Abstract
Introduction:
In 1998, the first licensed rotavirus vaccine was associated with intussusception, an unexpected adverse event, following reports of this condition to an adverse event reporting system. This rotavirus vaccine was withdrawn from the market and newer rotavirus vaccines have been extensively evaluated for an association with intussusception.
Areas covered:
We review the different study designs that have been used both pre- and post licensure to evaluate the association of rotavirus vaccines with intussusception and discuss the pros and cons of each design. Each of these study designs has their own strengths and weaknesses and the choice of the design often depends on the objective and the timing of the study and the resources available. For post-licensure monitoring of rotavirus vaccines, the self-controlled case-series design has become the most commonly used design to monitor this association.
Expert opinion:
Use of this common study design has enabled comparison of findings across diverse settings. As new rotavirus vaccines enter the market, use of the self-controlled case-series design will enable examination of this association in a timely manner.
Keywords: Rotavirus vaccine, intussusception, rotavirus, adverse event following immunization
1. Introduction
Following its discovery in 1973, rotavirus quickly became recognized as the most common cause of severe, dehydrating diarrhea in young children globally. In less than 10 years, the first rotavirus vaccine entered clinical trials [1] and in 1998, 25 years after the discovery of the virus, the first licensed rotavirus vaccine (RotaShield [RRV-TV]; Wyeth Lederle Vaccines) was recommend for routine use in the United States by the Advisory Committee on Immunization Practices (ACIP) [2]. RRV-TV is a 3-dose, attenuated, live oral tetravalent rotavirus vaccine based on a rhesus rotavirus strain. In clinical trials, the vaccine was shown to be highly effective in preventing severe rotavirus diarrhea [2]. The vaccine was generally well tolerated in pre-licensure trials with adverse events including fever and abdominal cramping [3]. While no statistically increased risk of intussusception following vaccination was found in the clinical trials, intussusception, a rare condition in which a portion of the bowel invaginates into another portion causing a blockage, was listed as a potential adverse reaction of RRV-TV and ACIP recommended post-licensure surveillance for intussusception [2]. Subsequent post-licensure evaluations found an elevated risk of intussusception following vaccination with any dose of RRV-TV with the greatest risk after the first dose [4,5]. These findings led to the withdrawal of RRV-TV from the market and from US immunization schedule in 1999 [6]. In a subsequent review of the data during a workshop in 2001, a consensus was reached that the attributable risk of intussusception due to RRV-TV was 1 excess case per 10,000 vaccinated children (range: 1 per 5,000–1 per 12,000) [7].
Despite the withdrawal of RRV-TV from the market, the substantial global burden of rotavirus disease encouraged continued development of rotavirus vaccines. Since RRV-TV had been associated with intussusception, newer rotavirus vaccines were closely evaluated for this rare adverse event. In this manuscript, we review the different study designs that have been used both pre- and post-licensure to evaluate the association between rotavirus vaccination and intussusception and discuss the pros and cons of each design.
2. Pre-licensure monitoring
Randomized controlled trials are considered the gold standard for evaluating new interventions. Subjects are screened for eligibility and then randomized into either a vaccine or placebo arm of the study. Randomized controlled trials are used to monitor both the efficacy and safety of new vaccines and their findings are important for vaccine licensure.
Across 11 pre-licensure trials of RRV-TV at varying dosage levels, 5 cases of intussusception in children 4–7 months of age were identified after the second or third doses of RRV-TV [8]. However, the rate of intussusception (0.05%) among the 10,054 vaccine recipients was not statistically increased over that of the 4,633 placebo recipients, where 1 case of intussusception occurred (0.02%; p = 0.45) [8]. Thus, while a possible signal for intussusception was detected in the clinical trials, the data were not clear enough to make firm conclusions because of the relatively small sample size.
For the first two new rotavirus vaccines (Rotarix [RV1], GlaxoSmithKline Biologicals and RotaTeq [RV5], Merck and Company), large randomized controlled trials were conducted to evaluate the safety of these vaccines with respect to intussusception [9,10]. These trials were substantially larger than those conducted for RRV-TV. The trial for RV1 included 63,255 infants in 11 Latin American countries and Finland with the primary hypothesis to evaluate the risk of intussusception within 31 days of either dose of vaccine [9]. Similarly, the trial for RV5 included 68,038 healthy infants from 11 countries in Europe and the United Sates and as a primary hypothesis, evaluated the risk of intussusception within 42 days of vaccination of any of the three doses [10]. Neither of these trials detected a significantly increased risk of intussusception following any dose of vaccine in the windows examined.
