Abstract
The incidence of hepatocellular carcinoma (HCC) is significantly lower in women than men, implying that estrogen receptors (ERs) may play an important role in this sex dimorphism. Recently, considerable progress has been made in expanding our understanding of the mechanisms of ERs in HCC. As one of the most important ERs, ERα functions as a tumor suppressor in the progression of HCC through various pathways, such as STAT3 signaling pathways, lipid metabolism-related signaling pathways, and non-coding RNAs. However, the function of ERα was reduced with the changes of some molecules in the liver, which may develop further into HCC and make it difficult to achieve an effective hormone treatment effect. Intriguingly, there are signs that individualized hormone therapy according to the activity of ERα will overcome this challenge. Based on these observations, it is particularly imperative to reassess and extend the function of ERα. In this review, we mainly elucidated molecular mechanisms associated with ERα in HCC and investigated the individualized hormone therapy based on these mechanisms, with the aim of providing new insights for HCC treatment.
Keywords: Hepatocellular carcinoma, Estrogen receptor, Molecular mechanism, Hormone therapy, Precision medicine
Graphical abstract
Introduction
Hepatocellular carcinoma (HCC) is the most common histological type of primary liver cancer, comprising 75–85% of cases.1 Viral infection, metabolic disorders and alcoholism are the major risk factors for HCC development.2 In addition, the disparity of incidence and prognosis in HCC suggests that sex is a key factor for HCC.3,4 Since it has been reported that oral contraceptives could increase the risk of hepatic neoplasms in the last century (1970s), the role of estrogen and estrogen receptors (ERs) in the progression of HCC has gradually been revealed. Subsequently, a large number of studies explored the therapeutic value of tamoxifen (TMX) or estrogen replacement therapy for HCC, triggering the debate on whether hormone therapy can be used for HCC.
TMX, a selective estrogen receptor modulator (SERM), is used as a first-line treatment for breast cancer through its ability to antagonize estrogen-dependent growth by binding ERs; yet, its efficiency for HCC remains controversial. Early clinical trials with small samples showed that TMX can lower the level of alpha-fetoprotein (AFP) and significantly prolong the survival of patients with inoperable HCC.5,6 However, this conclusion was doubted by the subsequent clinical trial which demonstrated that TMX has no efficiency in improving the survival of patients with advanced HCC.7 TMX treatment of HCC has since reached an impasse. Compared with TMX therapy, the effects of estrogen replacement therapy of HCC are relatively clear. It has been reported that estrogen replacement therapy was mainly used to treat female perimenopausal syndrome, and it has effects on advanced HCC to some extent, especially for certain specific populations.8 However, estrogen replacement therapy possesses certain side effects, and how to maximize the benefits from this kind of hormone therapy in HCC needs to be further explored.
In addition to the understanding of hormone therapy, the molecular mechanisms of ERs in HCC have been revealed frequently in recent years, with the mechanisms of ERα being the most extensively reported ones. In spite of few studies showing that ERα has tumor-promoting effects on HCC, the estrogen-mediated inhibition of HCC is widely recognized. ERα is considered to regulate inflammation, iron homoeostasis, energy metabolism and other processes to protect the liver.9–12 Moreover, a multitude of studies have shown that the expression of ERα in primary HCC tissues is decreased compared to normal liver tissues or the adjacent tissues, which indirectly confirmed the suppressive effects of ERα in HCC.13–15 In light of this anti-tumor effect of ERα in HCC, reactivating ERα signaling can offer a new therapeutic strategy in HCC prevention and treatment.
Up to now, the therapeutic value of targeting ERα in HCC has not reached a consensus. This review focuses on the regulatory mechanisms of ERα in HCC and seeks out the potential initiative of hormone therapy for HCC.
Structure and mechanism of ERα
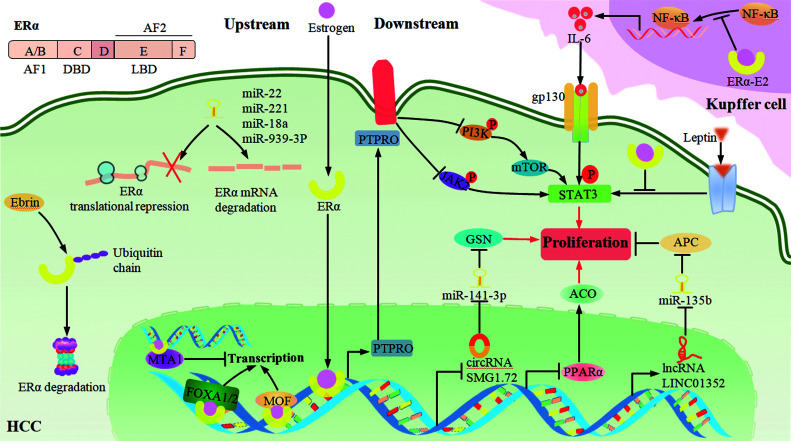
ERα is the first discovered type of ERs, consisting of 595 amino acid residues. On account of its molecular mass being 66 kDa, it is also called ERα66. ERα is encoded by the ESR1 gene and functionally divided into five parts, including the amino-terminal ligand-dependent transcription activation function 1 (AF-1) domain, DNA binding domain, a hinge region which participates in DNA binding and nuclear localization, ligand binding domain, and carboxyl-terminal ligand-dependent transcription activation function 2 (AF-2) domain (Fig. 1).
Fig. 1. Signaling pathways of ERα involved in HCC.
The human ERα (ERα66) gene consists of five functional domains (AF-1, DBD, Hinge, LBD, AF-2). Upstream pathways: MOF and FOXA1/2 enhance, while MTA1, miRNAs (miR-22, miR-221, miR-18a, miR-939-3p) and Erbin attenuate the effects of ERα in different stages. Downstream pathways: ERα promotes or inhibits a string of STAT3 signaling mediated by PTPRO, NF-κB, and leptin. In addition, ERα also inhibits circRNA-SMG1.72/miR-141-3p/GSN and PPARα/ACO, and promotes the LINC01352-miR-135b/APC signaling axis through direct genomic response. ACO, acyl-CoA oxidase; AF-1, amino-terminal ligand-dependent transcription activation function 1; AF-2, carboxyl-terminal ligand-dependent transcription activation function 2; APC, adenomatous polyposis coli; circRNAs, circular RNAs; DBD, DNA binding domain; ERα, estrogen receptor α; GSN, gelsolin; HCC, hepatocellular carcinoma; LBD, ligand binding domain; lncRNAs, long non-coding RNAs. MTA1, metastasis-associated 1; PPARα, peroxisome proliferator-activated receptor α; PTPRO, protein tyrosine phosphatase receptor type O.
