Abstract
Aplasia cutis congenita (ACC) is a rare group of congenital disorders characterised by focal or widespread absence of skin, predominantly affecting the scalp. A Malay female infant was born at 37 weeks with extensive ACC, affecting 37% of total body surface area, including her scalp and trunk. There is no consensus on the management of ACC given the rarity and variable presentation. A multi-disciplinary team comprising neonatologists, paediatric dermatologists, plastic surgeons and medical laboratory scientists at the skin bank, employed a more aggressive surgical approach with the aim of avoiding potentially catastrophic morbidity, including sagittal sinus haemorrhage and brain herniation. Out of several surgical options, the team used a staged artificial dermal matrix (Integra) and cultured epithelial autograft application, followed by regular wound dressing, and eventually allowed the child to achieve complete epithelialisation of her trunk, and most of scalp before she was discharged from hospital.
Keywords: dermatology, neonatal and paediatric intensive care, plastic and reconstructive surgery
Background
Aplasia cutis congenita (ACC) is a rare group of congenital disorders characterised by focal or widespread absence of skin, predominantly affecting the scalp, although other areas can be involved. It was first described and reported by Cordon.1 ACC is a rare condition with its precise incidence unknown but thought to be around 0.5–1 in 10 000 births.2 The exact pathogenesis is unknown, although several theories have been proposed including neural tube defect, vascular compromise from placental insufficiency, intra-uterine infections, genetic mutations, teratogens and ischaemic events related to fetus papyraceus.3
The presentation of ACC is varied and several classification systems have been proposed. Frieden4 classified ACC based on its body area affected, associated abnormalities, inheritance pattern and aetiology. Evers et al5 attempted to classify into four groups based on aetiology: (1) chromosomal changes, (2) monogenic group, which includes autosomal dominant, recessive and X-linked genetic mutations associated with ACC, (3) teratogenic/exogenous causes and (4) unknown group, which includes ACC with multiple (2) congenital defects or single (1) congenital defect with uncertain cause. Silberstein et al6 proposed to classify according to defect size, layers involved, and whether there are associated exposed veins. Given the varied presentation of ACC, while many cases fit into current classification systems, there have been exceptions. In a 25-year retrospective classification of 56 patients with ACC, 2 patients could not be classified according to the Frieden classification.7
ACC has reportedly been associated with significant morbidity. ACC patients with large scalp defects, often associated with skull defects and exposed dura or superior sagittal sinus have reportedly suffered morbidity or demised from catastrophic haemorrhage8 9 and/or suffered meningitis.
Diagnosis is mostly clinical. In terms of the management of ACC, given the rarity and variability in terms of the site and extent of ACC, there is currently no consensus. Management approaches3 include conservative treatment, which involves dressing of the aplastic skin until complete re-epithelialisation, and surgical management, which include excision and closure, skin grafting, local flaps and tissue expansion.
We present a case of very extensive ACC, involving 37% body surface area (BSA), including the scalp and trunk, and discuss our management approach, which we believe has contributed to the avoidance of potentially catastrophic morbidity including superior sagittal sinus haemorrhage and brain herniation.
Case presentation
Our patient was born at a gestational age of 37 weeks 3 days via elective lower section Caesarean section for breech presentation, to a 40-year-old gravida 5 para 5 mother whose pregnancy was complicated by intra-uterine growth retardation and uteroplacental insufficiency with raised umbilical artery pressure index >95th centile, which was noted at 36 weeks 5 days gestation. There was no other significant maternal medical history of note including recent infection nor rash. Her mother was also not on any medication. Antenatal screening was unremarkable.
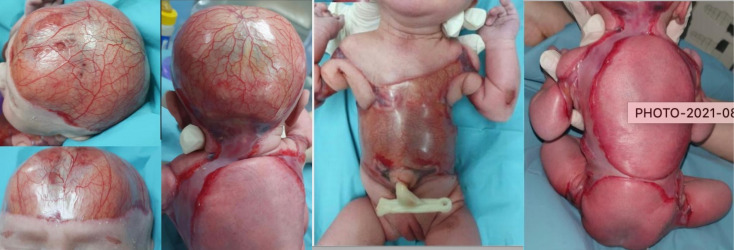
At birth, she required continuous positive airway pressure for 15 min, but was promptly weaned to room air thereafter. She was noted to have large areas of skin defect over her scalp (sparing her face), anterior and postero-lateral aspects of her trunk, as well as circumferential bands at the upper aspect of both arms and thighs. Total BSA involvement was taken to be 37% (figure 1). There were no blisters seen. No limb abnormalities were found on examination. In view of the extensive skin defect, she was transferred to the neonatal intensive care unit on room air in a closed transport incubator.
Figure 1.
At birth, the baby was noted to have a membranous covering in lieu of normal skin affecting her scalp, trunk and four limbs.
