Abstract
Background
Protothecosis is a rare infection in humans and animals caused by the achlorophyllic algae Prototheca species. More than half of the protothecosis cases are cutaneous infections, and most cases are observed in immunocompromised individuals.
Case presentation
We report a case of Prototheca wickerhamii infection in the mucosa of the pharynx in a 53-year-old immunocompetent woman with an incidentally found mass lesion at the left tongue base. Histopathological findings of the mass lesion suggested cryptococcosis, but P. wickerhamii was identified from the oropharynx scrape culture based on DNA sequencing. After surgical resection, fosfluconazole treatment was initiated, and subsequently, treatment was switched to topical amphotericin B. The residual mass lesion did not deteriorate during the 4-month antifungal treatment and 1-year observational period.
Conclusions
Prototheca species can be easily misdiagnosed as yeasts because of their morphological and pathological similarities. Prototheca, in addition to Cryptococcus should be considered if slow-growing, large Gram-positive organisms are encountered. Lactophenol cotton blue staining of the colony helps distinguish these organisms. Further study is needed to determine the appropriate treatment according to the infection focus.
Keywords: Prototheca wickerhamii, Protothecosis, Larynx, Cryptococcus, Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry
Background
Protothecosis is a rare infection caused by Prototheca species in both humans and animals. These organisms are achlorophyllic algae that are ubiquitous in the environment and animal intestinal flora. The genus Prototheca is divided into eight species, and the most common causative species of human infection are Prototheca wickerhamii and Prototheca zopfi [1]. Since the first case of human protothecosis in 1964, approximately 200 cases have been reported [2–4]. The clinical forms of protothecosis are classified into three types: cutaneous disease, olecranon bursitis, and disseminated disease [3, 5, 6]. More than half of the cases are cutaneous infections, and immunosuppression is a predisposing factor for human protothecosis [2–4].
We report a case of P. wickerhamii infection in the pharynx of an immunocompetent patient, successfully treated with a combination of surgical resection and medical therapy.
Case presentation
A 53-year-old Japanese woman with no significant medical history other than chronic gastritis, diagnosed by upper gastrointestinal endoscopy 6 years previously, presented to our hospital with a mass in the larynx that appeared to be malignant. She had a 1-year history of a dull feeling in her throat and cough. Three months earlier, she had been diagnosed with anisakiasis at a local clinic and had been incidentally found to have mass lesion of approximately 7-mm in diameter at the left tongue base, by upper gastrointestinal endoscopy. One month earlier, follow-up nasopharyngoscopy had revealed no changes in the mass lesion, and an endoscopic biopsy had been performed. Squamous cell carcinoma was suspected pathologically, and the patient was referred to the department of otorhinolaryngology at our hospital for further evaluation.
An endoscopic biopsy was also performed in our outpatient clinic (Fig. 1a), but the biopsy specimens only showed atypical epithelium, and the scrape culture was negative. Intravenous contrast-enhanced computed tomography (CT) of the neck and thorax was unremarkable, except for bilateral cervical lymphadenopathy.
Fig. 1.

Macroscopic appearance of protothecosis in the pharyngeal mucosa. a Endoscopic image taken at our outpatient clinic showing a polypoid mass at the left tongue base. b Intraoperative close-up image showing a reddish, smooth, and pedunculated mass without adjacent dysplastic mucosa
For further evaluation, an excision biopsy under general anesthesia was performed (Fig. 1b). Histopathological examination of a hematoxylin and eosin-stained biopsy specimen showed granulomatous tissue consisting mainly of histiocytes and multinucleated giant cells (Fig. 2a). Some histiocytes had phagocytized the encapsulated yeast-like organisms that were invading the epithelium. There were also scant neutrophils, but no micro-abscesses were found. The walls of the mass were positive on staining with Grocott’s methenamine silver (Fig. 2b and c). These findings suggested cryptococcosis; therefore, she was referred to the Department of Infectious Disease for the treatment of the residual mass lesion.
Fig. 2.

