quality of life, and exercise capacity after adjusting for covariates. We performed 2 sensitivity analyses. We used the LLN for FVC to define restriction and also used multiple imputation to account for missing data in the multivariate analyses.(9) The associations between restriction, FVC percent predicted, and mortality were assessed using a Fine-Gray subdistribution hazard competing risks model to account for LT as a competing risk.(10) Cumulative incidence function curves were generated to illustrate the cumulative incidence of competing risks of death and LT in patients, stratified by the median FVC percent predicted for the cohort (84.4%). We also analyzed the effect of restriction and FVC percent predicted on posttransplant mortality using a Cox proportional hazards model. A P value <0.05 was considered significant. All data analysis was performed in SAS, version 9.4 (SAS Institute, Cary, NC). Figures for generalized additive models were generated in R, version 3.6.1 (R Foundation for Statistical Computing, Vienna, Austria). The institutional review boards at all study sites approved the study: University of Pennsylvania, Office of Regulatory Affairs Protocol number 816361; Mayo Clinic institutional review board protocol 12-007715; and University of Texas Committee for the Protection of Human Subjects protocol HSC-MS-12-0481. All authors had access to the study data and reviewed and approved all statistical analyses as well as the resulting final manuscript.
Results
The cohort included 425 patients. We excluded 12 (2.8%) patients who did not have spirometry, 47 (11.1%) with obstructive lung disease, and 23 (5.4%) who did not have available chest imaging, leaving 343 (80.7%) in the study sample for this analysis (Fig. 1). A total of 63 (18.4%; 95% confidence interval [CI], 14.4%–22.9%) patients had restriction as defined by FVC <70% predicted, and 167/342 (48.8%; 95% CI, 43.4%–54.3%) patients had restriction as defined by an FVC below the LLN.
FIG. 1.
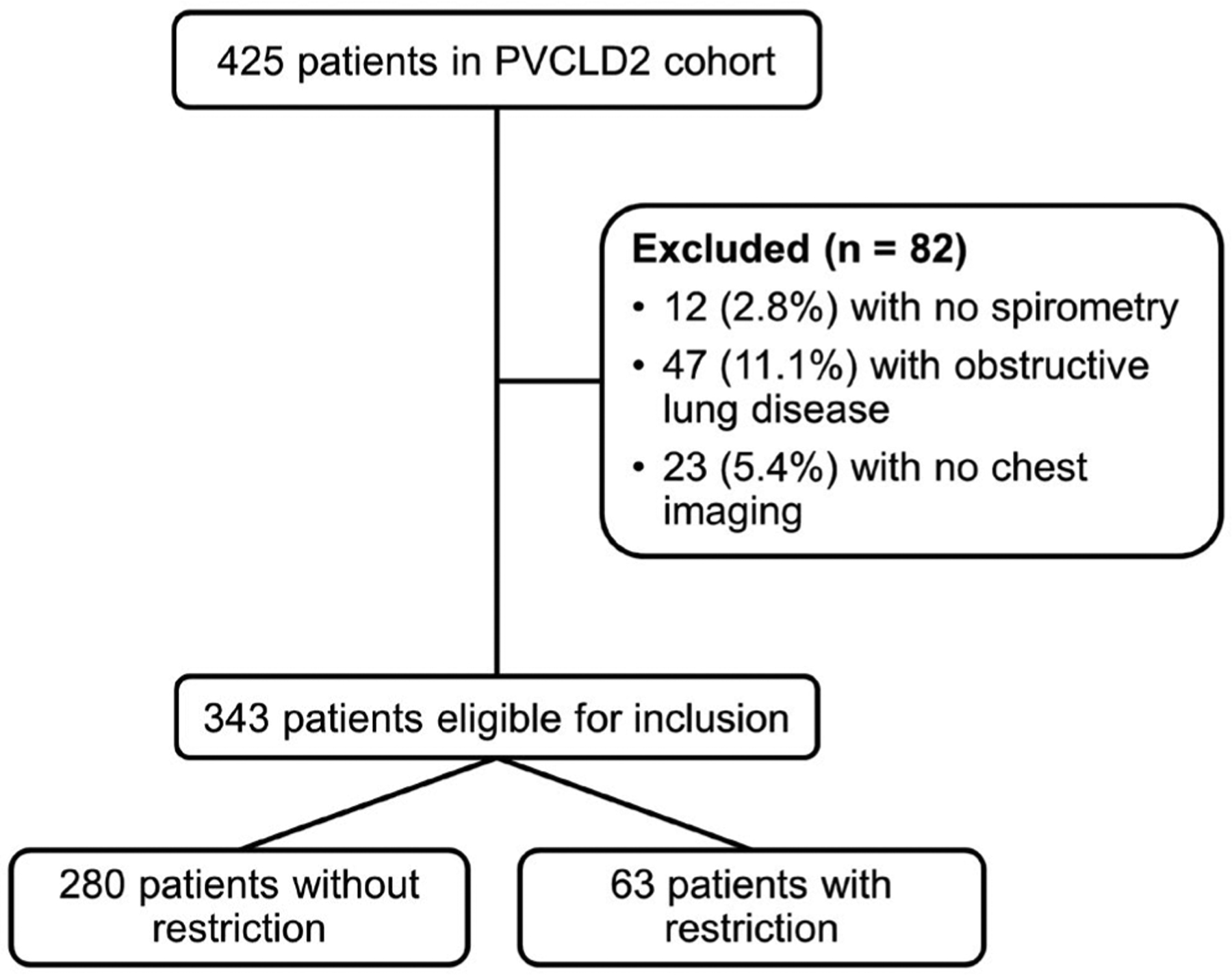
The selection of study sample.
