Abstract
In the present study, we analyzed the possible relationship between interferon (IFN) sensitivity-determining region (ISDR) sequence variation of various hepatitis C virus (HCV) subtypes and serum HCV titers in Indonesian patients without IFN treatment. The viremia titers (mean ± standard deviation) of HCV subtype 1b (HCV-1b) isolates with low (three or fewer) and high (four or more) numbers of ISDR mutations were 5.4 ± 0.6 and 4.2 ± 0.9 log10 RNA copies/ml, respectively, with the difference between the two groups being statistically significant (P < 0.01). Similarly, the viremia titers of HCV-1c isolates with low and high numbers of ISDR mutations were 5.3 ± 0.6 and <3.0 ± 0.0 log10 RNA copies/ml, respectively, with the difference between the two groups being statistically significant (P < 0.01). Also, the virus titers of HCV-2a isolates with low and high numbers of ISDR mutations were 4.3 ± 0.7 and 3.5 ± 0.4 log10 RNA copies/ml, respectively, with the difference between the two groups being statistically significant (P < 0.01). Thus, our results demonstrated that virus load in Indonesian patients infected with HCV-1b, HCV-1c, or HCV-2a correlated inversely with the number of mutations in the ISDR sequence, implying the possibility that the ISDR sequence plays an important role in determining the levels of HCV viremia.
Hepatitis C virus (HCV) readily establishes a chronic persistent infection that often results in chronic hepatitis and more deteriorating disease such as liver cirrhosis and hepatocellular carcinoma (14). HCV is phylogenetically classified into at least six clades (formerly called genotypes), each of which can be further divided into a number of subtypes (4, 26, 30). We have previously reported the prevalence of each HCV subtype, including HCV subtype 1c (HCV-1c) (formerly referred to as HCV-1d), among various clinical populations in Surabaya, Indonesia (13, 31). HCV-1c has been found almost exclusively in Indonesia (12, 13, 23) and shown to be associated with high viral load and poor prognosis (18, 31).
Interferon (IFN) is the most successful therapeutic agent for the treatment of chronic hepatitis C, although less than half of the patients treated with IFN show sustained responses with eradication of the virus. It is now recognized that HCV viral load in the serum and the HCV genotype and/or quasispecies complexity as well as sequence diversity of particular regions of the viral genome may predict the effectiveness of IFN therapy (2, 3, 10, 16, 25, 27). Lower pretreatment serum HCV RNA levels have been shown to be associated with a better response to IFN therapy. Patients infected with HCV-1b tend to exhibit poor IFN responsiveness compared with those infected with HCV-2a. Enomoto et al. (6, 7) first demonstrated that amino acid mutations of the nonstructural protein 5A (NS5A) of HCV-1b in a region between residues 2209 and 2248 were associated with improved responsiveness to IFN in Japanese patients, and the region has therefore been designated as the IFN sensitivity-determining region (ISDR). This observation was subsequently confirmed by other research groups mostly in Japan (2, 3, 21, 28, 34). However, several reports from Europe and the United States failed to show the correlation between ISDR mutations and IFN responsiveness (5, 11, 22, 32), challenging the ISDR hypothesis. The IFN-mediated antiviral activity is executed in part by the double-stranded RNA-activated protein kinase (PKR), which has been suggested to form a complex with NS5A through a region, designated the PKR-binding region, that spans the ISDR and the adjacent 26 residues (9). In the present study, we have investigated whether the PKR-binding region of HCV-1b, -1c, and -2a plays a role in determining the levels of viremia in patients without IFN treatment.
MATERIALS AND METHODS
Serum samples.
Sera were obtained from the Red Cross Blood Transfusion Center, Surabaya, Indonesia, and from patients with chronic liver disease at Dr. Soetomo Hospital, Faculty of Medicine, Airlangga University, Surabaya, Indonesia. They were tested for anti-HCV antibodies by enzyme-linked immunosorbent assay (UBI HCV EIA [United Biologicals, Inc., New York, N.Y.]; Ortho HCV Ab ELISA Test II [Ortho Diagnostics, Inc., Tokyo, Japan]) and for hepatitis B surface antigen (subtypes ad and ay) by using AUSAB EIA (Abbott Laboratories, Diagnostics Division). Sera that were positive for anti-HCV antibodies and negative for hepatitis B surface antigen were used for further analysis. A total of 57 HCV isolates obtained from 57 individuals (23 isolates of HCV-1b, 15 isolates of HCV-1c, and 19 isolates of HCV-2a) were analyzed. Table 1 summarizes the number, sex, and age of the subjects, and mean HCV viremia titers for each HCV subtype with low (three or fewer) and high (four or more) numbers of mutations in the ISDR (see below). The grouping of HCV isolates on the basis of low (three or fewer) and high (four or more) numbers of ISDR mutations has been reported (2, 3, 6, 7).