Histopathological findings of protothecosis in the excision biopsy specimen. a Hematoxylin and Eosin staining of the lesion showing granulomatous inflammation. b, c Grocott–Gomori methenamine silver staining of mass lesion biopsy specimen showing encapsulated yeast-like organisms
On physical examination, the patient was afebrile, and her vital signs were normal. Head and neck examination revealed no enlarged lymph nodes, and no meningeal signs. Examination of the pharynx revealed no pharyngeal edema or exudate. She had no skin lesions. The blood test results were unremarkable. An HIV antibody/antigen combination test result was negative. A neutrophil function test was not performed because she did not have a history of recurrent or severe bacterial infection, suggesting that her neutrophil function was normal. Chest CT revealed no pulmonary findings of note. A serum Cryptococcus antigen test (Bio-Medical Laboratories, Inc.), using a latex agglutination method was negative. Serum beta D glucan was not measured. Based on these findings and the histopathology, she was provisionally diagnosed with possible non-meningeal, non-pulmonary cryptococcosis. The scrape culture of the residual lesion at the base of the tongue was repeated, and then fosfluconazole treatment (6 mg/kg bodyweight/day) was initiated as treatment for localized cryptococcosis.
After 3 days of incubation of the separation culture that targeted Cryptococcus from the scrape specimen, white to pale purple-colored small colonies grew on the XM-Candida agar plate (Nissui Pharmaceutical Co., Ltd., Tokyo, Japan) cultured at 35 °C in aerobic conditions (Fig. 3a). The VITEK® 2 COMPACT Microbial Detection System (version 8.01 database: SYSMEX bioMérieux Co., Ltd., Tokyo, Japan) based on the biochemical reaction method with yeast identification card identified the colonies as P. wickerhamii. Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS, using the MALDI Biotyper version 4.0.0.1 database; Bruker Daltonik, Germany) did not identify the colonies initially, but in the re-examination, it identified the colonies as P. wickerhamii with low probability (score 1.451). Lactophenol cotton blue staining of the colony revealed tightly packed endospores within the sporangia distinctive of P. wickerhamii [7] (Fig. 3b).
