Abstract
Objective:
The optic nerve is a frequent site for involvement in multiple sclerosis (MS). Optical coherence tomography (OCT) detects thinning of the retinal nerve fiber layer (RNFL) in eyes of patients with MS and those meeting criteria for clinically- or radiologically-isolated demyelinating syndromes. Current international diagnostic criteria for MS do not include the optic nerve as an imaging lesion site despite the high prevalence of acute optic neuritis (ON), or occult optic neuropathy, among early MS and clinically isolated syndrome (CIS) patients;as well as most MS patients over the course of the disease. We sought to determine optimal thresholds for inter-eye difference in peripapillary RNFL thickness that are most predictive of a unilateral optic nerve lesion.
Methods:
We analyzed Spectral-domain (SD-) OCT data for 31 healthy volunteers and 124 patients with MS at a single center as part of an ongoing collaborative investigation of visual outcomes. Inter-eye differences in peripapillary (360°) RNFL thickness were calculated as the absolute value of the difference. First, we determined the 95th percentile value of inter-eye difference for the healthy volunteers. This value was applied to the convenience sample group of MS patients as a validation cohort determining how well this threshold could distinguish patients with vs. without a history of unilateral ON. The relation of inter-eye differences in peripapillary RNFL thickness to binocular low-contrast letter acuity (LCLA) scores was also examined.
Results:
Among healthy volunteer participants (n=31), the 95th percentile value for inter-eye difference (upper boundary of expected for normal controls) was 6.0 microns. This value was applied to the convenience sample group of MS patients (n=124, validation cohort). Positive predictive value, negative predictive value, sensitivity and specificity for identifying MS patients with a history of unilateral ON were calculated for the 6-micron threshold value in a 2×2 table analysis with the application of chi-square tests (P<0.0001). The 6-micron threshold was predictive of worse binocular low-contrast acuity scores at 2.5% (P=0.03) and 1.25% (P=0.002 by linear regression analyses). A receiver operating characteristic (ROC) curve analysis demonstrated an optimal inter-eye difference threshold of 5 microns for identifying unilateral ON in the MS cohort.
Conclusion:
An inter-eye difference of 5–6 microns in RNFL thickness is a robust structural threshold for identifying the presence of a unilateral optic nerve lesion in MS.
Keywords: optic neuritis (ON), multiple sclerosis (MS), retinal nerve fiber layer (RNFL), quality of life (QOL), optical coherence tomography (OCT)
The visual pathways are frequent sites of involvement in multiple sclerosis (MS). For 20% of patients with MS, acute optic neuritis (ON) is the first clinical manifestation. Fifty percent of patients experience ON at some point in the course of their disease, and optic neuropathy is present post-mortem in 95% (1–7). At 15 years after entry into the optic neuritis treatment trial (ONTT), 72% of participants with acute ON and acute and chronic brain white matter lesions on MRI at baseline developed clinically-definite MS (CDMS, defined as two clinically-manifest demyelinating events separated in time and space). In the same trial, only 25% of patients without MRI white matter lesions at baseline developed CDMS over the same follow-up period (8). The Controlled High Risk Avonex Multiple Sclerosis Prevention Study (CHAMPS), the ONTT and other cohorts have demonstrated that patients with optic nerve and brain lesions have a similar risk of developing CDMS compared to those with brainstem or spinal cord lesions (8–12).
MS is a demyelinating disease of the central nervous system that is now established using the recently revised 2017 McDonald Criteria (13–16). Heavily based upon brain MR imaging, existing lesion sites that qualify towards an MS diagnosis include periventricular, juxtacortical/ cortical or infratentorial brain lesions or spinal cord lesions; these require proof of dissemination in space and time for an MS diagnosis to be confirmed. Acute or chronic optic nerve lesions are still considered only by clinical criteria and there are no recognized imaging criteria for this lesion site for diagnosis of MS (15, 16). This is true despite the high prevalence of ON among early MS and clinically isolated syndrome (CIS) patients.
Optical coherence tomography (OCT) is a rapid, inexpensive, well-tolerated and noninvasive test that can detect both symptomatic and subclinical optic neuropathies using measures of peripapillary retinal nerve fiber layer (RNFL, includes ganglion cell layer axons) thickness. Spectral-domain OCT is a high-resolution imaging technique that detects thinning of the RNFL and the macular ganglion cell + inner plexiform layer (GCIP) with great precision and reproducibility in eyes of patients with MS. Peripapillary RNFL thinning has been documented to occur even early in the course of MS, as well as in CIS or radiologically-isolated syndrome patients, and in eyes of MS patients without a history of acute ON (17–24).
OCT measurements showing RNFL thinning are associated with impaired visual function, reduced quality of life, increased global disability and more marked degrees of brain atrophy in MS (24–41). Further, the magnitude of RNFL thinning in MS is particularly correlated with eyes targeted by the disease process in MS; most specifically in the context of acute ON, where such affected eyes can demonstrate up to 20–25-micron irreversible losses of RNFL thickness following a single acute ON attack. This occurs over a latency as short as three months from the onset of symptoms to the nadir of the layer thickness (42–44). Nonetheless, even mild loss of the RNFL (4 to 6 microns) has been associated with clinically meaningful loss of low contrast visual acuity (7 to 10 letters of low contrast acuity at the 1.25% and 2.5% levels) (45). The threshold degree of inter-eye difference in RNFL thickness that best identifies patients with a history of acute unilateral ON (46), and thus predicts an optic nerve lesion, has not been established.
The purpose of our study was to determine optimal thresholds for inter-eye difference in RNFL thickness as measured by OCT that are most predictive of a history of acute unilateral ON and that can thus identify the presence of an optic nerve lesion.
METHODS
Study Participants
Participants included patients aged 18 years or older who were part of an ongoing collaborative investigation of visual outcomes in MS (n=124). Among those with MS, 45/124 (36%) had a history of acute ON, with none suffering attacks in both eyes. A cohort of healthy volunteers (n=31) was also included for determining the 95th percentile (upper limit of expected value) for inter-eye difference in RNFL thickness in disease-free individuals. Patients with ocular co-morbidities or other conditions that could affect RNFL thinning or inter-eye asymmetry not related to MS were excluded. By virtue of the nature of our convenience sample of patients with MS with and without a history of acute unilateral ON (both were included in our cohort), nearly all were examined clinically by a neuro-ophthalmologist. As such, the diagnosis of acute unilateral ON was confirmed clinically using standard diagnostic criteria of history of painful, acute monocular visual loss. Patients with a history of acute ON in both eyes were excluded.
