Abstract
Airway smooth muscle thickening, a key characteristic of chronic asthma, is largely attributed to increased smooth muscle cell proliferation and reduced smooth muscle apoptosis. Polo-like kinase 1 (Plk1) is a serine/threonine protein kinase that participates in the pathogenesis of airway smooth muscle remodeling. Although the role of Plk1 in cell proliferation and migration is recognized, its function in smooth muscle apoptosis has not been previously investigated. Caspase-9 (Casp9) is a key enzyme that participates in the execution of apoptosis. Casp9 phosphorylation at Ser-196 and Thr-125 is implicated in regulating its activity in cancer cells and epithelial cells. Here, exposure of human airway smooth muscle (HASM) cells to platelet-derived growth factorfor 24 hours enhanced the expression of Plk1 and Casp9 phosphorylation at Ser-196, but not Thr-125. Overexpression of Plk1 in HASM cells increased Casp9 phosphorylation at Ser-196. Moreover, the expression of Plk1 increased the levels of pro-Casp9 and pro-Casp3 and inhibited apoptosis, demonstrating a role of Plk1 in inhibiting apoptosis. Knockdown of Plk1 reduced Casp9 phosphorylation at Ser-196, reduced pro-Casp9/3 expression, and increased apoptosis. Furthermore, Casp9 phosphorylation at Ser-196 was upregulated in asthmatic HASM cells, which was associated with increased Plk1 expression. Knockdown of Plk1 in asthmatic HASM cells decreased Casp9 phosphorylation at Ser-196 and enhanced apoptosis. Together, these studies disclose a previously unknown mechanism that the Plk1-Casp9/3 pathway participates in the controlling of smooth muscle apoptosis.
Keywords: caspases, protein kinase, smooth muscle, apoptosis
Clinical Relevance
We discover that Plk1 regulates airway smooth muscle apoptosis by affecting caspase-9 phosphorylation. This finding provides necessary knowledge to investigate pathogenesis of respiratory diseases such as asthma.
Tissue homeostasis is regulated by the balance between cell proliferation and cell death. Increased cell proliferation and/or reduced cell death leads to enlargement of organs or tissues, which is associated with pathogenesis of various diseases such as cancer and airway remodeling. Chronic asthma is characterized by airway smooth muscle layer thickening, which exacerbates airway narrowing during asthma attacks (1–3). Airway smooth muscle remodeling is largely attributed to increased smooth muscle cell proliferation (1, 4–6) and reduced smooth muscle apoptosis (7). Although there is considerable information regarding smooth muscle cell proliferation, the mechanisms that control smooth muscle apoptosis remain to be elucidated.
Apoptosis is a form of programmed cell death that is important for embryonic development and tissue homeostasis. Caspase-9 (Casp9) is a key enzyme that participates in the execution of apoptosis. Many proapoptotic signals stimulate release of cytochrome c from mitochondria into the cytosol, in which it binds to Apaf-1 (apoptotic protease activating factor-1), inducing recruitment and proteolytic processing/activation of pro-Casp9. Once activated, Casp9 then cleaves and activates the downstream effector pro-Casp3, initiating a cascade of additional caspase activation that culminates in cell death (8).
Phosphorylation of Casp9 has been proposed to inhibit its activation in cancer and epithelial cells. In Hela cells, treatment with epidermal growth factor (EGF) or a protein kinase C activator increases the extracellular signal-regulated kinase (ERK)-mediated phosphorylation of Casp9 at Thr-125, which inhibits the activation of Casp9 and may contribute to tumorigenesis (9). In addition, Casp9 phosphorylation at Ser-196 inhibits Casp9 activation in epithelial cells expressing a mutant Ras, which is mediated by Akt (10). However, other studies do not support the role of Akt at this site phosphorylation (8).
Polo-like kinase 1 (Plk1) is a serine/threonine protein kinase that participates in the pathogenesis of airway smooth muscle remodeling. Plk1 regulates the proliferation and migration of various cell types, including airway smooth muscle cells (5, 11–13). Smooth muscle conditional knockout of Plk1 attenuates allergen-induced airway smooth muscle thickening (11). The role of Plk1 in apoptosis has not been previously investigated.
In this study, we find that Plk1 mediates Casp9 phosphorylation at Ser-196, which affects Casp9/3 expression and apoptosis in human airway smooth muscle (HASM) cells. The Plk1–Casp9/3 pathway is upregulated in asthmatic HASM cells.