Fig. 3.
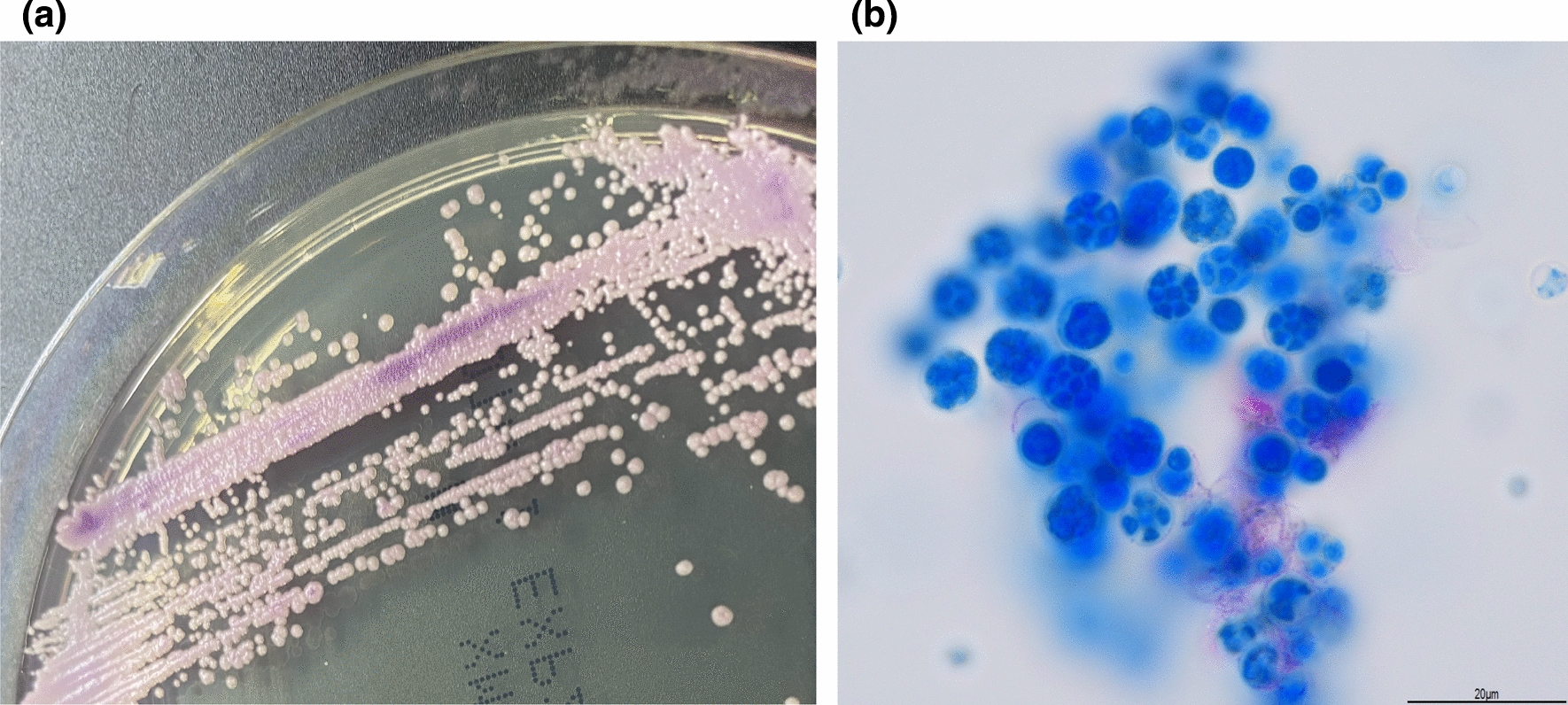
Microbiological findings of the Prototheca isolate. a Colony appearance of the Prototheca isolate grew on XM-Candida agar plate (Nissui Pharmaceutical Co., Ltd., Tokyo, Japan) at 35 °C in aerobic conditions for 3 days. b Lactophenol cotton blue staining of the Prototheca colony showing tightly packed endospores within a sporangium. (Pictured over cover glass at 1000× magnification using an oil immersion lens)
DNA was extracted and polymerase chain reaction (PCR) method was performed using primers to amplify the internal transcribed spacer region and the D1/D2 domain of the large subunit ribosomal DNA gene. Sequence analysis of the amplicons showed no significant results, suggesting genetic polymorphism. Cloning was performed, and base sequences showing high homology with P. wickerhamii genes were detected.
Therefore, a diagnosis of laryngeal protothecosis was established. The minimum inhibitory concentration (MIC) results using the Frozen Plate for Antifungal Susceptibility Testing of Yeasts, Eiken (Eiken Chemical Co., Ltd., Tokyo) were as follows: amphotericin B, 1 µg/mL; fluconazole, > 64 µg/mL; itraconazole, 4 µg/mL; voriconazole, 1 µg/mL; miconazole, > 16 µg/mL; flucytosine, > 64 µg/mL; and micafungin, > 16 µg/mL. Empiric fosfluconazole treatment was discontinued after 10 weeks because the size of the residual mass lesion did not change. Amphotericin B syrup (1 mL, 4 times a day) was initiated and continued for 6 weeks instead of intravenous amphotericin B treatment because the patient was asymptomatic and could not take time off from work to be admitted to hospital for intravenous amphotericin B treatment. Although we considered additional and definitive resection after the patient was diagnosed with Prototheca infection, we decided against it because we anticipated that it would be difficult to remove the lesion with safety margins because the vertical margin was not clearly determined on macroscopic examination, and resection carried a risk of causing difficulties with speech and swallowing. The residual mass lesion did not deteriorate during the antifungal treatment or the post-treatment one-year follow-up period.