For purposes of accuracy and homogeneity of technique of all OCT measurements, data were obtained by a single, highly-expert technologist, from a single study site (NYU) for patients with MS and healthy volunteers. The ‘history of acute ON’ designation for this study was specifically defined by a patient-reported exacerbation consistent with inflammatory demyelinating characteristics, consistent with the diagnosis of acute optic neuritis. Fellow eye history and evidence of fellow-eye ON were exclusion criteria. Further, the inception of the acute ON syndrome had to be more than 3 months prior to the onset of testing for the current investigation. Healthy volunteers (controls) with no history or evidence of neurologic or ophthalmic disease were recruited among faculty, staff, students, and family members of the research study participants. Study protocols were approved by the New York University School of Medicine Institutional Review Board (IRB); all participants provided written informed consent.
Optical Coherence Tomography (OCT)
Spectral-domain OCT imaging was performed by a trained technologist for participants in the convenience sample cohort of MS patients and controls using either Spectralis OCT (Heidelberg Engineering, Heidelberg, Germany) or Cirrus OCT (Model 4000, Carl Zeiss Meditec, Dublin, CA). At the inception of the cohort, only Cirrus OCT was available; most subsequent scans were performed using Spectralis. Peripapillary RNFL thickness for 360° around the optic disc was measured on Spectralis using a 3.5 mm diameter circle centered on the optic disc. On the Cirrus OCT, the ONH Cube 200×200 scan was used to measure peripapillary RNFL thickness. All scans were reviewed to meet quality control standards. The OSCAR-IB guidelines (47) for quality control were followed for Spectralis OCT scans. All scans that were not centered or segmented properly, had a signal strength <7, or any algorithm failure on Cirrus OCT were excluded.
Low-Contrast Letter Acuity
Low-contrast letter acuity (LCLA) was measured monocularly for each eye and binocularly with both eyes together using low-contrast Sloan letter charts at both 2.5% and 1.25% contrast levels, in a retro-illuminated light box at 2 meters’ distance (Precision Vision, LaSalle, IL). Scores were calculated as the numbers of letters read correctly with a maximum score of 70 letters per chart (14 lines with five letters per line). Sloan LCLA charts are standardized with 5 letters of equal difficulty per line and equal spacing between lines (45).
Statistical Analyses
Statistical analyses were performed using Stata 15.0 (Stata Corp, College Station, TX). Mean and median values for inter-eye differences in peripapillary RNFL thickness were calculated for all study participants at a single visit. The 95th percentile for RNFL thickness inter-eye difference for the healthy control group was calculated. The absolute value of this difference was used for analyses since such differences in healthy controls are not expected to demonstrate laterality. The control group inter-eye difference 95th percentile value was examined as a threshold in 2×2 table analyses to determine sensitivity, specificity and predictive values for this value to distinguish MS patients in the convenience sample cohort who had a history of acute unilateral ON vs. those with no acute ON history. This 95th percentile value and two standard deviations from the control group mean inter-eye difference (absolute value), represents an estimate of the largest expected value for inter-eye difference in a healthy population. Chi-square tests were used to examine the association of inter-eye difference at or above the threshold values with greater likelihood of acute unilateral ON history among the patients in the MS cohort.
A receiver operating characteristic (ROC) curve was constructed to identify the cutoff value for inter-eye RNFL thickness difference that optimizes both sensitivity and specificity for distinguishing MS patients with vs. without a history of acute unilateral ON. This cutpoint corresponds to a 45-degree tangent line intersection and is mathematically equivalent to the point at which sensitivity and specificity are closest together. The relation of binocular visual function tests, including high- and low-contrast acuity, to inter-eye differences in RNFL thickness was examined using linear regression models, accounting simultaneously for age.
RESULTS
Among 124 patients with MS and 31 healthy volunteers in the convenience sample cohort, peripapillary RNFL thickness was similar between right and left eyes (92.7±10.5 microns vs. 93.1±10.5 microns for controls; 86.1±16.6 microns vs. 85.2±17.6 microns for patients with MS). This is important for both controls and patients with MS in demonstrating that there is no laterality (right vs. left eye inter-eye difference) that could be present due to chance.
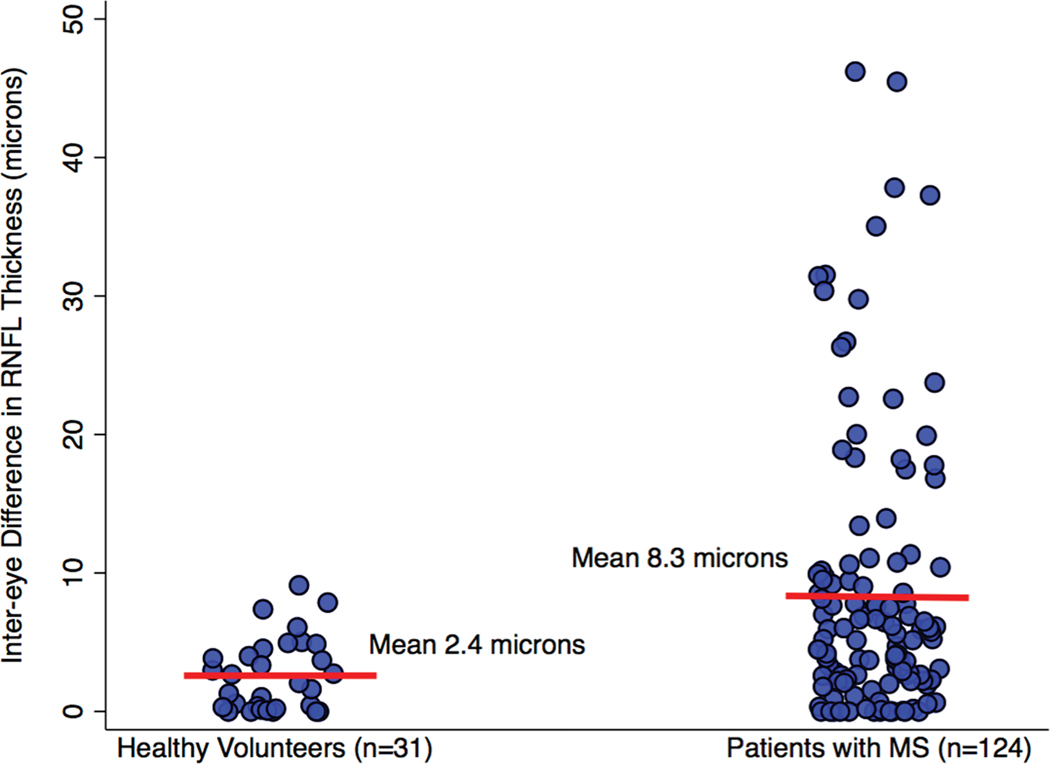
Absolute values for average inter-eye differences in RNFL thickness were greater among patients with MS compared to healthy controls in the convenience sample cohort (Table 1, Fig. 1). Affected eyes of patients with MS had a mean peripapillary RNFL thickness of 77.9±20.0 microns; this value in unaffected eyes of MS patients was 88.6±17.6 microns. Healthy controls, in contrast, had a mean RNFL thickness of 92.9±10.4 microns.