Results
Effects of Platelet-derived Growth Factor, IL-13, and IL-33 on Expression of Plk1 in Smooth Muscle Cells
Plk1 plays a role in the progression of airway smooth muscle thickening. Because platelet-derived growth factor (PDGF)-BB, IL-13, and IL-33 have been implicated in asthma pathology (12, 14, 15), we evaluated whether they affect the expression of Plk1 in smooth muscle cells. HASM cells were treated with 10 ng/ml PDGF-BB or IL-13 or IL-33 for 24 hours. IB analysis was used to assess the expression of Plk1. Exposure to PDGF-BB significantly enhanced the expression of Plk1 in HASM cells (Figures 1A and 1B). However, treatment with IL-13 or IL-33 did not affect Plk1 expression (Figures 1A and 1B). The results suggest that these cytokines differentially regulate Plk1 expression in smooth muscle cells.
Figure 1.
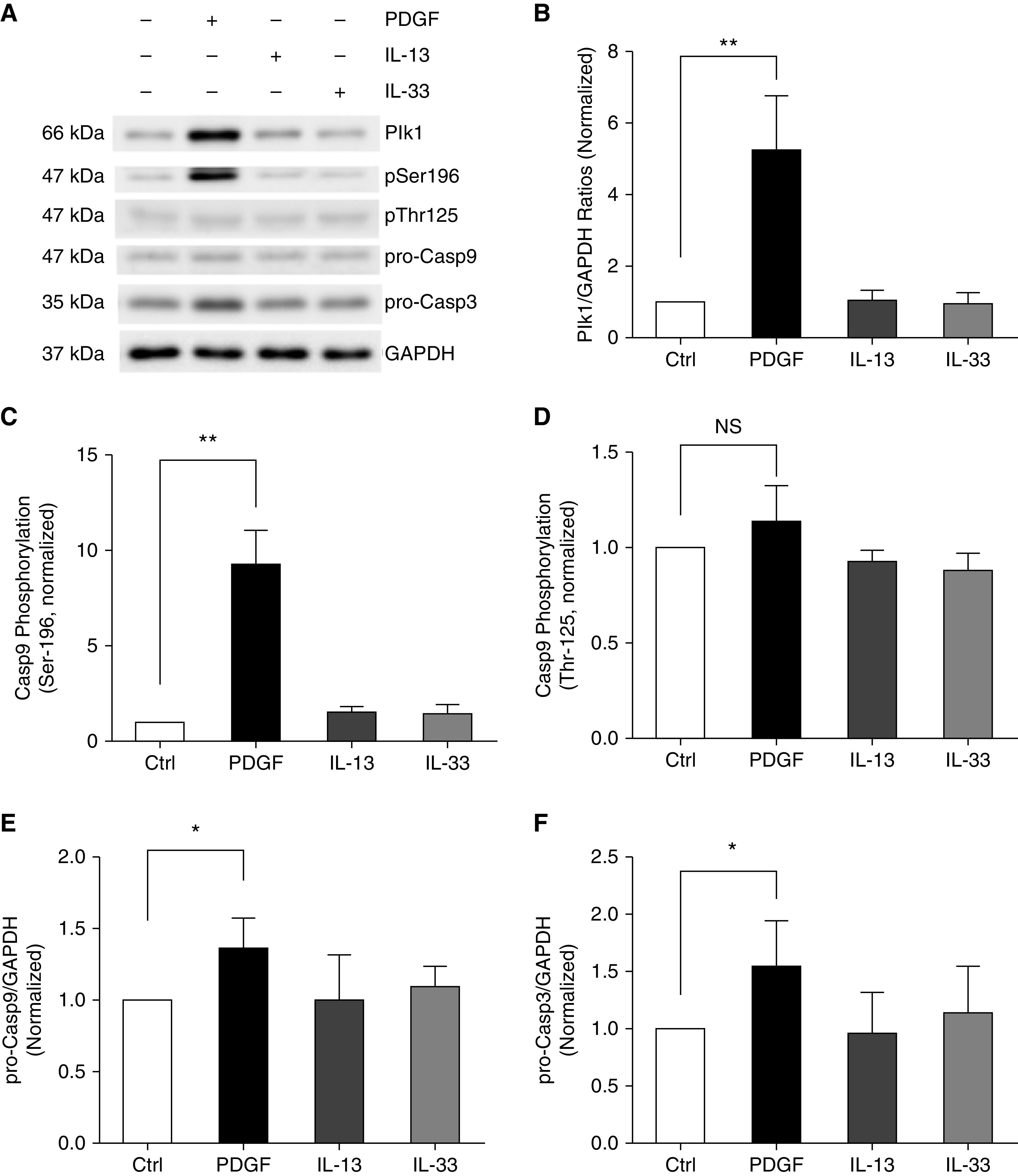
Platelet-derived growth factor (PDGF) exposure increases the expression of Polo-like kinase 1 (Plk1), Caspase-9 (Casp9) phosphorylation at Ser-196, and pro-Casp9 and pro-Casp3 concentrations in human airway smooth muscle (HASM) cells. (A) Representative IBs illustrating the effects of PDGF-BB, IL-13, and IL-33 on Plk1, Casp9 phosphorylation, pro-Casp9, and pro-Casp3. HASM cells were treated with 10 ng/ml PDGF-BB, IL-13, and IL-33 for 24 hours, or they were not treated, followed by IB analysis. (B–F) Protein ratios of Plk1/GAPDH (B), Casp9 phosphorylation at Ser-196 (C), Casp9 phosphorylation at Thr-125 (D), pro-Casp9 concentration (E), and pro-Casp3 concentration (F) are normalized to untreated cells, respectively. PDGF-BB, but not IL-13 or IL-33, enhances Plk1 expression, Casp9 phosphorylation at Ser-196, pro-Casp9 level, and pro-Casp3 level. However, PDGF-BB, IL-13, and IL-33 do not affect Casp9 phosphorylation at Thr-125. Data are mean values of four nonasthmatic cell cultures from three donors. Error bars indicate SD. *P < 0.05 and **P < 0.01. One-way ANOVA was used for statistical analysis. Ctrl = control; NS = not significant.
PDGF Promotes Phosphorylation of Casp9 at Ser-196, but Not at Thr-125
Phosphorylation of Casp9 at Ser-196 or Thr-125 has been proposed to inhibit its activation in epithelial cells or cancer cells (9, 10). Thus, we tested the possibility that these cytokines may affect Casp9 phosphorylation at these two residues. Treatment with 10 ng/ml PDGF-BB for 24 hours enhanced Casp9 phosphorylation at Ser-196, but not Thr-125 (Figures 1A, 1C, and 1D). However, IL-13 or IL-33 did not affect Casp9 phosphorylation at these two sites (Figures 1A, 1C, and 1D). PDGF-BB treatment also increased the concentration of pro-Casp9 and pro-Casp3 (Figures 1A, 1E, and 1F).
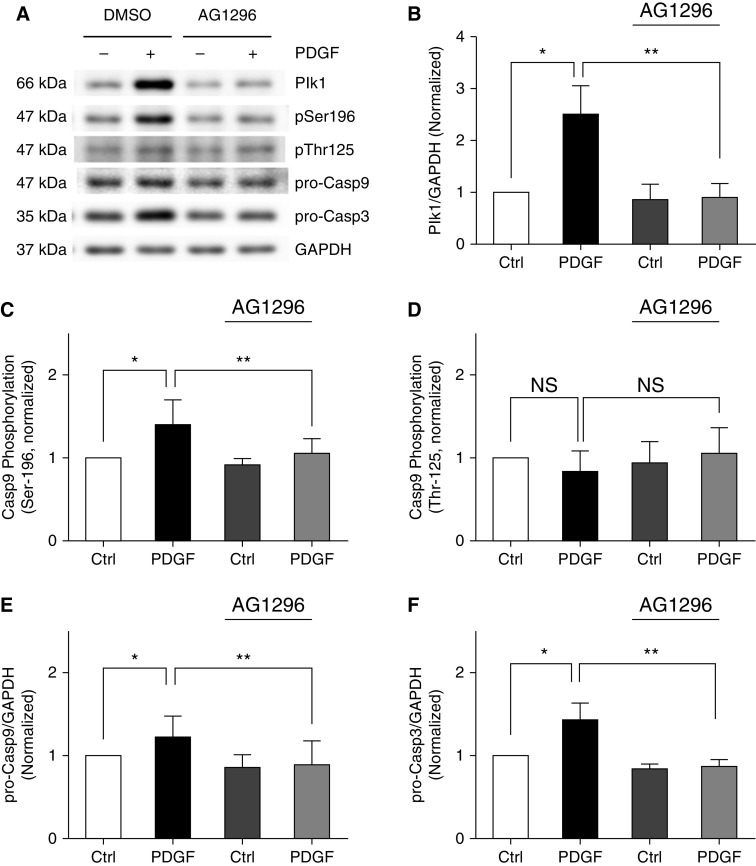
PDGF-induced Plk1 Expression and Casp9 Phosphorylation at Ser-196 Are PDGF Receptor Dependent
We evaluated the effects of the PDGF receptor inhibitor AG1296 on Plk1 expression and the Casp9/3 pathway. Treatment with AG1296 significantly reduced Plk1 expression, Casp9 phosphorylation at Ser-196, and pro-Casp9 and pro-Casp3 concentrations (Figure 2). Treatment with AG1296 did not affect Casp9 phosphorylation at Thr-125. The results suggest that the effects of PDGF on Plk1 and Casp9 phosphorylation at Ser-196 are dependent on PDGF receptor.
Figure 2.
PDGF-induced upregulation of Plk1–Casp9/3 pathway is PDGF receptor dependent. HASM cells were treated with 10 ng/ml PDGF for 24 hours in the presence or absence of 10 μM AG1296. Dimethyl sulfoxide (DMSO) was used as vehicle. (B–F) Protein ratios of Plk1/GAPDH (B), Casp9 phosphorylation at Ser-196 (C), Casp9 phosphorylation at Thr-125 (D), pro-Casp9 concentration (E), and pro-Casp3 concentration (F) are normalized to Ctrl cells not treated with AG1296, respectively. Data are mean values of five to six nonasthmatic cell cultures from three donors. Error bars indicate SD. *P < 0.05 and **P < 0.01. Two-way ANOVA was used for statistical analysis.
Growth Factor-induced Casp9 Phosphorylation at Ser-196 Is Time Dependent
To further assess the role of growth factors in this cellular process, we determined Casp9 phosphorylation at Ser-196 in HASM cells treated with 10 ng/ml PDGF-BB for 5 minutes to 48 hours. Short-term treatment with PDGF-BB (5 min to 2 h) did not significantly increase Plk1 protein expression and Casp9 Ser-196 phosphorylation. However, Plk1 expression and Casp9 phosphorylation at Ser-196 began to increase at 6 hours, peaked at 24 hours, and reduced at 48 hours after PDGF treatment (Figure 3A).
Figure 3.
