Abstract
A previously described monoclonal antibody (MAb)-based competitive-inhibition enzyme-linked immunosorbent assay (cELISA) was modified to optimize performance, and the assay was validated in various defined cattle populations for detection of serum antibody to Neospora caninum, a major cause of bovine abortion. Modifications to the cELISA included capturing native N. caninum antigen with a parasite-specific MAb (MAb 5B6-25) and directly conjugating the competitor MAb (MAb 4A4-2), with both MAbs binding different epitopes of a conserved, immunodominant 65-kDa tachyzoite surface antigen. The assay was validated using three serum sets, a “gold standard” set of 184 cow sera defined by fetal histopathology and N. caninum immunohistochemistry and by maternal N. caninum indirect fluorescence assay (IFA) at a 1:200 serum dilution, a relative standard set of 330 cow sera defined by IFA alone, and a set of 4,323 cow sera of unknown N. caninum status. A test cutoff of 30% inhibition was identified. The diagnostic sensitivity was 97.6%, and diagnostic specificity was 98.6% for the gold standard abortion-defined sera. The diagnostic sensitivity was 96.4%, and diagnostic specificity was 96.8% for the relative standard IFA-defined sera. Testing of the 4,323 bovine sera of unknown N. caninum status revealed a distinct bimodal distribution and steep sigmoid frequency curve with only 1.8% of samples within 5% of the test cutoff, indicating a sharp discrimination between test-positive and test-negative samples. In summary, the modified N. caninum cELISA provided a simple, rapid, and versatile method to accurately identify N. caninum infection status in cattle using a single cutoff value.
Infection with Neospora caninum, an apicomplexan protozoan parasite, is reported as a significant cause of economic loss in dairy and beef cattle herds worldwide due primarily to abortion and reduced reproductive efficiency but also to poor milk production, increased culling, and poor feed efficiency (2, 10, 23, 24, 26). Similar to Toxoplasma gondii, N. caninum has a two-host herbivore-carnivore life cycle. Domestic dogs are identified to date as a definitive parasite host capable of shedding infective oocysts (9, 14, 18). The diagnosis of N. caninum-induced abortion in individual cattle is based upon examination of fetal tissues for histological lesions (3), for tachyzoites by immunohistochemistry (13), or for parasite DNA by PCR (4). Validated N. caninum-specific serological assays are necessary for accurate herd-based abortion diagnosis (25) and for population-based epidemiological investigations of disease transmission, disease risk factors, and identification of additional definitive and intermediate hosts.
Many serologic tests for detecting N. caninum antibodies are described for cattle, including indirect fluorescence assay (IFA), Western blot assay, agglutination assay, and various enzyme-linked immunosorbent assays (ELISA) based upon either whole or partially purified native N. caninum antigen or recombinant N. caninum antigen (1, 8, 10), antibody avidity (7), or competitive inhibition with parasite-specific monoclonal antibodies (MAbs) (5). The purpose of this paper is to report validation data for a new competitive-inhibition ELISA (cELISA) closely based upon a previously described MAb-based cELISA that detects serum antibody to a 65-kDa immunodominant N. caninum tachyzoite surface antigen (5). The newly formatted cELISA was modified by capturing N. caninum native 65-kDa antigen with a newly described MAb (MAb 5B6-25) and by directly conjugating the competitor MAb previously described (5). The captured-antigen cELISA has decreased nonspecific antibody binding and allowed the use of undiluted test sera to increase both assay specificity and sensitivity. Direct conjugation of the competitor MAb was done to increase the versatility of the test for use in multiple animal species. Both captured N. caninum 65-kDa ELISA plates and MAb conjugate are commercially available (VMRD Inc.).
The original description of the N. caninum 65-kDa MAb-based cELISA reported various development and standardization data, including the specificity of MAb 4A4-2 for N. caninum, optimal concentrations of antigen and MAb, the analytical specificity using a panel of sera from animals with experimental infections with related protozoa, and testing a small panel of N. caninum defined bovine sera (5). In the present analysis, the newly formatted cELISA was more thoroughly validated by determining the optimal test cutoff; by determining the diagnostic sensitivity, specificity, and accuracy using large sets of defined reference sera; and by long-term monitoring of assay performance. The goal was to test the discriminatory ability of the cELISA on both high-titer sera, obtained from cows aborting N. caninum-infected fetuses, and low-titer sera, obtained from random herd samples.
MATERIALS AND METHODS
Parasites and N. caninum antigen preparation.
Polystyrene (96-well) plates coated with captured N. caninum antigen were obtained commercially (VMRD Inc., Pullman, Wash.). Native parasite antigen (NSo) was obtained from tachyzoites of the NC-1 isolate of N. caninum (11) as previously described (5). Tachyzoites from the RH strain of T. gondii and bradyzoites from two isolates of Sarcocystis cruzi were processed similarly for dot blot assay. For plate coating, a previously unpublished N. caninum MAb, MAb 5B6-25, was used for antigen capture. The MAb was generated by immunizing BALB/c mice with sonicated N. caninum antigen in Freund's complete adjuvant as previously described (5).
