Abstract
Obstacles continue to hinder in vitro studies of the gastric human pathogen Helicobacter pylori, including difficulty culturing the organism in the absence of serum or blood, rapid loss of viability following exponential growth due to autolysis, and the necessity for using high starting inocula. We demonstrate that H. pylori grows in the chemically defined broth medium Ham's F-12 nutrient mixture (F-12) in the absence of fetal bovine serum (FBS); this represents a breakthrough for studies in which serum components or proteins interfere with interpretation of results. Cultures can be continually passaged in fresh, FBS-free F-12 medium at an initial inoculum of only ∼103 CFU/ml. All H. pylori strains (n = 21), including fresh clinical isolates, grew in serum-free F-12. H. pylori grew poorly in the related medium, F-10, unless additional zinc was supplied. Enhanced growth of H. pylori in F-12 broth was obtained by addition of bovine serum albumin (BSA) (1 mg/ml), β-cyclodextrin (200 μg/ml), or cholesterol (50 μg/ml). H. pylori also grew in several simplified versions of F-12 broth lacking glucose and most vitamins but containing hypoxanthine, pyruvate, and all 20 amino acids. On F-12 medium solidified with agar, H. pylori only grew when BSA (98% pure; 1 mg/ml), cholesterol (50 μg/ml), β-cyclodextrin (200 μg/ml), or FBS (2 to 4%) was added; addition of urea and phenol allowed colorimetric detection of urease activity. Thus, F-12 agar plus cholesterol or β-cyclodextrin represents the first transparent chemically defined agar and the first urease indicator agar for H. pylori. Several lines of evidence suggested that BSA itself is not responsible for H. pylori growth enhancement in F-12 containing BSA or FBS. Taken together, these innovations represent significant advances in the cultivation and recovery of H. pylori using chemically defined media. Use of F-12 or its derivatives may lead to improved understanding of H. pylori metabolism, virulence factors, and transmission, and result in improved recovery and identification of H. pylori from clinical specimens.
The human gastric pathogen Helicobacter pylori is regarded as a highly fastidious organism, typically requiring 3 days of growth on complex media containing blood or serum in a low-oxygen atmosphere. This fastidiousness may be responsible for our current inability to reproducibly culture H. pylori from environmental sources and clinical specimens, thereby preventing us from fully understanding H. pylori transmission and accurately diagnosing infection. Furthermore, use of complex media to grow H. pylori may not mimic the conditions encountered by H. pylori in vivo, and specific nutrient requirements cannot be readily assessed using a complex medium. It is now clear that all strains require arginine, leucine, isoleucine, valine, phenylalanine, methionine, and histidine, and most H. pylori strains require alanine and serine (23, 25). However, most other H. pylori nutrient requirements are still poorly understood. Therefore, a chemically defined medium must be developed for growth of H. pylori to improve our understanding of metabolism, physiology, virulence factors, and transmission.
Previously, researchers have developed chemically defined media for growing H. pylori (1, 23, 25, 26), but four problems exist. Firstly, bovine serum albumin (BSA) is often added to chemically defined media to improve the growth of H. pylori (1, 25). BSA, a major serum protein, complicates experiments in which a chemically defined medium is desired. Moreover, it is not currently clear that the BSA itself is responsible for the growth enhancement, since large concentrations of BSA (1 to 5 mg/ml) are typically used (1, 25). No attempt has been made to directly determine whether BSA itself or a protein contaminant in commercial preparations of BSA has the growth-enhancing capability for H. pylori. Secondly, these chemically defined media only support minimal bacterial growth (∼1 to 2 logs), which is typically followed by rapid autolysis (1, 23, 25, 26), making it difficult for these media to be widely adopted for routine culture of H. pylori. Thirdly, genetic differences in H. pylori strains (2, 13) may lead to some strains failing to grow in currently available chemically defined media (23, 25). Finally, high starting inocula of >106 viable CFU/ml are required for growth in currently available chemically defined media (1, 23, 25, 26). This high inoculum requirement may preclude the ability to detect low numbers of viable H. pylori organisms from environmental sources or in vivo clinical specimens such as feces.
In the present study, we identified a superior chemically defined medium, Ham's F-12 nutrient mixture (7), to grow, store, and recover H. pylori in the absence of serum. We also report the first description of a solid transparent chemically defined medium for the growth of H. pylori and provide suggestive evidence that BSA itself is not the growth-enhancing component in serum.
MATERIALS AND METHODS
Bacterial strains.
H. pylori strains 26695 (provided by Kate A. Eaton, Ohio State University, Columbus) (29), SS1 (provided by Adrian Lee, University of New South Wales, Sydney, Australia), 43504 (American Type Culture Collection, Manassas, Va.), UMAB41 (isolated from a patient with peptic ulcer disease at the University of Maryland School of Medicine) (11, 21), HPDJM17 (low-passage clinical isolate from a patient with gastritis at the University of Maryland School of Medicine), and 3401 (provided by Keith T. Wilson, University of Maryland School of Medicine) were used in this study. Additionally, 15 minimally passaged fresh clinical isolates were also used (provided by Richard M. Peek, Vanderbilt University, Nashville, Tenn.).
Media and chemicals.
Unless otherwise stated, all media and supplements were from Gibco BRL. Media used in this study are as follows: Ham's F-10 nutrient broth mixture with glutamine (catalog no. 11550-043); Ham's F-12 nutrient powder mixture lacking bicarbonate (catalog no. 21700-075); Ham's F-12 nutrient broth mixture with glutamine (catalog no. 11765-054); McCoy's 5A medium with glutamine (catalog no. 16600-082); RPMI medium 1640 with glutamine without methionine (catalog no. 11876-026); RPMI medium 1640 without glutamine (catalog no. 21870-076); minimum essential medium (MEM), Eagle's with Earle's salts and glutamine (catalog no. 100G-500; Biofluids, Inc., Rockville, Md.); CMRL medium 1066 (CMRL) without glutamine (catalog no. 11530-037); basal medium eagle (BME) with Earle's salts, without glutamine (catalog no. 21010-046); Dulbecco's MEM (DMEM)–F-12 at 1:1 (vol/vol), with 15 mM HEPES buffer, glutamine, and pyridoxine HCl (catalog no. 11330-032); DMEM with high glucose, glutamine, and pyridoxine HCl, without pyruvate (catalog no. 11965-092); medium 199 with Earle's salts, glutamine, and bicarbonate (catalog no. 11043-023); MEM amino acids solution (catalog no. 11130-051); MEM nonessential amino acids solution (catalog no. 11140-050); MEM vitamin mixture (100×; catalog no. 11120-052); chemically defined lipid concentrate (catalog no. 11905-031); FBS (heat-inactivated, 56°C, 30 min; Sigma); cholesterol (catalog no. 3045, Sigma); β-cyclodextrin (cycloheptaamylose; catalog no. C-4767; Sigma); BSA (98 or ≥99% pure; catalog no. A7906 or A-7638, respectively; Sigma). Other compounds were from Sigma.