No large randomized controlled trials to examine rotavirus vaccine safety with respect to intussusception have been conducted in Africa or Asia nor have such trials been conducted with other newly available rotavirus vaccines (ROTAVAC, Bharat Biotech and Rotasiil, Serum Institute of India) in any setting [11–16]. Each of these clinical trials in Africa and Asia, which were powered for an efficacy endpoint, enrolled fewer than 10,000 infants total. Thus, while clinical trial data for rotavirus vaccines can be reassuring that there is not a high level of risk of a rare adverse event such as intussusception, they cannot not rule out smaller levels of risk in a diverse range of settings.
3. Post-licensure monitoring
3.1. Passive adverse event reporting
Adverse event monitoring systems are designed to accept spontaneous reports of adverse events temporally associated with vaccination including those that are unexpected and not just those conditions that are pre-defined. These adverse events may or may not be caused by vaccination. Such systems are an integral part of monitoring a country’s immunization program as an early warning system to identify potential vaccine safety problems (or ‘signals’).
The Vaccine Adverse Events Reporting System (VAERS) is a national passive surveillance system in the United Sates for reporting of adverse events following immunization. Within a year after national routine introduction of RRV-TV in the United States, VAERS recorded 15 spontaneous reports of intussusception among the approximately 1 million infants who had received RRV-TV by 27 May 1999 [17]. Because of likely severe under-reporting due to inadequate awareness of intussusception as a potential adverse event with RRV-TV, even these small numbers raised a concern about an association between RRV-TV and intussusception. The US rotavirus vaccination program was subsequently suspended in July 1999 while this potential association was further investigated. Numerous other studies including a national case-control study, a self-controlled case-series analysis, and a retrospective cohort analysis were launched and these studies confirmed and better quantified this risk.
Following introduction of RV5 in the United States in 2006, early review of data reported to VAERS from February 2006 to September 2007 did not show an increase in age-adjusted intussusception reporting rates compared to baseline rates but an apparent clustering of intussusception cases in the first week after vaccination was observed and further monitoring was recommended [18]. A subsequent analysis of VAERS reports from 2006–2012 after more than 47 million doses of RV5 were administered in the United States found a statistically increased risk of intussusception in the 3–6 day period after dose 1 of RV5 compared to the 0–2 day period after dose 1 of RV5 (reporting ratio: 3.75, 95% CI: 1.90–7.39) [19]. No increased risk of intussusception was detected after the second or third dose of RV5. With RV1, which was introduced in the United States in 2008 and had a comparatively low number of doses administered by mid-2012, intussusception appeared to cluster in the 3–6 days after each dose but there were too few reports for more detailed analyses [19]. A later analysis of VAERS data through 2014 showed a significant daily reporting ratio comparing days 3–6 with days 0–2 for the first dose of RV1 (reporting ratio: 7.5, 95% CI: 2.3–24.6) but no increased risk was detected after the second dose of RV1 although significant clustering was observed in the 2–7 days after this dose [20].
A similar analysis of worldwide spontaneous reporting data for RV5 found a significantly increased risk of intussusception in the 3–7 day period following the first dose compared to the 15–30 day period (IR: 3.45, 95% CI: 1.84–6.55) but no significantly elevated risk of intussusception was detected after the second or third dose [21].
3.2. Cohort studies
Like randomized controlled trials, cohort studies require large patient populations to be monitored over time to evaluate the risk of intussusception with respect to rotavirus vaccination. Successive cohorts of infants are followed to identify cases of intussusception and vaccine receipt is also captured and recorded. Rates of intussusception among vaccinated children can be compared either to pre-vaccine introduction rates or to rates of contemporary infants that had not received rotavirus vaccination.
Several cohorts of insured children have been monitored in the United States for the association between rotavirus vaccination and intussusception. The Vaccine Safety Datalink (VSD), which analyzed data from managed care facilities, and the Post-Licensure Rapid Immunization Safety Monitoring Program (PRISM), which evaluated data from health insurance plans, use ICD-9 codes to identify potential intussusception cases and link them to vaccination data [5,18,22–24]. VSD data were used to confirm the risk of intussusception following RRV-TV and both systems were used to examine the risk of IS following RV1 and RV5. Despite their large size of over 1 million children each for the more recent evaluations, both systems required multiple birth cohorts of children to achieve sufficient power to examine this risk.
Following the withdrawal of RRV-TV in the United States, VSD data were used to conduct a retrospective cohort analysis [5]. Of the 463,277 children who were included in the cohort, 56,253 had received at least one dose of RRV-TV. An increased risk of intussusception (RR: 16.0, 95% CI: 5.5–46.7) was observed in the 3–7 days after any dose with the greatest risk in the 3–7 days after the first dose (RR: 30.4, 95% CI: 8.8–104.9).
