Abstract
Background context:
The development of muscle fat infiltration (MFI) in the neck muscles is associated with poor functional recovery following whiplash injury. Custom software and time-consuming manual segmentation of magnetic resonance imaging (MRI) is required for quantitative analysis and presents as a barrier for clinical translation.
Purpose:
The purpose of this work was to establish a qualitative MRI measure for MFI and evaluate its ability to differentiate between individuals with severe whiplash-associated disorder (WAD), mild or moderate WAD, and healthy controls.
Study Design/Setting:
This is a cross-sectional study.
Patient Sample:
Thirty-one subjects with WAD and 31 age- and sex-matched controls were recruited from an ongoing randomized controlled trial.
Outcome Measures:
The cervical multifidus was visually identified and segmented into eighths in the axial fat/water images (C4-C7). Muscle fat infiltration was assessed on a visual scale: 0 for no or marginal MFI, 1 for light MFI, and 2 for distinct MFI. The participants with WAD were divided in two groups: mild or moderate and severe based on Neck Disability Index % scores.
Methods:
The mean regional MFI was compared between the healthy controls and each of the WAD groups using the Mann-Whitney U-test. Receiver operator characteristic (ROC) analyses were carried out to evaluate the validity of the qualitative method.
Results:
Twenty (65%) patients had mild or moderate disability and 11 (35%) were considered severe. Inter- and intra- rater reliability was excellent when grading was averaged by level or when frequency of grade 2 was considered. Statistically significant differences (p<0.05) in regional MFI were particularly notable between the severe WAD group and healthy controls. The ROC curve, based on detection of distinct MFI, showed an area-under-the curve of 0.768 (95% CI 0.59–0.94) for discrimination of WAD participants.
Conclusions:
These preliminary results suggest a qualitative MRI measure for MFI is reliable and valid, and may prove useful towards the classification of WAD in radiology practice.
INTRODUCTION
Whiplash associated disorders (WAD) from a motor vehicle collision (MVC) are a major health problem where approximately 50% may never fully recover [1] and 25–30% demonstrate severe pain-related disability [2], neck muscle degeneration [3–8], sensory and motor disturbances [9–13], muscle weakness [14] and altered patterns of activation [14, 15], and psychological distress [5, 7, 9, 16–18]. Current best multimodal management options (e.g. physical therapies, pharmacological agents, and psychological regimens) have not substantially influenced rates of recovery [19–21]. Furthermore, no link between persistent symptoms and a pathoanatomical lesion has been consistently identified with currently available imaging techniques [7].
Previous work has identified an increased expression of muscle fat infiltration (MFI) in the neck muscles of those with severe WAD and signs of post-traumatic stress [5, 7]. Individuals with mild or recovered symptoms and those with chronic idiopathic neck pain do not express the same increase in MFI [22], suggesting that the development of MFI could represent one physiologic marker underlying the transition to persistent pain and disability after a whiplash injury.
The radiological identification of muscle degeneration may help improve the clinical characterization of the WAD condition [23]. However, quantitative methods require costly commercial, or custom-developed, software for processing magnetic resonance imaging (MRI) and computing the MFI, making these methods unrealistic for clinical translation. Qualitative MRI grading methods of pathology requiring expert visual inspection or determination from the radiologist have, however, historically been better received [24–26].
It is recognized that in the absence of overt structural or neurological clinical findings in the emergent care setting, the current imaging guidelines do not provide specific recommendations for the assessment and subsequent performance of imaging for those with acute whiplash injury [27]. Most individuals presenting with neck pain after a MVC with WAD require no further imaging once medically screened. However, emerging evidence from three different cultures (Australia, United States, and Sweden) [5, 7, 28] suggest that advanced imaging may assist in the risk-based assessment and possible prediction of severe WAD. A practical MRI method for MFI grading in the cervical spine could facilitate translation of whiplash research to direct clinical care, leading to improved 1) characterization of recovery trajectories, 2) treatment planning, and hopefully 3) functional outcomes on a patient-by-patient basis.
The purpose of this work was to develop and evaluate a qualitative grading method for MFI in the cervical multifidii and to assess its ability to characterize muscle changes in a discrete number of patients with WAD.
Methods
Thirty-one participants (14 men, 17 women, mean age 41.5 ± 10.9, range 20.7–62.7 years) with chronic (>6 month) WAD and 31 healthy controls, matched for age and sex (14 men, 17 women, mean age 41.5 ± SD 10.6, range 22.2–61.8 years) were included in this study. The participants with WAD were consecutively recruited from an on-going randomized controlled trial (RCT) comparing neck-specific exercise with or without a behavioral approach to general physical activity with chronic WAD [29, 30]. The MRI scans were performed prior to any intervention, at the initial stage of the RCT.
The inclusion criteria to participate in the RCT were: age 18–63 years; WAD grade II after a whiplash injury at least six months, but not more than three years, in duration (Quebec Task Force grade II includes neck complaints and musculoskeletal sign(s) [31]); and scores of at least ≥ 20% on the 10-item Neck Disability Index (NDI, 0–100%) [32].
The exclusion criteria were: contraindications for MRI; known or suspected physical pathology, including myelopathy, spinal tumor, spinal infection or on-going malignancy; spinal fracture or subluxation; previous cervical spine surgery; neck pain that caused a >1 month, full-time, absence from work in the year prior to the traumatic event; signs of traumatic brain injury at the time of enrollment; generalized or more dominant pain elsewhere in the body; diseases or other injuries that might prevent full participation in the study; known drug abuse; pregnancy; and insufficient knowledge of the Swedish language (inability to answer the questionnaires).
The age- and sex-matched healthy controls with no neck pain were recruited from a convenience sample of university and hospital staff. Exclusion criteria for the healthy controls included present or past neck problems, trauma to the neck, neck or lower back pain, rheumatologic or neurological disease, and generalized myalgia as well as contraindications for MRI.
The participants with WAD were further divided into a mild or moderate disability group (20 ≤ NDI < 40%) and a severe WAD group (NDI ≥ 40%) [33]. For demographic details, see Table 1. The regional ethical review board approved the study (DNR: 2011/262–32). All participants provided written informed consent.
Table 1.
Demographics of all participants
| Healthy Controls | Mild/Moderate WAD (NDI 20%−40%) |
Severe WAD (NDI ≥ 40 %) |
||
|---|---|---|---|---|
| n | 31 | 20 | 11 | |
| Age (years) | Mean | 41.5 | 39.2 | 45.7 |
| SD | 10.6 | 11.5 | 8.5 | |
| Min | 20 | 20 | 34 | |
| Max | 61 | 62 | 58 | |
| BMI (kg/m2) | Mean | 24.4 | 25.5 | 25.8 |
| SD | 3.2 | 4.1 | 3.4 | |
| Min | 19.7 | 19.1 | 20.3 | |
| Max | 34.5 | 33.8 | 32.3 | |
| NDI (%) | Mean | N/A | 27.3 | 51.3 |
| SD | N/A | 6.8 | 10.2 | |
| Min | N/A | 10 | 40 | |
| Max | N/A | 38 | 68 | |
| Time since injury (months) | Mean | N/A | 20.1 | 14.5 |
| SD | N/A | 9.8 | 7.2 | |
| Min | N/A | 7 | 6 | |
| Max | N/A | 36 | 32 |
There was no significant difference between the three groups regarding age and BMI (all p>0.05).
