To the Editor:
Distal airways appear to be involved in idiopathic pulmonary fibrosis (IPF) pathogenesis, even in regions of minimal fibrosis (1,2). Our previous work has demonstrated that distal airway epithelial cultures from patients with IPF recapitulate in vivo distal airways and exhibit significant biophysical and structural defects in vitro (3). We have shown that IPF epithelia display a phenotypical delay in the jamming transition, which is characterized as a transition within an epithelium from a fluid or migratory (unjammed) to a solid or nonmigratory (jammed) phase (3–6). Our previous work demonstrated that control distal airway epithelia undergo the jamming transition around Day 8 of air–liquid interface (ALI) culture. However, IPF distal airway epithelia persist in an unjammed phase past Day 14 of culture and persisted until approximately Day 28 of ALI (3). We identified several pathways differentially regulated in the unjammed phase by comparing bulk RNA-sequencing data from distal airway epithelia from control donors (N = 3) and those with IPF (N = 4) at ALI Day 4, Day 8, and Day 14. Genes downregulated across the jamming transition in control cells (between Days 4 and 8) demonstrated an enrichment for focal adhesions, cellular cytoskeleton, stress fiber formation, and endocytosis consistent with a shift from a migratory to nonmigratory state. This gene profile was conserved when comparing control (jammed) and IPF (unjammed) distal epithelia at Days 8 and 14 of ALI. Interestingly, pathway analysis of these genes demonstrated an enrichment for integrin, ERBB, and focal adhesion kinase (FAK) signaling.
Integrin signaling dynamically interacts with all of the enriched pathways and is critical for epithelial integrity, stemness, and migration (7, 8). A central downstream regulator of integrin-dependent epithelial migration is activation of SRC family kinases (SFK) and FAK (8). Integrin activation signals to SFK and FAK to regulate cell migration (8). Achieving this highly dynamic state in an epithelial tissue requires intricate coordination of endocytosis for protein recycling and extracellular signal-regulated kinase (ERK) for continued gene transcription (8). This raised the question if inhibition of these targets could modulate IPF distal epithelial biophysics and gene signatures.
We obtained distal airway epithelial cells (airway internal diameter <2 mm) from control (N = 4) and IPF (N = 6) lungs that were not suitable for transplantation. Donors were matched on the basis of age, sex, smoking history, and MUC5B variant status. Distal epithelial cultures were expanded to passage 2 before experimentation. At passage 2, cells were seeded on a 24-Transwell plate coated with type I collagen and maintained in submerged conditions for 5–7 days before establishing ALI as previously reported (3).
Control and IPF distal airway epithelial cells were sent for bulk RNA sequencing on ALI Days 4, 8, and 14. Differential expression was assessed utilizing DESeq2 (Bioconductor) with pathway enrichment analysis performed with Enrichr. Benjamini-Hochberg false discovery rate-adjusted P values less than 0.05 were considered significant (9, 10). RNA-sequencing data have been deposited to the National Center for Biotechnology Information Gene Expression Omnibus and are accessed at GSE17600.
Experiments were performed as previously published (3). Briefly, time-lapse microscopy was utilized on ALI Day 14 for IPF distal airway epithelia. Cells were imaged in a stage incubator (37°C and 5% CO2) consecutively for 48 hours after inhibitor treatment. Root mean squared velocity (VRMS) and shape index were obtained through forward integration of velocity fields with particle image velocimetry and apical cell segmentation, respectively.
Transepithelial electrical resistance (TEER) was utilized as a proxy for barrier function and cell viability. TEER was compared with baseline values for each well prior to treatment and measured every 24 hours following treatment for up to 48 hours. Gene expression was assessed utilizing TaqMan assays with each point representing the average of a single donor Transwell run in triplicate.
