Abstract
Patients with Cushing disease (CD) may present with both chronic and acute perioperative complications that necessitate multidisciplinary care. This review highlights several objectives for these patients before and after transsphenoidal surgery. Preoperative management includes treatment of electrolyte disturbances, cardiovascular comorbidities, prediabetes/diabetes, as well as prophylactic consideration(s) for thromboembolism and infection(s). Preoperative medical therapy (PMT) could prove beneficial in patients with severe hypercortisolism or in cases of delayed surgery. Some centers use PMT routinely, although the clinical benefit for all patients is controversial. In this setting, steroidogenesis inhibitors are preferred because of rapid and potent inhibition of cortisol secretion. If glucocorticoids (GCs) are not used perioperatively, an immediate remission assessment postoperatively is possible. However, perioperative GC replacement is sometimes necessary for clinically unstable or medically pretreated patients and for those patients with surgical complications. A nadir serum cortisol of less than 2 to 5µg/dL during 24 to 74 hours postoperatively is generally accepted as remission; higher values suggest nonremission, while a few patients may display delayed remission. If remission is not achieved, additional treatments are pursued. The early postoperative period necessitates multidisciplinary awareness for early diagnosis of adrenal insufficiency (AI) to avoid adrenal crisis, which may also be potentiated by acute postoperative complications. Preferred GC replacement is hydrocortisone, if available. Assessment of recovery from postoperative AI should be undertaken periodically. Other postoperative targets include decreasing antihypertensive/diabetic therapy if in remission, thromboprophylaxis, infection prevention/treatment, and management of electrolyte disturbances and/or potential pituitary deficiencies. Evaluation of recovery of thyroid, gonadal, and growth hormone deficiencies should also be performed during the following months postoperatively.
Keywords: Cushing, perioperative management, cardiovascular, thromboprophylaxis, infection, remission
Cushing syndrome (CS), comprising all etiologies of cortisol excess, is associated with important morbidities including cardiovascular (CV), thromboembolic, metabolic, infectious, musculoskeletal, psychiatric, and other complications [1-4]. Cushing disease (CD), the most frequent cause of endogenous CS, is due to an adrenocorticotropin (ACTH)-producing corticotroph pituitary adenoma leading to cortisol overproduction. Mortality in patients with untreated CS is high and mostly due to CV, thromboembolic, and infectious causes [1, 2, 5]. The management of patients with CS is complex and requires clinicians to address multiple aspects of this multimorbid condition from the moment of initial diagnosis.
The first line treatment for most patients with CD is transsphenoidal pituitary surgery (TSS), which generally achieves remission in 70% to 90% of microadenoma cases but is lower for macroadenomas [3, 4]. Surgery should ideally be performed in a high-volume center by an experienced pituitary surgeon [3, 4]. While surgery is expected to induce remission and improve cortisol-related morbidities, the perioperative period, from the time of diagnosis until at least 3 months postoperatively, is marked by the highest rate of morbidity and mortality [6, 7]. Therefore, it is crucial to assess and treat hypertension, hypokalemia, hyperglycemia/diabetes, as well as assess the risk of thromboembolic and infectious complications and use preventive measures to reduce this risk. Notably, the risk of complications such as stroke, thromboembolism, and sepsis could persist even during long-term remission [8].
Furthermore, besides management of complications, the immediate postoperative period is critical in assessment for surgical success. Adrenal insufficiency (AI) indicates postoperative remission, and requires adequate glucocorticoid (GC) replacement to prevent severe GC withdrawal symptoms or even adrenal crisis [3]. Additionally, sodium derangements are common and require close monitoring and treatment [3, 9, 10].
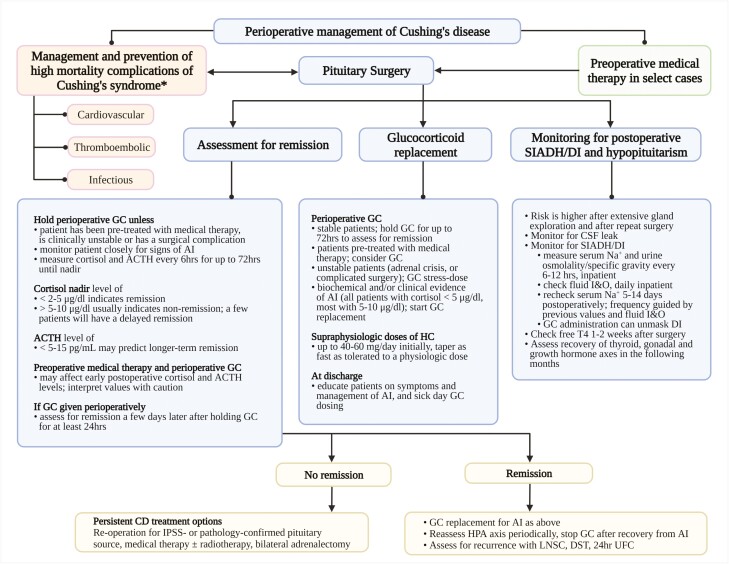
In this review, we discuss in detail the aforementioned aspects of care. We aim to provide comprehensive and evidence-based information to help clinicians with perioperative care of patients with CD (Fig. 1 [4, 9, 11, 12]).
Figure 1.
Suggested algorithm for perioperative management of Cushing disease. AI, adrenal insufficiency; DI, diabetes insipidus; DST, dexamethasone suppression test; GC, glucocorticoids, HPA, hypothalamic-pituitary-adrenal; I&O, intake and output; IPSS, inferior petrosal sinus sampling; LNSC, late-night salivary cortisol; Na+, sodium; SIADH, syndrome of inappropriate antidiuretic hormone; UFC, urinary free cortisol. *See Tables 1 and 2. Adjust antihypertensive and antihyperglycemic regimen proactively if remission is achieved postoperatively. Urine osmolality/specific gravity may be checked daily and/or as needed paired with serum sodium every 6 to 12 hours depending on local protocols.
Management Considerations for Patients With Cushing Disease Before Pituitary Surgery
Case 1
A 54-year-old woman presents with centripetal weight gain, skin thinning, easy bruising, poor wound healing, hypertension, and muscle weakness. She has a history of a stress foot fracture, complicated by deep vein thrombosis (DVT) after surgical repair. Her 24-hour urinary free cortisol (UFC) is 480 µg/d (< 45 µg/d), serum cortisol of 15 µg/dL on a 1-mg dexamethasone suppression test (DST; normal < 1.8 µg/dL), a late-night salivary cortisol of 1.114; 0.564 µg/dL (< 0.112 µg/dL), and ACTH of 55 (normal < 45 pg/mL). Pituitary magnetic resonance imaging scanning shows a 4-mm pituitary adenoma. Inferior petrosal sinus sampling confirms a pituitary source. Transsphenoidal surgery is recommended.
Should This Patient Be Anticoagulated? When and for How Long?
Hypercortisolism creates a prothrombotic state by altering multiple factors of coagulation cascade, regulators of fibrinolysis, and platelet function [1, 2, 13, 14]. Several studies have shown that procoagulation factors (eg, factor VIII, fibrinogen, von Willebrand factor) exhibit increased activity resulting in shortened activated partial thromboplastin time and compensatory increase in coagulation inhibitors (protein C, protein S, and antithrombin III) [13-17]. Platelets, thromboxane A2, and thrombin–antithrombin complexes are also increased, while fibrinolytic capacity is decreased [13, 14, 18]. In addition, chronic endothelial damage and atherosclerosis observed in patients with CS as well as in obesity, diabetes, and hypertension also contribute to thrombogenesis [19-22]. Abnormal coagulation parameters are not always present in CS cases and they do not correlate with the degree of hypercortisolemia or risk of thrombosis [14, 16, 17]. Therefore, they cannot be reliably used in clinical practice to assess the need for anticoagulation. Instead, the risk of thrombosis is assessed based on current knowledge of epidemiology of DVT/pulmonary embolism in CS as well as the individual’s medical history and clinical scenario [2].
The risk of venous thromboembolism (VTE) in patients with CS is roughly 18 times higher than in the general population [14]. The risk appears to be higher during the first year after diagnosis, peaking 2 to 3 months postoperatively [5, 6, 23], and VTE rates in the postoperative period could be up to 20% [18]. While surgery itself is a VTE risk factor because of reduced ambulation and changes in the coagulation system [24], an acute cortisol drop that occurs in the postoperative period has been implicated as a cause of this increased risk [2, 14]. Withdrawal of GC anti-inflammatory action could further enhance a procoagulatory state. Bilateral adrenalectomy (BLA) has been associated with even higher odds of VTE, though it is not known whether BLA itself or the underlying disease (eg, severe CS or presence of cancer) is responsible for this observation [2, 23].
Retrospective studies have shown a reduction in VTE events when using several weeks of postoperative thromboprophylaxis with heparin, low-molecular-weight heparin, and warfarin in patients with CS [25, 26]. In one study, a 2-week treatment with unfractionated heparin followed by 4 months of warfarin resulted in a 3-fold reduction of VTE events (6% in treated vs 20% in the untreated group), though retrospective design and changes in practice over time may have affected the results [26]. Importantly, no statistically significant bleeding events were reported [25, 26]. A recent consensus on management of CS provided expert recommendation for anticoagulation, advising the consideration of it for all patients with the highest risk of VTE [4]. As such, patients with a history of VTE, thrombophilic states, severe hypercortisolism, use of estrogen/testosterone, prolonged preoperative or postoperative hospitalization, and high postoperative cortisol levels or GC overreplacement should be considered for anticoagulation [4]. Holding estrogen/testosterone supplementation perioperatively has also been suggested [4]. In addition, clinicians should take into account other factors that may increase VTE risk, such as active cancer, smoking, immobilization, and older age. However, risk of bleeding and intracranial hemorrhage should be assessed individually in a multidisciplinary fashion.
