Abstract
We cloned the AFMP1 gene, which encodes the first antigenic cell wall galactomannoprotein in Aspergillus fumigatus. AFMP1 codes for a protein, Afmp1p, of 284 amino acid residues, with a few sequence features that are present in Mp1p, the antigenic cell wall mannoprotein in Penicillium marneffei that we described previously, as well as several other cell wall proteins of Saccharomyces cerevisiae and Candida albicans. It contains a serine- and threonine-rich region for O glycosylation, a signal peptide, and a putative glycosylphosphatidyl inositol attachment signal sequence. Specific anti-Afmp1p antibody was generated with recombinant Afmp1p protein purified from Escherichia coli to allow further characterization of Afmp1p. Afmp1p has a high affinity for Galanthus nivalis agglutinin, a characteristic indicative of a mannoprotein. Furthermore, it was recognized by a rat monoclonal antibody against the galactofuran side chain of galactomannan, indicating that it is a galactomannoprotein. Ultrastructural analysis by immunogold staining indicated that Afmp1p is present in the cell walls of the hyphae and conidia of A. fumigatus. Finally, it was observed that patients with aspergilloma and invasive aspergillosis due to A. fumigatus develop a specific antibody response against Afmp1p. This suggested that the recombinant protein and its antibody may be useful for serodiagnosis in patients with aspergilloma or invasive aspergillosis, and the protein may represent a good cell surface target for host humoral immunity.
Since the last decade, Aspergillus spp. have been gaining prominence as opportunistic pathogens. In immunocompetent hosts, Aspergillus spp. rarely causes serious illnesses except for aspergilloma in patients with preexisting chronic lung diseases. On the other hand, invasive aspergillosis is one of the most important infectious causes of mortality in patients with hematological malignancies and bone marrow transplant (BMT) recipients, with an incidence of 6% in our recent study with 230 BMT recipients (35). Furthermore, up to 2.5% of solid organ transplant recipients, 12% of patients with AIDS, and 40% of patients with chronic granulomatous disease could be affected by this infection (12). The mortality rate in patients with invasive aspergillosis with pulmonary involvement and persistent neutropenia was 95% (8). Of all the known Aspergillus spp., Aspergillus fumigatus is the most common species associated with human disease.
The successful management of invasive aspergillosis is hampered by difficulties in establishing a diagnosis. The “gold standard” for making a diagnosis is to obtain a positive culture of A. fumigatus and to demonstrate histological evidence of mycelial invasion from tissue specimens obtained by biopsy. Due to the very sick nature of these patients and often the presence of bleeding diathesis, tissue biopsy is often not possible or acceptable by patients. Although commercial kits for antigen detection assays with a monoclonal antibody against the galactomannan antigen extract are available for clinical use, no antigen detection kit based on recombinant antigens of Aspergillus is available for the serodiagnosis of invasive aspergillosis. Recombinant antibody and antigen detection tests may offer higher sensitivities, specificities, and reproducibilities. Moreover, tests with recombinant antigens and generated antibodies are easy to standardize.
We have previously described the cloning and characterization of a highly antigenic cell wall mannoprotein (Mp1p) in Penicillium marneffei (2), and have shown that an enzyme-linked immunosorbent assay based on recombinant Mp1p is very useful for the serodiagnosis of penicilliosis marneffei (3, 4). Since there are no recombinant antigen-based kits for the serodiagnosis of A. fumigatus infections, it would be logical to search for the Mp1p homolog in A. fumigatus and examine its potential for use for serodiagnostic purposes.
Here we report on the cloning of the AFMP1 gene, which encodes an antigenic cell wall galactomannoprotein of A. fumigatus (Afmp1p). Sequence analysis reveals that Afmp1p is homologous to Mp1p. Indirect immunofluorescence and immunoelectron microscopy studies indicate that Afmp1p is specifically located in the cell walls of A. fumigatus. Finally, our results show that patients with invasive A. fumigatus infections develop high levels of specific antibody against Afmp1p, suggesting that Afmp1p may represent a good cell surface target for host humoral immunity.
MATERIALS AND METHODS
Strains and growth conditions.
The A. fumigatus strain isolated from a BMT recipient (strain UPN158) was used throughout the study. A 1-μl suspension of conidia obtained by flushing the surface of A. fumigatus colonies grown on Sabouraud agar at 37°C for 4 days was used to inoculate 25 ml of Czapek Dox medium (Difco) in a 500-ml conical flask at 37°C in a gyratory shaker. A 2-day-old culture was harvested for RNA extraction. Escherichia coli XL-1 Blue and SOLR, from Stratagene (La Jolla, Calif.), were used for screening of the cDNA library and for phage-to-plasmid conversion.
Generation of antibodies.
To produce a polyclonal guinea pig antibody, 10 ml of mycelial sediment from a 1-day-old culture was washed three times in phosphate-buffered saline (PBS; 13.7 mM sodium chloride, 0.27 mM potassium chloride, 1 mM phosphate buffer [pH 7.4]) and was suspended in PBS with 0.05% phenol to a turbidity of McFarland no. 3 standard. An equal volume of complete Freund's adjuvant was mixed with 500 μl of mycelial suspension, and the mixture was injected intramuscularly into a guinea pig's thigh. Incomplete Freund's adjuvant was used in subsequent immunizations, and a total of four inoculations were completed in 2 months.
To prepare antibodies specific for Afmp1p, 250 μg of purified glutathione S-transferase (GST)–Afmp1p recombinant protein (250 μl) was mixed with an equal part of complete Freund's adjuvant, and the mixture was injected subcutaneously into rabbits and guinea pigs. Incomplete Freund's adjuvant was used in subsequent injections. Serum samples were taken 2 weeks after the third injection.