To assess the signaling regulation of the biophysical dysfunction, we inhibited four processes throughout the integrin signaling axis: 1) integrin activation (β1), 2) clathrin-mediated endocytosis, 3) SFK signaling, and 4) ERK or FAK activation. Day 14 distal airway epithelia were transferred to a starvation media 24 hours before treatment. Media was then supplemented with a single inhibitor for 2–4 hours, followed by replacement of the starvation media. We found that brief exposure to inhibitors was sufficient to modify the biophysical state without significantly impairing barrier function of the epithelium, as was the case with extended inhibitor treatment (Figure 1A). Untreated IPF distal epithelia maintained a high VRMS, indicating that the epithelial layer is in the unjammed phase. However, upon inhibiting β1 integrin, endocytosis, SFK, ERK, or FAK signaling, the IPF epithelia rapidly entered the jammed, nonmigratory phase. The distal epithelia persisted in a jammed phase for longer than 48 hours of imaging (Figure 1A). The entrance into the jammed phase owing to integrin, endocytic, SFK, ERK, or FAK inhibition was also illustrated in a dynamic shift in epithelial cell shape (Figure 1B). The shape index (q) is a predictive jamming measurement of the perimeter-to-area ratio for the apical surface of the epithelium. Tissues with q > 3.81 are predicted to be in the unjammed phase, whereas tissues with q < 3.81 are predicted to be in the jammed phase. IPF epithelia at Day 14 of ALI have an average q well above this threshold. However, 48 hours after inhibition of β1 integrin, endocytosis, SFK, ERK, or FAK, the IPF epithelial q is less than 3.81, indicating the acquisition of a cobblestone-like epithelial structure and the jamming transition. Notably, entrance into the jammed state via signaling inhibition did not diminish epithelial integrity as measured by the TEER. Instead, barrier function continued to increase with treatment similar to that of the untreated control and IPF epithelial cells indicating the maintenance of the epithelium (Figure 1C).