Following RV5 introduction in the United States in 2006, no statistically increased risk of intussusception was found after any dose of RV5 in the initial VSD analyses with data from May 2006 to September 2007 and May 2006 to May 2008 [18,24]. Similarly, analysis of VSD data from May 2006 to March 2013 when approximately 1.3 million total doses had been administered to cohort members found no increased number of cases within 7 days of vaccination with RV5 after all doses or the first dose when compared to the expected number of cases based on historical rates [22]. However, in a similar analysis of VSD data from April 2008 to March 2013, when 208,000 doses of RV1 had been administered to VSD cohort members, a significantly higher number of intussusception cases occurred within 7 days of either RV1 dose when compared to the expected number based on background historical rates [22]. Compared with the risk after RV5, an increased risk of intussusception was observed after RV1 (RR: 9.4, 95% CI: 1.4–103.8) [22]. In the PRISM cohort where 1.3 million doses of RV5 and 100,000 doses of RV1 had been given to cohort members by September 2011, an increased risk (IRR: 9.1, 95% CI: 2.2–38.6) was observed in the 1–7 days post dose 1 of RV5 compared to the 22–42 day control interval in a self-controlled risk interval analysis [23]. No statistically increased risk of intussusception was identified after the second or third dose of RV5. These findings were confirmed by a secondary cohort analysis. No increased risk of intussusception following either dose of RV1 was identified in primary analysis for the PRISM cohort but the power of these analyses were low given that fewer doses of RV1 had been administered.
3.3. Case-control evaluations
A case-control evaluation is another study design that can be used to assess the association between rotavirus vaccination and intussusception. These evaluations compare the odds of recent rotavirus vaccine receipt among children with intussusception with the odds of recent rotavirus vaccine receipt among children without intussusception.
The case-control design has been successfully implemented to evaluate the association between rotavirus vaccines and intussusception including a large national case-control study in the United States assessing RRV-TV receipt and risk of intussusception and post-licensure evaluations of RV1 in Mexico and Brazil and of RV1 and RV5 in Australia following the introduction of these newer rotavirus vaccines into their national immunization programs [4,25,26].
The nation-wide case-control evaluation of RRV-TV in the United States found an elevated risk of intussusception in the 3–14 days following any dose of RRV-TV (aOR: 10.6, 95% CI: 5.7–19.6), with a higher risk in the 3–14 days after the first dose (aOR: 21.7, 95% CI: 9.6–48.9) than after the second dose (aOR: 3.3, 95% CI: 1.1–9.8), and the highest risk in the 3–7 days following dose 1 (aOR: 37.2; 95% CI: 12.6–110.1) [4]. These timely results provided some of the first evidence of the association between rotavirus vaccination and intussusception.
Case-control evaluations were also some of the first post-licensure evaluations of RV1 and RV5 in early introducing countries. In Mexico, a case-control evaluation enrolling cases from 16 hospitals in 10 states and age-matched controls from the community found an increased risk of intussusception in the 1–7days after the first dose of RV1 (OR: 5.9, 95% CI: 2.6–13.0) but no increased risk in the 1–7 days after the second dose [25]. A similar evaluation in Brazil with cases enrolled from 53 hospitals in 7 states and age-matched community controls found no increased risk of intussusception in the 1–7 days after the first dose of RV1 but a small increased risk following the second dose (OR: 2.6, 95% CI: 1.3–5.2) [25]. In Australia, a matched case-control evaluation in which controls were selected from the national immunization registry found an increased risk of intussusception in the 1–7 days after the first doses of both RV1 (OR: 15.6, 95% CI: 3.4–72.6) and RV5 (OR: 11.7, 95% CI: 3.2, 43.4) and the 8–21 days after the second doses of RV1 (OR: 6.5, 95% CI: 1.7–24.2) and RV5 (OR: 4.7, 95% CI: 1.8–12.0) [26].
3.4. Self-controlled case-series evaluations
The self-controlled case-series method is a modified cohort design that uses only cases to calculate the relative incidence of an event in a defined time period after a transient exposure compared with another time period [27]. In studies that have used this design, intussusception cases have been identified through active surveillance using a standardized case definition and receipt and timing of rotavirus vaccination was confirmed by either the child’s vaccination card or vaccine clinic records.