TABLE 1.
Demographic, clinical and OCT data for MS patients and healthy volunteers
| Convenience Sample Cohort | Eyes of Patients with MS and History of Unilateral ON (n=90 Eyes) | |||
|---|---|---|---|---|
| Healthy Volunteers (n=31) | Patients with MS (n=124) | Unaffected Eyes of Patients with History of Acute ON (n=45) | Affected Eyes (History of Acute ON, n=45) | |
| Age, years, mean ± SD | 30 ± 9 | 44 ± 12 | 42 ± 11 | 42 ± 11 |
| Sex, n (% female) | 17 (55%) | 87 (70%) | 34 (76%) | 34 (76%) |
| Relapsing-remitting MS, n (%) | -- | 96 (78%) | -- | -- |
| Disease duration, years, median (range) | -- | 7 (0–30) | -- | -- |
| History of acute unilateral ON, n (%) | -- | 45 (46%) | -- | -- |
| RNFL thickness, microns, mean ± SD, median (range) | 92.9 ± 10.4 93.5 (61.5–116.5) | 85.7 ± 15.8 85.5 (46.5–114.0) | 86.6 ± 17.6 90 (46 – 121) | 77.9 ± 20.0 76 (43 – 123) |
| Inter-eye difference in RNFL thickness, microns, mean ± SD, median (range) | 2.4 ± 1.9 2.0 (0.0–10.0) | 8.3 ± 9.8 5.0 (0.0–45.0) | -- | 13.2 ± 12.6 9.0 (1.0 – 45.0) |
OCT, optical coherence tomography; MS, multiple sclerosis; SD, standard deviation; ON, optic neuritis; RNFL, retinal nerve fiber layer.
FIG. 1.
Scatter plot demonstrating inter-eye differences in RNFL thickness for healthy controls and for patients with MS. Means are indicated by the red bars.
Among healthy controls in the convenience sample cohort, the 95th percentile value for inter-eye difference in RNFL thickness (absolute value) was 6.0 microns. This threshold was examined in 2×2 table analyses as shown in Table 2. In these analyses, convenience sample cohort MS patients served as a validation cohort for examining the thresholds for inter-eye difference in RNFL thickness.
TABLE 2.
Results of 2×2 table analyses of inter-eye difference thresholds for peripapillary RNFL thickness for distinguishing patients with MS in the convenience sample cohort with vs. without a history of acute ON
| Sensitivity | Specificity | Positive Predictive Value | Negative Predicative Value | P-value* | Relative Risk of Acute ON History | |
|---|---|---|---|---|---|---|
| Inter-eye difference in RNFL thickness ≥ 5 microns | 76% | 75% | 72% | 78% | P<0.0001 | 3.4 (95% CI 1.9, 5.8) |
| Inter-eye difference in RNFL thickness ≥ 6 microns | 62% | 81% | 74% | 72% | P<0.0001 | 2.6 (95% CI 1.7, 4.1) |
RNFL, retinal nerve fiber layer; ON, optic neuritis
P-value from chi-square test
Sensitivity = probability of having an RNFL inter-eye difference at or above the threshold value if the patient has a unilateral ON history
Specificity = probability of having an RNFL inter-eye difference below the threshold value if the patient does not have a unilateral ON history
Positive Predictive Value = probability of having a history of acute unilateral ON if the RNFL inter-eye difference is at or above the threshold value
Negative Predictive Value = probability of not having a history of acute unilateral ON if the RNFL inter-eye difference is below the threshold value
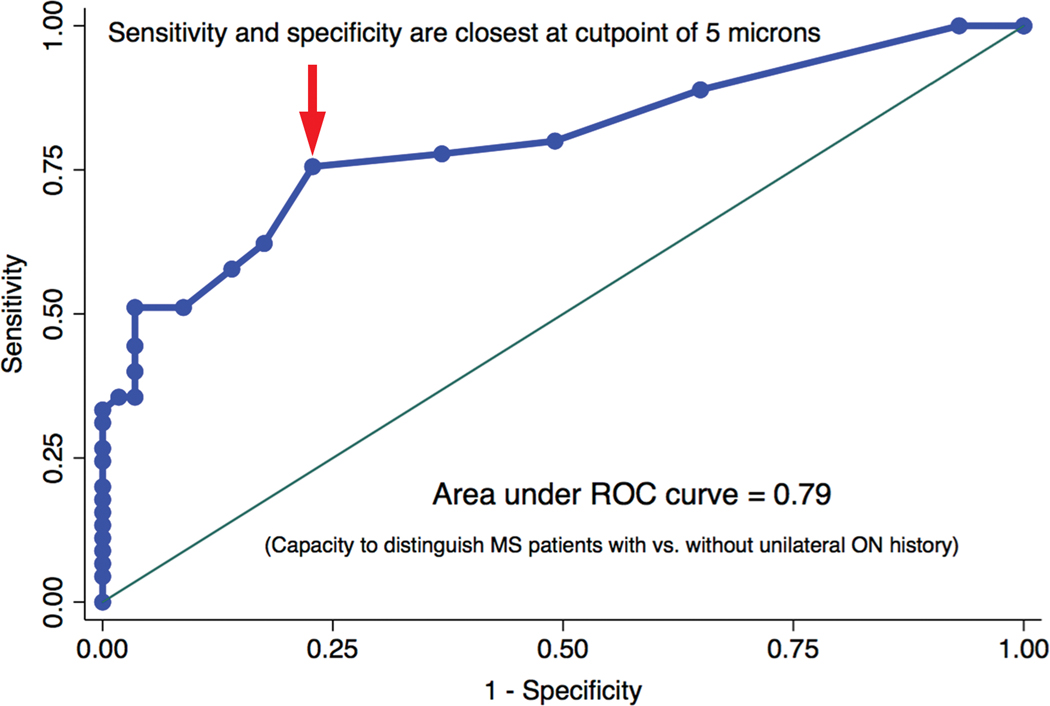
Receiver operating characteristic (ROC) curve analysis examining RNFL thickness inter-eye difference as a continuous variable demonstrated an optimal threshold of 5 microns; this is the value on the ROC curve that optimizes both sensitivity and specificity for distinguishing patients with MS with vs. without a history of acute unilateral ON in the convenience sample cohort (Fig. 2). The 5-micron threshold was also included in the 2×2 table analyses shown in Table 2. Analyses in Table 2 confirmed that sensitivity, specificity, positive predictive value and negative predictive value were most consistent at the 5-micron threshold for inter-eye difference in RNFL thickness. Relative risk of a patient with MS having an acute unilateral ON history vs. not having an ON history was also greatest using the 5-micron cutoff (relative risk 3.4; 95% CI 1.9, 5.8; P<0.0001, chi-square test). Inter-eye differences in RNFL thickness above the thresholds examined in Table 2 were associated with greater likelihood of a patient having an acute unilateral ON history (P≤0.0001, chi-square tests).
FIG. 2.
