Abstract
Advancements in methods, technology, and our understanding of the pathobiology of lung injury have created the need to update the definition of experimental acute lung injury (ALI). We queried 50 participants with expertise in ALI and acute respiratory distress syndrome using a Delphi method composed of a series of electronic surveys and a virtual workshop. We propose that ALI presents as a “multidimensional entity” characterized by four “domains” that reflect the key pathophysiologic features and underlying biology of human acute respiratory distress syndrome. These domains are 1) histological evidence of tissue injury, 2) alteration of the alveolar–capillary barrier, 3) presence of an inflammatory response, and 4) physiologic dysfunction. For each domain, we present “relevant measurements,” defined as those proposed by at least 30% of respondents. We propose that experimental ALI encompasses a continuum of models ranging from those focusing on gaining specific mechanistic insights to those primarily concerned with preclinical testing of novel therapeutics or interventions. We suggest that mechanistic studies may justifiably focus on a single domain of lung injury, but models must document alterations of at least three of the four domains to qualify as “experimental ALI.” Finally, we propose that a time criterion defining “acute” in ALI remains relevant, but the actual time may vary based on the specific model and the aspect of injury being modeled. The continuum concept of ALI increases the flexibility and applicability of the definition to multiple models while increasing the likelihood of translating preclinical findings to critically ill patients.
Keywords: lung injury, respiratory distress syndrome, pneumonia, hypoxia, extravascular lung water
Contents
Overview
-
Introduction
Defining the Goal of Experimental ALI Models
Modeling the Multidimensional Aspect of Experimental ALI
-
Methods
Selection of Participants
Delphi Approach
Virtual Discussion
Post-Meeting Survey
-
Results
Histological Evidence of Tissue Injury
Alteration of the Alveolar–Capillary Barrier
Presence of an Inflammatory Response
Evidence of Physiological Dysfunction
Recommended Definition of Experimental ALI
Time Criterion as a Part of the Definition of Experimental ALI
Discussion
Conclusions
Overview
The purpose of this report is to update the definition of acute lung injury (ALI) in model systems, revise the relevant measurements that describe the main features of ALI, and reassess the role of time as part of the definition. The ultimate goal is to provide a current framework for defining experimental ALI, which can serve as a standard for the field. The key findings of this workshop are as follows:
-
•
Experimental ALI encompasses a continuum of models ranging from those focusing on gaining specific mechanistic insights to those primarily concerned with preclinical testing of novel therapeutics or interventions.
-
•
We suggest that mechanistic studies may justifiably focus on a single domain of lung injury, but models must document alterations of at least three of the four domains to qualify as “experimental ALI.” For preclinical testing of novel therapeutics or interventions, fulfillment of all four domains is recommended.
-
•
Demonstrating alterations in a domain requires at least one measurement identified as “relevant” for that domain.
-
•
We propose that a time criterion defining “acute” in ALI remains relevant, but the actual time may vary based on the specific model and the aspect of injury being modeled.
Introduction
There is significant variability in what researchers consider ALI in an animal or model system. This variability makes it difficult to compare data from different studies and assess rigor and transparency, and it may be a barrier to accurate “bench-to-bedside” translation. Therefore, there is a major need to agree on what constitutes ALI in animals. In 2011, the American Thoracic Society (ATS) published a Workshop Report that used a Delphi approach to identify the main features and measurements defining experimental ALI (1). Since then, advances in imaging, genetic tools, “omics” technologies, and cellular biology have provided new insights into lung injury both in preclinical models and in humans (2–6). These advancements have created a need for a careful reexamination of how ALI is measured and defined, with the goal of facilitating optimal translation of preclinical observations to clinical medicine. The purpose of this update to the 2011 Workshop report is to refine the definition of ALI in model systems, revise the relevant measurements that describe the main features of ALI, and reassess the role of time as part of the definition. The ultimate goal is to provide an updated framework for defining experimental ALI, which can serve as a standard for the field.
Defining the Goal of Experimental ALI Models
A major question regarding experimental models of ALI is what is being modeled. Originally described in 1967 as a case series of 12 patients, acute respiratory distress syndrome (ARDS) was characterized clinically by acute onset of tachypnea, worsening hypoxemia, impaired lung compliance, and widespread alveolar opacities on radiographic imaging (7). Since its original description, the definition of ARDS remains clinical and has evolved to the current 2012 Berlin consensus definition that focuses on “feasibility, reliability, validity and objective evaluation of its performance” (8). The Berlin definition defines the time component of acute onset as “occurring within 7 days of exposure to a recognized predisposing event.” It also includes risk factors to consider when ascertaining the origin of edema, categorizes ARDS severity based on physiologic dysfunction or hypoxemia, and provides more explicit criteria for bilateral airspace opacities on radiographic imaging (8). Although animal models are central to studying this clinically defined syndrome, each of the model systems have limitations that need to be considered depending on the goal of the study. For example, small animal models may not replicate all the clinical features of ARDS but are tractable systems that can address mechanisms of disease and provide the framework for rational therapeutic design. Large animal models are less malleable to mechanistic studies but may reproduce clinical features of ARDS and therefore serve as important models for preclinical therapeutic testing (9).
Animal models of ALI have played a fundamental role in the development of key effective interventions for the treatment of ARDS. For example, the concept that overexpansion of alveolar spaces was due to excessive tidal volumes or inspiratory pressures was initially derived from animal studies and eventually led to low tidal volume ventilation (10–15). Similarly, animal studies of the impact of gravity on pulmonary perfusion during lung injury provided the initial rationale for prone ventilation in ARDS (16). However, challenges exist in the direct application of criteria used to define ARDS in humans, and one must first determine the goal of the study to identify the optimal model of experimental ALI (Figure 1). For instance, rodent models of ALI rarely use hypoxemia as a defining criterion despite it being the primary criterion of physiologic dysfunction in humans. This is partly because of the technical challenges of obtaining an arterial blood gas in small animals, which is more feasible in large animal models. In contrast, rodent models may serve the goal of investigating genetic variants and predisposing conditions more readily than large animal models (Figure 1). Although patients with ARDS can be supported with invasive or noninvasive mechanical ventilation, mechanical ventilation is possible in rodent models of experimental ALI for only short periods of time and is not a practical approach to study the resolution phase of injury in either small or large animal models. Despite these limitations, animal models remain essential to advance the understanding of the biology of ALI, and working toward uniformity on what constitutes ALI in a preclinical experimental model may accelerate progress related to specific therapeutics in ARDS.
Figure 1.
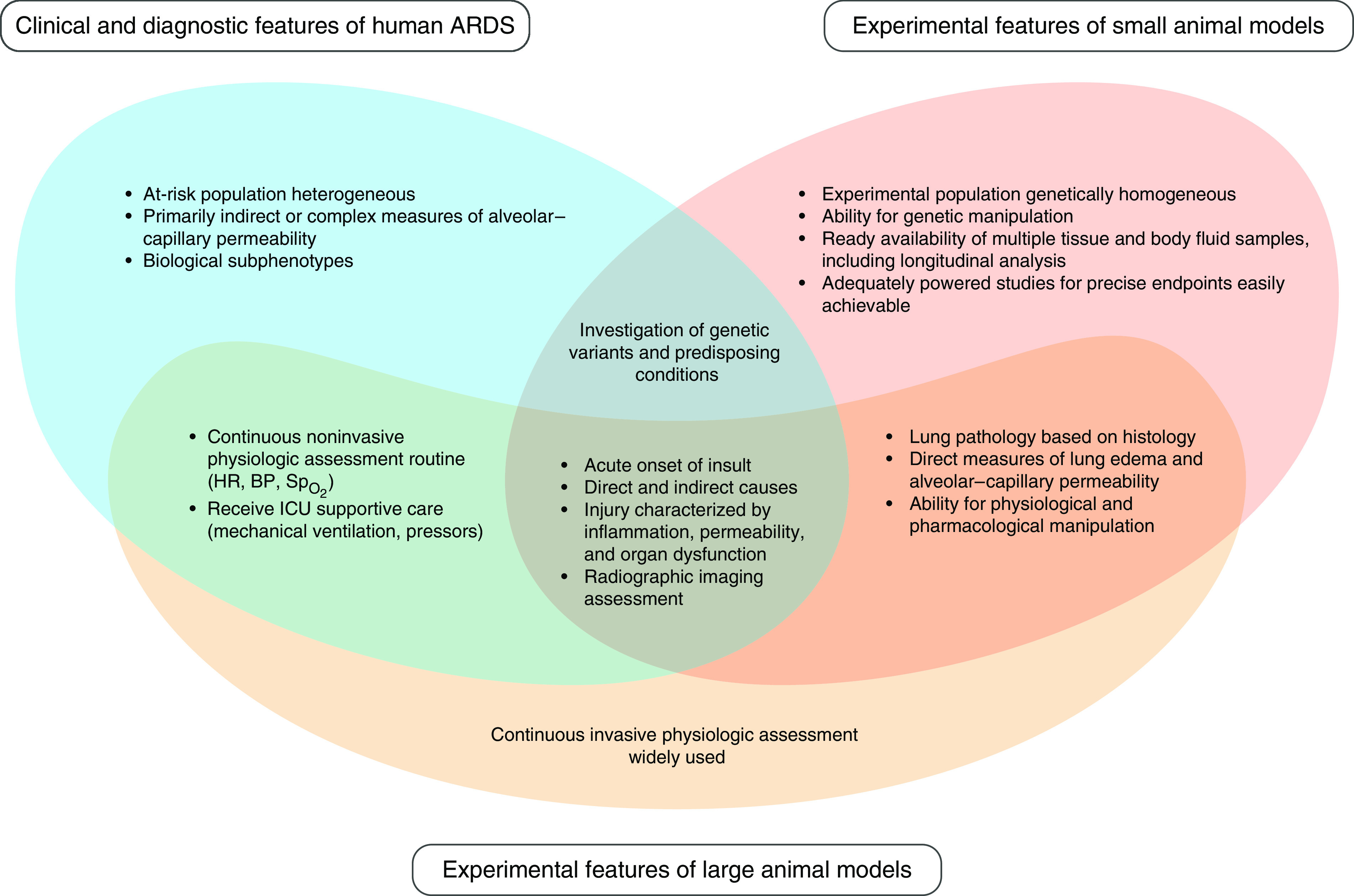
Modeling clinical features of ARDS for mechanistic analyses and therapeutic interventions. ARDS = acute respiratory distress syndrome; BP = blood pressure; HR = heart rate; = pulse oximetry.