Significant differences were noted in NDI (%) scores between severe and mild/moderate WAD (p<0.001).
There was no significant difference in time since injury between severe and mild/moderate WAD (p=0.113).
MRI Protocol
Magnetic resonance images were acquired with a Philips Ingenia 3.0 T scanner (Philips Health Care, Best, the Netherlands) using the built in phased-array posterior coil, a 32-channel head coil and an anterior flexible coil placed adjacent to the head coil. The participants were imaged in the supine, headfirst position. A 3D gradient-echo Dixon sequence was used with out-of-phase and in-phase echo times of 3.66 ms and 7.24 ms respectively. The echo times were chosen to enable the production of high-resolution images. The repetition time, TR, was 10 ms and the flip angle was 10° with a total acquisition time of 9.07 minutes. The images covered cervical segmental levels C2-C7 and were angled so that the in-plane images were parallel to each disc space and perpendicular to the long axis of the cervical musculature. The acquired image resolution was 0.75×0.75×0.75 mm3. Phase sensitive reconstruction was used to acquire fat and water separated image [34, 35].
Analysis of Muscle Fat Infiltration in Cervical Multifidus
In each participant the bilateral cervical multifidii muscles were identified and visually segmented by one blinded operator using the following scheme:
Cervical levels (C4-C7) were identified from fat/water separated sagittal slices for reference, and MFI was assessed using the axial fat/water MRIs (see Figure 1). Once the superior level of the vertebral body on the axial slice for each cervical level had been identified, four additional caudal slices were included, generating a slab consisting of five slices through each vertebra, C4-C7.
For each cervical level and side, the anatomy of the multifidii muscles was identified and visually divided in eight equally sized regions in two rows of four. Regions 1–4 were adjacent to the vertebra. Regions 1 and 5 were most medial, closest to spinous processes, whereas regions 4 and 8 were more lateral and closest to the facet joint (see Figure 2. Top, Bottom).
Finally, MFI in each one-eighth section was assessed on a visual scale based on the fat/water MRIs according to: 0 for normal muscle or some fatty streaks, 1 for less than 50% MFI, and 2 for 50% or greater MFI (see Figure 3. Left, Middle, and Right).
Figure 1.
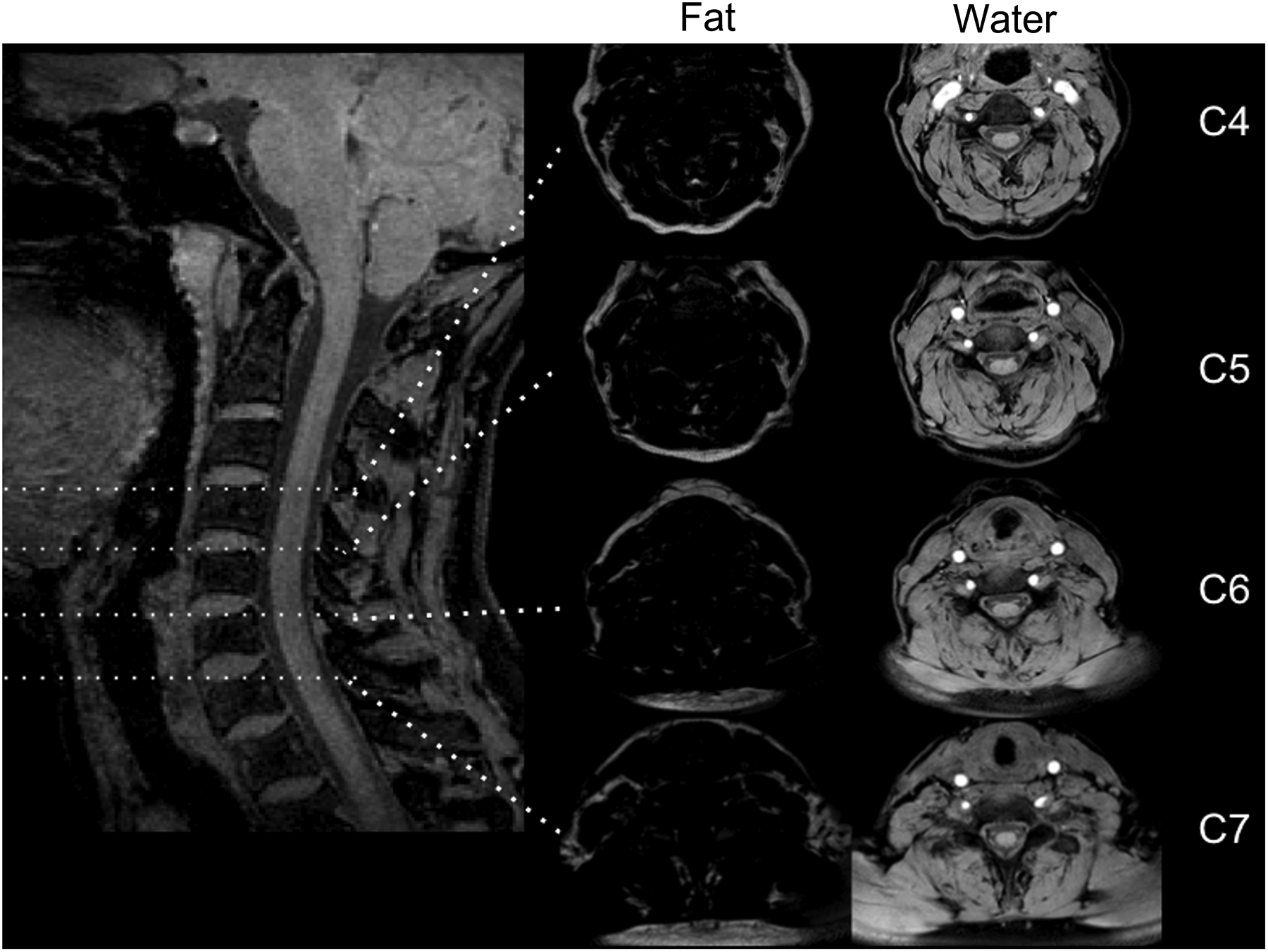
Left: Sagittal MR slice with vertebral level C4, C5, C6, and C7 marked. Middle: Axial Fat image at vertebral level C4, C5, C6, and C7. Right: Corresponding axial water image to the right. The image is from a 54-year old healthy female control.
Figure 2.