Receiver operating characteristic (ROC) curve examining inter-eye difference in RNFL thickness as a continuous variable for distinguishing patients with MS with vs. without a history of acute unilateral ON. The optimal threshold value at which sensitivity and specificity are closest together for inter-eye RNFL thickness difference is 5 microns; this value is represented by the point of inflection on the ROC curve indicated by the arrow. The area under the ROC curve is the probability that inter-eye difference in RNFL thickness will correctly classify MS patients with vs. without an acute unilateral ON history. ROC curve areas range between 0.5 (capacity to distinguish no better than flipping a fair coin) and 1.0 (perfect ability to distinguish).
Within the convenience sample MS cohort, the threshold of 6 microns or greater inter-eye difference in RNFL thickness was associated with worse binocular LCLA scores at 2.5% (P=0.03) and 1.25% (P=0.002, linear regression analyses, accounting for age). Specifically, patients with inter-eye differences in RNFL thickness at or above 6 microns had worse scores for binocular LCLA. At the even lower threshold of 5 microns, RNFL thickness inter-eye difference at or above this value was associated with worse binocular LCLA scores at 2.5% (P=0.03) and 1.25% (P=0.009). Among patients with a history of unilateral ON, inter-eye differences in monocular low-contrast letter acuity were associated with greater peripapillary RNFL inter-eye differences, accounting for age (P=0.03, linear regression for 2.5% contrast level).
DISCUSSION
Results of this study demonstrate that inter-eye differences of 5 to 6 microns in peripapillary RNFL thickness are predictive of a unilateral optic nerve lesion in MS. Prior studies have demonstrated abnormal average RNFL thickness values using the 5th percentile in the normative database on time-domain OCT to be 60% sensitive in detecting a history of acute ON, and 77% sensitive in identifying MS patients with a history of severe ON (48). Severe ON was defined as those subjects who had ON with a visual acuity ≤20/200 at onset and poor visual recovery (<20/40). In a study using spectral-domain OCT, RNFL thickness was abnormal in 68% of eyes with a history of ON more than 3 months prior to scanning (49). Using fundus photography and indirect ophthalmoscopy, another study showed that 89% of eyes with history of ON had clinically evident abnormalities of the RNFL, and that 68% of MS patients had clinical evidence suggesting thinning of the RNFL in the absence of acute ON history (50). In post-mortem investigations of the optic nerve in MS, 94–99% of patients, irrespective of a history of acute ON, were found to have RNFL thinning and optic nerve lesions pathologically (6, 7). However, the use of normative database cutoffs may underestimate the occurrence of optic neuropathy compared to inter-eye thresholds.
Costello et al. (51) showed a significant difference between eyes with a history of ON (mean 86.1 microns)) and the fellow eye (mean 109.3 microns)) 6 months after an episode of acute ON. Similarly, Klistorner et al. (44) showed that 6 months following an episode of unilateral ON, the affected eye had reduced RNFL thickness (mean 87.5 microns) compared to fellow eye (mean 105 microns). Similar degrees of RNFL inter-eye difference following acute unilateral ON were demonstrated in a systematic review and meta-analysis of 27 studies by Petzold et al (52) that included time-domain OCT data (estimate for average inter-eye difference 14.57 (95% CI 16.50 to 12.63). The I2 value (I-squared is the percentage of total variation across studies in a meta-analysis that is due to heterogeneity rather than chance) for this composite average for all studies was 81%, appropriately reflecting the heterogeneity inherent in ON and MS disease. Importantly, the average inter-eye difference publishes by Petzold et al was similar to the ON vs. fellow eye differences noted in the RENEW trial (53). Collectively, these studies and others emphasize the degree of RNFL thinning that frequently results from an optic nerve lesion in patients with MS and CIS.