Clinical samples and experimental design.
Cattle sera were submitted to the Washington Animal Disease Diagnostic Laboratory at Washington State University for routine diagnostic investigation. Sera were grouped into three separate test groups according to N. caninum status (Table 1). The sera originated from commercial dairy and beef herds in the Pacific Northwest region of the United States in Washington, Idaho, and Oregon (groups 1, 2, and 3) and from the Eastern Seaboard region of the United States in Virginia and Maryland (portion of group 2). All sera were stored at −70°C.
TABLE 1.
Summary of bovine sera examined by MAb cELISA for N. caninum antibodies
| Group | N. caninum status | No. of sera tested |
|---|---|---|
| 1 | Abortion-defined positivea | 42 |
| Abortion-defined negativeb | 142 | |
| 2 | IFA-defined positivec | 170 |
| IFA-defined negatived | 160 | |
| 3 | Unknown statuse | 4,323 |
N. caninum abortion positive: IFA positive at a serum dilution of 1:200 and originating from dam aborting N. caninum-infected fetus as determined by histopathology and N. caninum-specific immunohistochemistry.
N. caninum abortion negative: negative by IFA at a serum dilution of 1:200 and originating from dams aborting fetuses without N. caninum-compatible histological lesions.
N. caninum IFA positive at a serum dilution of 1:200; N. caninum abortion status unknown.
N. caninum IFA negative at a serum dilution of 1:200; N. caninum abortion status unknown.
N. caninum IFA or abortion status unknown.
Group 1 sera, used as a “gold standard” for validation, consisted of N. caninum-positive and N. caninum-negative sera defined by IFA and abortion status as determined by histopathology and immunohistochemistry of aborted fetal tissues. Aborted fetuses were submitted to the Washington Animal Disease Diagnostic Laboratory as part of an abortion diagnosis kit which included histopathology (brain, heart, liver, lung, kidney, placenta, thymus, adrenal gland, thyroid gland, spleen, and skeletal muscle), bacterial culture and virus isolation from fresh tissue pool, and maternal serology for antibodies to abortofacient pathogens (N. caninum, infectious bovine rhinotracheitis herpesvirus, bovine virus diarrhea virus, Leptospira spp., and Brucella abortus). N. caninum-positive aborted fetuses in group 1 had histopathological changes consistent with N. caninum infection and tachyzoites within affected tissues detectable by N. caninum-specific immunohistochemistry. Maternal sera were obtained within 2 weeks following abortion. For the purposes of the present study, histopathological changes compatible with N. caninum abortion had to be present, at a minimum, in the brain and heart (3, 29). The lesions consisted of moderate or severe multifocal necrosis and gliosis in the brain associated with nonsuppurative encephalitis and moderate or severe nonsuppurative myocarditis. Parasite infection was confirmed by immunohistochemical demonstration of tachyzoites in fetal brain using hyperimmune goat anti-N. caninum serum (VMRD Inc.). Group 1 N. caninum-negative aborted fetuses did not have microscopic lesions compatible with N. caninum infection.
Group 2 sera, used as relative standards for validation, were defined as N. caninum positive or negative by IFA alone as described previously (5), using commercially available, acetone-fixed, N. caninum tachyzoite slides (VMRD Inc.). The abortion status of dams contributing individual samples was not known. The sera originated both from herds experiencing N. caninum abortion problems, as epizootic or enzootic abortion, and from herds not experiencing abortion problems.
Group 3 sera, used for monitoring assay validation, had undefined N. caninum status by any method but originated from cattle herds experiencing abortion problems. The sera were submitted over a 3-year period (1998 to 2000) to the Washington Animal Disease Diagnostic Laboratory for routine abortion screen serology.
The specificity and sensitivity of detecting N. caninum serum antibodies by cELISA were determined from both group 1 and group 2 sera using the following formulas: sensitivity = (number of cELISA test-positive sera/number of reference positive sera) × 100; specificity = number of cELISA test-negative sera/number of reference negative sera) × 100); accuracy = [(number of cELISA test-positive sera + number of cELISA test-negative sera)/(number of reference positive sera + number of reference negative sera)] × 100. The frequency distribution and bivariate scattergram used to analyze group 3 sera determined how sharply the cELISA discriminated between test-positive and test-negative samples.
Histopathology and immunohistochemistry
Fetal tissues were fixed in 10% neutral buffered formalin, paraffin embedded, and processed for routine histopathology and immunohistochemistry as previously described (16). Positive control tissue consisted of formalin-fixed brain tissue from BALB/c mice experimentally inoculated with the NC-1 strain of N. caninum (15). Negative controls consisted of replacement of the primary antibody with a similar dilution of normal goat serum on all examined tissues.