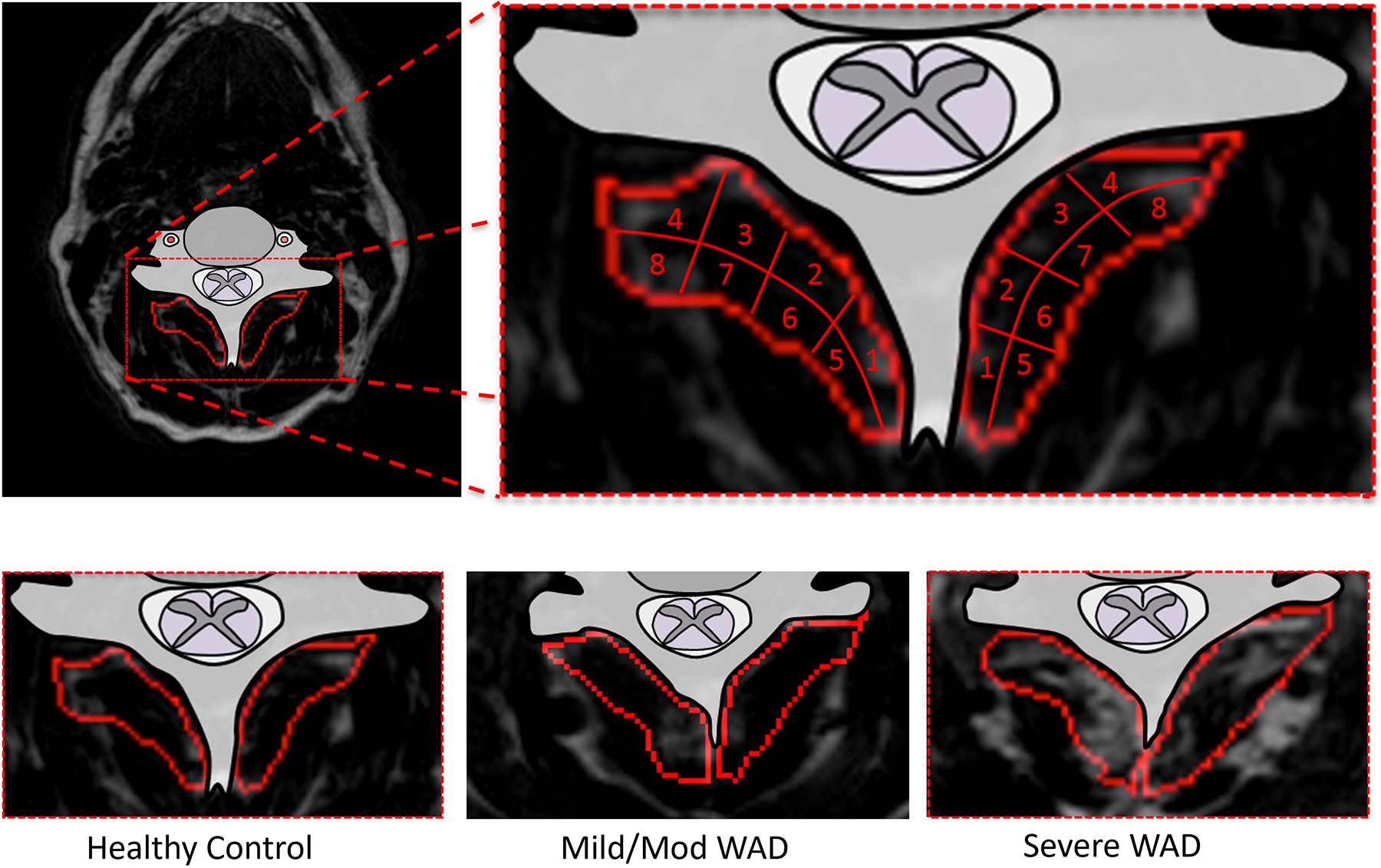
Top: Fat MR image with the multifidus muscle outlined. The muscle is then divided into eight regions for visual fat interpretation. Bottom: Example fat images of a healthy control, mild or moderate WAD, and a severe WAD participant.
Figure 3.
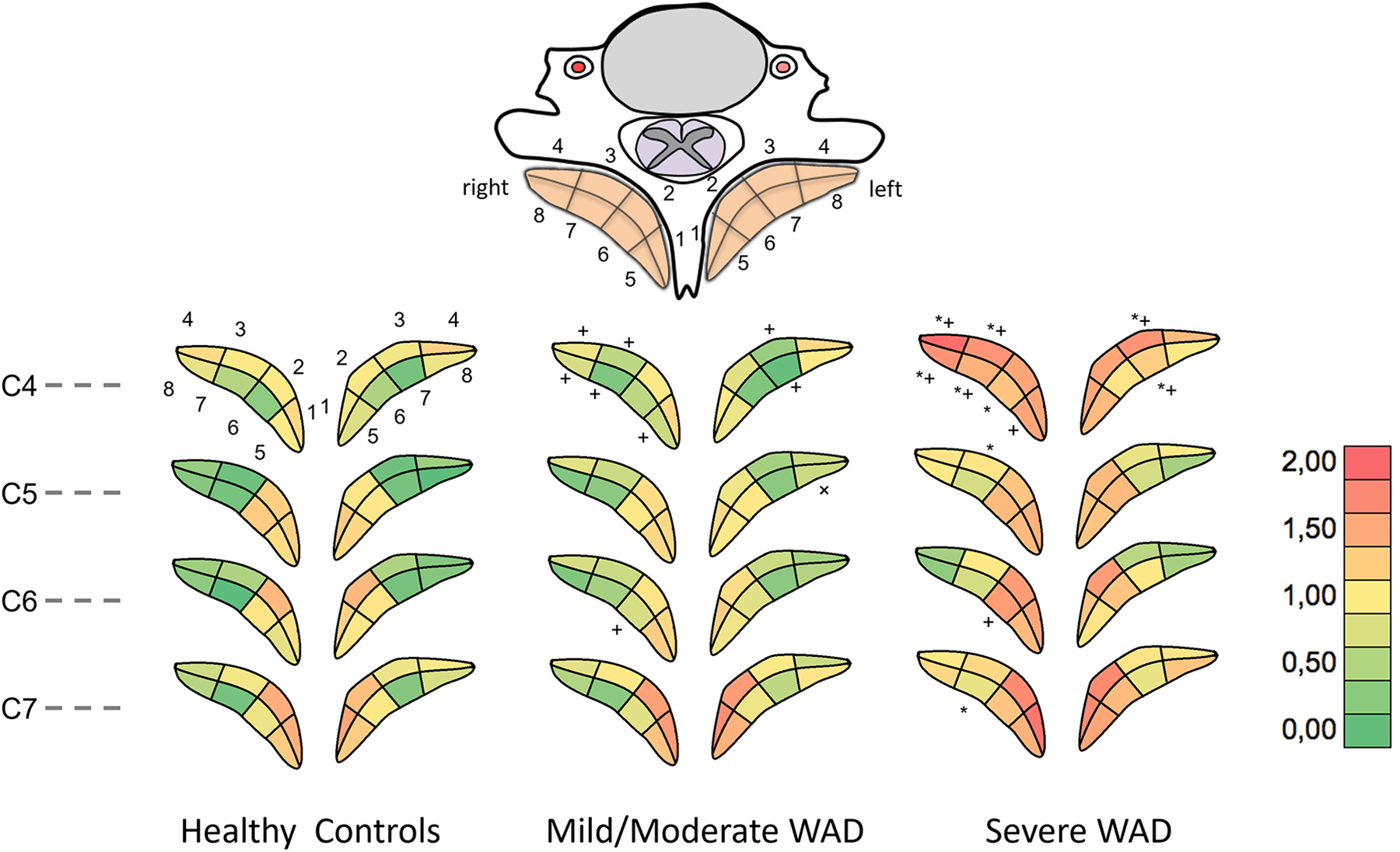
Average MFI for each region and cervical level for (Left) Healthy controls, (Middle) Mild or moderate WAD, (Right) Severe WAD. * p<0.03 Severe WAD compared to healthy controls, + p<0.02 Mild or moderate WAD compared to severe WAD.