Modeling the Multidimensional Aspect of Experimental ALI
An important contribution of the 2011 workshop was the description of experimental ALI as a “multidimensional entity” that is characterized by four main “domains,” which reflect the key pathophysiologic features and underlying biology of human ARDS. These domains are 1) histological evidence of tissue injury, 2) alteration of the alveolar–capillary barrier, 3) the presence of an inflammatory response, and 4) evidence of physiologic dysfunction (1). The “acute” component of ALI was defined as “24 hours from the initial injurious intervention.” We convened the current workshop to update the ALI definition by answering the following questions: 1) Should a time criterion continue to be included in the definition of experimental ALI, and, if so, what should that time be? 2) What is the minimum number of domains that should show alterations to determine that ALI has occurred? 3) What are the measurements that determine that one of these domains has been altered? To address these questions, we queried a broad and diverse range of international experts in the field of ALI and ARDS, using a Delphi approach.
Methods
Selection of Participants
Recommendations for workshop panelists were solicited from participants of the 2011 workshop report as well as from the Assembly Chair and Planning Committee Chair of the ATS Assemblies that cosponsored the workshop (Allergy, Immunology, and Inflammation; Critical Care; Environmental, Occupational, and Population Health; Pediatrics; Pulmonary Circulation; Pulmonary Infections and Tuberculosis; Respiratory, Cell, and Molecular Biology; and Respiratory Structure and Function). A list of 50 participants was finalized to include those working in the fields of experimental ALI and human models of ARDS as well as to have representation from diverse geographical regions, genders, and seniority levels (Table E1 in the data supplement). For each domain, a lead was identified to collate and coordinate survey responses from each round and report back to the committee chairs. Potential conflicts of interest were disclosed and managed in accordance with the policies and procedures of the ATS.
Delphi Approach
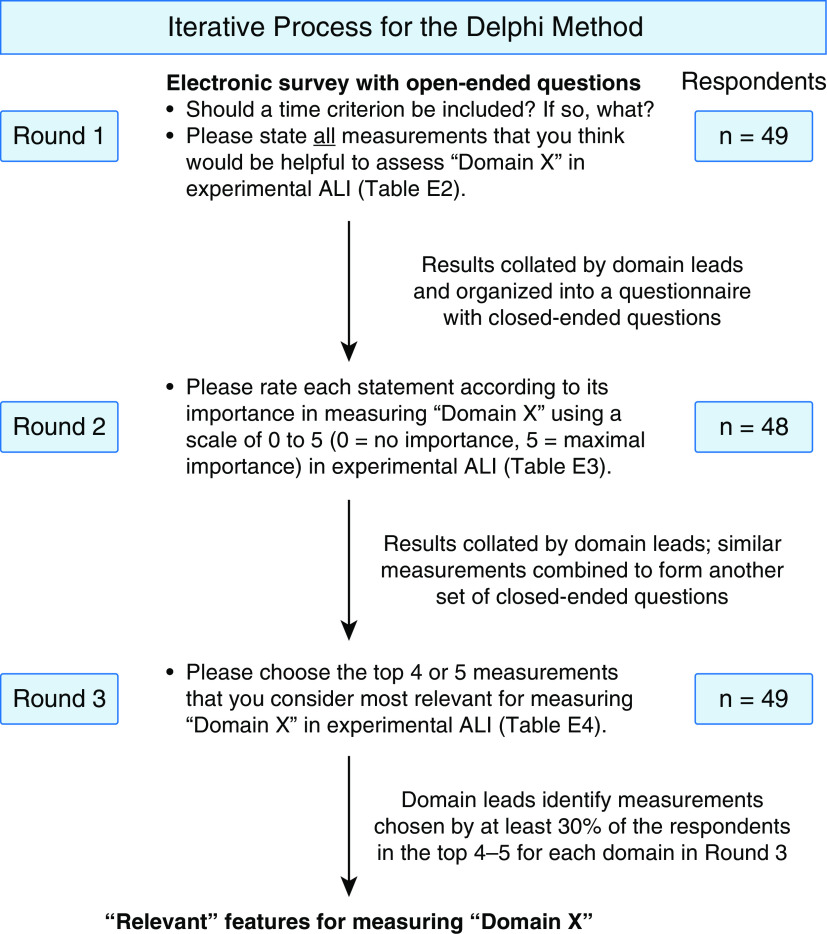
Similar to the 2011 workshop, a Delphi method was used to solicit measurements of experimental ALI (Figure 2). In Round 1 (Table E2), the 50 participants were asked to complete an electronic survey, wherein they stated all the measurements that they thought would be helpful to assess 1) histological evidence of tissue injury, 2) alteration of the alveolar–capillary barrier, 3) presence of an inflammatory response, and 4) evidence of physiologic dysfunction. Here, we refer to “features” as a measurement or group of measurements that address a specific component of a domain. Additionally, we asked the participants whether a time criterion should be included in the definition of experimental ALI and, if yes, to suggest a time. This question was asked based on feedback from workshop participants that the time criterion of 24 hours was too short for some models of lung injury (e.g., viral pneumonia) (17).
Figure 2.
Delphi method for determining measurements for experimental acute lung injury (ALI). Schematic representation of the three rounds of the Delphi method used to arrive at the measurements of experimental ALI. Domain X represents any one of the four domains: 1) histologic evidence of injury, 2) disruption of the alveolar–capillary barrier, 3) presence of an inflammatory response, and 4) evidence of physiologic dysfunction. The complete list of questions and their answers is provided in Tables E2–E4, corresponding to each round. The number of respondents in each round have been provided on the right-hand side of the figure. The figure was created using www.biorender.com
All the responses obtained as a part of the first round were collated by the domain leads and organized into a questionnaire for Round 2 (Table E3). Specifically, in Round 2, participants were provided with a list of all the measurements obtained from Round 1 under each of the four original domains in the 2011 workshop report. Participants were asked to rate each measurement according to importance using a scale of 0–5 (0 = minimal importance, 5 = maximal importance). As a part of the “histological evidence of tissue injury” domain, measurements were grouped by anatomical location: 1) alveolar spaces, 2) alveolar epithelium, 3) vasculature, 4) alveolar septae, and 5) interstitium. As a part of the “alteration of the alveolar–capillary barrier” domain, measurements were grouped under 1) endothelial injury or dysfunction, 2) epithelial injury or dysfunction, 3) lung edema, and 4) transfer of plasma or lung constituents across the barrier. As a part of the “presence of an inflammatory response” domain, measurements were grouped under 1) soluble mediator profiles, 2) inflammatory cellular composition and characteristics, and 3) consequences of inflammation. Finally, as a part of the “evidence of physiologic dysfunction” domain, measurements were grouped under 1) gas exchange, 2) lung mechanics, 3) vital signs, and 4) other aspects of the physiological domain.
In Round 3, answers to the questions from Round 2 were collated and presented to the participants (Table E4). Participants were asked to choose the top 4–5 features that they considered “most relevant” to measure each domain. Those features selected as “most relevant” by 30% or more of the respondents were considered “relevant” for that domain for the purpose of this workshop. We noted that a 30% cutoff resulted in at least five measurements ranked as “most relevant” in each of the four domains by the panelists. This approach would maximize the numbers of relevant measurements available to the community and maintain consistency in the number of relevant measurements across the domains, thereby increasing flexibility in application to many experimental ALI models.
Virtual Discussion
The original proposal included an in-person meeting, but because of the coronavirus disease (COVID-19) pandemic, this was replaced by virtual approaches. First, a video containing a presentation of the results was prerecorded by the domain leads and shared with the participants. Certain questions that were perceived by the domain leads to be important for the appropriate interpretation of the document were also included in this presentation (Table E5). Subsequently, two 90-minute virtual sessions in which the domain leads presented the results were held in the same week. Participants were encouraged to give their opinions about the questions in a live, online setting. All participants were asked to attend at least one of these sessions.
Post-Meeting Survey
The participants were subsequently emailed a post-meeting survey composed of the same questions that were discussed at the virtual meeting (Table E6). A free-text box was also provided for participants to provide feedback on any aspects of the project, including the methodology and/or the results. These results were tabulated by the domain leads and have been incorporated into the manuscript to demonstrate the extent of agreement on each of the questions.
Results
The concept of four domains reflecting the key pathophysiologic features and underlying biology of experimental ALI were retained from the 2011 workshop report (Table 1). Additionally, the time criterion defining “acute” was retained based on responses from the participants. Key results for each domain and the time criterion are presented below.
Table 1.
Main Features of Experimental ALI
| Main Features | |
|---|---|
| Rapid onset (with a defined period of time, specific to the model utilized) plus*: | |
| Histological evidence of tissue injury | |
| Alteration of the alveolar–capillary barrier | |
| Presence of an inflammatory response | |
| Evidence of physiological dysfunction |
Definition of abbreviation: ALI = acute lung injury.
To state ALI has occurred, at least one accepted “relevant” measurement under at least three out of four domains should be reported.