Discussion and conclusions
Protothecosis has been classified into three types of clinical forms: cutaneous infections, olecranon bursitis, and disseminated infections. More than half of protothecosis cases are cutaneous infections [3, 5, 6]. The majority of cases occur in immunosuppressed individuals [3, 4], while olecranon bursitis can occur in immunocompetent individuals after some types of penetrating trauma to the elbow [6]. The main underlying conditions of protothecosis are local or systemic steroid use, hematologic malignancy or cancer, diabetes mellitus, acquired immunodeficiency syndrome, solid organ transplantation, alcoholism, and peritoneal dialysis [3]. We describe a rare case of protothecosis in the pharyngeal mucosa of an immunocompetent patient.
Although there have been approximately 200 reports of protothecosis [2], to our knowledge, there have been only two previous reports of protothecosis in the field of otorhinolaryngology: a nasopharyngeal ulceration complicating prolonged endotracheal intubation in 1992 [8] and protothecosis of the larynx [9]. The laryngeal infection occurred in an immunocompetent individual, but a branchiogenic cyst was observed close to the site of the infection, and it was suspected that the inflamed cyst might have provided a portal of entry for Prototheca species. Unlike the two previous cases, our patient did not have any pre-existing mucosal defects or anatomical abnormalities. She had undergone upper gastrointestinal endoscopy 6 years before the diagnosis but there have been no reports of Prototheca algae being introduced to the pharynx by endoscopy; thus, the source of the infection is unclear. The patient’s blood test results and medical history did not suggest an immune-compromised state; however, it has been reported that qualitative factors may play a greater role than quantitative factors in neutrophil defense against P. wickerhamii [10]. Thus, it is difficult to evaluate the actual level of immunity against Prototheca species.
The diagnosis of protothecosis is conventionally based on morphological and biochemical tests of the isolated organism and histopathological tests of the affected tissues [1, 3]. The colony characteristics of Prototheca species are similar to those of yeasts such as Candida species or Cryptococcus species [5, 11] and there have been case reports that mistakenly identified Prototheca species as yeasts [12–14]. The morphological characteristics of Prototheca species are due to their life cycle, where they reproduce asexually by releasing numerous sporangiospores [5, 11]. They can be distinguished from yeasts if typical morula forms containing sporangiospores are observed with lactophenol cotton blue staining [12]. However, we were unable to isolate P. wickerhamii from the oropharynx scrape culture during the initial attempt. There are two possible reasons for our failure to isolate Prototheca species: First, they are easily overgrown by bacteria when the culture is taken from contaminated sites, such as the pharynx. Second, most Prototheca species require incubation at 30 °C for 72 h, whereas some slow-growing strains require incubation at 25 °C for up to 8 days; thus, they can be missed using standard culture methods [3, 11].
In the histopathological examination, characteristic morula forms are helpful for diagnosing protothecosis, but there can be a lack of such findings due to the period of their life cycle. The external capsule of Prototheca species and the wall of yeasts stain positive with Grocott’s methenamine silver and periodic acid-Schiff stains [15]. Thus, they may be mistaken for Candida or Cryptococcus species, as we experienced.
As discussed above, Prototheca species can be easily misdiagnosed as yeasts because of their morphological and pathologic similarities. In our case, VITEK2 and MALDI-TOF MS testing were useful for making the diagnosis. Although the results are not reproducible, we established a differential diagnosis of protothecosis. Recently, molecular characterization of ribosomal DNA has been exploited for intraspecies identification of Prototheca species. Previous studies have shown that comprehensive analysis by PCR of the internal transcribed spacer region and the large subunit D1/D2 domain is useful for species identification [16, 17]. In this case, we confirmed the diagnosis of protothecosis based on DNA sequencing, combined with morphological, histopathological, and biochemical findings.