As part of characterizing epitopes bound by N. caninum MAbs 5B6-2 and 4A4-2, some N. caninum-infected mouse brains also were immunostained with dilutions of concentrated hybridoma supernatants followed by biotinylated rabbit anti-mouse immunoglobulin G and peroxidase-conjugated streptavidin-biotin complex as described above.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotting. (i) Dot blot.
Sonicated antigens of N. caninum, T. gondii, or S. cruzi were absorbed to nitrocellulose sheets using a dot blot manifold and probed with MAb 5B6-25 to determine N. caninum specificity as previously described (5).
(ii) SDS-PAGE and Western blotting.
N. caninum antigen (NSo) was separated by SDS-PAGE under denaturing conditions, transferred to nitrocellulose, and probed with N. caninum antibody as previously described to determine the specificity of MAb 5B6-25 compared to MAb 4A4-2 and N. caninum-positive mouse serum (5). Immunoblotting assay controls consisted of replacement of the primary antibody with buffer alone (secondary reagent control) and inclusion of a lane containing a concentration-matched, isotype-matched irrelevant MAb (isotype control).
IFA for detection of antibodies to N. caninum.
Group 1 and 2 bovine sera were tested for the presence of N. caninum antibodies by IFA as previously described (5) but using commercially available slides containing acetone-fixed N. caninum tachyzoites (VMRD Inc.). The cutoff for a positive test was a serum dilution of 1:200. Some test sera were diluted only to 1:200, and some test sera were diluted twofold in phosphate-buffered saline (PBS) starting at a dilution of 1:50. One investigator (S.A.) read IFA slides without knowledge of N. caninum status. Sera were considered positive if the entire surface of the tachyzoite was fluorescent. All IFAs included known positive and negative bovine sera as controls.
cELISA.
The cELISA procedure was modified from previously reported techniques (5). The primary differences included the use of N. caninum 65-kDa captured antigen, undiluted test sera, and direct conjugate (directly conjugated competitor MAb 4A4-2). Fifty microliters of undiluted test bovine serum was added to individual wells of 96-well plates containing captured N. caninum 65-kDa antigen (VMRD Inc.) and incubated 60 min at room temperature. A small subset of serum samples from group 2 sera was diluted twofold in PBS from 1:2 to 1:128 for correlation studies with IFA titer. Following thorough washing (three times) in wash buffer (0.1 M PBS, pH 7.2, containing 0.05% Tween 20), 50 μl of peroxidase-conjugated N. caninum MAb 4A4-2 (VMRD Inc.), diluted 1:30, was added to each well and incubated for 20 min at room temperature. The optimal concentration of MAb 4A4-2 was determined previously by limiting-dilution titration of MAb 4A4-2 (5). The amount of bound MAb 4A4-2 was measured by adding 50 μl of TM-Blue substrate (BioFX Laboratories, Inc., Owings Mills, Md.) to each well and incubating in the dark for 20 min. The reaction was stopped by adding 50 μl of 1% NaF per well, and the optical density at 620 nm (OD620) was measured with a Dynatech MR-5000 ELISA plate reader. Each cELISA included controls of (i) two wells of known positive bovine sera (as identified by IFA and immunoblotting); (ii) MAb 4A4-2 conjugate alone (to determine maximal OD620 of MAb 4A4-2); and (iii) four wells of pooled negative reference bovine sera, pooled from 10 cows identified as negative for N. caninum antibodies by IFA and immunoblotting at a 1:10 dilution. After initial test validation with group 1 sera, each test run had a mean OD for negative controls of >0.5 and <1.2 and a mean percent inhibition for positive control of ≥30%. The percent inhibition of MAb 4A4-2 binding to captured NSo was calculated as 100 − [(OD of test sera/mean OD of negative reference sera) × 100].
RESULTS
Characteristics of N. caninum MAb 5B6-25.
Monoclonal antibody 5B6-25 was identified as immunoglobulin G1 by radial immunodiffusion and bound diffusely to the exterior surface of viable N. caninum tachyzoites as indicated by IFA (not shown). Dot blot assay revealed binding of MAb 5B6-25 at low concentrations (0.05 μg/ml) to sonicated N. caninum antigen (NSo), while binding to sonicated antigens of T. gondii or S. cruzi was not observed (not shown). Western blot analysis revealed MAb 5B6-25 bound to a single N. caninum tachyzoite antigen with a molecular mass of 65 kDa, similar to N. caninum competitor MAb 4A4-2 (not shown) (5). Immunohistochemistry analysis on formalin-fixed, paraffin-embedded brain from experimentally infected mice revealed immunoreactivity with MAb 5B6-25 but no immunoreactivity with MAb 4A4-2 (not shown).