Histological Evidence of Tissue Injury
Most of the histological features of ALI proposed by the panel (Table 2) represent individual aspects of tissue injury, but one of them deserves specific mention: a “validated histologic score,” ranked number 2 overall and recommended by 63% of respondents. However, there is no validated score at the present time with demonstrated intra- and interobserver reproducibility. This situation has not changed from 2011, when such a score did not yet exist. In the prior report, an example of a potential score was proposed, but many readers understood it as a “recommended score” rather than a “proposal for a score.” Therefore, we purposely do not include any scores in the present update. Instead, the issue of histological scoring and injury quantification was further discussed at the virtual meeting, and in the post-meeting survey, panelists were asked, “how should histologic injury be quantified” (Table E6, Q.2)? The majority (57%) of respondents answered that a blinded assessment of lung injury features in several nonoverlapping fields with a clear methodologic description of each measure was preferred. Other respondents (36%) stated that the field should develop a validated lung injury score to be used in histology, followed by a smaller group of respondents stating that no quantification is necessary (5%). Automated assessment using image analysis software was suggested by one respondent (2%).
Table 2.
Measurements of Histological Evidence of Tissue Injury
| Domain Recommendations | n (%) |
|---|---|
| Filling of the alveolar space with proteinaceous alveolar fluid and debris* | 40 (82) |
| A validated histologic injury score* | 31 (63) |
| Evidence of alveolar epithelial injury (cell death, epithelial denudation, or ATII proliferation)* | 28 (57) |
| Neutrophil infiltration of the alveolar space* | 26 (53) |
| Thickening of alveolar septae and/or interstitial edema* | 25 (51) |
| Diffuse alveolar damage pattern* | 21 (43) |
| Hyaline membranes or presence of fibrin or derivates in the airspaces* | 20 (41) |
| Evidence of intraalveolar hemorrhage or extravasated red cells | 14 (29) |
| Evidence of capillary and/or endothelial cell death | 13 (27) |
| Neutrophil infiltration of alveolar septae or interstitium | 11 (23) |
| Perivascular inflammation, including intravascular accumulation of neutrophils | 8 (16) |
| Perivascular edema or cuffing | 3 (6) |
| Hepatization | 2 (4) |
| Loss of tight junctions | 2 (4) |
| Presence of microthrombi | 1 (2) |
Definition of abbreviation: ATII = Type II alveolar epithelial cell.
Features or measurements that were considered as being “most relevant” to the domain by 30% or more of the respondents.
In addition to a validated histologic score, other features ranked “most relevant” to the domain by the highest number of panelists (number 1 and number 3, respectively) were “filling of the alveolar space with proteinaceous alveolar fluid and debris” (82% of respondents) and “evidence of alveolar epithelial injury” (57% of respondents). Each of these reflect consequences of destruction of the alveolar–capillary barrier and flooding of the alveoli with protein-rich fluid. Interestingly, “neutrophilic infiltration” and “interstitial edema” were both ranked lower but remained above the 30% cutoff required for a “recommended measurement.” We speculate that this may reflect the perception that these two assessments are less specific for ALI; for example, “interstitial edema” can also be seen in cardiogenic pulmonary edema. Finally, the other two measurements recommended by 30% or more of the respondents and therefore considered “most relevant” were “diffuse alveolar damage pattern” (43%) and “hyaline membranes or presence of fibrin derivatives in the airspaces” (41%).
Alteration of the Alveolar– Capillary Barrier
Disruption of the alveolar–capillary barrier is a central feature of ARDS, leading to flooding of the airspace with protein-rich fluid. Loss of barrier integrity differentiates pulmonary edema caused by ARDS and edema from cardiogenic causes. Experimental measures of alveolar–capillary barrier dysfunction reflect the major pathophysiologic changes that result from barrier loss. The feature considered “most relevant” by the highest number of panelists was the direct measurement of high concentrations of albumin or, alternatively, IgM or another large-molecular-weight plasma protein, which nearly 90% of the panel considered to be in the top five “most relevant” measures of alveolar–capillary barrier dysfunction (Table 3). Other direct measures of leakage of plasma components into the airspace or interstitium were also considered “most relevant” and included elevated BAL total protein, Evan’s blue dye–labeled albumin and protein accumulation in lung homogenate, rate of accumulation of a tagged marker in the airspace, and transport of a large-molecular-weight substance. Although Evan’s blue dye is commonly used as a tracer because it binds tightly to albumin and other proteins, thus reflecting transit of these proteins into the airspace, unbound Evan’s blue is only 0.98 kD and acts as a small molecule. When using this method, caution should be taken to ensure that free Evan’s blue is not present. Endothelial-specific permeability can be assessed by the measurement of the filtration coefficient, but this measure is technically challenging and is not available in all laboratories. Another feature considered “most relevant” to the domain by ⩾30% of the panelists was assessment of pulmonary edema accumulation as measured by lung wet weight to dry lung weight or body weight ratio. Currently, the ability to quantify edema readily and easily is a unique feature of animal models. Although extravascular lung water can be measured with thermodilution techniques (18), these methods have limited clinical applicability and are not as accurate as gravimetric methods (19).
Table 3.
Measurements of Alteration of the Alveolar–Capillary Barrier
| Domain Recommendations | n (%) |
|---|---|
| Elevated BAL albumin, IgM, or other large circulating protein* | 44 (90) |
| Increased lung wet-to-dry weight ratio, lung wet weight to body weight ratio, or extravascular lung water* | 38 (78) |
| Elevated BAL total protein* | 30 (61) |
| Evan’s blue dye accumulation in lung homogenate* | 24 (49) |
| Pulmonary vascular permeability index and/or filtration coefficient* | 21 (43) |
| Rate of accumulation of tagged marker (fluorescent probe, I-131 albumin, etc.) in the airspace* | 20 (41) |
| Transport of large-molecular-weight substance (∼70 kD or larger, e.g., dextran)* | 18 (37) |
| Accumulation of airspace-injected tracers into the circulation | 9 (18) |
| Circulating markers of epithelial and/or airway injury (e.g., RAGE, SP-D, KL-6) | 9 (18) |
| Increased markers of ATI or ATII injury in the airspace | 9 (18) |
| Hemorrhage and/or RBCs in airspace | 8 (16) |
| Elevated BAL RAGE | 7 (14) |
| Transport of a very large (∼300 kD) tracer across barrier | 7 (14) |
| Surfactant function | 1 (2) |
Definition of abbreviations: ATI = Type I alveolar epithelial cells; KL-6 = Kreb von den Lungen-6; RAGE = receptor for advanced glycation end products; RBCs = red blood cells; SP-D = surfactant protein-D.
Features or measurements that were considered as being “most relevant” to the domain by 30% or more of the respondents.
Measurements that less than 30% of panelists considered “most relevant” included markers of alveolar epithelial injury (e.g., BAL or plasma RAGE [receptor for advanced glycation end products] or SP-D [surfactant protein-D]), surfactant function, and evidence of large defects in the alveolar–capillary barrier (e.g., red blood cells in the airspace and transfer of very large-molecular-weight proteins across the barrier). Although these measures were not considered “most relevant” to the domain by ⩾30% of the panelists, they may be highly relevant to specific studies of alveolar epithelial injury, surfactant dysregulation, and hemorrhage into the airspace. In a follow-up questionnaire, we asked the panel if, in aggregate, measures of alveolar epithelial injury should be added as a measure that would fulfill this domain for purposes of defining ALI (Table E6, Q.3). The majority of respondents (52%) were of the opinion that epithelial injury alone should not be used to fulfill this domain. It was noted that although epithelial injury is a prominent feature of both ARDS and experimental ALI, there can be some degree of epithelial injury without significant breakdown of the alveolar–capillary barrier (20–22). Thus, epithelial injury alone is insufficient as a measure of alveolar–capillary barrier dysfunction. Overall, measures in this domain reflect the barrier breakdown and extravascular accumulation of lung fluid and protein that are key features of human ARDS.
Presence of an Inflammatory Response
Nearly all respondents (96%) chose “increase in chemokine and cytokine expression in the BAL or lung tissue” as a relevant measurement indicating the presence of an inflammatory response (Table 4). Soluble mediators stated by respondents included chemokines such as IL-8 (CXCL8) or its murine homolog chemokines KC (CXCL1) and MIP-2 (macrophage inflammatory protein-1 or CXCL2), MCP-1 (monocyte chemotactic protein-1 or CCL2), and MCP-3 (CCL7) as well as cytokines such as IL-6, TNF-α (tumor necrosis factor–α), IL-1β, IL-18, sTNFR1 (soluble TNF receptor 1), and IL-10. The majority of respondents (55%) recommended measuring these inflammatory mediators as examples indicating the presence of an inflammatory response, and there was broad agreement that this list of mediators is neither comprehensive nor specific to lung injury per se (Table E6, Q.5). Another feature that was considered “most relevant” to the domain by many panelists (86%) was an increase in neutrophil numbers, as quantified by absolute cell numbers or by the protein content of NE (neutrophil elastase) or MPO (myeloperoxidase) in BAL or lung tissue. The next feature considered “most relevant” to the domain (61%) was an increase in leukocyte subpopulations of inflammatory monocytes, macrophages, and/or lymphocytes in the BAL or lung tissue. An increase in neutrophil activity, as measured by NE or MPO activity assay in the supernatant of BAL or lung tissue, was viewed as “most relevant” by almost half of the respondents (49%). A key distinction between measuring the enzymes NE or MPO, versus their activity, is that enzymatic levels serve as a proxy of neutrophil numbers, whereas the activity, such as in BAL supernatants, represents the proteolytic action of enzymes released from activated cells during injury (23, 24).
Table 4.