Our data suggest strongly that OCT thresholds of RNFL thickness can identify the presence of an optic nerve lesion in MS. Specifically, an inter-eye difference threshold of 5 microns in our study optimized sensitivity, specificity and predictive values, while a 6-micron cutoff represented the upper boundary expected among healthy controls without MS. Such a threshold for detection of unilateral optic nerve involvement, as calculated using 2×2 table and ROC curve analyses, is necessarily lower than the average degree of inter-eye RNFL thickness difference observed following acute ON.
MRI abnormalities may be difficult to detect in the optic nerve that is asymptomatic. In one study of asymptomatic subjects, 54% of patients had an abnormal VEP indicative of subclinical ON, while none of these individuals demonstrated an optic nerve lesion on dedicated MRI assessments of the anterior visual pathway (54). OCT is far more sensitive than MRI in detecting optic nerve damage and, importantly, the test-retest variability of peripapillary RNFL thickness measurements for spectral-domain OCT is within 2–3 microns.
In a recent consensus article assessing new data on the application of MRI techniques to aid in the diagnosis of MS by the MAGNIMS group, the presence of an optic nerve lesion was recommended for inclusion in the criteria for dissemination in space by expert consensus of the study group. The group recommended clinical examination, MR imaging, OCT measurement of RNFL thickness and VEP latency as factors that could all provide evidence for the rigorous confirmation of an optic nerve lesion (55). Adding evidence of an optic nerve lesion to the diagnostic criteria for MS may further refine the accuracy of diagnosis. In a recent analysis of MS misdiagnoses, Soloman et al. (56) found that 34 (31%) of 79 patients experienced morbidity resulting from misdiagnosis of MS (e.g. such patients were either not treated sufficiently, or were instead treated incorrectly). As such, the detection of an occult optic neuropathy in the clinical setting will not only facilitate MS diagnosis, but may improve outcomes for vision if specific agents can be shown to facilitate protection and repair of the optic nerve.
Further studies will need to determine the role for RNFL as well as ganglion cell layer (GCL) thinning by OCT in evaluating patients with bilateral optic nerve involvement, including bilateral and asymmetric thinning from a previous baseline value. Examining patients with clinically isolated syndromes also will be particularly valuable in determining the role for RNFL and GCL inter-eye asymmetry in predicting the timing and nature of future clinical demyelinating events. Adding OCT-based thresholds for an optic nerve lesion to the MS diagnostic criteria may be helpful in refining the individual components that currently establish the diagnosis of MS.
Acknowledgments
S.L. Galetta has received consulting fees from Biogen. T.C. Frohman has received speaker fees from Novartis, Genzyme and Acorda. E.M. Frohman has received consulting fees from Novartis, Genzyme, Accorda and Teva. P.A. Calabresi has received consulting fees from Vertex and Biogen and has received research funding from Biogen, Novartis, Annexon, Teva and MedImmune; he is co-chairman of the scientific advisory board of OCTIMS study. C. Castrillo-Viguera is an employee of Biogen. D. Cadavid is an employee of Fulcrum Therapeutics. L.J. Balcer has received consulting fees from Biogen. The remaining authors report no conflicts of interest.
Study Funding: National Multiple Sclerosis Society RG 4649A5/1
Footnotes
Statement of Authorship
Category 1:
a. Conception and design
Rachel C. Nolan; Steven L. Galetta, MD; Laura J. Balcer, MD, MSCE
b. Acquisition of data
Rachel C. Nolan; Teresa C. Frohman, PA-C; Elliot M. Frohman, MD, PhD; Peter A. Calabresi, MD; Laura J. Balcer, MD, MSCE
c. Analysis and interpretation of data
Rachel C. Nolan; Laura J. Balcer, MD, MSCE
Category 2:
a. Drafting the manuscript
Rachel C. Nolan; Steven L. Galetta, MD; Laura J. Balcer, MD, MSCE
b. Revising it for intellectual content
Rachel C. Nolan; Steven L. Galetta, MD; Teresa C. Frohman, PA-C; Elliot M. Frohman, MD, PhD; Peter A. Calabresi, MD; Carmen Castrillo-Viguera, MD; Diego Cadavid, MD; Laura J. Balcer, MD, MSCE
Category 3:
a. Final approval of the completed manuscript
Rachel C. Nolan; Steven L. Galetta, MD; Teresa C. Frohman, PA-C; Elliot M. Frohman, MD, PhD; Peter A. Calabresi, MD; Carmen Castrillo-Viguera, MD; Diego Cadavid, MD; Laura J. Balcer, MD, MSCE
REFERENCES
- 1.Sorensen TL, Frederiksen JL, Bronnum-Hansen H, Petersen HC. Optic neuritis as onset manifestation of multiple sclerosis: a nationwide, long-term survey. Neurology. 1999;53:473–478. [DOI] [PubMed] [Google Scholar]
- 2.Balcer LJ. Clinical practice. Optic neuritis. N Engl J Med. 2006;354:1273–1280. [DOI] [PubMed] [Google Scholar]
- 3.Foroozan R, Buono LM, Savino PJ, Sergott RC. Acute demyelinating optic neuritis. Curr Opin Ophthalmol. 2002;13:375–380. [DOI] [PubMed] [Google Scholar]
- 4.Frohman EM, Frohman TC, Zee DS, McColl R, Galetta S. The neuro-ophthalmology of multiple sclerosis. Lancet Neurol. 2005;4:111–121. [DOI] [PubMed] [Google Scholar]