Figure 1.
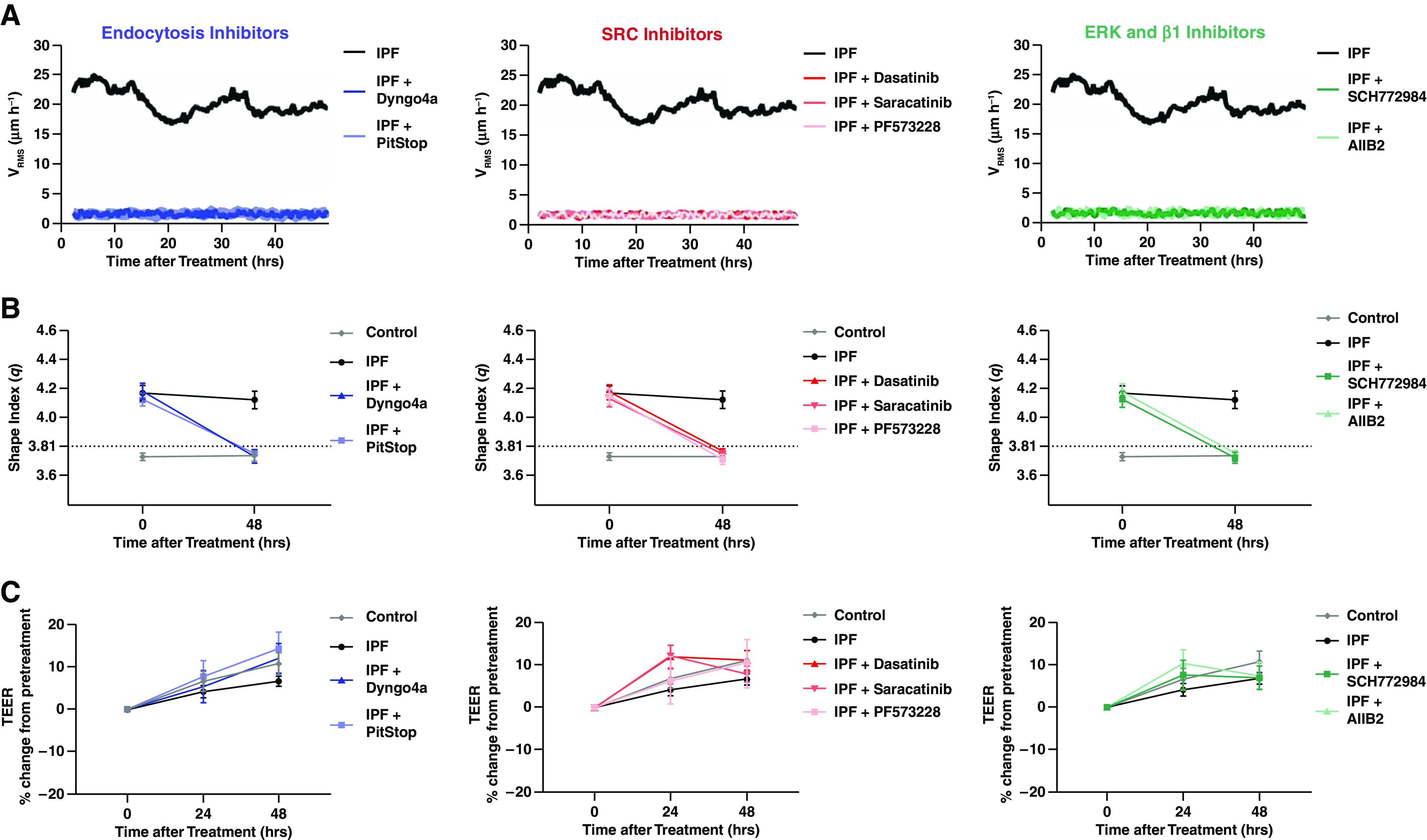
Inhibition of integrin signaling axis rescues biophysical dysfunction in idiopathic pulmonary fibrosis (IPF) distal airway epithelia. Control and IPF distal airway epithelial cells were treated with dimethylsulfoxide as a treatment carrier control. To inhibit the integrin-related signaling axis, IPF distal airway epithelial cells were treated at air–liquid interface Day 15 with endocytosis (Dyngo4a 1 μm and PitStop 10 μm), SRC family kinases (dasatinib 10 μm and saracatinib 1 μm), focal adhesion kinase (PF573228 5 μm), extracellular signal-regulated kinase (SCH772984 500 nM), or an Integrin β1 (AIIB2 20 μg/ml) inhibitor and imaged continuously for 48 hours. Inhibition demonstrated the entrance into the jammed phase by (A) root-mean-square velocity (VRMS) and (B) the perimeter-to-area cell shape ratio. Inhibitor treatment did not reduce barrier function (C) as measured by the transepithelial electrical resistance (TEER). Shown: mean ± 95% confidence interval for control (N = 4) and IPF (N = 6) with at least three technical replicates.
Next, we tested if inhibition of integrin, endocytosis, SFK, ERK, or FAK in IPF epithelia could rescue aberrant expression of integrin and extracellular matrix-related genes. We found that inhibition of IPF epithelia diminished aberrant integrin (ITGB1, ITGB4, and ITGB6) gene expression after 48 hours (Figure 2). Additionally, we observed that integrin β1, β4, and α6 laminin binding partners LAMA5, LAMB1, and LAMC1 (laminin-511) transcript expression decreased 48 hours after IPF epithelial inhibition (Figure 2).
Figure 2.