Cloning of AFMP1 gene.
The MP1 gene, which encodes a highly antigenic cell wall mannoprotein in P. marneffei, was cloned and characterized previously (2). By performing BLAST analysis with the National Center for Biotechnology Information server at the National Library of Medicine (Bethesda, Md.) and a server at Stanford University containing databases of the complete Saccharomyces cerevisiae genome, a short A. fumigatus expressed sequence tag (EST; GenBank accession no. AA12162.1) for which no information has previously been presented in any form was found.
Total A. fumigatus RNA was isolated from 25 ml of A. fumigatus cells with TRIzol reagent (Gibco BRL). Poly(A)+ RNA was obtained with a QuickPrep Micro mRNA purification kit (Pharmacia, Uppsala, Sweden), based on the conventional oligo(dT) cellulose method. The mRNA was then used to construct a bacteriophage lambda ZAP cDNA expression library (Stratagene). The library had at least 106 independent phage plaques, with more than 95% containing inserts with an average size of 1 kb.
Hybridization probes were produced by amplifying the A. fumigatus cDNA library with 0.5 μM primers specific to the immunoglobulin E (IgE) binding protein sequence (primers AMPF1 [5′-TCTCCTCCTACAACGGTGGT-3′] and primer AMPR1 [5′-AGAGGTCAGAGCCAGAGCAT-3′]; Gibco BRL, Rockville, Md.). The PCR mixture (100 μl) contained denatured A. fumigatus DNA, PCR buffer (10 mM Tris-HCl [pH 8.3], 50 mM KCl, 3 mM MgCl2, 0.01% gelatin), each deoxynucleoside triphosphate at a concentration of 200 μM, and 2.5 U of Taq polymerase (Perkin-Elmer Cetus, Norwalk, Conn.). The sample was amplified in 25 cycles of 94°C for 1.5 min, 55°C for 1.5 min, and 72°C for 1.5 min, with a final extension at 72°C for 8 min, in an automated thermal cycler (Perkin-Elmer Cetus, Gouda, The Netherlands). A total of 100 ng of the agarose gel-purified 136-bp amplified product was labeled with [32P]dATP (Amersham, Buckinghamshire, United Kingdom) by using the Prime-It II Random Primer Labeling kit according to the manufacturer's instructions (Stratagene).
Approximately 50,000 plaques of the cDNA library were screened with the labeled hybridization probe according to the manufacturer's instructions. Nine positive phage clones were isolated, and their cDNA inserts were excised with ExAssist helper phage in SOLR cells (Stratagene), yielding pBluescript SK (pBSK) plasmids containing the inserts.
DNA sequencing.
DNA sequencing was carried out by using vector primers of pBSK (T3 and T7) and additional primers designed from the sequencing data of the first round of the sequencing reaction (primer LPW270 [5′-AAGGTCCCTGAGTCCCTCTC-3′] and primer LPW271 [5′-CCGAGGGAGTAGCTGAAGTG-3′]; Gibco BRL). Bidirectional sequencing was performed with an Applied Biosystems automatic sequencer. The DNA sequence was analyzed with a Genetics Computer Group (Madison, Wis.) program (version 8.0). BLAST analysis was performed with the National Center for Biotechnology Information server at the National Library of Medicine and a server at Stanford University containing databases of the complete S. cerevisiae genome. The searches were done at both the protein and the DNA levels.
Expression and purification of recombinant Afmp1p protein from E. coli.
To produce a fusion plasmid for protein purification, primers were used to amplify the AFMP1 gene from plasmid pBSK-AFMP1. The sequence coding for amino acid residues 18 to 284 of Afmp1p was amplified and cloned into the BamHI and EcoRI sites of expression vector pGEX-2T in frame and downstream of the GST coding sequence. The GST-Afmp1p fusion protein was expressed and purified with a GST Gene Fusion System (Pharmacia) according to the manufacturer's instructions. Approximately 10 mg of purified protein was routinely obtained from 1 liter of E. coli carrying the fusion plasmid.
In vitro translation of AFMP1 and immunoprecipitation assays.
Afmp1p was translated in vitro with the TNT coupled reticulocyte lysate system (Promega, Madison, Wis.). Briefly, the reaction was set up with 1 μg of plasmid pcDNA3-AFMP1 (subcloned from pBSK-AFMP1), 4 μl of [35S]methionine (1,000 Ci/mmol at 10 mCi/ml), reaction buffer, an amino acid mixture without methionine, RNA polymerase, and 25 μl of rabbit reticulocyte lysate. The mixture was incubated at 30°C for 2 h, and 0.5 μl of the protein translated in vitro was analyzed in a sodium dodecyl sulfate (SDS)–10% polyacrylamide gel. The protein translated in vitro was immunoprecipitated with preimmunized guinea pig serum, the serum of a guinea pig immunized with A. fumigatus, sera from patients with aspergilloma, sera from acute myeloid leukemia patients with invasive aspergillosis, sera from patients with candidemia, sera from patients with P. marneffei infection, sera from patients with E. coli bacteremia, or sera from healthy blood donors. All sera were diluted 1:1,000. Immune complexes were separated on an SDS–10% polyacrylamide gel, followed by fluorography.
Mannoprotein detection assay.