Commonly used thromboprophylaxis agents include low-molecular-weight heparin, fodaparinux, and direct oral anticoagulants (Table 1 [2, 27, 28]). Aspirin, unfractionated heparin, and warfarin are rarely used. VTE prophylaxis may be initiated 24 to 48 hours after surgery and continued for 2 to 6 weeks [2, 4]. Longer regimens of 2 to 3 months should be considered for patients with the highest thrombotic risk, particularly after BLA [2, 5]. Some clinicians initiate thromboprophylaxis immediately after the diagnosis of CS if the risk of VTE is considered high. In this case, anticoagulation should be held before the surgery and the timing coordinated with the surgeon [4]. Additionally, early ambulation and intermittent compression devices are recommended during the postoperative period [2, 29].
Table 1.
Thromboprophylaxis agents
| Agent class | Examples | Comments |
|---|---|---|
| Low-molecular-weight heparin | Enoxaparin | – Subcutaneous daily administration |
| Dalteparin | – Dose adjustment is necessary for renal impairment and weight | |
| Tinzaparin | ||
| Unfractionated heparin | – Subcutaneous twice or thrice daily administration | |
| – May not be available in the outpatient setting | ||
| – No adjustment needed for renal impairment | ||
| Factor Xa inhibitor | Fondaparinux | – Subcutaneous daily administration |
| – Sometimes used in patients with heparin induced thrombocytopenia | ||
| – Contraindicated in severe renal impairment | ||
| Direct anticoagulants | Rivaroxaban | – Oral administration |
| Dabigatran | – Rivaroxaban is approved for acutely ill hospitalized medical patients and for orthopedic patients (knee and hip replacement)a | |
| Apixaban | – Dabigatran is approved for hip replacement and apixaban for knee and hip replacementa | |
| – Not used in severe renal impairment | ||
| – Levoketoconazole increases levels of dabigatran (avoid combination) [27] | ||
| – Ketoconazole increases levels of rivaroxaban (avoid combination), dabigatran, and apixaban (consider therapy modification) [28] | ||
| Acetyl salicylic acid | Aspirin | – Very rarely used in nonorthopedic patients |
| – May be used if other agents are contraindicated or not available | ||
| – Consider concomitant use of gastrointestinal prophylaxis with a proton-pump inhibitor | ||
| Warfarin | – Very rarely used for routine thromboprophylaxis; concurrent use of ketoconazole results in increased level of warfarin [28] |
Mechanical prophylaxis using intermittent pneumatic compression or elastic stockings in addition to pharmacologic agents for higher-risk patients.
a Food and Drug Administration–approved indications.
Case 1 continued
Given a prior history of DVT, this patient is started on Lovenox (enoxaparin) shortly after diagnosis and held 2 days before surgery and reinitiated 3 days after with continuation for 6 weeks. No thromboembolic events or bleeding complications are encountered.
How Can Cardiovascular Risk Be Reduced Perioperatively? Does This Patient Need a Preoperative Cardiology Evaluation?
Chronic hypercortisolism elevates CV risk via a combination of pathophysiological mechanisms; development of insulin resistance and diabetes by inducing visceral fat accumulation, decreasing insulin secretion and incretin action, and by inducing lipolysis and free fatty acid release [30-32]. These changes, along with endothelial damage and chronic inflammation, are major contributors to atherosclerosis and CV disease [33, 34]. Hypercortisolism also induces hypertension by activating mineralocorticoid receptor(s), the renin-angiotensin-aldosterone system, and the sympathetic nervous system [2, 35]. In addition, mineralocorticoid receptor activation leads to hypokalemia, which can cause QT interval prolongation and serious heart arrhythmias [36, 37].
CV events occur more frequently around the time of CS diagnosis and postoperatively [5, 6]. Therefore, heightened attention to the recognition and active management of cardiometabolic comorbidities should reduce perioperative risk. Many patients with CS are already being treated with antihypertensive, antihyperglycemic, and lipid-lowering medications at the time of a CS diagnosis; however, treatment is not always optimized. Though comorbidities typically improve with CS remission, hazard ratios for myocardial infarction and stroke remain elevated compared to the general population (hazard ratio = 3.7 and 2.0 for myocardial infarction and stroke in patients with CS) [6].
Treatment of hypertension in patients with CS includes the use of mineralocorticoid antagonists, angiotensin-converting-enzyme inhibitors, angiotensin receptor 2 blockers, and β-blockers [2]. Loop diuretics are helpful in edematous states, but may further worsen hypokalemia and potassium replacement is often necessary in moderate-to-severe CS cases. Of note, medical therapy with mifepristone, metyrapone, or osilodrostat may cause or worsen hypokalemia, hypertension, and peripheral edema in some patients. Mifepristone blocks GC receptors, thus cortisol levels often increase during treatment, and these excessive levels could activate mineralocorticoid receptors [38]. Metyrapone and osilodrostat block 11-β-hydroxylase (converts 11-deoxycortisol into cortisol and 11-deoxycortisone into corticosterone) and aldosterone synthase (converts corticosterone into aldosterone). Accumulation of deoxycorticosterone and subsequent activation of mineralocorticoid receptors can induce hypertension in some patients [39]. However, patients with controlled and partially controlled mean UFC on osilodrostat in the LINC3 study had improvement in their blood pressure at week 24, whereas patients with uncontrolled mean UFC did not. Interestingly, at week 48, improvement in blood pressure occurred regardless of UFC status [40, 41].
Mineralocorticoid blockade with spironolactone or eplerenone could be effective at controlling these side effects [38, 40, 42]. Postoperatively, if remission is achieved, it is often necessary to promptly reduce or discontinue antihypertensive medications as well as potassium supplements.
The identification and treatment of CV cortisol-related comorbidities such as coronary artery disease or heart failure necessitates a proactive approach, based on personalized risk and on the clinical situation (more rarely observed in young patients), and if suspected, the patient should be referred to cardiology for risk evaluation. It is unknown whether screening with echocardiogram or stress testing is needed in asymptomatic patients with CS, particularly before surgery. Aspirin is used for secondary prevention of CV events and is also effective in primary prevention of coronary artery disease [43-45]; as such it may be considered for high-risk patients preoperatively if surgery is delayed or postoperatively in persistent CD patients who are at increased risk of CV events. However, this should be balanced with the risk of bleeding. Statins have an established benefit of CV risk reduction, and initialization in high-risk patients is recommended [46].
Treatment of hyperglycemia and diabetes is essential and should not be delayed. Mild hyperglycemia may be ameliorated with metformin. Sodium-glucose cotransporter 2 (SGLT-2) and glucagon-like-1 (GLP-1) could be particularly beneficial in patients with CS who also have CV disease [47], but severe hyperglycemia should be addressed by insulin therapy. In more severe cases, such as hospitalized patients with CS, intravenous insulin infusion may be required for hyperglycemia control [2, 48] (Table 2 [27, 28, 39, 44, 47-55]). In patients with postoperative remission, proactive reduction of antihyperglycemic medications is necessary because of the risk of hypoglycemia.
Table 2.
Management considerations for cardiovascular and metabolic complications in Cushing syndrome
| Complication | Agent | Considerations | Drug-drug interactionsa |
|---|---|---|---|
| DM | Metformin | First-line agent in all patients unless contraindicated | Levoketoconazole increases levels of metformin [27] |
| Beneficial in insulin resistant states [47, 49, 50, 55] | - Monitor | ||
| SGLT-2 inhibitors | Demonstrated CV benefit for DM2 patients with atherosclerotic CV disease or kidney disease [44, 47, 49, 50, 55] | ||
| Risk of genitourinary infections might be higher in CS | |||
| Risk of fractures | |||
| GLP-1 agonist | Demonstrated CV benefit for DM2 patients with atherosclerotic CV disease | ||
| May be beneficial in CS given impaired insulin secretion/incretin effect induced by glucocorticoids [48, 49, 55] | |||
| DPP4 inhibitors | May be beneficial in CS given impaired insulin secretion/incretin effect induced by glucocorticoids [48, 49, 55] | Ketoconazole may increase levels of saxagliptin [54] | |
| - Monitor | |||
| Sulfonylureas | May be less effective in CS | Mifepristone may increase levels of sulfonylureas [53] - Monitor |
|
| Thiazolinediones | Cause fluid retention and contraindicated in heart failure | Ketoconazole increases levels of pioglitazone [54] | |
| Side effects may outweigh insulin-sensitizing benefit | - Monitor | ||
| Insulin | Use for severe hyperglycemia and when rapid reduction of blood glucose is necessary | ||
| -Monitor hypertension | Mineralocorticoid | Block effects of cortisol and cortisol/aldosterone precursors at MR receptor | Ketoconazole increases levels of eplerenone [54] |
| Receptor blockers | Effective for hypokalemia, edema, and hypertension in CS and during treatment with mifepristone, metyrapone, and osilodrostat | - Contraindicated | |
| Additional cardiovascular benefits [44, 47] | Mifepristone increases levels of eplerenone [52] | ||
| - Avoid | |||
| ACE inhibitors/ARBs | Target activated RAAS system in CS | Ketoconazole increases levels of losartan [54] | |
| Reduce risk of CV events in patients with DM and atherosclerotic disease [44] | - Monitor | ||
| Potential benefit for hypokalemia | Mifepristone increases levels of losartan [52] | ||
| Thiazide diuretics | Reduce risk of CV events in patients with DM [44] | - Monitor | |
| May worsen hypokalemia | |||
| Loop diuretics | Beneficial in edematous states May worsen hypokalemia |
||
| Dihydropyridine calcium channel blockers | Reduce risk of CV events in patients with DM | Ketoconazole increases levels of nifedipine [28] | |
|
- Consider therapy modification |
|||
| Mifepristone may increase levels of carvedilol [52] | |||
| - Monitor | |||
| Ketoconazole increases levels of amlodipine [28] | |||
| β-Blockers | May be used for patients with previous MI, active angina, or heart failure | Osilodrostat may increase levels of carvedilol and propranolol [39] | |
| Hyperlipidemia | Statins | First-line in all patients with DM and atherosclerotic disease | Ketoconazole and levoketoconazole increase levels of simvastatin and lovastatin [27, 54] |
| For patients with DM but without established atherosclerotic disease, use based on ADA guidelines [44] | - Contraindicated | ||
| Ketoconazole and Levoketoconazole increase levels of atorvastatin [27, 28] | |||
| - Monitor | |||
| Mifepristone increases levels of simvastatin and lovastatin [53] | |||
| - Contraindicated | |||
| Osilodrostat increases level of simvastatin [51] | |||
| - Monitor | |||
| Mifepristone increases level of atorvastatin [52] | |||
| - Monitor | |||
| Ezetimibe | Add-on therapy to maximally tolerated statin in patients with very high CV risk [44] | ||
| PCSK9 inhibitors | Add-on therapy to maximally tolerated statin in patients with very high CV risk [44] | ||
| Antithrombotic therapy | Aspirin (or clopidogrel if allergy to aspirin) | Secondary prevention in patients with DM and atherosclerotic CV disease [44] Primary prevention in patients with DM who are at increased CV risk (after risk-and-benefit discussion) [44] |
Ketoconazole decreases effects of clopidogrel [54]; consider alternative |
| Hypokalemia | Potassium replacement | Oral in most cases, intravenous in severe cases | |
| Mineralocorticoid | Block effects of cortisol and cortisol/aldosterone precursors at MR receptor | Ketoconazole increases levels of eplerenone [54] | |
| Receptor blockers | Effective for hypokalemia in CS and during treatment with mifepristone, metyrapone, and osilodrostat | - Contraindicated | |
| Mifepristone increases levels of eplerenone [52] | |||
| - Avoid | |||
| ACE inhibitors/ARBS | Potential benefit for hypokalemia | Ketoconazole increases level of losartan [54] | |
| - Monitor | |||
| Mifepristone increases levels of losartan [52] | |||
| - Monitor | |||
| Screening for CV disease | Multidisciplinary evaluation in conjunction with PCP, cardiology and preoperative medicine | ||
| History, symptoms (eg, dyspnea, chest pain, edema), physical exam | |||
| Resting EKG preoperatively; additional CV testing and imaging may be needed based on clinical evaluation | |||
| CV risk assessment using established tools (eg, revised cardiac risk index, American College of Surgeons surgical risk calculator) prior to surgery | |||
| Referral to cardiology for established CV disease and/or if CV disease is highly suspected |
Abbreviations: ACE, angiotensin-converting enzyme; ADA, American Diabetes Association; ARB, angiotensin II receptor blocker; CS, Cushing syndrome; CV, cardiovascular; DM, diabetes mellitus; DM2, type 2 diabetes mellitus; EKG, electrocardiogram; GLP-1, glucagon-like peptide 1; MI, myocardial infarction; MR, mineralocorticoid receptor; PCP, primary care provider; RAAS, renin-angiotensin-aldosterone system; SGLT-2, sodium-glucose cotransporter 2.