Apgar scores were 3 at 1 min, and 9 at 5 min. Screening tests showed that she did not have glucose-6-phosphate dehydrogenase deficiency, congenital hypothyroidism nor hearing impairment.
The patient was small for gestational age, weighing 2140 g.
Treatment
A multi-disciplinary team comprising the neonatologist, dermatologist, plastic surgeon, hand surgeon and neurosurgeon was promptly engaged to manage this patient.
The patient was managed similarly to a paediatric burns patient in view of extensive areas of skin defect. She was nursed in a closed incubator with strict infection prevention measures in view of the high risk of infection. Empiric antibiotic coverage was started on the second day of life. There was meticulous attention to fluid balance to ensure she remained euvolemic. Dressings were performed in an aseptic technique to minimise fluid losses and prevent infection while awaiting definitive plan
Both conservative and surgical approaches were considered. On top of the inherent risks associated with conservative management of ACC, such as wound infection, electrolyte abnormalities and delayed wound healing, this baby was considered to be at high risk of potentially catastrophic haemorrhage,8 9 meningitis and even brain herniation10 due to the large scalp defect. As such, coverage for the scalp was prioritised. Surgical options for skin coverage in a neonate are limited, and her extensive ACC of total body surface area (TBSA) of 37% precluded the options of excision and closure, local flaps, tissue expansion and skin grafting for expedient coverage. The use of autografts11 was limited by donor site availability and risks of donor site morbidity. Alternatives such as allografts12–14 also have increased risk of rejection and infection. Decision was hence made for cultured epithelial autograft (CEA)15–19 but it would only be ready after 3 weeks of cell culture by the skin bank.
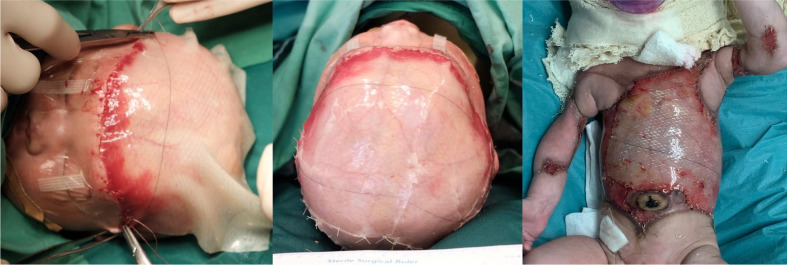
As a bridging measure, staged artificial dermal matrix (ADM)16 20–24 in the form of Integra was applied to her scalp on the third day of life to protect her scalp and provide a scaffold for cellular ingrowth while awaiting CEA, followed by application to the remaining involved areas on her trunks and limbs on day 10 of life under general anaesthesia (figure 2). A small amount of skin was harvested from her right groin for CEA preparation and the donor site was closed primarily at the same setting.
Figure 2.
Application of Integra artificial dermal matrix to scalp, trunk and upper limbs.
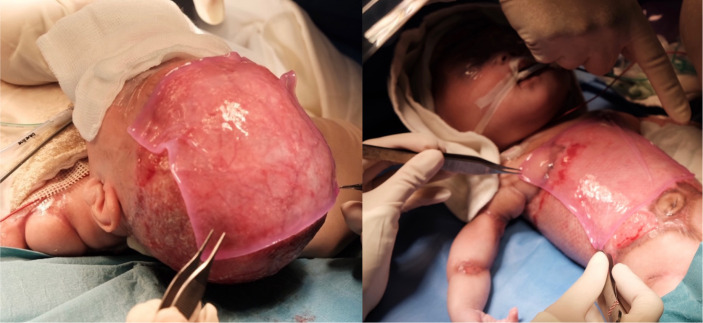
The first application of CEA occurred on day 29 of life when it became available (figure 3). She required two further applications of CEA on day 102 of life and day 143 of life to both her scalp and trunk.
Figure 3.
Application of cultured epithelial autograft.