Growth conditions.
Unless otherwise specified, H. pylori strains were incubated at 37°C under an atmosphere of 5% CO2 and 100% humidity without aeration. Strains were passaged every 2 to 3 days on the complex medium Campylobacter agar (Becton Dickinson) containing 10% (vol/vol) defibrinated sheep blood (CBA) or Trypticase soy agar containing10% (vol/vol) defibrinated sheep blood. Mueller-Hinton broth (MHB) containing FBS was used as a complex liquid medium. H. pylori strains were confirmed by Gram staining, colony morphology on CBA, and urease positivity.
Simplified versions of F-12 broth.
Two simplified versions of F-12 were used: F-12m and TT7. F-12m (for F-12 “minus”) is F-12 broth lacking lipids, glucose, hypoxanthine, pyruvate, putrescine, thymidine, glycine, lysine, and all vitamins except thiamine. This medium was specially formulated by Gibco BRL. TT7 contains 1× Hanks' balanced salt solution (contains glucose, NaCl, KCl sodium and potassium phosphate buffer, magnesium, and calcium), bicarbonate (1.18 mg/ml), ZnSO4 · 7H2O (1 μg/ml), FeSO4 · 7H2O (1 μg/ml), 1× MEM amino acids solution, and 1× MEM nonessential amino acids solution. Following addition of the amino acids solutions, the pH was adjusted to 7.0 with NaOH and filter sterilized. Additional compounds were added to F-12m or TT7 as indicated (see Table 6 and Fig. 5).
TABLE 6.
Compounds tested for their ability to permit growth of H. pylori in simplified serum-free chemically defined broths F-12m or TT7a
| Role | Compound |
|---|---|
| Required for growth | Amino acidsbc |
| Hypoxanthineb | |
| Pyruvateb | |
| Enhances growth | Bicarbonateb |
| BSA, 98% | |
| Cholesterol | |
| β-Cyclodextrin | |
| FBS | |
| Zincb | |
| No growth or not required | Biotinb |
| Cholineb | |
| Folic acidb | |
| Glucoseb | |
| Inositolb | |
| Linoleic acidb | |
| Lipid concentrated | |
| Lipoic acidb | |
| Putrescineb | |
| Thiamineb | |
| Thymidineb | |
| Riboflavinb | |
| Vitamin B12b |
H. pylori strain 26695 was grown in the simplified chemically defined broths F-12m or TT7 (see Materials and Methods) plus the compounds listed. Concentrations used were the same as those found in F-12 broth (in the microgram-per-milliliter range). Growth or no growth was assessed by increased turbidity after 2 to 7 days of incubation at 37°C in a humidified atmosphere containing 5% CO2.
This compound is normally present in F-12.
Amino acids used were MEM amino acids solution and MEM nonessential amino acids solution at a 1× final concentration. Specific amino acids were not tested.
Chemically defined lipid concentrate (Gibco BRL).
FIG. 5.
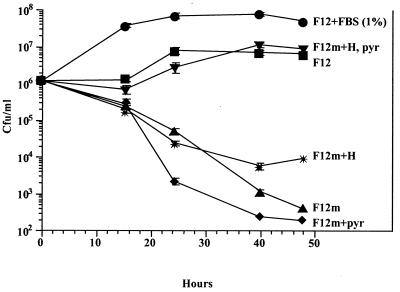
H. pylori grows in a simplified chemically defined medium. H. pylori strain 26695 was inoculated into F12m medium (see Materials and Methods) plus the compounds listed and incubated at 37°C in a humidified atmosphere containing 5% CO2. At different times, portions were serially diluted in PBS (pH 7.4) and plated in quadruplicate on CBA plates for enumeration of CFU. Error bars denote standard deviations and are usually smaller than the size of the graph symbols. H, hypoxanthine (4.77 μg/ml); pyr, pyruvate (110 μg/ml).
Preparation of F-12 chemically defined agar.
A dry powder concentrate of F-12 was dissolved in distilled water and filter sterilized through 0.22-μm-pore-diameter filters as a 2× stock. Bacto Agar (Difco) was made at 30 g/liter and autoclaved. When cooled to 55°C, agar was added 1:1 (vol/vol) to the 2× F-12 stock, and the medium was mixed and poured into standard petri dishes.
Modifications to F-12 chemically defined agar.
A lawn of H. pylori (106 to 107 CFU) was plated on serum-free F-12 agar and several sterile 6-mm-diameter filter paper disks were added. Various compounds were spotted onto the disks. Alternatively, compounds were added prior to pouring of agar into the plates. Plates were incubated for 2 to 7 days at 37°C in an atmosphere of 5% CO2 and 100% humidity. The same medium containing FBS (20 to 25 μl) served as a positive control for growth.
Fractionation and treatment of FBS and BSA.
FBS and BSA were centrifuged (3,000 × g) through a membrane filter with a 100-kDa cutoff according to the manufacturer's instructions to yield retentate (enriched for proteins >100 kDa) and filtrate (enriched for proteins <100 kDa) fractions (Centricon Plus 80; Amicon). The filtrate was concentrated by centrifuging through a membrane filter with a 10-kDa cutoff (Centriprep 10; Amicon). For other experiments, unfractionated FBS was boiled for 15 min or treated with proteinase K (100 μg/ml) for 0 to 240 min followed by boiling.
Protein determinations.
Protein concentrations of the BSA and FBS extracts were determined using the bicinchoninic acid assay method (Pierce Chemical Company, Rockford, Ill.), according to the manufacturer's 30-min protocol. BSA supplied by the manufacturer was used as the standard.
SDS-PAGE analysis of proteins.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was conducted by the method of Laemmli (14), using a 10% resolving gel. Gels were stained with Coomassie brilliant blue R-250. The same amount of protein was loaded per lane within an experiment, as indicated in the figure legends.
Plating for viable CFU.
Suspensions of H. pylori grown in various media were serially diluted 10-fold in duplicate into sterile phosphate-buffered saline (PBS) (pH 7.4) and plated in quadruplicate for viable counts on CBA. Data are presented as log CFU/milliliter ± standard deviation.
Recovery of H. pylori from gerbil feces.
Fresh gerbil feces were seeded with H. pylori plus F-12 broth containing 5% FBS plus six antimicrobials to suppress normal flora bacteria: nalidixic acid (10 μg/ml), vancomycin (10 μg/ml), amphotericin B (2 μg/ml), bacitracin (30 μg/ml), polymyxin B (10 U/ml), and trimethoprim (10 μg/ml). As a confirmation test for H. pylori, urea (1 mM), phenol red (35 μg/ml), and six antimicrobials (see above) were added to F-12 agar plates to create urease indicator plates. H. pylori, which is urease positive, hydrolyzes the urea in the medium to produce ammonium, which raises the pH and converts the phenol red indicator from yellow-orange to pink-red.