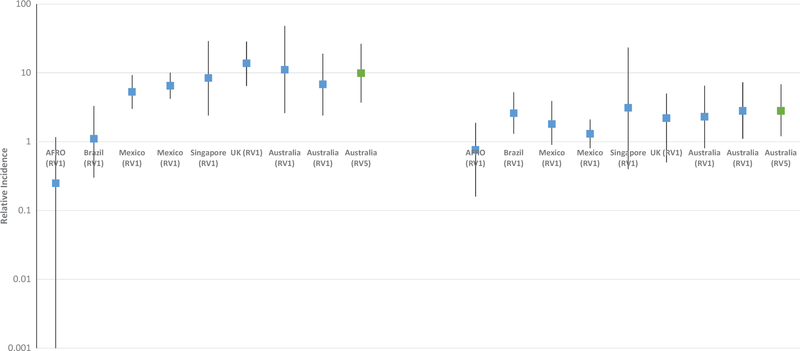
The self-controlled case-series design is time and resource efficient and has become the most common method for evaluating the risk of intussusception following rotavirus vaccination. It has successfully been implemented in a variety of developed and developing countries [25,26,28–32]. An increased risk of intussusception was detected following rotavirus vaccination in most, but not all, of these evaluations. (Figure 1)
Figure 1.
Relative incidence of intussusception in the 1–7 days following dose 1 and dose 2 of rotavirus vaccine by country.
As this study design has not been as widely used as some of the traditional designs for evaluating adverse events associated with vaccination, some of the initial self-controlled case-series analyses were conducted in parallel with case-control evaluations. In Mexico, an increased risk of intussusception was observed in the 1–7 days after the first dose of RV1 (IR: 5.3; 95% CI: 3.0–9.3) but not after dose 2 and in Brazil, no increased risk was identified after dose 1 but a small increased risk was identified in the 1–7 days after dose 2 (IR: 2.6; 95% CI: 1.3–5.7) [25]. These findings mirrored those of the concurrent case-control evaluations in these two countries [25]. A separate self-controlled case-series evaluation in Mexico also found similar results with an increased risk of intussusception in the 0–6 days following dose 1 of RV1 (RI: 6.49; 95% 4.17–10.09) [29]. An increased risk of intussusception in the 1–7 days after dose 1 of RV1 was also observed in several high income countries including England (IR: 13.81; 95% CI: 6.44–28.32), Singapore (IR: 8.4; 95% CI: 2.4, 29), and Australia (IR: 6.8; 95% CI: 2.4–19) [26,30,33]. Similarly, an increased risk of intussusception in the 1–7 days after dose 1 of RV5 in Australia was also observed (IR: 9.9; 95% CI: 3.7, 26.4) [26]. In a self-controlled case-series analysis with pooled data from seven lower income countries in sub-Saharan Africa, no increased risk of intussusception was observed in the 1–7 days following dose 1 or dose 2 of RV1 [31]. In the self-controlled case-series analyses from high and middle-income countries, the risk in the 1–7 days following dose 2 was mixed with increased risks observed in Brazil and Australia but not in Mexico, Singapore, or England [25,26,29,30,33].
3.5. Ecologic trends
Use of surveillance data is often proposed to monitor trends in an adverse event over time. Cases are identified through either active or passive surveillance. Individual level data, including vaccination status, may not be available. Such evaluations provide an ecologic look at trends in intussusception pre- and post-rotavirus vaccine introduction but findings often require confirmation with more rigorous study designs.
An ecologic analysis of intussusception admissions in the United States did not detect a population level increase in intussusception admissions among infants <1 year of age during the time of RRV-TV use although low coverage limited the power of the evaluation [34]. However, a small increase in intussusception admissions was noted in infants 45–210 days of age, the targeted age-range for the first dose of RRV-TV. Following the introduction of RV1 and RV5 in the United States, population level trends in the rate of intussusception hospitalizations pre- and post-rotavirus vaccine introduction were again examined using International Classification of Disease (ICD) coding of hospital discharges [35,36]. No population level increase in intussusception hospitalization rates was observed among infants <12 months of age. However, an increased rate was observed post-vaccine introduction among children 8–11 weeks of age, when the majority of first doses are given in the United States, but the findings were not completely consistent with increasing vaccine coverage in the population and thus required other study designs to confirm.
4. Pros and cons of each study design
Numerous study designs have been used to monitor the association of rotavirus vaccine with intussusception in both the pre- and post-vaccine licensure periods. Each of these study designs has their own strengths and weaknesses (Table 1) and the choice of the design often depends on the objective and the timing of the evaluation and the resources available.
Table 1.