She required parenteral antibiotics (cloxacillin for 10 days, and gentamicin for 2 days) from the second day of life for presumed sepsis as her initial infective markers were elevated (C reactive protein 11.4 mg/L, immature: total neutrophil ratio 0.56). She subsequently received another course of parenteral antibiotics (piperacillin-tazobactam for 9 days, followed by amoxicillin-clavulanate for 16 days) for Integra infection, and a further course later on (vancomycin for 5 days, ceftazidime for 9 days) after the first application of CEA. Systemic antibiotics was stopped when her wounds were noted to be clean clinically on day 42 of life. The patient also received fluconazole as fungal prophylaxis from the second to seventh weeks of life, when her peripherally inserted central catheter was no longer required and removed
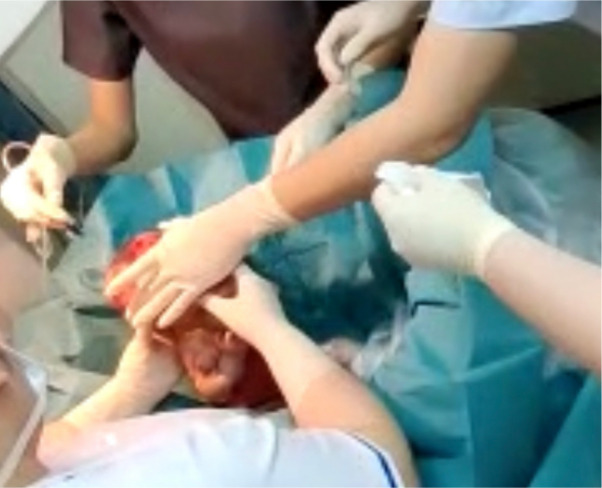
Dressings and sterile baths using saline (figure 4) were performed at highly regular intervals every 2–3 days throughout her hospitalisation and involved the use of bacteriostatic nanocrystalline silver dressings, such as Acticoat (Smith and Nephew) and Mepilex Ag (Molyncke). The use of potentially cytotoxic topicals and dressings were temporarily stopped prior to and after each CEA application due to the possible cytotoxic effects of silver in wound healing.15
Figure 4.
Sterile bath using warmed saline.
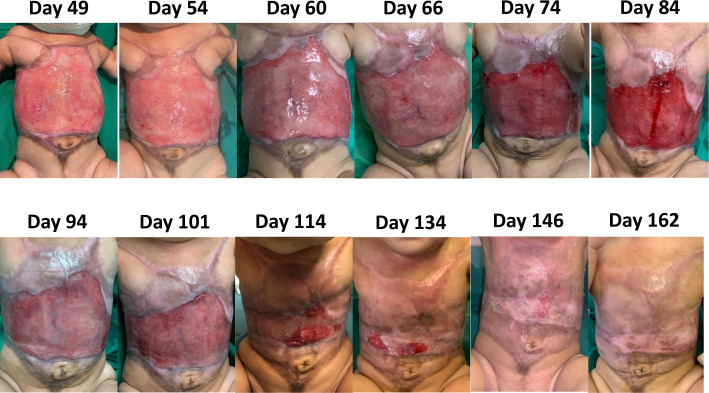
Following the third and last application of CEA on day 143 of life to both her scalp and anterior trunk, she managed to achieve complete epithelialisation of her trunk skin 9 days later, and her trunk could be exposed on day 157 of life.
During her hospitalisation, she was also given Infatrini (1 kcal/mL) formula in view of poor weight gain, and also started on weaning diet when she approached 6 months of age.
In terms of investigations for associated anomalies, she underwent a skull X-ray early on which did not show any calvarial skull defects that other babies with scalp ACC reportedly had.3
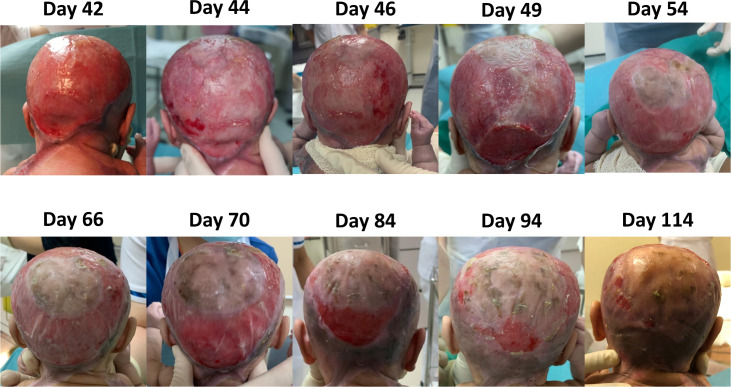
At the point of discharge from the hospital (day 187 of life), she had achieved complete epithelialisation of her trunk and near complete epithelialisation of her scalp (see figures 5 and 6). She still had a small residual wound on her crown, which was about 1.5% BSA, which then required regular dressing at the specialist outpatient clinic.
Figure 5.
Progressive epithelialisation of her scalp.
Figure 6.
Progressive epithelialisation of her trunk.
Outcome and follow-up
We continued with regular wound dressings for her head in the outpatient setting, three times a week.
Discussion
ACC is a rare condition with many subtypes and varied presentations in terms of extent, and location.3 4 6 7 25
In terms of the extent of involvement, to the best of the authors’ knowledge, our patient possibly has one of the largest extent in the reported literature, among surviving patients with ACC. Although there are numerous case reports of ACC, only a handful describe ‘extensive ACC’, including seven that involve the scalp,21 26–31 four that affect the trunk,32–35 and three that affect both the head and trunk. Among these, only the latter three36–38 are nearly as extensive as our patient, with only one of them having survived.38 This particular patient was similar to our patient, and had both scalp and trunk involvement, although the exact extent in terms of BSA was not reported.