Whole-cell A. fumigatus lysate or a lyticase wash extract was immunoprecipitated with preimmunized guinea pig serum, serum from a guinea pig immunized with A. fumigatus, or serum from a guinea pig immunized with purified Afmp1p. Immune complexes were again separated on an SDS–10% polyacrylamide gel and were then electroblotted onto a nitrocellulose membrane. The blots were incubated with digoxigenin (DIG)-labeled Galanthus nivalis agglutinin (Roche Biochemicals, Mannheim, Germany) which recognizes the terminal mannose. The resulting DIG-labeled mannoconjugates were detected with the addition of anti-DIG antibody conjugated with alkaline phosphatase and the chromogenic substrate 4-nitroblue tetrazolium chloride–5-bromo-4-chloro-3-indoylphosphate.
Galactomannan detection assay.
Whole-cell A. fumigatus lysate was immunoprecipitated with preimmunized guinea pig serum or serum from a guinea pig immunized with purified Afmp1p, and immune complexes were separated and electroblotted as described above. One blot was incubated with rat antigalactomannan monoclonal antibody (a gift from Jean-Paul Latge). The resulting antigen-antibody interaction was detected by the addition of rabbit anti-rat antibody conjugated to horseradish peroxidase (Zymed Laboratories Inc., South San Francisco, Calif.) and detected via enhanced chemiluminescence fluorescence (Amersham Life Science, Buckinghamshire, United Kingdom). The other blot was incubated with serum from a rabbit immunized with purified Afmp1p. The resulting antigen-antibody interaction was detected by the addition of goat anti-rabbit antibody conjugated to horseradish peroxidase and was detected by enhanced chemiluminescence fluorescence.
Indirect immunofluorescence staining.
In the indirect immunofluorescence staining assay, A. fumigatus mold cells were harvested and washed twice in PBS. The cells were deposited on Teflon-coated slides, air dried, and fixed in cold acetone for 10 min. Rabbit serum with antibodies specific for Afmp1p was added to the fixed cells, and the mixture was incubated in a humidity chamber at 37°C for 45 min. Rabbit serum with antibodies directed against the whole cell of A. fumigatus and a preimmune rabbit serum were used as the positive and negative controls, respectively. The cells were then washed with PBS, air dried, and incubated with affinity-purified fluorescein isothiocyanate-conjugated anti-rabbit IgG (DAKO A/S, Glostrup, Denmark) at 37°C for 45 min. The cells were mounted and observed under UV light.
Immunogold staining and electron microscopy.
A. fumigatus mycelia and conidia were harvested and washed twice in PBS. The cells and conidia were fixed in filtered PBS containing 4% (wt/vol) paraformaldehyde and 2% (vol/vol) glutaraldehyde for 30 min at room temperature, followed by dehydration in a graded series of ethanol and embedment in acrylic resin (LR White; Sigma). Ultrathin sections were cut and mounted onto 200-mesh gold grids for immunostaining.
For immunostaining, sections were blocked for 20 min in 3% (wt/vol) bovine serum albumin, fraction V (BSA; Sigma). Rabbit anti-Afmp1p serum (diluted 1:80 in PBS with 3% BSA) was added and incubated with the cell sections for 2 h, with preimmune rabbit serum used as the negative control. After the sections were washed in PBS containing 0.1% Tween 20, they were incubated in 1% TBSA (20 mM Tris [pH 8.2], 1% BSA) containing 1:20-diluted goat anti-rabbit immunoglobulin G conjugated with gold particles of 10 nm in diameter (Amersham). After the sections were washed in 1% TBSA, they were counterstained with uranyl acetate and lead citrate. Electron microscopy work was performed with a JEOL 100SX transmission electron microscope at 80 kV.
Nucleotide sequence accession number.
The nucleotide sequence of the AFMP1 gene has been deposited with GenBank under accession no. AY007312.
RESULTS
Cloning of AFMP1.
About 50,000 independent phage plaques were screened with the amplified DNA fragments of the EST of A. fumigatus (GenBank accession no. AA12162.1). Nine positive plaques were selected, purified, and converted into plasmids. When induced with isopropyl-β-d-thiogalactopyranoside, four of the nine isolates produced protein bands of about 32 kDa that were recognized by the guinea pig hyperimmune serum on a Western blot (data not shown). PCR and partial sequence analysis of the four clones revealed a single gene of about 0.85 kb which was named AFMP1 (for Aspergillus fumigatus mannoprotein 1). (A U.S. patent application [docket no. 609920] was filed on 2 May 2000.)
Sequence analysis of AFMP1.
Bidirectional DNA sequencing of AFMP1 revealed that the cDNA contained a single open reading frame encoding 284 amino acid residues with a predicted molecular mass of 31.4 kDa. The DNA and predicted protein sequences are shown in Fig. 1.
FIG. 1.
DNA and amino acid sequences of Afmp1p. AFMP1 cDNA contains a single open reading frame that encodes 284 amino acid residues and that has a predicted molecular mass of 31.4 kDa. The N-terminal cleavable signal peptide of 17 amino acids is underlined. The C-terminal cleavable glycosylphosphatidyl inositol signal peptide of 18 amino acids is double underlined. An 84-amino-acid serine- and threonine-rich region is indicated in italics, indicating that this protein may have many O-glycosylation sites. The asterisk indicates the stop codon, and the arrow indicates a potential cleavage site.