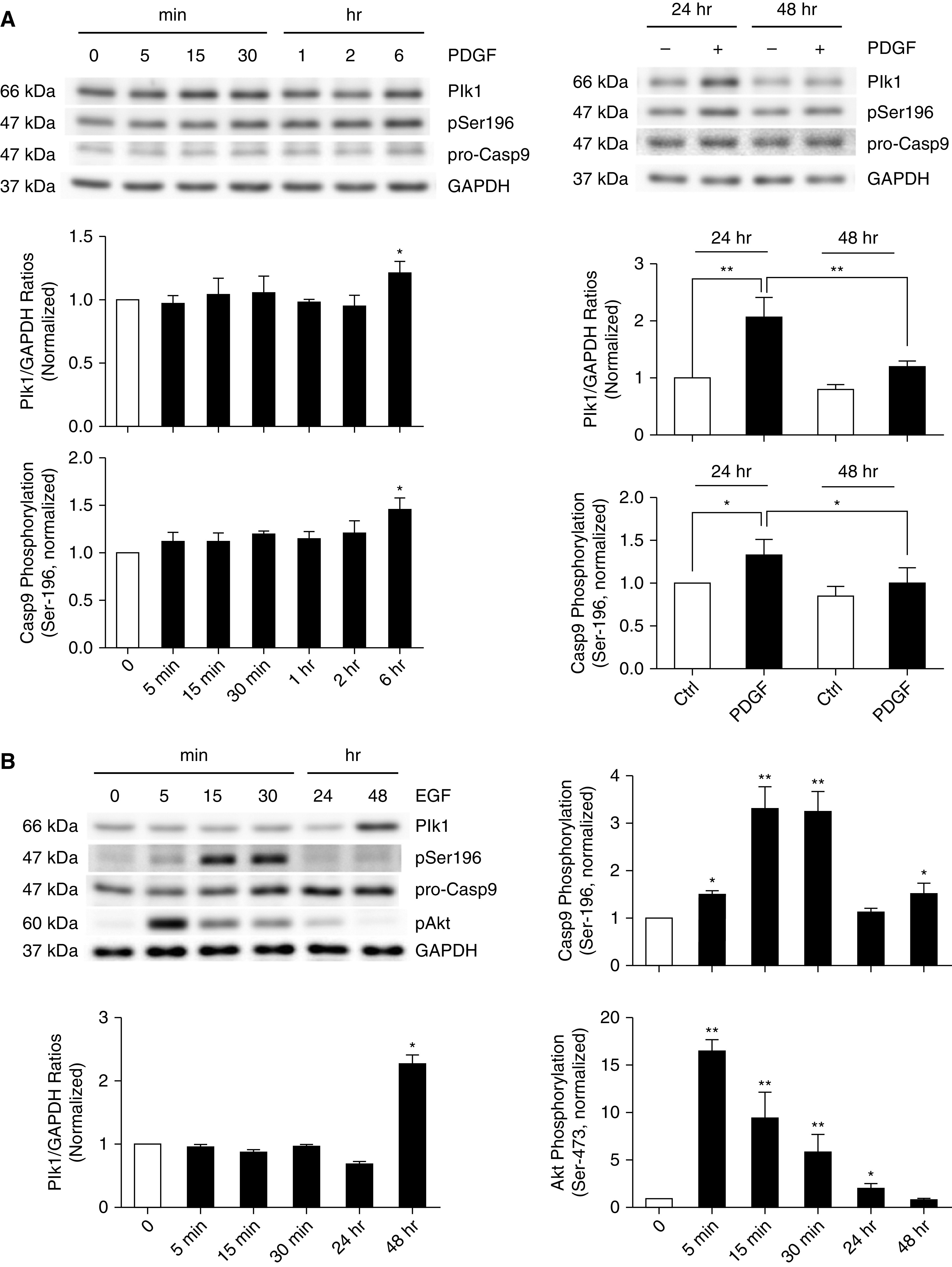
Time course of Plk1 expression and Casp9 phosphorylation at Ser-196 in response to stimulation with PDGF and epidermal growth factor. (A and B) HASM cells were treated with 10 ng/ml PDGF (A) or epidermal growth factor (B) for different time points. Protein phosphorylation and expression were evaluated by IB. Plk1 expression and Casp9 phosphorylation at Ser-196 are time dependent. Data are mean values of four to six nonasthmatic cell cultures from three donors. Error bars indicate SD. *P < 0.05 and **P = 0.01. One-way ANOVA was used for statistical analysis.
Because EGF has also been implicated in asthma pathogenesis, we treated HASM cells with 10 ng/ml for 5 minutes to 48 hours. EGF treatment for 5 minutes to 24 hours did not significantly enhance Plk1 expression (Figure 3B). In addition, treatment with EGF enhanced Casp9 Ser-196 phosphorylation at 5–30 minutes. Because Akt has been implicated in Casp9 phosphorylation at Ser-196 (10), we also determined Akt phosphorylation in these cells. Akt phosphorylation was increased as early as 5 minutes after EGF treatment (Figure 3B). These results are consistent with the observation that Akt mediates Casp9 phosphorylation at Ser-196 in epithelial cells (10). Furthermore, EGF exposure for 48 hours increased both the expression of Plk1 and Casp9 phosphorylation at Ser-196 (Figure 3B).
Overexpression of Plk1 Induces Phosphorylation of Casp9 at Ser-196 in Smooth Muscle Cells
As exposure to PDGF-BB coordinately increases Plk1 expression and Casp9 phosphorylation at Ser-196, we questioned whether Plk1 mediates Casp9 phosphorylation at this site. We transfected cells with plasmids encoding Plk1 and verified Plk1 overexpression by using IB analysis (Figures 4A and 4B). The overexpression of Plk1 enhanced the phosphorylation degree of Casp9 at Ser-196 (Figure 4A, Lanes 1 and 2, and Figure 4C). Furthermore, Plk1 overexpression increased the concentrations of pro-Casp9 and pro-Casp3 (Figure 4A, Lanes 1 and 2, and Figures 4D and 4E), suggesting a role of Plk1 in expression of Casp9 and Casp3. In addition, we also assessed whether PDGF-BB treatment affects Casp9 phosphorylation in cells overexpressing Plk1. Treatment with PDGF-BB for 24 hours did not affect Plk1 expression, Casp9 phosphorylation at Ser-196, or Casp9/3 expression in cells overexpressing Plk1 (Figure 4A, Lanes 2 and 4, and Figures 4B–4E).
Figure 4.