Comparison of different study designs used to monitor the safety of rotavirus vaccines with respect to intussusception.
| Study Design | Strengths | Weaknesses |
|---|---|---|
| Randomized Controlled Trials | • Randomization minimizes selection bias and helps distribute potential confounders equally • Rigorous follow-up of patients and strict adherence to protocol |
• Large sample sizes required for rare events and/or low level risks • Costly • May not reflect real-world conditions |
| Passive Adverse Event Reporting | • Can capture new or unexpected events | • Expected or baseline rate of the condition, the number of children vaccinated, and completeness of reporting often unknown |
| Cohort Studies | • Known catchment population enables calculation of rates • Closely monitor for cases of intussusception and record vaccination status for individual cases |
• Requires large sample size for rare adverse events • Costly • Susceptible to changes in care/treatment over time • Cohort may not be representative of entire population |
| Case-Control Evaluations | • Smaller sample size required • Resource efficient |
• Potential bias due to control selection and confounders • Study team not blinded to case/control status • Logistical challenges in enrolling controls |
| Self-Controlled Case-Series Evaluations | • Smaller sample size required • Resource efficient • Controls for individual level confounders |
• Complex analytic methods |
| Ecologic Trends | • Relatively straight-forward to implement | • Trends susceptible to improved surveillance and increased awareness over time especially with rare events |
4.1. Randomized controlled trials
In clinical trials, randomization minimizes selection bias and helps to distribute potential confounders equally between the different arms of the study. Such trials are conducted under optimal conditions with close follow-up of patients, timely administration of vaccines, and prompt reporting of potential adverse events. However, use of randomized controlled trials to assess rare adverse events such as the association between rotavirus vaccination and intussusception can be challenging. Randomized controlled trials are time and resource intensive and when the outcome is rare, the sample size needed to detect small increases in risk can be prohibitive. Despite the large size of the trials for RV1 and RV5, they were only powered to detect a risk similar to that seen with RRV-TV in the 4–6 weeks after any dose. The evaluation of dose-specific risk or shorter duration risk windows was not possible with the given sample sizes. Furthermore, randomized controlled trials often do not reflect the real-world conditions in which vaccines are administered. Rates of baseline intussusception vary by location and children in different populations may respond differently to the vaccine. Also, children may present late for vaccination and have a broader array of underlying conditions compared with children enrolled in randomized controlled trials.
4.2. Passive adverse event reporting
Passive adverse event reporting systems are flexible and allow reporting of any adverse event following immunization and not just those events that have been pre-specified. However, interpretation of these data can be challenging as the expected or baseline rate of the condition, the number of children vaccinated, and the completeness of reporting are often unknown. In the United States, the initial signal of an increased risk of intussusception following RRV-TV was generated by VAERS, a national passive reporting system that relies on spontaneous reports of potential adverse events, but the ultimate confirmation of this signal required further evaluation using other study designs. Thus, while such systems have the capacity to detect signals for new or unexpected adverse events, these signals will need to be more rigorously examined with other study designs.
4.3. Cohort studies
Cohort studies, with their known catchment population, enables the direct calculation of rates, close monitoring of the population for intussusception, and better capture of vaccination data. Taking advantage of existing cohorts, such as members of managed care organizations or health insurance plans, can increase the efficiency and decrease costs of such studies. However, the sample size required to detect a small increased risk of intussusception associated with rotavirus vaccination is still large and the insured population may not be representative of the entire population. If the standard of care changes over time (e.g. a shift from inpatient care to emergency department care over time), then historical rates may result in an under or overestimate of the risk of intussusception following rotavirus vaccination. Furthermore, such large cohorts are often not available in resource-limited settings.
4.4. Case-control evaluations
Case-control evaluations require smaller sample sizes than those needed for randomized controlled trials and cohort studies and thus, can be resource efficient. However, case-control evaluations have some limitations including the potential for bias due to control selection and confounders. The study team is not blinded to the case or control status of the subject and therefore special care is needed to ensure that all intussusception cases are enrolled and not just those that have been recently vaccinated and that equal effort is expended in obtaining the vaccination histories of cases and controls. Logistical challenges in enrolling of controls and in ensuring that the controls are representative of the population that gave rise to the cases may also be present.
4.5. Self-controlled case-series evaluations
The self-controlled case-series method combines the power and simplicity of a cohort study with the economy of a case-control evaluation. Only data from case-patients are used in these analyses. To ensure that there is no bias, cases must be identified independent of their vaccination status. As each case effectively acts as its own control, this method intrinsically controls for individual level confounders such as sex, socioeconomic status, and genetics. Age-adjustment is possible to accounting for the varying risk of intussusception in the first year of life but narrow age bands should be used to account for the rapidly changing background risk of intussusception by age. The method can also account for contraindications against further doses of rotavirus vaccine if a child experiences an episode of intussusception [37]. While the data are easy to collect, the analytic methods require careful skill. In relation to the other study designs, this design is relatively new. However, concurrent case-control and self-controlled case-series analyses in several populations have yielded comparable results [25,26].