Protein sequence analysis suggested that Afmp1p is likely a fungal cell wall protein. BLAST analysis was performed to search for homologs that might suggest the potential biological functions of AFMP1. Careful examination of the Afmp1p sequence revealed that it has several features that are similar to Mp1p of P. marneffei and several other fungal cell wall proteins (1, 14, 15, 23, 25, 26, 29, 32), suggesting that Afmp1p is most likely a fungal cell wall protein. Similar to Mp1p, Afmp1p has a putative N-terminal signal peptide found in most secretory proteins (26, 34). It also has a putative C-terminal glycosylphosphatidyl inositol membrane attachment signal sequence that is commonly used for cytoplasmic membrane attachment (5, 31) and that has been implicated in fungal cell wall assembly (9). After processing of Afmp1p, it should have 267 amino acid residues with a predicted molecular mass of 29.5 kDa as a polypeptide.
Afmp1p is expected to be a glycosylated protein, since it has potential O-glycosylation sites. These sites are found in an 84-amino-acid serine- and threonine-rich region in its C-terminal half, which is similar to Mp1p as well as some cell wall proteins of S. cerevisiae and Candida albicans (1). However, unlike Mp1p, which contains two potential N-glycosylation sites, Afmp1p does not contain any potential N-glycosylation sites.
Expression and purification of Afmp1p and production of Afmp1p-specific antibodies.
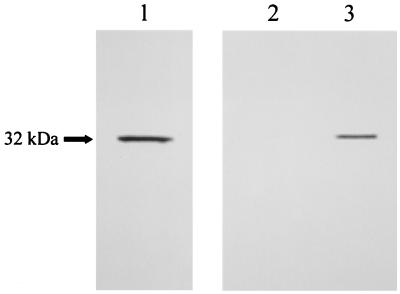
To identify the Afmp1p protein, AFMP1 cDNA was transcribed and translated in vitro with the TNT coupled reticulocyte lysate system in the presence of [35S]methionine. The resulting protein was run on an SDS-polyacrylamide gel, and a protein band of about 32 kDa was visualized (Fig. 2, lane 1). This 32-kDa protein was specifically immunoprecipitated with guinea pig anti-A. fumigatus serum (Fig. 2, lane 3) but not with preimmune serum (Fig. 2, lane 2), confirming that Afmp1p is an immunogenic protein of A. fumigatus in guinea pigs.
FIG. 2.
In vitro translation of AFMP1. AFMP1 was translated in vitro with Promega's rabbit reticulocyte lysate, and a band of about 32 kDa could be detected (lane 1). Afmp1p was immunoprecipitated with guinea pig immune serum against A. fumigatus cells (lane 3) but not with preimmune serum (lane 2).
To investigate the biochemical properties, cellular localization, and biological function of Afmp1p, as well as its potential role in fungal pathogenesis and antifungal immunity, specific antibodies against this protein were produced. The purified GST-Afmp1p protein was used to immunize guinea pigs and rabbits to generate specific antisera. The antibodies were then examined on enzyme-linked immunosorbent assay plates coated with Afmp1p recombinant protein. Titers of greater than 10,000 were obtained, and the antibodies were used for subsequent studies as anti-Afmp1p antibodies.
Carbohydrates associated with Afmp1p.
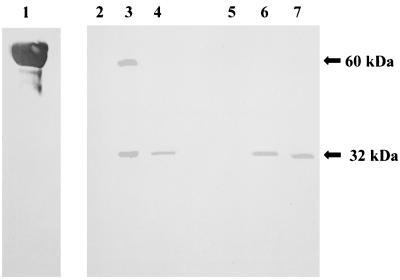
Since mannoproteins are the major cell wall components of S. cerevisiae, C. albicans, and P. marneffei, we determined whether Afmp1p is also a mannoprotein. G. nivalis agglutinin was used for these studies because it has a high affinity for terminal mannose. The protein at about 32 kDa was the primary G. nivalis agglutinin-reactive band detected when a cell lysate obtained by sonication or a lyticase wash extract was immunoprecipitated with sera obtained from guinea pigs immunized with whole cells of A. fumigatus (Fig. 3, lanes 3 and 6) or GST-Afmp1p (Fig. 3, lanes 4 and 7) but not with preimmune guinea pig serum (Fig. 3, lanes 2 and 5). This indicated that Afmp1p is a mannoprotein. Additionally, a band of about 60 kDa was observed when a cell lysate obtained by sonication was immunoprecipitated with guinea pig serum after immunization with A. fumigatus whole cells (Fig. 3, lane 3). This may represent glycosylated Afmp1p in A. fumigatus with a different level of glycosylation.
FIG. 3.
Detection of mannoprotein after immunoprecipitation of two protein fractions, a cell lysate obtained by sonication and a lyticase wash extract, with guinea pig preimmune serum, serum after immunization with A. fumigatus whole cells, and serum after immunization with GST-Afmp1p. Carboxypeptidase was used as the mannoprotein positive control (lane 1). A band of about 32 kDa could be detected when a cell lysate obtained by sonication or a lyticase wash extract was immunoprecipitated with guinea pig serum after immunization with A. fumigatus whole cells (lanes 3 and 6) or serum after immunization with GST-Afmp1p (lanes 4 and 7) but not with preimmune serum (lanes 2 and 5).
In addition, a band at approximately 32 kDa that was recognized by both an antigalactomannan monoclonal antibody (Fig. 4, lane 2) and antibody against Afmp1p (Fig. 4, lane 4) was detected when a whole-cell A. fumigatus lysate was immunoprecipitated with serum from a guinea pig immunized with purified Afmp1p but not with serum obtained from a guinea pig before immunization (Fig. 4, lanes 3 and 5), indicating that Afmp1p is a galactomannoprotein.
FIG. 4.
