Abstract
Background:
Research on the adverse effects of mindfulness-based programs (MBPs) has been sparse and hindered by methodological imprecision.
Methods:
The 44-item Meditation Experiences Interview (MedEx-I) was used by an independent assessor to measure meditation-related side effects (MRSE) following three variants of an 8-week program of mindfulness-based cognitive therapy (n = 96). Each item was queried for occurrence, causal link to mindfulness meditation practice, duration, valence, and impact on functioning.
Results:
Eighty-three percent of the MBP sample reported at least one MRSE. Meditation-related adverse effects (MRAEs) with negative valences or negative impacts on functioning occurred in 58% and 37% of the sample, respectively. Lasting bad effects occurred in 6–14% of the sample and were associated with signs of dysregulated arousal (hyperarousal and dissociation).
Conclusion:
Meditation practice in MBPs is associated with transient distress and negative impacts at similar rates to other psychological treatments.
Keywords: mindfulness, meditation, adverse effects, harms monitoring
While mindfulness-based programs (MBPs) have emerged as a promising treatment for a range of conditions with comparable efficacy to established psychological treatments (Goldberg et al., 2018; Hofmann, Sawyer, Witt, & Oh, 2010), very little is known about negative or adverse effects (Baer, Crane, Miller, & Kuyken, 2019). While distressing and functionally impairing effects of meditation have been reported in textual sources, clinical literature, and multiple research studies (Lindahl, Britton, Cooper, & Kirmayer, 2019), adverse event monitoring in MBPs remains inadequate and inconsistent, producing widely varying frequency estimates depending on how adverse events are defined and measured. As a result, the widespread dissemination of MBPs into schools, hospitals, prisons and mobile apps has proceeded without sufficient information about potential harms. The current study aims to clarify the nature and frequency of meditation-related adverse effects (MRAEs) in MBPs by implementing 25 updated harms assessment recommendations of what to measure (severity, types of events, expectedness) and how to measure (mode, independence, patient-based, relatedness) (Ioannidis et al., 2004; Lineberry et al., 2016; NIH, 2016; Rozental et al., 2018). See Table 1 for a list of harms monitoring recommendations addressed in this paper.
Table 1.
Harms Monitoring Recommendations
| # | Issue | Description and Recommendation | How the Current Study Addresses |
|---|---|---|---|
|
| |||
| What to Measure | |||
|
|
|||
| Degree of Harm |
|||
| 1 | Severity | Studies limited to serious AEs inadequate; include all clinically-relevant events | 3 tiered approach: valence, impact, LBEs |
| 2 | Duration | Clinically relevant events can have different durations | Durations of all events assessed, LBEs are reported according to three different durations of negative impacts |
| 3 | Transient distress vs. harm | Differentiate transient distress from negative impact on life and functioning | Separate ratings for valence and impact |
| Types of Events |
|||
| 4 | Deterioration of target symptoms inadequate | Misses novel or unexpected symptoms | MedEx-I measures wide range of meditation-related side effects |
| 5 | General deterioration vs multiple domains | Treatments can improve some symptoms in some domains while making other worse | MedEx-I measures across 6 different domains |
| 6 | Treatment specific | Different treatment have different types of adverse effects | MedEx-I based on previous research of meditation-related challenges |
| 7 | Expectedness | Prior research of meditation can inform what types of adverse effects may be expected | MedEx-I based on previous research of meditation-related challenges, including >40 published reports |
|
|
|||
| How to Measure | |||
|
|
|||
| Mode (How) |
|||
| 8 | Active vs. passive monitoring | Accurate estimates require active monitoring; passive monitoring underestimates AEs | MedEx-I is active and systematic; all participants were queried |
| 9 | Open-ended vs. specific questions | Open-ended questions under-estimate frequencies; query specific symptoms | MedEx-I contains both open-ended and specific questions |
| 10 | Detailed case reports | Detailed case reports are more informative than group comparisons to detect harms signals | MedEx-I is a detailed qualitative interview embedded in a prospective trial |
| 11 | Hawthorn effect-like scripting | Repeatedly asking questions about specific experiences can make them more likely to happen or be reported | MedEx-I was administered retrospectively as last assessment |
| Mode (Who) |
|||
| 12 | Independence | Researchers/clinicians underestimate harms; use an independent assessor | MedEx-I was conducted by independent assessor unrelated to trial |
| 13 | Diverse perspectives | Different participants can view the same experience in different ways | Multivalent (positive, negative, neutral/mixed) ratings of valence and impact |
| Relatedness |
|||
| 14 | Prior published reports | Use reports of adverse effects of the treatment agent | MedEx-I is consistent with more than 40 published reports of MRAEs |
| 15 | Expert judgment | Causal attribution to treatment by experts | MedEx-I is based on interviews with meditation teachers who attributed the cause to meditation |
| 16 | Subjective attribution | Causal attribution to the treatment by the subject | MedEx-I asks about experiences that the participant attributes to meditation |
| 17 | Temporal proximity (challenge) | Event occurs during or immediately following treatment agent | MedEx-I queries if experience occurred during or immediately following meditation practice |
| 18 | Exacerbation | Exacerbation of pre-existing symptoms during or immediately following treatment agent | MedEx-I queries if pre-existing symptoms got worse during or immediately following meditation practice |
| 19 | De-challenge | Decrease when treatment is reduced | [established in VCE study, see Lindahl 2017] |
| 20 | Re-challenge | Re-appearance when treatment agent is reinstated | [established in VCE study, see Lindahl 2017] |
| 21 | Dose-response, biological gradient | Greater exposure leads to higher incidence | Statistical correlations with meditation practice |
| 22 | Intra-subjective consistency | Occurrence of same event following treatment on more than one occasion in the same individual | [established in VCE study, see Lindahl 2017] |
| 23 | Inter-subjective consistency | Occurrence of same event following treatment in different individuals | [established in VCE study, see Lindahl 2017] |
| 24 | Specificity | Rule out alternate causes | Experiences that did not appear for first time or worsen during program were not counted |
Adverse Effects: What to Measure?
The World Health Organization (WHO) International Classification for Patient Safety uses the term side effect to indicate any effect of a treatment that was not the intended goal or that deviates from package labeling or advertising (Edwards & Aronson, 2000; WHO, 2010). Side effects that are negatively valenced or “subjectively unpleasant” are called adverse effects (AEs) and may vary in degree of harm (WHO, 2010, p. 16). Harm is any physical, psychological, or social suffering or impairment in functioning, and is measured on a continuum (WHO, 2010).
Degree of harm is determined by AE severity and duration and any resulting treatment implications (WHO, 2010). AE reporting in MBP trials has typically been limited to extremely severe or “serious” AEs that are “fatal or life threatening, resulting in significant incapacity” because only serious AEs are required to be reported (FDA, 2010; Wong, Chan, Zhang, Lee, & Tsoi, 2018). However, new guidelines recommend “broaden[ing] adverse event reporting beyond what is mandated by regulators” to include clinically relevant events that influence treatment decisions, tolerability, adherence, functioning and quality of life (Lineberry et al., 2016, p3). This new definition includes not only events of moderate severity that require countermeasures (including reducing dose of treatment), or involve impairment in at least one domain of functioning, but also mild events (transient distress) that require no intervention. While mild events have been considered “nuisance,” expected or necessary for progress (Baer et al., 2019; Peterson, Roache, Raj, Young-McCaughan, & Consortium, 2013), they still affect risk-benefit assessment, treatment tolerability and quality of life, and therefore remain clinically relevant (Linden, 2013; Lineberry et al., 2016).
Although duration is related to harm, there is no specific or required duration that makes an event clinically relevant or harmful (Lineberry et al., 2016; WHO, 2010). Instead, duration interacts with other contextual factors to constitute harm. Higher levels of impairment or risk require shorter durations for clinical relevance (for example, suicidality is serious regardless of duration). Conversely, mild events (headaches) that last for months also constitute harm. Thus, degree of harm is best understood as a combination of duration, distress, impairment of functioning or quality of life, and risk to self/other.
In the context of MBPs, definitions of AEs typically include “deteriorations” on pre-existing target outcomes (Baer et al., 2019; Hirshberg, Goldberg, Rosenkranz, & Davidson, 2020; Wong et al., 2018). However, this approach fails to capture unexpected or novel, treatment-emergent effects (Dimidjian & Hollon, 2010; Linden, 2013). Similarly, global, summed or averaged deterioration scores also obscure the fact that treatments can improve some symptoms and some domains of functioning while making others worse (Dimidjian & Hollon, 2010; Lilienfeld, 2007). For this reason, current guidelines recommend assessing different domains of functioning independently (Dimidjian & Hollon, 2010; Lineberry et al., 2016).
Current guidelines recommend assessing all potential AEs that are linked to the central mechanism of action for a treatment (e.g., meditation) (Dimidjian & Hollon, 2010; Lineberry et al., 2016; Mayo-Wilson et al., 2019), which requires knowledge of previously published reports about MRAEs. Undesirable side effects and risks of meditation have been documented in more than 40 scientific reports [for reviews see (Baer et al., 2019; Kuijpers, van der Heijden, Tuinier, & Verhoeven, 2007; Lindahl et al., 2019; Lustyk, Chawla, Nolan, & Marlatt, 2009; Van Dam et al., 2018)]. Many MRAEs are listed as potential risks in MBP guidelines (Kuyken, Crane, & Williams, 2012; Santorelli et al., 2017) and are linked to known mechanisms of action of MBPs. For example, the MBP mechanism of increased body awareness and/or activation of the insula cortex can be associated with increased anxiety, panic and flashbacks; the MBP mechanisms of decentering, or increased psychological distance from experience, and prefrontal control over the amygdala can be associated with disembodiment, affective blunting and dissociation (Britton, 2019).
Available frequency estimates of MRAEs have varied widely, depending on how AEs were defined and measured. A recent meta-analysis of meditation studies found that AE rates ranged from 4–33% depending on study design (Farias, Maraldi, Wallenkampf, & Lucchetti, 2020). In MBP trials, non-systematic and passive monitoring of serious AEs produced rates of <1% (Wong et al., 2018), systematic queries of “unpleasant experiences” produced rates of 67–73% (Baer et al., 2020), and “proportion of participants with increased symptoms” produced rates of 15–44% (Hirshberg et al., 2020). In addition, some RCTs have found that average symptom severity significantly worsened in MBP arms compared to controls (Britton, Haynes, Fridel, & Bootzin, 2010; Johnson, Burke, Brinkman, & Wade, 2016; Lomas et al., 2017; Reynolds, Bissett, Porter, & Consedine, 2017). However, none of these studies formally assessed the relationship of AEs to meditation practice. Systematic queries of meditation-related AEs (MRAEs) that were “particularly bad or frightening” or “unwanted” produced MRAE rates of 20–25% (Anderson, Suresh, & Farb, 2019; Cebolla, Demarzo, Martins, Soler, & Garcia-Campayo, 2017; Schlosser, Sparby, Voros, Jones, & Marchant, 2019). More common, less serious MRAEs that have been reported in surveys of meditators who meditate less than an hour per day include increased depression, anxiety or panic; re-experiencing of traumatic memories; dissociation; executive dysfunction; headaches or body pain, insomnia and social impairment (Cebolla et al., 2017; Farias et al., 2020; Lindahl, Fisher, Cooper, Rosen, & Britton, 2017; Lomas, Cartwright, Edginton, & Ridge, 2014). More serious MRAEs including mania, psychosis, and suicidality have also been reported, often in the contexts of intensive retreats (>5 hrs/day) or in conjunction with pre-existing psychopatholog (Kuijpers et al., 2007; Kuyken et al., 2012; Lindahl et al., 2017; Yorston, 2001).