4.6. Ecologic trends
Ecologic studies that monitor trends in intussusception without individual level data on vaccination status can be used to determine if an increased risk of intussusception is observed at the population level following rotavirus vaccination. While ecologic analyses of surveillance data have been successfully used to document the impact of rotavirus vaccines on all-cause and rotavirus diarrhea hospitalizations in many settings [38], the use of surveillance to monitor trends over time of a rare adverse event can be challenging. For rare adverse events like intussusception, small, short-term increases in risk are hard to observe at the population level. Such ecologic evaluations assume that the baseline rate of the event should remain constant during the pre- and post-vaccination periods. However, with rare adverse events like intussusception, improved surveillance or increased awareness of the condition over time can result in an increased detection of cases independent of any association with the vaccine but that may be falsely attributed to vaccination. Similarly, missing just a few cases of a rare adverse event by a surveillance system can have a big impact on findings.
5. Expert opinion
Rotavirus vaccines have had a tremendous impact on rotavirus disease burden with documented declines in diarrheal deaths and rotavirus hospitalizations in countries all over the world that are using the vaccine [38]. By the end of 2018, over 90 countries had introduced rotavirus vaccine into their national immunization programs. Rotavirus vaccines continue to be recommended for routine use globally as the well-documented benefits of the vaccine in terms of reduced morbidity and mortality far outweigh the small increased risk of intussusception [39]. Age restrictions were initially recommended for the administration of rotavirus vaccine to ensure that all doses were administered on a timely basis when the risk of natural intussusception was low [40]. However, as the benefits and risks of rotavirus vaccines have been better quantified in post-licensure evaluations and the benefit risk ratio found to be quite favorable toward rotavirus vaccination, removal of age restrictions has been recommended, particularly in high rotavirus disease burden settings [39].
The exact mechanism by which rotavirus vaccine triggers intussusception is not well understood but different properties of the vaccine virus may contribute to a differential risk. The rhesus human reassortant strain that was used in the RotaShield vaccine resulted in a stronger immune reaction to the vaccine than has been seen with the newer generation of rotavirus vaccines currently available. Thus, as new rotavirus vaccines, including the Indian-manufactured vaccines that were recently prequalified for use by the World Health Organizations, are introduced into national immunization programs, examining their association with intussusception is important. The development of non-replicating, parenterally delivered rotavirus vaccines may diminish this risk altogether but such vaccines are much further down the vaccine pipeline [41].
While the risk of intussusception does not appear to differ substantially for the two currently available rotavirus vaccines, the risk of intussusception following rotavirus vaccination does appear to vary by setting. In most high and middle-income countries where post-licensure evaluations have been performed, a small increased risk of intussusception mainly in the 1–7 days after the first dose has been observed. A similar increase in risk was not seen in a pooled analysis of data from seven lower income countries in Africa. Reasons for these differences in risk are unknown but may be related to possible differences in vaccine virus replication, oral polio vaccine use, nutrition, diet, and genetics. Rotavirus vaccines continue to be introduced in a variety of settings and these introductions offer additional setting in which the risk of intussusception following rotavirus vaccination can be examined.
Since the first unanticipated association between rotavirus vaccination and intussusception was detected, researchers have employed numerous study designs to better quantify this association. Each of these study designs has its own strengths and limitations, especially when monitoring rare adverse events. For post-licensure monitoring of rotavirus vaccines, the self-controlled case-series design has become the most commonly used design to monitor this association. The use of a common study design has enabled comparison of findings across diverse settings. As new rotavirus vaccines enter the market, use of the self-controlled case-series design will enable examination of this association in a timely manner and producing findings that can be compared across settings.
Article highlights.
Rotavirus vaccines have been associated with a small increased risk of intussusception in some settings.
Numerous study designs have been used to examine the association between rotavirus vaccination and intussusception.
The choice of study design depends on the objective and timing of the study and the resources available.
The self-controlled case-series design has become the most commonly used design to monitor the association between rotavirus vaccination and intussusception.
As new rotavirus vaccines become available, the self-controlled case-series design should be used to monitor the association between rotavirus vaccination and intussusception.
Acknowledgments
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Footnotes
Reviewer Disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Midthun K, Kapikian AZ. Rotavirus vaccines: an overview. Clin Microbiol Rev. 1996;9:423–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rotavirus vaccine for the prevention of rotavirus gastroenteritis among children. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 1999;48:1–20. [PubMed] [Google Scholar]
- 3.Joensuu J, Koskenniemi E, Vesikari T. Symptoms associated with rhesus-human reassortant rotavirus vaccine in infants. Pediatr Infect Dis J. 1998;17:334–340. [DOI] [PubMed] [Google Scholar]
- 4. Murphy TV, Gargiullo PM, Massoudi MS, et al. Intussusception among infants given an oral rotavirus vaccine. N Engl J Med. 2001;344:564–572.11207352 • Original paper which associated rotavirus vaccination with intussusception.