ERα commonly refers to nuclear ERα which is recognized by estrogen at the ligand binding domain in cytoplasm. Mechanistically, ERα exposes its DNA binding region after dissociating from heat shock protein, and then directly acts on the estrogen response elements (EREs) to regulate the transcription of target genes or indirectly interact with some transcription factors, such as AP-1, SP1, NF-κB, in a protein-protein manner to regulate the transcription of their target genes.16 Different from nuclear ERα, membrane-localized ERα mainly triggers the protein-kinase cascades to enable gene regulation.17
Signaling pathways of ERα
Upstream signaling pathways
The activity of ERα is indispensable for its anti-tumor effects and hormone therapy of HCC. However, it has been reduced by the aberration of numerous upstream regulators, such as the weakening of MOF and FOXA1/2, and the enhancement of a series of putative cancer-related microRNAs (miRNAs), metastasis-associated protein 1 (MTA1) and Erbin. These changes together contribute to the low activity of ERα in HCC and the deficiency of hormone therapy (Fig. 1).
Positive regulators of ERα
MOF, which was found to have lower expression in HCC tissues, is identified as an liver protective factor.18 It stabilizes ERα by participating in the acetylation of ERα at K266, K268 and K299, hindering ERα ubiquitination and co-activating ERα target genes to augment its anti-tumor effects.19 FOXA1/2 are members of the forkhead box protein family, which bind to the relatively compact chromatin of the ERα target genes, and then enhance the interaction between ERα and its target genes.20 Furthermore, single nucleotide polymorphisms (SNPs) at FOXA2 binding sites and the FOXA1 nonsynonymous variant have frequently been observed in association with the development of HCC, which impairs their interaction with ERα and leads to the loss of ERα protective roles, especially in females.21,22
Negative regulators of ERα
Studies have shown that there are many putative upstream cancer-related miRNAs involved in regulating expression of ERα in HCC, such as miR-22, miR-221, miR-18a and miR-939-3p; these miRNAs have been demonstrated as overexpressed in HCC tissues and they can specifically recognize the ERα mRNA 3′-untranslated region and then trigger the degradation and the translation inhibition of ERα mRNA, which ultimately promotes the progress of HCC. It is worth mentioning that this interaction between miR-22 and ERα is more pronounced in males.23–26 MTA1 is a co-repressor of ERα, and there is the bidirectional regulation between ERα and MTA1. Not only can ERα decrease the expression of MTA1 to protect liver, but overexpressed MTA1 can reduce the transcriptional activity of ERα to promote the progress of HCC.27 In addition, Erbin is overexpressed in HCC; functionally, it inactivates ERα nuclear translocation and leads to the proteasomal degradation of ERα by increasing E3 ubiquitin ligase-mediated ubiquitination, which eventually contributes to the progression of HCC. Intriguingly, the interaction between Erbin and ERα is more pronounced in males, and downregulating Erbin expression enhances the sensitivity of HCC cells to TMX.28
Downstream signaling pathways
Clinical trials about ERα have been reported sporadically over the last decade, while some basic research about mechanisms of ERα in HCC had been reported successively (Fig. 1). Elucidating these mechanisms will help reveal the targets and screen the beneficiaries of hormone therapy for HCC, pointing towards the direction for individualized therapy and combination therapy.
STAT3 signaling
STAT3 is activated by a variety of cytokines, such as IL-6, epidermal growth factor (EGF) and hepatocyte growth factor, and plays an important role in the development of tumors through regulating downstream target genes in the form of a transcription factor. Recently, a number of studies have shown that ERα is an upstream repressor of STAT3 in HCC, which can mediate multiple pathways to inhibit HCC progression.
The NF-κB signaling pathway plays an important role in the development of HCC, and its abnormal activation can induce the chronic inflammation that ultimately results in HCC.29 ERα physically interacts with NF-κB, thereby inhibiting activation of the IL-6/STAT3 pathway.30 On the foundation of this work, Naugler et al.31 found that in the HCC mouse models induced by diethylnitrosamine (DEN), the concentration of serum IL-6 and the activities of STAT3 as well as the abilities of tumor cell proliferation and necrosis were at a high level in males, as opposed to those in females. Furthermore, the administration of the ERα-specific agonist 1,3,5-tris(4-hydroxyphenyl)-4-propyl-1H-pyrazole (PPT) in male mice can block the secretion of IL-6 from Kupffer cells by interacting with NF-κB and thereby reduce the concentration of IL-6 in the circulation, which eventually inhibits the progression of HCC. The above results demonstrate that ERα has the ability to protect liver from damage by inhibiting the NF-κB/IL-6/STAT3 pathway in vivo, and to lower the concentration of IL-6 and elicit milder underlying liver damage in females who have the higher ERα activity naturally. For this reason, the sexual dimorphism of IL-6 concentration regulated by ERα might be considered as one basis for determining the potential beneficiaries of HCC.
Protein tyrosine phosphatase receptor type O (PTPRO) is a transmembrane protein. It inhibits the progression of various types of tumors by resorting to its intracellular protein tyrosine phosphatase activity, which is responsible for catalyzing tyrosine dephosphorylation,32,33 and there is no exception in HCC to this trend.34 Hou et al.13 showed that ERα can up-regulate PTPRO, initiating the dephosphorylation of JAK2 and PI3K by interacting with EREs at the PTPRO gene promoter; this eventually reduces the activity of STAT3, which then promotes HCC cell proliferation. This work confirmed the protective role of ERα in hepatocellular carcinogenesis and revealed a new therapeutic target (PTPRO), which can be used to develop targeted drugs, and gave insights into the mechanisms of hormone therapy for HCC.