There was no difference between patients with and without restriction in regard to age, sex, or race (Table 1). Patients with restriction were less likely to have a diagnosis of hepatitis C and had higher MELD-Na scores (indicating worse liver disease severity). Patients with restriction were more likely to have a history of encephalopathy, ascites, and hepatic hydrothorax, and they were less likely to have hepatocellular carcinoma. Comorbid medical illnesses were similar, and there was no difference in smoking history or body mass index (BMI) between the groups. Patients with restriction had a higher heart rate but otherwise similar physical examination characteristics, and they had higher INR and lower albumin, alanine aminotransferase (ALT), and aspartate aminotransferase (AST). Other laboratory tests, ABG tests, and echocardiograms were similar between the 2 groups.
TABLE 1.
Patient Demographics, Comorbidities, and Liver Disease Characteristics
| Characteristic | No Restriction Group (n = 280) | Restriction Group (n = 63) | P Value |
|---|---|---|---|
| Age, years | 56.1 ± 9.3 | 55.5 ± 9.5 | 0.6 |
| Sex, male | 187 (66.8) | 35 (55.6) | 0.09 |
| Race | 0.31 | ||
| Non-Hispanic white | 195 (69.6) | 40 (63.5) | |
| Black | 29 (10.4) | 4 (6.3) | |
| Asian | 3 (1.1) | 0 (0.0) | |
| Hispanic white | 46 (16.4) | 17 (27.0) | |
| Other | 7 (2.5) | 2 (3.2) | |
| Liver disease etiology* | |||
| Hepatitis C virus | 118 (42.1) | 18 (28.6) | 0.047 |
| Alcohol | 107 (38.2) | 24 (38.1) | 0.99 |
| NAFLD | 61 (21.8) | 16 (25.4) | 0.53 |
| Autoimmune | 9 (3.2) | 3 (4.8) | 0.47 |
| Primary biliary cholangitis | 15 (5.4) | 6 (9.5) | 0.21 |
| Primary sclerosing cholangitis | 13 (4.6) | 1 (1.6) | 0.48 |
| Cryptogenic cirrhosis | 17 (6.1) | 4 (6.4) | >0.99 |
| Liver disease severity | |||
| MELD-Na score† | 14.6 ± 5.6 | 16.8 ± 6.6 | 0.006 |
| Complications of liver disease | |||
| Encephalopathy | 150 (53.6) | 45 (71.4) | 0.01 |
| Esophageal varices | 191 (68.2) | 42 (66.7) | 0.81 |
| Ascites, by history | 188 (67.1) | 53 (84.1) | 0.008 |
| Hepatic hydrothorax, by history | 29 (10.4) | 13 (20.6) | 0.02 |
| Medical history | |||
| Hepatocellular carcinoma | 96 (34.3) | 10 (15.9) | 0.004 |
| Hypertension | 128 (45.7) | 27 (42.9) | 0.68 |
| Hyperlipidemia | 56 (20.0) | 19 (30.2) | 0.08 |
| Hypothyroidism | 39 (13.9) | 9 (14.3) | 0.94 |
| Depression | 86 (30.7) | 16 (25.4) | 0.4 |
| Congestive heart failure | 14 (5.0) | 4 (6.4) | 0.75 |
| Diabetes mellitus | 103 (36.8) | 25 (39.7) | 0.67 |
| Obstructive sleep apnea | 43 (15.4) | 9 (14.3) | 0.83 |
| Asthma | 27 (9.6) | 7 (11.1) | 0.72 |
| COPD | 14 (5.0) | 4 (6.4) | 0.75 |
| Chronic bronchitis | 22 (7.9) | 4 (6.4) | 0.79 |
| Sarcoidosis | 3 (1.1) | 3 (4.8) | 0.08 |
| Interstitial lung disease | 8 (2.9) | 1 (1.6) | >0.99 |
| Smoking history | |||
| Current or former smoker‡ | 165 (59.1) | 37 (58.7) | 0.95 |
| Current smoker | 40 (24.2) | 8 (21.6) | 0.74 |
NOTE: Data expressed as mean ± SD or n (%). Bold values are significant.
Patients may have had more than 1 liver disease etiology.
No restriction group, n = 275.
No restriction group, n = 279.
Patients with restriction had lower FVC and FEV1 with higher FEV1/FVC ratios and were more likely to have pleural effusions on imaging (Table 2). The prevalence and severity of ascites on abdominal imaging was similar between the 2 groups. Of 62 patients with restriction who had both chest and abdominal imaging for review, 20 had ascites and pleural effusions, 17 had ascites alone, and 4 had pleural effusions alone. One-third of patients with restriction had neither pleural effusions nor ascites. Among those without pleural effusions or ascites, 3 had interstitial changes, and 15 were obese. A total of 6 patients out of 62 (9.7%) had no identifiable cause of restriction. Among all patients, pleural effusions were associated with a significantly lower FVC percent predicted (73.9% ± 19.2% versus 86.1% ± 15.4%; P < 0.001). A clinical history of ascites was also associated with significant reductions in FVC percent predicted (81.3% ± 16.8% versus 89.6% ± 15.6%; P < 0.001). Higher MELD-Na score (odds ratio [OR], 1.06 per 1 point increase in MELD-Na score; 95% CI, 1.02–1.11; P = 0.007), the presence of pleural effusions (OR, 3.59; 95% CI, 1.96–6.58; P < 0.001), and a history of ascites (OR, 2.59; 95% CI, 1.26–5.33; P = 0.01) were associated with the presence of restriction. In the multivariate analysis including these variables, pleural effusions were the only factor that remained associated with restriction (OR, 2.75; 95% CI, 1.44–5.24; P = 0.002).
TABLE 2.