Statistical analysis
Multifidus MFI was compared between healthy controls and the two WAD groups for each one-eighth region individually and each level as a whole. First, grade frequencies were calculated by summing all grades of ‘0’, ‘1’, and ‘2’ in each individual subject and compared using a Kruskal-Wallis test. In a second analysis, the Kruskal-Wallis test was used to compare each of the eight regions at each cervical level (C4-C7) separately. Where significance was found in either test, pair-wise post-hoc tests with Bonferroni-correction for multiple comparisons were used to compare all groups.
Inter- and intra-rater reliability for the multifidus MFI grading was performed using images from 5 randomly selected participants. Two raters (UA and RA) analyzed these images in a blinded fashion for the segmented MFI grades with a number of reliability comparisons across level and side: 1) comparing each grade individually, 2) comparing the summed grades (0,1, and 2) by level, 3) comparing total score by individual, and 4) comparing the number of grade 2 scores (e.g. distinct fat).
Furthermore, results from the qualitative MFI method were compared to NDI scores to evaluate the method’s ability to discriminate between individuals with mild or moderate and severe WAD. The classifiers were based on (Test 1) diffuse grades (summed for 1 and 2 MFI scores), and (Test 2) a distinct grade (only grade of 2). In both methods, the muscle was divided into eight regions by side and level (right or left; C4-C7) and fat-infiltration was assessed in all eight regions as 0, 1, or 2. In diagnostic Test 1, all grades of 1 and 2 were summed for discriminating mild or moderate and severe WAD. In Test 2, the total number of 2’s (distinct MFI) for discriminating mild or moderate and severe WAD were counted. Receiver Operator Characteristic (ROC) analyses were carried out for both tests and area-under-the-curve was calculated.
Results
The demographics of the participants are detailed in Table 1.
The inter- and intra-rater reliability is presented in Tables 2 and 3, respectively. Comparing the grade for each region individually, repeatability results demonstrate low inter-rater reliability with a Kappa score of 0.34 (95% confidence interval [CI] 0.26–0.43) and a low to moderate intra-rater reliability with a Kappa score of 0.49 (95% CI 0.41–0.57). The comparisons using summed grades (by level and side and across individuals) demonstrated high inter- and intra- rater reliability with intra-class correlation values ranging between 0.67 and 0.88.
Table 2.
Inter-rater Reliability
| ICC (95% CI) | Kappa (95% CI) | Cronbach alpha | Reliability | |
|---|---|---|---|---|
| Each grade individually (0, 1, 2) | N/A | 0.34 (0.26–0.43) | N/A | Low |
| Summed grade by level and side | 0.67 (0.46–0.81) | N/A | 0.81 | High |
| Summed grades by individual | 0.76 (−0.15–0.97) | N/A | 0.86 | High |
| Number of grade ‘2’ scores | 0.82 (0.03–0.98) | N/A | 0.90 | High |
Table 3.
Intra-rater Reliability
| ICC (95% CI) | Kappa (95% CI) | Cronbach alpha | Reliability | |
|---|---|---|---|---|
| Each grade individually (0, 1, 2) | N/A | 0.49 (0.41–0.57) | N/A | Low/moderate |
| Summed grade by level and side | 0.77 (0.60–0.87) | N/A | 0.87 | High |
| Summed grades by individual | 0.88 (0.22–0.99) | N/A | 0.93 | High |
| Number of grade ‘2’ scores | 0.84 | N/A | 0.92 | High |
The average MFI scores (0,1,2) for each region on each cervical level for all groups, as well as the frequency of each MFI score across groups and levels, are displayed in Figure 3, Left, Middle, and Right, Figure 4, and Table 4, respectively. Statistically significant differences for MFI frequencies across all levels combined were detected for a grade of ‘0’ (p=0.03), and grades of ‘2’ (p=0.02). Post-hoc tests showed significant differences in ‘0’ grades between healthy controls and severe WAD (NDI ≥ 40%) (p=0.03), number of grades of ‘2’ between healthy controls and severe WAD (p=0.03), as well as mild or moderate WAD and severe WAD (p=0.02).
Figure 4.