a Summary of major drug-drug interactions.
How Can one Lower This Patient’s Risk of Infection, Including Pneumocystis jirovecii?
CS causes immunosuppression and increases the risk of a variety of infections, both typical and opportunistic bacterial, viral, and fungal [1, 6]. Similar to CV and thromboembolic complications, the incidence of infections is highest during active hypercortisolism and the first 3 months after surgery [5, 6]. Urinary tract infections, skin and soft-tissue infections, pneumonia, and sepsis are the most frequent types [5].
Pneumocystis jirovecii pneumonia (PJP) is a rare, life-threatening opportunistic infection with a high mortality rate [1, 2, 56]; risk is highest during rapid cortisol decline either after surgery or on initiation of medical therapy [56]. The pathophysiology behind this is thought to be immune reconstitution syndrome, manifesting as a vigorous response to the infectious organisms colonizing the lungs of an immunocompromised patient [56]. It presents with substantial hypoxia, dyspnea, fever, and ground-glass opacities on imaging. If not promptly recognized and treated, PJP can be fatal, and therefore clinicians should be aware of the possibility of this rare complication. Treatment of PJP is with trimethoprim-sulfamethoxazole, usually in combination with prednisone [57-59].
There is no current guideline on the prevention of PJP in patients with CS. Based on published case reports and retrospective series, the risk of PJP is the highest in those with severe CS, particularly when UFC is greater than 20 times the upper limit of normal (ULN) [56]. However, milder cases of CS (UFC ~ 5 × ULN) with PJP have been described [56]. It has been previously suggested that PJP prophylaxis be considered for patients with UFC greater than 10 times the ULN and patients with other risk factors for immunodeficiency [2]. Prophylaxis, if considered, should be initiated before surgery or cortisol-lowering therapy and continued for at least 2 weeks or until the patient is no longer considered immunosuppressed [2]. The most commonly used agent is the trimethoprim-sulfamethoxazole double-strength tablet (160/800 mg) or single-strength tablet (80/400 mg) administered daily. Side effects include fever, rash, agranulocytosis, nausea/vomiting, and elevated transaminases. There is a risk of QT prolongation and hepatotoxicity if administered concurrently with ketoconazole. Other prophylaxis regimens are also available [57, 59].
A few cases of SARS-CoV-2 infection in patients with CS have been reported in the literature, varying from asymptomatic to fatal [14]. The severity of COVID-19 disease may depend on the degree of hypercortisolemia and comorbidities and further worsened by hypercoagulability associated with both conditions [14]. Of note, computed tomography scan appearances of SARS-CoV-2 infection (ground-glass opacities) may be similar to those of PJP [60]. It is important not to overlook PJP as cases of concurrent SARS-CoV-2 infection and PJP as well as post–COVID-19 development of PJP have been reported [61, 62].
Because hyperglycemia is one of the major risk factors for infection, optimal glucose control is paramount. Continuous insulin infusion with frequent blood sugar monitoring may need to be initiated in severely ill hospitalized patients or postoperatively, when tighter glucose control is usually necessary [2, 48].
Perioperative antibiotic prophylaxis is frequently used to reduce the risk of meningitis and sinusitis after TSS for pituitary adenomas, though there are no controlled studies in this area [63, 64]. Cerebrospinal fluid leak, comorbidities (particularly those observed in CD cases), preexisting sinus disease, nasal packing, and other factors increase the risk of infection, and researchers support perioperative antibiotics in such situations [64].
Last, vaccination against SARS-CoV-2, pneumococcus, influenza, and herpes zoster are recommended in all eligible patients [3, 65]. However, live vaccines should not be administered to immunocompromised individuals [66].
Are There any Benefits in Starting Medical Therapy to Treat Cushing Disease Preoperatively?
Once a diagnosis is established, pituitary surgery by an experienced surgeon should ideally be performed shortly. However, some patients may experience delays lasting weeks because of a lack of access to surgery or other factors, or may be considered “too ill” to undergo surgery [4, 67]. Furthermore, in light of the COVID-19 pandemic, many patients had their surgery further delayed [68, 69]. When surgery cannot be performed within a reasonable period of time, preoperative medical therapy (PMT) can be considered [4]. The goal of PMT is to reduce hypercortisolemia or block GC receptor(s) to reduce symptoms and CS complications such as hyperglycemia/diabetes, hypertension, hypokalemia, infection, and psychiatric symptoms [67]. Some centers use cortisol-lowering therapy routinely, with a rationale of improving postoperative recovery and wound healing and reducing infection risk [67, 70]. Other potential proposed benefits include reduction of intraoperative bleeding (though not confirmed) and reduction in the degree of postoperative AI [67, 70].
Despite PMT’s potential advantages, the data on outcomes, either perioperative or postoperative, are scarce and heterogeneous. One retrospective study found a lower VTE rate in medically pretreated patients before TSS compared to treatment-naive patients (2.5% vs 7.2%) [71]. On the other hand, data from a large European registry showed no difference in perioperative mortality or prevalence of postsurgical morbidities, including thromboembolism, within 6 months postoperatively between the groups of patients with CS who received and did not receive PMT [67]. Of note, patients who received PMT were “sicker” as determined by their higher blood pressure, muscle weakness, and skin changes; UFC data were lacking in this study. Future prospective studies would help clarify the role of routine PMT.
If medical therapy is considered, most clinicians select adrenal steroidogenesis inhibitors, as they have a rapid onset of action (except mitotane) and the ability to suppress cortisol in a dose-dependent manner [39]. Currently available steroidogenesis inhibitors approved by the US Food and Drug Administration and European Medical Agency include osilodrostat for the treatment of patients with CD and CS, respectively. Approved in Europe, but used off-label in the United States, are metyrapone and ketoconazole. Etomidate, an anesthetic with cortisol-inhibiting properties, is available in an intravenous form and is used for severe, life-threatening hypercortisolism as a bridge therapy to definitive surgical therapy [3, 4, 72]. Mitotane is rarely used in CD cases [39]. Other classes of medications include somatostatin-receptor ligands (pasireotide) and dopamine agonists (cabergoline), which potentially reduce ACTH production by the corticotroph adenoma. However, they are rarely used preoperatively given their lower efficacy compared with adrenal steroidogenesis inhibitors. In addition, because pituitary-targeted therapies have been shown to reduce adenoma size [73, 74], there is a theoretical risk that very small adenomas can become even more difficult to identify during surgery. The GC receptor blocker mifepristone has also been used in selected cases with severe diabetes as preoperative treatment [3, 4, 72]; however, it is usually not first line. Of note, high doses of dexamethasone are needed to overcome cortisol receptor blockade [38] if used preoperatively to avoid intraoperative AI.
Retrospective data show that the preoperative use of steroidogenesis inhibitors ketoconazole, metyrapone, or both is able to achieve cortisol normalization in approximately 40% to 50% of patients at an average of 3 o 4 months [75, 76]. A rapid reduction (~ 70%) in UFC occurred within 1 month of treatment with metyrapone at a median dose of 1000 mg/d [77]. Ketoconazole also has a rapid onset of action and induces rapid decrease in cortisol at doses of 600 to 800 mg/d [78]. Clinical improvement (hypertension, diabetes, hypokalemia) was noted in approximately 50% of patients treated with ketoconazole preoperatively during a median of 4 months [75]. Treatment with metyrapone similarly results in clinical improvement [70, 76, 77]. Osilodrostat has been studied mostly in patients who had failed TSS, but showed rapid UFC improvement. Therefore, it could be envisioned to work in a preoperative setting, also. A prospective, open-label, phase 2 trial of 19 patients treated with osilodrostat demonstrated that 84.2% of patients had normalized UFC at 10 weeks [79]. Median time to normalization of UFC was 41 days in a phase 3 trial, and clinical improvement was noted within the first 12 weeks of treatment [40].