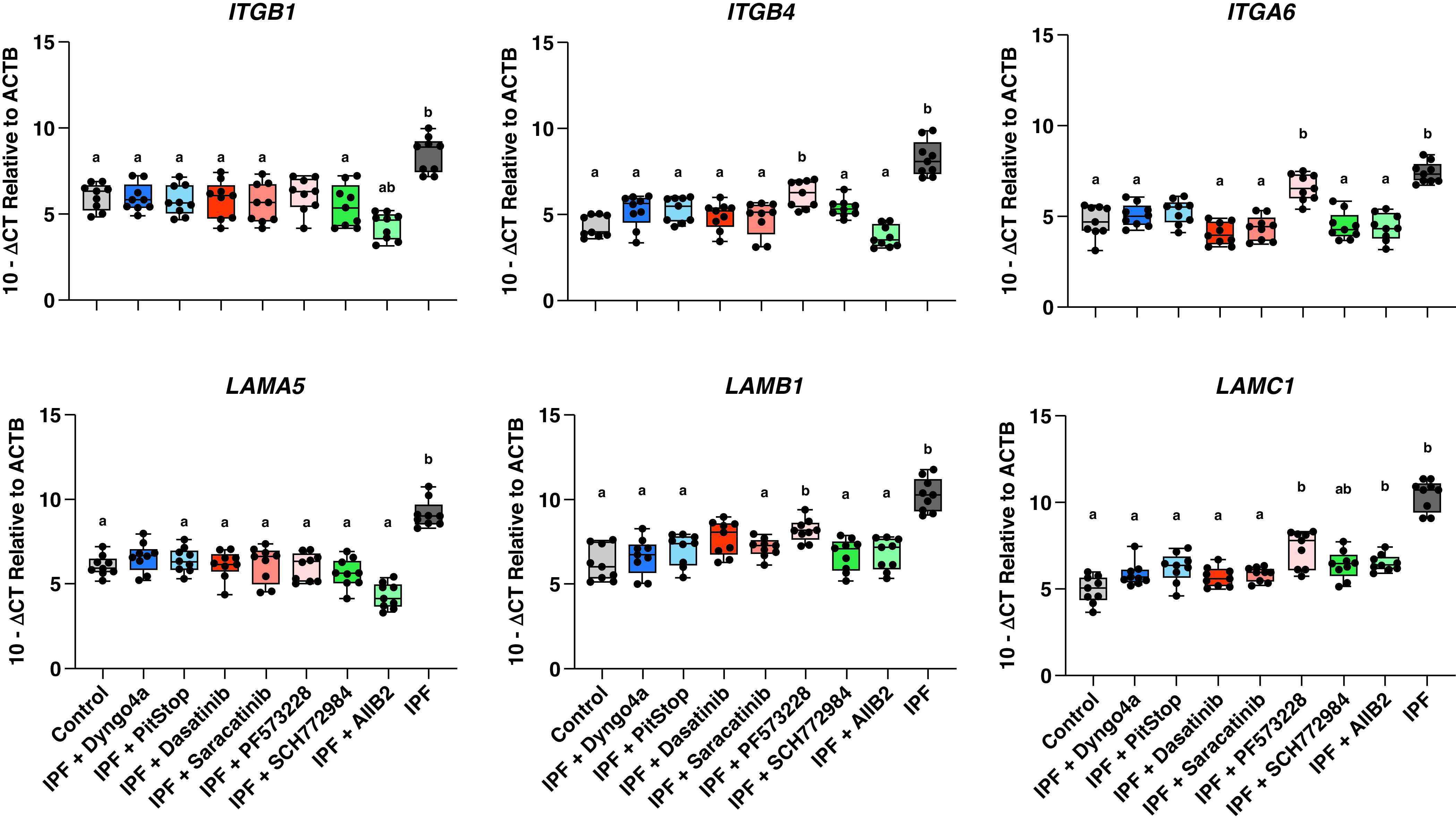
Inhibition of integrin signaling axis reduces gene expression of integrin-matrix binding partners. Control and IPF distal airway epithelial cells were treated with DMSO as a treatment carrier control. IPF distal airway epithelial cells were treated at air–liquid interface (ALI) Day 15 with endocytosis (Dyngo4a 1μm and PitStop 10 μm), SRC family kinases (dasatinib 10 μm and saracatinib 1 μm), focal adhesion kinase (PF573228 5 μm), extracellular signal-regulated kinase (SCH772984 500 nM), or an integrin β1 (AIIB2 20 μg/ml) inhibitor. Treatment of the cultures began on Day 15 of ALI, and RNA was collected on Day 17 of ALI. Inhibitor treatment of IPF distal airway epithelia reduced ITGB1, ITGB4, and ITGA6 gene expression across nearly all conditions back to control-like levels. Additionally, the gene expression of integrin-matrix binding partner laminin-511, composed of LAMA5, LAMB1, and LAMC1, was reduced after inhibitor treatment. Shown: mean ± 95% confidence interval for control (N = 3) and IPF (N = 3) with at least three technical replicates. A Welch and Brown-Forsythe one-way ANOVA was performed to identify statistical significance with a representing P < 0.05 compared with IPF untreated and b representing P < 0.05 compared with control untreated.