We have classified our patient as Frieden Group V ACC. Frieden4 classified ACC into nine groups in 1986. Group I ACC, the most common type, is localised on the scalp with no other associated anomalies. Group II is scalp involvement associated with limb abnormalities. Group III is scalp involvement associated with epidermal nevus, neurological and ophthalmic abnormalities. Group IV is ACC accompanied by embryological deformities, and can affect the scalp, abdomen, lumbar and any other sites. Group V is ACC associated with fetus papyraceus or placental infarction with the extended absence of the skin on the trunk or limbs. Group VI ACC is divided into localise on extremities or widespread on extremities and torso accompanied by epidermolysis bullosa (EB). Group VII is ACC on extremities without EB. Group VIII is ACC associated with teratogens or varicella and herpes simplex infections, and can affect scalp locally or any other areas. Group IX is ACC associated with malformation syndromes. Based on Frieden’s classification, Groups I to III only involve the scalp and were excluded because our patient had extensive truncal involvement. Group VII only involved the extremities and was excluded too.
Groups IV, V, VI and VIII are ACC that affect the trunk, and were thus considered for our patient. Group IV ACC is less likely as it is accompanied by other congenital malformations, such as meningomyelocele, spinal dysraphia, cranial stenosis, omphalocele and gastroschisis, which were not present in our patient. Group VI ACC is classically associated with EB or blistering, which were not present in our patient. Group VIII ACC is caused by specific teratogens, but there was no antenatal history of varicella or herpes simplex infection nor any significant medication history during pregnancy. Therefore, type VIII is unlikely.
As our patient did not have limb abnormalities, it was unlikely to be Adams-Oliver syndrome.12 13 39 Absence of blistering and EB also made Bart Syndrome40 unlikely.
The above-mentioned cases of extensive ACC with truncal involvement were classified as Group V ACC,32–35 38 even though two of them, namely Boente et al32 and Effendi et al,38 did not have a history of fetus papyraceous or vanishing twin, as was the case in our patient. In these cases, the lesion had a highly characteristic, symmetric distribution pattern that encircled the umbilicus and formed an ‘H’ pattern on the anterior or posterior trunk, which was very similar to the presentation in our patient. As such, we have classified our patient as having Group V ACC, although without fetus papyraceous.
In terms of investigations for associated congenital anomalies, apart from a normal skull X-ray, she did not undergo further imaging including MRI of her brain as her neurological examination was unremarkable and she was developmentally age appropriate. Moreover, a cohort study of 69 patients with scalp ACC who underwent neuroimaging concluded there was a low risk of detecting central nervous system abnormality.41 Her physical examination and clinical course were otherwise unremarkable, and she hence did not undergo other investigations including echocardiography and ultrasound imaging of her abdomen, which was suggested in a review article.3
Diagnosis of ACC is usually made clinically in the postnatal period, as was the case in our patient. The antenatal fetal anomaly and subsequent obstetric scans for our patient were unremarkable apart from evidence of uteroplacental insufficiency. This was congruent with the literature showing antenatal diagnosis was rare, with only four cases42–45 detected on obstetric scan. Cambiaghi et al42 reported that a patient with scalp ACC was found on an ultrasonographic examination at 27 weeks’ gestation to have a well-defined, exophytic, parieto-occipital tumour which did not involve the underlying endocranial structures. Liu et al44 reported antenatal diagnosis of truncal ACC during an ultrasonographic examination at 28 weeks of gestation when strong echoes were absent for some of the abdominal circumference and the liver appeared to be exposed. Bick et al46 suggested there was an association between ACC and elevated concentrations of alpha-fetoprotein and presence of acetyl-cholinesterase in amniotic fluid, although it is not standard practice to measure acetyl-cholinesterase levels in the amniotic fluid.
There is no consensus in the management of extensive ACC. Different management strategies have been proposed, including conservative31 47–50 and surgical approaches.12 15 20 21 24 51–53
Harvey et al48 have proposed the adoption of a conservative approach to the management of ACC of the scalp. In this case series, 13 out of 17 cases of ACC were managed conservatively, which involved regular wound dressings and use of systemic antibiotics. Two out of the four cases that were managed surgically, in the form of split skin graft and rotation flaps, developed complications in the form graft failure and partial flap failure. However, we note the majority of these 17 ACC cases had less extensive ACC, in the form of isolated scalp vertex or parieto-occipital involvement, with none of the cases having entire scalp involvement, as was the case in our patient.
In addition, it is pertinent to note that cases of extensive scalp ACC have also been reported to be associated with the life-threatening complications, including superior sagittal sinus haemorrhage8 9 and brain herniation.10 Given these potentially fatal complications, our multi-disciplinary team decided on a surgical approach, in order to achieve urgent and prompt coverage of her scalp.
With regards to the available options for skin coverage in our patient, autografts were preferred over parental allografts due to lower risks of rejection and higher graft take rates. However, in this instance our patient had extensive ACC affecting TBSA 37%, and it was not possible to obtain sufficient autograft without significant donor site morbidity. We were able to circumvent this lack of normal skin with the staged use of CEA, which requires 3 weeks for culture in our inhouse laboratory.