Interestingly, the Study of levOketocoNazole In Cushing’s Syndrome (SONICS) included almost one-third of patients naive to all treatments for CS, but efficacy in normalizing UFC was somewhat lower (~ 40% using similar criteria with previous studies) [80].
If a very rapid control of hypercortisolism is desired, higher doses of steroidogenesis inhibitors are needed, thus potentially increasing the risk of AI. One approach to prevent AI is to administer replacement doses of hydrocortisone or dexamethasone concurrently with steroidogenesis inhibitors. This is known as the block-and-replace strategy and it allows the achievement of eucortisolemia, particularly in severe cases. On the other hand, lower starting doses of steroidogenesis inhibitors (eg, osilodrostat 1-2 mg twice daily, metyrapone 250 mg 4 times daily, or ketoconazole 200 mg twice daily) and slower titration is preferred in patients with less severe disease as cortisol withdrawal symptoms and AI are common even with typical doses [39].
Medical therapy is frequently associated with various other side effects. Ketoconazole and levoketoconazole may cause gastrointestinal distress, hepatotoxicity, and QT interval prolongation, especially if coadministered with other QT-prolonging drugs [39]. Metyrapone and osilodrostat, but also mifepristone, could cause hypokalemia, edema, and hypertension. Given an unclear benefit of routine PMT and potential adverse effects, PMT should be offered on an individual basis, mainly if the surgery is delayed, not feasible, or if hypercortisolism is severe or life-threatening [4].
Low and undetectable postoperative cortisol is generally interpreted as evidence of remission, while “normal” levels are interpreted as a marker of persistent disease, as discussed later. Importantly, PMT may affect postsurgery cortisol levels. It has been suggested that preoperative cortisol normalization helps suppressed corticotropes to recover, thus leading to less postoperative AI [67, 81]. In the European Registry on Cushing’s Syndrome (ERCUSYN) registry, patients who received PMT were less frequently reported to have low or undetectable cortisol levels compared to a group that received surgery only (60% vs 69% respectively, P = .01) [67]. However, this has not been confirmed in other studies, which showed either no difference in remission or even improved remission rates after PMT [76, 82, 83].
Management Considerations Immediately After Pituitary Surgery
Case 1 continued
The patient undergoes an uncomplicated transsphenoidal resection of the pituitary mass. Pathology confirms corticotroph adenoma. The patient’s postoperative cortisol levels are 2.6, 1.7, 1.2, and 1.0 µg/dL, and ACTH levels are 6, 5, 4, and 4 pg/mL, measured every 6 hours starting postoperative day 1. The patient is started on hydrocortisone replacement 20 mg 2 times daily with subsequent gradual dose taper.
How Should one Assess for Disease Remission in the Immediate Postoperative Period?
Disease remission immediately postoperatively is assessed by measuring nadir serum cortisol, which usually occurs within 48 hours after surgery if no perioperative GC was administered, but this is variable [84, 85]. Since normal corticotroph cells are suppressed in CD, secondary AI after complete removal of the tumor is regarded as evidence of remission, and can continue for months and even years [3, 12]. Most studies report a high likelihood of durable remission if postoperative nadir serum cortisol is less than 2 µg/dL (< 55 nmol/L) [4, 12, 85-87]. A cortisol nadir of 2 to 5 µg/dL (55-138 nmol/L) has also been considered as remission in many studies; the likelihood of recurrence appears to be similar in most studies to that of patients with cortisol of less than 2 µg/dL (10% in the long term in some studies) [81, 88, 89]. A cortisol greater than 5 to 10 µg/dL signifies nonremission; however, some patients experience a delayed remission [90]. Indeed, cortisol nadir on day 3 has been sometimes observed, suggesting that monitoring for up to 72 hours without GC maybe needed, especially if early reoperation is considered [85]. Nevertheless, earlier hypocortisolemia of less than or equal to 2 µg/dL, before 21 postoperative hours, appeared to more accurately predict 1-year remission in one study [91]. Last, because medical pretreatment of CD can alter postoperative cortisol levels, normal levels in those who received PMT could be misleading.
Measurement of ACTH is often incorporated into early postoperative assessment(s); however, its role is less clearly defined. A postoperative nadir ACTH value lower than 15 to 20 pg/mL (3.3-4.4 pmol/L) has been described as a prognostic marker for remission [92, 93]. A combination postoperative nadir cortisol of less than 2 µg/dL and ACTH of less than 5 pg/mL had a 100% positive predictive value for remission in one study [86]. In the same study, persistent disease was be predicted by an ACTH greater than 15 pg/mL (87.5% positive predictive value) [86].
A 24-hour UFC level of less than 10 to 20 μg/d (< 28-56 nmol/d) suggests remission; however, it may be difficult to obtain accurate 24-hour UFC in the immediate postoperative period, particularly if GC is given perioperatively, and very few centers use this currently [94, 95]. Late-night salivary cortisol may be more useful in patients with normal 24-hour UFC preoperatively, cyclic CS, and those pretreated medically as their morning serum cortisol may not fall drastically after TSS [95]; the DST is considered less reliable in the assessment of remission especially because cortisol cutoff is not agreed on [96]. While some of these tests are not routinely used in the immediate postoperative period in the United States, differences in local practice exist worldwide as reflected in the International Pituitary Society Delphi Survey [97].
Delayed normalization of 24-hour UFC and DST has been also described. In a study of 620 cases of TSS for CD, 5.6% of patients with early postoperative normal or elevated 24-hour UFC had a delayed cortisol decrease and remission achieved at 38 days (SD = 50 d) postoperatively [90]. Another study evaluating long-term outcomes of 426 patients with CD found that 4% of patients who failed to adequately suppress cortisol after a 2-mg DST in the early postoperative period were in remission 6 months postoperatively [98]. Therefore, initial postoperative hormonal evaluation might be misleading and expectant management and follow-up testing for 6 to 12 weeks [90], depending on the clinical and biochemical picture, might be needed to avoid unnecessary early reoperation.
Which Factors Predict Remission and Recurrence? Is There a Role of Pathological Confirmation?
A recent meta-analysis (88 studies) confirmed consistent remission rates over the last 4 decades, 83% in microadenomas and 68% in macroadenomas [3, 87]. Factors that affect remission rates include adenoma size, invasiveness, presence of adenoma on imaging, and ACTH+ adenoma on pathology, number of surgeries, the surgeon’s experience, and others [3, 87]. Postoperative remission is lower in patients with larger and invasive macroadenomas (Knosp grades 3 and 4) and in patients with microadenomas that lack a surgical pseudocapsule [99, 100]. Macroadenomas have higher Ki-67 and p53 expression and are more commonly sparsely granulated [99]. In addition, low expression of O-6-methylguanine-DNA methyltransferase (MGMT) is associated with larger tumors, higher p53, and lower probability for early clinical remission [101]. On the other hand, one study showed that remission may be higher in patients with USP8-mutated alleles [102].
While the presence of ACTH staining on pathology confirms CD, a pathology sample may not always be adequate, raising the question of whether the tumor was actually removed. Patients with microadenomas who achieve postoperative hypocortisolemia and require GC in the absence of a tumor on imaging and/or confirmative pathology are considered to have achieved surgical remission [103]. However, patients with persistent hypercortisolemia and no adenoma on pathology either had their adenoma missed during surgery or the diagnosis of CD may need to be reconsidered (consider ectopic CS or pseudo-Cushing). Most patients with CD have a Crooke hyaline change in nontumorous corticotrophs on pathology, and the absence of Crooke change has been associated with reduced chance of remission [104]. Also, double pituitary adenomas are also very rarely reported in patients with CD and may be the cause of nonremission [105].
Patients who achieve remission postoperatively may develop a disease recurrence/relapse during the following months and years. In those who achieve early remission, there is no immediate postoperative test that best predicts the risk of recurrence [4, 12]. Some studies have suggested that a desmopressin test could potentially play a role [4, 12]. A corticotropin-releasing hormone test early in the postoperative period does not yield adequate information on recurrence likelihood [88].
What Glucocorticoid Regimen Is Used Postoperatively?
GC replacement regimens for patients with CD perioperatively vary from center to center, with 2 protocols dominating clinical approaches; however, none has been systematically studied: 1) intraoperative and early postoperative GC, and 2) no intraoperative and early postoperative GC until documentation of low nadir cortisol or development of signs of hypoadrenalism. The first approach is used to avoid the risk of AI perioperatively in patients not monitored on intensive therapy floors (GCs are withheld a few days later to measure morning or serial serum cortisol levels during the first postoperative week, with timelines differing, depending on local practices) [10, 95]. Nevertheless, patients with CD have a considerably activated hypothalamic-pituitary-adrenal (HPA) axis and cortisol levels could remain high during the first 10 hours following surgery (this is expected given the half-life of cortisol) [106]. Based on this, the second approach in many centers is not to administer any GCs until biochemical remission is confirmed or clinical symptoms of AI develop [29, 84]. Cortisol levels are measured every 6 hours for 24 to 72 hours postoperatively [10, 85, 95, 107], though some centers measure only morning cortisol on days 1 to 2 [29, 94]. Close monitoring for signs/symptoms of hypoadrenalism (eg, hypotension, lightheadedness, weakness, nausea, and abdominal pain) and a fast laboratory turnaround of serum cortisol levels are required.
Distinguishing between GC withdrawal from AI can be difficult [108]; therefore multidisciplinary clinician and nursing awareness about the possibility of adrenal crisis and prompt management is warranted. Given a reported high incidence of postoperative acute complications (especially infections) in patients with CD, special attention should be paid to patients with acute postoperative complications who may acutely develop AI [5].
Patients who had PMT could have lower cortisol levels and will often receive a GC stress dose intravenously perioperatively to prevent an adrenal crisis. Steroidogenesis inhibitors should be stopped several days before surgery to minimize the risk of AI and interference with postoperative cortisol assessment for remission [29].