Adverse Effects: How to Measure?
CONSORT guidelines define safety as “substantive evidence of an absence of harm,” and not “when there is simply absence of evidence of harm” (Ioannidis et al., 2004). In pharmacology treatments, the most detailed harms assessments occur in early preclinical and basic science phases of treatment development (Phase 0–1) in the form of case reports, dose-response curves and observational studies before proceeding to randomized controlled trials (RCTs) (Gitlin, 2013). MBPs, however, have largely omitted in-depth harms assessments in both treatment development and RCTs. Thus, despite CONSORT requirements (Moher, Schulz, & Altman, 2001), and compared to 100% of pharmacology trials (Vaughan, Goldstein, Alikakos, Cohen, & Serby, 2014), less than 20% of meditation trials actively measure adverse effects (Wong et al., 2018).
The majority of MBP programs rely on passive monitoring—that is, spontaneous participant reports. However, it is well known that research participants and psychotherapy clients are unlikely to spontaneously report negative treatment reactions because of demand characteristics (the desire to please the therapist or researcher) (Horigian, Robbins, Dominguez, Ucha, & Rosa, 2010; Nichols & Maner, 2008). As a result, relying on passive monitoring may underestimate AE frequency by more than 20-fold (Kramer, 1981). AE accuracy can be improved by active and systematic monitoring (Horigian et al., 2010), but only if active monitoring uses scales that assess specific symptoms, which have more sensitive detection rates than either open-ended queries (Allen, Chandler, Mandimika, Leisegang, & Barnes, 2018; Bent, Padula, & Avins, 2006) or passive monitoring (Talbot & Aronson, 2012).
Treatment providers and researchers are not good sources of harms estimates. Providers often overestimate their success rates, underestimate harms and fail to recognize deteriorations when they occur (Hatfield, McCullough, Frantz, & Krieger, 2010; Walfish, McAlister, O’Donnell, & Lambert, 2012). Providers tend to dismiss patient complaints and their credibility as reliable reporters (Crichton, Carel, & Kidd, 2017), and deny that patient-reported symptoms were caused by the treatment, even for known side effects (Golomb, McGraw, Evans, & Dimsdale, 2007). Providers are also prone to the fallacy that “worsening is to be expected and is a positive sign that the therapy is working,” (Hannan et al., 2005, p. 156) even though less than 10% of deteriorations result in positive treatment response and intervening on deteriorating patients improves treatment outcomes (Lambert et al., 2003). Researchers may be motivated to downplay AEs because of reporting burden or because continued funding depends on treatment success (Ioannidis, 2009). Researcher conflicts of interest have been found to significantly predict fewer AEs in the MBP arm (Wong et al., 2018). Thus, because researchers and providers are reliably biased, recent guidelines recommend patient-based reports elicited by an independent party for identifying sensitive or socially undesirable information such as negative reactions to treatment (Dimidjian & Hollon, 2010; Fowler, 1998; Weissman et al., 2008). While patient-centered assessments protect against motivated minimization by researchers and providers, different patients may view the same experience in different ways (Dimidjian & Hollon, 2010; Rozental et al., 2018). One patient may experience crying or traumatic re-experiencing as destabilizing, another as part of healing. Thus, it is necessary to assess the valence and impact of each experience for each patient independently.
Contrary to the assumption that RCTs always convey the best evidence, they are not the best design for detecting AEs (Hammad, Pinheiro, & Neyarapally, 2011; Vandenbroucke, 2006), and have instead been called “the gold standard way to miss adverse events” (Healy & Mangin, 2019, p. 1). RCTs are powered for efficacy but underpowered for detecting AEs, which are typically rare, outlier events (Edwards, 2012; Hammad et al., 2011; Lineberry et al., 2016) that are easily obscured by lack of patient or assessor blinding, lack of intent-to-treat analyses or author conflict of interest (Chou et al., 2010; Chou, Fu, Carson, Saha, & Helfand, 2007; Hammad et al., 2011). These limitations of RCTs to detect AEs or make causal inferences are further compounded in behavioral intervention studies, including MBPs, where harms assessment and methodological rigor lags far behind pharmaceutical trials (Goldberg et al., 2017; Jonsson, Alaie, Parling, & Arnberg, 2014; Wong et al., 2018). Widespread use of waitlist controls and lack of patient blinding results in global overestimation of treatment efficacy and underestimation of AEs (Hrobjartsson, Emanuelsson, Skou Thomsen, Hilden, & Brorson, 2014). Waitlist controls are prone to nocebo effects and cannot be used to estimate base rates of AEs without treatment (Cuijpers, Reijnders, Karyotaki, de Wit, & Ebert, 2018; Freedland et al., 2019; Furukawa et al., 2014; Van Dam & Galante, 2020).
Rather than relying on RCTs, regulatory agencies such as the National Institutes of Health (NIH), the World Health Organization (WHO), and the Food and Drug Administration (FDA) rely instead on Phase 0–1 or post-market observational studies and detailed case reports to identify treatment-related harms and make safety policy decisions (CIOMS, 2010; Moore, Singh, & Furberg, 2012; Singh & Loke, 2012; Talbot & Aronson, 2012). Rather than trying to infer causality mathematically based on group averages, causality can be more confidently and thoroughly established by assessing each event in each person with multiple causality criteria (Edwards, 2012; Hauben & Aronson, 2007). Standard causality assessment criteria are listed in Table 1 (relatedness items 14–24) (Agbabiaka, Savovic, & Ernst, 2008; NIH, 2016; Turner, 1984; WHO, 2016). Since MBP development skipped this phase, it has been recommended that MBPs be “sent back” to Phases 0–1 to assess potential AEs properly (Dimidjian & Segal, 2015, p. 605). The Lancet Psychiatry Commission on psychological treatments recommend recouping Phase 0–1 level safety information by embedding in-depth qualitative interviews about AEs into clinical trials (Holmes et al., 2018).
Based upon these recommendations for the assessment of AEs, the current study has several related aims. We provide an example of a harms assessment that incorporates the updated guidelines for harms assessments, with special attention to issues pertinent to behavioral interventions in general and MBPs in particular (See Table 1). In the current paper, we use the term meditation-related side effect (MRSE) to refer to all meditation effects that are unintended and meditation-related adverse effect (MRAE) to refer to meditation effects with negative valence or negative impacts. By using an empirically-derived taxonomy of MRSEs (Lindahl et al., 2017), the study aims to clarify the nature and frequency of MRSEs and MRAEs in the context of 8-week mindfulness-based programs. By asking each participant to rate the valence of each MRSE that occurred, the study can clarify which side effects are experienced as “adverse” (i.e. are MRAEs) on a patient-centered case-by-case basis. By taking a three-tiered approach to severity and degree of harm that incorporates valence, impact and duration, the study is able to differentiate transient distress, negative impact MRAEs and “lasting bad effects.” By identifying specific types of MRSEs that are associated with lasting bad effects, the study aims to help meditators, meditation teachers and MBP providers identify potentially problematic MRSEs that may warrant attention, corrective feedback and/or intervention. By evaluating the performance of open-ended versus specific questions, the study can provide information on how method of measurement impacts frequency rates. By assessing MRAE rates across MBP variants, the study can begin to investigate whether frequencies are dependent on type(s) of meditation practice.
Methods
Participants
The target sample was intended to represent Americans seeking mindfulness meditation training for the management or alleviation of clinical, sub-clinical and transdiagnostic expressions of affective disturbances, including anxiety, depression and stress (Morone, Moore, & Greco, 2017; Santorelli et al., 2017). Participants were English-speaking individuals, age 18–65, with mild-severe levels of depression and persistently high levels of negative affect. Following MBP guidelines (Kuyken et al., 2012; Santorelli et al., 2017), exclusion criteria included lifetime history of bipolar, psychotic, borderline or antisocial personality disorders; repeated self-harm or organic brain damage; current depression in the extremely severe range or active suicidal ideation; current panic, post-traumatic stress disorder, obsessive-compulsive disorder, eating disorder or substance abuse; current psychotherapy; a regular meditation practice; or modification of antidepressant medication in the last two months. See Britton et al. (2018) for details.
Setting and oversight
The registered clinical trial (clinicaltrials.gov #NCT01831362) took place at Brown University between November 2012 and March 2016. The study was approved and supervised by the Brown University Institutional Review Board (IRB), an independent Data Safety Monitoring Board (DSMB) and the National Center for Complementary and Integrative Health’s (NCCIH) Office of Clinical and Regulatory Affairs (OCRA) in accordance with the World Medical Association Declaration of Helsinki. Participants were recruited through community flyers advertising mindfulness meditation for stress, anxiety, and depression. Eligible participants provided written, informed consent, and did not receive financial compensation. All adverse events, both serious and non-serious, were reported to the IRB, DSMB and OCRA according to NCCIH reporting requirements.
Design and Training Programs
As reported in Britton et al. (2018), the treatment programs were three variants of Mindfulness-Based Cognitive Therapy (MBCT): open monitoring (OM), focused attention (FA), and standard MBCT. Standard MBCT combines components of cognitive behavioral therapy and Mindfulness-Based Stress Reduction (MBSR) using a group-based psychoeducational format (Segal, Williams, & Teasdale, 2002) and employs a combination of both OM and FA meditation techniques. The OM variant includes only OM meditation where participants bring unbiased, receptive and open attention to their experience without focusing on any single object. The FA variant, by contrast, included only FA meditation where participants focus attention on an anchor (like the breath) during meditation. Detailed descriptions of treatment development and validation with session-by-session treatment manuals can be found in Britton et al. (2018). All treatments met for three hours once per week for eight weeks, with a full-day silent retreat during week seven. Prescribed formal meditation practice homework was 45 min/day, six days/week, with additional informal practice as needed. Participants received basic training in targeted practices (FA, OM, or the combination in MBCT) during weeks 1–4 and then learned how to apply these practices to regulate negative emotions in weeks 5–8. All treatments were equivalent for program structure, duration, instructor training/fidelity, and participant compliance (e.g., attendance, meditation amount) (Britton et al., 2018).
Four meditation teachers taught the MBPs: three men and one woman. All instructors had graduate degrees (3 PhDs, 1 MA, 2 clinical) and more than 20 years of personal meditation experience in one or more meditation traditions. Three were trained MBSR and/or MBCT instructors; three had training as Buddhist meditation teachers. Treatment adherence was 93.3% (kappa = .71) as assessed by adapted versions of the MBCT adherence scale (Britton et al., 2018; Segal, Teasdale, Williams, & Gemar, 2002).