Detection of galactomannan after immunoprecipitation of whole-cell A. fumigatus lysate with serum obtained from a guinea pig before immunization or after immunization with GST-Afmp1p. Purified galactomannan extracted from A. fumigatus was used as the positive control (lane 1). A band of about 32 kDa could be detected with an antigalactomannan monoclonal antibody (lane 2) or antibody against purified Afmp1p (lane 4) when A. fumigatus lysate was immunoprecipitated with guinea pig serum after immunization with GST-Afmp1p but not with guinea pig preimmune serum (lanes 3 and 5).
Examination of distribution of Afmp1p in A. fumigatus by indirect immunofluorescence and electron microscopy.
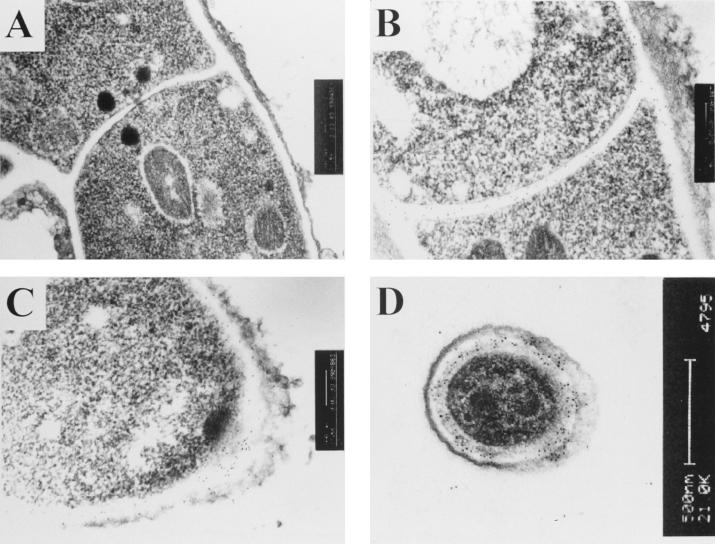
To examine the distribution of Afmp1p in the hyphae, fixed sections of A. fumigatus cells were examined by indirect immunofluorescence light microscopy and immunogold staining with rabbit anti-Afmp1p antibody and transmission electron microscopy. The indirect immunofluorescence assay showed that Afmp1p was specifically located on the surfaces of A. fumigatus hyphae (data not shown). In addition, electron micrographs demonstrated that the protein was evenly spread throughout the entire thickness of the hyphal cell walls, hyphal septa, and walls of conidiospores of A. fumigatus (Fig. 5B, C, and D), while preimmune rabbit serum showed no staining (Fig. 5A).
FIG. 5.
Immunoelectron micrographs of an A. fumigatus hyphal septum stained with preimmune rabbit serum (A) and a hyphal septum (B), a tip of a hypha (C), and a conidiospore (D) stained with rabbit anti-Afmp1p antibody.
Detection of Afmp1p antibody in infected patients.
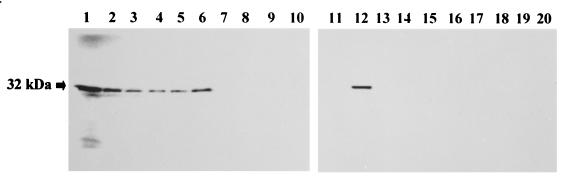
The presence of specific anti-Afmp1p antibody in the sera of infected patients was investigated by immunoprecipitation with Afmp1p translated in vitro. Afmp1p was specifically immunoprecipitated by sera from patients with aspergilloma or invasive aspergillosis (Fig. 6, lanes 3 to 6) at a level similar to that noted for the guinea pig hyperimmune serum (Fig. 6, lanes 2 and 12). No precipitated Afmp1p was seen with sera from healthy blood donors (Fig. 6, lanes 7 to 10 and 17 to 20), patients with documented C. albicans fungemia (Fig. 6, lanes 13 and 14), patients with documented P. marneffei infections (Fig. 6, lanes 15 and 16), or patients with E. coli bacteremia (data not shown).
FIG. 6.
Specific immunoprecipitation of Afmp1p by sera of patients with A. fumigatus infections. A band of about 32 kDa (arrow) was the Afmp1p translated in vitro (lane 1). Positive control sera (guinea pig immune serum, lanes 2 and 12), a negative control serum (preimmune guinea pig immune serum (lane 11), sera from patients positive for Afmp1p protein antibodies (two patients with aspergilloma [lanes 3 and 4] and two patients with invasive aspergillosis [lanes 5 and 6]), sera from healthy blood donors (lanes 7 to 10 and 17 to 20), and sera from patients infected with C. albicans (lanes 13 and 14) and P. marneffei (lanes 15 and 16) were tested.
DISCUSSION
The cell wall of Aspergillus mainly consists of polysaccharides and, to a lesser extent, proteins. The most abundant polysaccharide is 1,3-β-glucan, which forms a skeleton on which other cell wall polysaccharides, such as galactomannan and chitin, are anchored. Concerning the synthesis of 1,3-β-glucans, the FKS gene, which encodes the catalytic subunit of the plasma membrane-bound 1,3-β-glucan synthase complex of Aspergillus, and the RHO1 gene, which encodes the regulatory GTP-activatable subunit, have been cloned (11, 18). For postsynthesis modifications, three glucanases, one endoglucanase, and two glucanosyltransferases have been identified. These are responsible for the elongation of 1,3-β-glucan chains and the branching of 1,3-β-glucans with 1,6-β-linkages. Concerning the cell wall proteins, seven glycosylphosphatidyl inositol proteins have been identified, two of which were cloned and sequenced. These include the glucan elongating glucanosyltransferase (Gel1p) and a phosphatase, but none of them is reported to be a cell wall galactomannoprotein (21).
