Abstract
Subacute thyroiditis (SAT) is a painful thyroiditis that often requires steroid therapy. Here, we report the first case of severe SAT in a patient who received the first dose of mRNA coronavirus disease 2019 (COVID-19) vaccination. A 34-year-old man without a viral prodrome felt a lump when swallowing 5 days after his first dose of mRNA-1273 (Moderna) vaccination. Ten days after vaccination, the patient visited the hospital and was advised to rest and take nonsteroidal anti-inflammatory drugs. He revisited the hospital 10 days later as symptoms aggravated with anterior neck pain, headache, fatigue, muscle weakness, and weight loss. Thyroid hormone levels and inflammatory markers were consistent with thyrotoxicosis. A thyroid ultrasound scan revealed typical SAT findings. His symptoms rapidly improved after receiving prednisone. A week later, the patient successfully completed his second dose of the vaccine. The thyroid function test results were nearly normal 1 month after the completion of the vaccination. We report this case to raise awareness of the occurrence of SAT after COVID-19 vaccination. As the risk of COVID-19 outweighs the minor risks of the vaccine, managing the side effects of the first vaccine dose is crucial to complete COVID-19 vaccination.
Keywords: COVID-19, COVID-19 Vaccines, mRNA-1273 Vaccine, Subacute Thyroiditis, Anterior Neck Pain
Graphical Abstract
INTRODUCTION
Subacute thyroiditis (SAT) is a painful thyroiditis presumed to be caused by viral infection or a post-viral inflammatory process. However, there are also reports of SAT after the administration of viral vaccinations, such as the seasonal flu vaccine.1 As the rate of vaccination against coronavirus disease 2019 (COVID-19) has risen worldwide, reports of COVID-19 vaccine-related SAT are increasing.2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17 However, previous reports did not mention the completion of the COVID-19 vaccine when the SAT developed after the first dose of vaccination. Here, we present the first case of severe SAT in Korea in a patient who had received the first dose of mRNA-1273 vaccine (Moderna) and successfully completed his second dose during the treatment. Further, we summarized 22 patients who were previously reported to develop SAT following COVID-19 vaccination.
CASE DESCRIPTION
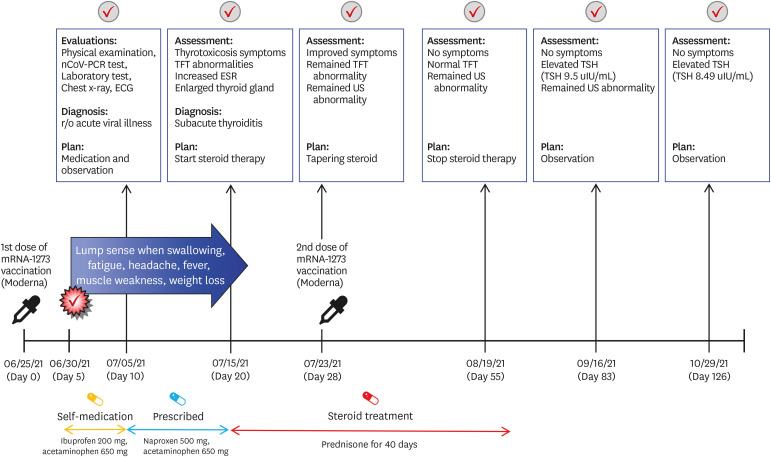
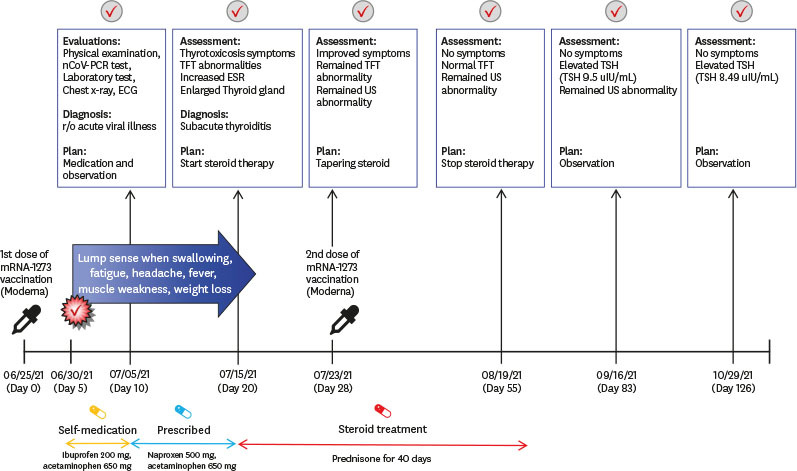