Measures
Baseline diagnostic status and exclusion criteria were established with the Structured Clinical Interview for DSM-IV for Axis I (SCID-I) and Axis II (SCID-II) disorders (Frist, 1997). Depression symptom severity was assessed with the clinician-administered Inventory of Depressive Symptomatology (IDS-C) (Rush, Gullion, Basco, Jarrett, & Trivedi, 1996) (kappa = 0.89) and was interpreted as follows: 12–23 mild, 24–36 moderate, 37–47 severe, ≥ 48 very severe.
The Meditation Experiences Interview (MedEx-I) Instrument Design
The Meditation Experiences Interview (MedEx-I) was derived from The Varieties of Contemplative Experience (VCE) research project, a mixed-methods study about meditation-related challenges based upon interviews with Buddhist meditation practitioners and meditation teachers (Lindahl et al., 2017). The VCE study yielded 59 types of meditation-related experiences that were described by meditators and teachers as unexpected, challenging, distressing and/or associated with impairment of functioning. Relatedness to meditation was established by employing eleven causality criteria (Agbabiaka et al., 2008; NIH, 2016; WHO, 2016). See Table 1 for details.
Because the VCE study and the current trial were concurrent, an earlier version of the VCE codebook was used as basis for the development of the MedEx-I used in the MBP. This “MedEx codebook” consisted of 44 categories across six domains. The affective domain (11 categories) included changes in the type, frequency, or intensity of emotions, such as anxiety, affective lability, blunting, suicidality and re-experiencing of traumatic memories. The cognitive domain (six categories) included experiences related to mental processes, and thought content, quality and frequency, such as executive dysfunction, delusions, racing or absence of thoughts or loss of concepts. The perceptual domain (eight categories) captured alterations in sensory processes, such as vision, hearing, interoception, and perception of time, and included perceptual hypersensitivity, distortions, and derealization. The sense of self domain (four categories) included self disturbances such as feelings of disembodiment, loss of sense of ownership or agency. The somatic domain (13 categories), included changes in bodily functioning or physiological processes, such as sleep, pain, appetite, digestion and involuntary movements. The social domain (two categories) included social and occupational impairment. Detailed descriptions, inclusion criteria, and exclusion criteria for each category of the MedEx-I codebook can be found in the Codebook S1 in the Supplemental Material available online.
Meditation Experiences Interview (MedEx-I) Procedure
The MedEx-I was administered to participants in the MBP as the last part of the final assessment at the 3-month follow-up interview (week 20). In order to meet the independence criterion, the MedEx-I was administered by an independent researcher (JL) who was otherwise unaffiliated with the clinical trial—i.e., who had no contractual relationship with the sponsor or AE reporting responsibilities with the human subjects protection oversight committees (IRB, OCRA, DSMB). The interviewer was the primary coder for the VCE study and possessed expert-level familiarity with the phenomenology of MedEx-I categories.
Interview questions. The MedEx-I featured three types of questions: 1) one initial open-ended question; 2) 44 category-specific questions; and 3) five follow-up questions. The interview commenced with the open-ended query: “Have you had any unexpected, unpleasant, adverse or challenging experiences as a result of mindfulness meditation practice during or following the program?” Under the overarching framework of subjective attribution to mindfulness meditation established in the open-ended question, category-specific queries asked about the presence of each of 44 MRSE categories in the MedEx-I codebook. Once the presence of a MRSE was established, five follow-up questions aimed to establish causality, relationship to specific practices, duration, valence and impact.
1). Pre-existing experience.
Participants were asked if they had experienced the codebook category prior to learning to meditate, in order to rule out experiences that could plausibly be unrelated to meditation practice. An experience counted as plausibly causally related to meditation (i.e., was coded as “present”) only if the experience emerged for the first time, or if it increased in frequency, intensity or duration during the mindfulness training program and was attributed to mindfulness meditation practice by the participant. Experiences that failed to meet these criteria were not counted.
2a). Practice-related.
“Did the experience occur during or immediately following meditation practice?”
2b). Specific practice.
If it occurred during/following meditation, participants were then asked, “Was the experience associated with a particular or specific practice or exercise?” In order to assess their unique contribution, experiences associated with the all-day retreat and the “working with difficulties” practice (where a difficult emotion is deliberately invited) were included but coded separately.
3). Duration.
The participant was asked to describe the duration or how long the experience lasted in terms of minutes, days, weeks, months or ongoing, and if it was limited to a meditation practice session or continued into daily life.
4). Valence.
Participants were asked to rate the valence or emotional tone of the experience when it was occurring as positive, negative or neutral/mixed. Experiences that changed valences—i.e., were initially negative but then became positive or vice versa—were classified as “mixed.”
5). Impact.
“Did the experience have a positive, negative or no impact on your life or functioning?” In contrast to valence, impact refers to the effect of the experience on daily life and domains of functioning (e.g., work, social, driving, decision-making), requirement for countermeasures (additional/modified treatment), or change in behavior (including willingness or ability to meditate).
Data Collection and Qualitative Coding
All interviews were recorded, transcribed and imported into NVivo software for qualitative data analysis and validation of categories. Qualitative coding was performed by the coders of the VCE study (JL, DC). False-positives (descriptions that did not meet criteria), were retained only as an index of the initial open-ended question performance, but otherwise were not included. One-third of the transcripts were coded by multiple coders to ensure inter-rater reliability (kappa =.77). Validated data were imported into SPSS for quantitative analysis.
Meditation Practice
Home meditation practice amount during and following treatment was monitored daily with an online survey, which queried both formal and informal practice amounts (frequency and duration). Formal practice included time set aside from daily life for meditation or use of audio recordings of guided meditation practices, while informal practice occurred unscheduled, during daily activities and without the use of audio recordings.
Data Analysis
Outcomes
Outcomes included descriptive statistics of the following:
MRSEs are all meditation-related experiences or MedEx-I-derived events independent of valence or impact.
Duration indicates the longest lasting MRSE in each participant by measure of minutes, days, weeks, months or ongoing.
MRAEs are MRSEs that are reported as having negative valence or negative impact on functioning and are arranged in three tiers: A negative valence MRAE is experienced as unpleasant while it is occurring, regardless of its impact on functioning. A negative impact MRAE results in a negative impact in functioning, requires countermeasures or a change in behavior. Lasting bad effects (LBEs) were defined as negative impact MRAEs with three possible durations: > 1 day (LBE>dy), > 1 week (LBE>wk), > 1 month (LBE>mo).
Clinically-relevant categories are MRSE categories that are constitutive and/or predictive of LBEs>wk. Constitutive categories were rated by participants as the cause of impairment, whereas predictive categories significantly predicted increased risk of LBEs from other categories. Risk ratios (RRs) were used to assess if the presence of any particular meditation-related side effect (MRSE) could signify an elevated risk for LBE>wk, (Siegerink & Rohmann, 2018). The risk ratio (RR) is calculated as the likelihood of LBE in someone who reported a specific category divided by the likelihood of LBEs in participants who have not reported that category. Significantly elevated risk for LBEs is signified if the standardized value of the Risk Ratio (z-score) has a p-value of <.05 (Sheskin, 2004). See Table S2 in the Supplemental Material available online for details.
Relationship to meditation practice In the current study, causal attribution to meditation was assessed in six ways (see Table 1): by querying experiences that 1) had previously established causal links to meditation; 2) emerged for the first time or intensified in the context of a meditation training program; 3) were subjectively attributed to meditation by the participant; 4) occurred during or immediately following meditation practice; 5) by comparing meditation practice amounts between LBE>wk and non-LBE>wk groups (Wilcoxon-Mann-Whitney-U); and 6) by Pearson correlations of practice amount and MRAE frequency.
Open-ended question performance was indexed by number of true (accurate) and false (inaccurate) positive (“yes”) and negative (“no”) responses to the open-ended question when compared to results of specific queries for each MedEx-I category.
Between-group differences To determine if the frequencies of MRSEs, MRAEs or LBEs differed across the three treatment variants, negative binomial regressions were used to model outcomes measured in number of events, and Firth’s penalized likelihood logistic regression was used to model outcomes that were assessed dichotomously (i.e. presence vs. absence) while reducing bias due to rare events. See Treatment Analyses S1 in the Supplemental Material available online for details.
Results
Participant Characteristics
Ninety-six of the 104 randomized participants (92%) completed treatment and all assessments. Eight participants dropped out: two before the first class, two after the second, three after the third and one after the seventh. Reasons for attrition included time commitment and scheduling issues (n = 5), moving away (n = 1), “not wanting to be in a study” (n = 1), and “increased stress” (n = 1). Participants attended 90% of all face-to face sessions, with an average of 8.1 (SD = 1.0) out of 9 sessions, with 91% attending the all-day silent retreat. During the 8-week treatment, participants practiced meditation at home for an average of 34 min/day, which represents 76% of the prescribed amount (45 min/day). Between the end of treatment and the 3-month follow-up assessment, participants’ average daily practice was 17 min/day (range = 0–67 min/day).
Participants were representative of Americans who use mindfulness meditation: predominantly white (97%), non-Hispanic (97%), middle-aged (mean age 40.4 years, SD = 12.9), female (73%), and educated (mean education 17.1 years, SD = 2.7) with clinical and subclinical levels of anxiety and depression (Morone et al., 2017). Forty percent of the sample met diagnostic criteria for major depression and 50% for generalized anxiety disorder. IDS scores indicated mild-moderate levels of depression, with one third (33%) of the sample taking antidepressant medication. Participant characteristics and adherence did not differ between treatment variants.
Treatment Efficacy
To provide a context of overall treatment efficacy, all three forms of mindfulness training produced large effect size improvements in IDS scores from baseline to post-treatment (ds = 1.48–1.65) and week 20 follow-up (ds = 1.34–1.57) with no differences between groups (Cullen et al., 2021).
MedEx-I
Available Data
Data for open-ended questions, MRSE and valence ratings were available for all 96 (100%) participants who completed the trial. Duration ratings were available for 90 participants (94%), impact ratings for 81 (84%), and LBE data for 78 (81%). Because denominators in percentages differ by outcome, they are explicitly reported, as specified by current guidelines (Ioannidis et al., 2004; Lineberry et al., 2016).
Replication of the Varieties of Contemplative Experience Study
Eighty percent (35/44) of the VCE phenomenology codebook categories replicated in the context of an MBP. Nine categories from the 44-item MedEx-I codebook did not replicate: delusions, hallucinations, synesthesia, anomalous recall, cardiac changes, fatigue, sleep paralysis, gastrointestinal problems, and occupational impairment. In addition, 26 events that were categorized as “other” in the MedEx-I would either become 5 new categories in the final 59-item VCE codebook (14 events), be included in expanded versions of existing categories (7 events), or remain uncategorized (5 events), yielding an 83% replication rate in relation to the final 59-item VCE phenomenology codebook.