In this report we describe the cloning of the complete open reading frame of the AFMP1 gene of A. fumigatus. Amplification and sequencing of this gene in three additional clinical isolates of A. fumigatus as well as A. fumigatus ATCC 28282 confirmed that this gene is present in other strains of A. fumigatus (data not shown). DNA sequence analysis of the gene revealed that it encodes a protein (Afmp1p) of 284 amino acid residues. Examination of the Afmp1p sequence identified several features that are common to Mp1p as well as cell wall proteins in other fungi (1, 14, 20, 23, 25, 26, 29, 32). These include an N-terminal signal peptide (34), a serine- and threonine-rich region in the C-terminal half that acts as a site for O glycosylation (1), and a C-terminal glycosylphosphatidyl inositol membrane attachment signal, which many proteins use to anchor themselves to eukaryotic cell membranes (5, 31). Contrary to Mp1p, Afmp1p does not possess any potential N-glycosylation sites, but it has a GAA site for cleavage by phospholipase. On release from the cell membrane into the cell wall, like other surface proteins, Afmp1p may fulfill many important physiological functions, such as cell-cell recognition, cell adhesion, and transport of ion and nutrients, or it may act as a receptor. By using G. nivalis agglutinin and monoclonal antibody against the galactofuran side chains of galactomannan, Afmp1p was documented to have terminal mannose residues and galactofuran side chains. It has been shown that the galactofuran side chains of the galactomannan in A. fumigatus were linked to a series of at least 10 mannose residues (13, 28), and a series of 10 mannose residues generally indicates that they are linked to the corresponding protein through N glycosylation. Since there are no potential N-glycosylation sites in Afmp1p, there may be concern that this is an apparent contradiction of our results. However, the galactofuran side chains in Afmp1p can simply be linked to other shorter polysaccharide chains that are in turn linked to Afmp1p through O glycosylation rather than to a series of mannose residues. This indicates that Afmp1p may contain terminal mannose residues and galactofuran side chains linked to different amino acids on Afmp1p.
Ultrastructural analysis by indirect immunofluorescence and immunogold staining with anti-Afmp1p antibody reveals that Afmp1p is specifically located in the cell walls of A. fumigatus hyphae. The similarity in both the anatomical localization and functional motifs among Afmp1p, Mp1p, and other fungal cell wall proteins may imply that similar cell wall proteins may be present in other fungi. Since it has been shown (as described previously) that Mp1p and Afmp1p (in this study) are important antigenic proteins in P. marneffei and A. fumigatus and are useful for the diagnosis of the corresponding infections, the cloning of similar genes in other pathogenic fungi may be very rewarding in terms of helping with the serological diagnosis of other fungal infections.
Contrary to Mp1p, which is an abundant mannoprotein in P. marneffei, Afmp1p may not be as abundant in A. fumigatus. When the P. marneffei cDNA library was screened with serum from a guinea pig immunized with P. marneffei, about 700 positive clones were observed, with 70% of the clones containing the MP1 gene. This suggests that MP1 mRNA, and hence Mp1p, is abundant in P. marneffei. On the other hand, when the A. fumigatus cDNA library was screened with serum from a guinea pig immunized with A. fumigatus, none of the positive clones contained the AFMP1 gene (data not shown). Furthermore, when the library was screened by hybridization in the present study, only four of nine positive clones were observed. These results indicate that the mRNA of AFMP1, and hence Afmp1p, is not as abundant in A. fumigatus. Interestingly, in the mannoprotein detection assay, in addition to the 32-kDa band, another band which also reacted with G. nivalis agglutinin was also observed. This may represent another form of glycosylation in this protein in A. fumigatus instead of a dimer, as the experiments were performed under complete denaturing conditions.
In this report, we have presented some limited data that patients with aspergilloma and some patients with invasive aspergillosis, but not healthy blood donors or patients with C. albicans or P. marneffei infections or E. coli bacteremia, possess antibodies against Afmp1p. Thus, it is quite possible that an enzyme-linked immunosorbent assay with purified Afmp1p or antibody against Afmp1p may enhance the sensitivity and specificity of the serological tests for antibody or antigen detection, respectively, in patients with A. fumigatus infections.
In addition to laboratory diagnosis, the Afmp1p protein may have a potential use as an immunomodulating glycopeptide in patients at risk of developing invasive aspergillosis. Mannoproteins have been implicated both in the activation of nonspecific immunity (24, 27, 33) and in the elicitation of cell-mediated immunity (19, 30), and on the basis of our results, they may also be closely associated with humoral immunity. Antibodies have been suggested to be an important defense against certain extracellular opportunistic fungi (7, 16, 17). It has been shown that antibodies against the mannan of C. albicans protect against intravenously injected C. albicans cells (10). Similarly, monoclonal antibodies against the capsular polysaccharide glycomannan of Cryptococcus neoformans prolonged survival when mice were inoculated with the fungus (6, 22). Since A. fumigatus is acquired by inhalation of infectious conidia, immunization could be administered through the mucosal route to stimulate the production of secretory IgA. The specific IgA could have neutralizing activity on the infectious conidia by preventing the adherence of the fungus to the surface of the host cells, which represents the very first step in fungal invasion. Furthermore, elevation of the antibody response might lead to lysis of the mold by stimulating the complement pathway and might facilitate phagocytosis of the mold by opsonization, thereby preventing infection.