TABLE 1.
Comparison between the number of ISDR mutations and serum HCV RNA titers for HCV subtypes 1b, 1c, and 2a
| HCV subtype | No. of ISDR mutations (mean ± SD) | No. of patients (male/female) | Mean age (yr) ± SD | HCV RNA titer (log10 copies/ml) |
|---|---|---|---|---|
| 1b | ≤3 (0.6 ± 0.6) | 20 (14/6) | 55.1 ± 9.3 | 5.4 ± 0.6a |
| ≥4 (7.0 ± 1.7) | 3 (1/2) | 59.5 ± 4.9 | 4.2 ± 0.9a | |
| 1c | ≤3 (0.6 ± 0.8) | 13 (11/2) | 52.3 ± 14.3 | 5.3 ± 0.6b |
| ≥4 (5.5 ± 0.7) | 2 (1/1) | 59.0 ± 1.4 | <3.0b | |
| 2a | ≤3 (1.0 ± 0.9) | 10 (9/1) | 56.0 ± 12.4 | 4.3 ± 0.7a |
| ≥4 (4.8 ± 0.8) | 9 (9/0) | 55.3 ± 13.4 | 3.5 ± 0.4a |
P < 0.01 (one-way ANOVA, Student's t test).
P < 0.01 (one-way ANOVA); P < 0.05 (Mann-Whitney test).
HCV subtype analysis.
RNA was extracted from the anti-HCV antibody-positive sera (60 μl each) using Trizol LS (Life Technologies, Gaithersburg, Md.) and reverse transcribed into cDNA using Rous-associated virus type 2 reverse transcriptase (Takara Shuzo, Co., Ltd., Kyoto, Japan) and a primer specific for a portion of the NS5B region of the HCV genome (167R), as described previously (13, 18, 31). The resultant cDNA was then amplified by nested PCR using Tth DNA polymerase (Toyobo Co., Ltd., Osaka, Japan) and appropriate sets of primers. Anti-HCV-negative sera or saline served as a negative control in the reverse transcription (RT)-PCR analysis to monitor the possible cross-contamination between the samples. Also, other standard precautions were taken to minimize possible cross-contamination. The amplified fragments were sequenced by using the Taq DiDeoxy Terminator Cycle Sequencing kit (Perkin-Elmer) and an ABI 373A DNA sequencer (Applied Biosystems, Inc.). Based on the sequence similarity to the reported sequences, each HCV isolate was assigned an HCV subtype.
Measurement of HCV viral load.
Levels of HCV viral load were assessed using a commercially available kit (Amplicor HCV Monitor Test, version 1.0; Roche Diagnostic Systems, Inc., Branchburg, N.J.) according to the manufacturer's instructions. The lowest detectable titer with this kit was 3.0 log10 RNA copies/ml. A viral load of 4.1 log10 RNA copies/ml or higher was regarded as a high virus titer, and that of 4.0 log10 RNA copies/ml or lower was regarded as a low titer, according to the titers for the high and low controls included in the kit.
Analysis of NS5A sequences.
RNA extracted from the sera (60 μl each) was reverse transcribed into cDNA by using Rous-associated virus type 2 reverse transcriptase (Takara Shuzo) and an appropriate primer (Fig. 1). The resultant cDNA was subjected to the first-round PCR over 35 cycles, with each cycle consisting of 1 min at 94°C, 1 min at 50°C, and 2 min at 72°C, followed by the second-round PCR under the same conditions described above. The primers used to analyze the entire NS5A region of HCV-1b, -1c, and -2a were selected on the basis of the sequences that had been reported to be conserved among HCV-1b and -1c or -2a. The sequences and positions of the primers used are shown in Fig. 1. The PCR products were electrophoresed in an agarose gel containing ethidium bromide and were visualized by UV illumination. Nucleotide sequences of the amplified fragments were determined with the Big Dye Deoxy Terminator Cycle Sequencing kit (Perkin-Elmer) and ABI 377 or ABI 310 DNA sequencer (Applied Biosystems, Inc.), and the amino acid sequences were deduced.