A 34-year-old male physician was admitted to the endocrinology outpatient clinic on the 20th day after the first dose of mRNA-1273 vaccination. He reported fatigue, headache, weakness in the lower extremities, sweating, and weight loss, which started 5 days after the first dose of the vaccine; the first symptom was a lump sensation when swallowing. The course of the vaccination and his symptoms are shown in Fig. 1. As the patient was a physician, he assumed that the symptoms were the common side effects of vaccination, such as neck lymphadenopathy, which was reported after mRNA-1273 vaccination. As symptoms worsened, he visited the infectious disease outpatient clinic on the 10th day after vaccination. At that time, his physical examination was unremarkable. The serum polymerase chain reaction assay, electrocardiography, chest radiography, and laboratory tests showed no evidence of COVID-19 or other known side effects of COVID-19 vaccination, such as myocarditis (Supplementary Table 1, Supplementary Figs. 1 and 2). He was advised to rest, and nonsteroidal anti-inflammatory drugs were prescribed; however, the symptoms persisted.
Fig. 1. Timeline of the symptoms, diagnosis and treatment of subacute thyroiditis after mRNA-1273 vaccination (Moderna).
nCoV-PCR = novel coronavirus polymerase chain reaction, ECG = electrocardiogram, TFT = thyroid function tests, US = ultrasound scan, ESR = erythrocyte sedimentation rate, TSH = thyroid-stimulating hormone.
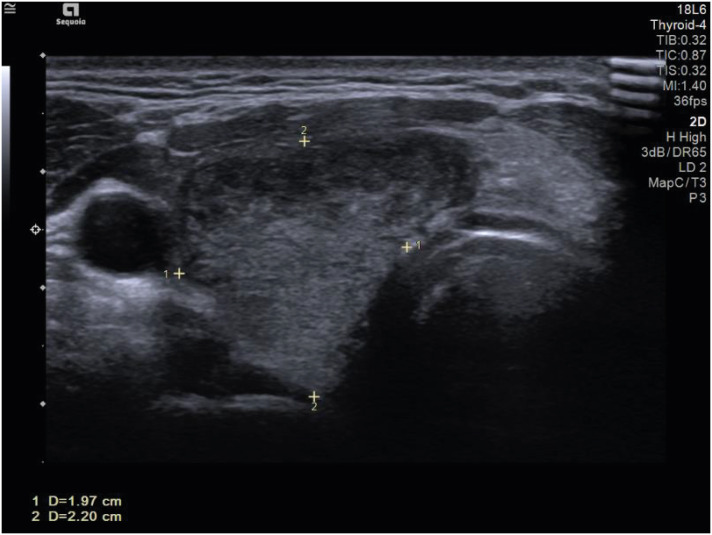
At presentation, his body temperature was 37.8°C, and he had sinus tachycardia (108/min). Physical examination revealed goiter, and pain was observed when it was touched. His thyroid hormone levels and inflammatory markers were consistent with thyrotoxicosis: thyroid-stimulating hormone (TSH) 0.005 µIU/mL (normal range, 0.4–4.8 µIU/mL), T3 2.82 ng/ml (normal range, 0.6–1.6 ng/mL), free T4 4.35 ng/dL (normal range, 0.8–1.71 ng/dL), C-reactive protein 6.54 mg/dL (normal range, 0–0.3 mg/dL), and sedimentation 79 mm/hr (normal range, 0–26 mm/hr). Thyroid autoantibody levels were normal: anti-microsome Ab < 9 IU/mL (normal range, 0.4–4.8 IU/mL), anti-thyroglobulin antibody 11.10 IU/mL (normal range, 0–115 10 IU/mL), and thyroid-stimulating immunoglobulin 0.1 IU/L (normal range, 0–1.5 IU/L). Thyroid ultrasonography (USG) demonstrated typical SAT findings, including an enlarged thyroid gland and heterogeneous echotexture (Fig. 2). Prednisone 30 mg was initiated, and his symptoms rapidly improved after medication. A week later, he received a second dose of the mRNA-1273 vaccine as scheduled while taking 22.5 mg of prednisone. He further had no symptoms associated with SAT and took prednisone for a total of 40 days, which was slowly tapered.