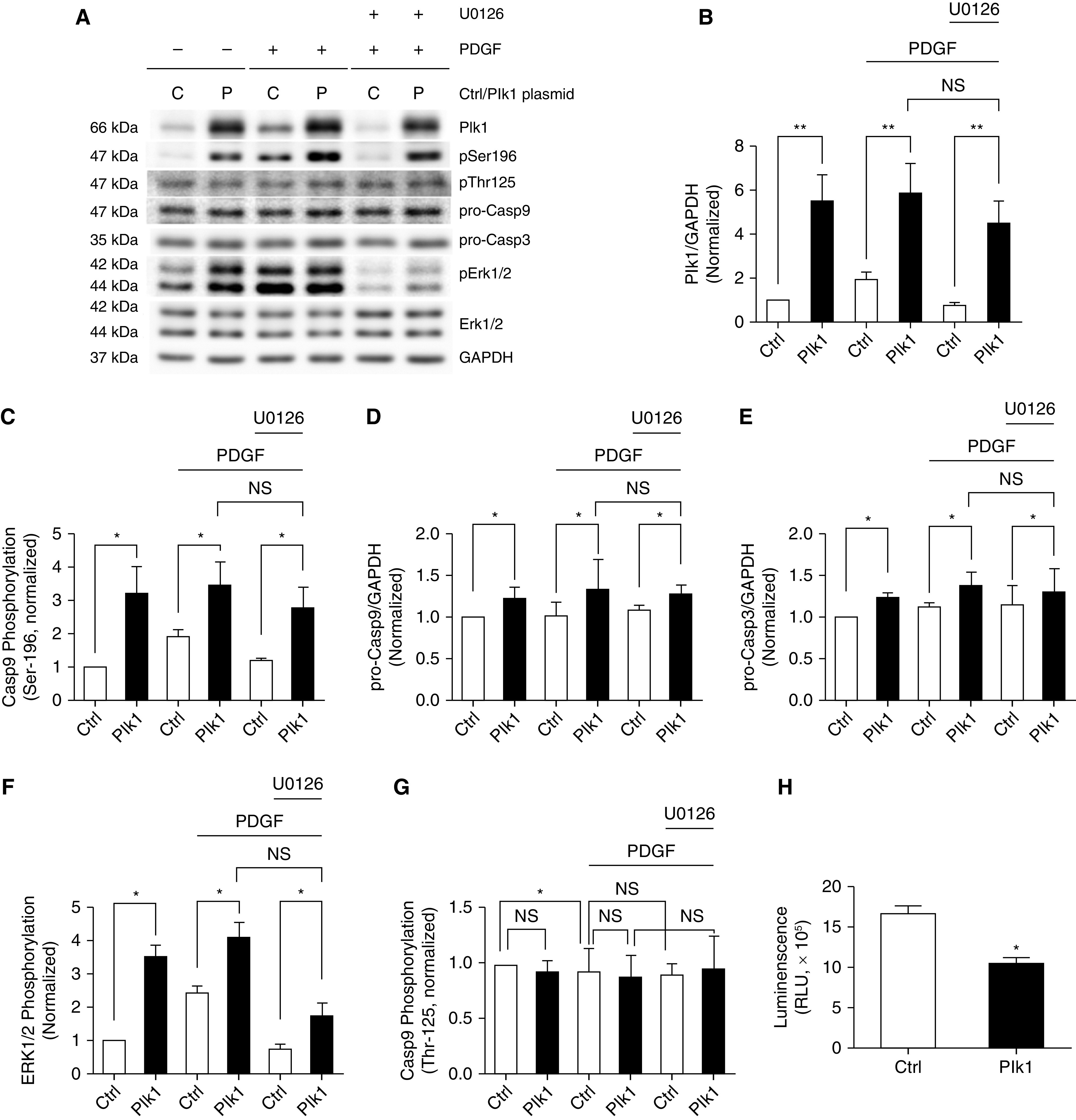
Overexpression of Plk1 enhances Casp9 phosphorylation at Ser-196, but not Thr-125 in HASM cells. (A) Representative IBs showing the effects of Plk1 overexpression on Casp9 phosphorylation at Ser-196/Thr-125, Casp9/3 expression and ERK1/2 phosphorylation. HASM cells were transfected with Ctrl plasmids or plasmids encoding Plk1 for 24 hours and serum starved for additional 24 hours. They were then treated with 10 ng/ml PDGF-BB for 24 hours in the presence or absence of 10 μM U0126. IB analysis was used to evaluate protein phosphorylation and expression. (B) Protein ratios of Plk1/GAPDH are normalized to Ctrl cells not treated with PDGF-BB or U0126. Plk1 overexpression is verified in these cells. (C–G) Casp9 phosphorylation at Ser-196 (C), pro-Casp9/3 (D and E), ERK1/2 phosphorylation (ERK1: Thr-202/Tyr-204; ERK2: Thr-185/Tyr-187) (F), and Casp9 phosphorylation at Thr-125 are normalized to Ctrl cells not treated with PDGF-BB or U0126 (G). (H) Apoptosis of HASM cells overexpressing Plk1 was evaluated using the Annexin V apoptosis assay kit. RLU = relative luciferase unit. Data are mean values of five to six nonasthmatic cell cultures from three donors. Error bars indicate SD. *P < 0.05 and **P < 0.01. Two-way ANOVA was used for statistical analysis.
Plk1-mediated Casp9 Phosphorylation at Ser-196 Is Not Dependent upon ERK1/2
Because ERK has been shown to regulate Casp9 phosphorylation at Thr-125 (9), we questioned whether the Plk1-mediated Casp9 phosphorylation at Ser-196 is affected by ERK. Overexpression of Plk1 or PDGF-BB treatment enhanced the phosphorylation of ERK1/2 (Figures 4A and 4F), which is consistent with our previous studies (5, 12). Moreover, we used the MEK1/2 inhibitor U0126 to attenuate ERK1/2 phosphorylation, which was verified by IB analysis (Figures 4A and 4F). However, treatment with U0126 did not affect Casp9 phosphorylation at Ser-196 and the expression of Casp9/3 (Figure 4A, Lanes 4 and 6, and Figures 4C–4E).
Overexpression of Plk1 Does Not Affect Phosphorylation of Casp9 at Thr-125 in Smooth Muscle Cells
Because Casp9 phosphorylation at Thr-125 has been implicated in tumorigenesis (9), we also assessed the effects of Plk1 expression on this site phosphorylation. Plk1 overexpression did not increase Casp9 phosphorylation at Thr-125 in the absence or presence ofPDGF-BB (Figures 4A and 4G). Furthermore, the inhibition of ERK1/2 phosphorylation by U0126 did not significantly affect this site phosphorylation (Figures 4A and 4G).
Overexpression of Plk1 Attenuates Apoptosis in HASM Cells
We also evaluated the effects of Plk1 overexpression on apoptosis by using the Annexin V apoptosis assay kit. Compared with control cells, the expression of Plk1 reduced the luminescence signals of cells (Figure 4H). The results suggest a negative role for Plk1 in apoptosis.