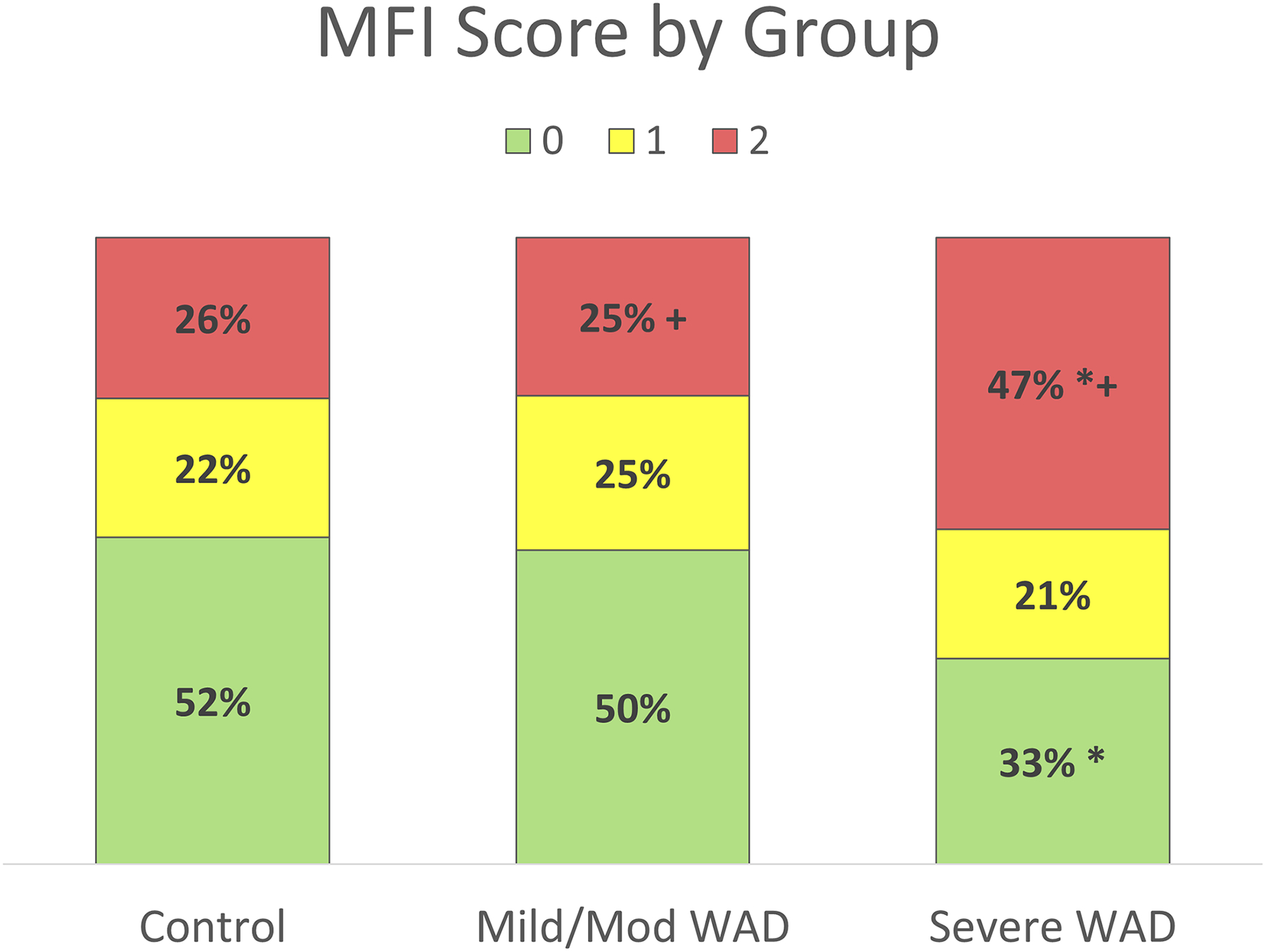
Frequency of MFI Scores across each group and level; Control, Mild or moderate WAD, severe WAD. * p = 0.03 Severe WAD compared to healthy controls, + p = 0.02 Mild or moderate compared to severe WAD.
Table 4.
Percentage MFI scores in each region by level and group
| Control (n=31) | Mild/moderate (n=20) | Severe (n=11) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 0 | 1 | 2 | 0 | 1 | 2 | ||
| C4 | Whole | 52% | 23% | 25% | 58% | 21% | 22% | 22% | 18% | 60% |
| 1 | 45% | 21% | 34% | 40% | 28% | 33% | 18% | 18% | 64% | |
| 2 | 47% | 24% | 29% | 45% | 33% | 23% | 18% | 18% | 64% | |
| 3 | 50% | 21% | 29% | 68% | 18% | 15% | 9% | 18% | 73% | |
| 4 | 35% | 27% | 37% | 50% | 13% | 38% | 14% | 9% | 77% | |
| 5 | 45% | 34% | 21% | 55% | 23% | 23% | 23% | 18% | 59% | |
| 6 | 71% | 16% | 13% | 73% | 13% | 15% | 41% | 14% | 45% | |
| 7 | 76% | 10% | 15% | 83% | 10% | 8% | 32% | 14% | 55% | |
| 8 | 47% | 34% | 19% | 48% | 30% | 23% | 23% | 32% | 45% | |
| C5 | Whole | 59% | 16% | 24% | 52% | 27% | 21% | 42% | 16% | 41% |
| 1 | 37% | 16% | 47% | 38% | 25% | 38% | 32% | 9% | 59% | |
| 2 | 37% | 29% | 34% | 40% | 35% | 25% | 32% | 14% | 55% | |
| 3 | 85% | 6% | 8% | 63% | 23% | 15% | 45% | 27% | 27% | |
| 4 | 73% | 15% | 13% | 48% | 40% | 13% | 41% | 32% | 27% | |
| 5 | 40% | 21% | 39% | 43% | 23% | 35% | 36% | 5% | 59% | |
| 6 | 42% | 16% | 42% | 40% | 38% | 23% | 27% | 18% | 55% | |
| 7 | 77% | 19% | 3% | 75% | 15% | 10% | 64% | 9% | 27% | |
| 8 | 84% | 8% | 8% | 68% | 20% | 13% | 59% | 18% | 23% | |
| C6 | Whole | 55% | 23% | 22% | 53% | 25% | 22% | 40% | 24% | 35% |
| 1 | 35% | 29% | 35% | 35% | 25% | 40% | 27% | 14% | 59% | |
| 2 | 19% | 32% | 48% | 45% | 18% | 38% | 14% | 18% | 68% | |
| 3 | 63% | 26% | 11% | 55% | 30% | 15% | 50% | 32% | 18% | |
| 4 | 74% | 15% | 11% | 63% | 20% | 18% | 64% | 27% | 9% | |
| 5 | 50% | 21% | 29% | 40% | 28% | 33% | 32% | 27% | 41% | |
| 6 | 40% | 29% | 31% | 50% | 33% | 18% | 14% | 36% | 50% | |
| 7 | 84% | 11% | 5% | 70% | 20% | 10% | 50% | 27% | 23% | |
| 8 | 73% | 21% | 6% | 68% | 25% | 8% | 73% | 14% | 14% | |
Figure 5 Left and Right shows the ROC curves for Test 1 and Test 2. Test 1 (sum of all grades) had an area-under-curve of 0.718 (CI 0.53 – 0.91). Test 2 (frequency of grade ‘2’ only) had an area-under-curve of 0.768 (CI 0.60 – 0.94). Accordingly, only considering distinct MFI (e.g. a grade of 2) showed higher predictive power compared to diffuse MFI for discriminating between the mild or moderate and severe WAD groups.
Figure 5.