GCs should be started not only for cortisol levels of less than 5 µg/dL [10] but also for levels of 5 to 10 µg/dL because these patients may have symptoms of AI or experience delayed remission in the following days [29, 84]. Patients with cortisol levels of 10 to 15 µg/dL may also require GC replacement on a case-by-case basis, particularly in case of a surgical complication or clinical manifestations of AI [29]. Subsequent retesting of morning serum cortisol can be performed 1 to 2 weeks after discharge and after withholding GCs for 24 hours. The dose of GC replacement also varies among centers. Endocrine Society guidelines recommend hydrocortisone at 10 to 12 mg/m2/d (or equivalent) in divided doses [3, 9, 10]. However, many centers prefer supraphysiologic replacement up to 20 mg of hydrocortisone 2 to 3 times daily with a 2- to 4-week taper afterward, as tolerated. Dexamethasone has a long half-life and should be avoided postoperatively because it may prolong HPA axis suppression [3].
Patients in nonremission can be considered for early repeated TSS, which may be successful in achieving at least short-term remission, but is associated with higher rates of postoperative hypopituitarism and cerebrospinal fluid leak when compared to the initial intervention [4, 109]. Other treatment options for persistent disease include medical therapy, radiation therapy, and BLA [4]; however, they are not the focus of this review and can be found detailed elsewhere [3, 4].
How Should one Monitor for Postoperative Hyponatremia, Diabetes Insipidus, and Other Pituitary Deficiencies?
Transsphenoidal surgery for any sellar or suprasellar lesion may lead to posterior pituitary dysfunction manifesting as hyponatremia or diabetes insipidus (DI). Hyponatremia is usually related to the syndrome of inappropriate antidiuretic hormone (SIADH), occurring in 5% to 30% of patients after pituitary surgery for all tumors [3, 107], with a peak incidence on days 4 to 7 (range, 3-12 d) [107, 110]. Some studies reported a higher incidence of hyponatremia after TSS for CD [111-113], while other risk factors include tumor size, hypopituitarism, female sex, and low body mass index [107, 114]. Postoperative fluid restriction has been shown to reduce the risk of hyponatremia and hospitalization after TSS for various pituitary lesions [115]. In one study, 1L fluid restriction effectively prevented readmissions for hyponatremia in patients with CD, who comprised 13.3% of the cohort [116], but this has to be balanced with avoiding dehydration. Of note, postoperative AI is also a risk factor for hyponatremia [114], and one could suggest that inadequate GC replacement may contribute to hyponatremia post TSS for CD, particularly in patients with delayed remission.
Transient postoperative DI has been reported in 4% to 30% of patients with sellar lesions, with 0.3% to 7.3% persisting long term [10, 117, 118]. Few studies have demonstrated a higher occurrence of postoperative DI in CD patients, attributing it to more extensive exploratory surgery and repeated surgery [107, 119]. DI usually develops within 24 to 48 hours postoperatively and resolves within 3 to 5 days [96]. Sometimes it is followed by transient hyponatremia and a subsequent return of DI, which is usually permanent (triphasic response).
To monitor for electrolyte disturbances, it is recommended to measure serum sodium every 6 to 12 hours, urine osmolality daily, and record inpatient fluid intake and urine output [10, 95]. When serum sodium is abnormal or shows a trend to move toward the extremes of the normal range, measurement of serum sodium paired with urine osmolality or specific gravity is necessary for correct diagnosis. In clinical practice, several centers, including ours, monitor paired serum sodium and urine osmolality or specific gravity routinely. Elevated urine output is often observed after large fluid administration intraoperatively, and should be differentiated from DI. A combination of high output of hypotonic urine (> 250-300 mL/h for 2 consecutive h) and high serum sodium (> 145 mmol/L) usually signifies DI [3, 9, 10].
DI is treated with desmopressin, for example, starting with 0.5 µg subcutaneously or intravenously or 50 to 100 µg orally and repeated as needed, rather than administering scheduled doses, unless persistent DI is clearly established. Scheduled doses should be avoided to reduce the risk of hyponatremia during the time when SIADH may occur [3, 9, 10]. Sodium should also be measured after discharge at least once on days 5 to 7, and as needed up to day 15 guided by the patient’s previous sodium levels, fluid intake, and urine output [3, 10]. Mild hyponatremia (125-135 mmol/L) can be managed with fluid restriction as outpatient, but severe and symptomatic hyponatremia requires evaluation and treatment in the hospital and possible administration of hypertonic saline with close monitoring to avoid overcorrection [10]. Sodium can be rapidly corrected by 4 to 6 mmol/L if the known value was normal less than 24 to 48 hours previously [120, 121]. Symptomatic hyponatremia of chronic or unknown duration requires a slow correction (≤ 8-10 mmol/L per 24 h) with small boluses of hypertonic saline or slow infusion rates and sodium monitoring every 2 hours [120, 121].
Patient education on postoperative water-electrolyte imbalances, symptoms that accompany them, and when to seek medical help is essential [3, 9, 10]. Close monitoring for increase urinary output after starting GCs is also needed as restarting GCs could unmask DI [3, 9, 10].
Assessment for anterior hypopituitarism is usually performed during the following 4 to 6 weeks. Abnormal thyroid function is frequent in CD cases [122], likely mediated by subnormal nocturnal thyrotropin, and is reversible in many patients after cure [123]. Since free thyroxine half-life is approximately 1 week, free thyroxine measurement is recommended at 1 to 2 weeks postoperatively [3, 29], and reevaluated 4 to 6 weeks thereafter. Testing for HPA axis recovery should be conducted periodically (starting 1-2 wk postoperatively and then every 4-6 wk) with morning serum cortisol or dynamic testing [3, 9, 10]. Assessment for growth hormone deficiency should be delayed until 6 to 12 months postoperatively because of the possibility of recovery of the growth hormone axis [4]. Postoperative pituitary magnetic resonance imaging within 1 to 3 months of successful TSS is also needed as a new baseline [3, 9, 10, 124].
Conclusion
CD has many comorbidities and has high mortality if not treated promptly and adequately. Hypercortisolemia is addressed by pituitary surgery in most patients, but preoperative medical therapy may be necessary in case of surgical delay or severe disease. Management and prevention of CV, thromboembolic, and infectious complications should be proactive and intensive, particularly during the first 3 months after diagnosis and surgery, when the incidence of complications is the highest. Postoperative remission is confirmed by low serum cortisol levels, though protocols for remission assessment and GC coverage vary across centers. Close monitoring for AI, hyponatremia, and/or DI is of paramount importance, and prompt management is required to prevent adverse outcomes.
Acknowledgments
The authors thank Shirley McCartney, PhD (OHSU), for editorial assistance and Fabienne Langlois, MD (Sherbrooke University, Canada), for discussions on protocol management and critical review.
Glossary
Abbreviations
- ACTH
adrenocorticotropin
- AI
adrenal insufficiency
- BLA
bilateral adrenalectomy
- CD
Cushing disease
- CS
Cushing syndrome
- CV
cardiovascular
- DI
diabetes insipidus
- DST
dexamethasone suppression test
- DVT
deep vein thrombosis
- GC
glucocorticoid
- HPA
hypothalamic-pituitary-adrenal
- PJP
Pneumocystis jirovecii pneumonia
- PMT
preoperative medical therapy
- SIADH
syndrome of inappropriate antidiuretic hormone
- (TSS
transsphenoidal pituitary surgery
- UFC
urinary free cortisol
- ULN
upper limit of normal
- VTE
venous thromboembolism
Financial Support
The authors received no financial support for the research, authorship, and/or publication of this article.
Author Contributions
All authors contributed to this manuscript by drafting sections, providing conceptual guidance, text review, and valuable content discussion.
Disclosures
E.V.V. has nothing to disclose. G.V. has received occasional lecture and/or consulting fees from HRA Pharma and Recordati, and is aresearch investigator for studies sponsored by Novartis, Recordati, and Corcept. M.F. has received research support to OHSU as a principal investigator from Recordati and Strongbridge and has received occasional scientific consulting fees from Recordati, HRA Pharma, and Sparrow.
Data Availability
Data sharing is not applicable to this article because no data sets were generated or analyzed during the present study.