RESULTS
Development of a chemically defined medium for the growth of H. pylori.
Our strategy to identify a chemically defined medium to support the growth of H. pylori included testing of various commercially available tissue culture media for bacterial growth. If such a medium were identified, it would provide H. pylori researchers with a readily available chemically defined medium that would be inexpensive and easy to use and may be compatible with cell lines. H. pylori strain 26695 was inoculated into various tissue culture media (DMEM, DMEM–F-12, RPMI 1640, BME, McCoy's 5A, MEM, CMRL, 199, KGM, F-10, and F-12) containing 2 to 5% FBS. Bacteria were incubated in tissue culture flasks (25 cm2) or 24-well plates without aeration at 37°C in an environment containing 5% CO2 and 100% humidity. The only medium to result in a significant increase in turbidity of H. pylori in 1 to 2 days was Ham's F-12 (data not shown); we did, however, obtain growth of H. pylori in F-10 upon extended incubation (see below). Two independent lots of RPMI medium 1640 did not yield growth under our conditions. Activated charcoal, a compound previously reported to allow growth of H. pylori (5, 26), did not result in improved growth of H. pylori under our conditions (data not shown).
H. pylori grows in F-12 broth in the absence of serum and at low starting inocula.
To determine whether H. pylori would grow in F-12 medium in the absence of FBS, H. pylori strain 26695 growing in F-12 broth plus 1% FBS was diluted 1:10,000 or 1:100,000 into fresh serum-free F-12 broth. At these dilutions, FBS carryover was negligible. It was found that H. pylori strain 26695 grew by 3 logs in serum-free F-12 within 2 to 3 days of incubation (Fig. 1). By 4 days, viability started to decline. All H. pylori strains (n = 21), including fresh clinical isolates (n = 16), grew in serum-free F-12 broth (data not shown). To eliminate the effects of nutrient carryover from F-12 containing FBS, H. pylori strain 26695 was continually passaged from serum-free F-12 to fresh serum-free F-12 (n = 10). H. pylori grew and remained viable throughout all of the study, encompassing about 3 weeks, indicating that serum is not absolutely required for H. pylori to grow in F-12 broth.
FIG. 1.
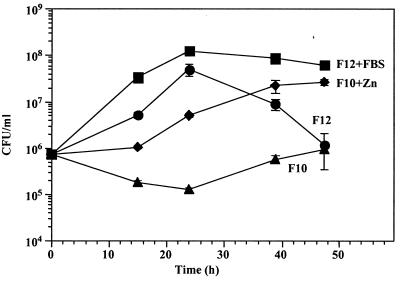
Growth of H. pylori in serum-free F-12 broth. Broth cultures of H. pylori strain 26695 grown in F-12 plus 1% FBS were diluted 1:10,000 or 1:100,000 into fresh serum-free F-12 broth and incubated at 37°C in a humidified atmosphere containing 5% CO2. At different times, portions were serially diluted in PBS (pH 7.4) and plated in duplicate on CBA plates for enumeration of CFU. Error bars denote standard deviations and are not shown if smaller than the graph symbols.
It is widely accepted that high inocula are required for growth of H. pylori in either chemically defined broth or in complex broth containing serum (1, 23, 25, 26). We found that this was not necessary with H. pylori grown in F-12. We initiated growth of H. pylori with an inoculum of only 103 viable CFU/ml and still obtained 3 logs of growth in the absence of FBS (Fig. 1). We were unable to obtain more than 6 × 107 viable CFU/ml in serum-free unsupplemented F-12.
H. pylori can be recovered from frozen stocks in F-12 broth.
Currently, researchers streak pure cultures of H. pylori from frozen stocks onto complex solid media containing blood, since the organism fails to grow from frozen stocks directly in complex broth containing serum or blood. Although the reason for this is unknown, we speculate that H. pylori is stressed during the freezing procedure. Indeed, H. pylori streaked from frozen stocks often requires extended incubation (>2 days) on solid medium to obtain growth. To investigate whether F-12 broth could recover H. pylori from frozen stocks directly, samples of frozen crystals of H. pylori strains 26695 and fresh clinical isolate HPDJM17 were suspended in PBS (pH 7.4) and equal volumes were added to either F-12 broth containing 5% FBS or the standard rich medium MHB containing 5% FBS. After 1 (26695) or 2 (HPDJM17) days of incubation, the F-12 medium became turbid and viable bacteria were recovered from F-12, whereas no bacteria were recovered in MHB plus FBS, even after 4 days (Table 1). Furthermore, H. pylori was recovered from frozen stocks in which F-12 containing 20% glycerol was used as the freezing medium. These results suggest that F-12 broth may be used for transport and storage of H. pylori.
TABLE 1.
Growth of H. pylori from frozen stock following direct inoculation into F-12 brotha
| Medium | Turbidity
|
Viable CFU
|
||
|---|---|---|---|---|
| Strain 26695 | HPDJM17 | 26695 | HPDJM17 | |
| F-12 + 5% FBS | Yes (by day 2) | Yes (by day 4) | Yes (by day 1) | Yes (by day 2) |
| MHB + 5% FBS | Nob | Nob | Nob | Nob |
Frozen crystals of H. pylori laboratory strains 26695 and a fresh clinical isolate, HPDJM17, were suspended in 200 μl of PBS to standardize the inocula. F-12 or MHB medium (1 ml) containing 5% FBS in a 24-well plate was inoculated with 25 μl of bacterial suspensions. The plate was incubated at 37°C with 5% CO2 and 100% humidity. Cultures were monitored daily for 4 days for visible turbidity (approximate optical density at 600 nm, ≥0.1) and for the ability to form colonies on CBA.
There was no evidence of turbidity or viable CFU at any time point.
Zinc is a crucial compound required in optimal concentrations for growth of H. pylori.
In early experiments in which we used various media to determine whether any supported growth of H. pylori, we included Ham's F-10 nutrient mixture, which is largely similar to Ham's F-12. A few major differences between F-12 and F-10 are that F-10 has significantly lower zinc concentrations (0.03 mg/liter in F-10 versus 0.86 mg/liter in F-12) and choline concentrations (0.7 mg/liter in F-10 versus 14.0 mg/liter in F-12), and that no putrescine or linoleic acid is present in F-10 in contrast with F-12. Despite the close similarities of F-12 and F-10, growth of H. pylori in F-10 broth was poor (Fig. 2). We did observe that after extended incubation H. pylori began to grow in serum-free F-10 broth. In preliminary experiments, we were unable to restore growth of H. pylori in F-10 to F-12 levels by supplementation of F-10 with choline, linoleic acid, or putrescine. Notably, if we supplemented F-10 with additional zinc to bring the concentration back to F-12 levels, growth of H. pylori was restored at a level comparable to F-12 growth conditions (Fig. 2). Addition of zinc to F-10 to yield concentrations of two- or fivefold above F-12 levels resulted in similar levels of H. pylori growth, but higher concentrations were toxic (data not shown). These results indicated that zinc is required at an optimal concentration and is a crucial metal ion for H. pylori growth.