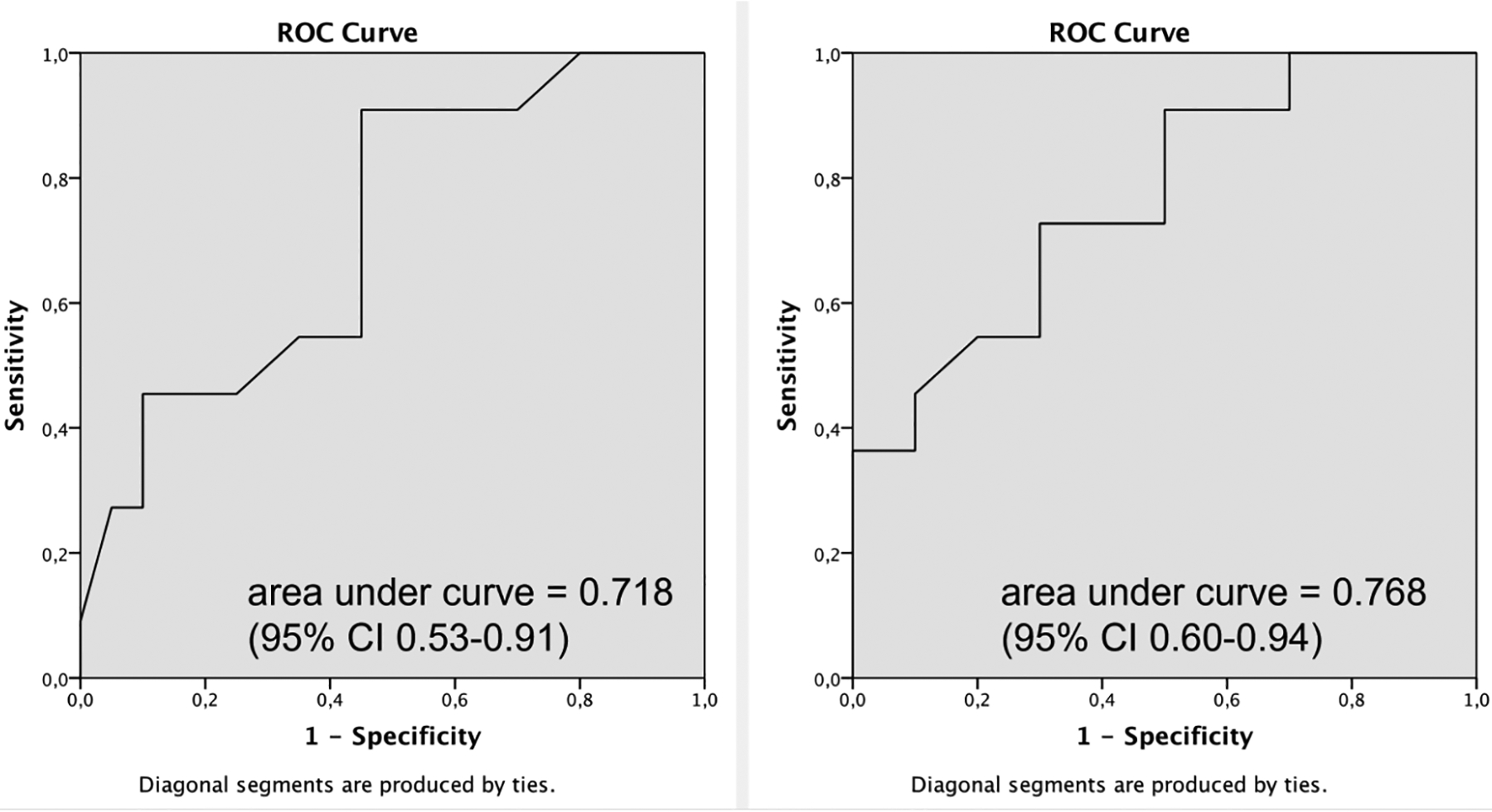
Results of ROC analyses discriminating mild or moderate WAD and severe WAD when, (Left) considering both diffuse (grades 0, 1, and 2) and distinct (only grade 2) MFI, and (Right) only considering distinct MFI.
DISCUSSION
Findings from this investigation support previous studies identifying increased MFI in the muscles of the cervical spine in those with higher levels of self-reported pain-related disability and psychological distress following an MVC [5, 7]. Furthermore, the findings are in agreement with previous quantitative work revealing a larger magnitude of MFI situated medially in the cervical multifidus muscle, adjacent to the spinous process and bony lamina [36].
It is reasonable to suspect that the magnitude and location of structural muscle changes could have biomechanical implications for persistent traumatic (and non-traumatic) neck disorders, as they have the potential to alter the internal forces in facet joints and moments produced by the muscles across those joints. Beyond the established association between MFI and WAD, the functional and biomechanical consequences of MFI are unknown. Future work should aim to determine if increases in MFI translate to observable deficits in sensorimotor output with clinical tests (e.g. range of motion, kinesthesia, strength, and endurance) [14].
While the qualitative method reported in this study might simplify radiological measurement of MFI, the underlying neurobiological mechanisms that support the development of MFI and its implications on functional recovery from whiplash remain largely unknown [3]. Regardless, emerging evidence from three countries (Australia [5], Sweden [28], and the United States [7]), with different insurance arrangements, suggests the expression of MFI may play a role in and possibly be associated with factors (age, higher pain-related disability, and the presence of hyperarousal symptoms) shown to influence recovery [37]. In short, interpreting MFI by averaging the grades over each level and side for each patient appears to decrease the variability across readers.
Fat infiltration of skeletal muscle has long been considered an indicator of muscle pathology [38]. It is thus plausible that functional outcomes in WAD are linked to healthy (or unhealthy) muscle composition [39]. However, our ability to effectively treat injured or affected muscles may rely on an improved understanding of the distribution and etiology of fat deposition across the age-spectrum [40]. Emerging work of the lumbar spine is providing foundation for an improved understanding of normative age-related decline in and measurement of muscles of the axial skeleton [41–46]. Nevertheless, a clear link between muscle morphometry and associated risk factors in WAD, including age, remains equivocal [3, 39, 47–50]. Accordingly, a major obstacle in the study of MFI is our collective lack of understanding on how (mechanical mechanisms), why (cellular processes), where (right vs. left side, poly- or mono-segmental), and when (pre-trauma muscle status vs. trauma exposure vs. normal aging) it occurs, making informed disease characterization, longitudinal evaluation, and therapeutic modulation difficult.
From a clinical standpoint, the patterns of MFI distribution in this preliminary work suggest a potential biomechanical consequence whereby deficits in motor function [14] may be partially explained by the overall content of MFI. While purely anecdotal at this stage, the potential influence that total MFI has on head/neck kinematics in day-to-day activities is worthy of further investigation, which is currently underway.
There are several limitations with this study. The graders were researchers familiar with MRI analysis of fat infiltration in skeletal muscles. Radiologists, surgeons, and physical medicine and rehabilitation clinicians should be included in future studies to better assess the clinical value of the measure. Additionally, the arguably small sample size prohibits our ability to draw strong conclusions. With larger sample sizes, we could investigate potential confounders, such as socioeconomic status, ongoing litigations related to cause of injury, age, and body mass index. Future studies should also include the potential longitudinal impacts on MFI following trauma, as well as the association to accidental findings, such as degenerative changes in the cervical spine. However, this preliminary cross-sectional study provides foundation for a larger prospective cohort study whereby clinicians provide the grades for MFI and any spatiotemporal changes thereof.
The ROC analyses presented in this paper support that higher levels of MFI (grade 2) might play an important role in characterization of the whiplash condition. These findings suggest that it may be possible to construct a diagnostic test based on qualitatively assessed distinct grades of MFI for discriminating WAD. Verifying the clinical usefulness of this qualitative MR measure estimating MFI in patients undergoing appropriate clinical scans as part of standard radiologic care needs to be investigated.