Performance of cELISA on abortion-defined N. caninum-positive and N. caninum-negative sera.
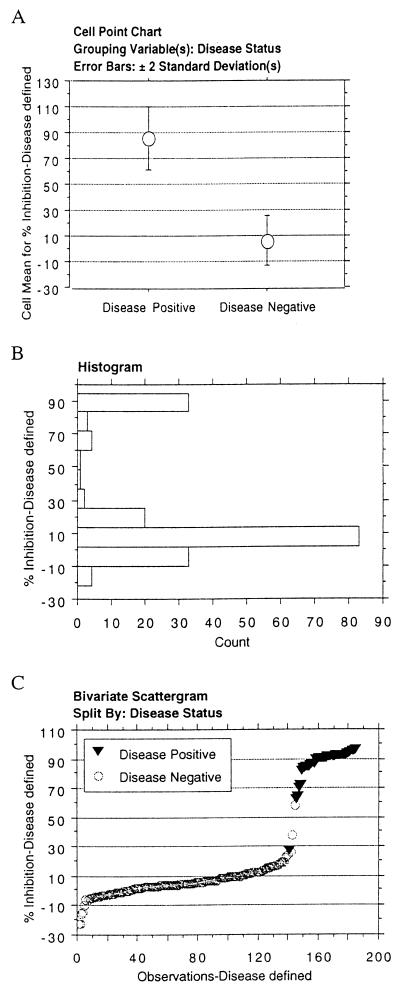
A test cutoff of 30% inhibition from pooled negative reference control sera was determined from analysis of cELISA test performance on group 1 abortion-defined N. caninum-positive and -negative sera (Table 1). Nonparametric statistical analysis (Mann-Whitney test) of mean percent inhibition revealed a significant difference between N. caninum-positive and N. caninum-negative groups (P ≤ 0.0001) (Fig. 1A). From this data analysis a 30% inhibition cutoff was chosen because the value was greater than 2 standard deviations of the mean percent inhibition of the gold standard negative sera. Histogram analysis of percent inhibition value from group 1 sera indicated a distinct bimodal distribution (Fig. 1B). A frequency distribution plot of percent inhibition was a steep sigmoid curve (Fig. 1C). Only 2.1% of samples (3 of 144) were within 5% of the 30% inhibition cutoff, indicating a sharp distinction between N. caninum disease-positive and -negative sera. The calculated test sensitivity was 97.6%, and test specificity was 98.6%; test accuracy was 97.8% (Table 2).
FIG. 1.
cELISA analysis of bovine sera defined by N. caninum abortion status (group 1). (A) Mean cELISA percent inhibition and variation of test-positive and test-negative sera. Error bars, 2 standard deviations. (B) Histogram of the distribution of cELISA percent inhibition values. (C) Frequency distribution of the cELISA percent inhibition values of N. caninum abortion-positive and N. caninum abortion-negative sera.
TABLE 2.
Performance of N. caninum MAb cELISA using abortion-defined N. caninum-positive and N. caninum-negative bovine sera
Performance of cELISA on IFA-defined N. caninum-positive and N. caninum-negative sera.
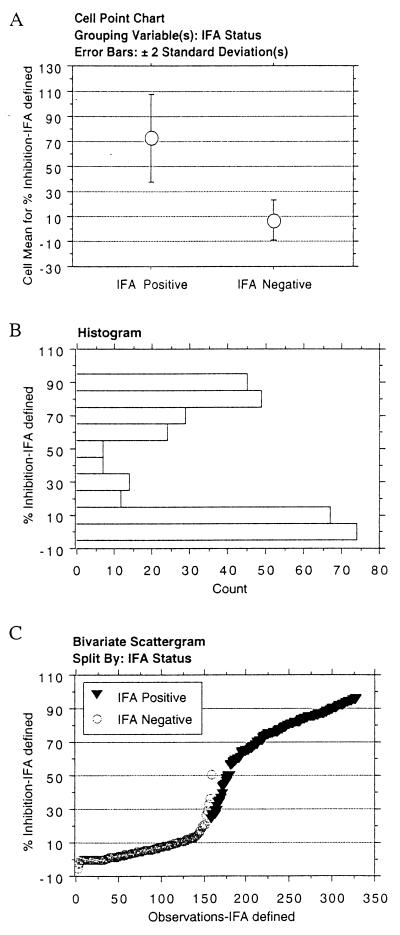
Disease-defined group 1 sera likely contained highly biased positive and negative samples (very-high-titer postabortion samples and negative samples) and may not represent the full spectrum of N. caninum antibody titers encountered in field investigations (19, 28). For this reason, we tested cELISA performance on group 2 sera (Table 1) obtained from cows in herds experiencing N. caninum abortions, but not necessarily originating from individual cows aborting N. caninum-infected fetuses, and from herds not experiencing abortions. This included cows with low titers (<1:800) by IFA that are not consistently identified as Neospora positive by Neospora ELISA. The titers of group 2 N. caninum IFA-positive sera ranged from 1:200 to 1:25,600; 21% of samples had serum IFA titers of 1:200 to 1:800. Nonparametric statistical analysis (Mann-Whitney test) revealed a significant difference between N. caninum IFA-positive and N. caninum IFA-negative groups (P ≤ 0.0001) (Fig. 2A). The 30% inhibition cutoff value was outside 2 standard deviations from the mean of both IFA-positive and IFA-negative sera. Histogram analysis of group 2 sera indicated a bimodal distribution of test results (Fig. 2B). The frequency distribution plot of percent inhibition for group 2 sera was a sigmoid curve slightly flatter than that for group 1 sera (Fig. 2C). Nevertheless, only 4.2% of samples (14 of 330) were within 5% of the 30% inhibition cutoff, indicating a sharp distinction between test-positive and test-negative sera. The calculated test sensitivity was 96.4%, and test specificity was 96.8%; test accuracy was 96.7% (Table 3).