There is no standard treatment for protothecosis. Many treatment strategies have been attempted, with variable clinical response [4]. A combination of medical and surgical approaches is most commonly used, and antifungal drugs, such as ketoconazole, itraconazole, fluconazole, conventional amphotericin B, and liposomal amphotericin B are the most commonly used antimicrobial agents [3]. Previous studies have shown that Prototheca species are normally susceptible to amphotericin B and variably susceptible to azoles [4, 18, 19]. The susceptibility to azoles could be explained by the presence of ergosterol in their cell membranes, and the absence of D-glucans in their cell walls could be the reason for resistance to echinocandins [19–21]. Our patient was successfully treated with excision biopsy and antifungal therapy, including fosfluconazole and topical amphotericin B, based on the MIC results. However, as there are no official guidelines and breakpoints for in vitro susceptibility testing of Prototheca species, it is difficult to interpret the results of disc diffusion zone diameters and MICs using automated systems or E-tests [22]. MIC testing is not always reproducible, and when it comes to treatment with azoles, in vitro susceptibility does not correlate with favorable clinical outcomes [4, 8]. It remains debatable how these results should be interpreted, but in vitro susceptibility testing could be helpful for choosing a better treatment regimen.
Here, we report a case of human protothecosis of the pharynx, and our results provide valuable information on the diagnosis and treatment of protothecosis in clinical practice.
Acknowledgements
We would like to thank Editage (www.editage.com) for English language editing.
Abbreviations
- CT
Computed tomography
- MALDI-TOF
Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry
- MIC
Minimum inhibitory concentration
- PCR
Polymerase chain reaction
Authors’ contributions
MY, M Ikeda and MA treated the patient, described the manuscript. IK and YO worked to identify the pathogen from clinical samples as clinical microbiologist. M Ikemura diagnosed pathological specimens and advised the manuscript. DJ, YK, KO, SO and KM were discussed about treatment of the patient and revised the manuscript. TU, SN, and YM were performed DNA sequencing for identification of the pathogen and revised the manuscript. All authors read and approved the final manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Availability of data and materials
The data supporting the conclusions of this article are included within the article.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Written informed consent was obtained from the patient for publication of their clinical details.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Jagielski T, Gawor J, Bakula Z, Decewicz P, Maciszewski K, Karnkowska A. cytb as a new genetic marker for differentiation of Prototheca species. J Clin Microbiol. 2018 doi: 10.1128/JCM.00584-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Inoue M, Miyashita A, Noguchi H, Hirose N, Nishimura K, Masuda M, et al. Case report of cutaneous protothecosis caused by Prototheca wickerhamii designated as genotype 2 and current status of human protothecosis in Japan. J Dermatol. 2018;45(1):67–71. doi: 10.1111/1346-8138.14010. [DOI] [PubMed] [Google Scholar]
- 3.Lass-Flörl C, Mayr A. Human protothecosis. Clin Microbiol Rev. 2007;20(2):230–42. doi: 10.1128/CMR.00032-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Todd JR, King JW, Oberle A, Matsumoto T, Odaka Y, Fowler M, et al. Protothecosis: report of a case with 20-year follow-up, and review of previously published cases. Med Mycol. 2012;50(7):673–89. doi: 10.3109/13693786.2012.677862. [DOI] [PubMed] [Google Scholar]