Fig. 2. Thyroid US image demonstrates enlarged thyroid gland (1.97 × 2.20 cm) with heterogeneous echotexture granuloma at the patient’s second visit to hospital.
US = ultrasound scan.
On the 55th day, his thyroid hormone level normalized (TSH, 0.288 µIU/mL; T3, 0.72 ng/mL; and free T3, 0.81 ng/dL), but the USG revealed an enlarged thyroid gland. On the 83rd day, the TSH level increased to 9.5 µIU/mL. As his free T4 level (0.85 ng/dL) was in normal range, he was observed without any medication and TSH level slightly decreased to 8.5 µIU/mL (day 126). The patient experienced symptoms associated with SAT.
Ethics statement
Informed consent for publication of clinical data was obtained from the case patient.
DISCUSSION
To the best of our knowledge, this is the first case of SAT caused by mRNA vaccination against COVID-19 in Korea. The patient developed SAT after the first dose of mRNA-1273 COVID-19 vaccination (Moderna) and completed the second dose while being treated for SAT.
SAT is presumed to be caused by a viral infection in which many patients have a viral prodrome typically 2 to 8 weeks beforehand. Adenovirus, enterovirus, influenza virus, coxsackievirus, mumps, measles, and other viruses have been reported as possible agents in SAT.18 Although the pathogenesis of SAT is not yet clearly defined, it is thought to be associated with genetically predisposed individuals who carry certain human leukocyte antigen (HLA) haplotypes.19 A unifying hypothesis is that an antigen from the viral infection binds to HLA-B35 molecules on macrophages, which activates cytotoxic T lymphocytes that damage thyroid follicular cells. Furthermore, cases of SAT are also seen after viral vaccinations, such as the seasonal flu vaccine.1 One is thought that viral antigens in the vaccine may damage thyroid cells, similar to infectious agents. The other is autoimmune/inflammatory syndrome induced by vaccine adjuvants.20
SAT has been reported following severe acute respiratory syndrome coronavirus 2 infection since the emergence of the COVID-19 pandemic,21,22 and more recently, cases of SAT associated with COVID-19 vaccination have increased rapidly. To date, a total of 16 reports with 22 SAT cases after vaccination against COVID-19 have been reported.2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17 The clinical features of each patient, including our patient, are summarized in Table 1. Six types of vaccines have been reported to be associated with SAT: AstraZeneca (6 cases),2,4,8,9,13 CoronaVac (5 cases),6,14,15 Pfizer-BioNTech (4 cases),5,7,11,16 Moderna (3 cases),2,12 Janssen (1 case),8 Covaxin (1 case)17 and cases that did not include the type of vaccine (3 cases).3,10 There were more cases of SAT after the first dose (13/23 cases) than after the second dose (10/23 cases). Most patients were female (18/23 cases), and patients ages ranged from 26 to 75 years (median, 40 years). The median number of days for the development of symptoms after vaccination was 7 days (range, 5–21 days). It took about median 2 weeks from the onset of symptoms to the diagnosis, but it ranged from 6 days to 10 weeks. Of the 23 patients, 13 required steroid therapy. As compared with 8 cases of SAT after influenza vaccine (time of exposures, 2 days to 8 weeks; treatment, steroid use among 4 cases),1 SAT associated with COVID-19 vaccine seems to develop around 7 days after the injection and commonly requires steroids. This may occur regardless of the vaccine type and shots. Females seem to be more vulnerable as they are known to be risk factors for typical SATs. It seems that it takes about 2 weeks to diagnose because the development of SAT after COVID-19 vaccination is not well known.