References
- 1. Pivonello R, Isidori AM, De Martino MC, Newell-Price J, Biller BM, Colao A. Complications of Cushing’s syndrome: state of the art. Lancet Diabetes Endocrinol. 2016;4(7):611-629. [DOI] [PubMed] [Google Scholar]
- 2. Varlamov EV, Langlois F, Vila G, Fleseriu M. Management of endocrine disease: cardiovascular risk assessment, thromboembolism, and infection prevention in Cushing’s syndrome: a practical approach. Eur J Endocrinol. 2021;184(5):R207-R224. [DOI] [PubMed] [Google Scholar]
- 3. Nieman LK, Biller BM, Findling JW, et al. Treatment of Cushing’s syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2015;100(8):2807-2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fleseriu M, Auchus R, Bancos I, et al. Consensus on diagnosis and management of Cushing’s disease: a guideline update. Lancet Diabetes Endocrinol. 2021;9(12):847-875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schernthaner-Reiter MH, Siess C, Micko A, et al. Acute and life-threatening complications in Cushing syndrome: prevalence, predictors, and mortality. J Clin Endocrinol Metab. 2021;106(5):e2035-e2046. [DOI] [PubMed] [Google Scholar]
- 6. Dekkers OM, Horváth-Puhó E, Jørgensen JOL, et al. Multisystem morbidity and mortality in Cushing’s syndrome: a cohort study. J Clin Endocrinol Metab. 2013;98(6):2277-2284. [DOI] [PubMed] [Google Scholar]
- 7. Valassi E, Tabarin A, Brue T, et al. High mortality within 90 days of diagnosis in patients with Cushing’s syndrome: results from the ERCUSYN registry. Eur J Endocrinol. 2019;181(5):461-472. [DOI] [PubMed] [Google Scholar]
- 8. Papakokkinou E, Olsson DS, Chantzichristos D, et al. Excess morbidity persists in patients with Cushing’s disease during long-term remission: a Swedish nationwide study. J Clin Endocrinol Metab. 2020;105(8):2616-2624. [DOI] [PubMed] [Google Scholar]
- 9. Fleseriu M, Hashim IA, Karavitaki N, et al. Hormonal replacement in hypopituitarism in adults: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2016;101(11):3888-3921. [DOI] [PubMed] [Google Scholar]
- 10. Prete A, Corsello SM, Salvatori R. Current best practice in the management of patients after pituitary surgery. Ther Adv Endocrinol Metab. 2017;8(3):33-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang F, Catalino MP, Bi WL, et al. Postoperative day 1 morning cortisol value as a biomarker to predict long-term remission of Cushing disease. J Clin Endocrinol Metab. 2021;106(1):e94-e102. [DOI] [PubMed] [Google Scholar]
- 12. Fleseriu M, Hamrahian AH, Hoffman AR, Kelly DF, Katznelson L; AACE Neuroendocrine and Pituitary Scientific Committee . American Association of Clinical Endocrinologists and American College of Endocrinology disease state clinical review: diagnosis of recurrence in Cushing disease. Endocr Pract. 2016;22(12):1436-1448. [DOI] [PubMed] [Google Scholar]
- 13. Isidori AM, Minnetti M, Sbardella E, Graziadio C, Grossman AB. Mechanisms in endocrinology: the spectrum of haemostatic abnormalities in glucocorticoid excess and defect. Eur J Endocrinol. 2015;173(3):R101-R113. [DOI] [PubMed] [Google Scholar]
- 14. Wagner J, Langlois F, Lim DST, McCartney S, Fleseriu M. Hypercoagulability and risk of venous thromboembolic events in endogenous Cushing’s syndrome: a systematic meta-analysis. Front Endocrinol (Lausanne). 2019;9:805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Casonato A, Pontara E, Boscaro M, et al. Abnormalities of von Willebrand factor are also part of the prothrombotic state of Cushing’s syndrome. Blood Coagul Fibrinolysis. 1999;10(3):145-151. [DOI] [PubMed] [Google Scholar]
- 16. Erem C, Nuhoglu I, Yilmaz M, et al. Blood coagulation and fibrinolysis in patients with Cushing’s syndrome: increased plasminogen activator inhibitor-1, decreased tissue factor pathway inhibitor, and unchanged thrombin-activatable fibrinolysis inhibitor levels. J Endocrinol Invest. 2009;32(2):169-174. [DOI] [PubMed] [Google Scholar]
- 17. Kastelan D, Dusek T, Kraljevic I, et al. Hypercoagulability in Cushing’s syndrome: the role of specific haemostatic and fibrinolytic markers. Endocrine. 2009;36(1):70-74. [DOI] [PubMed] [Google Scholar]
- 18. Van Zaane B, Nur E, Squizzato A, et al. Hypercoagulable state in Cushing’s syndrome: a systematic review. J Clin Endocrinol Metab. 2009;94(8):2743-2750. [DOI] [PubMed] [Google Scholar]
- 19. Akaza I, Yoshimoto T, Tsuchiya K, Hirata Y. Endothelial dysfunction associated with hypercortisolism is reversible in Cushing’s syndrome. Endocr J. 2010;57(3):245-252. [DOI] [PubMed] [Google Scholar]
- 20. Engin A. Endothelial dysfunction in obesity. Adv Exp Med Biol. 2017;960:345-379. [DOI] [PubMed] [Google Scholar]
- 21. Kaur R, Kaur M, Singh J. Endothelial dysfunction and platelet hyperactivity in type 2 diabetes mellitus: molecular insights and therapeutic strategies. Cardiovasc Diabetol. 2018;17(1):121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Konukoglu D, Uzun H. Endothelial dysfunction and hypertension. Adv Exp Med Biol. 2017;956:511-540. [DOI] [PubMed] [Google Scholar]
- 23. Suarez MG, Stack M, Hinojosa-Amaya JM, et al. Hypercoagulability in Cushing syndrome, prevalence of thrombotic events: a large, single-center, retrospective study. J Endocr Soc. 2020;4(2):bvz033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Adams GL, Manson RJ, Turner I, Sindram D, Lawson JH. The balance of thrombosis and hemorrhage in surgery. Hematol Oncol Clin North Am. 2007;21(1):13-24. [DOI] [PubMed] [Google Scholar]
- 25. Barbot M, Daidone V, Zilio M, et al. Perioperative thromboprophylaxis in Cushing’s disease: what we did and what we are doing? Pituitary. 2015;18(4):487-493. [DOI] [PubMed] [Google Scholar]
- 26. Boscaro M, Sonino N, Scarda A, et al. Anticoagulant prophylaxis markedly reduces thromboembolic complications in Cushing’s syndrome. J Clin Endocrinol Metab. 2002;87(8):3662-3666. [DOI] [PubMed] [Google Scholar]
- 27. Recorlev (levoketoconazole). Xeris Pharmaceuticals; 2021. Accessed January 5, 2022. https://www.xerispharma.com/pdf/recorlev-fact-sheet.pdf
- 28. Ketoconazole. Lexi-drugs. Last Updated March 9, 2019. Accessed January 5, 2022. online.lexi.com
- 29. Barbot M, Ceccato F, Lizzul L, et al. Perioperative multidisciplinary management of endoscopic transsphenoidal surgery for sellar lesions: practical suggestions from the Padova model. Neurosurg Rev. 2020;43(4):1109-1116. [DOI] [PubMed] [Google Scholar]
- 30. Arnaldi G, Scandali VM, Trementino L, Cardinaletti M, Appolloni G, Boscaro M. Pathophysiology of dyslipidemia in Cushing’s syndrome. Neuroendocrinology. 2010;92(Suppl 1):86-90. [DOI] [PubMed] [Google Scholar]
- 31. Ferrau F, Korbonits M. Metabolic syndrome in Cushing’s syndrome patients. Front Horm Res. 2018;49:85-103. [DOI] [PubMed] [Google Scholar]
- 32. Scaroni C, Zilio M, Foti M, Boscaro M. Glucose metabolism abnormalities in Cushing syndrome: from molecular basis to clinical management. Endocr Rev. 2017;38(3):189-219. [DOI] [PubMed] [Google Scholar]
- 33. Hasenmajer V, Sbardella E, Sciarra F, Minnetti M, Isidori AM, Venneri MA. The immune system in Cushing’s syndrome. Trends Endocrinol Metab. 2020;31(9):655-669. [DOI] [PubMed] [Google Scholar]
- 34. Neary NM, Booker OJ, Abel BS, et al. Hypercortisolism is associated with increased coronary arterial atherosclerosis: analysis of noninvasive coronary angiography using multidetector computerized tomography. J Clin Endocrinol Metab. 2013;98(5):2045-2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Barbot M, Zilio M, Scaroni C. Cushing’s syndrome: overview of clinical presentation, diagnostic tools and complications. Best Pract Res Clin Endocrinol Metab. 2020;34(2):101380. [DOI] [PubMed] [Google Scholar]
- 36. Yelamanchi VP, Molnar J, Ranade V, Somberg JC. Influence of electrolyte abnormalities on interlead variability of ventricular repolarization times in 12-lead electrocardiography. Am J Ther. 2001;8(2):117-122. [DOI] [PubMed] [Google Scholar]
- 37. Stewart PM, Walker BR, Holder G, O’Halloran D, Shackleton CH. 11 beta-Hydroxysteroid dehydrogenase activity in Cushing’s syndrome: explaining the mineralocorticoid excess state of the ectopic adrenocorticotropin syndrome. J Clin Endocrinol Metab. 1995;80(12):3617-3620. [DOI] [PubMed] [Google Scholar]
- 38. Fleseriu M, Molitch ME, Gross C, Schteingart DE, Vaughan TB III, Biller BMK. A new therapeutic approach in the medical treatment of Cushing’s syndrome: glucocorticoid receptor blockade with mifepristone. Endocr Pract. 2013;19(2):313-326. [DOI] [PubMed] [Google Scholar]
- 39. Varlamov EV, Han AJ, Fleseriu M. Updates in adrenal steroidogenesis inhibitors for Cushing’s syndrome–a practical guide. Best Pract Res Clin Endocrinol Metab. 2021;35(1):101490. [DOI] [PubMed] [Google Scholar]
- 40. Pivonello R, Fleseriu M, Newell-Price J, et al. Efficacy and safety of osilodrostat in patients with Cushing’s disease (LINC 3): a multicentre phase III study with a double-blind, randomised withdrawal phase. Lancet Diabetes Endocrinol. 2020;8(9):748-761. [DOI] [PubMed] [Google Scholar]
- 41. Pivonello R, Fleseriu M, Newell-Price J, et al. Effect of osilodrostat on clinical signs, physical features and health-related quality of life (HRQoL) by degree of mUFC control in patients with Cushing’s disease (CD): results from the LINC 3 study 2021. Presented at: 2021 AACE Virtual Annual Meeting, May 26-29, 2021. [Google Scholar]
- 42. Nieman LK. Hypertension and cardiovascular mortality in patients with Cushing syndrome. Endocrinol Metab Clin North Am. 2019;48(4):717-725. [DOI] [PubMed] [Google Scholar]