FIG. 2.
cELISA analysis of bovine sera defined by N. caninum IFA status (group 2). (A) Mean cELISA percent inhibition and variation of test-positive and test-negative sera. Error bars, 2 standard deviations. (B) Histogram of the distribution of cELISA percent inhibition values. (C) Frequency distribution of the cELISA percent inhibition values of N. caninum IFA-positive and N. caninum IFA-negative sera.
TABLE 3.
Performance of N. caninum MAb cELISA using IFA-defined N. caninum-positive and N. caninum-negative bovine sera
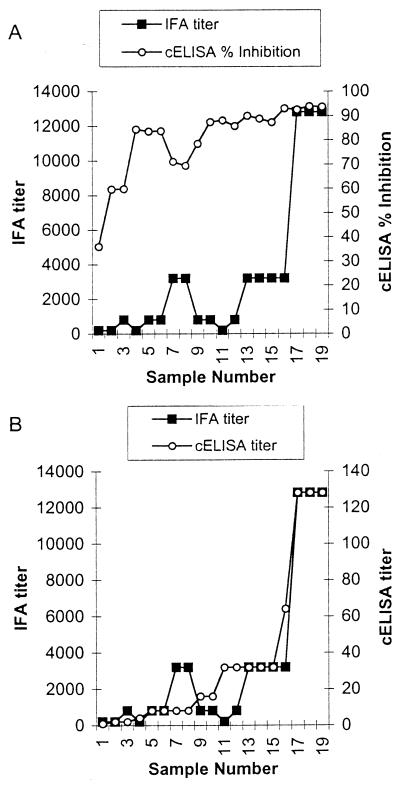
Of the 330 samples in group 2, 154 had complete IFA titer data, and for these the correlation between cELISA values and IFA titers was examined. Although the concordance between IFA and cELISA in distinguishing test-positive and test-negative results was high (as shown above and in Table 3), there was poor correlation between the cELISA percent inhibition value or raw OD value and the IFA titer (r2 = 0.22) (Fig. 3A). However, when a subset of test sera (IFA titers ranging from 1:200 to 1:12,800) were diluted twofold in the cELISA from 1:2 to 1:128, there was excellent correlation in rank between the cELISA titer value and the IFA titer value (r2 = 0.90) (Fig. 3B).
FIG. 3.
Comparison of IFA titer and cELISA percent inhibition (A) or IFA titer and cELISA titer (B). IFA titer is the highest serum dilution with diffuse tachyzoite fluorescence. cELISA titer is the highest serum dilution with ≥30% inhibition.
Performance of cELISA on field sera of unknown N. caninum status.
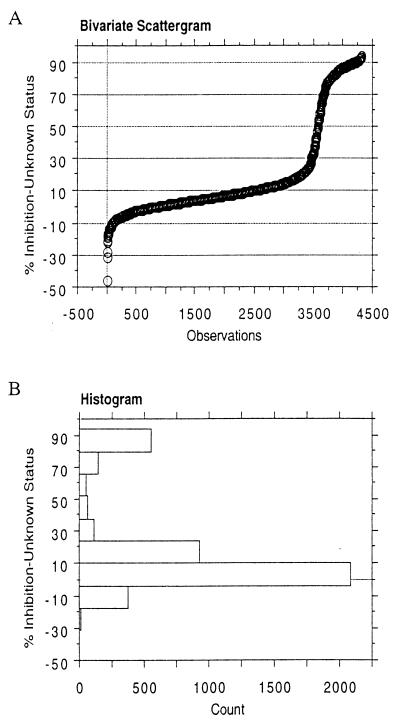
The performance of cELISA on field samples of unknown N. caninum status was evaluated with 4,323 bovine sera submitted to the Washington Animal Disease Diagnostic Laboratory over a 3-year period (1998 to 2000) (Table 1, group 3). The large majority of samples originated from herds experiencing abortions. A frequency distribution plot of percent inhibition revealed a steep sigmoid curve and a clear distinction between test-positive and test-negative sera (Fig. 4A), similar to the curve obtained using abortion-defined group 1 N. caninum-positive and -negative sera (Fig. 1C). Only 1.8% (79 of 4,323) of samples were within 5% of the 30% inhibition cutoff value, indicating clear discrimination between test-positive and test-negative results. Histogram analysis of group 3 sera indicated a bimodal distribution of test results, representing the clear distinction between test-positive and test-negative samples (Fig. 4B).