ACKNOWLEDGMENTS
This work was partly supported by a Research Grant Council grant, the AIDS Trust Fund, the Innovation and Technology Fund, and the Committee of Research and Conference Grant, The University of Hong Kong.
We thank Jean-Paul Latge for providing us with the rat antigalactomannan monoclonal antibody.
REFERENCES
- 1.Bailey D A, Feldmann P J, Bovey M, Gow N A, Brown A J. The Candida albicans HYR1 gene, which is activated in response to hyphal development, belongs to a gene family encoding yeast cell wall proteins. J Bacteriol. 1996;178:5353–5360. doi: 10.1128/jb.178.18.5353-5360.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cao L, Chan C M, Lee C, Wong S S, Yuen K Y. MP1 encodes an abundant and highly antigenic cell wall mannoprotein in the pathogenic fungus Penicillium marneffei. Infect Immun. 1998;66:966–973. doi: 10.1128/iai.66.3.966-973.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cao L, Chan K M, Chen D, Vanittanakom N, Lee C, Chan C M, Sirisanthana T, Tsang D N, Yuen K Y. Detection of cell wall mannoprotein Mp1p in culture supernatants of Penicillium marneffei and in sera of penicilliosis patients. J Clin Microbiol. 1999;37:981–986. doi: 10.1128/jcm.37.4.981-986.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cao L, Chen D L, Lee C, Chan C M, Chan K M, Vanittanakom N, Tsang D N, Yuen K Y. Detection of specific antibodies to an antigenic mannoprotein for diagnosis of Penicillium marneffei penicilliosis. J Clin Microbiol. 1998;36:3028–3031. doi: 10.1128/jcm.36.10.3028-3031.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caras I W, Weddell G N. Signal peptide for protein secretion directing glycophospholipid membrane anchor attachment. Science. 1989;243:1196–1198. doi: 10.1126/science.2466338. [DOI] [PubMed] [Google Scholar]
- 6.Casadevall A. Antibody immunity and invasive fungal infections. Infect Immun. 1995;63:4211–4218. doi: 10.1128/iai.63.11.4211-4218.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cassone A, Conti S, De Bernardis F, Polonelli L. Antibodies, killer toxins and antifungal immunoprotection: a lesson from nature? Immunol Today. 1997;18:164–169. doi: 10.1016/s0167-5699(97)84662-2. [DOI] [PubMed] [Google Scholar]
- 8.Denning D W, Stevens D A. Antifungal and surgical treatment of invasive aspergillosis: review of 2121 published cases. Rev Infect Dis. 1990;12:1147–1201. doi: 10.1093/clinids/12.6.1147. [DOI] [PubMed] [Google Scholar]
- 9.De Nobel H, Lipke P N. Is there a role for GPIs in yeast cell-wall assembly? Trends Cell Biol. 1994;4:42–45. doi: 10.1016/0962-8924(94)90003-5. [DOI] [PubMed] [Google Scholar]
- 10.Han T, Cutler J E. Antibody response that protects against disseminated candidiasis. Infect Immun. 1995;63:2714–2719. doi: 10.1128/iai.63.7.2714-2719.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kelly R, Register E, Hsu M J, Kurtz M, Nielsen J. Isolation of a gene involved in 1,3-β-glucan synthesis in Aspergillus nidulans and purification of the corresponding protein. J Bacteriol. 1996;178:4381–4391. doi: 10.1128/jb.178.15.4381-4391.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Latge J P. Aspergillus fumigatus and aspergillosis. Clin Microbiol Rev. 1999;12:310–350. doi: 10.1128/cmr.12.2.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Latge J P, Kobayashi H, Debeaupuis J P, Diaquin M, Sarfati J, Wieruszeski J M, Parra E, Bouchara J P, Fournet B. Chemical and immunological characterization of the extracellular galactomannan of Aspergillus fumigatus. Infect Immun. 1994;62:5424–5433. doi: 10.1128/iai.62.12.5424-5433.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lipke P N, Wojciechowicz D, Kurjan J. AG alpha 1 is the structural gene for the Saccharomyces cerevisiae alpha-agglutinin, a cell surface glycoprotein involved in cell-cell interactions during mating. Mol Cell Biol. 1989;9:3155–3165. doi: 10.1128/mcb.9.8.3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maertens J, Verhaegen J, Demuynck H, Brock P, Verhoef G, Vandenberghe P, Van Eldere J, Verbist L, Boogaerts M. Autopsy-controlled prospective evaluation of serial screening for circulating galactomannan by a sandwich enzyme-linked immunosorbent assay for hematological patients at risk for invasive aspergillosis. J Clin Microbiol. 1999;37:3223–3228. doi: 10.1128/jcm.37.10.3223-3228.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matthews R, Hodgetts S, Burnie J. Preliminary assessment of a human recombinant antibody fragment to hsp90 in murine invasive candidiasis. J Infect Dis. 1995;171:1668–1671. doi: 10.1093/infdis/171.6.1668. [DOI] [PubMed] [Google Scholar]
- 17.Matthews R C, Burnie J P, Howat D, Rowland T, Walton F. Autoantibody to heat-shock protein 90 can mediate protection against systemic candidosis. Immunology. 1991;74:20–24. [PMC free article] [PubMed] [Google Scholar]
- 18.Mazur P, Baginsky W. In vitro activity of 1,3-β-d-glucan synthase requires the GTP-binding protein Rho1. J Biol Chem. 1996;271:14604–14609. doi: 10.1074/jbc.271.24.14604. [DOI] [PubMed] [Google Scholar]