- 43. Santilli F, Simeone P. Aspirin in primary prevention: the triumph of clinical judgement over complex equations. Intern Emerg Med. 2019;14(8):1217-1231. [DOI] [PubMed] [Google Scholar]
- 44. American Diabetes Association. 10. Cardiovascular disease and risk management: Standards of Medical Care in Diabetes–2021. Diabetes Care. 2021;44(Suppl 1)S125-–S150.. [DOI] [PubMed] [Google Scholar]
- 45. Al-Sofiani ME, Derenbecker R, Quartuccio M, Kalyani RR. Aspirin for primary prevention of cardiovascular disease in diabetes: a review of the evidence. Curr Diab Rep. 2019;19(10):107. [DOI] [PubMed] [Google Scholar]
- 46. Chou R, Dana T, Blazina I, Daeges M, Jeanne TL. Statins for prevention of cardiovascular disease in adults: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2016;316(19):2008-2024. [DOI] [PubMed] [Google Scholar]
- 47. American Diabetes Association. 9. Pharmacologic approaches to glycemic treatment: Standards of Medical Care in Diabetes–2021. Diabetes Care. 2021;44(Suppl 1):S111-S124. [DOI] [PubMed] [Google Scholar]
- 48. Baroni MG, Giorgino F, Pezzino V, Scaroni C, Avogaro A. Italian Society for the Study of Diabetes (SID)/Italian Endocrinological Society (SIE) guidelines on the treatment of hyperglycemia in Cushing’s syndrome and acromegaly. J Endocrinol Invest. 2016;39(2):235-255. [DOI] [PubMed] [Google Scholar]
- 49. Sharma A, Vella A. Glucose metabolism in Cushing’s syndrome. Curr Opin Endocrinol Diabetes Obes. 2020;27(3):140-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Pernicova I, Kelly S, Ajodha S, et al. Metformin to reduce metabolic complications and inflammation in patients on systemic glucocorticoid therapy: a randomised, double-blind, placebo-controlled, proof-of-concept, phase 2 trial. Lancet Diabetes Endocrinol. 2020;8(4):278-291. [DOI] [PubMed] [Google Scholar]
- 51. Osilodrostat. IBM Micromedex. Last Modified January 12, 2022. Accessed January 5, 2022. www.micromedexsolutions.com
- 52. Mifepristone. Lexi-drugs. Last Updated February 1, 2022. Accessed January 5, 2022. online.lexi.com
- 53. Mifepristone. IBM Micromedex. Last Modified December 22, 2021. Accessed January 5, 2022. www.micromedexsolutions.com
- 54. Ketoconazole. IBM Micromedex. Last Modified January 31, 2022. Accessed January 5, 2022. www.micromedexsolutions.com
- 55. Arnaldi G, Mancini T, Tirabassi G, Trementino L, Boscaro M. Advances in the epidemiology, pathogenesis, and management of Cushing’s syndrome complications. J Endocrinol Invest. 2012;35(4):434-448. [DOI] [PubMed] [Google Scholar]
- 56. van Halem K, Vrolijk L, Pereira AM, de Boer MGJ. Characteristics and mortality of Pneumocystis pneumonia in patients with Cushing’s syndrome: a plea for timely initiation of chemoprophylaxis. Open Forum Infect Dis. 2017;4(1):ofx002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Cooley L, Dendle C, Wolf J, et al. Consensus guidelines for diagnosis, prophylaxis and management of Pneumocystis jirovecii pneumonia in patients with haematological and solid malignancies, 2014. Intern Med J. 2014;44(12b):1350-1363. [DOI] [PubMed] [Google Scholar]
- 58. Fishman JA. Pneumocystis jiroveci. Semin Respir Crit Care Med. 2020;41(1):141-157. [DOI] [PubMed] [Google Scholar]
- 59. Fishman JA, Gans H; Infectious Diseases Community of Practice. Pneumocystis jiroveci in solid organ transplantation: guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin Transplant. 2019;33(9):e13587. [DOI] [PubMed] [Google Scholar]
- 60. Anggraeni AT, Soedarsono S, Soeprijanto B. Concurrent COVID-19 and Pneumocystis jirovecii pneumonia: the importance of radiological diagnostic and HIV testing. Radiol Case Rep. 2021;16(12):3685-3689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Chong WH, Saha BK, Chopra A. Narrative review of the relationship between COVID-19 and PJP: does it represent coinfection or colonization? Infection. 2021;49(6):1079-1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Gentile I, Viceconte G, Lanzardo A, et al. Pneumocystis jirovecii pneumonia in non-HIV patients recovering from COVID-19: a single-center experience. Int J Environ Res Public Health. 2021;18(21):11399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Little AS, White WL. Prophylactic antibiotic trends in transsphenoidal surgery for pituitary lesions. Pituitary. 2011;14(2):99-104. [DOI] [PubMed] [Google Scholar]
- 64. Moldovan ID, Agbi C, Kilty S, Alkherayf F. A systematic review of prophylactic antibiotic use in endoscopic endonasal transsphenoidal surgery for pituitary lesions. World Neurosurg. 2019;128:408-414. [DOI] [PubMed] [Google Scholar]
- 65. Vogel F, Reincke M. Endocrine risk factors for COVID-19: endogenous and exogenous glucocorticoid excess. Rev Endocr Metab Disord. 2021:1-18. doi:10.1007/s11154-021-09670-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Rubin LG, Levin MJ, Ljungman P, et al. Infectious Diseases Society of America . 2013 IDSA clinical practice guideline for vaccination of the immunocompromised host. Clin Infect Dis. 2014;58(3):309-318. [DOI] [PubMed] [Google Scholar]
- 67. Valassi E, Franz H, Brue T, et al. ERCUSYN Study Group . Preoperative medical treatment in Cushing’s syndrome: frequency of use and its impact on postoperative assessment: data from ERCUSYN. Eur J Endocrinol. 2018;178(4): 399-409. [DOI] [PubMed] [Google Scholar]
- 68. Fleseriu M, Buchfelder M, Cetas JS, et al. Pituitary society guidance: pituitary disease management and patient care recommendations during the COVID-19 pandemic–an international perspective. Pituitary. 2020;23(4):327-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Newell-Price J, Nieman LK, Reincke M, Tabarin A. Endocrinology in the time of COVID-19: management of Cushing’s syndrome. Eur J Endocrinol. 2020;183(1):G1-G7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. van den Bosch OFC, Stades AME, Zelissen PMJ. Increased long-term remission after adequate medical cortisol suppression therapy as presurgical treatment in Cushing’s disease. Clin Endocrinol (Oxf). 2014;80(2):184-190. [DOI] [PubMed] [Google Scholar]
- 71. Stuijver DJF, van Zaane B, Feelders RA, et al. Incidence of venous thromboembolism in patients with Cushing’s syndrome: a multicenter cohort study. J Clin Endocrinol Metab. 2011;96(11):3525-3532. [DOI] [PubMed] [Google Scholar]
- 72. Constantinescu SM, Driessens N, Lefebvre A, Furnica RM, Corvilain B, Maiter D. Etomidate infusion at low doses is an effective and safe treatment for severe Cushing’s syndrome outside intensive care. Eur J Endocrinol. 2020;183(2):161-167. [DOI] [PubMed] [Google Scholar]
- 73. Theodoropoulou M, Reincke M. Tumor-directed therapeutic targets in Cushing disease. J Clin Endocrinol Metab. 2019;104(3):925-933. [DOI] [PubMed] [Google Scholar]
- 74. Lacroix A, Gu F, Schopohl J, et al. Pasireotide treatment significantly reduces tumor volume in patients with Cushing’s disease: results from a phase 3 study. Pituitary. 2020;23(3):203-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Castinetti F, Guignat L, Giraud P, et al. Ketoconazole in Cushing’s disease: is it worth a try? J Clin Endocrinol Metab. 2014;99(5):1623-1630. [DOI] [PubMed] [Google Scholar]
- 76. Valassi E, Crespo I, Gich I, Rodríguez J, Webb SM. A reappraisal of the medical therapy with steroidogenesis inhibitors in Cushing’s syndrome. Clin Endocrinol (Oxf). 2012;77(5):735-742. [DOI] [PubMed] [Google Scholar]
- 77. Ceccato F, Zilio M, Barbot M, et al. Metyrapone treatment in Cushing’s syndrome: a real-life study. Endocrine. 2018;62(3):701-711. [DOI] [PubMed] [Google Scholar]
- 78. Loli P, Berselli ME, Tagliaferri M. Use of ketoconazole in the treatment of Cushing’s syndrome. J Clin Endocrinol Metab. 1986;63(6):1365-1371. [DOI] [PubMed] [Google Scholar]
- 79. Fleseriu M, Pivonello R, Young J, et al. Osilodrostat, a potent oral 11β-hydroxylase inhibitor: 22-week, prospective, phase II study in Cushing’s disease. Pituitary. 2016;19(2):138-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Fleseriu M, Pivonello R, Elenkova A, et al. Efficacy and safety of levoketoconazole in the treatment of endogenous Cushing’s syndrome (SONICS): a phase 3, multicentre, open-label, single-arm trial. Lancet Diabetes Endocrinol. 2019;7(11):855-865. [DOI] [PubMed] [Google Scholar]
- 81. Biller BMK, Grossman AB, Stewart PM, et al. Treatment of adrenocorticotropin-dependent Cushing’s syndrome: a consensus statement. J Clin Endocrinol Metab. 2008;93(7):2454-2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Invitti C, Pecori Giraldi F, de Martin M, Cavagnini F. Diagnosis and management of Cushing’s syndrome: results of an Italian multicentre study. Study Group of the Italian Society of Endocrinology on the Pathophysiology of the Hypothalamic-Pituitary-Adrenal Axis. J Clin Endocrinol Metab. 1999;84(2):440-448. [DOI] [PubMed] [Google Scholar]
- 83. Pereira AM, van Aken MO, van Dulken H, et al. Long-term predictive value of postsurgical cortisol concentrations for cure and risk of recurrence in Cushing’s disease. J Clin Endocrinol Metab. 2003;88(12):5858-5864. [DOI] [PubMed] [Google Scholar]
- 84. AbdelMannan D, Selman WR, Arafah BM. Peri-operative management of Cushing’s disease. Rev Endocr Metab Disord. 2010;11(2):127-134. [DOI] [PubMed] [Google Scholar]
- 85. Mayberg M, Reintjes S, Patel A, et al. Dynamics of postoperative serum cortisol after transsphenoidal surgery for Cushing’s disease: implications for immediate reoperation and remission. J Neurosurg. 2018;129(5):1268-1277. [DOI] [PubMed] [Google Scholar]