Physical Examination, Laboratory Results, and Other Testing
| No Restriction Group | Restriction Group | ||||
|---|---|---|---|---|---|
| Characteristic | n | Value | n | Value | P Value |
| Physical examination | |||||
| Weight, kg | 279 | 89.2 ± 21.8 | 63 | 92.6 ± 21.0 | 0.27 |
| Height, cm | 279 | 170.9 ± 9.2 | 63 | 170.2 ± 9.2 | 0.59 |
| Waist circumference, cm | 265 | 104.5 ± 16.9 | 58 | 105.4 ± 15.2 | 0.71 |
| BMI, kg/m2 | 279 | 30.5 ± 6.8 | 63 | 32.1 ± 7.7 | 0.1 |
| Obese (BMI >30 kg/m2) | 279 | 124 (44.4) | 63 | 34 (54.0) | 0.17 |
| Systolic BP, mm Hg | 279 | 122.2 ± 17.4 | 63 | 119.4 ± 20.0 | 0.27 |
| Diastolic BP, mm Hg | 279 | 68.6 ± 11.1 | 63 | 67.7 ± 13.1 | 0.57 |
| Respiratory rate, breaths per minute | 278 | 15.6 ± 3.5 | 63 | 17.0 ± 5.9 | 0.07 |
| Heart rate, bpm | 279 | 72.4 ± 13.6 | 63 | 76.8 ± 14.4 | 0.02 |
| Seated SpO2, % | 279 | 97.1 ± 3.0 | 63 | 97.0 ± 3.2 | 0.78 |
| Supine SpO2, % | 274 | 97.4 ± 2.6 | 62 | 96.9 ± 3.2 | 0.26 |
| Clubbing | 279 | 23 (8.2) | 63 | 5 (7.9) | 0.94 |
| Cyanosis | 279 | 10 (3.6) | 63 | 3 (4.8) | 0.71 |
| Spider angioma | 278 | 93 (33.5) | 63 | 22 (34.9) | 0.82 |
| Asterixis | 279 | 13 (4.7) | 63 | 0 (0.0) | 0.14 |
| Jaundice | 279 | 92 (33.0) | 63 | 22 (34.9) | 0.77 |
| Laboratory results | |||||
| Creatinine, mg/dL | 279 | 1.18 ± 0.42 | 63 | 1.35 ± 0.73 | 0.09 |
| Platelets, 1000/μL | 278 | 100.1 ± 56.9 | 63 | 101.5 ± 56.9 | 0.91 |
| Hemoglobin, g/dL | 280 | 12.2 ± 2.0 | 63 | 11.7 ± 2.2 | 0.05 |
| INR | 276 | 1.4 ± 0.3 | 63 | 1.5 ± 0.4 | 0.02 |
| Albumin, g/dL | 279 | 3.2 ± 0.6 | 62 | 2.9 ± 0.6 | 0.002 |
| Total bilirubin, mg/dL | 279 | 2.8 ± 2.5 | 63 | 3.0 ± 2.3 | 0.57 |
| ALT, units/L | 279 | 55.5 ± 44.1 | 63 | 38.2 ± 21.7 | <0.001 |
| AST, units/L | 279 | 75.5 ± 63.0 | 63 | 56.5 ± 39.5 | 0.003 |
| ABG | |||||
| pH | 271 | 7.44 ± 0.04 | 61 | 7.45 ± 0.05 | 0.16 |
| PaCO2, mm Hg | 271 | 34.5 ± 4.8 | 61 | 34.9 ± 5.5 | 0.58 |
| PaO2, mm Hg | 270 | 87.0 ± 14.8 | 61 | 83.6 ± 14.2 | 0.1 |
| A-a gradient, mm Hg | 270 | 20.4 ± 14.2 | 61 | 23.3 ± 14.4 | 0.15 |
| Echocardiogram | |||||
| Left atrial volume, mL | 246 | 76.2 ± 23.4 | 50 | 71.4 ± 26.4 | 0.19 |
| Stroke volume, mL | 257 | 91.6 ± 24.5 | 59 | 88.8 ± 26.3 | 0.4 |
| Cardiac output, L/minute | 257 | 6.2 ± 1.9 | 59 | 6.2 ± 2.0 | 0.97 |
| LV ejection fraction, % | 276 | 63.3 ± 5.4 | 63 | 63.6 ± 5.4 | 0.76 |
| RVSP, mm Hg | 191 | 32.2 ± 10.7 | 45 | 33.5 ± 13.2 | 0.49 |
| Intrapulmonary vascular dilatation* | 227 | 129 (56.8) | 57 | 29 (50.9) | 0.42 |
| Spirometry | |||||
| FVC, L | 280 | 3.74 ± 0.94 | 63 | 2.39 ± 0.62 | <0.001 |
| FVC percent predicted | 280 | 89.5 ± 11.9 | 63 | 58.3 ± 10.6 | <0.001 |
| FEV1, L | 280 | 2.90 ± 0.67 | 63 | 1.94 ± 0.53 | <0.001 |
| FEV1 percent predicted | 280 | 89.9 ± 11.3 | 63 | 61.0 ± 12.1 | <0.001 |
| FEV1/FVC | 280 | 78.0 ± 5.5 | 63 | 81.5 ± 5.5 | <0.001 |
| Imaging | |||||
| Pleural effusion | 280 | 41 (14.6) | 63 | 24 (38.1) | <0.001 |
| Interstitial lung disease | 280 | 33 (11.8) | 63 | 7 (11.1) | 0.88 |
| Pulmonary artery enlargement | 280 | 11 (3.9) | 63 | 2 (3.2) | >0.99 |
| Cardiomegaly | 280 | 18 (6.4) | 63 | 6 (9.5) | 0.38 |
| Ascites | 274 | 138 (50.4) | 62 | 37 (59.7) | 0.19 |
| Exercise testing | |||||
| 6MWD, m | 241 | 407.2 ± 95.9 | 53 | 337.3 ± 97.3 | <0.001 |
| SpO2 at end of walk, % | 241 | 96.1 ± 4.5 | 53 | 95.1 ± 5.6 | 0.23 |
| Heart rate at end of walk, bpm | 241 | 94.9 ± 17.6 | 53 | 91.1 ± 19.9 | 0.16 |
| Borg score ≥2 after walk | 241 | 145 (60.2) | 53 | 36 (67.9) | 0.29 |
NOTE: Data expressed as mean ± SD or n (%). Bold values are significant.