- 5.Arnold AC. Evolving management of optic neuritis and multiple sclerosis. Am J Ophthalmol. 2005;139:1101–1108. [DOI] [PubMed] [Google Scholar]
- 6.Toussaint D, Perier O, Verstappen A, Bervoets S. Clinicopathological study of the visual pathways, eyes, and cerebral hemispheres in 32 cases of disseminated sclerosis. J Clin Neuroophthalmol. 1983;3:211–220. [PubMed] [Google Scholar]
- 7.Ikuta F, Zimmerman HM. Distribution of plaques in seventy autopsy cases of multiple sclerosis in the United States. Neurology. 1976;26:26–28. [DOI] [PubMed] [Google Scholar]
- 8.Group ONS. Multiple sclerosis risk after optic neuritis: final optic neuritis treatment trial follow-up. Archives of neurology. 2008;65:727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simon JH, Kinkel RP, Kollman C, O’Connor P, Fisher E, You X, Hyde R, Group CI. Ten-year follow-up of the ‘minimal MRI lesion’ subgroup from the original CHAMPS Multiple Sclerosis Prevention Trial. Mult Scler. 2015;21:415–422. [DOI] [PubMed] [Google Scholar]
- 10.Brex PA, Ciccarelli O, O’Riordan JI, Sailer M, Thompson AJ, Miller DH. A longitudinal study of abnormalities on MRI and disability from multiple sclerosis. N Engl J Med. 2002;346:158–164. [DOI] [PubMed] [Google Scholar]
- 11.Tintore M, Rovira A, Rio J, Nos C, Grive E, Tellez N, Pelayo R, Comabella M, Montalban X. Is optic neuritis more benign than other first attacks in multiple sclerosis? Ann Neurol. 2005;57:210–215. [DOI] [PubMed] [Google Scholar]
- 12.Kuhle J, Disanto G, Dobson R, Adiutori R, Bianchi L, Topping J, Bestwick JP, Meier UC, Marta M, Dalla Costa G, Runia T, Evdoshenko E, Lazareva N, Thouvenot E, Iaffaldano P, Direnzo V, Khademi M, Piehl F, Comabella M, Sombekke M, Killestein J, Hegen H, Rauch S, D’Alfonso S, Alvarez-Cermeno JC, Kleinova P, Horakova D, Roesler R, Lauda F, Llufriu S, Avsar T, Uygunoglu U, Altintas A, Saip S, Menge T, Rajda C, Bergamaschi R, Moll N, Khalil M, Marignier R, Dujmovic I, Larsson H, Malmestrom C, Scarpini E, Fenoglio C, Wergeland S, Laroni A, Annibali V, Romano S, Martinez AD, Carra A, Salvetti M, Uccelli A, Torkildsen O, Myhr KM, Galimberti D, Rejdak K, Lycke J, Frederiksen JL, Drulovic J, Confavreux C, Brassat D, Enzinger C, Fuchs S, Bosca I, Pelletier J, Picard C, Colombo E, Franciotta D, Derfuss T, Lindberg R, Yaldizli O, Vecsei L, Kieseier BC, Hartung HP, Villoslada P, Siva A, Saiz A, Tumani H, Havrdova E, Villar LM, Leone M, Barizzone N, Deisenhammer F, Teunissen C, Montalban X, Tintore M, Olsson T, Trojano M, Lehmann S, Castelnovo G, Lapin S, Hintzen R, Kappos L, Furlan R, Martinelli V, Comi G, Ramagopalan SV, Giovannoni G. Conversion from clinically isolated syndrome to multiple sclerosis: A large multicentre study. Mult Scler. 2015;21:1013–1024. [DOI] [PubMed] [Google Scholar]
- 13.Polman CH, Reingold SC, Banwell B, Clanet M, Cohen JA, Filippi M, Fujihara K, Havrdova E, Hutchinson M, Kappos L, Lublin FD, Montalban X, O’Connor P, Sandberg-Wollheim M, Thompson AJ, Waubant E, Weinshenker B, Wolinsky JS. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011;69:292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frohman EM, Racke MK, Raine CS. Multiple sclerosis--the plaque and its pathogenesis. N Engl J Med. 2006;354:942–955. [DOI] [PubMed] [Google Scholar]
- 15.Thompson AJ, Banwell BL, Barkhof F, Carroll WM, Coetzee T, Comi G, Correale J, Fazekas F, Fiippi M, Freedman MS, Fujihara K, Galetta SL, Hartung HP, Kappos L, Lublin FD, Marrie RA, Miller AE, Miller DH, Montalban X, Mowry EM, Soelberg-Sorensen P, Tintore M, Traboulsee AL, Trojano M, Uitdehhaag BMJ, Vukusic S, Waubant E, Weinshenker BG, Reingold SC, Cohen JA. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald Criteria. Lancet Neurol 2017, in press. [DOI] [PubMed] [Google Scholar]
- 16.Galetta SL, Balcer LJ. The optic nerve should be included as one of the typical CNS regions for establishing dissemination in space when diagnosing MS – Yes. Mult Scler 2017; Oct 1 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 17.Gelfand JM, Goodin DS, Boscardin WJ, Nolan R, Cuneo A, Green AJ. Retinal axonal loss begins early in the course of multiple sclerosis and is similar between progressive phenotypes. PLoS One. 2012;7:e36847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Costello F, Hodge W, Pan YI, Freedman M, DeMeulemeester C. Differences in retinal nerve fiber layer atrophy between multiple sclerosis subtypes. J Neurol Sci. 2009;281:74–79. [DOI] [PubMed] [Google Scholar]
- 19.Costello F, Hodge W, Pan YI, Eggenberger E, Freedman MS. Using retinal architecture to help characterize multiple sclerosis patients. Can J Ophthalmol. 2010;45:520–526. [DOI] [PubMed] [Google Scholar]
- 20.Oberwahrenbrock T, Schippling S, Ringelstein M, Kaufhold F, Zimmermann H, Keser N, Young KL, Harmel J, Hartung H-P, Martin R. Retinal damage in multiple sclerosis disease subtypes measured by high-resolution optical coherence tomography. Multiple sclerosis international. 2012;2012:530305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oberwahrenbrock T, Ringelstein M, Jentschke S, Deuschle K, Klumbies K, Bellmann-Strobl J, Harmel J, Ruprecht K, Schippling S, Hartung HP, Aktas O, Brandt AU, Paul F. Retinal ganglion cell and inner plexiform layer thinning in clinically isolated syndrome. Mult Scler. 2013;19:1887–1895. [DOI] [PubMed] [Google Scholar]