Measurements of an Inflammatory Response
| Domain Recommendations | n (%) |
|---|---|
| Increase in chemokines or cytokines in BAL or lung tissue (e.g., CXCL1/2, MCP-1, MCP-3, IL-6, IL-1β, TNF-α, TNFR1, IL-18, IL-10, etc.)* | 47 (96) |
| Increase in neutrophil numbers in BAL or in lung tissue (absolute numbers or by neutrophil elastase or myeloperoxidase content)* | 42 (86) |
| Increase in inflammatory monocyte and macrophage (and/or lymphocyte) subpopulations in BAL or lung tissue* | 30 (61) |
| Increase in neutrophil activity as measured by elastase or myeloperoxidase in supernatant of BAL or lung tissue* | 24 (49) |
| Endothelial cell adhesion molecule expression or mediator release (e.g., sICAM-1, sVCAM-1, Ang-2, vWF)* | 15 (31) |
| Transcriptomic signatures that mirror human gene expression | 12 (25) |
| Soluble DAMPs: extracellular ATP, HMGB1, or extracellular DNA | 7 (14) |
| Increased proteolysis (e.g., MMPs, elastase, other proteases) | 7 (14) |
| Changes in acute response genes (e.g., Egr1) | 5 (10) |
| Inflammasome activation | 5 (10) |
| Mitochondrial dysfunction | 1 (2) |
| Neutrophil extracellular traps | 1 (2) |
Definition of abbreviations: Ang-2 = angiopoietin-2; DAMPs = damage associated molecular patterns; Egr1 = early growth receptor 1; HMGB1 = high mobility group box 1; MCP = monocyte chemotactic protein; MMPs = matrix metalloproteinases; sICAM-1 = soluble intercellular adhesion molecule-1; sVCAM-1 = soluble vascular cell adhesion molecule-1; TNF-α = tumor necrosis factor–α; TNFR1 = tumor necrosis factor receptor 1; vWF = von Willebrand Factor.
Features or measurements that were considered as being “most relevant” to the domain by 30% or more of the respondents.
Different from the original workshop report, structural cells such as endothelial cells were noted as important cell types involved in the inflammatory response of experimental ALI. Over 30% of respondents indicated that endothelial cell adhesion molecule expression or release of mediators such as sICAM-1 (soluble intercellular adhesion molecule), sVCAM-1 (soluble vascular cell adhesion molecule), Ang-2 (angiopoietin-2), and vWF (von Willebrand Factor) are relevant measurements of endothelial injury. The majority of respondents (55%) recommended providing measurements of one or more of these mediators (e.g., sICAM-1, sVCAM-1, Ang-2, vWF), recognizing that the list is neither exhaustive nor specific to lung injury per se (Table E6, Q.5). When asked in what compartment (e.g., blood, lung tissue, airspace compartment) should endothelial cell adhesion molecule expression or mediator release be measured, the majority of respondents (52%) stated that the compartment should not be specified, whereas the rest of the respondents were split among the blood, lung tissue, and airspace compartments (Table E6, Q.4).
Measurements that less than 30% of panelists considered “most relevant” included transcriptomic signatures that mirror those found in clinical ARDS; soluble damage associated molecular patterns such as extracellular ATP, extracellular DNA, and HMGB1 (high mobility group box 1); proteases such as matrix metalloproteases and elastase; changes in acute response genes; inflammasome activation; mitochondrial dysfunction; and neutrophil extracellular traps (Table 4). The relevant measurements of this domain reflect a broader recognition of the changing composition of the inflammatory cellular profile depending on the experimental animal model and the phase of injury being studied. In addition, the inflammatory response domain now includes endothelial cell adhesion molecule expression or endothelial mediator release as a relevant measurement.
Evidence of Physiological Dysfunction
Physiological dysfunction, defined here not only as impaired alveolar–capillary gas exchange, lung mechanics, or alveolar fluid clearance but also as systemic manifestations of ALI and notably altered appearance of lung tissue on radiographic imaging, is the cardinal feature of clinical ARDS. The 2012 Berlin definition of ARDS is primarily based on impaired oxygenation and evidence of bilateral opacities on chest imaging (8). As such, different measures of gas exchange, including partial pressure of arterial oxygen, ratio of partial pressure of arterial oxygen to fractional inspired oxygen, oxygen saturation, alveolar–arterial oxygen gradient, partial pressure of carbon dioxide, diffusing capacity of carbon monoxide, or ventilation–perfusion mismatch, were commonly named by all respondents (Table 5). Of these, arterial blood gas measurements of oxygenation were ranked by ⩾30% of the panelists as being “most relevant” to the domain. It was, however, noted by several respondents that although arterial blood gases are probably the “ideal” measurement, blood gas analyses in small rodent models require specific instrumentation (e.g., small animal blood gas analyzer) and experience in blood sampling. Furthermore, arterial blood sampling for blood gas analysis is commonly a terminal procedure in mice unless an indwelling arterial catheter has been placed; even in the latter case, the number of possible blood samplings remains limited by the small murine blood volume. As such, noninvasive assessment of oxygenation by pulse oximetry may provide a reasonable alternative (albeit signal quality deteriorates commonly in hemodynamic shock) and was accordingly also considered by nearly half the respondents as one of the top four “most relevant” measures of physiological dysfunction. Second to changes in gas exchange, impaired lung mechanics were considered “most relevant” by the highest number of panelists. Of these, changes in lung or respiratory system compliance were considered the “most relevant,” followed by changes in dead space, airway and/or tissue resistance, total or inspiratory lung capacity, or atelectasis. Several respondents highlighted the forced oscillation technique as a widely accepted, accurate measure of lung mechanics in small rodents. Third, a change in alveolar fluid clearance was considered by more than half of the respondents as one of the top four “most relevant” features of physiological dysfunction, although it was pointed out that quantification of alveolar fluid clearance, particularly in rodent models, is performed in only a few specialized laboratories. Measures of impaired breathing such as increased respiratory rate, visualized difficulties in breathing, or increased minute ventilation were also considered as one of the top four “most relevant” measures. Notably, the applicability of this criterion will depend on the model of ALI and its time course (i.e., impaired breathing will be typically observed after longer time periods in spontaneously breathing animals).
Table 5.
Measurements of Physiological Dysfunction
| Domain Recommendations | n (%) |
|---|---|
| Arterial blood gas measurements of oxygenation* | 42 (86) |
| Lung and/or respiratory compliance and/or elastance* | 38 (78) |
| Alveolar fluid clearance* | 26 (53) |
| Noninvasive measurements of oxygenation* | 23 (47) |
| Respiratory rate, difficulty breathing, and minute ventilation* | 18 (37) |
| Appearance of lung tissue in lung imaging | 13 (27) |
| Dead space and/or partial pressure of carbon dioxide | 9 (18) |
| Weight loss | 7 (14) |
| Systemic illness and/or systemic organ dysfunction | 7 (14) |
| Quantity of pathogen | 7 (14) |
| Systemic hemodynamics | 6 (12) |
| Temperature | 0 (0) |
Features or measurements that were considered as being “most relevant” to the domain by 30% or more of the respondents.
Measurements that less than 30% of panelists considered “most relevant” included the quantity of pathogen (in pneumonia, sepsis, or peritonitis models), systemic illness and/or systemic organ dysfunction, weight loss, systemic hemodynamics (for monitoring vital signs), or temperature. Notably, the low ranking for some of these features (e.g., pathogen quantity) may be attributable to individual models used, as numerous animal models of ALI, such as ventilator-associated lung injury, bleomycin, oleic acid, or acid aspiration, are sterile in nature and do not involve the administration of infectious pathogens. Another measurement that less than 30% of panelists considered “most relevant” was the appearance of lung tissue on radiographic imaging. As this measurement constitutes one of the cardinal features of ARDS in humans, we asked respondents why lung imaging may be less relevant in animal models than in humans (Table E6, Q.6). Most respondents (84%) conceded that imaging is an important tool to document ALI in large animals. Yet in small animals, in which the bulk of ALI research is performed, imaging is considered as a potentially powerful and evolving technology that is, however, not widely used or available. It is noteworthy that relevant measurements of physiological dysfunction received much broader support as compared with the 2011 definition, which only listed hypoxemia and an increased alveolar–arterial oxygen difference as “very relevant” features. In contrast, in addition to impaired gas exchange, impaired lung mechanics, alveolar fluid clearance, and signs of increased respiratory effort (e.g., elevated respiratory rate, difficulty breathing, and minute ventilation) were now also considered as “most relevant” features by at least 30% of respondents.
Recommended Definition of Experimental ALI
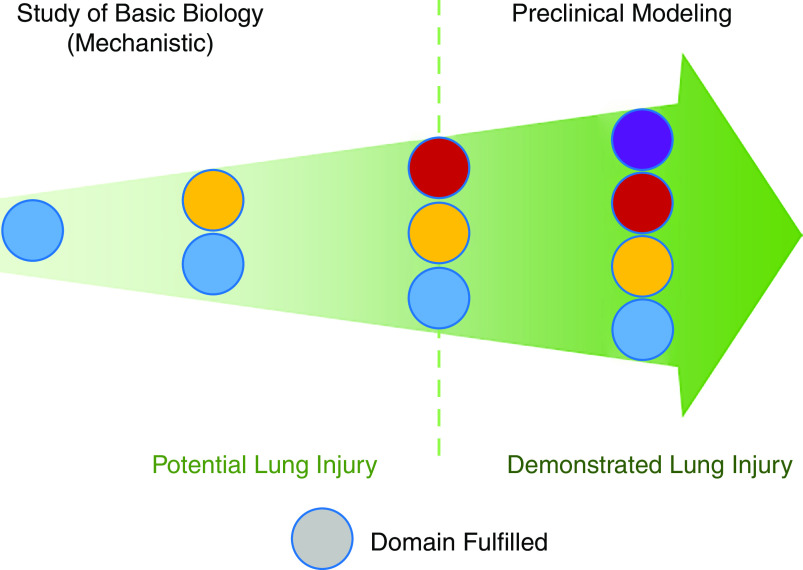
The 2011 workshop report recommended that alterations in at least three of the four domains should be present to determine that ALI has occurred in an experimental model (Table 1). In this revised report, we propose that experimental ALI encompasses a continuum of models ranging from those focusing on gaining specific mechanistic insights to models that are largely concerned with preclinical testing of therapeutics or promising interventions (Figure 3). This new framework acknowledges that mechanistic studies may justifiably focus on one or two domains of lung injury (i.e., “potential lung injury”; Figure 3). However, to fully qualify as “experimental ALI” (i.e., “demonstrated lung injury”; Figure 3), a model should demonstrate alterations in at least three of the four domains, reflecting the multidimensional aspects of human ARDS (Table 1). We propose that demonstrating alterations in a domain requires at least one measurement identified as “relevant” for that domain (Tables 2–5). “Relevant measurements” are those that were ranked among the “top 4–5 most relevant measurements” by at least 30% of the respondents (Tables 2–5). Tables 2–5 also list measurements that did not reach the 30% cutoff. These measurements may be included or reported in support of changes in a domain, but at least one of the “relevant” measurements should also be reported to fulfill that domain. As the goal of experimental modeling advances from studying basic mechanisms to preclinical drug testing and the need to ensure translation to clinical studies increases, we suggest demonstrating alterations of all four domains using at least one “relevant measurement” per domain (Figure 3).