Patients with a patent foramen ovale were excluded from the analysis.
Patients with restriction had significantly lower 6MWDs (Table 2). Restriction was associated with a lower 6MWD after adjustment for age, sex, race, BMI, MELD-Na score, and pleural effusions (least squares mean, 342.0 [95% CI, 316.6–367.4] m versus 395.7 [95% CI, 381.2–410.2] m; P < 0.001; Fig. 2A). FVC percent predicted as a continuous variable was also associated with 6MWD in the multivariate analysis (P < 0.001; Fig. 2B).
FIG. 2.
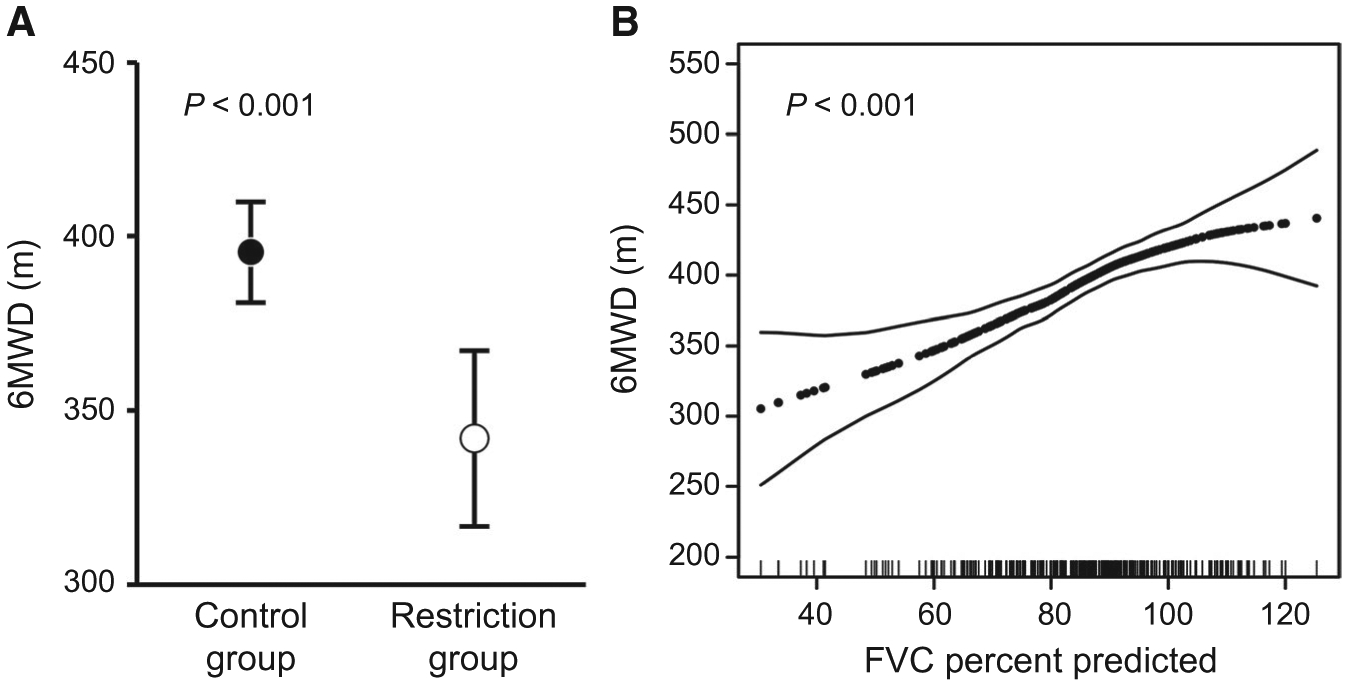
Restriction and exercise capacity. (A) Least squares mean for 6MWD in patients with and without restriction after adjustment for age, sex, race, BMI, MELD-Na scores, and pleural effusions. Markers show the point estimates, and whiskers show the 95% CI. (B) Generalized additive model plots depicting the relationship between FVC percent predicted and 6MWD after adjusting for age, sex, race, BMI, MELD-Na scores, and pleural effusions.
Patients with restriction were much more likely to report dyspnea and orthopnea and had worse World Health Organization (WHO) functional class (Table 3). They had significantly lower scores on all physical domains of the SF-36 as well as the social functioning and vitality domain, indicating worse quality of life. After adjustment for age, sex, race, BMI, MELD-Na scores, and pleural effusions, restriction was significantly and independently associated with dyspnea (OR, 2.69; 95% CI, 1.46–4.95; P = 0.002) and lower physical component summary (PCS) SF-36 scores (worse quality of life; least squares mean, 34.1 [95% CI, 31.5–36.7] versus 38.2 [95% CI, 36.6–39.7]; P = 0.004; Fig. 3A). FVC percent predicted as a continuous variable was independently associated with PCS scores in the multivariate analysis as well (P < 0.001; Fig. 3B).
TABLE 3.