- 22.Lange AP, Zhu F, Sayao A-L, Sadjadi R, Alkabie S, Traboulsee AL, Costello F, Tremlett H. Retinal nerve fiber layer thickness in benign multiple sclerosis. Multiple Sclerosis Journal. 2013;19:1275–1281. [DOI] [PubMed] [Google Scholar]
- 23.Pulicken M, Gordon-Lipkin E, Balcer LJ, Frohman E, Cutter G, Calabresi PA. Optical coherence tomography and disease subtype in multiple sclerosis. Neurology. 2007;69:2085–2092. [DOI] [PubMed] [Google Scholar]
- 24.Talman LS, Bisker ER, Sackel DJ, Long DA, Galetta KM, Ratchford JN, Lile DJ, Farrell SK, Loguidice MJ, Remington G. Longitudinal study of vision and retinal nerve fiber layer thickness in multiple sclerosis. Annals of neurology. 2010;67:749–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gordon-Lipkin E, Chodkowski B, Reich DS, Smith SA, Pulicken M, Balcer LJ, Frohman EM, Cutter G, Calabresi PA. Retinal nerve fiber layer is associated with brain atrophy in multiple sclerosis. Neurology. 2007;69:1603–1609. [DOI] [PubMed] [Google Scholar]
- 26.Raphael BA, Galetta KM, Jacobs DA, Markowitz CE, Liu GT, Nano-Schiavi ML, Galetta SL, Maguire MG, Mangione CM, Globe DR. Validation and test characteristics of a 10-item neuro-ophthalmic supplement to the NEI-VFQ-25. American journal of ophthalmology. 2006;142:1026–1035. e1022. [DOI] [PubMed] [Google Scholar]
- 27.Walter SD, Ishikawa H, Galetta KM, Sakai RE, Feller DJ, Henderson SB, Wilson JA, Maguire MG, Galetta SL, Frohman E. Ganglion cell loss in relation to visual disability in multiple sclerosis. Ophthalmology. 2012;119:1250–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fisher JB, Jacobs DA, Markowitz CE, Galetta SL, Volpe NJ, Nano-Schiavi ML, Baier ML, Frohman EM, Winslow H, Frohman TC. Relation of visual function to retinal nerve fiber layer thickness in multiple sclerosis. Ophthalmology. 2006;113:324–332. [DOI] [PubMed] [Google Scholar]
- 29.Martinez-Lapiscina EH, Arnow S, Wilson JA, Saidha S, Preiningerova JL, Oberwahrenbrock T, Brandt AU, Pablo LE, Guerrieri S, Gonzalez I, Outteryck O, Mueller AK, Albrecht P, Chan W, Lukas S, Balk LJ, Fraser C, Frederiksen JL, Resto J, Frohman T, Cordano C, Zubizarreta I, Andorra M, Sanchez-Dalmau B, Saiz A, Bermel R, Klistorner A, Petzold A, Schippling S, Costello F, Aktas O, Vermersch P, Oreja-Guevara C, Comi G, Leocani L, Garcia-Martin E, Paul F, Havrdova E, Frohman E, Balcer LJ, Green AJ, Calabresi PA, Villoslada P. Retinal thickness measured with optical coherence tomography and risk of disability worsening in multiple sclerosis: a cohort study. Lancet Neurol. 2016;15:574–584. [DOI] [PubMed] [Google Scholar]
- 30.Saidha S, Syc SB, Durbin MK, Eckstein C, Oakley JD, Meyer SA, Conger A, Frohman TC, Newsome S, Ratchford JN. Visual dysfunction in multiple sclerosis correlates better with optical coherence tomography derived estimates of macular ganglion cell layer thickness than peripapillary retinal nerve fiber layer thickness. Multiple Sclerosis Journal. 2011;17:1449–1463. [DOI] [PubMed] [Google Scholar]
- 31.Sabadia SB, Nolan RC, Galetta KM, Narayana KM, Wilson JA, Calabresi PA, Frohman EM, Galetta SL, Balcer LJ. 20/40 or Better Visual Acuity After Optic Neuritis: Not as Good as We Once Thought? J Neuroophthalmol. 2016;36: 369–376. [DOI] [PubMed] [Google Scholar]
- 32.Costello F, Coupland S, Hodge W, Lorello GR, Koroluk J, Pan YI, Freedman MS, Zackon DH, Kardon RH. Quantifying axonal loss after optic neuritis with optical coherence tomography. Ann Neurol. 2006;59:963–969. [DOI] [PubMed] [Google Scholar]
- 33.Mowry EM, Loguidice MJ, Daniels AB, Jacobs DA, Markowitz CE, Galetta SL, Nano-Schiavi ML, Cutter GR, Maguire MG, Balcer LJ. Vision related quality of life in multiple sclerosis: correlation with new measures of low and high contrast letter acuity. J Neurol Neurosurg Psychiatry. 2009;80:767–772. [DOI] [PubMed] [Google Scholar]
- 34.Sakai RE, Feller DJ, Galetta KM, Galetta SL, Balcer LJ. Vision in multiple sclerosis: the story, structure-function correlations, and models for neuroprotection. J Neuroophthalmol. 2011;31:362–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sepulcre J, Murie-Fernandez M, Salinas-Alaman A, Garcia-Layana A, Bejarano B, Villoslada P. Diagnostic accuracy of retinal abnormalities in predicting disease activity in MS. Neurology. 2007;68:1488–1494. [DOI] [PubMed] [Google Scholar]
- 36.Toledo J, Sepulcre J, Salinas-Alaman A, Garcia-Layana A, Murie-Fernandez M, Bejarano B, Villoslada P. Retinal nerve fiber layer atrophy is associated with physical and cognitive disability in multiple sclerosis. Mult Scler. 2008;14:906–912. [DOI] [PubMed] [Google Scholar]
- 37.Behbehani R, Al-Hassan AA, Al-Khars A, Sriraman D, Alroughani R. Retinal nerve fiber layer thickness and neurologic disability in relapsing-remitting multiple sclerosis. J Neurol Sci. 2015;359:305–308. [DOI] [PubMed] [Google Scholar]
- 38.El Ayoubi NK, Ghassan S, Said M, Allam J, Darwish H, Khoury SJ. Retinal measures correlate with cognitive and physical disability in early multiple sclerosis. J Neurol. 2016;263:2287–2295. [DOI] [PubMed] [Google Scholar]