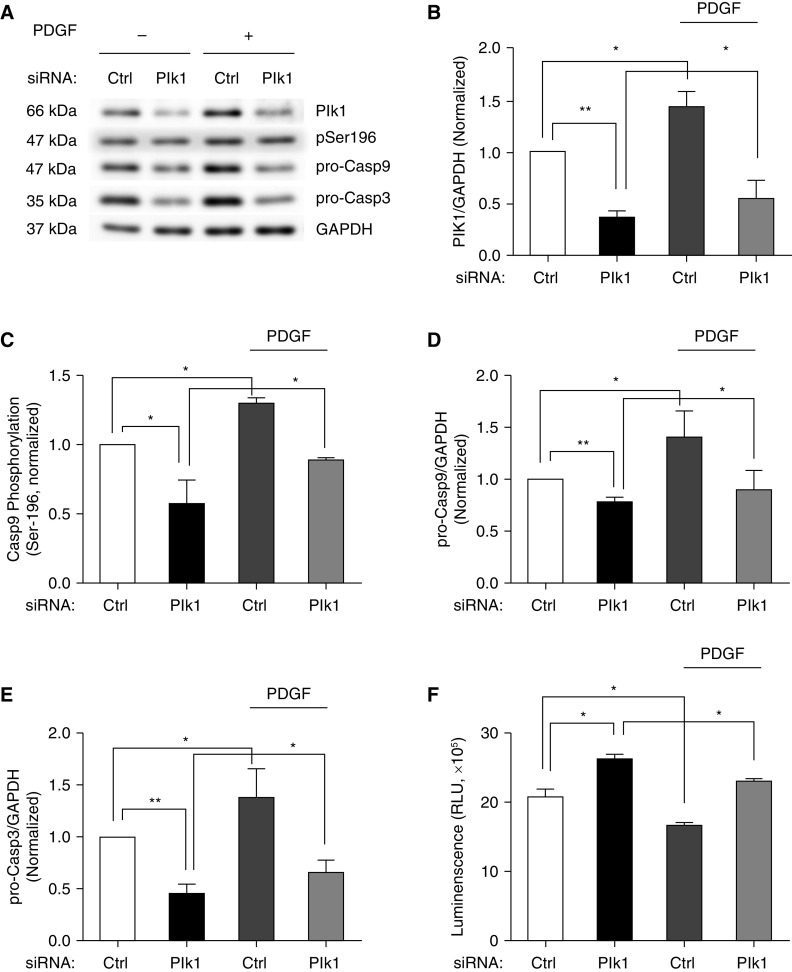
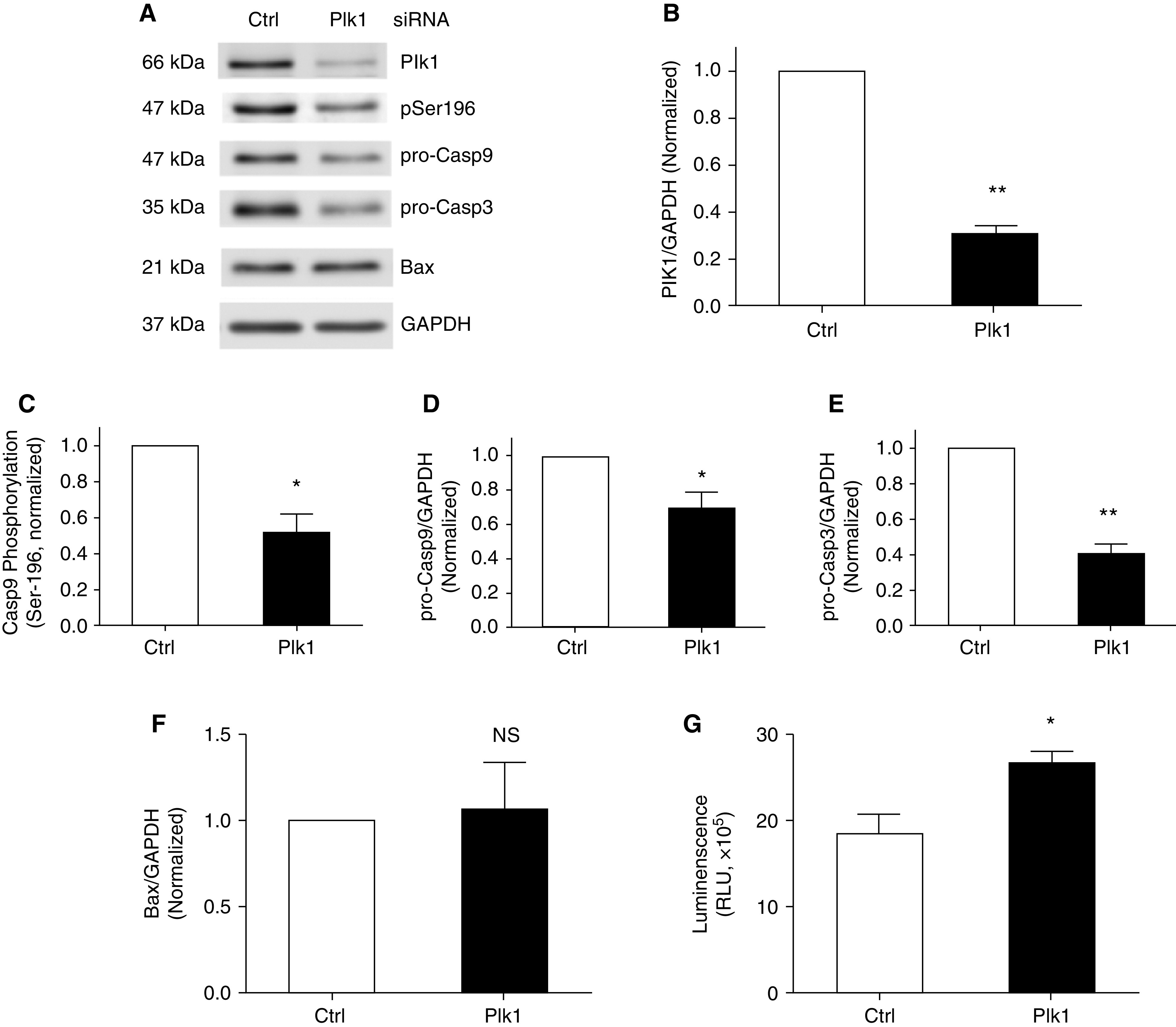
Plk1 Knockdown Affects Casp9 Phosphorylation at Ser-196 and Concentrations of Pro-Casp9/3 in HASM Cells
To further assess the role of Plk1, we silenced Plk1 protein expression using siRNA and evaluated the consequences of Plk1 knockdown (KD). We noticed that Plk1 KD reduced Casp9 phosphorylation at Ser-196 and the expression of pro-Casp9/3 (Figures 5A–5E). However, Plk1 KD did not affect the expression of Bax (Bcl-2-associated X protein) (Figure E1 in the data supplement), which regulates Casp9 via cytochrome c (9). In addition, Plk1 KD increased apoptosis of these cells as evidenced by the Annexin V assay (Figure 5F). Furthermore, PDGF treatment rescued Casp9 phosphorylation at Ser-196, concentrations of pro-Casp9/3, and apoptosis in Plk1 KD cells (Figure 5). The results suggest that Plk1 mediates PDGF-induced impact on the Casp9/3 pathway and apoptosis.
Figure 5.
Knockdown (KD) of Plk1 affects Casp9 phosphorylation at Ser-196 and concentrations of Casp9/3 in HASM cells. (A) Representative IBs illustrating the effects of Plk1 KD on the levels of Casp9 phosphorylation at Ser-196, pro-Casp9, and pro-Casp3. Ctrl and Plk1 KD cells were treated with or without 10 ng/ml PDGF for 24 hours, followed by IB analysis. (B) Protein ratios of Plk1/GAPDH are normalized to cells treated with Ctrl siRNA. Plk1 KD is verified in these cells. (C–E) Plk1 KD reduces Casp9 phosphorylation at Ser-196 (C) and the concentrations of pro-Casp9 (D) and pro-Casp3 (E). (F) Apoptosis of HASM cells was evaluated using the Annexin V apoptosis assay kit. Data are mean values of four to six nonasthmatic cell cultures from three donors. Error bars indicate SD. *P < 0.05 and **P < 0.01. Two-way ANOVA was used for statistical analysis.
Phosphorylation of Casp9 at Ser-196 Is Higher in Asthmatic HASM Cells
Because apoptosis has been implicated in airway smooth muscle remodeling and Casp9 is a key enzyme to initiate apoptosis, we questioned whether the phosphorylation level of Casp9 is altered in asthmatic HASM cells using IB analysis. We noticed that Plk1 expression and the phosphorylation degree of Casp9 at Ser-196 were higher in asthmatic HASM cells as compared with nonasthmatic HASM cells (Figures 6A–6C). Moreover, the concentrations of pro-Casp9 and pro-Casp3 were greater in asthmatic HASM cells (Figures 6A, 6D, and 6E). However, the phosphorylation degree of Casp9 at Thr-125 was not significantly different between asthmatic and nonasthmatic HASM cells (Figures 6A and 6F). Furthermore, we found that Plk1 protein levels were higher in asthmatic human bronchial tissues as compared with nonasthmatic human bronchial tissues (Figure E2).
Figure 6.