Frequencies of MRSEs, MRAEs and LBEs
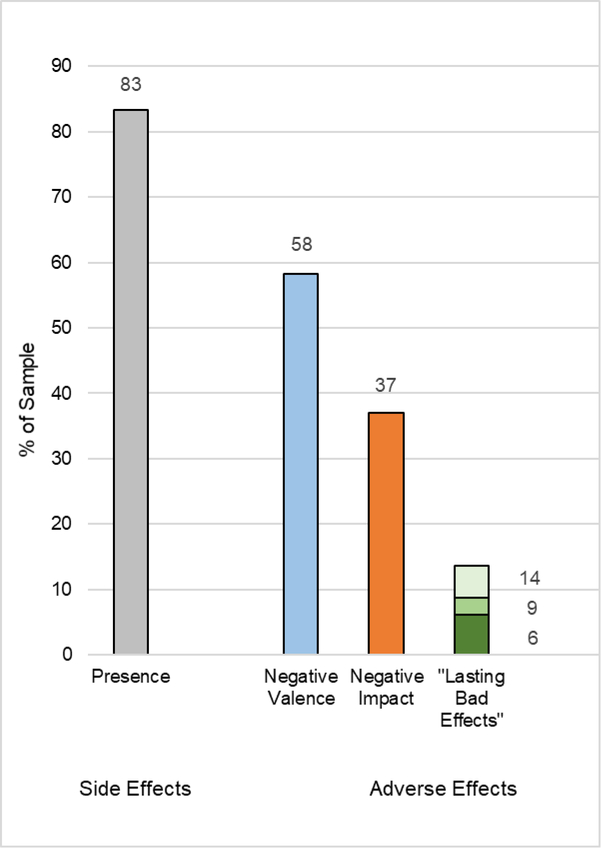
Figure 1 and Table 2 display MRSEs, MRAEs and LBEs frequencies. Total number of events, proportion of sample with one or more events, mean, SD and ranges are displayed for the overall sample and for each treatment separately. Across all participants, a total of 266 MRSEs or “events” were reported. Eighty-three percent (80/96) of the sample reported experiencing at least one MRSE, with the majority (62.5%) reporting multiple MRSEs. The mean number of MRSEs per person was 2.8 (SD = 2.6, range = 0–13). It is important to note that not all MRSEs were “negative” or “adverse events,” as some events were neutral, mixed or positive in either valence, impact or both. Fifty-eight percent (56/96) of the sample reported at least one MRAE with a negative valence, and 27% (26/96) experienced more than one (range = 0–7). Thirty-seven percent (30/81) of the sample reported at least one MRAE with a negative impact, and 16% (13/81) reported more than one (range = 0–5). For the majority of participants (56.7%), the longest MRSEs lasted less than 1 hour; for 7.8% less than 1 day; for 7.8% less than 1 week; for 3.3% 1 week to 1 month; and for 6.6%, MRSEs lasted 1 to 5 months or were ongoing at the time of interview. Lasting Bad Effects (LBEs) or negative impact MRAEs with durations of 1 day to 1 week were reported by 11 participants (14.1%), with durations of 1 week to 1 month by seven participants (9.0%), and with durations of 1 to 5 months or ongoing by five participants (6.4%). Frequencies of MRSEs, MRAEs and LBEs did not significantly differ between treatment groups in any omnibus or pairwise comparisons (see Table 2 and Treatment Analyses S1 in the Supplemental Material available online for details).
Figure 1.
Frequencies of Meditation-Related Side Effects and Adverse Effects
Note. Percent of the sample that reported the presence of any meditation-related side effect (MRSE) (grey). Meditation-related adverse effects (MRAE) are displayed in 3 tiers: MRAEs with negative valence (blue), MRAEs with negative impact (orange) and “lasting bad effects,” or MRAEs with negative impacts lasting >1 month (dark green), >1 week (green), or > 1 day (light green).
Table 2.
Frequencies of MRSEs, MRAEs and LBEs
| Variable | Total | MBCT | OM | FA | χ 2 a | p b |
|---|---|---|---|---|---|---|
|
| ||||||
| Meditation-Related Side Effects (MRSE) | ||||||
|
| ||||||
| N | 96 | 30 | 31 | 35 | ||
| # MRSEs | 266 | 92 | 63 | 111 | ||
| % sample with 1+ MRSE | 83.33 | 83.33 | 87.10 | 80.00 | 0.54 | 0.763 |
| mean # events/person (SD) | 2.77 (2.64) | 3.07 (3.40) | 2.03 (1.40) | 3.17 (2.66) | 4.27 | 0.119 |
| Range, # events/person | 0–13 | 0–13 | 0–5 | 0–9 | ||
|
| ||||||
| Meditation-Related Adverse Effects (MRAE) | ||||||
|
| ||||||
| Negative Valence Events |
||||||
| N | 96 | 30 | 31 | 35 | ||
| # events | 109 | 35 | 29 | 45 | ||
| % sample with 1+ event | 58.33 | 60.00 | 54.84 | 60.00 | 0.22 | 0.895 |
| mean # events/person (SD) | 1.14 (1.41) | 1.17 (1.60) | 0.94 (1.21) | 1.29 (1.43) | 1.10 | 0.578 |
| Range, # events/person | 0–7 | 0–5 | 0–5 | 0–7 | ||
| Negative Impact Events |
||||||
| N | 81 | 25 | 24 | 32 | ||
| # negative impact events | 53 | 15 | 9 | 29 | ||
| % sample with 1+ event | 37.04 | 32.00 | 29.17 | 46.88 | 2.11 | 0.348 |
| mean # events/person (SD) | 0.65 (1.07) | 0.60 (1.00) | 0.38 (0.65) | 0.91 (1.33) | 2.23 | 0.328 |
| Range, # events/person | 0–5 | 0–3 | 0–2 | 0–5 | ||
| Lasting Bad Effects (LBEs) |
||||||
| N | 78 | 25 | 21 | 32 | ||
| LBE > 1day (% of sample) | 14.10 | 16.00 | 9.52 | 15.63 | 0.41 | 0.815 |
| LBE>1 week (% of sample) | 8.97 | 4.00 | 4.76 | 15.63 | 2.24 | 0.326 |
| LBE>1 month (% of sample) | 6.41 | 4.00 | 4.76 | 9.38 | 0.52 | 0.773 |
Chi-square values for omnibus tests for differences between conditions. See Treatment Analyses S1 in the Supplemental Material available online for details
p statistics for χ2 omnibus tests
Clinically-relevant Categories
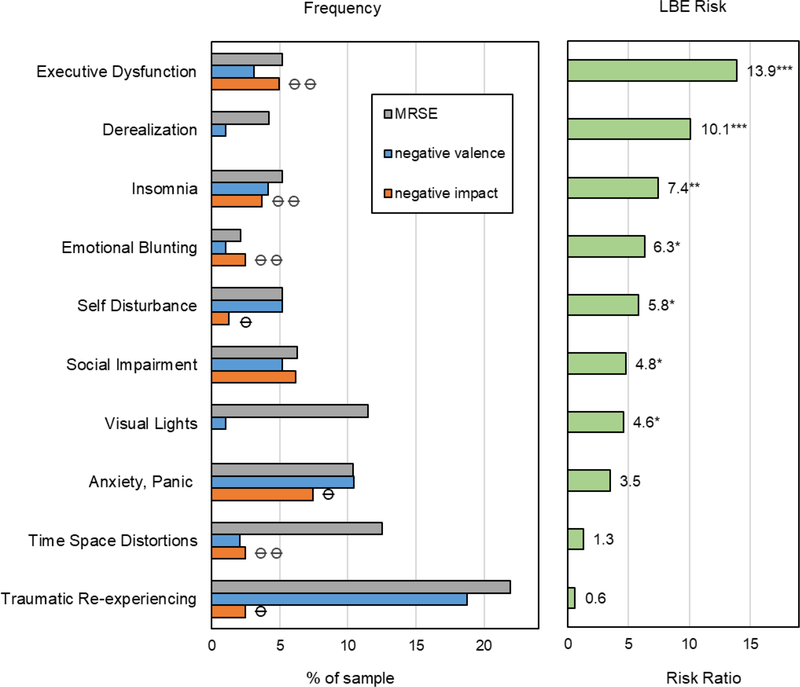
Frequencies of MRSEs, negative valence MRAEs and negative impact MRAEs for each MedEx-I category can be found in Table S1 in the Supplemental Material online. Figure 2 displays MedEx-I categories that were constitutive and/or predictive of LBEs. Executive dysfunction, insomnia, emotional blunting and self-disturbance were reported in less than 5% of the sample and were both constitutive and predictive of LBEs, increasing the risk of LBEs by 6 to 14-fold. Anxiety, time-space distortions and traumatic re-experiencing were reported in 10–25 % of the sample and while they could constitute LBEs, they were not predictive of LBEs because most instances were not associated with enduring impairment. Derealization, social impairment and visual lights were predictive but not constitutive of LBEs. See Table S2 in the Supplemental Material available online for details.
Figure 2.
Clinically-Relevant Categories that are Constitutive and/or Predictive of LBEs
Note: a. Frequencies of MRSEs (grey), negative valence MRAEs (blue) and negative impact MRAEs (orange); ⦵ categories that constitute LBEs > 1 week; ⦵ ⦵ categories that constitute LBEs> 1 month. b. LBE risk is displayed as a risk ratio (RR) that compares the probability of LBE>wk in the presence vs absence of a category. For example, LBEs are 10 times more likely when derealization is present then when it is absent. Significant predictors of LBE>wk are denoted with * p<.05, **p <.005 *** p <.0001. See Table S2 in the Supplemental Material available online for details.
Relatedness to Meditation Practice
All 266 events met minimal causality criteria on account of either occurring for the first time or increasing in frequency, duration, or intensity during the mindfulness program, and being subjectively attributed to meditation by the participant. Some common experiences such as fatigue, cardiovascular changes, and gastrointestinal symptoms were reported but failed to meet these causality criteria and were therefore not counted or included in the analysis.
Data on relationship to mindfulness meditation practice follow-up questions were available for 84% (225/266) of events. For 198 events (88%), participants reported that the experience occurred during or immediately following mindfulness meditation practice and for 140 of those events (62%), participants were able to identify specific practices or exercises associated with the experiences. The majority of events occurred during daily home practice or class, while a small minority of events occurred in the context of the all-day retreat (6.2%) or the “working with difficulty” practice (6.2%). For 27 events (12%), participants reported that the experience was more of a general or cumulative effect of participating in the program rather than during practice, although many of these were experiences that could not have occurred during meditation (e.g. nightmares, social impairment).
The LBE>wk group did not differ in the amount (minutes) of formal mindfulness meditation practice either during or following treatment. However, the LBE>wk group did show a trend toward more informal practice minutes during treatment (70 min vs. 53 min/week; p = .075) as well as significantly more frequent informal practice sessions following treatment (13.5 vs. 4.3 times/week, p =.028). Frequency of informal practice following treatment was significantly correlated with number of negative impact MRAEs (r = .213, p =.037).