Conclusion
This study provides further evidence of increased MFI within the cervical multifidii muscles of individuals with persistent WAD when compared to those with milder symptoms and healthy controls. The qualitative MFI grading method utilizing the frequency of distinct fat infiltration (grade 2) was shown to have high inter- and intra- rater reliability as well as a high predictive power for discriminating between the mild or moderate and severe WAD groups. Prospective studies assessing clinical usefulness of this qualitative MR method for grading MFI in patients undergoing appropriate clinical scans as part of standard radiologic care are needed. This investigation could enable the translation of qualitative/quantitative reads of MFI to clinical radiology to facilitate improvement in the biopsychosocial classification and interdisciplinary management of patients at risk of, or those with, poor functional recovery following whiplash injury.
References
- 1.Carroll LJ, Holm LW, Hogg-Johnson S, et al. Course and prognostic factors for neck pain in whiplash-associated disorders (WAD): results of the Bone and Joint Decade 2000–2010 Task Force on Neck Pain and Its Associated Disorders. Spine (Phila Pa 1976). 2008;33(4 Suppl):S83–92. [DOI] [PubMed] [Google Scholar]
- 2.Sterling M, Hendrikz J, Kenardy J, et al. Assessment and validation of prognostic models for poor functional recovery 12 months after whiplash injury: a multicentre inception cohort study. Pain. 2012;153(8):1727–34. [DOI] [PubMed] [Google Scholar]
- 3.Elliott J. Are there implications for morphological changes in neck muscles after whiplash injury? Spine (Phila Pa 1976). 2011;1(36(25 Suppl)):S205–10. Review. [DOI] [PubMed] [Google Scholar]
- 4.Elliott J, Jull G, Noteboom JT, Darnell R, Galloway G, Gibbon WW. Fatty infiltration in the cervical extensor muscles in persistent whiplash-associated disorders: a magnetic resonance imaging analysis. Spine (Phila Pa 1976). 2006;31(22):E847–55. [DOI] [PubMed] [Google Scholar]
- 5.Elliott J, Pedler A, Kenardy J, Galloway G, Jull G, Sterling M. The temporal development of Fatty infiltrates in the neck muscles following whiplash injury: an association with pain and posttraumatic stress. PLoS One. 2011;6(6):e21194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elliott JM, O’Leary S, Sterling M, Hendrikz J, Pedler A, Jull G. Magnetic resonance imaging findings of fatty infiltrate in the cervical flexors in chronic whiplash. Spine (Phila Pa 1976). 2010;35(9):948–54. [DOI] [PubMed] [Google Scholar]
- 7.Elliott JM, Courtney DM, Rademaker A, Pinto D, Sterling MM, Parrish TB. The Rapid and Progressive Degeneration of the Cervical Multifidus in Whiplash: A MRI study of Fatty Infiltration. Spine (Phila Pa 1976). 2015;40(12):E694–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elliott J, Sterling M, Noteboom JT, Treleaven J, Galloway G, Jull G. The clinical presentation of chronic whiplash and the relationship to findings of MRI fatty infiltrates in the cervical extensor musculature: a preliminary investigation. Eur Spine J. 2009;18(9):1371–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sterling M, Jull G, Kenardy J. Physical and psychological factors maintain long-term predictive capacity post-whiplash injury. Pain. 2006;122(1–2):102–8. [DOI] [PubMed] [Google Scholar]
- 10.Passatore M, Roatta S. Influence of sympathetic nervous system on sensorimotor function: whiplash associated disorders (WAD) as a model. Eur J Appl Physiol. 2006;98(5):423–49. [DOI] [PubMed] [Google Scholar]
- 11.Treleaven J, Jull G, Lowchoy N. The relationship of cervical joint position error to balance and eye movement disturbances in persistent whiplash. Man Ther. 2005;11(2):99–106. [DOI] [PubMed] [Google Scholar]
- 12.Periera MJ, Jull GA, Treleaven J. Self-Reported Driving Habits in Subjects With Persistent Whiplash-Associated Disorder: Relationship to Sensorimotor and Psychologic Features. Arch Phys Med Rehabil. 2008;89:1097–102. [DOI] [PubMed] [Google Scholar]
- 13.Treleaven J. Dizziness, unsteadiness, visual disturbances, and postural control: implications for the transition to chronic symptoms after a whiplash trauma. Spine (Phila Pa 1976). 2011;36(25 Suppl):S211–7. [DOI] [PubMed] [Google Scholar]
- 14.Schomacher J, Farina D, Lindstroem R, Falla D. Chronic trauma-induced neck pain impairs the neural control of the deep semispinalis cervicis muscle. Clin Neurophysiol. 2012;123(7):1403–8. [DOI] [PubMed] [Google Scholar]
- 15.Falla D, Bilenkij G, Jull G. Patients with chronic neck pain demonstrate altered patterns of muscle activation during performance of a functional upper limb task. Spine (Phila Pa 1976). 2004;29(13):1436–40. [DOI] [PubMed] [Google Scholar]
- 16.Sterling M, Kenardy J. Physical and psychological aspects of whiplash: Important considerations for primary care assessment. Man Ther. 2008;13(2):93–102. [DOI] [PubMed] [Google Scholar]
- 17.Craig A, Tran Y, Guest R, et al. Psychological impact of injuries sustained in motor vehicle crashes: systematic review and meta-analysis. BMJ Open. 2016;6(9):e011993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guest R, Tran Y, Gopinath B, Cameron ID, Craig A. Psychological distress following a motor vehicle crash: A systematic review of preventative interventions. Injury. 2016;47(11):2415–23. [DOI] [PubMed] [Google Scholar]
- 19.Jull G, Kenardy J, Hendrikz J, Cohen M, Sterling M. Management of acute whiplash: a randomized controlled trial of multidisciplinary stratified treatments. Pain. 2013;154(9):1798–806. [DOI] [PubMed] [Google Scholar]
- 20.Lamb SE, Gates S, Williams MA, et al. Emergency department treatments and physiotherapy for acute whiplash: a pragmatic, two-step, randomised controlled trial. Lancet. 2013;381(9866):546–56. [DOI] [PubMed] [Google Scholar]
- 21.Michaleff ZA, Maher CG, Lin CW, et al. Comprehensive physiotherapy exercise programme or advice for chronic whiplash (PROMISE): a pragmatic randomised controlled trial. Lancet. 2014;384(9938):133–41. [DOI] [PubMed] [Google Scholar]
- 22.Elliott J, Sterling M, Noteboom JT, Darnell R, Galloway G, Jull G. Fatty infiltrate in the cervical extensor muscles is not a feature of chronic, insidious-onset neck pain. Clin Radiol. 2008;63(6):681–7. [DOI] [PubMed] [Google Scholar]
- 23.Elliott JM, Noteboom JT, Flynn TW, Sterling M. Characterization of acute and chronic whiplash-associated disorders. J Orthop Sports Phys Ther. 2009;39(5):312–23. [DOI] [PubMed] [Google Scholar]
- 24.Pfirrmann CW, Metzdorf A, Zanetti M, Hodler J, Boos N. Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine (Phila Pa 1976). 2001;26(17):1873–8. [DOI] [PubMed] [Google Scholar]
- 25.Dudli S, Fields AJ, Samartzis D, Karppinen J, Lotz JC. Pathobiology of Modic changes. Eur Spine J. 2016;25(11):3723–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Swami VG, Katlariwala M, Dhillon S, Jibri Z, Jaremko J. Magnetic Resonance Imaging in Patients With Mechanical Low Back Pain Using a Novel Rapid-Acquisition Three-Dimensional SPACE Sequence at 1.5-T: A Pilot Study Comparing Lumbar Stenosis Assessment With Routine Two-Dimensional Magnetic Resonance Sequences. Can Assoc Radiol J. 2016;67(4):368–78. [DOI] [PubMed] [Google Scholar]
- 27.Newman J, Weissman B, Angevine P, al. e. Chronic Neck Pain. Appropriateness Criteria 2013. 2013 [Accessed December 21, 2015.]; Available from: https://acsearch.acr.org/docs/69426/Narrative/..