- 5.Min Z, Moser SA, Pappas PG. Prototheca wickerhamii algaemia presenting as cholestatic hepatitis in a patient with systemic lupus erythematosus: a case report and literature review. Med Mycol Case Rep. 2012;2:19–22. doi: 10.1016/j.mmcr.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yagnik K, Bosse R, Reppucci J, Butts R, Islam S, Cannella AP. Case report: Olecranon Bursitis due to Prototheca wickerhamii in an immunocompromised patient. Am J Trop Med Hyg. 2019;100(3):703–5. doi: 10.4269/ajtmh.18-0818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bandaranayake TD, Paniz Mondolfi A, Peaper DR, Malinis MF. Prototheca wickerhamii algaemia: an emerging infection in solid organ transplant recipients. Transpl Infect Dis. 2015;17(4):599–604. doi: 10.1111/tid.12407. [DOI] [PubMed] [Google Scholar]
- 8.Iacoviello VR, DeGirolami PC, Lucarini J, Sutker K, Williams ME, Wanke CA. Protothecosis complicating prolonged endotracheal intubation: case report and literature review. Clin Infect Dis. 1992;15(6):959–67. doi: 10.1093/clind/15.6.959. [DOI] [PubMed] [Google Scholar]
- 9.Hirata A, Tamagawa M, Urakawa K, Kakimaru T, Obayashi C. Protothecosis at the larynx. Jpn J Med Tech. 1997;46(10):1465–7. [Google Scholar]
- 10.Torres HA, Bodey GP, Tarrand JJ, Kontoyiannis DP. Protothecosis in patients with cancer: case series and literature review. Clinical Microbiol Infect. 2003;9(8):786–92. doi: 10.1046/j.1469-0691.2003.00600.x. [DOI] [PubMed] [Google Scholar]
- 11.Mohd Tap R, Sabaratnam P, Salleh MA, Abd Razak MF, Ahmad N. Characterization of Prototheca wickerhamii isolated from disseminated algaemia of kidney transplant patient from Malaysia. Mycopathologia. 2012;173(2-3):173–8. doi: 10.1007/s11046-011-9469-8. [DOI] [PubMed] [Google Scholar]
- 12.McMullan B, Muthiah K, Stark D, Lee L, Marriott D. Prototheca wickerhamii mimicking yeast: a cautionary tale. J Clin Microbiol. 2011;49(8):3078–81. doi: 10.1128/JCM.00487-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nwanguma V, Cleveland K, Baselski V. Fatal Prototheca wickerhamii bloodstream infection in a cardiac allograft recipient. J Clin Microbiol. 2011;49(11):4024–5. doi: 10.1128/JCM.05305-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dion WM. Bovine mastitis due to Prototheca zopfi. Can Vet J. 1979;20(8):221–2. [PMC free article] [PubMed] [Google Scholar]
- 15.Whipple KM, Wellehan JF, Jeon AB, Sabatino BR, Frasca S, Jr, Popov VL, et al. Cytologic, histologic, microbiologic, and electron microscopic characterization of a canine Prototheca wickerhamii infection. Vet Clin Pathol. 2020;49(2):326–32. doi: 10.1111/vcp.12864. [DOI] [PubMed] [Google Scholar]
- 16.Hirose N, Nishimura K, Inoue-Sakamoto M, Masuda M. Ribosomal internal transcribed spacer of Prototheca wickerhamii has characteristic structure useful for identification and genotyping. PLoS ONE. 2013;8(11):e81223. doi: 10.1371/journal.pone.0081223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Masuda M, Hirose N, Ishikawa T, Ikawa Y, Nishimura K. Prototheca miyajii sp. nov., isolated from a patient with systemic protothecosis. Int J Syst Evol MicroBiol. 2016;66(3):1510–20. doi: 10.1099/ijsem.0.000911. [DOI] [PubMed] [Google Scholar]
- 18.Miura A, Kano R, Ito T, Suzuki K, Kamata H. In vitro algaecid effect of itraconazole and ravuconazole on Prototheca species. Med Mycol. 2019 doi: 10.1093/mmy/myz119. [DOI] [PubMed] [Google Scholar]
- 19.Tortorano AM, Prigitano A, Dho G, Piccinini R, Daprà V, Viviani MA. In vitro activity of conventional antifungal drugs and natural essences against the yeast-like alga Prototheca. J Antimicrob Chemother. 2008;61(6):1312–4. doi: 10.1093/jac/dkn107. [DOI] [PubMed] [Google Scholar]
- 20.Linares MJ, Solís F, Casal M. In vitro activity of voriconazole against Prototheca wickerhamii: comparative evaluation of sensititre and NCCLS M27-A2 methods of detection. J Clin Microbiol. 2005;43(5):2520–2. doi: 10.1128/JCM.43.5.2520-2522.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sud IJ, Feingold DS. Lipid composition and sensitivity of Prototheca wickerhamii to membrane-active antimicrobial agents. Antimicrob Agents Chemother. 1979;16(4):486–90. doi: 10.1128/AAC.16.4.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khan ID, Sahni AK, Sen S, Gupta RM, Basu A. Outbreak of Prototheca wickerhamii algaemia and sepsis in a tertiary care chemotherapy oncology unit. Med J Armed Forces India. 2018;74(4):358–64. doi: 10.1016/j.mjafi.2017.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data supporting the conclusions of this article are included within the article.