Lipid metabolism-related pathways
Leptin is a peptide hormone secreted by white adipose tissue. It binds to specific receptors and prevents the development of obesity by regulating various physiological and biochemical processes, such as glucose and lipid metabolisms and food intake.35 Nevertheless, leptin is always at a higher level in obese people, and it is considered to induce HCC by activating JAK/STAT3, PI3K/AKT and other signaling pathways.36 Shen et al.37 documented that ERs may be involved in opposing leptin-induced hepatocellular carcinogenesis. In detail, ERβ inhibits HCC mainly through activating the suppressor of cytokine signaling 3 (SOCS3)/STAT3 and p38/MAPK two pathways, while ERα inhibits HCC principally through enhancing ERK signaling and diminishing the leptin-induced STAT3 signaling without affecting SOCS3.
Peroxisome proliferator-activated receptor α (PPARα) is a ligand-dependent nuclear receptor, which is mainly involved in reducing fat accumulation and maintaining the stability of the intracellular environment.38 However, excessive energy combustion triggered by sustained activation of PPARα may lead to the proliferation of liver parenchymal cells and increase the risk of liver cancer in obese mice.39,40 Chang-Lee and colleagues41 demonstrated that ERβ can down-regulate PPARα and its downstream genes through interacting with EREs of the PPARα gene, which consequently blocks the progress of HCC. Besides, Jeng et al.42 showed that when PPARα was overexpressed in Hep3B cells and HCC tissues, not only ERβ but also ERα mediated inhibition of PPARα. In this process, ERα directly binds to EREs of the PPARα gene to reduce its transcription and further regulate the PPARα target acyl-CoA oxidase (ACO), cyclin Dl, P27 and others, thereby blocking cancer cell proliferation and promoting apoptosis.
The above studies suggested that ERα has as its responsibility of inhibiting tumor progression and protecting HCC patients by regulating leptin-induced signaling and the lipid metabolism-related receptor PPARα. Enhancing these processes may bring some benefit to HCC patients, especially those who have lipid metabolism disorders.
Noncoding RNAs
miRNAs, circular RNAs (circRNAs) and long non-coding RNAs (lncRNAs) are the canonical family members of noncoding RNAs that are deficient in protein-coding function. They can be involved in the control of gene expression and affect multiple biological processes in specific ways.
The downstream ERα-mediated signaling pathways with miRNA involvement in HCC have been extensively studied, such as ERα enhancement of the p53-mediated regulation of miR-23a expression and further augmentation of caspase-3/7 activity to induce apoptosis of HCC cells.43 Besides, ERα can regulate circRNA to exert a tumor suppressor effect. Xiao et al.44 showed that ERα inhibits HCC through mediating the circRNA-SMG1.72/miR-141-3p/Gelsolin (GSN) signaling pathway. Specifically, ERα binds to the ERE of circRNA-SMG1.72 host gene, thus weakening the expression and sponge role of circRNA-SMG1.72 for miR-141-3p. This indirectly augments the activity of miR-141-3p and ultimately inhibits the invasion of HCC by promoting the degradation of miR-141-3p-regulated GSN mRNA.
Furthermore, in hepatitis B virus (HBV)-related HCC, the transcription factor ERα can bind to the promoter of the lncRNA LINC01352 by forming a complex with HBx. Functioning as a tumor suppressor, LINC01352 prevents the miR-135b-mediated suppression of adenomatous polyposis coli (APC) by sponging miR-135b and then repressing the Wnt/β-catenin signaling pathway. These findings demonstrate that the LINC01352-miR-135b-APC axis regulated by the ERα/HBx complex acts as an important signaling crosstalk factor for tumor progression, which may offer a theoretical basis for HBV-related HCC.45
These non-coding RNAs that are involved in the anti-tumor effect of ERα in HCC may give clues to the possibility for developing new diagnostic and therapeutic approaches.
Hormone therapy of HCC
There are various therapeutic approaches for HCC, but therapeutic satisfaction and practical effects still leave much to be desired. Hormone therapy plays an indispensable role in some specific tumors, due to its lower price and fewer adverse reactions, but there are still controversies in HCC. Considering the current research status and the value of TMX as well as the estrogen replacement therapy in HCC, more attention should be paid to these therapeutic schemes (Table 1).6,8,46–52
Table 1. Landmark trials of hormone therapy of HCC.
| Treatment | Main population | Comparison group | Salient findings | Reference |
|---|---|---|---|---|
| TMX (10 mg bid) | Patients with advanced HCC | No treatment | TMX improved survival in patients with advanced HCC and was well tolerated | Martínez 19946 |
| TMX (10 mg tid) plus triptorelin (3.75 mg monthly) | Patients with HCC | Flutamide plus triptorelin or placebo | TMX significantly prolongs survival and the TVDT in unresectable HCC | Manesis 199546 |
| TMX (60 mg bid) | Patients with inoperable HCC | TMX (30 mg bid) or placebo | TMX does not prolong survival in patients with inoperable HCC and has an increasingly negative impact with increasing dose | Chow 200247 |
| TMX (20 mg daily) | Patients with advanced HCC | Symptomatic treatment | TMX is not effective in prolonging survival of patients | Barbare 200548 |
| TMX (80 mg daily) | Patients with inoperable HCC, wild-type ER | Megestrol for patients with inoperable HCC, variant ER | Treatment with TMX is strongly dependent on the type of ER present in the HCC | Villa 199650 |
| TMX (20 mg bid) plus octreotide (0.6 mg daily) | Patients with inoperable HCC, ER-positive | 5-Fluorouracil plus mitomycin C | TMX plus octreotide is superior to 5-fluorouracil plus mitomycin C in the patients with ER-positive status | Pan 200351 |
| TMX (40 mg daily) plus sorafenib (400 mg daily) | Patients who are intolerant to or progressed during sorafenib therapy | Full-dose sorafenib therapy (800 mg daily) | TMX could produce some clinical benefit at sorafenib progression in advanced HCC | Ottaviano 201752 |
| HRT | Women with HCC (mostly menopausal) | Healthy women with no treatment | HRT reduced risk of HCC and increased overall survival times of patients | Hassan 20178 |
| HRT | Women with HCC (most menopausal) | Patients’ female relatives without HCC | Use of HRT was associated with a lower risk of HCC | Yu 200349 |
ER, estrogen receptor; HCC, hepatocellular carcinoma; HRT, hormone replacement therapy; TVDT, tumor volume doubling time; TMX, tamoxifen.