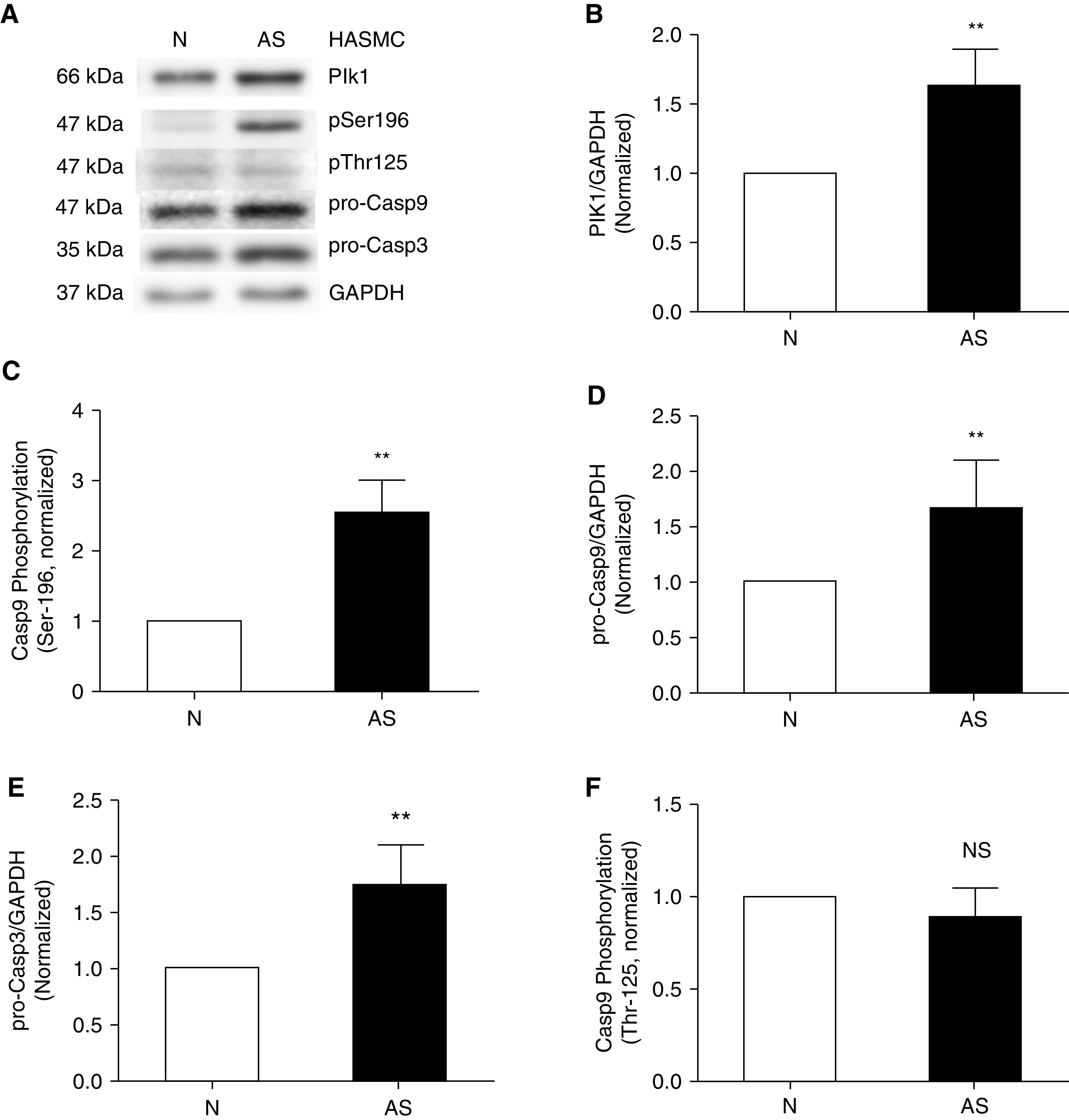
The Plk1-Casp9/3 pathway is upregulated in asthmatic HASM cells. (A) Representative IBs illustrating Plk1 expression, Casp9 phosphorylation, and pro-Casp9/3 concentrations in nonasthmatic and asthmatic HASM cells. (B–F) Protein expression and phosphorylation in asthmatic (AS) cells are compared with nonasthmatic (N) cells. Casp9 phosphorylation is determined using pSer-196 or pThr-125/total pro-Casp9 ratios. Data are mean values of four asthmatic cell cultures from three donors. Error bars indicate SD. **P < 0.01. Student’s t-test was used for statistical analysis.
Plk1 Regulates Phosphorylation of Casp9 at Ser-196 and Concentrations of pro-Casp9/3 in Asthmatic HASM Cells
Next, we determined whether Plk1 has a role in mediating Casp9 in asthmatic HASM cells. Treatment with Plk1 siRNA reduced the expression of Plk1 in asthmatic HASM cells (Figures 7A and 7B). Moreover, Plk1 KD reduced Casp9 phosphorylation at Ser-196 and pro-Casp9 and pro-Casp3 concentrations (Figures 7A and 7C–7E). However, Plk1 KD did not affect the expression of Bax in asthmatic HASM cells (Figures 7A and 7F). Furthermore, apoptosis was increased in asthmatic Plk1 KD HASM cells (Figure 7G). The results suggest that Plk1 regulates Casp9 phosphorylation at Ser-196, the expression of pro-Casp9/3, and apoptosis in asthmatic HASM cells.
Figure 7.
Role of Plk1 in Casp9 phosphorylation at Ser-196 and apoptosis in asthmatic HASM cells. (A) Representative IBs illustrating the effects of Plk1 KD on the degree of Casp9 phosphorylation at Ser-196 and concentrations of pro-Casp9, pro-Casp3, and Bax in asthmatic HASM cells. Asthmatic HASM cells were treated with Ctrl siRNA or Plk1 siRNA for 48 hours, followed by IB analysis. (B) Protein ratios of Plk1/GAPDH are compared with asthmatic cells treated with Ctrl siRNA. Treatment with Plk1 siRNA reduces Plk1 expression in asthmatic HASM cells. (C–E) Casp9 phosphorylation at Ser-196 and ratios of pro-Casp9/GAPDH or pro-Casp3/GAPDH are compared with Ctrl asthmatic cells. (F) Ratios of Bax/GAPDH are compared with Ctrl asthmatic cells. Bax levels are not affected by Plk1 KD. (G) Apoptosis of asthmatic HASM cells treated with Ctrl siRNA or Plk1 siRNA was evaluated using the Annexin V apoptosis assay kit. Data are mean values of four to six cell cultures from three donors. Error bars indicate SD. *P < 0.05 and **P < 0.01. Student’s t test was used for statistical analysis.
Discussion
Airway smooth muscle thickening in severe asthma is attributed in part to reduced smooth muscle apoptosis (7). The mechanisms that control smooth muscle apoptosis are largely unknown. Here, we present evidence to suggest that Plk1 plays an important role in regulating Casp9 phosphorylation at Ser-196 and apoptosis in nonasthmatic and asthmatic HASM cells.
PDGF-BB, IL-13, and IL-33 have been implicated in asthma pathology (12, 14, 15). In this study, treatment with PDGF-BB, but not IL-13 or IL-33, increased the expression of Plk1 in HASM cells (Figure 1). Moreover, exposure to PDGF-BB, but not IL-13 or IL-33, increased Casp9 phosphorylation at Ser-196 and pro-Casp9 and pro-Casp3 concentrations. The results suggest that these cytokines differentially affect the expression of Plk1 and Casp9 phosphorylation at Ser-196 in smooth muscle cells. In addition, we found that exposure to EGF also upregulated Plk1 expression and Casp9 phosphorylation at Ser-196. Thus, growth factors may participate in modulating the Plk1-Casp9/3 pathway in HASM cells.
The mechanism by which growth factors (PDGF and EGF) upregulate Plk1 is currently unknown. Because miR-509 regulates Plk1 expression in proliferating smooth muscle cells (12), it is possible that the growth factor upregulates Plk1 abundance by inhibiting miR-509. In addition, it is known that Akt and ERK1/2 can activate mTORC1, which promotes protein translation (16). Stimulation with PDGF/EGF activates Akt and ERK1/2 in smooth muscle (5, 12, 17, 18). Thus, it is likely that stimulation with growth factors may activate mTORC1 via Akt/ERK1/2, which enhances Plk1 translation. Future studies are required to test the possibilities.
Casp9 phosphorylation at Ser-196 is catalyzed by Akt in epithelial cells (10), which is supported by our current study (Figure 3B). More importantly, the overexpression of Plk1 in smooth muscle cells enhanced the phosphorylation degree of Casp9 at Ser-196 (Figure 4). Our results are consistent with analysis of substrate consensus sequence for Plk1. In general, the consensus phosphorylation motif for Plk1 is (E/D)-X-[pS/pT]- (I/L/V/M)-X-(E) (19). The amino acid sequence near Ser-196 on Casp9 is EKLRRRFSpSLHFMVE (NCBI reference sequence: NP_001220.2), which possesses the consensus phosphorylation motif for Plk1. However, the amino acid sequence near Thr-125 (EVLRPEpTPRPVDIG) lacks an L and an E in the C-terminal flanking region. Thus, it is not surprising that Plk1 did not mediate Casp9 phosphorylation at Thr-125 (Figure 4). In addition, Plk1 overexpression enhanced the expression of pro-Casp9/3 and inhibited apoptosis in smooth muscle cells (Figure 4). Plk1 KD reduced Casp9 phosphorylation at Ser-196 and the amount of pro-Casp9/3 and enhanced apoptosis in smooth muscle cells (Figure 5). Therefore, Plk1 mediates Casp9 phosphorylation at Ser-196, which may attenuate apoptosis in smooth muscle cells.