Our findings are twofold: 1) biophysical dysfunction in the distal airway can be rescued via modulation of integrin-directed signaling, and 2) more broadly that the unjammed phase of IPF epithelia can be regulated via integrin and integrin-related signaling pathways. The targeting of the airway epithelium, integrin, and integrin-related signaling represent a pathologically relevant axis for disease intervention. These data expand our knowledge of IPF airway epithelial biology, identifying novel molecular targets while establishing a role for altered airway epithelial matrix biology in IPF pathogenesis. Future studies are needed to understand the relevant matrix-integrin complexes in disease and migration as well as differential downstream targets to modulate disease phenotypes.
Footnotes
Supported by National Heart, Lung, and Blood Institute grants R01-HL097163, P01-HL092870, and UH3-HL123442; National Institute on Aging grant T32-AG000279; and U.S. Department of Defense grant W81XWH-17-1-0597.
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Verleden SE, Tanabe N, McDonough JE, Vasilescu DM, Xu F, Wuyts WA, et al. Small airways pathology in idiopathic pulmonary fibrosis: a retrospective cohort study. Lancet Respir Med. 2020;8:573–584. doi: 10.1016/S2213-2600(19)30356-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Seibold MA, Wise AL, Speer MC, Steele MP, Brown KK, Loyd JE, et al. A common MUC5B promoter polymorphism and pulmonary fibrosis. N Engl J Med . 2011;364:1503–1512. doi: 10.1056/NEJMoa1013660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Stancil IT, Michalski JE, Davis-Hall D, Chu HW, Park J-A, Magin CM, et al. Pulmonary fibrosis distal airway epithelia are dynamically and structurally dysfunctional. Nat Commun . 2021;12:4566. doi: 10.1038/s41467-021-24853-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Park JA, Kim JH, Bi D, Mitchel JA, Qazvini NT, Tantisira K, et al. Unjamming and cell shape in the asthmatic airway epithelium. Nat Mater . 2015;14:1040–1048. doi: 10.1038/nmat4357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mitchel JA, Das A, O’Sullivan MJ, Stancil IT, DeCamp SJ, Koehler S, et al. In primary airway epithelial cells, the unjamming transition is distinct from the epithelial-to-mesenchymal transition. Nat Commun . 2020;11:5053. doi: 10.1038/s41467-020-18841-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mongera A, Rowghanian P, Gustafson HJ, Shelton E, Kealhofer DA, Carn EK, et al. A fluid-to-solid jamming transition underlies vertebrate body axis elongation. Nature . 2018;561:401–405. doi: 10.1038/s41586-018-0479-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Reed NI, Jo H, Chen C, Tsujino K, Arnold TD, DeGrado WF, et al. The αvβ1 integrin plays a critical in vivo role in tissue fibrosis. Sci. Transl. Med. . 2015;7:288ra279. doi: 10.1126/scitranslmed.aaa5094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cooper J, Giancotti FG. Integrin signaling in cancer: mechanotransduction, stemness, epithelial plasticity, and therapeutic resistance. Cancer Cell . 2019;35:347–367. doi: 10.1016/j.ccell.2019.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen EY, Tan CM, Kou Y, Duan Q, Wang Z, Meirelles GV, et al. Enrichr: interactive and collaborative HTML5 gene list enrichment analysis tool. BMC Bioinformatics . 2013;14:128. doi: 10.1186/1471-2105-14-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kuleshov MV, Jones MR, Rouillard AD, Fernandez NF, Duan Q, Wang Z, et al. Enrichr: a comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res . 2016;44:W90-7. doi: 10.1093/nar/gkw377. [DOI] [PMC free article] [PubMed] [Google Scholar]