Tamoxifen – growing in doubts
In the past decades, researchers held different views about the clinical use of TMX for HCC. On the one hand, according to a small sample-sized clinical study, TMX significantly prolonged the life cycle of patients with advanced HCC than that without any interventions.6 A further controlled trial evaluated the effectiveness of TMX in HCC patients, and found that compared with the placebo group, the survival and tumor volume doubling time of TMX-treated patients were significantly prolonged.46 Moreover, basic research disclosed that TMX suppresses HCC by downregulating survivin at the transcriptional and post-transcriptional levels, as well as by inhibiting volume-activated chloride currents and the activity of cyclin-dependent kinase 5.53–55 Therefore, TMX is considered effective for HCC patients. On the other hand, the beneficial therapeutic potential of TMX for HCC patients has been questioned. A multicenter randomized controlled study containing 329 patients was developed to assess the effect of high-dose TMX compared with placebo, and showed that even a moderate dose of TMX has a negative impact on the survival of advanced HCC patients.47 Another investigation showed that TMX seems to benefit patients without severe liver insufficiency, but for patients with advanced HCC, TMX is almost ineffective.48 However, it is noteworthy that the above studies did not incorporate ER expression into the standard of patient selection, which may have resulted in the above different outcomes.
Hormone replacement therapy (HRT)
HRT is another alternative of hormone therapy, and the effectiveness of estrogen replacement therapy in HCC has been proven to some extent. Estrogen is at a high level in women during pregnancy or before menopause. In order to verify the influence of reproductive factors on HCC, a large sample-sized case-control study has been conducted between HCC patients and their relatives, and showed that the longer the period of the administration of estrogen, full-term pregnancies and age at natural menopause, the lower risk of adult women suffering from HCC.49 Recently, another case-control study of female patients with HCC showed that estrogen replacement therapy effectively inhibited hepatitis C virus- or HBV-related HCC, and long-term application of estrogen replacement therapy significantly prolonged the survival of menopausal patients.8 In addition, studies have found that the anti-cancer effect of estrogen is mainly exerted by stimulating ERα, and it was reversed after knocking down ERα in HepG2 cells.56 However, evidence from several lines of research suggest estrogen may increase the risk of breast cancer and endometrial cancer in female patients, and may bring some troubles to male patients. Hence, how to accurately apply estrogen replacement therapy to HCC needs to be discussed in many aspects.
Individualized treatment – breaking through the bottleneck of HCC hormone therapy potentially
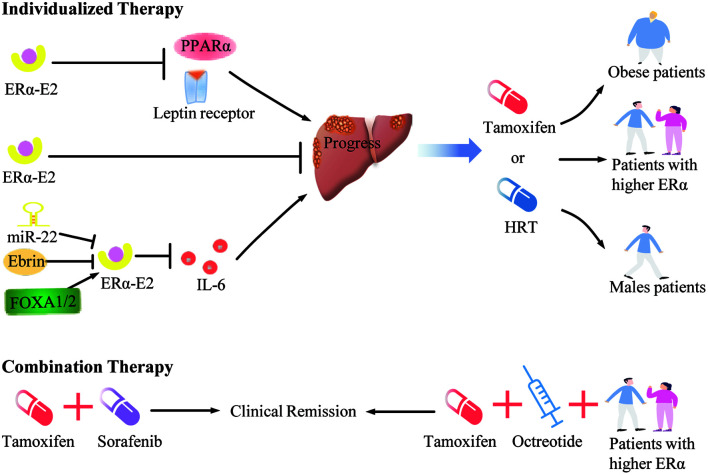
In view of the mechanisms of ERα in HCC, ERα-selective stimulation was postulated to achieve better results in the treatment of HCC. RNA sequencing was established in a human HCC-derived HepG2 cell line following treatment of control, estradiol (E2) and the ERα-specific agonist PPT; the results indicated that E2 or PPT suppressed the HepG2 cell transcriptome involving cellular and metabolic processes, which provided insight into the protective effects of an ERα-specific agonist in HCC development.57 However, agents selectively agonizing ERα for HCC are limited to the basic research realm, neither TMX nor estrogen replacement therapy can precisely and arbitrarily regulate ERα. Therefore, it is especially indispensable to accurately classify HCC patients underlying ERα expression and molecular mechanisms in order to find the potential candidate of hormone therapy.
The sensitivity of TMX for HCC is believed to have a positive relationship with the expression of ERα.28 Considering this positive relationship and anti-HCC effect of ERα, it is plausible that TMX and estrogen can be used to cure HCC to a certain extent. Nevertheless, considering the impacts of ER variants and upstream negative regulatory molecules, ERα is found only in a limited percentage of HCC patients,58,59 and this may contribute to the non-response or low-response to hormone therapy. It’s worth noting that there are already indications that treating HCC patients after classification may help to overcome the difficulties of hormone therapy. Villa et al.50 divided HCC patients into two groups according to the expression of wild-type ERα and the variant ERΔ5, then administrated them with TMX and megestrol (opposing estrogen action through ER-independent mechanisms) respectively, and found that the tumor volume of patients with wild-type ER was significantly reduced, while tumors with the ERΔ5 type decreased only temporarily and then continued to increase. A further research investigation of ER-positive patients confirmed that the combination of TMX and octreotide are superior to the combination of 5-fluorouracil and mitomycin C in terms of computed tomography imaging features, levels of AFP and accumulative survival rates;51 yet, a following study seems to have neglected ER classification of patients and failed to confirm the efficiency of TMX and octreotide.60 These collective results suggest that hormone therapy may be beneficial to HCC patients with wild-type ERs, rather than for patients with tumors bearing variant ERs. Although the number of samples included in these studies is limited, the conception of classified treatment opens the possibility for developing individualized precision treatments for HCC patients.