Figure 3.
The “continuum” framework. Experimental ALI encompasses a continuum of models ranging from those focusing on the study of basic biology for gaining specific mechanistic insights to those that are largely concerned with preclinical modeling of therapeutics or promising interventions. This new framework acknowledges that mechanistic studies may justifiably focus on one or two domains of lung injury (i.e., “potential lung injury”). However, to fully qualify as “experimental ALI” (i.e., “demonstrated lung injury”), a model should demonstrate alterations in at least three of the four domains, reflecting the multidimensional aspects of human ARDS (Table 1).
Time Criterion as a Part of the Definition of Experimental ALI
Nearly three-quarters of respondents considered that the definition of experimental ALI should include a time criterion to define “acute.” However, the time range for this criterion was broad. Among those who responded “yes,” nearly one-third proposed the range to be within 24 hours from the onset of exposure to the stimulus. The next most common answer was 72 hours (14%). Approximately 10% of panelists recommended “up to 7 days,” and 4% proposed “up to 10 days.” This was considered by the panel to be important to account for in models that progress slowly (e.g., viral infection or bleomycin) and also to parallel the clinical definition of ARDS (17, 25). A majority of panelists (62%) considered that the time criterion should vary based on the insult resulting in ALI, including the model, dose, and use of adjunctive therapies (e.g., antibiotics).
Given the broad range of responses for the time criterion, participants were asked for further comments after the discussion of results at the virtual meeting. When asked, “By what time point should injury be evident to meet the definition of ALI?”, none of the respondents selected that “the original 24-hour window is sufficient” (Table E6, Q.1.). Approximately 38% felt that the time point can be up to 7 days of the inciting injury, although a majority (62%) responded that there should be no fixed time criterion, as the time depends on the model system being used.
Discussion
We used a Delphi approach to query an international panel of 50 experts in the field and provide key changes to the 2011 definition by introducing a continuum concept and revising the time criterion. An unintended consequence of the prior workshop as voiced by several panelists was that it was often used to invalidate highly mechanistic studies and suggest that they were not relevant to ALI. However, experimental research on ALI spans a continuum ranging from studies focusing on specific mechanisms to studies primarily concerned with preclinical testing of novel therapeutics or promising interventions (Figure 3). Accordingly, the stringency of the requirements to identify lung injury in an experimental model should also follow a continuum that matches study goals. This new framework acknowledges that mechanistic studies may justifiably focus on a single aspect or domain of lung injury, but in those cases, they would suffice as “potential lung injury.” However, for a model to qualify as “experimental ALI,” we recommend demonstrating alterations in at least three of the four domains of lung injury (Table 1). For preclinical testing of novel therapeutics or interventions, fulfillment of all four domains is recommended. Thus, although the definition of ALI remains binary, the fulfilment of domains represents a continuum based on the purpose of the model, with a greater number of domains fulfilled increasing the confidence of demonstrating ALI in the model. Additionally, our revised definition decreases the number of “relevant measurements” required to fulfil an ALI domain to only one, thus increasing flexibility for the field. This “continuum” concept seeks to improve bench-to-bedside translation without imposing excessive burden to researchers doing highly mechanistic, focused studies. This concept also emphasizes the need for experimental ALI to reflect the multidimensional aspects of human ARDS.
We were also able to build on feedback from the workshop participants that the original time criterion of 24 hours for defining “acute” was too restrictive. We continue to recommend a time criterion in the definition of experimental ALI, but we now recommend against a fixed time cutoff required to define “acute.” Instead, we acknowledge that the time cutoff may vary depending on the specific injury-causing agent, the model, doses studied, and any use of adjunctive therapies (e.g., antibiotics). We foresee our revision facilitating additional rigor in the field while allowing for newer, experimental models to evolve, which would more accurately represent ARDS in humans (17).
The four “domains” of experimental ALI emerged iteratively during the Delphi process of the 2011 workshop (1), and, therefore, we decided to keep these domains as a conceptual framework for our project. In the current workshop, we did not ask for reference values for any measurement, realizing that these are model specific and may differ between laboratories depending on techniques used. Additionally, we deliberately chose not to specifically discuss any individual lung injury models. There were several reasons for this. First, there are many excellent review papers on this topic (26–29). Second, we wanted to provide a flexible conceptual framework that could be applied to different lung injury models. Third, we wanted to encourage innovation in ALI model development (9). We reasoned that providing a list of models, or even examples, might be limiting for investigators wishing to develop new ways of modeling ALI. We hope our approach will increase the flexibility and applicability of the proposed definition for ALI.
“Histological evidence of tissue injury” has been considered as the most relevant defining feature of experimental ALI (1). However, the definition of ARDS is entirely clinical and does not include pathology (8). It has been repeatedly demonstrated that histological patterns other than diffuse alveolar damage can be seen in patients who otherwise meet the current Berlin definition of ARDS (30–32). Therefore, in the experimental setting, it may be important to avoid defining a single histological pattern as “diagnostic” or “required” to establish that ALI is present in an animal and instead focus on specific pathological features that are relevant for the hypothesis being tested in the study. For example, a study investigating whether deleting a particular gene impairs neutrophil recruitment may find it useful to focus on different assessments of the histological distribution of neutrophils in the lungs. In contrast, a study investigating a potentially novel therapeutic may want to emphasize the coexistence of several different histological measurements of lung injury (Table 2). Thus, an important difference in the present recommendations compared with the 2011 workshop is that the specific measurements of histological injury should be tailored to the specific scientific question being asked in each study. Members of the panel emphasized that histologic features of lung injury should be assessed in a rigorous manner that minimizes the potential for technical bias. We refer the reader to literature discussing important technical considerations (33).
Throughout the Delphi process, there was much discussion of the best way to assess histologic lung injury. A “validated histologic score” ranked highly among the list of features for assessing tissue injury despite the lack of a validated score that serves as a standard. This highlights the urgent need for an unbiased measurement schema in experimental ALI. Resolving this issue is beyond the scope of the current workshop, and we hope that the lung injury field works to develop a validated histologic score that can be broadly applied by ALI investigators. We acknowledge that histological scores may not be equally suitable for all ALI models, and some models may require specific histological criteria (34, 35). There is ongoing work in digital imaging and analysis to develop unbiased, automated computer-based scoring systems for lung injury, either involving computerized pixel-counting algorithms or the use of deep neural networks. Even if successful, these approaches require sophisticated computational and imaging-capture resources, which may be beyond the reach of most investigators. Simpler semiquantitative scores relying mostly on a microscope and basic image-analysis software are sufficient for most purposes.
There is broader recognition that the cellular composition of the inflammatory response may not be exclusively neutrophilic and instead differs in terms of the model system used and the phase of injury. This is exemplified by the inclusion of “increases in inflammatory monocyte and macrophage (and/or lymphocyte) subpopulations in BAL or lung tissue” as a relevant feature, likely reflecting the importance of these infiltrating cell types in models such as bacterial- or viral-induced lung injury as well as in sterile injury (36–40). The inclusion of “endothelial cell adhesion or activation markers” as a relevant feature of the inflammation domain is also new from the original workshop report and reflects a wider understanding of the activated endothelium as an important component of the inflammatory response (41, 42). However, the compartment in which endothelial cell adhesion or activation markers should be measured was not specified, as there was a recommendation that the report should provide general “good practice” guidelines for the field without being overly restrictive. In addition, we acknowledge that the presence of inflammatory features is not specific to ALI and may reflect the underlying insult that led to ALI rather than ALI itself (e.g., in pneumonia or sepsis).
Measurement of alterations in alveolar–capillary barrier permeability can be divided into two main groups: measures of increased extravascular lung water and measures of increased permeability of the alveolar– capillary barrier. These two main aspects have not changed substantially since the 2011 workshop report. Here, measurement of endogenous molecules or tagged tracers in the airspace or measurement of lung wet-to-dry weight ratios remain among the top features that fulfill this domain. One interesting new addition is the suggestion, made by many panelists, of measuring markers of lung epithelial injury (such as RAGE). Although none of these markers reached the >30% threshold and panelists did not feel that a measure of epithelial injury alone fulfills this domain, their inclusion reflects the growing appreciation of their potential to provide insight into the development of ALI and ARDS (43, 44). As our understanding of epithelial biology and specific subpopulations of lung epithelial cells continues to grow, future recommendations for modeling alveolar–capillary barrier dysfunction may become more nuanced.
Although many of the features from the 2011 workshop were still ranked as “most relevant” in our current report, two additional physiological features are now considered to demonstrate physiological dysfunction satisfactorily. First, impaired lung function, specifically reduced respiratory compliance or increased elastance, was considered one of the “most relevant” features to the domain by at least 30% of the respondents. A sizeable proportion (about one-fourth) of the respondents who suggested lung function measurements recommended the use of the forced oscillation technique rather than body plethysmography, in line with the reported higher sensitivity and specificity of the former technique in small rodent models of airway disease (45–47). Second, impaired alveolar fluid clearance is now included as a relevant parameter of physiological dysfunction, although it was noted that the measurement of alveolar fluid clearance by single or double indicator dilution techniques (48) is challenging in mice and rats and, as such, is not widely available. Overall, physiological measurements remain technically demanding, yet they provide the unique advantage that their absolute values can commonly be compared across studies, which would help comparisons across different laboratories or versus historical data.