- 5.Kramarz P, France EK, Destefano F, et al. Population-based study of rotavirus vaccination and intussusception. Pediatr Infect Dis J. 2001;20:410–416. [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease C, Prevention. Withdrawal of rotavirus vaccine recommendation. MMWR Morb Mortal Wkly Rep. 1999;48:1007. [PubMed] [Google Scholar]
- 7.Peter G, Myers MG, National Vaccine Advisory C, National Vaccine Program O. Intussusception, rotavirus, and oral vaccines: summary of a workshop. Pediatrics. 2002;110:e67. [DOI] [PubMed] [Google Scholar]
- 8.Rennels MB, Parashar UD, Holman RC, et al. Lack of an apparent association between intussusception and wild or vaccine rotavirus infection. Pediatr Infect Dis J. 1998;17:924–925. [DOI] [PubMed] [Google Scholar]
- 9.Ruiz-Palacios GM, Perez-Schael I, Velazquez FR, et al. Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N Engl J Med. 2006;354:11–22. [DOI] [PubMed] [Google Scholar]
- 10.Vesikari T, Matson DO, Dennehy P, et al. Safety and efficacy of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine. N Engl J Med. 2006;354:23–33. [DOI] [PubMed] [Google Scholar]
- 11.Armah GE, Sow SO, Breiman RF, et al. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in sub-Saharan Africa: a randomised, double-blind, placebo-controlled trial. Lancet. 2010;376:606–614. [DOI] [PubMed] [Google Scholar]
- 12.Madhi SA, Cunliffe NA, Steele D, et al. Effect of human rotavirus vaccine on severe diarrhea in African infants. N Engl J Med. 2010;362:289–298. [DOI] [PubMed] [Google Scholar]
- 13.Zaman K, Dang DA, Victor JC, et al. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in Asia: a randomised, double-blind, placebo-controlled trial. Lancet. 2010;376:615–623. [DOI] [PubMed] [Google Scholar]
- 14.Bhandari N, Rongsen-Chandola T, Bavdekar A, et al. Efficacy of a monovalent human-bovine (116E) rotavirus vaccine in Indian infants: a randomised, double-blind, placebo-controlled trial. Lancet. 2014;383:2136–2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Isanaka S, Guindo O, Langendorf C, et al. Efficacy of a low-cost, heat-stable oral rotavirus vaccine in niger. N Engl J Med. 2017;376:1121–1130. [DOI] [PubMed] [Google Scholar]
- 16.Kulkarni PS, Desai S, Tewari T, et al. A randomized Phase III clinical trial to assess the efficacy of a bovine-human reassortant pentavalent rotavirus vaccine in Indian infants. Vaccine. 2017;35:6228–6237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Centers for Disease C, Prevention. Intussusception among recipients of rotavirus vaccine–United States, 1998–1999. MMWR Morb Mortal Wkly Rep. 1999;48(27): 577–581. [PubMed] [Google Scholar]
- 18.Haber P, Patel M, Izurieta HS, et al. Postlicensure monitoring of intussusception after RotaTeq vaccination in the United States, February 1, 2006, to September 25, 2007. Pediatrics. 2008;121:1206–1212. [DOI] [PubMed] [Google Scholar]
- 19.Haber P, Patel M, Pan Y, et al. Intussusception after rotavirus vaccines reported to US VAERS, 2006–2012. Pediatrics. 2013;131:1042–1049. [DOI] [PubMed] [Google Scholar]
- 20.Haber P, Parashar UD, Haber M, et al. Intussusception after monovalent rotavirus vaccine-United States, Vaccine Adverse Event Reporting System (VAERS), 2008–2014. Vaccine. 2015;33:4873–4877. [DOI] [PubMed] [Google Scholar]
- 21.Escolano S, Hill C, Tubert-Bitter P. Intussusception risk after RotaTeq vaccination: evaluation from worldwide spontaneous reporting data using a self-controlled case series approach. Vaccine. 2015;33:1017–1020. [DOI] [PubMed] [Google Scholar]
- 22.Weintraub ES, Baggs J, Duffy J, et al. Risk of intussusception after monovalent rotavirus vaccination. N Engl J Med. 2014;370:513–519. [DOI] [PubMed] [Google Scholar]
- 23.Yih WK, Lieu TA, Kulldorff M, et al. Intussusception risk after rotavirus vaccination in U.S. infants. N Engl J Med. 2014;370:503–512. [DOI] [PubMed] [Google Scholar]
- 24.Belongia EA, Irving SA, Shui IM, et al. Real-time surveillance to assess risk of intussusception and other adverse events after pentavalent, bovine-derived rotavirus vaccine. Pediatr Infect Dis J. 2010;29:1–5. [DOI] [PubMed] [Google Scholar]
- 25. Patel MM, Lopez-Collada VR, Bulhoes MM, et al. Intussusception risk and health benefits of rotavirus vaccination in Mexico and Brazil. N Engl J Med. 2011;364:2283–2292.21675888 • Early study showing association of newer rotavirus vaccines with intussuscpetion using both self-controlled case-series analysis and case-control evaluation.