Conclusions
In the work of Villa and colleagues,50 HCC patients were distinguished by wild-type ERα and ERΔ5 phenotypes, and the treatment effectiveness of TMX was confirmed in the wild-type phenotype patients. Therefore, the application of hormone therapy may be mostly dependent on the classification of ERα, and screening or expanding HCC patients with higher ERα expression may be conducive to improving the hormonal therapy sensitivity. Besides, the targets of hormone therapy are not only ERα (ERα66) but also ERβ, ERα36 and others, and the therapy effects of these receptors are not completely identical.61 For instance, ERβ can inhibit HCC by blocking the oncogenic actions of leptin and transcription of PPARα, promoting pyroptosis mediated by the NOD-like receptor pyrin domain containing-3 inflammasome.37,41,62 Unlike ERβ, ERα36, one variant of ERα, was found to be highly expressed in HCC tissues and can promote the EGF receptor/Src/ERK signaling pathway to exert anti-tumor effects after E2 treatment.63 The G protein-coupled estrogen receptor (GPER) should also be mentioned, although its functions are still highly controversial.64–66 Thus, in the condition of the present understanding for ERs in HCC, the hormone therapy for HCC patients with higher expression of ERβ and lower expression of ERα36 should be considered into the ER classification system likewise to develop a comprehensive individualized hormone therapy regimen of HCC.
Identifying the target population who will benefit most from this hormone treatment is another important approach. The impacts caused by the changes of IL-6, FOXA1/2, miR-22 and Erbin showed the distinct sex dimorphism in HCC tissues. Higher circulating concentrations of IL-6 which can be reduced by ERα presenting in males, rather than females, suggests that TMX or estrogen mimics may carry more potential for benefit in males.31 FOXA1/2 protect females from HCC and promote HCC in males following hepatocarcinogenesis, which seems to indicate the importance of the hormone therapy for females; yet, it is also noteworthy that mutations of the FOXA1 and FOXA2 binding sites are significantly frequent in HCC tissue and have little effect on the attenuation of ERα transcriptional activity in males (as opposed to females);21,22 so, treating male patients who carry the above-described mutations with hormone therapy is worth investigating. miR-22 as well as Erbin exert stronger negative regulatory effects on ERα in male mice (vs. female mice), giving the opportunity to enhance the efficiency of hormone therapy for males after their inhibition.23,28 Other than that, aberrations of leptin-mediated pathways and PPARα transcriptional activity are most common in obese HCC patients, which emphasizes the potential value of hormone therapy for them.37,42 In sum, we hypothesized that males or obese HCC patients may be the population most likely to benefit from TMX and estrogen replacement therapy. Besides, based on the fact that a slice of upstream factors can negatively regulate ERα activity, trying hormone therapy in combination with inhibiting aberrations of ERα upstream regulators may be an entry point to expand the potential-benefit population.
It is important to acknowledge the limitations of hormone therapy alone for HCC. Early research tried the combination of hormonal therapy with other agents and partly confirmed the superiority of it (vs. monotherapy).60,67 Sorafenib, the first-line targeted therapy for unresectable HCC patients, combined with TMX, enabled patients to achieve a substantial overall disease control rate.52 Whether the drugs from among the present first-line treatment spectrum, e.g. oxaliplatin, can achieve the additive effect with the combination of individualized hormonal therapy is worth investigating. Although the current status of hormone therapy of HCC is not optimistic, the further research on combination therapy that we postulated above will aid in providing a novel therapeutic strategy for the treatment of patients with HCC.
Considering the aforementioned molecular mechanisms and potential therapeutic strategy, we believe that hormone therapy under the guidance of precision medicine and combination therapy will help to address the limitation of hormone therapy alone for HCC patients.
Abbreviations
- circRNAs
circular RNAs
- E2
estradiol
- ER
estrogen receptor
- EREs
estrogen response elements
- HBV
hepatitis B virus
- HCC
hepatocellular carcinoma
- MTA1
metastasis-associated protein 1
- miRNAs
microRNAs
- PTPRO
protein tyrosine phosphatase receptor type O
- PPARα
peroxisome proliferator-activated receptor α
- PPT
1,3,5-tris(4-hydroxyphenyl)-4-propyl-1H-pyrazole
- TMX
tamoxifen
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Yang JD, Hainaut P, Gores GJ, Amadou A, Plymoth A, Roberts LR. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol. 2019;16(10):589–604. doi: 10.1038/s41575-019-0186-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.White DL, Thrift AP, Kanwal F, Davila J, El-Serag HB. Incidence of hepatocellular carcinoma in all 50 United States, from 2000 through 2012. Gastroenterology. 2017;152(4):812–820.e5. doi: 10.1053/j.gastro.2016.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rich NE, Murphy CC, Yopp AC, Tiro J, Marrero JA, Singal AG. Sex disparities in presentation and prognosis of 1110 patients with hepatocellular carcinoma. Aliment Pharmacol Ther. 2020;52:701–709. doi: 10.1111/apt.15917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Farinati F, Salvagnini M, de Maria N, Fornasiero A, Chiaramonte M, Rossaro L, et al. Unresectable hepatocellular carcinoma: a prospective controlled trial with tamoxifen. J Hepatol. 1990;11(3):297–301. doi: 10.1016/0168-8278(90)90211-9. [DOI] [PubMed] [Google Scholar]