FIG. 1.
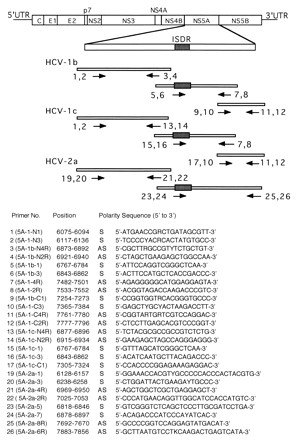
Schematic representation of the HCV genome and primers for RT-PCR for analysis of NS5A. The ISDR is depicted by a shaded rectangle. Empty bars represent amplified cDNA fragments, and the numbers and arrows along them indicate primers used for RT-PCR. Sequences, positions, and polarities of the primers are shown on the bottom. The sequences are numbered as described previously (15, 23, 24). Abbreviations: S, sense; AS, antisense; K, T or G; R, A or G; W, A or T; Y, C or T.
Statistical analysis.
The data obtained were statistically analyzed by one-way analysis of variance (ANOVA) and Student's t test. When appropriate, the nonparametric Mann-Whitney test was also used. A P value of <0.05 was considered significant.
Nucleotide sequence accession numbers.
The nucleotide sequence data reported in this paper have been submitted to the DDBJ/EMBL/GenBank nucleotide sequence databases with the accession numbers AB056520 through AB056569.
RESULTS
Levels of viremia in patients infected with HCV-1b, -1c, or -2a.
Levels of viremia of HCV-1b isolates ranged from 3.6 to 6.3 (5.3 ± 0.8) log10 RNA copies/ml (values are presented throughout as means ± standard deviations [SD]). The titers of HCV-1c isolates ranged from <3.0 to 6.0 (5.0 ± 1.0) log10 RNA copies/ml. The titers of HCV-2a isolates ranged from <3.0 to 5.8 (3.9 ± 0.7) log10 RNA copies/ml. The apparent difference in the viremia titers between HCV-2a and the other subtypes is likely due to the fact that the Amplicor HCV Monitor Test, version 1.0, underestimates the viral load for HCV genotypes other than genotype 1 (19).
Correlation between HCV viremia levels and the number of ISDR mutations in HCV-1b, -1c, and -2a.
We determined deduced amino acid sequences of the entire NS5A protein of representative isolates with high and low virus titers for HCV-1b, -1c, and -2a. When compared with each of the reference strains—HCV-1bJ (15) for HCV-1b, HC-G9 (23) for HCV-1c, and HC-J6 (24) for HCV-2a—the HCV isolates tested possessed 15 to 30 amino acid substitutions in NS5A excluding the PKR-binding region; however, those substitutions outside the PKR-binding region did not appear to correlate with serum HCV RNA titers (data not shown).