FIG. 2.
Requirement of zinc for H. pylori growth in F-12. Broth cultures of H. pylori strain 26695 grown in F-12 broth plus 1% FBS were diluted 1:100 into fresh serum-free F-12 broth (0.86 mg of zinc/liter), F-10 broth (0.03 mg of zinc/liter), F-10 broth plus ZnSO4 · 7H2O (1 mg/liter), or F-12 broth plus FBS (1%) and incubated at 37°C in a humidified atmosphere containing 5% CO2. At different times, portions were serially diluted in PBS (pH 7.4) and plated in duplicate on CBA for enumeration of CFU. Error bars denote standard deviations. Data are representative of three experiments.
H. pylori grows on serum-free F-12 agar or in F-12 broth in the presence of BSA, cholesterol, β-cyclodextrin, or FBS.
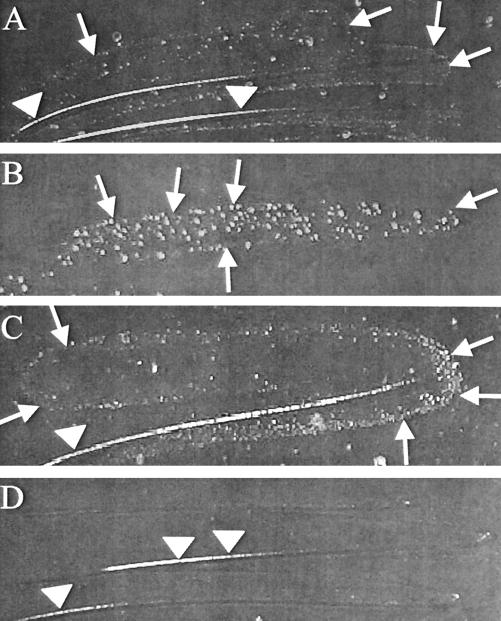
To determine whether H. pylori would grow on F-12 solid medium, we made serum-free F-12 agar plates as described in Materials and Methods. Unlike in serum-free F-12 broth (Fig. 1), H. pylori was unable to reproducibly grow on serum-free F-12 solid medium after 2 to 5 days (Fig. 3D) but sometimes formed tiny colonies after extended incubation (7 to 10 days). However, if serum-free F-12 agar was supplemented with FBS (2 to 4%) (data not shown), β-cyclodextrin (200 μg/ml) (Fig. 3A), BSA (1 mg/ml) (Fig. 3B), or cholesterol (50 μg/ml) (Fig. 3C), H. pylori grew in 2 to 5 days. Other H. pylori strains, including fresh clinical isolates, also grew, but only when one of those components was added (data not shown). It was determined that there was a dose dependency on BSA, since dropping the BSA concentration below 1 mg/ml resulted in poor growth (750 μg/ml) or no growth (below 750 μg/ml) (Table 2). There was also a dose dependency on β-cyclodextrin, since higher concentrations inhibited growth and lower concentrations failed to support growth at all. Since FBS renders the medium complex rather than chemically defined, and BSA is a protein with other minor contaminating proteins (see below), only the addition of cholesterol or β-cyclodextrin allowed growth of H. pylori on a chemically defined solid medium. To our knowledge, this is the first description of a transparent chemically defined solid medium for the growth of H. pylori.
FIG. 3.
Growth of H. pylori on serum-free F-12 agar supplemented with BSA, cholesterol, or β-cyclodextrin. H. pylori strain 26695 was streaked onto serum-free F-12 agar containing β-cyclodextrin (200 μg/ml) (A), BSA (1 mg/ml) (B), cholesterol (50 μg/ml) (C), or no supplement (D). Supplements were added prior to pouring of plates. Plates were incubated for 5 days at 37°C in a humidified atmosphere containing 5% CO2. Pictures were taken using a digital camera, and images were equally adjusted for brightness and contrast in Adobe Photoshop. Arrows denote edge of growth. Arrowheads denote streak line reflected by light. Magnification, approximately ×2.
TABLE 2.
Growth of H. pylori on serum-free F-12 agar in the presence of cholesterol, BSA, or β-cyclodextrina
| Compound | Amt (μg) | Growthc |
|---|---|---|
| None | − | |
| FBS | ND | +++ |
| Cholesterol | 50 to 1000 | +++ |
| BSA, 98% | 3,000 | +++ |
| BSA, 98% | 1,500 | +++ |
| BSA, 98% | 750 | − |
| BSA, 98% | 375 | − |
| β-Cyclodextrin | 200 | +++ |
H. pylori strain 26695 (∼108 CFU/ml) was plated onto serum-free F-12 agar. To this medium, the compounds listed above were added to filter disks, and the plates were incubated for 2 to 7 days at 37°C in a humidified atmosphere containing 5% CO2. Shown is one experiment of three conducted, all with identical results.
ND, not determined. Volume added was 25 μl.
Symbols: +++, excellent growth (lawn to high density of isolated colonies); −, no growth.
Addition of other components to serum-free F-12 agar, such as acetamide, ascorbic acid, choline, creatine, folic acid, formamide, glucose, glutathione (oxidized form), hydantoin, linoleic acid, lipoic acid, putrescine, urea, uridine, and vitamin B12 did not promote growth of H. pylori as determined by spotting these compounds over a lawn of H. pylori on serum-free F-12 agar plates (Table 3 [also see Table 6]). We also did not observe any growth of H. pylori on serum-free F-12 agar on which the serum protein human or bovine lactoferrin or bovine apolipoprotein A1 was added.
TABLE 3.