Symptoms and Quality of Life
| Characteristi | No Restriction Group (n = 279) | Restriction Group (n = 63) | P Value |
|---|---|---|---|
| Symptoms | |||
| Dyspnea | 90 (32.3) | 37 (58.7) | <0.001 |
| Orthopnea* | 10 (3.6) | 7 (11.1) | 0.01 |
| Platypnea* | 8 (2.9) | 1 (1.6) | 1.0 |
| Angina | 16 (5.7) | 7 (11.1) | 0.12 |
| Edema | 137 (49.1) | 36 (57.1) | 0.25 |
| Syncope | 3 (1.1) | 1 (1.6) | 0.56 |
| WHO functional class | <0.001 | ||
| 1 | 95 (34.1) | 12 (19.0) | |
| 2 | 129 (46.2) | 25 (39.7) | |
| 3–4 | 55 (19.7) | 26 (41.3) | |
| Quality of life (SF-36)† | |||
| PCS | 38.1 ± 10.4 | 32.1 ± 8.8 | <0.001 |
| Physical role | 47.8 ± 31.2 | 33.8 ± 29.4 | 0.001 |
| Physical functioning | 54.1 ± 28.6 | 37.2 ± 24.4 | <0.001 |
| Bodily pain | 53.7 ± 26.6 | 42.2 ± 21.3 | 0.003 |
| General health perception | 41.2 ± 22.7 | 32.8 ± 18.3 | 0.002 |
| Mental component summary | 47.2 ± 10.5 | 46.1 ± 11.0 | 0.49 |
| Emotional role | 67.3 ± 30.3 | 61.1 ± 33.5 | 0.15 |
| Social functioning | 62.1 ± 29.6 | 53.6 ± 27.9 | 0.04 |
| Mental health | 7163 ± 19.2 | 67.9 ± 20.1 | 0.18 |
| Vitality | 42.9 ± 22.3 | 36.7 ± 21.0 | 0.046 |
NOTE: Data expressed as mean ± SD or n (%). Bold values are significant.
No restriction group, n = 278.
No restriction group, n = 277.
FIG. 3.
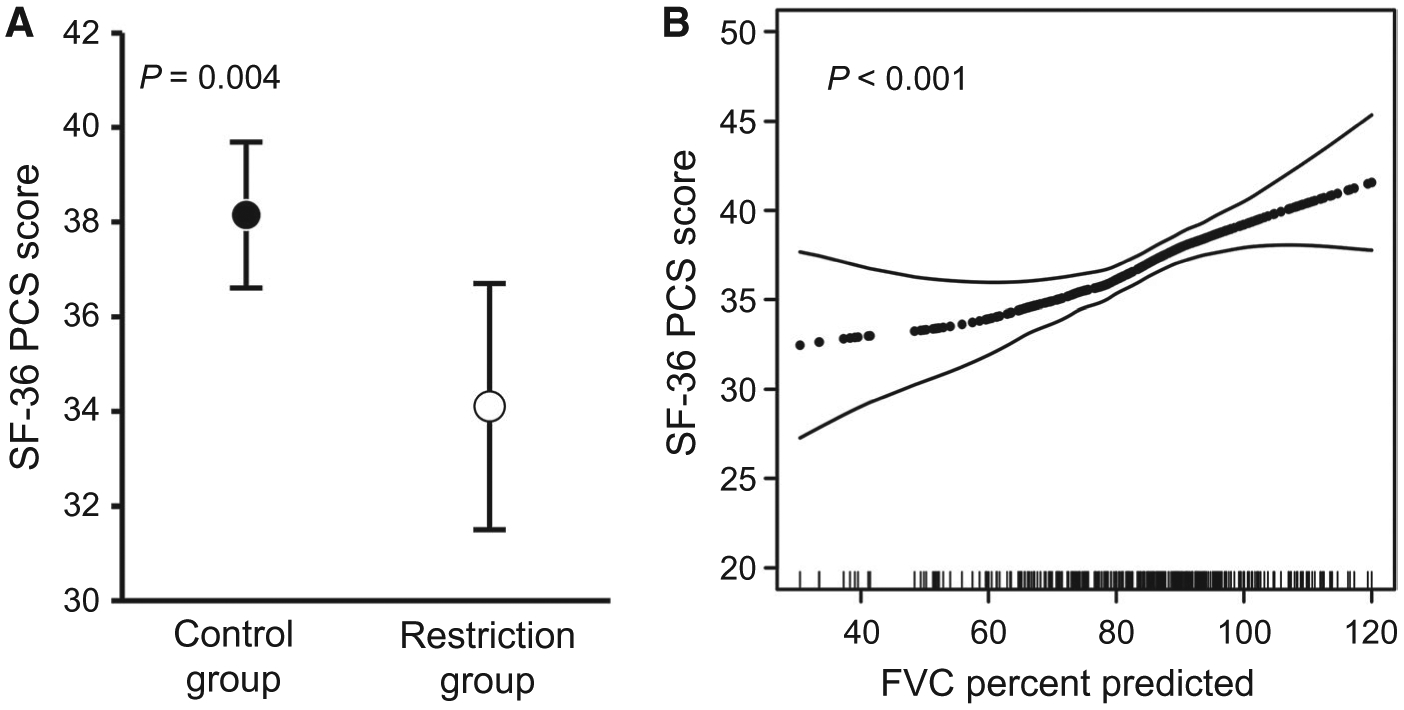
Restriction and physical quality of life. (A) Least squares mean for PCS scores in the control group and in patients with restriction after adjustment for age, sex, race, BMI, MELD-Na scores, and pleural effusions. The markers show the point estimates, and whiskers show the 95% CI. (B) Generalized additive model plots depicting the relationship between FVC percent predicted and PCS scores after adjusting for age, sex, race, BMI, MELD-Na scores, and pleural effusions.
In the sensitivity analyses, restriction defined as an FVC below the LLN was significantly associated with 6MWD (least squares mean 349.7 [95% CI, 331.7–367.7] versus 410.7 [95% CI, 394.8–426.6] m; P < 0.001) after adjustment for age, sex, race, BMI, MELD-Na score, and pleural effusions. FVC below the LLN was associated with dyspnea (OR, 2.14; 95% CI, 1.37–3.35; P < 0.001) in univariate analysis but not in the multivariate analysis (OR, 1.53; 95% CI, 0.92–2.54; P = 0.10) and was significantly associated with PCS scores (least squares mean, 35.3; 95% CI, 33.4–37.2 versus 38.9, 95% CI 37.2–40.6; P = 0.002) in the multivariate analysis. Using multiple imputation to account for missing data, restriction was similarly associated with lower 6MWD (parameter estimate, −51.2; 95% CI, −77.9 to −24.5 m; P < 0.001) and PCS scores (parameter estimate, −4.0; 95% CI, −6.8 to −1.3; P = 0.004) in the multivariate analysis.