- 28.Karlsson A, Dahlqvist Leinhard O, West J, et al. An Investigation of Fat Infiltration of the Multifidus Muscle in Patients with Severe Neck Symptoms Associated with Chronic Whiplash Associated Disorder. J Orthop Sports Phys Ther. 2016;46(10):886–93. [DOI] [PubMed] [Google Scholar]
- 29.Ludvigsson ML, Peterson G, O’Leary S, Dedering A, Peolsson A. The Effect of Neck-specific Exercise With, or Without a Behavioral Approach, on Pain, Disability, and Self-Efficacy in Chronic Whiplash-associated Disorders A Randomized Clinical Trial. Clin J Pain. 2015;31(4):294–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peolsson A, Ludvigsson ML, Overmeer T, et al. Effects of neck-specific exercise with or without a behavioural approach in addition to prescribed physical activity for individuals with chronic whiplash-associated disorders: a prospective randomised study. Bmc Musculoskel Dis. 2013;14(311.). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spitzer W, Skovron M, Salmi L, et al. Scientific Monograph of Quebec Task Force on Whiplash Associated Disorders: redefining “Whiplash “and its management. Spine. 1995;20:1–73. [PubMed] [Google Scholar]
- 32.Vernon H, Mior S. The Neck Disability Index: A study of reliability and validity. J Manipulative Physiol Ther. 1991;14:409–15. [PubMed] [Google Scholar]
- 33.Miettinen T, Leino E, Airaksinen O, Lindgren KA. The possibility to use simple validated questionnaires to predict long-term health problems after whiplash injury. Spine. 2004;29(3):E47–E51. [DOI] [PubMed] [Google Scholar]
- 34.Rydell J, Knutsson H, Pettersson J, al. e. Phase sensitive reconstruction for water/fat separation in MR imaging using inverse gradient. International Conference on Medical Image Computing and Computer-Assisted Intervention. 2007;10:210–8. [DOI] [PubMed] [Google Scholar]
- 35.Romu T, Dahlqvist Leinhard O, Dahlström N, M. B. Robust Water Fat Separated Dual-Echo MRI by Phase-Sensitive Reconstruction. Magn Reson Med. 2016. [DOI] [PubMed] [Google Scholar]
- 36.Abbott R, Pedler A, Sterling M, et al. The Geography of Fatty Infiltrates Within the Cervical Multifidus and Semispinalis Cervicis in Individuals With Chronic Whiplash-Associated Disorders. J Orthop Sports Phys Ther. 2015;45(4):281–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ritchie C, Hendrikz J, Jull G, Elliott J, Sterling M. External validation of a clinical prediction rule to predict full recovery and ongoing moderate/severe disability following acute whiplash injury. J Orthop Sports Phys Ther. 2015;45(4):242–50. [DOI] [PubMed] [Google Scholar]
- 38.Fritz RC, Domroese ME, Carter GT. Physiological and anatomical basis of muscle magnetic resonance imaging. Phys Med Rehabil Clin N Am. 2005;16(4):1033–51, x. [DOI] [PubMed] [Google Scholar]
- 39.Elliott JM, Kerry R, Flynn T, Parrish T. Content not quantity is a better measure of muscle degeneration in whiplash. Manual Ther. 2013;18(6):578–82. [DOI] [PubMed] [Google Scholar]
- 40.Davies TM, Cornwall J, Sheard PW. Modelling dichotomously marked muscle fibre configurations. Stat Med. 2013;32(24):4240–58. [DOI] [PubMed] [Google Scholar]
- 41.Crawford RJ, Filli L, Elliott JM, et al. Age- and Level-Dependence of Fatty Infiltration in Lumbar Paravertebral Muscles of Healthy Volunteers. AJNR Am J Neuroradiol. 2015;37(4):742–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Valentin S, Licka T, Elliott J. Age and side-related morphometric MRI evaluation of trunk muscles in people without back pain. Manual Ther. 2015;20(1):90–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sions JM, Coyle PC, Velasco TO, Elliott JM, Hicks G. Multifidi Muscle Characteristics and Physical Function Among Older Adults With and Without Chronic Low Back Pain. Arch Phys Med Rehabil. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Crawford RJ, Volken T, Valentin S, Melloh M, Elliott JM. Rate of lumbar paravertebral muscle fat infiltration versus spinal degeneration in asymptomatic populations: an age-aggregated cross-sectional simulation study. Scoliosis Spinal Disord. 2016;11:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Crawford RJ, Cornwall J, Abbott R, Elliott J. Manually defining regions of interest when quantifying paravertebral muscles fatty infiltration from axial magnetic resonance imaging: a proposed method for the lumbar spine with anatomical cross-reference. BMC Musculoskelet Disord. 2017;18(25). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ni Mhuiris Á, Volken T, Elliott JM, Hoggarth MA, Samartzis D, Crawford R. Reliability of quantifying the spatial distribution of fatty infiltration in lumbar paravertebral muscles using a new segmentation method for T1-weighted MRI. BMC Musculoskelet Disord. 2016;17(234). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Anderson SE, Boesch C, Zimmermann H, et al. Are There Cervical Spine Findings at MR Imaging That Are Specific to Acute Symptomatic Whiplash Injury? A Prospective Controlled Study with Four Experienced Blinded Readers. Radiology. 2012;262(2):567–75. [DOI] [PubMed] [Google Scholar]
- 48.Matsumoto M, Ichihara D, Okada E, et al. Cross-sectional area of the posterior extensor muscles of the cervical spine in whiplash injury patients versus healthy volunteers - 10year follow-up MR study. Injury. 2012;43(6):912–6. [DOI] [PubMed] [Google Scholar]
- 49.Ulbrich EJ, Aeberhard R, Wetli S, et al. Cervical muscle area measurements in whiplash patients: Acute, 3, and 6 months of follow-up. J Magn Reson Imaging. 2012;36(6):1413–20. [DOI] [PubMed] [Google Scholar]
- 50.De Pauw R, Coppieters I, Kregel J, De Meulemeester K, Danneels L, Cagnie B. Does muscle morphology change in chronic neck pain patients? - A systematic review. Man Ther. 2016;22:42–9. [DOI] [PubMed] [Google Scholar]