- 19.Mencacci A, Torosantucci A, Spaccapelo R, Romani L, Bistoni F, Cassone A. A mannoprotein constituent of Candida albicans that elicits different levels of delayed-type hypersensitivity, cytokine production, and anticandidal protection in mice. Infect Immun. 1994;62:5353–5360. doi: 10.1128/iai.62.12.5353-5360.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moukadiri I, Armero J, Abad A, Sentandreu R, Zueco J. Identification of a mannoprotein present in the inner layer of the cell wall of Saccharomyces cerevisiae. J Bacteriol. 1997;179:2154–2162. doi: 10.1128/jb.179.7.2154-2162.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mouyna I, Fontaine T, Vai M, Monod M, Fonzi W A, Diaquin M, Popolo L, Hartland R P, Latge J P. Glycosylphosphatidylinositol-anchored glucanosyltransferases play an active role in the biosynthesis of the fungal cell wall. J Biol Chem. 2000;275:14882–14889. doi: 10.1074/jbc.275.20.14882. [DOI] [PubMed] [Google Scholar]
- 22.Mukherjee S, Lee S C, Casadevall A. Antibodies to Cryptococcus neoformans glucuronoxylomannan enhance antifungal activity of murine macrophages. Infect Immun. 1995;63:573–579. doi: 10.1128/iai.63.2.573-579.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nuoffer C, Jeno P, Conzelmann A, Riezman H. Determinants for glycophospholipid anchoring of the Saccharomyces cerevisiae GAS1 protein to the plasma membrane. Mol Cell Biol. 1991;11:27–37. doi: 10.1128/mcb.11.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Palma C, Serbousek D, Torosantucci A, Cassone A, Djeu J Y. Identification of a mannoprotein fraction from Candida albicans that enhances human polymorphonuclear leukocyte (PMNL) functions and stimulates lactoferrin in PMNL inhibition of candidal growth. J Infect Dis. 1992;166:1103–1112. doi: 10.1093/infdis/166.5.1103. [DOI] [PubMed] [Google Scholar]
- 25.Roy A, Lu C F, Marykwas D L, Lipke P N, Kurjan J. The AGA1 product is involved in cell surface attachment of the Saccharomyces cerevisiae cell adhesion glycoprotein a-agglutinin. Mol Cell Biol. 1991;11:4196–4206. doi: 10.1128/mcb.11.8.4196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saporito-Irwin S M, Birse C E, Sypherd P S, Fonzi W A. PHR1, a pH-regulated gene of Candida albicans, is required for morphogenesis. Mol Cell Biol. 1995;15:601–613. doi: 10.1128/mcb.15.2.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scaringi L, Marconi P, Boccanera M, Tissi L, Bistoni F, Cassone A. Cell wall components of Candida albicans as immunomodulators: induction of natural killer and macrophage-mediated peritoneal cell cytotoxicity in mice by mannoprotein and glucan fractions. J Gen Microbiol. 1988;134(Pt 5):1265–1274. doi: 10.1099/00221287-134-5-1265. [DOI] [PubMed] [Google Scholar]
- 28.Stynen D, Safati J, Goris A, Prevost M C, Lesourd M, Kamphuis H, Darras V, Latge J P. Rat monoclonal antibodies against Aspergillus galactomannan. Infect Immun. 1992;60:2237–2245. doi: 10.1128/iai.60.6.2237-2245.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Teunissen A W, Holub E, van der H J, van den Berg J A, Steensma H Y. Sequence of the open reading frame of the FLO1 gene from Saccharomyces cerevisiae. Yeast. 1993;9:423–427. doi: 10.1002/yea.320090413. [DOI] [PubMed] [Google Scholar]
- 30.Torosantucci A, Bromuro C, Gomez M J, Ausiello C M, Urbani F, Cassone A. Identification of a 65-kDa mannoprotein as a main target of human cell-mediated immune response to Candida albicans. J Infect Dis. 1993;168:427–435. doi: 10.1093/infdis/168.2.427. [DOI] [PubMed] [Google Scholar]
- 31.Udenfriend S, Kodukula K. How glycosylphosphatidylinositol-anchored membrane proteins are made. Annu Rev Biochem. 1995;64:563–591. doi: 10.1146/annurev.bi.64.070195.003023. [DOI] [PubMed] [Google Scholar]
- 32.van der Vaart J M, Caro L H, Chapman J W, Klis F M, Verrips C T. Identification of three mannoproteins in the cell wall of Saccharomyces cerevisiae. J Bacteriol. 1995;177:3104–3110. doi: 10.1128/jb.177.11.3104-3110.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vecchiarelli A, Puliti M, Torosantucci A, Cassone A, Bistoni F. In vitro production of tumor necrosis factor by murine splenic macrophages stimulated with mannoprotein constituents of Candida albicans cell wall. Cell Immunol. 1991;134:65–76. doi: 10.1016/0008-8749(91)90331-5. [DOI] [PubMed] [Google Scholar]
- 34.von Heijne G. A new method for predicting signal sequence cleavage sites. Nucleic Acids Res. 1986;14:4683–4690. doi: 10.1093/nar/14.11.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yuen K Y, Woo P C, Ip M S, Liang R H, Chiu E K, Siau H, Ho P L, Chen F F, Chan T K. Stage-specific manifestation of mold infections in bone marrow transplant recipients: risk factors and clinical significance of positive concentrated smears. Clin Infect Dis. 1997;25:37–42. doi: 10.1086/514492. [DOI] [PubMed] [Google Scholar]