The number of nontransplant-related hospitalizations were similar in patients with (median, 1; IQR, 0–3) and without (median, 1; IQR, 0–3) restriction (P = 0.49). Restriction was not associated with mortality in unadjusted analysis (subdistribution hazard ratio [HR], 1.44; 95% CI, 0.80–2.60; P = 0.22) or after adjusting for age and MELD-Na score (subdistribution HR, 1.36; 95% CI, 0.74–2.49; P = 0.3), but lower FVC percent predicted as a continuous variable was significantly associated with a higher risk of death (subdistribution HR, 1.16; 95% CI, 1.04–1.27 per 10-point decrease; P = 0.01). After adjusting for age and MELD-Na score, the relationship was similar (subdistribution HR, 1.16; 95% CI, 1.02–1.27 per 10-point decrease in FVC percent predicted; P = 0.03). When the cohort was stratified by the median FVC percent predicted, LT candidates with an FVC percent predicted below the median had a higher cumulative incidence of death and a similar cumulative incidence of LT (Fig. 4). Among 141 patients who underwent LT, there were 12 posttransplant deaths. Neither restriction (HR, 0.38; 95% CI, 0.05–2.90; P = 0.35) nor FVC percent predicted (HR, 1.02; 95% CI, 0.99–1.05; P = 0.30) were significantly associated with posttransplant mortality.
FIG. 4.
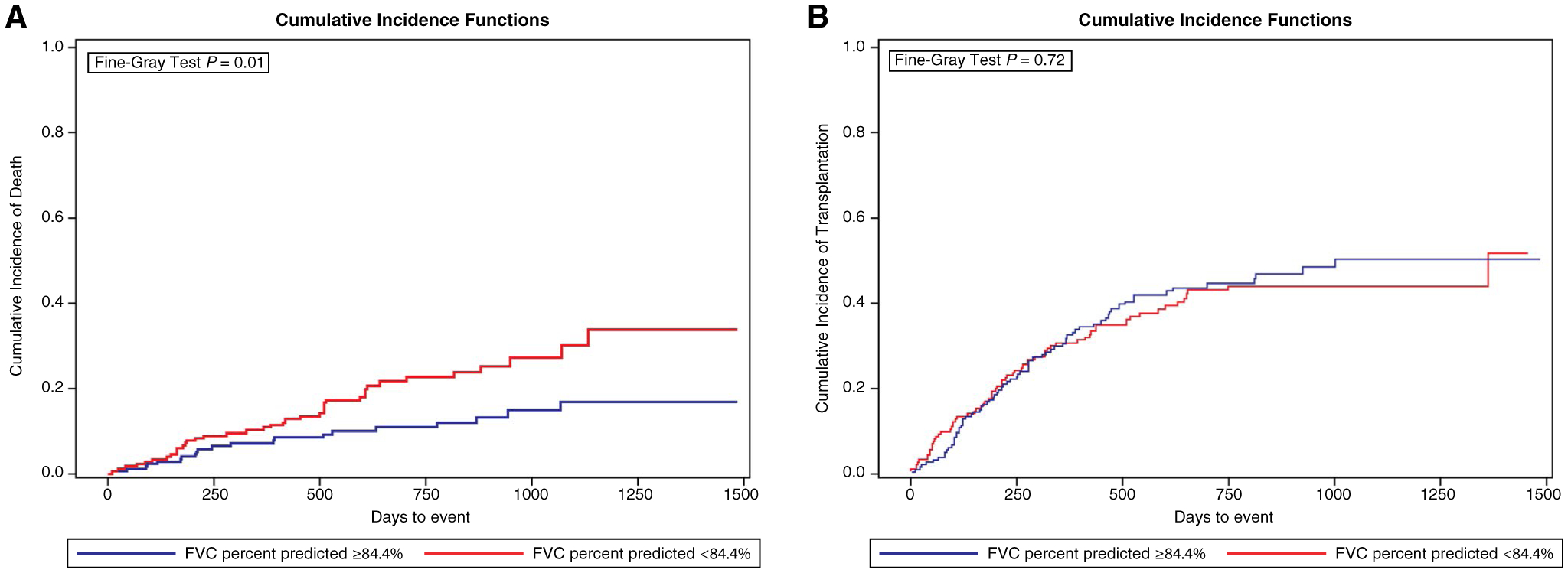
FVC percent predicted and cumulative incidence of death and transplantation. LT candidates with an FVC percent predicted below the median (84.4%) had (A) a higher cumulative incidence of death (P = 0.01) and (B) a similar cumulative incidence of transplantation (P = 0.72).
Discussion
In this study, we found that restrictive ventilatory deficits were common in LT candidates and were associated with worse exercise capacity and physical quality of life and increased dyspnea independent of other factors. We also found that lower FVC percent predicted was associated with a higher risk of death, even after adjusting for other factors such as age and MELD-Na score. Although spirometry is often performed in patients undergoing LT evaluation, the specific causes of restriction in these patients and the clinical impact of restriction were not previously known. The results of our study highlight the importance and clinical impact of restrictive lung disease in LT candidates.