In the interim, we needed a temporary solution for coverage and to allow more robust CEA take. Options explored included artificial skin substitutes15 17–19 51 and parental allografts,12–14 both of which have been used for ACC in the literature. We decided to proceed with CEA to minimise morbidity in the baby’s parents, reduce risk of rejection and to circumvent ethical considerations. The use of Integra has been described in two ACC patients.20 21 It is a non-cellular artificial matrix comprising an outer silicone layer that simulates the epidermis and an inner three-dimensional porous matrix of cross-linked collagen and glycosaminoglycan of bovine origin which acts as a dermal regeneration template. Following migration of endothelial cells and fibroblasts, the neodermis that is formed is purportedly, histologically and functionally, very similar to normal dermis. The use of CEA involves cultivating autologous keratinocytes for the purpose of autografting and has more commonly been used for paediatric burns patients with large wounds.15 In burns patients, the CEA would be placed directly on an allodermis, whereas in our case, we have placed the CEA directly on the Integra ADM.
Subsequently, with each application of CEA, it was evident that epithelisation was expedited and achieved (see figures 5 and 6).
In terms of prognosis, based on the favourable outcome of the patient reported by Effendi et al, we expect our patient to eventually achieve complete epithelialisation. Even after complete epithelialisation, the patient will need follow-up as complications including skin break-down and scar contractures remain clinical possibilities.
Learning points.
The management of aplasia cutis congenita (ACC) should take into consideration the extent and location of ACC.
Where there is extensive scalp ACC, consideration should be given for surgical management to provide prompt coverage, to prevent the potentially life-threatening complications of superior sagittal sinus haemorrhage and brain herniation.
Regular wound dressings, including the use of bacteriostatic dressings, sterile baths and strict infection control are critical for wound healing in extensive ACC.
Acknowledgments
Associate Professor Ong Yee Siang is a senior plastic surgeon who was involved in the management of the patient, and approved of the final manuscript. Dr Poon Woei Bing is a senior neonatologist who was involved in the management of the patient, and also approved of the final manuscript.
Footnotes
Contributors: AN was the primary physician who took care of the patient throughout the 6 months of hospitalisation and wrote the manuscript. CH is the plastic surgeon who meticulously applied the artificial dermal matrix, followed by cultured epithelial autograft, and carried out the subsequent wound dressings with AWCC. AWCC of the Singapore General Hospital Skin Bank provided the cultured epithelial autograft. MK assisted with the diagnosis of Aplasia Cutis Congenita, provided advice on management and edited the manuscript. Dr PWB and OYS were involved in the management of the patient, and approved of the final manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Case reports provide a valuable learning resource for the scientific community and can indicate areas of interest for future research. They should not be used in isolation to guide treatment choices or public health policy.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Consent obtained from parent(s)/guardian(s)
References
- 1.Cordon M. Extrait d’une lettre au sujet de trios enfants de la meme mere nes avec partie des extremites denuee de peau. J Med Chir Pharm 1767;26:556–8. [Google Scholar]
- 2.Browning JC. Aplasia cutis congenita: approach to evaluation and management. Dermatol Ther 2013;26:439–44. 10.1111/dth.12106 [DOI] [PubMed] [Google Scholar]
- 3.Humphrey SR, Hu X, Adamson K, et al. A practical approach to the evaluation and treatment of an infant with aplasia cutis congenita. J Perinatol. 2018;38:110–7. 2017. 10.1038/jp.2017.142 [DOI] [PubMed] [Google Scholar]