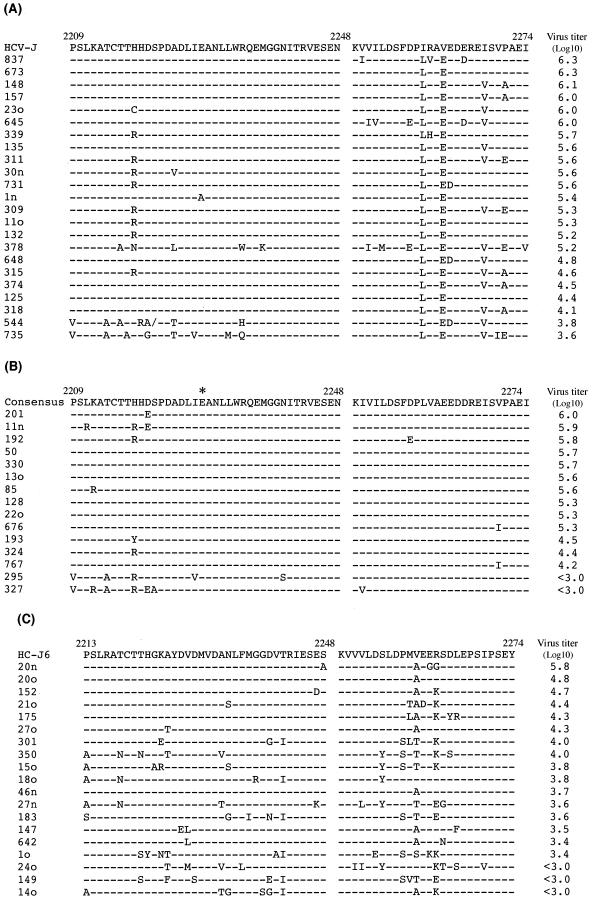
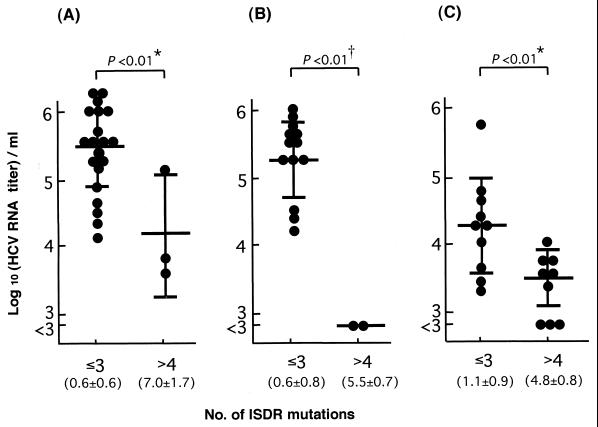
We then looked at the amino acid sequences of the PKR-binding region, including the ISDR. When compared with the sequence of HCV-1bJ (15), a standard sequence for IFN resistance (7), both of two HCV-1b isolates with low virus titers had seven or eight mutations in the ISDR, with one of them having even a single amino acid deletion. On the other hand, all but one isolate with high virus titers had two mutations or less in the ISDR (Fig. 2A). A certain degree of sequence variation was observed in the carboxy-terminal portion of the PKR-binding region for HCV-1b, but no correlation was observed between serum HCV titers and the sequence variation outside the ISDR. The HCV-1b isolates were divided into two groups: those with low (three or fewer) and high (four or more) numbers of mutations in the ISDR sequence, according to the criteria reported previously (2, 3, 6, 7), and serum HCV RNA titers were plotted (Fig. 3A and Table 1). All of the 20 isolates with low numbers of ISDR mutations showed viremia levels of ≥4.1 (5.4 ± 0.6) log10 RNA copies/ml. On the other hand, two of three isolates with high numbers of ISDR mutations showed viremia levels of <4.0 log10 RNA copies/ml, and the mean titer ± SD of this group was 4.2 ± 0.9 log10 RNA copies/ml. The difference in the mean virus titers between the two groups was statistically significant (P < 0.01).
FIG. 2.
Sequence alignment of amino acid residues of the PKR-binding region, including the ISDR, of HCV-1b, -1c, and -2a isolates. (A) Sequence alignment of the PKR-binding region (positions 2209 to 2274), including the ISDR (positions 2209 to 2248), of HCV-1b isolates. Dashes indicate residues identical to those in the reference sequences. A slash indicates an amino acid deletion. Serum HCV RNA titers are shown on the right. (B) Sequence alignment of the PKR-binding region (positions 2209 to 2274), including the ISDR (positions 2209 to 2248), of HCV-1c isolates. The reference consensus sequence differs from the HC-G9 sequence (23) by a single residue at position 2228 (∗), where only HC-G9 has threonine while all of the other HCV-1c isolates analyzed have glutamic acid. (C) Sequence alignment of the PKR-binding region (positions 2213 to 2274), including the ISDR (positions 2213 to 2248), of HCV-2a isolates.
FIG. 3.
Comparison between serum HCV RNA titers and the number of ISDR mutations of HCV-1b (A), HCV-1c (B), and HCV-2a isolates (C). Each circle represents the HCV RNA titer of each isolate. The mean values and SD of HCV RNA titers are shown by long and short horizontal bars, respectively. The virus titer of <3.0 was regarded as 3.0 when the mean values and SD were calculated. The mean numbers and SD of the ISDR mutations for groups of low (three or fewer) and high (four or more) numbers of mutations are shown in parentheses. ∗, P < 0.01 by one-way ANOVA and Student's t test; †, P < 0.01 by one-way ANOVA and P < 0.05 by Mann-Whitney test.