In this multicenter prospective cohort study, restriction defined as an FVC <70% predicted was present in 18.4% of LT candidates. Prior single-center prospective and retrospective cohort studies have described a prevalence of 21% and 27%, respectively, when restriction was defined as a total lung capacity <80% predicted.(4,11) Our study was a prospective multicenter study with spirometry as part of the study protocol, resulting in greater generalizability. Additionally, because spirometry is quicker and easier to obtain than total lung capacity and because FVC is routinely used for diagnosis and risk stratification for other diseases, our findings suggest that spirometry represents an important tool in the evaluation of LT candidates that is associated with clinically meaningful outcomes. Restriction defined as an FVC below the LLN was present in almost 50% of the cohort, demonstrating how common abnormal lung function is in LT candidates. Because this definition was less strongly associated with symptoms, however, it may be too sensitive for use in the LT candidate population. Although current guidelines do not recommend routine spirometry in the evaluation of LT candidates, it is important for the transplant care team to be aware of the prevalence and impact of restrictive lung disease because restriction can also affect posttransplant outcomes, such as ventilator time, length of stay, and postoperative pulmonary complications.(4,12)
Restriction was associated with worse liver disease severity and complications of liver disease, such as encephalopathy, ascites, and hepatic hydrothorax. The mechanism of the association between encephalopathy and restriction is not known, but it may be due to difficulty performing spirometric maneuvers or weakness in the setting of end-stage liver disease. As discussed later in this section, ascites and pleural effusions may have a direct impact on pulmonary function. Interestingly, restriction was also associated with lower levels of transaminases and albumin. The reasons for this are not known, but lower ALT levels have been associated with frailty and sarcopenia in elderly patients.(13) Therefore, ALT may represent a biomarker of frailty in LT candidates.
Restriction in the setting of liver disease is commonly attributed to ascites and/or pleural effusions, but we were surprised to find that one-third of patients with restriction had neither ascites nor pleural effusions. We also quantified the relative effect of both ascites and pleural effusions on FVC percent predicted and found that pleural effusions were associated with large and independent reductions in FVC percent predicted, indicating that extrathoracic fluid accumulation from ascites has less of an impact on lung function. Obesity was not significantly associated with restriction, and interstitial lung disease was relatively uncommon. Another unmeasured factor that might have contributed to a restrictive deficit was respiratory muscle weakness. Reduced FVC, particularly in the absence of usual causes of restriction, such as pleural effusions or ascites, may be a marker of weakness and frailty in LT candidates.
Restriction was independently associated with worse exercise capacity. A minimally important difference for 6MWD in other diseases is considered to be approximately 30 m.(14,15) In our study, patients with restriction walked 70 m less on 6MWD testing. Even after adjusting for other factors, patients with restriction had a 6MWD that was 54 m lower than those with normal FVC, and this relationship persisted in our sensitivity analyses. The 6MWD is not only a marker of exercise capacity and physical function but is also an important prognostic indicator in end-stage liver disease. Reduced 6MWD is associated with worse mortality in LT candidates and an increased risk of postoperative pulmonary complications.(2,16,17) Importantly, the association of restriction with reduced 6MWD was independent of liver disease severity and the presence of pleural effusions, suggesting it is an important test regardless of the etiology of restriction.
Restriction was associated with increased dyspnea, orthopnea, worse functional class, and worse quality of life. Quality of life is an important prognostic marker in liver disease and is inversely associated with frailty.(18,19) Similar to restriction, frailty is common in LT candidates and associated with worse quality of life and exercise capacity.(17,18,20) Although it is possible that frailty confounds the relationships between restriction, quality of life, and exercise capacity, spirometry as a simple test clearly provides important information with clinically meaningful correlates.
Restriction was not associated with increased hospitalizations or worse survival, but lower FVC percent predicted (modeled as a continuous variable) was associated with an increased risk of death. The cumulative incidence of death was also higher in patients with a lower FVC when the cohort was stratified by the median FVC percent predicted. One potential explanation for the lack of significant association between restriction (defined as an FVC <70% predicted) and survival is that the smaller sample size in the restriction group in this analysis resulted in a loss of power to detect a difference between the 2 groups. Across the continuum of FVC values, however, we did find that lower FVC percent predicted was associated with an increased risk of death, even after adjustment for age and MELD-Na scores. Although the relationship between FVC and survival may be confounded by other variables, this finding highlights the clinical importance of assessing FVC using spirometry in LT candidates. Lastly, restriction was not associated with an increased risk of posttransplant death, but our power to detect a significant difference in posttransplant outcomes was limited by the smaller sample size.
Our study had several limitations. We did not plan for other pulmonary function tests, such as total lung capacity, diffusing capacity for carbon monoxide, and maximal inspiratory and expiratory pressures, that could have better characterized the causes of restriction. Additionally, imaging was performed as clinically indicated within the year prior to study enrollment; therefore, it may not have temporally correlated with study tests and was not interpreted in a blinded or standardized fashion. We also did not collect data on posttransplant outcomes, such as postoperative pulmonary complications, duration of mechanical ventilation, intensive care unit, and hospital length of stay, so we do not know how spirometric restriction would impact these outcomes. Lastly, we do not know whether restriction represents a modifiable variable that can improve with interventions, such as pulmonary rehabilitation, thoracentesis, or paracentesis, and whether improvements in FVC percent predicted would result in improved quality of life and exercise capacity.
In conclusion, restriction and reduced FVC is common in LT candidates; can be present in the absence of an obvious cause, such as ascites or pleural effusions; and is independently associated with worse exercise capacity, quality of life, and survival. Future prospective studies to determine the effect of restriction on posttransplant outcomes and the effect of interventions, such as pulmonary rehabilitation, on restriction, quality of life, and exercise capacity in LT candidates are warranted.
Acknowledgments
The research reported in this publication was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under award numbers R01HL113988 and K24 HL103844 and by the Mayo Clinic Department of Medicine Catalyst Award for Advancing in Academics.