- 4.Frieden IJ. Aplasia cutis congenita: a clinical review and proposal for classification. J Am Acad Dermatol 1986;14:646–60. 10.1016/S0190-9622(86)70082-0 [DOI] [PubMed] [Google Scholar]
- 5.Evers ME, Steijlen PM, Hamel BC. Aplasia cutis congenita and associated disorders: an update. Clin Genet 1995;47:295–301. 10.1111/j.1399-0004.1995.tb03968.x [DOI] [PubMed] [Google Scholar]
- 6.Silberstein E, Pagkalos VA, Landau D, et al. Aplasia cutis congenita: clinical management and a new classification system. Plast Reconstr Surg 2014;134:766e–74. 10.1097/PRS.0000000000000638 [DOI] [PubMed] [Google Scholar]
- 7.Sathishkumar D, Ogboli M, Moss C. Classification of aplasia cutis congenita: a 25-year review of cases presenting to a tertiary paediatric dermatology department. Clin Exp Dermatol 2020;45:994–1002. 10.1111/ced.14331 [DOI] [PubMed] [Google Scholar]
- 8.GLASSON DW, DUNCAN GM. Aplasia cutis congenita of the scalp: delayed closure complicated by massive hemorrhage. In: Plastic and reconstructive surgery. Hagerstown, MD: Lippincott Williams & Wilkins, 19631985: 423–5. http://nus.summon.serialssolutions.com/2.0.0/link/0/eLvHCXMwtV3da9swEBdJB2Owh320LOva6WkvwcGxZFse7GG0XQfdRiENG30xkiKvgdYJc1vo0_713UmynGQdYw97MUbEH9HvfHc63f2OEJaM4mhDJ6hUCZEqbAcnlNKxkEoZ0J2SMzEbK2O7vyWTc3Z0ws57vZ9taUw3-F-RhzHAHitp_wH9cFMYgHOQATiCFMDxdzm41xydgoPc0rLa9a_njL01w8YVRVu2JvhGQljAJzAvbY3lUMPrNZifDg-ZX8s2raABeJcYUUCiyTtwXPXlAuONXZq6826vwD_Hh11gUu-PC_k9yNLxJ2SVtIkGh6NhiPYcTr8cvLejxyMfrZ25Qj2RbsYl7uGXsHGJ-SUm_6zqZJaAMXA8tiPj1XBSRDzha3radVjx8shWlC53FcvefnNXSL1Orb1h8kIiYgELSDFO3yDP-tVsrq_fmTqaTvrkQcJZhnrzREzCrlTOMtcRw7_xmi_zeClx3ivXD-XPCxbruJw9IdtdSSc9DdLxlPRM_Yw8_OwzK56Trx5sasGmAWy6qCiATS3Yb6mHmnqo6QrUVN1RDzXtoN4m0w9HZwcfI990I9LoTcP_0kLFpspNxbXRTBXg52RVnhlw_YXOjJSFVnCiTcxVjuz8aREXDPQ-XKRztkO26kVtXhDKxszAj8FqaMa5YrJIdZJLXaSSy5ibARm301cuHbdKuZITgazJvETRAkMUI4st-JMDsrc2z-FCj-SAvG7nvQRFibtfsjaLm6bEHeiCx_mA7Dg4wqXgkoO8sZd_ufUuedTJ-SuyBd-q2SP9-qbZJ_38m9i3wvILri2Qkg [DOI] [PubMed] [Google Scholar]
- 9.Johnson R, Offiah A, Cohen MC. Fatal superior sagittal sinus hemorrhage as a complication of aplasia cutis congenita: a case report and literature review. Forensic Sci Med Pathol 2015;11:243–8. 10.1007/s12024-014-9645-5 [DOI] [PubMed] [Google Scholar]
- 10.Tröbs R-B, Barenberg K, Hemminghaus M, et al. Herniation of the brain after conservative treatment of a large congenital skull defect in an infant with Adams-Oliver syndrome. J Pediatr Surg 2010;45:2064–7. 10.1016/j.jpedsurg.2010.06.029 [DOI] [PubMed] [Google Scholar]
- 11.Duan X, Yang GE, Yu D, et al. Aplasia cutis congenita: a case report and literature review. Exp Ther Med 2015;10:1893–5. 10.3892/etm.2015.2737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Henderson TOW, Thomas GPL, Wall SA. Parental allografts in the management of Adams-Oliver syndrome. Childs Nerv Syst 2013;29:1223–4. 10.1007/s00381-013-2174-9 [DOI] [PubMed] [Google Scholar]
- 13.Udayakumaran S, Mathew J, Panikar D. Dilemmas and challenges in the management of a neonate with Adams-Oliver syndrome with infected giant aplasia cutis lesion and exsanguination: a case-based update. Childs Nerv Syst 2013;29:535–41. 10.1007/s00381-012-1999-y [DOI] [PubMed] [Google Scholar]
- 14.Verhelle NAC, Heymans O, Deleuze JP, et al. Abdominal aplasia cutis congenita: case report and review of the literature. J Pediatr Surg 2004;39:237–9. 10.1016/j.jpedsurg.2003.10.021 [DOI] [PubMed] [Google Scholar]
- 15.Sood R, Balledux J, Koumanis DJ, et al. Coverage of large pediatric wounds with cultured epithelial autografts in congenital nevi and burns: results and technique. J Burn Care Res 2009;30:576–86. 10.1097/BCR.0b013e3181ac02de [DOI] [PubMed] [Google Scholar]
- 16.Chung KH, Kim TK, Cho BC, et al. Surgical treatment of aplasia cutis congenita with acellular dermal graft and cultured epithelial autograft. Dermatol Surg 2009;35:546–9. 10.1111/j.1524-4725.2009.01087.x [DOI] [PubMed] [Google Scholar]
- 17.Barillo DJ, Nangle ME, Farrell K. Preliminary experience with cultured epidermal autograft in a community hospital burn unit. J Burn Care Rehabil 1992;13:158–65. 10.1097/00004630-199201000-00035 [DOI] [PubMed] [Google Scholar]