- 26.Carlin JB, Macartney KK, Lee KJ, et al. Intussusception risk and disease prevention associated with rotavirus vaccines in Australia’s National immunization program. Clin Infect Dis. 2013;57:1427–1434. [DOI] [PubMed] [Google Scholar]
- 27. Whitaker HJ, Hocine MN, Farrington CP. The methodology of self-controlled case series studies. Stat Methods Med Res. 2009;18:7–26.18562396 • Paper explaining the self-controlled case-series methodology.
- 28.Stowe J, Andrews N, Ladhani S, et al. The risk of intussusception following monovalent rotavirus vaccination in England: A self-controlled case-series evaluation Ref. No: JVAC-D-16–01124. Vaccine. 2016;34:6115. [DOI] [PubMed] [Google Scholar]
- 29.Velazquez FR, Colindres RE, Grajales C, et al. Postmarketing surveillance of intussusception following mass introduction of the attenuated human rotavirus vaccine in Mexico. Pediatr Infect Dis J. 2012;31:736–744. [DOI] [PubMed] [Google Scholar]
- 30.Yung CF, Chan SP, Soh S, et al. Intussusception and monovalent rotavirus vaccination in Singapore: self-controlled case series and risk-benefit study. J Pediatr. 2015;167:163–8e1. [DOI] [PubMed] [Google Scholar]
- 31.Tate JE, Mwenda JM, Armah G, et al. Evaluation of intussusception after monovalent rotavirus vaccination in Africa. N Engl J Med. 2018;378:1521–1528. [DOI] [PubMed] [Google Scholar]
- 32.Quinn HE, Wood NJ, Cannings KL, et al. Intussusception after monovalent human rotavirus vaccine in Australia: severity and comparison of using healthcare database records versus case confirmation to assess risk. Pediatr Infect Dis J. 2014;33:959–965. [DOI] [PubMed] [Google Scholar]
- 33.Stowe J, Andrews N, Ladhani S, et al. The risk of intussusception following monovalent rotavirus vaccination in England: A self-controlled case-series evaluation. Vaccine. 2016;34:3684–3689. [DOI] [PubMed] [Google Scholar]
- 34.Simonsen L, Morens D, Elixhauser A, et al. Effect of rotavirus vaccination programme on trends in admission of infants to hospital for intussusception. Lancet. 2001;358:1224–1229. [DOI] [PubMed] [Google Scholar]
- 35.Tate JE, Yen C, Steiner CA, et al. Intussusception rates before and after the introduction of rotavirus vaccine. Pediatrics. 2016;138(3): pii: e20161082. doi: 10.1542/peds.2016-1082. Epub 2016 Aug 24. [DOI] [PubMed] [Google Scholar]
- 36.Yen C, Tate JE, Steiner CA, et al. Trends in intussusception hospitalizations among US infants before and after implementation of the rotavirus vaccination program, 2000–2009. J Infect Dis. 2012;206:41–48. [DOI] [PubMed] [Google Scholar]
- 37.Farrington CP, Whitaker HJ, Hocine MN. Case series analysis for censored, perturbed, or curtailed post-event exposures. Biostatistics. 2009;10:3–16. [DOI] [PubMed] [Google Scholar]
- 38.Burnett E, Jonesteller CL, Tate JE, et al. Global impact of rotavirus vaccination on childhood hospitalizations and mortality from diarrhea. J Infect Dis. 2017;215:1666–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rotavirus vaccines. WHO position paper - January 2013. Wkly Epidemiol Rec. 2013;88:49–64. [PubMed] [Google Scholar]
- 40. World Health Organization. Rotavirus vaccines. Wkly Epidemiol Rec. 2007;82:285–295.17691162 • World Health Organization position paper recommending use of rotavirus vaccines and the removal of age restrictions for their administration.
- 41.Kirkwood CD, Ma LF, Carey ME, et al. The rotavirus vaccine development pipeline. Vaccine. 2017:pii: S0264–410X(17)30410–3. doi: 10.1016/j.vaccine.2017.03.076. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]