- 6.Martínez CFJ, Tomás A, Donoso L, Enríquez J, Guarner C, Balanzó J, et al. Controlled trial of tamoxifen in patients with advanced hepatocellular carcinoma. J Hepatol. 1994;20(6):702–706. doi: 10.1016/s0168-8278(05)80138-2. [DOI] [PubMed] [Google Scholar]
- 7.Liu CL, Fan ST, Ng IO, Lo CM, Poon RT, Wong J. Treatment of advanced hepatocellular carcinoma with tamoxifen and the correlation with expression of hormone receptors: a prospective randomized study. Am J Gastroenterol. 2000;95(1):218–222. doi: 10.1111/j.1572-0241.2000.01688.x. [DOI] [PubMed] [Google Scholar]
- 8.Hassan MM, Botrus G, Abdel-Wahab R, Wolff RA, Li D, Tweardy D, et al. Estrogen replacement reduces risk and increases survival times of women with hepatocellular carcinoma. Clin Gastroenterol Hepatol. 2017;15(11):1791–1799. doi: 10.1016/j.cgh.2017.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shi L, Feng Y, Lin H, Ma R, Cai X. Role of estrogen in hepatocellular carcinoma: is inflammation the key? J Transl Med. 2014;12:93. doi: 10.1186/1479-5876-12-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muhammad JS, Bajbouj K, Shafarin J, Hamad M. Estrogen-induced epigenetic silencing of FTH1 and TFRC genes reduces liver cancer cell growth and survival. Epigenetics. 2020;15(12):1302–1318. doi: 10.1080/15592294.2020.1770917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shen M, Xu M, Zhong F, Crist MC, Prior AB, Yang K, et al. A multi-omics study revealing the metabolic effects of estrogen in liver cancer cells hepG2. Cells. 2021;10(2):455. doi: 10.3390/cells10020455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O’Brien MH, Pitot HC, Chung SH, Lambert PF, Drinkwater NR, Bilger A. Estrogen receptor-α suppresses liver carcinogenesis and establishes sex-specific gene expression. Cancers (Basel) 2021;13(10):2355. doi: 10.3390/cancers13102355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hou J, Xu J, Jiang R, Wang Y, Chen C, Deng L, et al. Estrogen-sensitive PTPRO expression represses hepatocellular carcinoma progression by control of STAT3. Hepatology. 2013;57(2):678–688. doi: 10.1002/hep.25980. [DOI] [PubMed] [Google Scholar]
- 14.Zhang J, Ren J, Wei J, Chong CC, Yang D, He Y, et al. Alternative splicing of estrogen receptor alpha in hepatocellular carcinoma. BMC Cancer. 2016;16(1):926. doi: 10.1186/s12885-016-2928-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen YF, Velmurugan BK, Wang HL, Tu CC, Che RJ, Chen MC, et al. Estrogen and ERα enhanced β-catenin degradation and suppressed its downstream target genes to block the metastatic function of HA22T hepatocellular carcinoma cells via modulating GSK-3β and β-TrCP expression. Environ Toxicol. 2017;32(2):519–529. doi: 10.1002/tox.22256. [DOI] [PubMed] [Google Scholar]
- 16.Fuentes N, Silveyra P. Estrogen receptor signaling mechanisms. Adv Protein Chem Struct Biol. 2019;116:135–170. doi: 10.1016/bs.apcsb.2019.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arnal JF, Lenfant F, Metivier R, Flouriot G, Henrion D, Adlanmerini M, et al. Membrane and nuclear estrogen receptor alpha actions: from tissue specificity to medical implications. Physiol Rev. 2017;97(3):1045–1087. doi: 10.1152/physrev.00024.2016. [DOI] [PubMed] [Google Scholar]
- 18.Poté N, Cros J, Laouirem S, Raffenne J, Negrão M, Albuquerque M, et al. The histone acetyltransferase hMOF promotes vascular invasion in hepatocellular carcinoma. Liver Int. 2020;40(4):956–967. doi: 10.1111/liv.14381. [DOI] [PubMed] [Google Scholar]
- 19.Wei S, Liu W, Sun N, Wu Y, Song H, Wang C, et al. MOF upregulates estrogen receptor α signaling pathway via its acetylase activity in hepatocellular carcinoma. Cancer Sci. 2021;112(5):1865–1877. doi: 10.1111/cas.14836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arruabarrena-Aristorena A, Maag JLV, Kittane S, Cai Y, Karthaus WR, Ladewig E, et al. FOXA1 mutations reveal distinct chromatin profiles and influence therapeutic response in breast cancer. Cancer cell. 2020;38(4):534–550.e9. doi: 10.1016/j.ccell.2020.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li Z, Tuteja G, Schug J, Kaestner KH. Foxa1 and Foxa2 are essential for sexual dimorphism in liver cancer. Cell. 2012;148(1-2):72–83. doi: 10.1016/j.cell.2011.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang S, Xiang C, Mou L, Yang Y, Zhong R, Wang L, et al. Trans-acting non-synonymous variant of FOXA1 predisposes to hepatocellular carcinoma through modulating FOXA1-ERα transcriptional program and may have undergone natural selection. Carcinogenesis. 2020;41(2):146–158. doi: 10.1093/carcin/bgz136. [DOI] [PubMed] [Google Scholar]
- 23.Jiang R, Deng L, Zhao L, Li X, Zhang F, Xia Y, et al. miR-22 promotes HBV-related hepatocellular carcinoma development in males. Clin Cancer Res. 2011;17(17):5593–5603. doi: 10.1158/1078-0432.CCR-10-1734. [DOI] [PubMed] [Google Scholar]
- 24.Chen JJ, Tang YS, Huang SF, Ai JG, Wang HX, Zhang LP. HBx protein-induced upregulation of microRNA-221 promotes aberrant proliferation in HBV related hepatocellular carcinoma by targeting estrogen receptor-α. Oncol Rep. 2015;33(2):792–798. doi: 10.3892/or.2014.3647. [DOI] [PubMed] [Google Scholar]
- 25.Li CL, Yeh KH, Liu WH, Chen CL, Chen DS, Chen PJ, et al. Elevated p53 promotes the processing of miR-18a to decrease estrogen receptor-α in female hepatocellular carcinoma. Int J Cancer. 2015;136(4):761–770. doi: 10.1002/ijc.29052. [DOI] [PubMed] [Google Scholar]