Abbreviations:
- 6MWD
6-minute walk distance
- A-a
alveolar-arterial
- ABG
arterial blood gas
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- ATS
American Thoracic Society
- BMI
body mass index
- BP
blood pressure
- bpm
beats per minute
- CI
confidence interval
- COPD
chronic obstructive pulmonary disease
- CT
computed tomography
- ERS
European Respiratory Society
- FEV1
forced expiratory volume in 1 second
- FVC
forced vital capacity
- HR
hazard ratio
- INR
international normalized ratio
- IQR
interquartile range
- LLN
lower limit of normal
- LT
liver transplantation
- LV
left ventricular
- MELD
Model for End-Stage Liver Disease
- MELD-Na
Model for End-Stage Liver Disease–sodium
- Na
serum sodium
- NAFLD
nonalcoholic fatty liver disease
- NHANES
National Health and Nutrition Survey
- OR
odds ratio
- PaCO2
partial pressure of arterial carbon dioxide
- PaO2
partial pressure of arterial oxygen
- PCS
physical component summary
- PVCLD2
Pulmonary Vascular Complications of Liver Disease 2
- RVSP
right ventricular systolic pressure
- SD
standard deviation
- SF-36
Short Form 36
- SpO2
oxygen saturation
- WHO
World Health Organization
REFERENCES
- 1).Alameri HF, Sanai FM, Al Dukhayil M, Azzam NA, Al-Swat KA, Hersi AS, Abdo AA. Six minute walk test to assess functional capacity in chronic liver disease patients. World J Gastroenterol 2007;13:3996–4001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2).Carey EJ, Steidley DE, Aqel BA, Byrne TJ, Mekeel KL, Rakela J, et al. Six-minute walk distance predicts mortality in liver transplant candidates. Liver Transpl 2010;16:1373–1378. [DOI] [PubMed] [Google Scholar]
- 3).Yadav A, Chang YH, Carpenter S, Silva AC, Rakela J, Aqel BA, et al. Relationship between sarcopenia, six-minute walk distance and health-related quality of life in liver transplant candidates. Clin Transplant 2015;29:134–141. [DOI] [PubMed] [Google Scholar]
- 4).Kia L, Cuttica MJ, Yang A, Donnan EN, Whitsett M, Singhvi A, et al. The utility of pulmonary function testing in predicting outcomes following liver transplantation. Liver Transpl 2016; 22:805–811. [DOI] [PubMed] [Google Scholar]
- 5).Forde KA, Fallon MB, Krowka MJ, Sprys M, Goldberg DS, Krok KL, et al. ; for Pulmonary Vascular Complications of Liver Disease 2 Study Group. Pulse oximetry is insensitive for detection of hepatopulmonary syndrome in patients evaluated for liver transplantation. Hepatology 2019;69:270–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6).Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc 1982;14:377–381. [PubMed] [Google Scholar]
- 7).Mahler DA, Rosiello RA, Harver A, Lentine T, McGovern JF, Daubenspeck JA. Comparison of clinical dyspnea ratings and psychophysical measurements of respiratory sensation in obstructive airway disease. Am Rev Respir Dis 1987;135:1229–1233. [DOI] [PubMed] [Google Scholar]
- 8).Hankinson JL, Bang KM. Acceptability and reproducibility criteria of the American Thoracic Society as observed in a sample of the general population. Am Rev Respir Dis 1991;143:516–521. [DOI] [PubMed] [Google Scholar]
- 9).Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med 1999;159:179–187. [DOI] [PubMed] [Google Scholar]
- 10).Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999;94:496–509. [Google Scholar]
- 11).Krowka MJ, Dickson ER, Wiesner RH, Krom RAF, Atkinson B, Cortese DA. A prospective study of pulmonary function and gas exchange following liver transplantation. Chest 1992; 102:1161–1166. [DOI] [PubMed] [Google Scholar]
- 12).Martin P, DiMartini A, Feng S, Brown R, Fallon M. Evaluation for liver transplantation in adults: 2013 practice guideline by the American Association for the Study of Liver Diseases and the American Society of Transplantation. Hepatology 2014;59: 1144–1165. [DOI] [PubMed] [Google Scholar]
- 13).Vespasiani-Gentilucci U, De Vincentis A, Ferrucci L, Bandinelli S, Antonelli Incalzi R, Picardi A. Low alanine aminotransferase levels in the elderly population: frailty, disability, sarcopenia, and reduced survival. J Gerontol A Biol Sci Med Sci 2018;73: 925–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14).Polkey MI, Spruit MA, Edwards LD, Watkins ML, Pinto-Plata V, Vestbo J, et al. Six-minute-walk test in chronic obstructive pulmonary disease: minimal clinically important difference for death or hospitalization. Am J Respir Crit Care Med 2013;187: 382–386. [DOI] [PubMed] [Google Scholar]
- 15).Swigris JJ, Wamboldt FS, Behr J, du Bois RM, King TE, Raghu G, Brown KK. The 6 minute walk in idiopathic pulmonary fibrosis: longitudinal changes and minimum important difference. Thorax 2010;65:173–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16).Magalhaes CBA, Nogueira IC, Marinho LS, Daher EF, Garcia JHP, Viana CFG, et al. Exercise capacity impairment can predict postoperative pulmonary complications after liver transplantation. Respiration 2017;94:272–278. [DOI] [PubMed] [Google Scholar]
- 17).Lai JC, Volk ML, Strasburg D, Alexander N. Performance-based measures associate with frailty in patients with end-stage liver disease. Transplantation 2016;100:2656–2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18).Derck JE, Thelen AE, Cron DC, Friedman JF, Gerebics AD, Englesbe MJ, Sonnenday CJ. Quality of life in liver transplant candidates: frailty is a better indicator than severity of liver disease. Transplantation 2015;99:340–344. [DOI] [PubMed] [Google Scholar]
- 19).Tanikella R, Kawut SM, Brown RS Jr., Krowka MJ, Reinen J, Dinasarapu CR, et al. Health-related quality of life and survival in liver transplant candidates. Liver Transpl 2010;16: 238–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20).Lai JC, Feng S, Terrault NA, Lizaola B, Hayssen H, Covinsky K. Frailty predicts waitlist mortality in liver transplant candidates. Am J Transplant 2014;14:1870–1879. [DOI] [PMC free article] [PubMed] [Google Scholar]