- 18.Meuli M, Raghunath M. Burns (Part 2). tops and flops using cultured epithelial autografts in children. Pediatr Surg Int 1997;12:471–7. 10.1007/BF01258705 [DOI] [PubMed] [Google Scholar]
- 19.Lo CH, Chong E, Akbarzadeh S, et al. A systematic review: current trends and take rates of cultured epithelial autografts in the treatment of patients with burn injuries. Wound Repair Regen 2019;27:693–701. 10.1111/wrr.12748 [DOI] [PubMed] [Google Scholar]
- 20.Trah J, Has C, Hausser I, et al. Integra®-Dermal regeneration template and split-thickness skin grafting: a therapy approach to correct aplasia cutis congenita and epidermolysis bullosa in Carmi syndrome. Dermatol Ther 2018;8:313–21. 10.1007/s13555-018-0237-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scotti A, Benanti E, Augelli F, et al. A case of large aplasia cutis congenita with underlying skull defect: effective surgical treatment with Integra® dermal regeneration template. Pediatr Neurosurg 2021;56:268–73. 10.1159/000512022 [DOI] [PubMed] [Google Scholar]
- 22.Bui D, Ikeda C. Reconstruction of aplasia cutis congenita (group V) of the trunk in a newborn. Plast Reconstr Surg. 2003;111:2119–20 10.1097/01.PRS.0000057071.85921.3D [DOI] [PubMed] [Google Scholar]
- 23.Smartt JM, Kim EM, Tobias AM, et al. Aplasia cutis congenita with calvarial defects: a simplified management strategy using acellular dermal matrix. Plast Reconstr Surg. 2008;121:1224–9 10.1097/01.prs.0000302588.95409.fe [DOI] [PubMed] [Google Scholar]
- 24.Herman O, Weiss J, Muhlbauer B, et al. Congenital aplasia cutis: nonsurgical treatment with a synthetic skin substitute. Plast Reconstr Surg 1989;83:886–8. [PubMed] [Google Scholar]
- 25.Schierz IAM, Giuffrè M, Del Vecchio A, et al. Recognizable neonatal clinical features of aplasia cutis congenita. Ital J Pediatr 2020;46:25. 10.1186/s13052-020-0789-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burkhead A, Poindexter G, Morrell DS. A case of extensive aplasia cutis congenita with underlying skull defect and central nervous system malformation: discussion of large skin defects, complications, treatment and outcome. J Perinatol 2009;29:582–4. 10.1038/jp.2008.250 [DOI] [PubMed] [Google Scholar]
- 27.Rocha D, Rodrigues J, Marques JS, et al. Aplasia cutis congenita: a conservative approach of a case with large, extensive skin, and underlying skull defect. Clin Case Rep 2015;3:841–4. 10.1002/ccr3.361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kantor J, Yan AC, Hivnor CM, et al. Extensive aplasia cutis congenita and the risk of sagittal sinus thrombosis. Arch Dermatol. 2005;141:554–6 10.1001/archderm.141.5.554 [DOI] [PubMed] [Google Scholar]
- 29.Torkamand F, Ayati A, Habibi Z, et al. Extensive aplasia cutis congenita associated with cephalocranial disproportion and brain extrusion. Childs Nerv Syst 2019;35:1629–32. 10.1007/s00381-019-04188-y [DOI] [PubMed] [Google Scholar]
- 30.Gómez M, Chiesura V, Noguera-Morel L, et al. Extensive intracranial arteriovenous malformation in a child with aplasia cutis congenita. Pediatr Dermatol 2015;32:e163–4. 10.1111/pde.12580 [DOI] [PubMed] [Google Scholar]
- 31.Starcevic M, Sepec MP, Zah V. A case of extensive aplasia cutis congenita: a conservative approach. Pediatr Dermatol 2010;27:540–2. 10.1111/j.1525-1470.2010.01266.x [DOI] [PubMed] [Google Scholar]
- 32.Boente MdelC, Frontini MdelV, Acosta MI, et al. Extensive symmetric truncal aplasia cutis congenita without fetus papyraceus or macroscopic evidence of placental abnormalities. Pediatr Dermatol 1995;12:228–30. 10.1111/j.1525-1470.1995.tb00164.x [DOI] [PubMed] [Google Scholar]
- 33.Chan RK, Liu AS, Rogers GF. Aplasia cutis congenita of the trunk associated with fetus papyraceous. J Craniofac Surg 2012;23:995–7. 10.1097/SCS.0b013e31824e27ac [DOI] [PubMed] [Google Scholar]