Plk1 consists of PBD (polo-box domain) and a kinase domain. In some cases, PBD of Plk1 binds to pS/pT motif of a substrate, which facilitates phosphorylation of additional pS/pT sites on a substrate (20). Because ERK is another kinase that has a role in mediating Casp9 phosphorylation at Thr-125 in Hela cells (9), this raises the possibility that ERK may prime Casp9 for Plk1-mediated Casp9 phosphorylation at Ser-196. However, the inhibition of ERK1/2 by U0126 did not affect Plk1-mediated Casp9 phosphorylation at Ser-196 (Figure 4). The results suggest that Plk1-mediated Ser-196 phosphorylation is not dependent on ERK1/2. Plk1 has been shown to catalyze substrate phosphorylation that is not dependent on activity of other kinases (21).
Although Plk1 affects Casp9 phosphorylation at Ser-196, it does not affect the expression of Bax in cells. It is not surprising because various proapoptotic signals promote the expression and/or interaction of Bax with mitochondria, which induces cytochrome c release and activates the Apaf-1/Casp9 pathway (8, 22).
Here, we found that the expression of Plk1 was upregulated in asthmatic HASM cells/tissues, which is consistent with our previous studies (12). Moreover, Casp9 phosphorylation at Ser-196 and the levels of pro-Casp9/3 were higher in asthmatic HASM cells (Figure 6). Furthermore, Plk1 KD reduced Casp9 phosphorylation at Ser-196 and enhanced apoptosis in asthmatic HASM cells (Figure 7). Thus, upregulation of Plk1 in asthmatic HASM cells leads to Casp9 phosphorylation at Ser-196, which subsequently inhibits apoptosis.
HASM cells derived from asthmatics proliferate faster than do cultured HASM cells derived from nonasthmatic subjects (12, 23). This intrinsic property is retained with extensive culture passage. In this study, asthmatic HASM was obtained from severe asthma patients who received treatment with bronchodilators and steroids. Plk1 upregulation in HASM may be affected by asthma severity and medication. Future studies are required to further investigate the possibilities.
In summary, we unveil a novel mechanism by which Plk1 inhibits apoptosis in smooth muscle cells. Plk1 mediates Casp9 phosphorylation at Ser-196, which attenuates apoptosis in HASM cells. In asthmatic HASM cells, the Plk1-Casp9/3 pathway is upregulated, which may contribute to the progression of airway smooth muscle thickening in severe asthma.
Methods
Cell Culture
HASM cells were prepared from human bronchi and adjacent tracheas obtained from the International Institute for Advanced Medicine as previously described (1, 24–28). This study was approved by the Albany Medical College Committee on Research Involving Human Subjects. The donor human lungs used to procure cells were not suitable for transplant, and not identifiable, thus studies were determined to be not human subjects research. Smooth muscle cells within passage 10 were used for the studies. Primary cells from three donors without asthma and three donors with asthma were used for the experiments. Clinical information of donors was included in Table 1. In addition, U0126 was dissolved in dimethyl sulfoxide.
Table 1.
Characteristics of Lung Donors
| Donor | Asthmatic? | Age, yr | Race and Ethnicity | Sex | Cause of Death | Bronchodilator (B) Steroid (S) |
|---|---|---|---|---|---|---|
| 1 | No | 18 | African American | Male | Head trauma | No |
| 2 | No | 28 | White | Female | Cardiac arrest | No |
| 3 | No | 58 | White | Male | Head trauma | No |
| 4 | Yes | 24 | White | Female | Anoxia | B and S |
| 5 | Yes | 46 | White | Male | Anoxia | B and S |
| 6 | Yes | 57 | African American | Male | Anoxia | B and S |
IB Analysis
Western blotting of cell lysis was performed using the experimental procedures as previously described (12, 24, 29–33). Anti-Plk1 (1:1000) was purchased from EMD Millipore (#05–844, L/N 2477015). Anti-Plk1 was validated by using corresponding KD cells. GAPDH antibody (1:1000) was purchased from Santa Cruz Biotechnology (Sc-32233, L/N K0315) and validated by assessing molecular weight of detected bands. Anti-p-Casp9 (Ser-196) (Thermo Fisher Scientific PA5–40222, L/N UD2758132) and anti-p-Casp9 (Thr-125) (Thermo Fisher Scientific PA5–37505, L/N UE2768101) were validated by assessing molecular weight of detected bands. Total anti-Casp9 (1:1000) was purchased from Cell Signaling (#9502S, L/N 8) and validated by assessing molecular weight of detected bands. Anti-Casp3 (1:1000) was purchased from Cell Signaling (#9662S, L/N 17) and validated by assessing molecular weight of detected bands. Anti-Bax was purchased from EMD Millipore (#ABC11, L/N 2435198) and validated by assessing molecular weight of detected bands. Finally, vendors have provided data sheets to show that antibodies were validated by positive controls.
The concentrations of proteins were quantified by scanning densitometry of IBs (Fuji Multigauge Software or GE IQTL software). The luminescent signals from all IBs were within the linear range.
Plasmids, siRNA, and Cell Transfection
Plk1 cDNA was cut off from pcDNA3 3 × Flag-Plk1 (5) and subcloned to pLenti-puro (Addgene #39481) at XhoI and ApaI sites. The construct has a CMV promoter, 3′UTR and poly A of BGH. Cells were transfected with pLenti-puro-Plk1 using FuGENE HD transfection reagent (Promega) according to the manual of the manufacture. They were then cultured in the growth medium followed by biochemical analysis. For Plk1 KD, HASM cells were transfected with control siRNA (Santa Cruz, #sc-37007, L/N K2320) or Plk1 siRNA (Santa Cruz, #sc-36277, L/N G0910) using the transfection protocol of the manufacture.
Assessment of Apoptosis by Annexin V Assay
Apoptosis was evaluated by using the Annexin V apoptosis assay kit (Promoga) according to the manual of manufacture.
Statistical Analysis
All statistical analysis was performed using Prism software (GraphPad Software). Differences between pairs of groups were analyzed by Student’s t test. Comparison among multiple groups was performed by one-way or two-way ANOVA followed by a post hoc test (Tukey’s multiple comparisons). Values of n refer to the number of experiments used to obtain each value. P < 0.05 was considered to be significant.
Footnotes
Supported by National Heart, Lung, and Blood Institute grants HL-110951, HL-130304, and HL-145392 from the National Institutes of Health (D.D.T.).
Author Contributions: G.L. performed biochemical and cellular experiments and drafted the manuscript. R.W. carried out cellular studies. D.D.T. conceived and coordinated the study and revised the manuscript. All authors reviewed the results and approved the final version of the manuscript.