- 26.Chen F, Ni X, Chen L, Wang X, Xu J. miR-939-3p promotes epithelial-mesenchymal transition and may be used as a prognostic marker in hepatocellular carcinoma. Oncol Lett. 2020;19(4):2727–2732. doi: 10.3892/ol.2020.11361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deng L, Yang H, Tang J, Lin Z, Yin A, Gao Y, et al. Inhibition of MTA1 by ERα contributes to protection hepatocellular carcinoma from tumor proliferation and metastasis. J Exp Clin Cancer Res. 2015;34:128. doi: 10.1186/s13046-015-0248-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu H, Yao S, Zhang S, Wang JR, Guo PD, Li XM, et al. Elevated expression of Erbin destabilizes ERα protein and promotes tumorigenesis in hepatocellular carcinoma. J Hepatol. 2017;66(6):1193–1204. doi: 10.1016/j.jhep.2017.01.030. [DOI] [PubMed] [Google Scholar]
- 29.Chen Q, Lu X, Zhang X. Noncanonical NF-κB signaling pathway in liver diseases. J Clin Transl Hepatol. 2021;9(1):81–89. doi: 10.14218/jcth.2020.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fan Y, Liu J, Miao J, Zhang X, Yan Y, Bai L, et al. Anti-inflammatory activity of the Tongmai Yangxin pill in the treatment of coronary heart disease is associated with estrogen receptor and NF-κB signaling pathway. J Ethnopharmacol. 2021;276:114106. doi: 10.1016/j.jep.2021.114106. [DOI] [PubMed] [Google Scholar]
- 31.Naugler WE, Sakurai T, Kim S, Maeda S, Kim K, Elsharkawy AM, et al. Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science. 2007;317(5834):121–124. doi: 10.1126/science.1140485. [DOI] [PubMed] [Google Scholar]
- 32.Dong H, Ma L, Gan J, Lin W, Chen C, Yao Z, et al. PTPRO represses ERBB2-driven breast oncogenesis by dephosphorylation and endosomal internalization of ERBB2. Oncogene. 2017;36(3):410–422. doi: 10.1038/onc.2016.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu Y, Li J, Wang P, Zhang Z, Wang X. LncRNA HULC promotes lung squamous cell carcinoma by regulating PTPRO via NF-κB. J Cell Biochem. 2019;120(12):19415–19421. doi: 10.1002/jcb.29119. [DOI] [PubMed] [Google Scholar]
- 34.Zhang W, Hou J, Wang X, Jiang R, Yin Y, Ji J, et al. PTPRO-mediated autophagy prevents hepatosteatosis and tumorigenesis. Oncotarget. 2015;6(11):9420–9433. doi: 10.18632/oncotarget.3353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Perakakis N, Farr OM, Mantzoros CS. Leptin in leanness and obesity: JACC state-of-the-art review. J Am Coll Cardiol. 2021;77(6):745–760. doi: 10.1016/j.jacc.2020.11.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saxena NK, Sharma D, Ding X, Lin S, Marra F, Merlin D, et al. Concomitant activation of the JAK/STAT, PI3K/AKT, and ERK signaling is involved in leptin-mediated promotion of invasion and migration of hepatocellular carcinoma cells. Cancer Res. 2007;67(6):2497–2507. doi: 10.1158/0008-5472.CAN-06-3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shen M, Shi H. Estradiol and estrogen receptor agonists oppose oncogenic actions of leptin in HepG2 cells. PLoS One. 2016;11(3):e0151455. doi: 10.1371/journal.pone.0151455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bougarne N, Weyers B, Desmet SJ, Deckers J, Ray DW, Staels B, et al. Molecular actions of PPARα in lipid metabolism and inflammation. Endocr Rev. 2018;39(5):760–802. doi: 10.1210/er.2018-00064. [DOI] [PubMed] [Google Scholar]
- 39.Brocker CN, Yue J, Kim D, Qu A, Bonzo JA, Gonzalez FJ. Hepatocyte-specific PPARA expression exclusively promotes agonist-induced cell proliferation without influence from nonparenchymal cells. Am J Physiol Gastrointest Liver Physiol. 2017;312(3):G283–G299. doi: 10.1152/ajpgi.00205.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang J, Jia Y, Fu T, Viswakarma N, Bai L, Rao MS, et al. Sustained activation of PPARα by endogenous ligands increases hepatic fatty acid oxidation and prevents obesity in ob/ob mice. FASEB J. 2012;26(2):628–638. doi: 10.1096/fj.11-194019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chang-Lee SN, Hsu HH, Shibu MA, Ho TJ, Tsai CH, Chen MC, et al. E(2)/ERβ inhibits PPARα to regulate cell-proliferation and enhance apoptosis in Hep3B-hepatocellular carcinoma. Pathol Oncol Res. 2017;23(3):477–485. doi: 10.1007/s12253-016-0136-8. [DOI] [PubMed] [Google Scholar]
- 42.Jeng LB, Velmurugan BK, Hsu HH, Wen SY, Shen CY, Lin CH, et al. Fenofibrate induced PPAR alpha expression was attenuated by oestrogen receptor alpha overexpression in Hep3B cells. Environ Toxicol. 2018;33(2):234–247. doi: 10.1002/tox.22511. [DOI] [PubMed] [Google Scholar]
- 43.Huang FY, Wong DK, Seto WK, Lai CL, Yuen MF. Estradiol induces apoptosis via activation of miR-23a and p53: implication for gender difference in liver cancer development. Oncotarget. 2015;6(33):34941–34952. doi: 10.18632/oncotarget.5472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xiao Y, Liu G, Sun Y, Gao Y, Ouyang X, Chang C, et al. Targeting the estrogen receptor alpha (ERα)-mediated circ-SMG1.72/miR-141-3p/Gelsolin signaling to better suppress the HCC cell invasion. Oncogene. 2020;39(12):2493–2508. doi: 10.1038/s41388-019-1150-6. [DOI] [PubMed] [Google Scholar]
- 45.Huang P, Xu Q, Yan Y, Lu Y, Hu Z, Ou B, et al. HBx/ERα complex-mediated LINC01352 downregulation promotes HBV-related hepatocellular carcinoma via the miR-135b-APC axis. Oncogene. 2020;39(18):3774–3789. doi: 10.1038/s41388-020-1254-z. [DOI] [PubMed] [Google Scholar]
- 46.Manesis EK, Giannoulis G, Zoumboulis P, Vafiadou I, Hadziyannis SJ. Treatment of hepatocellular carcinoma with combined suppression and inhibition of sex hormones: a randomized, controlled trial. Hepatology. 1995;21(6):1535–1542. doi: 10.1002/hep.1840210610. [DOI] [PubMed] [Google Scholar]