Compounds tested for their ability to permit growth of H. pylori on serum-free F-12 broth and agara
| Result | Compound (concn) |
|---|---|
| Growth | Bicarbonateb (1.2 mg/ml) |
| BSA, 98% (1 mg/ml) | |
| BSA, ≥99%c (1 mg/ml) | |
| Cholesterol (50 μg/ml) | |
| β-Cyclodextrin (200 μg/ml) | |
| FBS (1 to 4%) | |
| No growth | Acetamide (2.5 mM) |
| Adenine (200 μg/ml) | |
| Ascorbic acid (200 μg/ml) | |
| Bovine apolipoprotein A1 (5 μg/ml) | |
| Bovine lactoferrin (5 μg/ml) | |
| Choline (200 μg/ml) | |
| Creatine (200 μg/ml) | |
| Folic acid (200 μg/ml) | |
| Formamide (2.5 mM) | |
| Glutathione (oxidized form) (200 μg/ml) | |
| Human lactoferrin (5 μg/ml) | |
| Hydantoin (2.5 mM) | |
| Nicotinic acid (200 μg/ml) | |
| Orotic acid (10 mM) | |
| Urea (2.5 mM) | |
| Uridine (200 μg/ml) |
H. pylori strain 26695 was grown on serum-free F-12 agar and in serum-free F-12 broth containing the compounds listed. For F-12 agar, stock solutions of compounds (generally 100×) were initially spotted onto filter paper disks (6-mm diameter) and the final concentration was estimated. Compounds shown to support growth on agar by the filter disk method were confirmed by incorporating the compound at a specific concentration in the agar medium prior to soldification. All of the compounds listed are not normally present in F-12. Growth or no growth was assessed after 2 to 7 days of incubation at 37°C in a humidified atmosphere containing 5% CO2.
F-12 powder mixture, which lacks bicarbonate, was used to make 1 × F-12 broth without agar. To this, bicarbonate was added at a final concentration of 1.18 mg/ml.
Growth on serum-free F-12 agar containing ≥99% BSA was poor compared to F-12 agar containing 98% BSA (data not shown).
In contrast with its growth on serum-free F-12 agar, H. pylori grew without additional supplementation in serum-free F-12 broth (Fig. 1). However, further enhancement of growth was achieved by supplementation of F-12 broth with cholesterol, BSA, FBS, or β-cyclodextrin (Tables 3 and 4). Based on dose-response curves (data not shown), we determined that the optimal concentrations for cholesterol, BSA, and β-cyclodextrin were 50 μg/ml, 1 mg/ml, and 200 μg/ml, respectively, similar to those found on F-12 agar plates.
TABLE 4.
Growth of H. pylori in serum-free F-12 broth in the presence of BSAa
| Compound | Concn | Growth (2 days) |
|---|---|---|
| None | − | |
| FBS | 5% | +++b |
| BSA, 98% | 5,000 μg/ml | +++ |
| BSA, 98% | 2,500 μg/ml | +++ |
| BSA, 98% | 1,000 μg/ml | ++ |
| BSA, 98% | 500 μg/ml | − |
H. pylori recovered from CBA was suspended in serum-free F-12 broth containing the compound and concentration listed. Growth was assessed by turbidity after 2 days.
Symbols: +++, excellent growth; ++, good growth; −, no growth.
Fractions of BSA or FBS depleted of proteins of >100 kDa fail to support growth of H. pylori on F-12 agar.
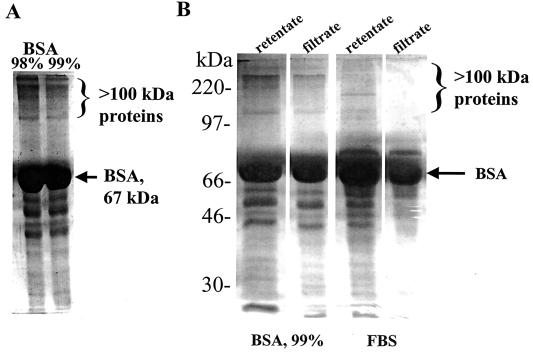
Since BSA (Fig. 3B) or FBS (Table 2) is required for the growth of H. pylori on serum-free F-12 agar and since both enhance the growth of H. pylori grown in serum-free F-12 broth (Table 4 and Fig. 2), we sought to determine the components of FBS and BSA that are growth enhancers. We (Tables 2 and 4) and others (1, 25) have observed that very high concentrations of BSA (1 mg/ml or higher) enhance growth of H. pylori. These high concentrations suggest that BSA per se may not represent the growth enhancer; rather, the enhancer might be a contaminating protein or a compound bound to BSA. Since BSA, an ∼67-kDa protein, is a major component of FBS, we propose that the same growth enhancer is in both BSA and FBS. SDS-PAGE analysis of 98 or ≥99% purified preparations of BSA revealed numerous minor contaminating proteins, some of which are >100 kDa (Fig. 4A). Therefore, we fractionated BSA and FBS, through a membrane filter with a 100-kDa cutoff and tested both the retentates, which are enriched for proteins of >100 kDa, and the filtrates, which are enriched for proteins of <100 kDa. By SDS-PAGE analysis, we found that the membrane filter easily allowed <100-kDa proteins in FBS and BSA to pass through, and thus the filtrates were reduced (BSA) or eliminated (FBS) for proteins of >100 kDa (Fig. 4B). However, the retentate contained proteins larger and smaller than 100 kDa, like the starting material. Equal amounts of these fractions were spotted onto serum-free F-12 agar on which H. pylori strain 26695 was plated. Growth of H. pylori cells did not occur at all with supplementation of F-12 agar with the BSA or FBS filtrates, whereas similar concentrations of the retentate fractions promoted growth (Table 5). Furthermore, H. pylori cells grew significantly better on serum-free F-12 agar around disks (6 mm diameter) impregnated with 98% BSA (1 mg) than with ≥99% BSA (1 mg) (data not shown). The growth-enhancing component was found to be resistant to boiling, since boiled FBS still supported growth of H. pylori on serum-free F-12 agar. Proteinase K treatment of FBS for >60 min led to reduction in growth enhancement of H. pylori on serum-free F-12 agar. Taken together, these results suggest that a >100-kDa heat-resistant, protease-sensitive protein in FBS and in commercial preparations of BSA is responsible for growth enhancement of H. pylori. Thus, BSA itself is not likely to be the main growth-enhancing compound in FBS.
FIG. 4.
Analysis of BSA and FBS. (A) SDS-PAGE analysis of 98 and ≥99% commercial preparations of BSA contain contaminating proteins of >100 kDa. Similar protein amounts (15 μg) were loaded in each lane. (B) Fractionation of BSA and FBS through a 100-kDa membrane cutoff. Similar protein amounts (20 μg) were loaded in each lane.
TABLE 5.
Lack of growth of H. pylori in F-12 with FBS or BSA fractions enriched for proteins of <100 kDaa
| Fractionb | Amt (μg) | Diameter (mm) of growth around disk containing:
|
|
|---|---|---|---|
| BSA | FBS | ||
| Retentate (>100 kDa enriched) | 500 | 12c | 11 |
| 1,000 | 14 | 15 | |
| 1,500 | 25 | 16 | |
| Filtrate (<100 kDa enriched) | 500 | NGd | ±e |
| 1,000 | NG | NG | |
| 1,500 | NG | ± | |
H. pylori strain 26695 (∼108 CFU/ml) was plated onto serum-free F-12 agar. BSA or FBS fractions were spotted at various concentrations onto filter paper disks (6-mm diameter) and incubated for 3 days. Data are representative of two experiments.