Open-Ended Question Performance
The open-ended question (“Have you had any unexpected, unpleasant, adverse or challenging experiences as a result of mindfulness meditation practice during or following the program?”) produced a roughly equal ratio of true positives (27%) to false positives (28%), but produced more than three times more false negatives (32%) than true negatives (10%). The open-ended question correctly identified only 26 of the 80 (32.5%) participants who experienced MRSEs, thus underestimating the true rate by nearly 70%.
Discussion
The current study employed updated harms assessment guidelines including an embedded qualitative interview in order to assess empirically-derived MRSEs and MRAEs in the context of an MBP. Results indicated a high degree of replication of MRSEs previously identified in a sample of practitioners of Buddhist meditation (Lindahl et al., 2017). More than 80% of categories replicating in the MBP and with more than 80% of the MBP sample reporting at least one MRSE. MRAEs with negative valences and negative impacts on functioning occurred in 58% and 37% of the sample, respectively. “Lasting bad effects” or MRAEs with lasting negative impacts were reported by 6–14% of the sample, depending on the duration. LBEs were associated with greater frequency of informal mindfulness practice and could be predicted by the presence of categories indicative of dysregulated arousal. Open-ended questions underestimated the prevalence of MRSEs by nearly 70%.
The current results demonstrated two forms of convergent validity with the VCE study. Eighty percent of the VCE categories replicated in the current study, which confirms that many of the MRSEs documented in practitioners of Buddhist meditation also occur in MBPs. Some of the categories that did not replicate represent some of the more severe experiences reported in the VCE study such as those related to psychosis (delusions and hallucinations) and occupational impairment. Other categories that did not replicate in the MBP were experiences that are extremely common and could not be established as being causally related to mindfulness meditation training. For example, fatigue, cardiac changes, and gastrointestinal complaints are experienced by most adults (Hinz et al., 2017). Exclusion of such commonly occurring symptoms from counting as MRSEs validates that the MedEx-I is not simply measuring symptoms that would have occurred without meditation (i.e., base rate level symptoms). Experiences that were categorized as “other” (i.e., those that were documented in the MBP but did not fit into existing categories of the MedEx-I) were subsequently identified in the VCE study sample and in the final version of the codebook. Similar to the Minnesota Multiphasic Personality Inventory (MMPI) F(p) scale, these items may serve as a validity check because they detect exaggeration or over-reporting of unusual experiences (Arbisi & Ben-Porath, 1995).
All events were counted as meditation-related only if they were new or worsening since beginning the mindfulness meditation program and were attributed to mindfulness meditation by the participant. The vast majority (>80%) of events occurred during or immediately following a mindfulness meditation practice. Only 6% of events occurred as a result of either the all-day retreat, or the working with difficulty practice. This challenges the idea that negative experiences are more likely to occur at higher practice intensities or when intentionally bringing attention toward difficult experiences (Baer et al., 2019). To the contrary, the majority of events occurred during daily home practice or during class. Amount and frequency of informal meditation practice during and following treatment was associated with more negative impact events and a higher likelihood of lasting bad effects, which indicates a dose-response relationship.
Nearly 60% of the sample experienced at least one MRAE with a negative valence, suggesting that at least some transient distressing experiences during meditation are the norm and should be expected for most participants. Similarly, Baer et al., (2020) found that 67–73% of MBP participants reported having unpleasant experiences associated with mindfulness practice during or following the course. Similarly high rates of transient mood deterioration (60–65%) have been found following a single session of group therapy for depression or anxiety (Schneibel et al., 2017).
Nearly 40% of participants reported at least one MRAE that had a negative impact on life outside of meditation practice, which is similar to the rates of new or worsening symptoms caused by psychotherapy when measured systematically with a questionnaire (42–51%) (Moritz et al., 2015; Rozental et al., 2019; Schermuly-Haupt, Linden, & Rush, 2018). Thus, while transient negative experiences during MBPs should be expected, they may also affect participants’ quality of life and functioning, require countermeasures or additional treatment, or affect their desire or ability to meditate.
“Lasting bad effects” or MRAEs with enduring negative impacts were reported by 6–14% of participants depending on whether “lasting” is defined as more than a month, more than a week, or more than a day. Similar rates of LBEs (3–14%) have also been reported in psychotherapy (Crawford et al., 2016; Hansen, Lambert, & Forman, 2002; Lambert, 2013). Thus, despite ambiguity in definitions, the rate of “lasting bad effects” that impair life or functioning from days to months in MBPs appears to be similar to other psychological treatments.
Duration has often been used to indicate severity of AEs and to differentiate transient discomfort from “disorders,” or problems that warrant clinical attention or interventions (APA, 2013; Baer et al., 2019; Lindahl, Cooper, Fisher, Kirmayer, & Britton, 2020). However, different symptoms require different durations to be considered clinically significant. For example, acute stress disorder requires a duration of three days, mania four days, depression two weeks, and PTSD one month (APA, 2013). Some symptoms, such as suicidality, hallucinations or delusions, warrant intervention regardless of duration because of the risk to self or others. In the current study, although MRAEs that lasted less than a day were not counted as LBEs, some short-duration MRAEs were nevertheless significant. At least three participants reported MRAEs that caused impairments in driving, which also poses a risk to self and others. Thus, while duration may be used as a rough guideline for when to intervene, short-duration MRAEs should not be discounted. As Crawford et al. (2016) explain, “Even when negative experiences do not turn out to be lasting, they are unpleasant for the patient and have the potential to erode the patient’s confidence in the therapist or therapy process and limit further engagement with the treatment” (p. 264).
Clinical Implications
The majority of MRAEs occurring in this study, particularly those with negative impacts, are consistent with signs of dysregulated arousal; i.e., hyperarousal and dissociation (Frewen & Lanius, 2006; Treleaven, 2018). Symptoms of hyperarousal (e.g., anxiety and insomnia) were some of the most likely to be appraised as negative in both valence and impact, and therefore may be more likely to be voluntarily reported and identified by teachers. Conversely, while dissociation symptoms (e.g., emotional blunting, derealization, and self-disturbance) were both less frequent and less likely to be appraised as negative, they were still associated with more than 5–10 times greater risk for LBEs. These results parallel findings from the VCE study, where greater attenuations in senses of self, although not always unpleasant, were associated with a greater impairment in functioning (Lindahl & Britton, 2019). This means that re-appraisal of dissociative symptoms via non-judgmental acceptance is not sufficient to prevent impairment in functioning and should not constitute the only response. Instead, training in how to recognize dissociative symptoms as potential indicators of the need for intervention, which have recently been added to some mindfulness teacher training programs (Britton, Lindahl, & Treleaven, 2017; Treleaven, 2018), may be important.
Research Implications
The deficient performance of the open-ended question parallels findings in psychotherapy research: AE rates are proportional to how well they are measured (Bent et al., 2006). Single open-ended questions about AEs in psychotherapy have yielded AE rates of 5–20%, but those rates rise to 40–60% when asked systematically with structured questionnaires about specific experiences (Moritz et al., 2015; Rheker, Beisel, Kraling, & Rief, 2017; Rozental et al., 2019; Schermuly-Haupt et al., 2018). In meditation studies, single open-ended questions about “unpleasant” or “unwanted” meditation effects have yielded rates of 25% of meditating samples (Cebolla et al., 2017; Schlosser et al., 2019). Given that the open-ended question in this study failed to detect more than two-thirds of the MRAEs detected by specific queries, more accurate estimates are probably in the 40–60% range, similar to psychotherapy. These findings highlight the need for a validated, meditation-specific questionnaire in order to produce accurate estimates.
In addition to mode of measurement, the frequency of adverse effects and whether they constitute harm depends on how the terms “adverse” and “harm” are defined. For example, Baer and colleagues found that when harm was defined as being “worse off, in any way, after the course, than you would have been if you hadn’t done the course,” 4–7% of MBP participants said they had been harmed (Baer et al., 2020, p. 3). When harm was defined as “sustained deterioration” (Baer et al., 2019; Duggan, Parry, McMurran, Davidson, & Dennis, 2014), as indicated by LBEs in the current study, harm rates were 6–14%. By contrast, the WHO defines harm on a continuum that includes all forms of suffering of any duration, including experiences that are “subjectively unpleasant” and/or clinically-relevant (WHO, 2010, p. 16). Following this definition would include all negative valence and impact events such that the current study’s rates of harm would be 40–60%, which are similar to psychotherapy. Until such definitions are harmonized across treatments and studies, differences in frequency, including declarations of “no adverse effects,” are likely artifacts of measurement or the lack thereof. In the meantime, providing precise and detailed descriptions of definitions and methods of measurement, as modeled in the current study, will help to clarify the nature and frequency of adverse effects.
Limitations
This study is the first to conduct a Phase 0–1 in-depth assessment of AEs in an MBP, which is only the first of many stages toward understanding MBP-related harms. While the current study met its objectives to assess the nature and frequency of MRAEs in an MBP, a number of questions remain. Predictors of MRAEs, including participant characteristics, type(s) or intensity of meditation practice, and teacher characteristics are all important questions.
Because “the same treatment can have both beneficial and harmful effects” (Dimidjian & Hollon, 2010, p. 22), it is important to consider the balance between benefits and harms in clinical decision-making. For example, in the current study, clinically relevant events associated with impaired functioning occurred within a context of overall efficacy on multiple outcomes (Cullen et al., 2021), high practice compliance and low attrition, which suggest that participants found the difficulties worth tolerating in light of expected or concurrent benefits, or in comparison to not receiving treatment.
Although the frequency of MRSEs, MRAEs and LBEs did not significantly differ between MBP variants, these statistical findings do not preclude the existence of practice-specific MRAEs or clinically meaningful differences. Instead these findings simply replicate earlier findings (Lindahl et al., 2017) that the types of meditation found in MBPs—concentration (FA) and insight (OM)— are capable of causing MRAEs. Given that different meditation practices produce different types of effects, they are also likely to produce different types of MRAEs, even if the overall frequency is similar. Future studies with larger, adequately powered samples and systematic MRAE assessment will be needed to address these important questions.
In addition, future research may want to address some of the limitations of the current study. For example, the current study queried only a subset of possible AEs: new or worsening symptoms of physical and psychological health that are associated with meditation practice. Similar to other psychological interventions (Rozental et al., 2018), MBPs may have additional unwanted effects, such as relationship ruptures, dependency, and time burden that may contribute to dissatisfaction and discontinuation (Anderson et al., 2019).
The current study can produce estimates about MRSEs and MRAEs that occur within the first 5 months of practicing less than an hour per day, but not associated with a longer timeframe or more intensive practice. While 25% of the VCE sample encountered meditation-related challenges within the first 50 hours or practicing less than 1 hour per day, the majority required more years of practice or more intensive practice such as meditation retreats (Lindahl et al., 2017). This suggests that the principle of a biological gradient, or that “greater exposure should lead to greater incidence of the effect” (Hill, 2015; Schunemann, Hill, Guyatt, Akl, & Ahmed, 2011), likely applies to MRAEs.