- 47.Chow PK, Tai BC, Tan CK, Machin D, Win KM, Johnson PJ, et al. High-dose tamoxifen in the treatment of inoperable hepatocellular carcinoma: a multicenter randomized controlled trial. Hepatology. 2002;36(5):1221–1226. doi: 10.1053/jhep.2002.36824. [DOI] [PubMed] [Google Scholar]
- 48.Barbare JC, Bouché O, Bonnetain F, Raoul JL, Rougier P, Abergel A, et al. Randomized controlled trial of tamoxifen in advanced hepatocellular carcinoma. J Clin Oncol. 2005;23(19):4338–4346. doi: 10.1200/JCO.2005.05.470. [DOI] [PubMed] [Google Scholar]
- 49.Yu MW, Chang HC, Chang SC, Liaw YF, Lin SM, Liu CJ, et al. Role of reproductive factors in hepatocellular carcinoma: impact on hepatitis B- and C-related risk. Hepatology. 2003;38(6):1393–1400. doi: 10.1016/j.hep.2003.09.041. [DOI] [PubMed] [Google Scholar]
- 50.Villa E, Dugani A, Fantoni E, Camellini L, Buttafoco P, Grottola A, et al. Type of estrogen receptor determines response to antiestrogen therapy. Cancer Res. 1996;56(17):3883–3885. [PubMed] [Google Scholar]
- 51.Pan DY, Qiao JG, Chen JW, Huo YC, Zhou YK, Shi HA. Tamoxifen combined with octreotide or regular chemotherapeutic agents in treatment of primary liver cancer: a randomized controlled trial. Hepatobiliary Pancreat Dis Int. 2003;2(2):211–215. [PubMed] [Google Scholar]
- 52.Ottaviano M, Palmieri G, Damiano V, Tortora M, Montella L. Rescue of sorafenib-pretreated advanced hepatocellular carcinoma with tamoxifen. Clinical Clin Res Trials. 2017;3(6):1–6. doi: 10.15761/CRT.1000200. [DOI] [Google Scholar]
- 53.Guo R, Huang Z, Shu Y, Jin S, Ge H. Tamoxifen inhibits proliferation and induces apoptosis in human hepatocellular carcinoma cell line HepG2 via down-regulation of survivin expression. Biomed Pharmacother. 2009;63(5):375–379. doi: 10.1016/j.biopha.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 54.Mao J, Yuan J, Wang L, Zhang H, Jin X, Zhu J, et al. Tamoxifen inhibits migration of estrogen receptor-negative hepatocellular carcinoma cells by blocking the swelling-activated chloride current. J Cell Physiol. 2013;228(5):991–1001. doi: 10.1002/jcp.24245. [DOI] [PubMed] [Google Scholar]
- 55.Wang F, Zhao W, Gao Y, Zhou J, Li H, Zhang G, et al. CDK5-mediated phosphorylation and stabilization of TPX2 promotes hepatocellular tumorigenesis. J Exp Clin Cancer Res. 2019;38(1):286. doi: 10.1186/s13046-019-1297-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xu H, Wei Y, Zhang Y, Xu Y, Li F, Liu J, et al. Oestrogen attenuates tumour progression in hepatocellular carcinoma. J Pathol. 2012;228(2):216–229. doi: 10.1002/path.4009. [DOI] [PubMed] [Google Scholar]
- 57.Shen M, Cao J, Shi H. Effects of estrogen and estrogen receptors on transcriptomes of HepG2 cells: a preliminary study using RNA sequencing. Int J Endocrinol. 2018;2018:5789127. doi: 10.1155/2018/5789127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Miceli V, Cocciadiferro L, Fregapane M, Zarcone M, Montalto G, Polito LM, et al. Expression of wild-type and variant estrogen receptor alpha in liver carcinogenesis and tumor progression. OMICS. 2011;15(5):313–317. doi: 10.1016/j.jsbmb.2016.05.023. [DOI] [PubMed] [Google Scholar]
- 59.Jeon Y, Yoo JE, Rhee H, Kim YJ, Il Kim G, Chung T, et al. YAP inactivation in estrogen receptor alpha-positive hepatocellular carcinoma with less aggressive behavior. Exp Mol Med. 2021;53(6):1055–1067. doi: 10.1038/s12276-021-00639-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Verset G, Verslype C, Reynaert H, Borbath I, Langlet P, Vandebroek A, et al. Efficacy of the combination of long-acting release octreotide and tamoxifen in patients with advanced hepatocellular carcinoma: a randomised multicentre phase III study. Br J Cancer. 2007;97(5):582–588. doi: 10.1038/sj.bjc.6603901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Baldissera VD, Alves AF, Almeida S, Porawski M, Giovenardi M. Hepatocellular carcinoma and estrogen receptors: Polymorphisms and isoforms relations and implications. Med Hypotheses. 2016;86:67–70. doi: 10.1016/j.mehy.2015.11.030. [DOI] [PubMed] [Google Scholar]
- 62.Wei Q, Zhu R, Zhu J, Zhao R, Li M. E2-Induced activation of the NLRP3 inflammasome triggers pyroptosis and inhibits autophagy in HCC cells. Oncol Res. 2019;27(7):827–834. doi: 10.3727/096504018x15462920753012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.You H, Meng K, Wang ZY. The ER-α36/EGFR signaling loop promotes growth of hepatocellular carcinoma cells. Steroids. 2018;134:78–87. doi: 10.1016/j.steroids.2018.02.007. [DOI] [PubMed] [Google Scholar]
- 64.Wei T, Chen W, Wen L, Zhang J, Zhang Q, Yang J, et al. G protein-coupled estrogen receptor deficiency accelerates liver tumorigenesis by enhancing inflammation and fibrosis. Cancer Lett. 2016;382(2):195–202. doi: 10.1016/j.canlet.2016.08.012. [DOI] [PubMed] [Google Scholar]
- 65.Qiu YA, Xiong J, Fu Q, Dong Y, Liu M, Peng M, et al. GPER-induced ERK signaling decreases cell viability of hepatocellular carcinoma. Front Oncol. 2021;11:638171. doi: 10.3389/fonc.2021.638171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chaturantabut S, Shwartz A, Evason KJ, Cox AG, Labella K, Schepers AG, et al. Estrogen activation of G-protein-coupled estrogen receptor 1 regulates phosphoinositide 3-kinase and mTOR signaling to promote liver growth in Zebrafish and proliferation of human hepatocytes. Gastroenterology. 2019;156(6):1788–1804.e13. doi: 10.1053/j.gastro.2019.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ganslmayer M, Ocker M, Kraemer G, Zopf S, Hahn EG, Schuppan D, et al. The combination of tamoxifen and 9cis retinoic acid exerts overadditive anti-tumoral efficacy in rat hepatocellular carcinoma. J Hepatol. 2004;40(6):952–956. doi: 10.1016/j.jhep.2004.02.004. [DOI] [PubMed] [Google Scholar]