BSA or FBS was centrifuged through a 100-kDa membrane cutoff. The retentate was found to not be enriched for proteins of <100 kDa, while the filtrate was enriched for proteins of <100 kDa.
Diameter includes the 6-mm-disk diameter. The growth around the BSA-containing disk was an irregular pattern of crowded but isolated colonies, whereas the growth around the FBS-containing disk was a near perfect circle of a lawn of heavy growth.
NG, no growth.
±, very faint haze of growth around edge of disk.
Pyruvate and hypoxanthine improve growth of H. pylori in simplified chemically defined broth, whereas many vitamins are toxic.
Comparisons of tissue culture medium formulations revealed that media containing more compounds than F-12 often yielded less H. pylori growth, even in the presence of serum. This led us to hypothesize that many substances in chemically defined tissue culture media might be toxic to H. pylori. To determine which compounds in F-12 broth were required versus those that were superfluous or toxic, two simplified derivatives of chemically defined media were made in which most vitamins and glucose were omitted. Both of these media, designated F-12m and TT7 (see Materials and Methods), supported the growth of H. pylori when serum, BSA, cholesterol, or β-cyclodextrin was separately added (Table 6 and Fig. 5). In the absence of serum and these compounds, growth could also be achieved in F-12m if both hypoxanthine and pyruvate were added back (Fig. 5). If either hypoxanthine or pyruvate were added to F-12m alone, viable counts declined (Fig. 5). Vitamins, such as folic acid and vitamin B12 did not enhance the growth of H. pylori in TT7 or F-12m (Table 6). Furthermore, addition of a concentrated MEM vitamin solution to F12 broth reduced H. pylori growth. These data suggest that H. pylori can grow in a simplified chemically defined medium and that superfluous vitamins may even be detrimental.
H. pylori can be recovered from gerbil fecal samples seeded with H. pylori on F-12 agar plates containing urea and phenol red.
To ascertain whether F-12 could be used to isolate H. pylori from a mixed culture, F-12 broth containing six antimicrobials (see Materials and Methods) and 5% FBS was seeded with H. pylori strain 26695 plus feces from uninfected gerbils. In the absence of feces, H. pylori grew in this medium by 3 to 4 days of incubation at 37°C in an atmosphere of 5% CO2 and 100% humidity (data not shown). After 5 days of growth, we were able to reproducibly recover urease-positive H. pylori from seeded gerbil feces by plating samples on F-12 agar containing 5% FBS, six antimicrobials, urea, and phenol red, whereas no H. pylori was recovered from unseeded fecal controls (data not shown). H. pylori turned this medium pink in several hours to several days, depending on the inoculum size. H. pylori morphology was also confirmed by Gram staining and microscopic observation. Virtually no contaminating microbial flora were recovered, suggesting this medium was selective for culturing H. pylori
F-12 can be used for H. pylori transformation experiments.
To determine whether F-12 agar containing 2% FBS is comparable to CBA for transformation experiments, we electroporated (18) strain 26695 of H. pylori with a nixA disruption plasmid in which a kanamycin resistance cassette was inserted into the SspI site of nixA as described previously (4). Following transformation, bacteria were plated either on F-12 containing 2% FBS or CBA. After 48 h, the bacteria were recovered and plated on F-12 containing 2% FBS plus kanamycin (5 μg/ml) or CBA plus kanamycin (5 μg/ml). We recovered similar numbers of transformants under both conditions (∼25 CFU per μg of DNA), indicating that F-12 is comparable to CBA in the direct selection of H. pylori transformants.
DISCUSSION
We first reported the use of Ham's F-12 plus FBS to coculture H. pylori with AGS gastric epithelial cells (28). Likewise, Miller-Podraza and colleagues used F-12 broth containing FBS to culture H. pylori (19, 20). It was not previously assessed, however, whether H. pylori could grow in this medium in the absence of serum, thereby achieving a novel chemically defined medium for the growth of H. pylori. Thus, this study represents the first detailed analysis of the use of F-12 medium to grow, recover, and store H. pylori. The optimum chemically defined medium in this study is F-12 broth containing BSA (98% pure; 1 mg/ml), cholesterol (50 μg/ml), or β-cyclodextrin (200 μg/ml).
F-12 broth can be used to culture H. pylori when inoculated directly from frozen stocks, without the need to first cultivate the organism on a solid medium. Additionally, low starting inocula (103 CFU/ml) may be used (Fig. 1). This finding questions the widely used practice in the H. pylori field of starting broth cultures of H. pylori at high densities (1, 23, 25, 26). This is also the first report, to our knowledge, of a solid chemically defined medium for the growth of H. pylori in which F-12 agar is supplemented with cholesterol or β-cyclodextrin (Fig. 3A and C).
H. pylori rapidly takes up cholesterol (3), a compound in serum, blood, and eucaryotic cell membranes. Interestingly, this cholesterol is glycosylated and comprises about 25% of the lipid membrane content of H. pylori (8, 10, 12), a unique feature among procaryotes. Since H. pylori does not appear to carry cholesterol biosynthesis genes in the genome (2, 29), H. pylori must obtain the cholesterol from the host gastric mucosa in vivo. However, both our study and that of Haque and colleagues (8) found that cholesterol is not absolutely required for H. pylori growth (Fig. 1). How cholesterol is incorporated and modified into H. pylori membranes is unclear, but the host protein apolipoprotein A1 may be involved (27). We confirmed that cholesterol is a growth-enhancing supplement after we discovered that H. pylori grown under acidic conditions (pH 5.7) interacts with the cholesterol-binding protein, apolipoprotein Al, from FBS (27). However, purified apolipoprotein A1 does not support growth of H. pylori on serum-free F-12 agar under the conditions used here. Recently, Albertson and colleagues have also found that addition of cholesterol improves the growth of H. pylori in the chemically defined medium RPMI 1640 (1). We did not add cholesterol to any of the culture media listed in Materials and Methods other than F-12.
As in our study, several other groups have reported growth enhancement of H. pylori when culture medium is supplemented with β-cyclodextrin (10, 16, 22, 24). However, it appears that not all preparations of β-cyclodextrins are able to support H. pylori growth (8, 16), perhaps explaining why one group was unable to obtain growth of H. pylori in media supplemented with β-cyclodextrin (30). The role of β-cyclodextrin in growth enhancement of H. pylori remains elusive but could increase viscosity and osmolarity or chelate toxic substances.
We found that zinc is a critical compound needed by H. pylori in optimal amounts for growth in F-12. We base this on the finding that H. pylori grows poorly in F-10 medium, which has very low zinc levels, unless the zinc levels in F-10 are restored to F-12 levels (Fig. 2). Zinc is essential for the activity of several enzymes in H. pylori, such as a metalloprotease (32) or ATPase (9). The metalloprotease is a 200-kDa outer membrane-associated enzyme with endoprotease activity for casein (32). The ATPase CadA is an ion pump with specificity for zinc, cobalt, and cadmium and has a possible role in modulating urease activity (9).