This article has a data supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1165/rcmb.2021-0192OC on October 27, 2021
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Wang R, Mercaitis OP, Jia L, Panettieri RA, Tang DD. Raf-1, actin dynamics, and abelson tyrosine kinase in human airway smooth muscle cells. Am J Respir Cell Mol Biol . 2013;48:172–178. doi: 10.1165/rcmb.2012-0315OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tang DD. Critical role of actin-associated proteins in smooth muscle contraction, cell proliferation, airway hyperresponsiveness and airway remodeling. Respir Res . 2015;16:134. doi: 10.1186/s12931-015-0296-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ammit AJ, Panettieri RA., Jr Airway smooth muscle cell hyperplasia: a therapeutic target in airway remodeling in asthma? Prog Cell Cycle Res . 2003;5:49–57. [PubMed] [Google Scholar]
- 4. Penn RB. Embracing emerging paradigms of G protein-coupled receptor agonism and signaling to address airway smooth muscle pathobiology in asthma. Naunyn Schmiedebergs Arch Pharmacol . 2008;378:149–169. doi: 10.1007/s00210-008-0263-1. [DOI] [PubMed] [Google Scholar]
- 5. Jiang S, Tang DD. Plk1 regulates MEK1/2 and proliferation in airway smooth muscle cells. Respir Res . 2015;16:93. doi: 10.1186/s12931-015-0257-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Page C, O’Shaughnessy B, Barnes P. Pathogenesis of COPD and asthma. Handb Exp Pharmacol . 2017;237:1–21. doi: 10.1007/164_2016_61. [DOI] [PubMed] [Google Scholar]
- 7. Bara I, Ozier A, Tunon de Lara JM, Marthan R, Berger P. Pathophysiology of bronchial smooth muscle remodelling in asthma. Eur Respir J . 2010;36:1174–1184. doi: 10.1183/09031936.00019810. [DOI] [PubMed] [Google Scholar]
- 8. Allan LA, Clarke PR. Apoptosis and autophagy: regulation of caspase-9 by phosphorylation. FEBS J . 2009;276:6063–6073. doi: 10.1111/j.1742-4658.2009.07330.x. [DOI] [PubMed] [Google Scholar]
- 9. Allan LA, Morrice N, Brady S, Magee G, Pathak S, Clarke PR. Inhibition of caspase-9 through phosphorylation at Thr 125 by ERK MAPK. Nat Cell Biol . 2003;5:647–654. doi: 10.1038/ncb1005. [DOI] [PubMed] [Google Scholar]
- 10. Cardone MH, Roy N, Stennicke HR, Salvesen GS, Franke TF, Stanbridge E, et al. Regulation of cell death protease caspase-9 by phosphorylation. Science . 1998;282:1318–1321. doi: 10.1126/science.282.5392.1318. [DOI] [PubMed] [Google Scholar]
- 11. Rezey AC, Gerlach BD, Wang R, Liao G, Tang DD. Plk1 mediates paxillin phosphorylation (ser-272), centrosome maturation, and airway smooth muscle layer thickening in allergic asthma. Sci Rep . 2019;9:7555. doi: 10.1038/s41598-019-43927-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Liao G, Wang R, Rezey AC, Gerlach BD, Tang DD. Microrna mir-509 regulates erk1/2, the vimentin network, and focal adhesions by targeting plk1. Sci Rep . 2018;8:12635. doi: 10.1038/s41598-018-30895-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schuldt A. Cell division: a timely exit for PLK1. Nat Rev Mol Cell Biol . 2013;14:194–195. doi: 10.1038/nrm3570. [DOI] [PubMed] [Google Scholar]
- 14. Gour N, Wills-Karp M. IL-4 and IL-13 signaling in allergic airway disease. Cytokine . 2015;75:68–78. doi: 10.1016/j.cyto.2015.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Altman MC, Lai Y, Nolin JD, Long S, Chen CC, Piliponsky AM, et al. Airway epithelium-shifted mast cell infiltration regulates asthmatic inflammation via IL-33 signaling. J Clin Invest . 2019;129:4979–4991. doi: 10.1172/JCI126402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Saxton RA, Sabatini DM. Mtor signaling in growth, metabolism, and disease. Cell . 2017;168:960–976. doi: 10.1016/j.cell.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Long J, Liao G, Wang Y, Tang DD. Specific protein 1, c-Abl and ERK1/2 form a regulatory loop. J Cell Sci . 2019;132:jcs222380. doi: 10.1242/jcs.222380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jia L, Wang R, Tang DD. Abl regulates smooth muscle cell proliferation by modulating actin dynamics and ERK1/2 activation. Am J Physiol Cell Physiol . 2012;302:C1026–C1034. doi: 10.1152/ajpcell.00373.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bibi N, Parveen Z, Rashid S. Identification of potential Plk1 targets in a cell-cycle specific proteome through structural dynamics of kinase and Polo box-mediated interactions. PLoS One . 2013;8:e70843. doi: 10.1371/journal.pone.0070843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Matthess Y, Raab M, Knecht R, Becker S, Strebhardt K. Sequential Cdk1 and Plk1 phosphorylation of caspase-8 triggers apoptotic cell death during mitosis. Mol Oncol . 2014;8:596–608. doi: 10.1016/j.molonc.2013.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lee K, Rhee K. PLK1 phosphorylation of pericentrin initiates centrosome maturation at the onset of mitosis. J Cell Biol . 2011;195:1093–1101. doi: 10.1083/jcb.201106093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pawlowski J, Kraft AS. Bax-induced apoptotic cell death. Proc Natl Acad Sci USA . 2000;97:529–531. doi: 10.1073/pnas.97.2.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Johnson PR, Roth M, Tamm M, Hughes M, Ge Q, King G, et al. Airway smooth muscle cell proliferation is increased in asthma. Am J Respir Crit Care Med . 2001;164:474–477. doi: 10.1164/ajrccm.164.3.2010109. [DOI] [PubMed] [Google Scholar]
- 24. Wang T, Wang R, Cleary RA, Gannon OJ, Tang DD. Recruitment of β-catenin to N-cadherin is necessary for smooth muscle contraction. J Biol Chem . 2015;290:8913–8924. doi: 10.1074/jbc.M114.621003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang R, Cleary RA, Wang T, Li J, Tang DD. The association of cortactin with profilin-1 is critical for smooth muscle contraction. J Biol Chem . 2014;289:14157–14169. doi: 10.1074/jbc.M114.548099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wang T, Cleary RA, Wang R, Tang DD. Glia maturation factor-γ phosphorylation at Tyr-104 regulates actin dynamics and contraction in human airway smooth muscle. Am J Respir Cell Mol Biol . 2014;51:652–659. doi: 10.1165/rcmb.2014-0125OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang T, Cleary RA, Wang R, Tang DD. Role of the adapter protein Abi1 in actin-associated signaling and smooth muscle contraction. J Biol Chem . 2013;288:20713–20722. doi: 10.1074/jbc.M112.439877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wang Y, Wang R, Tang DD. Ste20-like kinase-mediated control of actin polymerization is a new mechanism for thin filament-associated regulation of airway smooth muscle contraction. Am J Respir Cell Mol Biol . 2020;62:645–656. doi: 10.1165/rcmb.2019-0310OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang Y, Rezey AC, Wang R, Tang DD. Role and regulation of Abelson tyrosine kinase in Crk-associated substrate/profilin-1 interaction and airway smooth muscle contraction. Respir Res . 2018;19:4. doi: 10.1186/s12931-017-0709-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li J, Wang R, Gannon OJ, Rezey AC, Jiang S, Gerlach BD, et al. Polo-like kinase 1 regulates vimentin phosphorylation at ser-56 and contraction in smooth muscle. J Biol Chem. 2016;291:23693–23703. doi: 10.1074/jbc.M116.749341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wang Y, Liao G, Wang R, Tang DD. Acetylation of Abelson interactor 1 at K416 regulates actin cytoskeleton and smooth muscle contraction. FASEB J . 2021;35:e21811. doi: 10.1096/fj.202100415R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wang R, Liao G, Wang Y, Tang DD. Distinctive roles of Abi1 in regulating actin-associated proteins during human smooth muscle cell migration. Sci Rep . 2020;10:10667. doi: 10.1038/s41598-020-67781-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wang Y, Liao G, Wang R, Tang DD. Acetylation of Abelson interactor 1 at K416 regulates actin cytoskeleton and smooth muscle contraction. FASEB J . 2021;35:e21811. doi: 10.1096/fj.202100415R. [DOI] [PMC free article] [PubMed] [Google Scholar]