The current sample was aimed at representing the average adult American mindfulness meditator and included individuals with stress, anxiety, and depression who were self-selected (meditation-seeking) and then carefully screened according to standard MBP exclusion criteria (Kuyken et al., 2012; Santorelli et al., 2017). However, the findings may not extend to individuals not seeking a meditation-based program (e.g., individuals who are randomly assigned or required as part of school or employment), to children or the elderly, to those with other physical or mental health conditions, or to MBPs that assess prospective participants through group orientation sessions rather than 2-hour individual clinical interviews. Since individuals from minority ethnic or otherwise marginalized groups are more likely to report lasting bad effects of psychological treatments (Crawford et al., 2016), it is likely that more diverse MBP samples will report higher rates of harms than the current (predominantly white) sample.
The current study measured only treatment completers and not those who dropped out. At least one participant left because of worsening symptoms, and since AEs tend to lead to treatment discontinuation (Warden et al., 2009), it is likely that the AE rates in the study would have been higher if data could have been attained from dropouts.
At the request of the sponsor, the MedEx-I was administered as the last assessment of the study, three months after treatment concluded. This timepoint was selected to minimize Hawthorne effect-based scripting, where repeatedly querying about AEs increases the likelihood of having or reporting them (Braunholtz, Edwards, & Lilford, 2001). However, there are limitations to retrospective recall that may result in underestimates of more distal experiences. Similarly, although meditation practice amounts were similar to other trials (Parsons, Crane, Parsons, Fjorback, & Kuyken, 2017), self-reported meditation adherence may be prone to reporting biases.
While the MedEx-I improved on safety monitoring practices by querying MRSEs by an independent assessor, a validated self-report questionnaire of the same content is still recommended for several reasons. The MedEx-I required hundreds of hours of in-person assessments and qualitative coding by specially trained experts and is therefore both impractical and non-feasible for most researchers or clinicians. In addition to meeting the updated harms assessment guidelines described above, patient-based, treatment-specific AE questionnaires are low cost, low burden, require no special training to administer, and are the only method that supports direct quantitative comparisons between studies. While many medical fields (Corso, Pucino, DeLeo, Calis, & Gallelli, 1992) have been using such standardized treatment-specific AE scales for decades, behavioral treatments have recently started to develop their own AE instruments (Linden, 2013; Parker, Fletcher, Berk, & Paterson, 2013; Rozental et al., 2018) in order to keep up with AE monitoring standards and journal requirements (Hopewell, Altman, Moher, & Schulz, 2008).
Conclusion
All treatments cause some harm some of the time, and multiple sources suggest that MBPs are no exception. The current study found that the active ingredient in MBPs, mindfulness meditation practice—including focused attention and open-monitoring practices alone or in combination—was associated with both transient distress and enduring negative impacts on life and functioning at similar rates to other psychological treatments. Principles of informed consent require that treatment choice be based in part on the balance of benefits to harms, and therefore can only be made if harms are adequately measured and known. The passive monitoring-based “don’t ask, don’t tell” approach to treatment-related harms is being replaced by updated guidelines and validated treatment-specific harms assessment across physical, pharmacological, psychological and behavioral interventions. The current study is an attempt to bring MBP harms monitoring up to the standards of other treatments so that providers can identify events that require monitoring and intervention in order to maximize the safety and efficacy of MBPs.
Supplementary Material
References
- Agbabiaka TB, Savovic J, & Ernst E (2008). Methods for causality assessment of adverse drug reactions: a systematic review. Drug Safety, 31(1), 21–37. [DOI] [PubMed] [Google Scholar]
- Allen EN, Chandler CI, Mandimika N, Leisegang C, & Barnes K (2018). Eliciting adverse effects data from participants in clinical trials. Cochrane Database Syst Rev, 1, MR000039. doi: 10.1002/14651858.MR000039.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson T, Suresh M, & Farb NA (2019). Meditation Benefits and Drawbacks: Empirical Codebook and Implications for Teaching. Journal of Cognitive Enhancement, 3, 207–220. doi: 10.1007/s41465-018-00119-y [DOI] [Google Scholar]
- APA. (2013). Diagnostic and Statistical Manual of Mental Disorders (5th ed.). Washington DC: American Psychiatric Association. [Google Scholar]
- Arbisi P, & Ben-Porath Y (1995). An MMPI-2 infrequent response scale for use with psychopathological populations: The infrequency-psychopathology scale, F(p). Psychological Assessment, 7(4), 424–431. [Google Scholar]
- Baer R, Crane C, Miller E, & Kuyken W (2019). Doing no harm in mindfulness-based programs: Conceptual issues and empirical findings. Clinical Psychology Review, 71, 101–114. doi: 10.1016/j.cpr.2019.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baer R, Crane C, Montero-Marin J, Phillips A, Taylor L, Tickell A, & Kuyken W (2020). Frequency of Self-reported Unpleasant Events and Harm in a Mindfulness-Based Program in Two General Population Samples. Mindfulness. doi: 10.1007/s12671-020-01547-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bent S, Padula A, & Avins AL (2006). Brief communication: Better ways to question patients about adverse medical events: a randomized, controlled trial. Annals of Internal Medicine, 144(4), 257–261. [DOI] [PubMed] [Google Scholar]
- Braunholtz DA, Edwards SJ, & Lilford RJ (2001). Are randomized clinical trials good for us (in the short term)? Evidence for a “trial effect”. Journal of Clinical Epidemiology, 54(3), 217–224. doi: 10.1016/s0895-4356(00)00305-x [DOI] [PubMed] [Google Scholar]
- Britton WB (2019). Can mindfulness be too much of a good thing? The value of a middle way. Current Opinion in Psychology, 28, 159–165. doi: 10.1016/j.copsyc.2018.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton WB, Davis JH, Loucks EB, Peterson B, Cullen BH, Reuter L, … Lindahl JR (2018). Dismantling Mindfulness-Based Cognitive Therapy: Creation and validation of 8-week focused attention and open monitoring interventions within a 3-armed randomized controlled trial. Behaviour Research and Therapy, 101, 92–107. doi: 10.1016/j.brat.2017.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton WB, Haynes PL, Fridel KW, & Bootzin RR (2010). Polysomnographic and subjective profiles of sleep continuity before and after mindfulness-based cognitive therapy in partially remitted depression. Psychosomatic Medicine, 72(6), 539–548. doi: 10.1097/PSY.0b013e3181dc1bad [DOI] [PubMed] [Google Scholar]
- Britton WB, Lindahl JR, & Treleaven D (2017). First, Do No Harm. 20-hour meditation safety training for mindfulness teachers and clinicians. UMASS Center for Mindfulness (CFM). Shrewsbury MA. [Google Scholar]
- Cebolla A, Demarzo M, Martins P, Soler J, & Garcia-Campayo J (2017). Unwanted effects: Is there a negative side of meditation? A multicentre survey. PLoS One, 12(9), e0183137. doi: 10.1371/journal.pone.0183137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou R, Aronson N, Atkins D, Ismaila AS, Santaguida P, Smith DH, … Moher D (2010). AHRQ series paper 4: assessing harms when comparing medical interventions: AHRQ and the effective health-care program. Journal of Clinical Epidemiology, 63(5), 502–512. doi: 10.1016/j.jclinepi.2008.06.007 [DOI] [PubMed] [Google Scholar]
- Chou R, Fu R, Carson S, Saha S, & Helfand M (2007). Methodological shortcomings predicted lower harm estimates in one of two sets of studies of clinical interventions. Journal of Clinical Epidemiology, 60(1), 18–28. doi: 10.1016/j.jclinepi.2006.02.021 [DOI] [PubMed] [Google Scholar]
- CIOMS. (2010). CIOMS Working Group XIII: Practical aspects of signal detection in pharmacovigilance. Geneva: Council for International Organizations of Medical Sciences (CIOMS). [Google Scholar]
- Corso DM, Pucino F, DeLeo JM, Calis KA, & Gallelli JF (1992). Development of a questionnaire for detecting potential adverse drug reactions. Annals of Pharmacotherapy, 26(7–8), 890–896. [DOI] [PubMed] [Google Scholar]
- Crawford MJ, Thana L, Farquharson L, Palmer L, Hancock E, Bassett P, … Parry GD (2016). Patient experience of negative effects of psychological treatment: results of a national survey. British Journal of Psychiatry, 208(3), 260–265. doi: 10.1192/bjp.bp.114.162628 [DOI] [PubMed] [Google Scholar]
- Crichton P, Carel H, & Kidd IJ (2017). Epistemic injustice in psychiatry. BJPsych Bulletin, 41(2), 65–70. doi: 10.1192/pb.bp.115.050682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuijpers P, Reijnders M, Karyotaki E, de Wit L, & Ebert DD (2018). Negative effects of psychotherapies for adult depression: A meta-analysis of deterioration rates. Journal of Affective Disorders, 239, 138–145. doi: 10.1016/j.jad.2018.05.050 [DOI] [PubMed] [Google Scholar]
- Cullen B, Eichel K, Lindahl JR, Rahrig H, Kini N, Flahive J, & Britton WB (2021). The contributions of focused attention and open monitoring in mindfulness-based cognitive therapy for affective disturbances: A 3-armed randomized dismantling trial. PLoS ONE, 16(1), Article e0244838. 10.1371/journal.pone.0244838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimidjian S, & Hollon SD (2010). How would we know if psychotherapy were harmful? American Psychologist, 65(1), 21–33. [DOI] [PubMed] [Google Scholar]
- Dimidjian S, & Segal ZV (2015). Prospects for a clinical science of mindfulness-based intervention. American Psychologist, 70(7), 593–620. doi: 10.1037/a0039589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duggan C, Parry G, McMurran M, Davidson K, & Dennis J (2014). The recording of adverse events from psychological treatments in clinical trials: evidence from a review of NIHR-funded trials. Trials, 15, 335. doi: 10.1186/1745-6215-15-335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards IR (2012). Considerations on causality in pharmacovigilance. International Journal of Risk & Safety in Medicine, 24(1), 41–54. doi: 10.3233/JRS-2012-0552 [DOI] [PubMed] [Google Scholar]