Table 1. Clinical features of patients previously reported to develop subacute thyroiditis after coronavirus disease 2019 vaccination.
| Author | Type of vaccine | Number of shots | Age/sex | Time of symptoms onset | Chief complaints | Symptom duration until diagnosis | Medication |
|---|---|---|---|---|---|---|---|
| Current | mRNA-1273 (Moderna) | 1st shot | 34/Male | 5 days | Anterior neck pain, fatigue, headache, fever, tachycardia, weight loss | 15 days | Acetaminophen naproxen |
| After diagnosis: prednisone 30 mg | |||||||
| Patient 12 | mRNA-1273 (Moderna) | 1st shot | 49/Female | 1 week | Headache, cervical sore throat | 3 weeks | Diclonfenac, prednisolone |
| Patient 212 | mRNA-1273 (Moderna) | 2nd shot | 42/Female | 5 days | Earache radiating down to the lateral and anterior neck and bilateral lower jaw | - | Nonsteroidal anti-inflammatory drugs, prednisone |
| Patient 35 | BNT162b2 (Pfizer-BioNTech) | 1st shot | 42/Female | 5 days | Sore throat, palpitations, tachycardia | 10 days | Prednisone, propranolol |
| Patient 416 | BNT162b2 (Pfizer-BioNTech) | 1st shot | 51/Female | 4 days | Nausea, mild anterior neck pain and fever | 11 days | Methylprednisolone |
| Patient 511 | BNT162b2 (Pfizer-BioNTech) | 2nd shot | 57/Female | 2 days | Anterior neck pain and swelling, fever | 12 days | Propranolol, ibuprofen, prednisone |
| Patient 67 | BNT162b2 (Pfizer-BioNTech) | 2nd shot | Middle-aged/Female | 2 weeks | Painful swelling of the thyroid gland, poor sleep, night sweats, hyper defecation and weight loss | - | Nonsteroidal anti-inflammatory drugs |
| Patient 73 | COVID-19 mRNA vaccine | 1st shot | 35/Female | 12 days | Acute neck pain radiating to the jaw and ear, fatigue, and rare palpitations | - | Prednisone |
| Patient 83 | COVID-19 mRNA vaccine | 2nd shot | 32/Female | 4 days | Neck pain radiating to the jaw and ear, and mild fatigue | - | Prednisone |
| Patient 99 | ChAdOx1 nCoV-19 (Oxford-AstraZeneca) | 1st shot | 55/Female | 3 weeks | Neck pain and swelling, headache, sore throat, generalized aches, palpitations | 4 weeks | Propranolol, ibuprofen, paracetamol |
| 6 weeks later: levothyroxine | |||||||
| Patient 1013 | ChAdOx1 nCoV-19 (Oxford-AstraZeneca) | 1st shot | 75/Male | 14 days | Pain and tenderness in front neck, shortness of breath, palpitations, insomnia, anxiety | 10 weeks | Ibuprofen |
| Patient 112 | ChAdOx1 nCoV-19 (Oxford-AstraZeneca) | 1st shot | 26/Female | 2 days | Cervical pain, fever, chill | 2 weeks | Ibuprofen, prednisone |
| Patient 128 | ChAdOx1 nCoV-19 (Oxford-AstraZeneca) | 1st shot | 73/Female | 11 days | Fever, neck pain | - | - |
| Patient 134 | ChAdOx1 nCoV-19 (Oxford-AstraZeneca) | 1st shot | 47/Female | 3 weeks | Fever, neck pain, restlessness, difficulty in swallowing and weight loss of 3 kg | 2 weeks | Propranolol |
| Patient 148 | ChAdOx1 nCoV-19 (Oxford-AstraZeneca) | 2nd shot | 39/Female | 4 days | Neck pain | - | - |
| Patient 156 | SARS-CoV-2 (CoronaVac) | 1st shot | 34/Female | 4 days | Anterior neck pain, fatigue, weight loss | - | Methylprednisolone, propranolol |
| Patient 166 | SARS-CoV-2 (CoronaVac) | 2nd shot | 35/Female | 4 days | Severe anterior neck pain, palpitation | 15 days | Methylprednisolone, propranolol |
| Patient 176 | SARS-CoV-2 (CoronaVac) | 2nd shot | 37/Female | 7 days | Mild anterior neck pain | - | Rarely need paracetamol |
| Patient 1814 | SARS-CoV-2 (CoronaVac) | 2nd shot | 67/Male | 10 days | Hypertension complaints, fever, weight loss, mild anterior neck pain | 7 days | Ibuprofen |
| Patient 1915 | SARS-CoV-2 (CoronaVac) | 2nd shot | 38/Female | 2 weeks | Swelling in the neck, pain, fatigue, loss of appetite and sweating in the evening | - | Naproxen sodium, propranolol |
| On the 30th day: levothyroxine | |||||||
| Patient 208 | JNJ-78436735 (Janssen) | 1st shot | 39/Male | 14 days | Fever, neck pain | - | - |