It has long been suggested that H. pylori is highly fastidious for growth requirements in vitro. However, our finding that H. pylori can grow in a simple chemically defined medium without proteins or serum (Fig. 5 and Table 6) challenges this view. The conclusion that H. pylori is highly fastidious may have been reached due to our lack of understanding of the growth requirements of this human pathogen. The original isolation of H. pylori (17, 31) occurred after extended (>5 day) incubation of plates inoculated with gastric biopsy specimens from humans. However, with the increasing understanding of H. pylori metabolism through both detailed analyses of metabolic enzymes, genomic analyses (6, 15), previous chemically defined medium formulations (1, 23, 25), and the present study, we now have an improved understanding of growth requirements. This study suggests that perhaps H. pylori may not be fastidious after all. However, it should be noted that we have not been successful in obtaining growth of H. pylori to high densities (optical density at 600 nm, >1.0) in F-12 broth under any condition, despite the addition of numerous compounds. Thus, this medium may still be missing compounds needed by H. pylori to grow to high densities; in complex broth such as MHB plus FBS, H. pylori can reach an optical density at 600 nm of >1.0.
Our finding that fractions of FBS or BSA depleted of proteins of >100 kDa completely fail to support growth of H. pylori on F-12 agar challenges the widely held notion that BSA is the H. pylori growth-enhancing compound in serum. We used preparations of BSA, an ∼67-kDa protein found in abundance in FBS, that are 98 or ≥99% pure. When analyzed by SDS-PAGE, these samples were shown to contain significant quantities of proteins of >100 kDa, but the 98% BSA preparation contains more of these contaminating proteins (Fig. 4A). Furthermore, the ≥99% purified preparation of BSA surprisingly supported less growth than the 98% preparation (data not shown), suggesting that the ≥99% BSA preparation has less of the growth-enhancing factor. This finding, coupled with the observations that growth of H. pylori in the presence of BSA requires very high concentrations (1 mg/ml), suggests that the growth-enhancing factor is not BSA at all, but rather a protein of >100 kDa that contaminates commercial preparations of BSA. Since BSA is purified from FBS, the same protein must also be present in FBS. Thus far, we have been unable to prepare fractions of FBS or BSA containing only proteins of >100 kDa, using any of the currently available 100-kDa-cutoff membrane filters from Amicon. Our future work will therefore focus on biochemically purifying BSA and FBS fractions containing proteins of >100 kDa to identify the growth enhancer for H. pylori. These data, however, do not rule out the possibility of synergy between the >100-kDa protein and BSA or another protein or a compound of <100 kDa, since it is possible for a multisubunit protein with individual subunits smaller than 100 kDa to be overlooked via denaturing SDS-PAGE.
Our data also suggest that F-12 or its derivatives may be employed for selectively recovering, growing, and identifying H. pylori from complex microbial populations. This is based on our ability to recover H. pylori from gerbil feces ex vivo by suppressing fecal flora microbes and including a urease indicator in F-12 agar. This medium could therefore be useful for recovering H. pylori from clinical specimens.
In summary, we have shown that F-12 broth and agar are outstanding chemically defined media for culturing H. pylori. Additionally, F-12 agar can be used to detect urease activity. These media could be broadly applied to clinical microbiology laboratories to culture and positively identify H. pylori from clinical specimens and environmental sources and could be useful for improved understanding of H. pylori metabolism, physiology, transmission, and virulence factors.
ACKNOWLEDGMENTS
T.L.T. and D.J.M. contributed equally to this work.
We thank George L. Mendz for insightful discussions on H. pylori physiology and metabolism.
This work was supported in part by Public Health Service grants AI25567 (to H.L.T.M.), AI10098 (a postdoctoral fellowship to D.J.M.), and DK59709-01 (a postdoctoral fellowship to T.L.T.) from the National Institutes of Health.
REFERENCES
- 1.Albertson N, Wenngren I, Sjöström J E. Growth and survival of Helicobacter pylori in defined medium and susceptibility to Brij 78. J Clin Microbiol. 1998;36:1232–1235. doi: 10.1128/jcm.36.5.1232-1235.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alm R, Ling L S, Moir D T, King B L, Brown E D, Doig P C, Smith D R, Noonan B, Guild B C, deJonge B L, Carmel G, Tummino P J, Caruso A, Uria-Nickelsen M, Mills D M, Ives C, Gibson R, Merberg D, Mills S D, Jiang Q, Taylor D E, Vovis G F, Trust T J. Genomic-sequence comparison of two unrelated isolates of the human gastric pathogen Helicobacter pylori. Nature. 1999;397:176–180. doi: 10.1038/16495. [DOI] [PubMed] [Google Scholar]
- 3.Ansorg R, Müller K-D, von Recklinghausen G, Nalik H P. Cholesterol binding of Helicobacter pylori. Zentbl Bakteriol. 1992;276:323–329. doi: 10.1016/s0934-8840(11)80538-4. [DOI] [PubMed] [Google Scholar]
- 4.Bauerfeind P, Garner R M, Mobley H L T. Allelic exchange mutagenesis of nixA in Helicobacter pylori results in reduced nickel transport and urease activity. Infect Immun. 1996;64:2877–2880. doi: 10.1128/iai.64.7.2877-2880.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cao P, McClain M S, Forsyth M H, Cover T L. Extracellular release of antigenic proteins by Helicobacter pylori. Infect Immun. 1998;66:2984–2986. doi: 10.1128/iai.66.6.2984-2986.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doig P, de Jonge B L, Alm R A, Brown E D, Uria-Nickelsen M, Noonan B, Mills S D, Tummino P, Carmel G, Guild B C, Moir D T, Vovis G F, Trust T J. Helicobacter pylori physiology predicted from genomic comparison of two strains. Microbiol Mol Biol Rev. 1999;63:675–707. doi: 10.1128/mmbr.63.3.675-707.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ham R G. Clonal growth of mammalian cells in a chemically defined, synthetic medium. Proc Natl Acad Sci USA. 1965;53:288–293. doi: 10.1073/pnas.53.2.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haque M, Hirai Y, Yokota K, Oguma K. Lipid profiles of Helicobacter pylori and Helicobacter mustelae grown in serum-supplemented and serum-free media. Acta Med Okayama. 1995;49:205–211. doi: 10.18926/AMO/30380. [DOI] [PubMed] [Google Scholar]