Our revised ALI framework has several advantages but also several caveats. First, an important consideration in interpreting these measurements is that although we used the term “relevant” to denote those measurements that reached the 30% cutoff, this does not imply the other measurements are irrelevant for measuring ALI in experimental models. Thus, our usage of the term “relevant” refers to the development of a minimum set of standard criteria of ALI agreed on by the surveyed panelists that should be feasible to all investigators and reported in studies of ALI. Second, because fulfilling each ALI domain requires only one “relevant measure,” a model could potentially fulfill our proposed definition of ALI but not be viewed as a “model of ALI” by some investigators. For example, a model demonstrating a high respiratory rate, high circulating levels of vWF, and thickened alveolar septae on histologic analysis would fulfill the definition of ALI but might not be considered as “ALI” by certain experts. Another potential controversy is that our proposed framework allows for the possibility that three of the four ALI domains be met without demonstrating either histological evidence of injury or increased permeability to protein. Some panelists considered that histological evidence of tissue injury should always be demonstrated to document “lung injury.” However, we deliberately created the current framework to be as flexible as possible, and we feel that flexibility in this regard is more important than a firm insistence on specific measures needed to fulfill ALI criteria. Interpreting the relevance of a particular ALI model will be up to the reader.
We also acknowledge that the domain concept has limitations. For example, not all measurements fit clearly within only one domain. Lung imaging, such as by computed tomography, was suggested as a measurement in all four domains. Because most respondents included it in the physiology domain, after extensive discussion, we elected to include imaging in that domain, but we recognize that this choice was arbitrary. There were additional controversies regarding lung imaging in experimental ALI. Although lung imaging did not reach the 30% cutoff required to be considered a “relevant measurement,” some panelists pointed out that it is of very high value in large animal models. We speculate that the failure of lung imaging to make the cutoff may be due to its lower applicability to small animal models, in which the bulk of ALI research is presently performed. We suspect that the relevance of lung imaging will increase and perhaps become an ALI domain of its own as the availability and resolution of imaging techniques such as micro–computed tomography, small animal magnetic resonance imaging, small animal lung ultrasound, and other methodologies increases (49).
Conclusions
In summary, this Workshop Report revises and updates the previous 2011 report by emphasizing that models of experimental ALI span a continuum ranging from those used in highly focused mechanistic studies on one end of the spectrum to those used in preclinical testing of novel therapeutics or promising interventions on the other end. Accordingly, the highly mechanistic studies concerned with only one domain of lung injury may focus on multiple measurements of that single domain. However, for a model to qualify as “experimental ALI,” it must demonstrate evidence of alterations in at least one relevant measure from at least three of the four ALI domains and thus reflect the multidimensional aspect of human ARDS. For preclinical testing of novel therapeutics or interventions, fulfillment of all four domains is recommended. This continuum framework increases the flexibility and applicability of the definition, simultaneously inviting innovation and model development while increasing rigor for preclinical studies, and hopefully increases the translational potential. In addition, this revised workshop retains a time criterion as a part of the definition of experimental ALI but emphasizes that the specific time required to define “acute” is model dependent. Although the four-domain framework has proven useful for defining experimental ALI, this framework will need to be reevaluated in future workshops as new technologies become available and concepts within the field evolve.
Acknowledgments
This official workshop report was prepared by an ad hoc subcommittee of the ATS Assembly on Allergy, Immunology, and Inflammation.
Members of the subcommittee are as follows:
Hrishikesh S. Kulkarni, M.D., M.S.C.I. (Co-Chair)1
Janet S. Lee, M.D. (Co-Chair)2,3*
Gregory P. Downey, M.D. (Co-Chair)4
Gustavo Matute-Bello, M.D. (Co-Chair)5,6,7*
Guillermo M. Albaiceta, M.D., Ph.D.8,9,10
William A. Altemeier, M.D.5,6
Antonio Artigas, M.D., Ph.D.11
Julie A. Bastarache, M.D.12*
Jason H. T. Bates, Ph.D.13
Carolyn S. Calfee, M.D., M.A.S.14,15
Charles S. Dela Cruz, M.D., Ph.D.16
Robert P. Dickson, M.D. 17
Joshua A. Englert, M.D. 18
Jeffrey I. Everitt, D.V.M. 19
Michael B. Fessler, M.D. 20
Andrew E. Gelman, Ph.D. 21
Kymberly M. Gowdy, Ph.D. 18
Steve D. Groshong, M.D., Ph.D. 22
Susanne Herold, M.D., Ph.D.23,24,25
Robert J. Homer, M.D., Ph.D. 26,27
Jeffrey C. Horowitz, M.D. 18
Connie C. W. Hsia, M.D. 28
Wolfgang M. Kuebler, M.D.29,30*
Kiyoyasu Kurahashi, M.D., Ph.D.31
Victor E. Laubach, Ph.D. 32
Mark R. Looney, M.D. 14,33
Rudolf Lucas, Ph.D.34,35
Nilam S. Mangalmurti, M.D. 36
Anne M. Manicone, M.D. 5,6
Thomas R. Martin, M.D. 6
Sadis Matalon, Ph.D.37
Michael A. Matthay, M.D. 14,15,38
Daniel F. Mc Auley, M.B.B.Ch. 39
Sharon A. Mc Grath- Morrow, M.B.A., M.D. 40,41
Joseph P. Mizgerd, Sc.D. 42
Stephanie A. Montgomery, D.V.M., Ph.D. 43
Bethany B. Moore, Ph.D. 17,44
Alexandra Noël, Ph.D.45
Carrie E. Perlman, Ph.D. 46
John P. Reilly, M.D., M.S.C.E. 36
Eric P. Schmidt, M.D. 47,48
Shawn J. Skerrett, M.D. 6
Tomeka L. Suber, M.D., Ph.D. 2,3
Charlotte Summers, B.M., Ph.D.49
Benjamin T. Suratt, M.D. 50
Masao Takata, M.D., Ph.D.51
Rubin Tuder, Ph.D.47
Stefan Uhlig, Ph.D.52,53
Martin Witzenrath, M.D.30,54,55
Rachel L. Zemans, M.D. 17,56
*Domain Leads.
1Division of Pulmonary and Critical Care Medicine, Department of Medicine and 21Department of Surgery, Washington University School of Medicine, St. Louis, Missouri; 2Acute Lung Injury Center of Excellence and 3Division of Pulmonary, Allergy and Critical Care Medicine, University of Pittsburgh, Pittsburgh, Pennsylvania; 4Division of Pulmonary, Critical Care and Sleep Medicine, Department of Medicine, and 22Division of Pathology, National Jewish Health, Denver, Colorado; 5Center for Lung Biology and 6Division of Pulmonary, Critical Care and Sleep Medicine, Department of Medicine, University of Washington, Seattle, Washington; 7Department of Veterans Affairs, VA Puget Sound Health Care System, Seattle, Washington; 8Centro de Investigación Biomédica en Red-Enfermedades Respiratorias, Madrid, Spain; 9Instituto de Investigación Sanitaria del Principado de Asturias, Hospital Universitario Central de Asturias, Oviedo, Spain; 10Instituto Universitario de Oncología del Principado de Asturias, Universidad de Oviedo, Oviedo, Spain; 11Corporacion Universitaria Parc Tauli, CIBER Enfermedades Respiratorias, Autonomous University of Barcelona, Sabadell, Spain; 12Division of Pulmonary, Critical Care and Sleep Medicine, Department of Medicine, Vanderbilt University Medical Center, Nashville, Tennessee; 13Department of Medicine, Larner College of Medicine, University of Vermont, Burlington, Vermont; 14Department of Medicine, 15Department of Anesthesia, 33Department of Laboratory Medicine, and 38Cardiovascular Research Institute, University of California, San Francisco, San Francisco, California; 16Section of Pulmonary, Critical Care and Sleep Medicine, Department of Internal Medicine, and 26Department of Pathology, Yale School of Medicine, New Haven, Connecticut; 17Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, and 56Cellular and Molecular Biology Program, Rackham School of Graduate Studies, University of Michigan, Ann Arbor, Michigan; 18Division of Pulmonary, Critical Care and Sleep Medicine, Department of Internal Medicine and The Dorothy M. Davis Heart and Lung Research Institute, The Ohio State University Wexner Medical Center, Columbus, Ohio; 19Department of Pathology, Duke University School of Medicine, Durham, North Carolina; 20Immunity, Inflammation and Disease Laboratory, National Institute of Environmental Health Sciences, NIH, Research Triangle Park, North Carolina; 23Department of Pulmonary and Critical Care Medicine and 24Infectious Diseases, University of Giessen Lung Center, Justus Liebig University, Giessen, Germany; 25German Center for Lung Research (DZL), Giessen, Germany; 27Laboratory Medicine Service, VA Connecticut HealthCare System, New Haven, Connecticut; 28Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, University of Texas Southwestern Medical Center, Dallas, Texas; 29Institute of Physiology, 54Department of Infectious Diseases and Respiratory Medicine, and 55Division of Pulmonary Inflammation, Charité–University Medicine Berlin, Berlin, Germany; 30German Center for Lung Research (DZL), Berlin, Germany; 31Department of Anesthesiology and Intensive Care Medicine, School of Medicine, International University of Health and Welfare, Chiba, Japan; 32Department of Surgery, University of Virginia School of Medicine, Charlottesville, Virginia; 34Vascular Biology Center, Department of Pharmacology and Toxicology, and 35Division of Pulmonary, Critical Care and Sleep Medicine, Department of Medicine, Medical College of Georgia at Augusta University, Augusta, Georgia; 36Division of Pulmonary, Allergy, and Critical Care, Department of Medicine, and 41Department of Pediatrics, Perelman School of Medicine, University of Pennsylvania, Philadelphia, Pennsylvania; 37Department of Anesthesiology and Perioperative Medicine, University of Alabama at Birmingham, Birmingham, Alabama; 39Wellcome-Wolfson Institute for Experimental Medicine, Queen’s University of Belfast, Belfast, United Kingdom; 40Division of Pulmonary and Sleep Medicine, Children’s Hospital of Philadelphia, Philadelphia, Pennsylvania; 42Pulmonary Center, Boston University School of Medicine, Boston, Massachusetts; 43Department of Pathology and Laboratory Medicine, University of North Carolina at Chapel Hill School of Medicine, Chapel Hill, North Carolina; 44Department of Microbiology and Immunology, University of Michigan Medical School, Ann Arbor, Michigan; 45Department of Comparative Biomedical Sciences, School of Veterinary Medicine, Louisiana State University, Baton Rouge, Louisiana; 46Department of Biomedical Engineering, Stevens Institute of Technology, Hoboken, New Jersey; 47Department of Medicine, University of Colorado Denver Anschutz Medical Campus, Aurora, Colorado; 48Denver Health Medical Center, Denver, Colorado; 49Department of Medicine, University of Cambridge, Cambridge, United Kingdom; 50Early Drug Development, Immunology and Inflammation, Sanofi, Boston, Massachusetts; 51Division of Anesthetics, Pain Medicine and Intensive Care, Imperial College London, London, United Kingdom; 52Faculty of Medicine, RWTH Aachen University, Aachen, Germany; and 53University Hospital Aachen, Aachen, Germany.