FIG. 4.
cELISA analysis of undefined bovine sera with unknown N. caninum antibody or abortion status (group 3). (A) Frequency distribution of the cELISA percent inhibition values. (B) Histogram of the distribution of cELISA percent inhibition values. Error bars, standard deviations.
DISCUSSION
A commercially available modification of a previously described MAb-based cELISA provided clear discrimination of defined N. caninum-positive and -negative bovine sera. The test groups of sera contained a full range of N. caninum antibody levels likely to be encountered in field investigations, from 1:200 to >1:25,000 as measured by IFA. The test performance, as shown by diagnostic sensitivity, diagnostic specificity, and test accuracy using a single cutoff point, was equal to or superior to those of other N. caninum serological assays using abortion-defined or neonatal precolostral antibody-defined gold standard reference sera (1, 17, 20, 21, 28). It was not necessary with the cELISA to change cutoff values in order to maximize diagnostic sensitivity and diagnostic specificity, a method suggested for other N. caninum serological assays to improve test performance depending upon the cattle population under study (postabortion, nonpostabortion, calves, whole herd, etc.) (1, 21, 22). Furthermore, ongoing validation with more than 4,300 bovine sera of unknown N. caninum infection status showed clear bimodal distribution of data and clear distinction of positive and negative results. These data revealed no necessity to modify the standard 30% cutoff point of the cELISA after extensive field testing.
According to the Office International Des Epizooties, validation of diagnostic assays for infectious diseases involves five stages: (i) determination of test feasibility; (ii) optimization and standardization of reagents and protocols; (iii) determination of assay performance; (iv) monitoring assay performance for validity; and (v) maintenance and extension of validation criteria (12). Stages i and ii for a MAb-based cELISA for detection of serum antibodies to N. caninum in cattle were reported previously and demonstrated that MAb 4A4-2 conjugate detected an epitope on N. caninum that was not present in members of the closely related Sarcocystidae family; that the epitope was immunodominant, as indicated by inducing a high antibody titer (>1:10,000) in infected cattle; and that MAb 4A4-2 was competitively inhibited by sera from a small sample of N. caninum-infected cows (5). The present data report on modifications of stage ii and implementation of stages iii and iv, validation of test performance (establishment of test cutoff, normalization of data, and calculation of diagnostic sensitivity and diagnostic specificity) and ongoing test monitoring. Although our data included only a total of 212 defined N. caninum-positive sera and 302 defined N. caninum-negative sera (group 1 and group 2 sera), numbers that fall below the Office International Des Epizooties standards of 300 known positive sera and 1,000 known negative sera for initial determination of diagnostic sensitivity and specificity, our sample numbers are above those of most other reports for performance of serological tests for N. caninum to allow for reasonable comparisons between tests (6, 20, 21, 27, 28).
Quantitative analysis of N. caninum cELISA percent inhibition values or raw OD values correlated poorly with individual IFA titers despite excellent qualitative concordance between the two assays in distinguishing positive from negative sera for several possible reasons. Most importantly, the titration of sera in cELISA results in a sigmoid curve of percent inhibition values, with plateaus at high and low antibody levels. The percent inhibition values in the high plateau occur with bovine sera with both high and low IFA titers, as indicated in Fig. 3A. This shows that competitive inhibition in the cELISA format reaches a maximum after which addition of more competing antibody does not significantly effect the OD or percent inhibition value. Thus, comparing the cELISA results with indirect ELISA or non-ELISA serological assays will draw erroneous conclusions if raw percent inhibition or OD values are compared with test values obtained by serum dilution. Secondly, IFA and cELISA may measure different biological properties of immunoglobulin and likely measure different total antibody populations. IFA is affected primarily by antibody quantity, whereas cELISA is affected by both quantity and binding affinity of serum antibody in order to effectively compete with MAb conjugate. Finally, serum antibodies that compete for binding to N. caninum antigen may be present at low levels, not high enough to affect IFA titer, yet still be efficiently detected by cELISA.