| Patient 2117 | COVAXIN (The Bharat Biotech COVID-19 Vaccine) | 1st shot | 34/Female | 5 days | Mild fever, palpitation, and radiating anterior neck pain | 6 days | Prednisone, propranolol |
| Patient 2210 | - | 2nd shot | 48/Male | 1 week | Neck swelling, throat discomfort, palpitations, fevers, and weight loss | 3 weeks | Nonsteroidal anti-inflammatory drugs, prednisone |
Our patient completed the second dose while receiving steroid treatment. With respect to safety, vaccination undergoing steroid treatment was concerned with the setting of live vaccination and at systemic doses equivalent to 2 mg/kg or a dose of 20 mg per day of prednisone equivalents for 2 or more weeks. Although our patient started taking 30 mg of prednisone a week ago, the vaccine did not employ live viruses, so we did not anticipate any problems. With respect to efficacy, previous data demonstrate that chronic high-dose steroids may impair vaccine-based immunity, but its efficacy is small.23 Since the symptoms caused by SAT were severe, our patient could not delay or stop the steroid treatment. The safety and efficiency of steroid use in COVID-19 vaccinations require further research.
More than 70% of the Korean population has received a COVID-19 vaccine and booster vaccination shots have recently begun. We report this case to raise awareness of the occurrence of SAT after COVID-19 vaccination as its symptoms can severely interfere with the patients’ daily lives. To the best of our knowledge, this is the first case of a patient who completed vaccination with appropriate treatment for SAT. SAT can be mistaken as a common side effect of COVID-19 vaccination. The general side effects of vaccination usually resolve within 2–3 days. If anterior neck pain with fatigue, fever, and muscle pain persists unlike the usual course of post-vaccination symptoms, clinicians should consider SAT as a cause.
Footnotes
Disclosure: The authors have no potential conflicts of interest to disclose.
- Conceptualization: Jhon M, Kang HC.
- Formal analysis: Jhon M, Oh TH.
- Investigation: Lee SH.
- Methodology: Jhon M.
- Writing - original draft: Jhon M, Lee SH.
- Writing - review & editing: Kang HC, Jhon M, Lee SH.
SUPPLEMENTARY MATERIALS
Serial laboratory data
EKG on presentation.
Chest X-ray on presentation. Chest X-ray for case patient shows normal finding.
References
- 1.Bragazzi NL, Hejly A, Watad A, Adawi M, Amital H, Shoenfeld Y. ASIA syndrome and endocrine autoimmune disorders. Best Pract Res Clin Endocrinol Metab. 2020;34(1):101412. doi: 10.1016/j.beem.2020.101412. [DOI] [PubMed] [Google Scholar]
- 2.Bornemann C, Woyk K, Bouter C. Case report: two cases of subacute thyroiditis following SARS-CoV-2 vaccination. Front Med (Lausanne) 2021;8:737142. doi: 10.3389/fmed.2021.737142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chatzi S, Karampela A, Spiliopoulou C, Boutzios G. Subacute thyroiditis after SARS-CoV-2 vaccination: a report of two sisters and summary of the literature. Hormones (Athens) 2021:1–3. doi: 10.1007/s42000-021-00332-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Das L, Bhadada SK, Sood A. Post-COVID-vaccine autoimmune/inflammatory syndrome in response to adjuvants (ASIA syndrome) manifesting as subacute thyroiditis. J Endocrinol Invest. 2021:1–3. doi: 10.1007/s40618-021-01681-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Franquemont S, Galvez J. Subacute thyroiditis after mRNA vaccine for COVID-19. J Endocr Soc. 2021;5(Suppl 1):A956–A957. [Google Scholar]