Acknowledgment
The authors thank Michelle Price for assistance with the creation of Figure 1. The also thank Dr. Kevin Wilson and the ATS staff, Kimberly Lawrence, John Harmon, and Miriam Rodriguez, for assistance throughout the process.
Footnotes
This official Workshop Report of the American Thoracic Society was approved December 2021
An Executive Summary of this document is available at http://www.atsjournals.org/doi/suppl/10.1165/rcmb.2021-0531ST.
This article has a data supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Author Disclosures: J.S.L. served as a consultant for Janssen; and received research support from the National Institutes of Health. G.P.D. served as a consultant for Angion Biomedica, Altimmune, LAM Foundation, and Lovelace Respiratory Institute; and received research support from the National Institutes of Health. G.M.-B. served as a consultant for Jensen. G.M.A. received research support from Instituto de Salud Carlos III; and has intellectual property/patent unsold for Pcsk9 inhibitors for specific uses with the Instituto de Investigación Sanitaria del Principado de Asturias. A.A. served on an advisory committee for Aerogen and Grifols; and received research support from Fisher & Paykel, Grifols, and Instituto de Salud Carlos III. J.H.T.B. served on an advisory committee for the Parker B. Francis Foundation; served as a consultant for Healthy Design LLC, Johnson & Johnson, and Oscillavent LLC; had ownership or investment interest in Oscillavent LLC; and received research support from the National Heart, Lung, and Blood Institute. C.S.C. served on an advisory committee for Quark Pharmaceuticals; served as a consultant for Bayer, Gen1e Life Sciences, Roche/Genentech, and Vasomune; and received research support from Bayer, National Institutes of Health, Roche/Genentech, and Quantum Leap Healthcare Collaborative. S.D.G. served as a consultant for Veracyte. J.C.H. served on an advisory committee for United Therapeutics. C.C.W.H. served as a consultant for Allena, Allynum, Applied Therapeutics, Dicerna, Oscillavent LLC, and Tricida; received research support from ATyr Pharmaceutical, Mallinckrodt, and the National Heart, Lung, and Blood Institute; had ownership or investment interest in Oscillavent LLC; and has a patent pending for device and method for lung measurement (US 20160007882 A1), variable ventilation as a diagnostic tool for assessing lung mechanical function (WO2015127377 A1), and ventilator and ventilator valve (PCT/US21/24537). R.L. received research support from the National Institutes of Health; and has intellectual property/patent unsold with Apeptico. T.R.M. served as a consultant for Bill & Melinda Gates Foundation, Boehringer Ingelheim, Novartis, and Vespina. S.M. served as editor in chief for American Physiological Society’s Physiological Reviews; and received research support from IMSA. M.A.M. served as a consultant for Citius, Gilead, Johnson & Johnson, and Novartis; and received research support from Roche/Genentech. D.F.M. served as a consultant for Bayer, Boehringer Ingelheim, Eli Lilly, GlaxoSmithKline, Novartis, and SOBI; and received research support from Faron Pharmaceuticals, Innovate UK, Medical Research Council, National Institute for Health Research, Northern Ireland HSC R&D Division, Randox, Vir Biotechnology, and Wellcome Trust. C.S. received research support from AstraZeneca/Medimmune and GlaxoSmithKline; and had ownership or investment interest in Exvastat Limited. B.T.S. is an employee of Sanofi-Genzyme; and received research support from the National Institutes of Health. M.T. received research support from NeRRe Therapeutics Ltd. R.L.Z. received research support from the National Institutes of Health. H.S.K., W.A.A., J.A.B., C.S.D.C., R.P.D., J.A.E., J.I.E., M.B.F., A.E.G., K.M.G., S.H., R.J.H., W.M.K., K.K., V.E.L., M.R.L., N.S.M., A.M.M., S.A.M.-M., J.P.M., S.A.M., B.B.M., A.N., C.E.P., J.P.R., E.P.S., S.J.S., T.L.S., R.T., S.U., and M.W. reported no commercial or relevant noncommercial interests.
References
- 1. Matute-Bello G, Downey G, Moore BB, Groshong SD, Matthay MA, Slutsky AS, et al. Acute Lung Injury in Animals Study Group An official American Thoracic Society workshop report: features and measurements of experimental acute lung injury in animals. Am J Respir Cell Mol Biol . 2011;44:725–738. doi: 10.1165/rcmb.2009-0210ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Laffey JG, Kavanagh BP. Fifty years of research in ARDS. Insight into acute respiratory distress syndrome. From models to patients. Am J Respir Crit Care Med . 2017;196:18–28. doi: 10.1164/rccm.201612-2415CI. [DOI] [PubMed] [Google Scholar]
- 3. Xin Y, Cereda M, Hamedani H, Pourfathi M, Siddiqui S, Meeder N, et al. Unstable inflation causing injury. Insight from prone position and paired computed tomography scans. Am J Respir Crit Care Med . 2018;198:197–207. doi: 10.1164/rccm.201708-1728OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ross JT, Nesseler N, Lee J-W, Ware LB, Matthay MA. The ex vivo human lung: research value for translational science. JCI Insight . 2019;4:e128833. doi: 10.1172/jci.insight.128833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Matthay MA, Arabi YM, Siegel ER, Ware LB, Bos LDJ, Sinha P, et al. Phenotypes and personalized medicine in the acute respiratory distress syndrome. Intensive Care Med . 2020;46:2136–2152. doi: 10.1007/s00134-020-06296-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Grant RA, Morales-Nebreda L, Markov NS, Swaminathan S, Querrey M, Guzman ER, et al. NU SCRIPT Study Investigators Circuits between infected macrophages and T cells in SARS-CoV-2 pneumonia. Nature . 2021;590:635–641. doi: 10.1038/s41586-020-03148-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ashbaugh DG, Bigelow DB, Petty TL, Levine BE. Acute respiratory distress in adults Lancet 1967. 2 319 323 4143721 [Google Scholar]
- 8. ARDS Definition Task Force. Ranieri VM. Rubenfeld GD. Thompson BT. Ferguson ND. Caldwell E. Fan E. et al. Acute respiratory distress syndrome: the Berlin definition. JAMA . 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 9. Semler MW, Bernard GR, Aaron SD, Angus DC, Biros MH, Brower RG, et al. Identifying clinical research priorities in adult pulmonary and critical care: NHLBI Working Group report. Am J Respir Crit Care Med . 2020;202:511–523. doi: 10.1164/rccm.201908-1595WS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Webb HH, Tierney DF. Experimental pulmonary edema due to intermittent positive pressure ventilation with high inflation pressures. Protection by positive end-expiratory pressure. Am Rev Respir Dis . 1974;110:556–565. doi: 10.1164/arrd.1974.110.5.556. [DOI] [PubMed] [Google Scholar]
- 11. Dreyfuss D, Basset G, Soler P, Saumon G. Intermittent positive-pressure hyperventilation with high inflation pressures produces pulmonary microvascular injury in rats. Am Rev Respir Dis . 1985;132:880–884. doi: 10.1164/arrd.1985.132.4.880. [DOI] [PubMed] [Google Scholar]
- 12. Kolobow T, Moretti MP, Fumagalli R, Mascheroni D, Prato P, Chen V, et al. Severe impairment in lung function induced by high peak airway pressure during mechanical ventilation. An experimental study. Am Rev Respir Dis . 1987;135:312–315. doi: 10.1164/arrd.1987.135.2.312. [DOI] [PubMed] [Google Scholar]
- 13. Tsuno K, Miura K, Takeya M, Kolobow T, Morioka T. Histopathologic pulmonary changes from mechanical ventilation at high peak airway pressures. Am Rev Respir Dis . 1991;143:1115–1120. doi: 10.1164/ajrccm/143.5_Pt_1.1115. [DOI] [PubMed] [Google Scholar]
- 14. Tremblay L, Valenza F, Ribeiro SP, Li J, Slutsky AS. Injurious ventilatory strategies increase cytokines and c-fos m-RNA expression in an isolated rat lung model. J Clin Invest . 1997;99:944–952. doi: 10.1172/JCI119259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Brower RG, Matthay MA, Morris A, Schoenfeld D, Thompson BT, Wheeler A, Acute Respiratory Distress Syndrome Network Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med . 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 16. Albert RK, Leasa D, Sanderson M, Robertson HT, Hlastala MP. The prone position improves arterial oxygenation and reduces shunt in oleic-acid-induced acute lung injury. Am Rev Respir Dis . 1987;135:628–633. doi: 10.1164/arrd.1987.135.3.628. [DOI] [PubMed] [Google Scholar]