- 86. Hameed N, Yedinak CG, Brzana J, et al. Remission rate after transsphenoidal surgery in patients with pathologically confirmed Cushing’s disease, the role of cortisol, ACTH assessment and immediate reoperation: a large single center experience. Pituitary. 2013;16(4):452-458. [DOI] [PubMed] [Google Scholar]
- 87. Stroud A, Dhaliwal P, Alvarado R, et al. Outcomes of pituitary surgery for Cushing’s disease: a systematic review and meta-analysis. Pituitary. 2020;23(5):595-609. [DOI] [PubMed] [Google Scholar]
- 88. Lindsay JR, Oldfield EH, Stratakis CA, Nieman LK. The postoperative basal cortisol and CRH tests for prediction of long-term remission from Cushing’s disease after transsphenoidal surgery. J Clin Endocrinol Metab. 2011;96(7):2057-2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Esposito F, Dusick JR, Cohan P, et al. Clinical review: early morning cortisol levels as a predictor of remission after transsphenoidal surgery for Cushing’s disease. J Clin Endocrinol Metab. 2006;91(1):7-13. [DOI] [PubMed] [Google Scholar]
- 90. Valassi E, Biller BM, Swearingen B, et al. Delayed remission after transsphenoidal surgery in patients with Cushing’s disease. J Clin Endocrinol Metab. 2010;95(2):601-610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Ironside N, Chatain G, Asuzu D, et al. Earlier post-operative hypocortisolemia may predict durable remission from Cushing’s disease. Eur J Endocrinol. 2018;178(3):255-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Abellán-Galiana P, Fajardo-Montañana C, Riesgo-Suárez P, Pérez-Bermejo M, Ríos-Pérez C, Gómez-Vela J. Prognostic usefulness of ACTH in the postoperative period of Cushing’s disease. Endocr Connect. 2019;8(9):1262-1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Czirják S, Bezzegh A, Gál A, Rácz K. Intra- and postoperative plasma ACTH concentrations in patients with Cushing’s disease cured by transsphenoidal pituitary surgery. Acta Neurochir (Wien). 2002;144(10):971-977. [DOI] [PubMed] [Google Scholar]
- 94. Araujo-Castro M, Pascual-Corrales E, Martínez San Millan JS, et al. Postoperative management of patients with pituitary tumors submitted to pituitary surgery. Experience of a Spanish Pituitary Tumor Center of Excellence. Endocrine. 2020;69(1):5-17. [DOI] [PubMed] [Google Scholar]
- 95. Fountas A, Lithgow K, Karavitaki N. Perioperative endocrinological management in patients with pituitary adenomas. In: Honegger J, Reincke M, Petersenn S, eds. Pituitary Tumors: a Comprehensive and Interdisciplinary Approach. Academic Press; 2021:421-427. [Google Scholar]
- 96. Pivonello R, De Leo M, Cozzolino A, Colao A. The treatment of Cushing’s disease. Endocr Rev. 2015;36(4):385-486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Tritos NA, Fazeli PK, McCormack A, et al. Pituitary Society Delphi Survey: an international perspective on endocrine management of patients undergoing transsphenoidal surgery for pituitary adenomas [published online ahead of print, July 20, 2021]. Pituitary. 2021;1-10. doi: 10.1007/s11102-021-01170-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Hofmann BM, Hlavac M, Martinez R, Buchfelder M, Müller OA, Fahlbusch R. Long-term results after microsurgery for Cushing disease: experience with 426 primary operations over 35 years. J Neurosurg. 2008;108(1):9-18. [DOI] [PubMed] [Google Scholar]
- 99. Witek P, Zieliński G, Szamotulska K, Maksymowicz M, Kamiński G. Clinicopathological predictive factors in the early remission of corticotroph pituitary macroadenomas in a tertiary referral centre. Eur J Endocrinol. 2016;174(4):539-549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Petersenn S, Beckers A, Ferone D, et al. Therapy of endocrine disease: outcomes in patients with Cushing’s disease undergoing transsphenoidal surgery: systematic review assessing criteria used to define remission and recurrence. Eur J Endocrinol. 2015;172(6):R227-R239. [DOI] [PubMed] [Google Scholar]
- 101. Witek P, Maksymowicz M, Szamotulska K, et al. MGMT expression in pituitary corticotroph adenomas and its relationship to clinical, pathological, and ultrastructural parameters in patients with Cushing’s disease. Folia Neuropathol. 2020;58(4):357-364. [DOI] [PubMed] [Google Scholar]
- 102. Wanichi IQ, de Paula Mariani BM, Frassetto FP, et al. Cushing’s disease due to somatic USP8 mutations: a systematic review and meta-analysis. Pituitary. 2019;22(4):435-442. [DOI] [PubMed] [Google Scholar]
- 103. Mallari RJ, Thakur JD, Barkhoudarian G, et al. Diagnostic pitfalls in Cushing’s disease impacting surgical remission rates; test thresholds and lessons learned in 105 patients. J Clin Endocrinol Metab. 2022;107(1):205-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Akirov A, Larouche V, Shimon I, et al. Significance of Crooke’s hyaline change in nontumorous corticotrophs of patients with Cushing disease. Front Endocrinol (Lausanne). 2021;12:620005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Ollivier M, Haissaguerre M, Ferriere A, Tabarin A. Should we avoid using ketoconazole in patients with severe Cushing’s syndrome and increased levels of liver enzymes? Eur J Endocrinol. 2018;179(5):L1-L2. [DOI] [PubMed] [Google Scholar]
- 106. Graham KE, Samuels MH, Raff H, Barnwell SL, Cook DM. Intraoperative adrenocorticotropin levels during transsphenoidal surgery for Cushing’s disease do not predict cure. J Clin Endocrinol Metab. 1997;82(6):1776-1779. [DOI] [PubMed] [Google Scholar]
- 107. Perez-Vega C, Tripathi S, Domingo RA, et al. Fluid restriction after transsphenoidal surgery for the prevention of delayed hyponatremia: a systematic review and meta-analysis. Endocr Pract. 2021;27(9):966-972. [DOI] [PubMed] [Google Scholar]
- 108. Hochberg Z, Pacak K, Chrousos GP. Endocrine withdrawal syndromes. Endocr Rev. 2003;24(4):523-538. [DOI] [PubMed] [Google Scholar]
- 109. Cardinal T, Zada G, Carmichael JD. The role of reoperation after recurrence of Cushing’s disease. Best Pract Res Clin Endocrinol Metab. 2021;35(2):101489. [DOI] [PubMed] [Google Scholar]
- 110. Cote DJ, Alzarea A, Acosta MA, et al. Predictors and rates of delayed symptomatic hyponatremia after transsphenoidal surgery: a systematic review [corrected]. World Neurosurg. 2016;88:1-6. [DOI] [PubMed] [Google Scholar]
- 111. Hensen J, Henig A, Fahlbusch R, Meyer M, Boehnert M, Buchfelder M. Prevalence, predictors and patterns of postoperative polyuria and hyponatraemia in the immediate course after transsphenoidal surgery for pituitary adenomas. Clin Endocrinol (Oxf). 1999;50(4):431-439. [DOI] [PubMed] [Google Scholar]
- 112. Sane T, Rantakari K, Poranen A, Tähtelä R, Välimäki M, Pelkonen R. Hyponatremia after transsphenoidal surgery for pituitary tumors. J Clin Endocrinol Metab. 1994;79(5): 1395-1398. [DOI] [PubMed] [Google Scholar]
- 113. Adams JR, Blevins LS Jr, Allen GS, Verity DK, Devin JK. Disorders of water metabolism following transsphenoidal pituitary surgery: a single institution’s experience. Pituitary. 2006;9(2):93-99. [DOI] [PubMed] [Google Scholar]
- 114. Hong YG, Kim SH, Kim EH. Delayed hyponatremia after transsphenoidal surgery for pituitary adenomas: a single institutional experience. Brain Tumor Res Treat. 2021;9(1):16-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Perez A, Jansen-Chaparro S, Saigi I, Bernal-Lopez MR, Miñambres I, Gomez-Huelgas R. Glucocorticoid-induced hyperglycemia. J Diabetes. 2014;6(1):9-20. [DOI] [PubMed] [Google Scholar]
- 116. Burke WT, Cote DJ, Iuliano SI, Zaidi HA, Laws ER. A practical method for prevention of readmission for symptomatic hyponatremia following transsphenoidal surgery. Pituitary. 2018;21(1):25-31. [DOI] [PubMed] [Google Scholar]
- 117. Agam MS, Wedemeyer MA, Wrobel B, Weiss MH, Carmichael JD, Zada G. Complications associated with microscopic and endoscopic transsphenoidal pituitary surgery: experience of 1153 consecutive cases treated at a single tertiary care pituitary center. J Neurosurg. 2018;130(5):1576-1583. [DOI] [PubMed] [Google Scholar]
- 118. Ammirati M, Wei L, Ciric I. Short-term outcome of endoscopic versus microscopic pituitary adenoma surgery: a systematic review and meta-analysis. J Neurol Neurosurg Psychiatry. 2013;84(8):843-849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Nemergut EC, Zuo Z, Jane JA Jr, Laws ER Jr. Predictors of diabetes insipidus after transsphenoidal surgery: a review of 881 patients. J Neurosurg. 2005;103(3):448-454. [DOI] [PubMed] [Google Scholar]
- 120. Ellison DH, Berl T. Clinical practice. The syndrome of inappropriate antidiuresis. N Engl J Med. 2007;356(20): 2064-2072. [DOI] [PubMed] [Google Scholar]
- 121. Buffington MA, Abreo K. Hyponatremia: a review. J Intensive Care Med. 2016;31(4):223-236. [DOI] [PubMed] [Google Scholar]
- 122. Mathioudakis N, Thapa S, Wand GS, Salvatori R. ACTH-secreting pituitary microadenomas are associated with a higher prevalence of central hypothyroidism compared to other microadenoma types. Clin Endocrinol (Oxf). 2012;77(6):871-876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Shekhar S, McGlotten R, Auh S, Rother KI, Nieman LK. The hypothalamic-pituitary-thyroid axis in Cushing syndrome before and after curative surgery. J Clin Endocrinol Metab. 2021;106(3):e1316-e1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Hinojosa-Amaya JM, Varlamov EV, McCartney S, Fleseriu M. Pituitary magnetic resonance imaging use in the posttreatment follow-up of secreting pituitary adenomas. In: Honegger J, Reincke M, Petersenn S, eds. Pituitary Tumors. Academic Press; 2021, 447-455. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article because no data sets were generated or analyzed during the present study.