- 6.İremli BG, Şendur SN, Ünlütürk U. Three cases of subacute thyroiditis following SARS-CoV-2 vaccine: postvaccination ASIA syndrome. J Clin Endocrinol Metab. 2021;106(9):2600–2605. doi: 10.1210/clinem/dgab373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jeeyavudeen MS, Patrick AW, Gibb FW, Dover AR. COVID-19 vaccine-associated subacute thyroiditis: an unusual suspect for de Quervain's thyroiditis. BMJ Case Rep. 2021;14(11):e246425. doi: 10.1136/bcr-2021-246425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee KA, Kim YJ, Jin HY. Thyrotoxicosis after COVID-19 vaccination: seven case reports and a literature review. Endocrine. 2021;74(3):470–472. doi: 10.1007/s12020-021-02898-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oyibo SO. Subacute thyroiditis after receiving the adenovirus-vectored vaccine for coronavirus disease (COVID-19) Cureus. 2021;13(6):e16045. doi: 10.7759/cureus.16045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patel KR, Cunnane ME, Deschler DG. SARS-CoV-2 vaccine-induced subacute thyroiditis. Am J Otolaryngol. 2022;43(1):103211. doi: 10.1016/j.amjoto.2021.103211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schimmel J, Alba EL, Chen A, Russell M, Srinath R. Letter to the editor: thyroiditis and thyrotoxicosis after the SARS-CoV-2 mRNA vaccine. Thyroid. 2021;31(9):1440. doi: 10.1089/thy.2021.0184. [DOI] [PubMed] [Google Scholar]
- 12.Plaza-Enriquez L, Khatiwada P, Sanchez-Valenzuela M, Sikha A. A case report of subacute thyroiditis following mRNA COVID-19 vaccine. Case Rep Endocrinol. 2021;2021:8952048. doi: 10.1155/2021/8952048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ratnayake GM, Dworakowska D, Grossman AB. Can COVID-19 immunisation cause subacute thyroiditis? Clin Endocrinol (Oxf) doi: 10.1111/cen.14555. Forthcoming 2021. DOI: 10.1111/cen.14555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Şahin Tekin M, Şaylısoy S, Yorulmaz G. Subacute thyroiditis following COVID-19 vaccination in a 67-year-old male patient: a case report. Hum Vaccin Immunother. 2021;17(11):4090–4092. doi: 10.1080/21645515.2021.1947102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saygılı ES, Karakilic E. Subacute thyroiditis after inactive SARS-CoV-2 vaccine. BMJ Case Rep. 2021;14(10):e244711. doi: 10.1136/bcr-2021-244711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Siolos A, Gartzonika K, Tigas S. Thyroiditis following vaccination against COVID-19: report of two cases and review of the literature. Metabol Open. 2021;12:100136. doi: 10.1016/j.metop.2021.100136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Soltanpoor P, Norouzi G. Subacute thyroiditis following COVID-19 vaccination. Clin Case Rep. 2021;9(10):e04812. doi: 10.1002/ccr3.4812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Desailloud R, Hober D. Viruses and thyroiditis: an update. Virol J. 2009;6(1):5. doi: 10.1186/1743-422X-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ohsako N, Tamai H, Sudo T, Mukuta T, Tanaka H, Kuma K, et al. Clinical characteristics of subacute thyroiditis classified according to human leukocyte antigen typing. J Clin Endocrinol Metab. 1995;80(12):3653–3656. doi: 10.1210/jcem.80.12.8530615. [DOI] [PubMed] [Google Scholar]
- 20.Watad A, David P, Brown S, Shoenfeld Y. Autoimmune/inflammatory syndrome induced by adjuvants and thyroid autoimmunity. Front Endocrinol (Lausanne) 2017;7:150. doi: 10.3389/fendo.2016.00150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brancatella A, Ricci D, Viola N, Sgrò D, Santini F, Latrofa F. Subacute thyroiditis after Sars-COV-2 infection. J Clin Endocrinol Metab. 2020;105(7):dgaa276. doi: 10.1210/clinem/dgaa276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ruggeri RM, Campennì A, Siracusa M, Frazzetto G, Gullo D. Subacute thyroiditis in a patient infected with SARS-COV-2: an endocrine complication linked to the COVID-19 pandemic. Hormones (Athens) 2021;20(1):219–221. doi: 10.1007/s42000-020-00230-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chakravarthy K, Strand N, Frosch A, Sayed D, Narra LR, Chaturvedi R, et al. Recommendations and guidance for steroid injection therapy and COVID-19 vaccine administration from the American Society of Pain and Neuroscience (ASPN) J Pain Res. 2021;14:623–629. doi: 10.2147/JPR.S302115. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Serial laboratory data
EKG on presentation.
Chest X-ray on presentation. Chest X-ray for case patient shows normal finding.