- 9.Herrmann L, Schwan D, Garner R, Mobley H L T, Haas R, Schafer K P, Melchers K. Helicobacter pylori cadA encodes an essential Cd(II)-Zn(II)-Co(II) resistance factor influencing urease activity. Mol Microbiol. 1999;33:524–536. doi: 10.1046/j.1365-2958.1999.01496.x. [DOI] [PubMed] [Google Scholar]
- 10.Hirae Y, Haque M, Yoshida T, Yokota K, Yasuda T, Oguma K. Unique cholesteryl glucosides in Helicobacter pylori: composition and structural analysis. J Bacteriol. 1995;177:5327–5333. doi: 10.1128/jb.177.18.5327-5333.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hu L-T, Mobley H L T. Purification and N-terminal analysis of urease from Helicobacter pylori. Infect Immun. 1990;58:992–998. doi: 10.1128/iai.58.4.992-998.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Inamoto Y, Hamanaka S, Hamanaka Y, Nagate T, Kondo I, Takemoto T, Okita K. Lipid composition and fatty acid analysis of Helicobacter pylori. J Gastroenterol. 1995;30:315–318. doi: 10.1007/BF02347505. [DOI] [PubMed] [Google Scholar]
- 13.Jiang Q, Hiratsuka K, Taylor D E. Variability of gene order in different Helicobacter pylori strains contributes to genome diversity. Mol Microbiol. 1996;20:833–842. doi: 10.1111/j.1365-2958.1996.tb02521.x. [DOI] [PubMed] [Google Scholar]
- 14.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 15.Marais A, Mendz G L, Hazell S L, Mégraud F. Metabolism and genetics of Helicobacter pylori: the genome era. Microbiol Mol Biol Rev. 1999;63:642–674. doi: 10.1128/mmbr.63.3.642-674.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marchini A, d'Apolito M, Massari P, Atzeni M, Copass M, Olivieri R. Cyclodextrins for growth of Helicobacter pylori and production of vacuolating cytotoxin. Arch Microbiol. 1995;164:290–293. doi: 10.1007/BF02529963. [DOI] [PubMed] [Google Scholar]
- 17.Marshall B. Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet. 1983;i:1273–1275. [PubMed] [Google Scholar]
- 18.McGee D J, Radcliff F J, Mendz G L, Ferrero R L, Mobley H L. Helicobacter pylori rocF is required for arginase activity and acid protection in vitro but is not essential for colonization of mice or for urease activity. J Bacteriol. 1999;181:7314–7322. doi: 10.1128/jb.181.23.7314-7322.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miller-Podraza H, Bergström J, Milh M A, Karlsson K-A. Recognition of glycoconjugates by Helicobacter pylori. Comparison of two sialic acid-dependent specificities based on haemmagglutination and binding to human erythrocyte glycoconjugates. Glycoconj J. 1997;14:467–471. doi: 10.1023/a:1018599401772. [DOI] [PubMed] [Google Scholar]
- 20.Miller-Podraza H, Milh M A, Bergström J, Karlsson K-A. Recognition of glycoconjugates by Helicobacter pylori: an apparently high-affinity binding of human polyglycosylceramides, a second sialic acid-based specificity. Glycoconj J. 1996;13:453–460. doi: 10.1007/BF00731478. [DOI] [PubMed] [Google Scholar]
- 21.Mobley H L T, Cortesia M J, Rosenthal L E, Jones B D. Characterization of urease from Campylobacter pylori. J Clin Microbiol. 1988;26:831–836. doi: 10.1128/jcm.26.5.831-836.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morshed M G, Karita M, Konishi H, Okita K, Nakazawa T. Growth medium containing cyclodextrin and low concentration of horse serum for cultivation of Helicobacter pylori. Microbiol Immunol. 1994;38:897–900. doi: 10.1111/j.1348-0421.1994.tb02143.x. [DOI] [PubMed] [Google Scholar]
- 23.Nedenskøv P. Nutritional requirements for growth of Helicobacter pylori. Appl Environ Microbiol. 1994;60:3450–3453. doi: 10.1128/aem.60.9.3450-3453.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Olivieri R, Bugnoli M, Armellini D, Bianciardi S, Rappuoli R, Bayeli P F, Abate L, Esposito E, de Gregorio L, Aziz J, Basagni C, Figura N. Growth of H. pylori in media containing cyclodextrins. J Clin Microbiol. 1993;31:160–162. doi: 10.1128/jcm.31.1.160-162.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reynolds D J, Penn C W. Characteristics of Helicobacter pylori growth in a defined medium and determination of its amino acid requirements. Microbiology. 1994;140:2649–2656. doi: 10.1099/00221287-140-10-2649. [DOI] [PubMed] [Google Scholar]
- 26.Schraw W, McClain M S, Cover T L. Kinetics and mechanisms of extracellular protein release by Helicobacter pylori. Infect Immun. 1999;67:5247–5252. doi: 10.1128/iai.67.10.5247-5252.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Slonczewski J L, McGee D J, Phillips J, Kirkpatrick C, Mobley H L T. pH dependent protein profiles of Helicobacter pylori analyzed by two-dimensional gels. Helicobacter. 2000;5:240–247. doi: 10.1046/j.1523-5378.2000.00037.x. [DOI] [PubMed] [Google Scholar]
- 28.Smoot D T, Resau J H, Naab T, Desbordes B C, Gilliam T, Bull-Henry K, Curry S B, Nidiry J, Sewchand J, Mills-Robertson K, Frontin K, Abebe E, Dillon M, Chippendale G R, Phelps P C, Scott V F, Mobley H L T. Adherence of Helicobacter pylori to cultured human gastric epithelial cells. Infect Immun. 1993;61:350–355. doi: 10.1128/iai.61.1.350-355.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tomb J-F, White O, Kerlavage A R, Clayton R A, Sutton G G, Fleischmann R D, Ketchum K A, Klenk H P, Gill S, Dougherty B A, Nelson K, Quackenbush J, Zhou L, Kirkness E F, Peterson S, Loftus B, Richardson D, Dodson R, Khalak H G, Glodek A, McKenney K, Fitzgerald L M, Lee N, Adams M D, Hickey E K, Berg D E, Gocayne J D, Utterback T R, Peterson J D, Kelley J M, Cotton M D, Weidman J M, Fujii C, Bowman C, Watthey L, Wallin E, Hayes W S, Borodovsky M, Karp P D, Smith H O, Fraser C M, Venter J C. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature. 1997;388:539–547. doi: 10.1038/41483. [DOI] [PubMed] [Google Scholar]
- 30.Walsh E J, Moran A P. Influence of medium composition on the growth and antigen expression of Helicobacter pylori. J Appl Microbiol. 1997;83:67–75. doi: 10.1046/j.1365-2672.1997.00164.x. [DOI] [PubMed] [Google Scholar]
- 31.Warren J R. Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet. 1983;i:1273–1275. [PubMed] [Google Scholar]
- 32.Windle H J P, Kelleher D. Identification and characterization of a metalloprotease activity from Helicobacter pylori. Infect Immun. 1997;65:3132–3137. doi: 10.1128/iai.65.8.3132-3137.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]