The specificity of the N. caninum cELISA in this report relies on the N. caninum-specific MAb conjugate 4A4-2. Formatting the assay for use with undiluted sera maximizes test sensitivity. Because the MAb 4A4-2 is directly conjugated, there is no need for species-specific linker reagents or secondary functions such as agglutination, which makes the assay simple, very rapid, and adaptable to sera from a broad range of test species. The use of cELISA in other species will require validation data similar to this report and may result in some adjustment of the percent inhibition cutoff value. MAb-based cELISA should be easy to standardize for large-scale epidemiological studies and disease-monitoring programs of multiple animal species.
ACKNOWLEDGMENTS
We acknowledge the technical expertise in the serology, immunohistochemistry, and histology sections at the Washington Animal Disease Diagnostic Laboratory. We also thank Travis McGuire for his critical review of the manuscript.
This research was supported in part by research grant US-2913-97 from The United States-Israel Binational Agricultural Research and Development Fund.
REFERENCES
- 1.Atkinson R, Harper P A, Reichel M P, Ellis J T. Progress in the serodiagnosis of Neospora caninum infections of cattle. Parasitol Today. 2000;16:110–114. doi: 10.1016/s0169-4758(99)01604-x. [DOI] [PubMed] [Google Scholar]
- 2.Barling K S, McNeill J W, Thompson J A, Paschal J C, McCollum F T, Craig T M, Adams L G. Association of serologic status for Neospora caninum with postweaning weight gain and carcass measurements in beef calves. J Am Vet Med Assoc. 2000;217:1356–1360. doi: 10.2460/javma.2000.217.1356. [DOI] [PubMed] [Google Scholar]
- 3.Barr B C, Anderson M L, Blanchard P C, Daft B M, Kinde H, Conrad P A. Bovine fetal encephalitis and myocarditis associated with protozoal infections. Vet Pathol. 1990;27:354–361. doi: 10.1177/030098589002700508. [DOI] [PubMed] [Google Scholar]
- 4.Baszler T V, Gay L J, Long M T, Mathison B A. Detection by PCR of Neospora caninum in fetal tissues from spontaneous bovine abortions. J Clin Microbiol. 1999;37:4059–4064. doi: 10.1128/jcm.37.12.4059-4064.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baszler T V, Knowles D P, Dubey J P, Gay J M, Mathison B A, McElwain T F. Serological diagnosis of bovine neosporosis by Neospora caninum monoclonal antibody-based competitive inhibition enzyme-linked immunosorbent assay. J Clin Microbiol. 1996;34:1423–1428. doi: 10.1128/jcm.34.6.1423-1428.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bjorkman C, Holmdahl O J, Uggla A. An indirect enzyme-linked immunoassay (ELISA) for demonstration of antibodies to Neospora caninum in serum and milk of cattle. Vet Parasitol. 1997;68:251–260. doi: 10.1016/s0304-4017(96)01076-x. [DOI] [PubMed] [Google Scholar]
- 7.Bjorkman C, Naslund K, Stenlund S, Maley S W, Buxton D, Uggla A. An IgG avidity ELISA to discriminate between recent and chronic Neospora caninum infection. J Vet Diagn Investig. 1999;11:41–44. doi: 10.1177/104063879901100106. [DOI] [PubMed] [Google Scholar]
- 8.Bjorkman C, Uggla A. Serological diagnosis of Neospora caninum infection. Int J Parasitol. 1999;29:1497–1507. doi: 10.1016/s0020-7519(99)00115-0. [DOI] [PubMed] [Google Scholar]
- 9.De Marez T, Liddell S, Dubey J P, Jenkins M C, Gasbarre L. Oral infection of calves with Neospora caninum oocysts from dogs: humoral and cellular immune responses. Int J Parasitol. 1999;29:1647–1657. doi: 10.1016/s0020-7519(99)00154-x. [DOI] [PubMed] [Google Scholar]
- 10.Dubey J P. Neosporosis in cattle: biology and economic impact. J Am Vet Med Assoc. 1999;214:1160–1163. [PubMed] [Google Scholar]
- 11.Dubey J P, Hattel A L, Lindsay D S, Topper M J. Neonatal Neospora caninum infection in dogs: isolation of the causative agent and experimental transmission. J Am Vet Med Assoc. 1988;193:1259–1263. [PubMed] [Google Scholar]
- 12.Jacobson R. OIE manual of standards for diagnostic tests and vaccines. 4th ed. Paris, France: Office International Des Epizooties, World Organization for Animal Health; 2000. Principles of validation of diagnostic assays for infectious diseases; pp. 15–23. [Google Scholar]