- Edwards IR, & Aronson JK (2000). Adverse drug reactions: definitions, diagnosis, and management. Lancet, 356(9237), 1255–1259. doi: 10.1016/S0140-6736(00)02799-9 [DOI] [PubMed] [Google Scholar]
- Farias M, Maraldi E, Wallenkampf KC, & Lucchetti G (2020). Adverse events in meditation practices and meditation-based therapies: a systematic review. Acta Psychiatrica Scandinavica, 142(5), 374–393. doi: 10.1111/acps.13225 [DOI] [PubMed] [Google Scholar]
- FDA. (2010). Safety Reporting Requirements for Human Drug and Biological Products and Safety Reporting Requirements for Bioavailability and Bioequivalence Studies in Humans. Federal Register 29 September 2010: Department of Health and Human Services, Food and Drug Administration; Retrieved from http://edocket.access.gpo.gov/2010/pdf/2010-24296.pdf. [PubMed] [Google Scholar]
- Fowler F (1998). Mode effects in a survey of Medicare prostate surgery patients. Public Opinion Quarterly, 62, 29–46. [Google Scholar]
- Freedland KE, King AC, Ambrosius WT, Mayo-Wilson E, Mohr DC, Czajkowski SM, … Social Science Clinical T (2019). The selection of comparators for randomized controlled trials of health-related behavioral interventions: recommendations of an NIH expert panel. Journal of Clinical Epidemiology, 110, 74–81. doi: 10.1016/j.jclinepi.2019.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frewen PA, & Lanius RA (2006). Toward a psychobiology of posttraumatic self-dysregulation: reexperiencing, hyperarousal, dissociation, and emotional numbing. Annals of the New York Academy of Sciences, 1071, 110–124. doi: 10.1196/annals.1364.010 [DOI] [PubMed] [Google Scholar]
- Frist M (1997). Structured Clinical Interview for DSM-IV Axis II Personality Disorders, (SCID-II). Washington, D.C.: American Psychiatric Press, Inc. [Google Scholar]
- Furukawa TA, Noma H, Caldwell DM, Honyashiki M, Shinohara K, Imai H, … Churchill R (2014). Waiting list may be a nocebo condition in psychotherapy trials: a contribution from network meta-analysis. Acta Psychiatrica Scandinavica, 130(3), 181–192. doi: 10.1111/acps.12275 [DOI] [PubMed] [Google Scholar]
- Gitlin LN (2013). Introducing a new intervention: an overview of research phases and common challenges. American Journal of Occupational Therapy, 67(2), 177–184. doi: 10.5014/ajot.2013.006742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg SB, Tucker RP, Greene PA, Davidson RJ, Wampold BE, Kearney DJ, & Simpson TL (2018). Mindfulness-based interventions for psychiatric disorders: A systematic review and meta-analysis. Clinical Psychology Review, 59, 52–60. doi: 10.1016/j.cpr.2017.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg SB, Tucker RP, Greene PA, Simpson TL, Kearney DJ, & Davidson RJ (2017). Is mindfulness research methodology improving over time? A systematic review. PLoS One, 12(10), e0187298. doi: 10.1371/journal.pone.0187298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golomb BA, McGraw JJ, Evans MA, & Dimsdale JE (2007). Physician response to patient reports of adverse drug effects: implications for patient-targeted adverse effect surveillance. Drug Safety, 30(8), 669–675. doi: 10.2165/00002018-200730080-00003 [DOI] [PubMed] [Google Scholar]
- Hammad TA, Pinheiro SP, & Neyarapally GA (2011). Secondary use of randomized controlled trials to evaluate drug safety: a review of methodological considerations. Clinical Trials, 8(5), 559–570. doi: 10.1177/1740774511419165 [DOI] [PubMed] [Google Scholar]
- Hannan C, Lambert MJ, Harmon C, Nielsen SL, Smart DW, Shimokawa K, & Sutton SW (2005). A lab test and algorithms for identifying clients at risk for treatment failure. Journal of Clinical Psychology, 61(2), 155–163. doi: 10.1002/jclp.20108 [DOI] [PubMed] [Google Scholar]
- Hansen N, Lambert M, & Forman E (2002). The psychotherapy dose-response effect and its implications for treatment delivery services. Clinical Psychology: Science and Practice, 9(3), 329–343. [Google Scholar]
- Hatfield D, McCullough L, Frantz SH, & Krieger K (2010). Do we know when our clients get worse? an investigation of therapists’ ability to detect negative client change. Clinical Psychology & Psychotherapy, 17(1), 25–32. doi: 10.1002/cpp.656 [DOI] [PubMed] [Google Scholar]
- Hauben M, & Aronson JK (2007). Gold standards in pharmacovigilance: the use of definitive anecdotal reports of adverse drug reactions as pure gold and high-grade ore. Drug Safety, 30(8), 645–655. doi: 10.2165/00002018-200730080-00001 [DOI] [PubMed] [Google Scholar]
- Healy D, & Mangin D (2019). Clinical judgments, not algorithms, are key to patient safety-an essay by David Healy and Dee Mangin. BMJ, 367, l5777. doi: 10.1136/bmj.l5777 [DOI] [PubMed] [Google Scholar]
- Hill AB (2015). The environment and disease: association or causation? Journal of the Royal Society of Medicine, 108(1), 32–37. doi: 10.1177/0141076814562718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinz A, Ernst J, Glaesmer H, Brahler E, Rauscher FG, Petrowski K, & Kocalevent RD (2017). Frequency of somatic symptoms in the general population: Normative values for the Patient Health Questionnaire-15 (PHQ-15). Journal of Psychosomatic Research, 96, 27–31. doi: 10.1016/j.jpsychores.2016.12.017 [DOI] [PubMed] [Google Scholar]
- Hirshberg MJ, Goldberg SB, Rosenkranz M, & Davidson RJ (2020). Prevalence of harm in mindfulness-based stress reduction. Psychological Medicine, 1–9. doi: 10.1017/S0033291720002834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann SG, Sawyer AT, Witt AA, & Oh D (2010). The effect of mindfulness-based therapy on anxiety and depression: A meta-analytic review. Journal of Consulting and Clinical Psychology, 78(2), 169–183. doi: 10.1037/a0018555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes EA, Ghaderi A, Harmer CJ, Ramchandani PG, Cuijpers P, Morrison AP, … Craske MG (2018). The Lancet Psychiatry Commission on psychological treatments research in tomorrow’s science. Lancet Psychiatry, 5(3), 237–286. doi: 10.1016/S2215-0366(17)30513-8 [DOI] [PubMed] [Google Scholar]
- Hopewell S, Altman DG, Moher D, & Schulz KF (2008). Endorsement of the CONSORT Statement by high impact factor medical journals: a survey of journal editors and journal ‘Instructions to Authors’. Trials, 9, 20. doi: 10.1186/1745-6215-9-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horigian VE, Robbins MS, Dominguez R, Ucha J, & Rosa CL (2010). Principles for defining adverse events in behavioral intervention research: lessons from a family-focused adolescent drug abuse trial. Clinical Trials, 7(1), 58–68. doi: 10.1177/1740774509356575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrobjartsson A, Emanuelsson F, Skou Thomsen AS, Hilden J, & Brorson S (2014). Bias due to lack of patient blinding in clinical trials. A systematic review of trials randomizing patients to blind and nonblind sub-studies. International Journal of Epidemiology, 43(4), 1272–1283. doi: 10.1093/ije/dyu115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ioannidis JP (2009). Adverse events in randomized trials: neglected, restricted, distorted, and silenced. Archives of Internal Medicine, 169(19), 1737–1739. doi: 10.1001/archinternmed.2009.313 [DOI] [PubMed] [Google Scholar]
- Ioannidis JP, Evans SJ, Gotzsche PC, O’Neill RT, Altman DG, Schulz K, & Moher D (2004). Better reporting of harms in randomized trials: an extension of the CONSORT statement. Annals of Internal Medicine, 141(10), 781–788. [DOI] [PubMed] [Google Scholar]
- Johnson C, Burke C, Brinkman S, & Wade T (2016). Effectiveness of a school-based mindfulness program for transdiagnostic prevention in young adolescents. Behaviour Research and Therapy, 81, 1–11. doi: 10.1016/j.brat.2016.03.002 [DOI] [PubMed] [Google Scholar]
- Jonsson U, Alaie I, Parling T, & Arnberg FK (2014). Reporting of harms in randomized controlled trials of psychological interventions for mental and behavioral disorders: A review of current practice. Contemporary Clinical Trials, 38(1), 1–8. doi: 10.1016/j.cct.2014.02.005 [DOI] [PubMed] [Google Scholar]
- Kramer MS (1981). Difficulties in assessing the adverse effects of drugs. British Journal of Clinical Pharmacology, 11 Suppl 1, 105S–110S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuijpers H, van der Heijden F, Tuinier S, & Verhoeven W (2007). Meditation-induced psychosis. Psychopathology, 40, 461–464. [DOI] [PubMed] [Google Scholar]
- Kuyken W, Crane W, & Williams JM (2012). Mindfulness-Based Cognitive Therapy (MBCT) Implementation Resources. Oxford University, University of Exeter, Bangor University. [Google Scholar]
- Lambert M, Whipple JL, Hawkins EJ, Vermeersch DA, Nielsen SL, & Smart DW (2003). Is it time for clinicians to routinely track patient outcome? A meta-analysis. Clinical Psychology: Science and Practice, 10, 288–301. [Google Scholar]
- Lambert MJ (2013). The efficacy and effectiveness of psychotherapy. In Lambert M (Ed.), Bergin and Garfield’s handbook of psychotherapy and behavior change (6th ed., pp. 69–218). New York, NY: Wiley. [Google Scholar]
- Lilienfeld SO (2007). Psychological treatments that cause harm. Perspectives on Psychological Science, 2(1), 53–70. doi: 10.1111/j.1745-6916.2007.00029.x [DOI] [PubMed] [Google Scholar]
- Lindahl JR, & Britton WB (2019). ‘I Have This Feeling of Not Really Being Here’: Buddhist Meditation and Changes in Sense of Self. Journal of Consciousness Studies, 26(7–8), 157–183. [Google Scholar]
- Lindahl JR, Britton WB, Cooper DJ, & Kirmayer L (2019). Challenging and Adverse Meditation Experiences: Toward a Person-Centered Approach In Farias M, Lalljee M, & Brazier D (Eds.), The Oxford Handbook of Meditation. Oxford: Oxford University Press. [Google Scholar]
- Lindahl JR, Cooper DJ, Fisher NE, Kirmayer L, & Britton W (2020). Progress or pathology? Differential diagnosis and Intervention criteria for meditation-related challenges: Perspectives from Buddhist meditation teachers and practitioners. Frontiers in Psychology. doi: 10.3389/fpsyg.2020.01905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl JR, Fisher NE, Cooper DJ, Rosen RK, & Britton WB (2017). The varieties of contemplative experience: A mixed-methods study of meditation-related challenges in Western Buddhists. PLoS One, 12(5), e0176239. doi: 10.1371/journal.pone.0176239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden M (2013). How to define, find and classify side effects in psychotherapy: from unwanted events to adverse treatment reactions. Clinical Psychology & Psychotherapy, 20(4), 286–296. doi: 10.1002/cpp.1765 [DOI] [PubMed] [Google Scholar]