- 34.Leung AKC, Leong KF, Lam JM. Extensive aplasia cutis congenita encircling the trunk associated with fetus papyraceus. Neerja Bharti, Bharti N, editors. Case Rep Pediatr 2020:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bourque S, Preloger E. Extensive aplasia cutis congenita with associated vanishing twin syndrome. J Pediatr 2015;167:772–772.e1. 10.1016/j.jpeds.2015.06.041 [DOI] [PubMed] [Google Scholar]
- 36.Morrell DS, Rubenstein DS, Briggaman RA, et al. Congenital pyloric atresia in a newborn with extensive aplasia cutis congenita and epidermolysis bullosa simplex. Br J Dermatol. 2000;143:1342–3 10.1046/j.1365-2133.2000.03929.x [DOI] [PubMed] [Google Scholar]
- 37.Park MS, Hahn SH, Hong CH, et al. Extensive form of aplasia cutis congenita: a new syndrome? J Med Genet 1998;35:609–11. 10.1136/jmg.35.7.609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Effendi RMRA, Nuraeni L, Diana IA, et al. Extensive type V aplasia cutis congenita without fetus papyraceus or placental infarction: a rare case. Clin Cosmet Investig Dermatol 2021;14:1413–8. 10.2147/CCID.S330160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Renfree KJ, Dell PC. Distal limb defects and aplasia cutis: Adams–Oliver syndrome. J Hand Surg Am 2016;41:e207–10. 10.1016/j.jhsa.2016.04.014 [DOI] [PubMed] [Google Scholar]
- 40.Kulalı F, Bas AY, Kale Y, et al. Type VI Aplasia Cutis Congenita: Bart’s Syndrome. Morita A, editor. Case Rep Dermatol Med 2015;2015:549825–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kuemmet TJ, Miller JJ, Michalik D, et al. Low risk of clinically important central nervous system dysraphism in a cohort study of 69 patients with isolated aplasia cutis congenita of the head. Pediatr Dermatol 2020;37:455–60. 10.1111/pde.14117 [DOI] [PubMed] [Google Scholar]
- 42.Cambiaghi S, Gelmetti C, Nicolini U. Prenatal findings in membranous aplasia cutis. J Am Acad Dermatol 1998;39:638–40. 10.1016/S0190-9622(98)70016-7 [DOI] [PubMed] [Google Scholar]
- 43.Maeda K, Kaji T, Hayahi A, et al. EP17.29: prenatal diagnosis of aplasia cutis congenita and congenital defect of scalp and skull. Ultrasound Obstet Gynecol 2019;54:341. 10.1002/uog.21470 [DOI] [Google Scholar]
- 44.Liu F, Chen X, Tu R, et al. Prenatal diagnosis of aplasia cutis congenita of the trunk. Int J Dermatol 2014;53:1269–71. 10.1111/j.1365-4632.2012.05732.x [DOI] [PubMed] [Google Scholar]
- 45.Jelin AC, Glenn OA, Strachowski L, et al. Membranous aplasia cutis congenita. J Ultrasound Med 2009;28:1393–6. 10.7863/jum.2009.28.10.1393 [DOI] [PubMed] [Google Scholar]
- 46.Bick DP, Balkite EA, Baumgarten A, et al. The association of congenital skin disorders with acetylcholinesterase in amniotic fluid. Prenat Diagn 1987;7:543–9. 10.1002/pd.1970070803 [DOI] [PubMed] [Google Scholar]
- 47.Barcot Z, Baskovic M, Car A, et al. Aplasia cutis congenita of the scalp: the success of conservative approach in treatment of a large defect. Indian J Paediatr Dermatol 2019;20:166–8. [Google Scholar]
- 48.Harvey G, Solanki NS, Anderson PJ, et al. Management of aplasia cutis congenita of the scalp. J Craniofac Surg 2012;23:1662–4. 10.1097/SCS.0b013e31826542de [DOI] [PubMed] [Google Scholar]
- 49.Ross DA, Laurie SW, Coombs CJ, et al. Aplasia cutis congenita: failed conservative treatment. Plast Reconstr Surg. 1995;95:124–9 10.1097/00006534-199501000-00020 [DOI] [PubMed] [Google Scholar]
- 50.Wexler A, Harris M, Lesavoy M. Conservative treatment of cutis aplasia. Plast Reconstr Surg. 1990;86:1066–71 10.1097/00006534-199012000-00003 [DOI] [PubMed] [Google Scholar]
- 51.Donati V, Arena S, Capilli G, et al. Reparation of a severe case of aplasia cutis congenita with engineered skin. Biol Neonate 2001;80:273–6. 10.1159/000047156 [DOI] [PubMed] [Google Scholar]
- 52.Winston KR, Ketch LL. Aplasia cutis congenita of the scalp, composite type: the criticality and inseparability of neurosurgical and plastic surgical management. Pediatr Neurosurg 2016;51:111–20. 10.1159/000442989 [DOI] [PubMed] [Google Scholar]
- 53.Bang RL, Ghoneim IE, Gang RK, et al. Treatment dilemma: conservative versus surgery in cutis aplasia congenita. Eur J Pediatr Surg 2003;13:125–9. 10.1055/s-2003-39562 [DOI] [PubMed] [Google Scholar]