- 13.Lindsay D S, Dubey J P. Immunohistochemical diagnosis of Neospora caninum in tissue sections. Am J Vet Res. 1989;50:1981–1983. [PubMed] [Google Scholar]
- 14.Lindsay D S, Dubey J P, Duncan R B. Confirmation that the dog is a definitive host for Neospora caninum. Vet Parasitol. 1999;82:327–333. doi: 10.1016/s0304-4017(99)00054-0. [DOI] [PubMed] [Google Scholar]
- 15.Lindsay D S, Lenz S D, Cole R A, Dubey J P, Blagburn B L. Mouse model for central nervous system Neospora caninum infections. J Parasitol. 1995;81:313–315. [PubMed] [Google Scholar]
- 16.Long M T, Baszler T V, Mathison B A. Comparison of intracerebral parasite load, lesion development, and systemic cytokines in mouse strains infected with Neospora caninum. J Parasitol. 1998;84:316–320. [PubMed] [Google Scholar]
- 17.Louie K, Sverlow K W, Barr B C, Anderson M L, Conrad P A. Cloning and characterization of two recombinant Neospora protein fragments and their use in serodiagnosis of bovine neosporosis. Clin Diagn Lab Immunol. 1997;4:692–699. doi: 10.1128/cdli.4.6.692-699.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McAllister M M, Dubey J P, Lindsay D S, Jolley W R, Wills R A, McGuire A M. Dogs are definitive hosts of Neospora caninum. Int J Parasitol. 1998;28:1473–1478. [PubMed] [Google Scholar]
- 19.Otter A, Jeffrey M, Scholes S F, Helmick B, Wilesmith J W, Trees A J. Comparison of histology with maternal and fetal serology for the diagnosis of abortion due to bovine neosporosis. Vet Rec. 1997;141:487–489. doi: 10.1136/vr.141.19.487. [DOI] [PubMed] [Google Scholar]
- 20.Packham A E, Sverlow K W, Conrad P A, Loomis E F, Rowe J D, Anderson M L, Marsh A E, Cray C, Barr B C. A modified agglutination test for Neospora caninum: development, optimization, and comparison to the indirect fluorescent-antibody test and enzyme-linked immunosorbent assay. Clin Diagn Lab Immunol. 1998;5:467–473. doi: 10.1128/cdli.5.4.467-473.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pare J, Hietala S K, Thurmond M C. An enzyme-linked immunosorbent assay (ELISA) for serological diagnosis of Neospora sp. infection in cattle. J Vet Diagn Investig. 1995;7:352–359. doi: 10.1177/104063879500700310. [DOI] [PubMed] [Google Scholar]
- 22.Schares G, Rauser M, Sondgen P, Rehberg P, Barwald A, Dubey J P, Edelhofer R, Conraths F J. Use of purified tachyzoite surface antigen p38 in an ELISA to diagnose bovine neosporosis. Int J Parasitol. 2000;30:1123–1130. doi: 10.1016/s0020-7519(00)00092-8. [DOI] [PubMed] [Google Scholar]
- 23.Thurmond M C, Hietala S K. Culling associated with Neospora caninum infection in dairy cows. Am J Vet Res. 1996;57:1559–1562. [PubMed] [Google Scholar]
- 24.Thurmond M C, Hietala S K. Effect of Neospora caninum infection on milk production in first-lactation dairy cows. J Am Vet Med Assoc. 1997;210:672–674. [PubMed] [Google Scholar]
- 25.Thurmond M C, Hietala S K, Blanchard P C. Herd-based diagnosis of Neospora caninum-induced endemic and epidemic abortion in cows and evidence for congenital and postnatal transmission. J Vet Diagn Investig. 1997;9:44–49. doi: 10.1177/104063879700900108. [DOI] [PubMed] [Google Scholar]
- 26.Waldner C L, Janzen E D, Ribble C S. Determination of the association between Neospora caninum infection and reproductive performance in beef herds. J Am Vet Med Assoc. 1998;213:685–690. [PubMed] [Google Scholar]
- 27.Williams D J, Davison H C, Helmick B, McGarry J, Guy F, Otter A, Trees A J. Evaluation of a commercial ELISA for detecting serum antibody to Neospora caninum in cattle. Vet Rec. 1999;145:571–575. doi: 10.1136/vr.145.20.571. [DOI] [PubMed] [Google Scholar]
- 28.Wouda W, Brinkhof J, van Maanen C, de Gee A L, Moen A R. Serodiagnosis of neosporosis in individual cows and dairy herds: a comparative study of three enzyme-linked immunosorbent assays. Clin Diagn Lab Immunol. 1998;5:711–716. doi: 10.1128/cdli.5.5.711-716.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wouda W, Moen A R, Visser I J, van Knapen F. Bovine fetal neosporosis: a comparison of epizootic and sporadic abortion cases and different age classes with regard to lesion severity and immunohistochemical identification of organisms in brain, heart, and liver. J Vet Diagn Investig. 1997;9:180–185. doi: 10.1177/104063879700900212. [DOI] [PubMed] [Google Scholar]