- Lineberry N, Berlin JA, Mansi B, Glasser S, Berkwits M, Klem C, … Laine C (2016). Recommendations to improve adverse event reporting in clinical trial publications: a joint pharmaceutical industry/journal editor perspective. BMJ, 355, i5078. doi: 10.1136/bmj.i5078 [DOI] [PubMed] [Google Scholar]
- Lomas T, Cartwright T, Edginton T, & Ridge D (2014). A qualitative summary of experiential challenges associated with meditation practice. Mindfulness, 1–13. doi: 10.1007/s12671-014-0329-8 [DOI] [Google Scholar]
- Lomas T, Medina JC, Ivtzan I, Rupprecht S, Hart R, & Eiroa-Orosa FJ (2017). The impact of mindfulness on well-being and performance in the workplace: an inclusive systematic review of the empirical literature. European Journal of Work and Organizational Psychology, 26(4), 492–513. doi: 10.1080/1359432X.2017.1308924 [DOI] [Google Scholar]
- Lustyk M, Chawla N, Nolan R, & Marlatt G (2009). Mindfulness Medtation Research: Issues of participant screening, safety procedures, and researcher training. Advances in Mind-Body Medicine, 24(1), 20–30. [PubMed] [Google Scholar]
- Mayo-Wilson E, Fusco N, Li T, Hong H, Canner JK, Dickersin K, & investigators M (2019). Harms are assessed inconsistently and reported inadequately part 1: systematic adverse events. Journal of Clinical Epidemiology, 113, 20–27. doi: 10.1016/j.jclinepi.2019.04.022 [DOI] [PubMed] [Google Scholar]
- Moher D, Schulz KF, & Altman D (2001). The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomized trials. JAMA, 285(15), 1987–1991. [DOI] [PubMed] [Google Scholar]
- Moore TJ, Singh S, & Furberg CD (2012). The FDA and new safety warnings. Archives of Internal Medicine, 172(1), 78–80. doi: 10.1001/archinternmed.2011.618 [DOI] [PubMed] [Google Scholar]
- Moritz S, Fieker M, Hottenrott B, Seeralan T, Cludius B, Kolbeck KGJ, & Nestoriuc Y (2015). No pain, no gain? Adverse effects of psychotherapy in obsessive–compulsive disorder and its relationship to treatment gains. Journal of Obsessive-Compulsive and Related Disorders, 5, 61–66. [Google Scholar]
- Morone NE, Moore CG, & Greco CM (2017). Characteristics of Adults Who Used Mindfulness Meditation: United States, 2012. Journal of Alternative and Complementary Medicine, 23(7), 545–550. doi: 10.1089/acm.2016.0099 [DOI] [PubMed] [Google Scholar]
- Nichols AL, & Maner JK (2008). The good-subject effect: investigating participant demand characteristics. Journal of General Psychology, 135(2), 151–165. doi: 10.3200/GENP.135.2.151-166 [DOI] [PubMed] [Google Scholar]
- NIH. (2016). Adverse Event and Serious Adverse Event Guidelines. In OHRP Guidance on Reviewing and Reporting Unanticipated Problems Involving Risks to Subjects or Others and Adverse Events, OHRP Guidance. National Insitutes of Health (NIH): Office for Human Research Protections, U.S. Department of health and Human Servcies. [Google Scholar]
- Parker G, Fletcher K, Berk M, & Paterson A (2013). Development of a measure quantifying adverse psychotherapeutic ingredients: the Experiences of Therapy Questionnaire (ETQ). Psychiatry Research, 206(2–3), 293–301. doi: 10.1016/j.psychres.2012.11.026 [DOI] [PubMed] [Google Scholar]
- Parsons CE, Crane C, Parsons LJ, Fjorback LO, & Kuyken W (2017). Home practice in Mindfulness-Based Cognitive Therapy and Mindfulness-Based Stress Reduction: A systematic review and meta-analysis of participants’ mindfulness practice and its association with outcomes. Behaviour Research and Therapy, 95, 29–41. doi: 10.1016/j.brat.2017.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson AL, Roache JD, Raj J, Young-McCaughan S, & Consortium SS (2013). The need for expanded monitoring of adverse events in behavioral health clinical trials. Contemporary Clinical Trials, 34(1), 152–154. doi: 10.1016/j.cct.2012.10.009 [DOI] [PubMed] [Google Scholar]
- Reynolds L, Bissett I, Porter D, & Consedine N (2017). A brief mindfulness intervention is associated with negative outcomes in a randomised controlled trial among chemotherapy patients. Mindfulness, 8(5), 1291–1303. [Google Scholar]
- Rheker J, Beisel S, Kraling S, & Rief W (2017). Rate and predictors of negative effects of psychotherapy in psychiatric and psychosomatic inpatients. Psychiatry Research, 254, 143–150. doi: 10.1016/j.psychres.2017.04.042 [DOI] [PubMed] [Google Scholar]
- Rozental A, Castonguay L, Dimidjian S, Lambert M, Shafran R, Andersson G, & Carlbring P (2018). Negative effects in psychotherapy: commentary and recommendations for future research and clinical practice. BJPsych Open, 4(4), 307–312. doi: 10.1192/bjo.2018.42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozental A, Kottorp A, Forsstrom D, Mansson K, Boettcher J, Andersson G, … Carlbring P (2019). The Negative Effects Questionnaire: psychometric properties of an instrument for assessing negative effects in psychological treatments. Behavioural and Cognitive Psychotherapy, 47(5), 559–572. doi: 10.1017/S1352465819000018 [DOI] [PubMed] [Google Scholar]
- Rush AJ, Gullion CM, Basco MR, Jarrett RB, & Trivedi MH (1996). The Inventory of Depressive Symptomatology (IDS): psychometric properties. Psychological Medicine, 26(3), 477–486. [DOI] [PubMed] [Google Scholar]
- Santorelli S, Meleo-Meyer F, Koerbel L, Kabat-Zinn J, Blacker M, Herbette G, & Fulwiler C (2017). Mindfulness-Based Stress Reduction (MBSR) Authorized Curriculum Guide. Center for Mindfulness in Medicine, Health Care, and Society (CFM), University of Massachusetts Medical School. [Google Scholar]
- Schermuly-Haupt M, Linden M, & Rush J (2018). Unwanted Events and Side Effects in Cognitive Behavior Therapy. Cognitive Therapy and Research, 42, 219–229. [Google Scholar]
- Schlosser M, Sparby T, Voros S, Jones R, & Marchant NL (2019). Unpleasant meditation-related experiences in regular meditators: Prevalence, predictors, and conceptual considerations. PLoS One, 14(5), e0216643. doi: 10.1371/journal.pone.0216643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneibel R, Wilbertz G, Scholz C, Becker M, Brakemeier EL, Bschor T, … Schmoll D (2017). Adverse events of group psychotherapy in the in-patient setting - results of a naturalistic trial. Acta Psychiatrica Scandinavica, 136(3), 247–258. doi: 10.1111/acps.12747 [DOI] [PubMed] [Google Scholar]
- Schunemann H, Hill S, Guyatt G, Akl EA, & Ahmed F (2011). The GRADE approach and Bradford Hill’s criteria for causation. Journal of Epidemiology and Community Health, 65(5), 392–395. doi: 10.1136/jech.2010.119933 [DOI] [PubMed] [Google Scholar]
- Segal Z, Teasdale J, Williams J, & Gemar M (2002). The Mindfulness-Based Cognitive Therapy Adherence Scale: Inter-rater reliability, adherence to and treatment distinctiveness. Clinical Psychology and Psychotherapy, 9, 131–138. [Google Scholar]
- Segal ZV, Williams JMG, & Teasdale JD (2002). Mindfulness-Based Cognitive Therapy for Depression: A New Approach to Preventing Relapse. [Google Scholar]
- Sheskin D (2004). Handbook of parametric and nonparametric statistical procedures (3rd ed.). Boca Raton: Chapman & Hall /CRC. [Google Scholar]
- Siegerink B, & Rohmann JL (2018). Impact of your results: Beyond the relative risk. Research and Practice in Thrombosis and Haemostasis, 2(4), 653–657. doi: 10.1002/rth2.12148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S, & Loke YK (2012). Drug safety assessment in clinical trials: methodological challenges and opportunities. Trials, 13, 138. doi: 10.1186/1745-6215-13-138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbot J, & Aronson J (Eds.). (2012). Stephens’ Detection and Evaluation of Adverse Drug Reactions: Principles and Practice (6th ed.). Oxford: Wiley-Blackwell. [Google Scholar]
- Treleaven D (2018). Trauma-sensitive mindfulness: Practices for safe and transformative healing. New York: Norton. [Google Scholar]
- Turner WM (1984). The Food and Drug Administration algorithm. Special workshop: Regulatory. Drug Information Journal, 18(3–4), 259–266. [DOI] [PubMed] [Google Scholar]
- Van Dam NT, & Galante J (2020). Underestimating harm in mindfulness-based stress reduction. Psychological Medicine, 1–3. doi: 10.1017/S003329172000447X [DOI] [PubMed] [Google Scholar]
- Van Dam NT, van Vugt MK, Vago DR, Schmalzl L, Saron CD, Olendzki A, … Meyer DE (2018). Mind the Hype: A Critical Evaluation and Prescriptive Agenda for Research on Mindfulness and Meditation. Perspectives on Psychological Science, 13(1), 36–61. doi: 10.1177/1745691617709589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenbroucke JP (2006). What is the best evidence for determining harms of medical treatment? Canadian Medical Association Journal 174(5), 645–646. doi: 10.1503/cmaj.051484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan B, Goldstein MH, Alikakos M, Cohen LJ, & Serby MJ (2014). Frequency of reporting of adverse events in randomized controlled trials of psychotherapy vs. psychopharmacotherapy. Comprehensive Psychiatry, 55(4), 849–855. doi: 10.1016/j.comppsych.2014.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walfish S, McAlister B, O’Donnell P, & Lambert MJ (2012). An investigation of self-assessment bias in mental health providers. Psychological Reports, 110(2), 639–644. doi: 10.2466/02.07.17.PR0.110.2.639-644 [DOI] [PubMed] [Google Scholar]
- Warden D, Trivedi MH, Wisniewski SR, Lesser IM, Mitchell J, Balasubramani GK, … Rush AJ (2009). Identifying risk for attrition during treatment for depression. Psychotherapy and Psychosomatics, 78(6), 372–379. doi: 10.1159/000235977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman JS, Schneider EC, Weingart SN, Epstein AM, David-Kasdan J, Feibelmann S, … Gatsonis C (2008). Comparing patient-reported hospital adverse events with medical record review: do patients know something that hospitals do not? Annals of Internal Medicine, 149(2), 100–108. [DOI] [PubMed] [Google Scholar]
- WHO. (2010). Conceptual Framework for the International Classification for Patient Safety. Geneva: World Health Organization. [Google Scholar]
- WHO. (2016). The use of the WHO-UMC system for standardized case causaility assessment. who-umc.org: World Health Organization (WHO), Uppsala Monitoring Centre [Google Scholar]
- Wong S, Chan J, Zhang D, Lee E, & Tsoi K (2018). The Safety of Mindfulness-Based Interventions: a Systematic Review of Randomized Controlled Trials. Mindfulness. doi: 10.1007/s12671-018-0897-0 [DOI] [Google Scholar]
- Yorston G (2001). Mania precipitated by meditation: a case report and literature review. Mental Health, Religion & Culture, 4, 209–214. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.