- 17. Kreye J, Reincke SM, Kornau HC, Sánchez-Sendin E, Corman VM, Liu H, et al. A therapeutic non-self-reactive SARS-CoV-2 antibody protects from lung pathology in a COVID-19 hamster model. Cell . 2020;183:1058–1069.e19. doi: 10.1016/j.cell.2020.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Krenn K, Lucas R, Croizé A, Boehme S, Klein KU, Hermann R, et al. Inhaled AP301 for treatment of pulmonary edema in mechanically ventilated patients with acute respiratory distress syndrome: a phase IIa randomized placebo-controlled trial. Crit Care . 2017;21:194. doi: 10.1186/s13054-017-1795-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Brown LM, Liu KD, Matthay MA. Measurement of extravascular lung water using the single indicator method in patients: research and potential clinical value. Am J Physiol Lung Cell Mol Physiol . 2009;297:L547–L558. doi: 10.1152/ajplung.00127.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bachofen M, Weibel ER. Structural alterations of lung parenchyma in the adult respiratory distress syndrome. Clin Chest Med . 1982;3:35–56. [PubMed] [Google Scholar]
- 21. Walker DC, Behzad AR, Chu F. Neutrophil migration through preexisting holes in the basal laminae of alveolar capillaries and epithelium during streptococcal pneumonia. Microvasc Res . 1995;50:397–416. doi: 10.1006/mvre.1995.1067. [DOI] [PubMed] [Google Scholar]
- 22. Burns AR, Smith CW, Walker DC. Unique structural features that influence neutrophil emigration into the lung. Physiol Rev . 2003;83:309–336. doi: 10.1152/physrev.00023.2002. [DOI] [PubMed] [Google Scholar]
- 23. Zhao Y, Olonisakin TF, Xiong Z, Hulver M, Sayeed S, Yu MT, et al. Thrombospondin-1 restrains neutrophil granule serine protease function and regulates the innate immune response during Klebsiella pneumoniae infection. Mucosal Immunol . 2015;8:896–905. doi: 10.1038/mi.2014.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Qu Y, Olonisakin T, Bain W, Zupetic J, Brown R, Hulver M, et al. Thrombospondin-1 protects against pathogen-induced lung injury by limiting extracellular matrix proteolysis. JCI Insight . 2018;3:e96914. doi: 10.1172/jci.insight.96914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Matute-Bello G, Frevert CW, Martin TR. Animal models of acute lung injury. Am J Physiol Lung Cell Mol Physiol . 2008;295:L379–L399. doi: 10.1152/ajplung.00010.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lama VN, Belperio JA, Christie JD, El-Chemaly S, Fishbein MC, Gelman AE, et al. Models of lung transplant research: a consensus statement from the National Heart, Lung, and Blood Institute workshop. JCI Insight . 2017;2:e93121. doi: 10.1172/jci.insight.93121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pulido L, Burgos D, García Morato J, Luna CM. Does animal model on ventilator-associated pneumonia reflect physiopathology of sepsis mechanisms in humans? Ann Transl Med . 2017;5:452. doi: 10.21037/atm.2017.11.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Domscheit H, Hegeman MA, Carvalho N, Spieth PM. Molecular dynamics of lipopolysaccharide-induced lung injury in rodents. Front Physiol . 2020;11:36. doi: 10.3389/fphys.2020.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Amarelle L, Quintela L, Hurtado J, Malacrida L. Hyperoxia and lungs: what we have learned from animal models. Front Med (Lausanne) . 2021;8:606678. doi: 10.3389/fmed.2021.606678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Thille AW, Esteban A, Fernández-Segoviano P, Rodriguez J-M, Aramburu J-A, Peñuelas O, et al. Comparison of the Berlin definition for acute respiratory distress syndrome with autopsy. Am J Respir Crit Care Med . 2013;187:761–767. doi: 10.1164/rccm.201211-1981OC. [DOI] [PubMed] [Google Scholar]
- 31. Lorente JA, Cardinal-Fernández P, Muñoz D, Frutos-Vivar F, Thille AW, Jaramillo C, et al. Acute respiratory distress syndrome in patients with and without diffuse alveolar damage: an autopsy study. Intensive Care Med . 2015;41:1921–1930. doi: 10.1007/s00134-015-4046-0. [DOI] [PubMed] [Google Scholar]
- 32. Cardinal-Fernández P, Lorente JA, Ballén-Barragán A, Matute-Bello G. Acute respiratory distress syndrome and diffuse alveolar damage. New insights on a complex relationship. Ann Am Thorac Soc . 2017;14:844–850. doi: 10.1513/AnnalsATS.201609-728PS. [DOI] [PubMed] [Google Scholar]
- 33. Hsia CCW, Hyde DM, Ochs M, Weibel ER, ATS/ERS Joint Task Force on Quantitative Assessment of Lung Structure An official research policy statement of the American Thoracic Society/European Respiratory Society: standards for quantitative assessment of lung structure. Am J Respir Crit Care Med . 2010;181:394–418. doi: 10.1164/rccm.200809-1522ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Patel KJ, Cheng Q, Stephenson S, Allen DP, Li C, Kilkenny J, et al. Emphysema-associated autoreactive antibodies exacerbate post-lung transplant ischemia-reperfusion injury. Am J Respir Cell Mol Biol . 2019;60:678–686. doi: 10.1165/rcmb.2018-0224OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gruber AD, Osterrieder N, Bertzbach LD, Vladimirova D, Greuel S, Ihlow J, et al. Standardization of reporting criteria for lung pathology in SARS-CoV-2-infected hamsters: what matters? Am J Respir Cell Mol Biol . 2020;63:856–859. doi: 10.1165/rcmb.2020-0280LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Herold S, Steinmueller M, von Wulffen W, Cakarova L, Pinto R, Pleschka S, et al. Lung epithelial apoptosis in influenza virus pneumonia: the role of macrophage-expressed TNF-related apoptosis-inducing ligand. J Exp Med . 2008;205:3065–3077. doi: 10.1084/jem.20080201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. D’Alessio FR, Tsushima K, Aggarwal NR, West EE, Willett MH, Britos MF, et al. CD4+CD25+Foxp3+ Tregs resolve experimental lung injury in mice and are present in humans with acute lung injury. J Clin Invest . 2009;119:2898–2913. doi: 10.1172/JCI36498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sun J, Madan R, Karp CL, Braciale TJ. Effector T cells control lung inflammation during acute influenza virus infection by producing IL-10. Nat Med . 2009;15:277–284. doi: 10.1038/nm.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Herold S, Tabar TS, Janssen H, Hoegner K, Cabanski M, Lewe-Schlosser P, et al. Exudate macrophages attenuate lung injury by the release of IL-1 receptor antagonist in gram-negative pneumonia. Am J Respir Crit Care Med . 2011;183:1380–1390. doi: 10.1164/rccm.201009-1431OC. [DOI] [PubMed] [Google Scholar]
- 40. Cardani A, Boulton A, Kim TS, Braciale TJ. Alveolar macrophages prevent lethal influenza pneumonia by inhibiting infection of type-1 alveolar epithelial cells. PLoS Pathog . 2017;13:e1006140. doi: 10.1371/journal.ppat.1006140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Andonegui G, Zhou H, Bullard D, Kelly MM, Mullaly SC, McDonald B, et al. Mice that exclusively express TLR4 on endothelial cells can efficiently clear a lethal systemic Gram-negative bacterial infection. J Clin Invest . 2009;119:1921–1930. doi: 10.1172/JCI36411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Teijaro JR, Walsh KB, Cahalan S, Fremgen DM, Roberts E, Scott F, et al. Endothelial cells are central orchestrators of cytokine amplification during influenza virus infection. Cell . 2011;146:980–991. doi: 10.1016/j.cell.2011.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Basil MC, Katzen J, Engler AE, Guo M, Herriges MJ, Kathiriya JJ, et al. The cellular and physiological basis for lung repair and regeneration: past, present, and future. Cell Stem Cell . 2020;26:482–502. doi: 10.1016/j.stem.2020.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hewitt RJ, Lloyd CM. Regulation of immune responses by the airway epithelial cell landscape. Nat Rev Immunol . 2021;21:347–362. doi: 10.1038/s41577-020-00477-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Vanoirbeek JAJ, Rinaldi M, De Vooght V, Haenen S, Bobic S, Gayan-Ramirez G, et al. Noninvasive and invasive pulmonary function in mouse models of obstructive and restrictive respiratory diseases. Am J Respir Cell Mol Biol . 2010;42:96–104. doi: 10.1165/rcmb.2008-0487OC. [DOI] [PubMed] [Google Scholar]
- 46. Berndt A, Leme AS, Williams LK, Von Smith R, Savage HS, Stearns TM, et al. Comparison of unrestrained plethysmography and forced oscillation for identifying genetic variability of airway responsiveness in inbred mice. Physiol Genomics . 2011;43:1–11. doi: 10.1152/physiolgenomics.00108.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bates JHT. CORP: measurement of lung function in small animals. J Appl Physiol (1985) . 2017;123:1039–1046. doi: 10.1152/japplphysiol.00243.2017. [DOI] [PubMed] [Google Scholar]
- 48. Weidenfeld S, Chupin C, Langner DI, Zetoun T, Rozowsky S, Kuebler WM. Sodium-coupled neutral amino acid transporter SNAT2 counteracts cardiogenic pulmonary edema by driving alveolar fluid clearance. Am J Physiol Lung Cell Mol Physiol . 2021;320:L486–L497. doi: 10.1152/ajplung.00461.2020. [DOI] [PubMed] [Google Scholar]
- 49. Grune J, Beyhoff N, Hegemann N, Lauryn JH, Kuebler WM. From bedside to bench: lung ultrasound for the assessment of pulmonary edema in animal models. Cell Tissue Res . 2020;380:379–392. doi: 10.1007/s00441-020-03172-2. [DOI] [PMC free article] [PubMed] [Google Scholar]