- 39.Ma SL, Shea JA, Galetta SL, Jacobs DA, Markowitz CE, Maguire MG, Balcer LJ. Self-reported visual dysfunction in multiple sclerosis: new data from the VFQ-25 and development of an MS-specific vision questionnaire. Am J Ophthalmol. 2002;133:686–692. [DOI] [PubMed] [Google Scholar]
- 40.Baier ML, Cutter GR, Rudick RA, Miller D, Cohen JA, Weinstock-Guttman B, Mass M, Balcer LJ. Low-contrast letter acuity testing captures visual dysfunction in patients with multiple sclerosis. Neurology. 2005;64:992–995. [DOI] [PubMed] [Google Scholar]
- 41.Garcia-Martin E, Rodriguez-Mena D, Herrero R, Almarcegui C, Dolz I, Martin J, Ara JR, Larrosa JM, Polo V, Fernandez J, Pablo LE. Neuro-ophthalmologic evaluation, quality of life, and functional disability in patients with MS. Neurology. 2013;81:76–83. [DOI] [PubMed] [Google Scholar]
- 42.Henderson AP, Altmann DR, Trip AS, Kallis C, Jones SJ, Schlottmann PG, Garway-Heath DF, Plant GT, Miller DH. A serial study of retinal changes following optic neuritis with sample size estimates for acute neuroprotection trials. Brain. 2010;133:2592–2602. [DOI] [PubMed] [Google Scholar]
- 43.Kupersmith MJ, Garvin MK, Wang JK, Durbin M, Kardon R. Retinal ganglion cell layer thinning within one month of presentation for optic neuritis. Mult Scler. 2015;22:641–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kupersmith MJ, Anderson S, Kardon R. Predictive value of 1 month retinal nerve fiber layer thinning for deficits at 6 months after acute optic neuritis. Multiple Sclerosis Journal. 2013:1352458513485149. [DOI] [PubMed] [Google Scholar]
- 45.Balcer LJ, Raynowska J, Nolan R, Galetta SL, Kapoor R, Benedict R, Phillips G, LaRocca N, Hudson L, Rudick R, Multiple Sclerosis Outcome Assessments C. Validity of low-contrast letter acuity as a visual performance outcome measure for multiple sclerosis. Mult Scler. 2017;23:734–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Klistorner A, Arvind H, Garrick R, Graham SL, Paine M, Yiannikas C. Interrelationship of optical coherence tomography and multifocal visual-evoked potentials after optic neuritis. Invest Ophthalmol Vis Sci. 2010;51:2770–2777. [DOI] [PubMed] [Google Scholar]
- 47.Tewarie P, Balk L, Costello F, Green A, Martin R, Schippling S, Petzold A. The OSCAR-IB consensus criteria for retinal OCT quality assessment. PLoS One. 2012;7:e34823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Naismith RT, Tutlam NT, Xu J, Shepherd JB, Klawiter EC, Song SK, Cross AH. Optical coherence tomography is less sensitive than visual evoked potentials in optic neuritis. Neurology. 2009;73:46–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Di Maggio G, Santangelo R, Guerrieri S, Bianco M, Ferrari L, Medaglini S, Rodegher M, Colombo B, Moiola L, Chieffo R, Del Carro U, Martinelli V, Comi G, Leocani L. Optical coherence tomography and visual evoked potentials: which is more sensitive in multiple sclerosis? Mult Scler. 2014;20:1342–1347. [DOI] [PubMed] [Google Scholar]
- 50.Elbol P, Work K. Retinal nerve fiber layer in multiple sclerosis. Acta Ophthalmol (Copenh). 1990;68:481–486. [DOI] [PubMed] [Google Scholar]
- 51.Costello F, Hodge W, Pan Y, Eggenberger E, Coupland S, Kardon R. Tracking retinal nerve fiber layer loss after optic neuritis: a prospective study using optical coherence tomography. Multiple Sclerosis. 2008;14:839–905. [DOI] [PubMed] [Google Scholar]
- 52.Petzold A, de Boer JF, Schippling S, Vermersch P, Kardon R, Green A, Calabresi PA, Polman C. Optical coherence tomography in multiple sclerosis: a systematic review and meta-analysis. The Lancet Neurology. 2010;9:921–932. [DOI] [PubMed] [Google Scholar]
- 53.Cadavid D, Balcer L, Galetta S, Aktas O, Ziemssen T, Vanopdenbosch L, Frederiksen J, Skeen M, Jaffe GJ, Butzkueven H, Ziemssen F, Massacesi L, Chai Y, Xu L, Freeman S, Investigators RS. Safety and efficacy of opicinumab in acute optic neuritis (RENEW): a randomised, placebo-controlled, phase 2 trial. Lancet Neurol. 2017;16:189–199. [DOI] [PubMed] [Google Scholar]
- 54.Sisto D, Trojano M, Vetrugno M, Trabucco T, Iliceto G, Sborgia C. Subclinical visual involvement in multiple sclerosis: a study by MRI, VEPs, frequency-doubling perimetry, standard perimetry, and contrast sensitivity. Invest Ophthalmol Vis Sci. 2005;46:1264–1268. [DOI] [PubMed] [Google Scholar]
- 55.Filippi M, Rocca MA, Ciccarelli O, De Stefano N, Evangelou N, Kappos L, Rovira A, Sastre-Garriga J, Tintorè M, Frederiksen JL, Gasperini C, Palace J, Reich DS, Banwell B, Montalban X, Barkhof F. MRI criteria for the diagnosis of multiple sclerosis: MAGNIMS consensus guidelines. The Lancet Neurology. 2016;15:292–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Solomon AJ, Bourdette DN, Cross AH, Applebee A, Skidd PM, Howard DB, Spain RI, Cameron MH, Kim E, Mass MK, Yadav V, Whitham RH, Longbrake EE, Naismith RT, Wu GF, Parks BJ, Wingerchuk DM, Rabin BL, Toledano M, Tobin WO, Kantarci OH, Carter JL, Keegan BM, Weinshenker BG. The contemporary spectrum of multiple sclerosis misdiagnosis: A multicenter study. Neurology. 2016;87:1393–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]