As for HCV-1c, no reference sequence for the ISDR was reported as representing IFN-resistant strains. We initially used HC-G9 (23) as a reference. With all the isolates sequenced in the present study, however, the residue at position 2228 was glutamic acid; it was threonine only with HC-G9. Therefore, we constructed the consensus reference sequence, which differs from that of HC-G9 by the single residue in the ISDR. When compared with the consensus sequence, the two isolates with low virus titers had five or six mutations in the ISDR, whereas all isolates with high virus titers had three mutations or less in the ISDR (Fig. 2B). Unlike HCV-1b isolates, the carboxy-terminal portion of the PKR-binding region was well conserved among all HCV-1c isolates tested. The HCV-1c isolates were divided into the two groups of low (three or fewer) and high (four or more) numbers of ISDR mutations, and serum HCV titers were plotted (Fig. 3B and Table 1). All of the 13 isolates with low numbers of ISDR mutations showed viremia levels of ≥4.2 (5.3 ± 0.6) log10 RNA copies/ml. On the other hand, both of the two isolates with high numbers of ISDR mutations showed viremia levels of <3.0 log10 RNA copies/ml, with the mean titer being at its highest 3.0 log10 RNA copies/ml. The difference in the mean virus titers between the two groups was statistically significant (P < 0.01).
When HCV-1b and HCV-1c isolates were combined, the viremia titer for those with low numbers of ISDR mutations (three or fewer) was 5.4 ± 0.6 log10 RNA copies/ml, whereas that for those with high numbers of ISDR mutations (four or more) was 3.7 ± 0.9 log10 RNA copies/ml, with the difference between the two groups being statistically significant (P < 0.01).
The sequences of HCV-2a isolates were compared with that of HC-J6, which had been used as a reference strain in a previous work (20). We noticed a tendency, similarly to the cases with HCV-1b and HCV-1c isolates, for serum HCV titers to be correlated inversely with the number of ISDR mutations (Fig. 2C). Again, HCV-2a isolates were divided into the two groups of low (three or fewer) and high (four or more) numbers of ISDR mutations, and serum HCV titers were plotted (Fig. 3C and Table 1). The virus titers (mean of HCV-2a isolates with low [three or fewer] and high [four or more] numbers of ISDR mutations) were 4.3 ± 0.7 and 3.5 ± 0.4 log10 RNA copies/ml, respectively, with the difference between the two groups being statistically significant (P < 0.01).
DISCUSSION
We previously observed that serum HCV RNA titers varied considerably with different isolates of the same HCV subtype in Indonesia, such as HCV-1b, -1c, and -2a (18). In the present study, we analyzed amino acid sequences of NS5A of those HCV isolates in order to see whether or not there is any correlation between serum HCV titers and NS5A sequences, especially the sequence of the ISDR and PKR-binding region. Our results clearly demonstrated that with all subtypes tested, i.e., HCV-1b, -1c, and -2a, the high number of ISDR mutations was associated with low HCV viral load in patients without IFN treatment. (Fig. 3). Pretreatment viral load was reported to correlate with IFN responsiveness of HCV-infected patients (2, 3). It is reasonable, therefore, to assume that ISDR sequence analysis can predict IFN responsiveness of HCV-infected Indonesian patients, as has been reported with Japanese patients (2, 3, 6, 7, 16).
Despite the consistent observations by Japanese research groups that the number of amino acid substitutions in ISDR correlates well with sensitivity to IFN therapy in patients infected with HCV-1b or HCV-2a, conflicting observations were reported in that there was no significant correlation between ISDR mutations and IFN responsiveness in patients infected with HCV-1b in Europe and the United States (5, 11, 22, 32). Recently, Nakano et al. (21) pointed out sequence variation among HCV-1b isolates, based on which they divided the HCV-1b isolates into three groups, J, NJ, and W. The same authors concluded that correlation between ISDR mutations and IFN responsiveness was observed only with the J group, the group representing approximately 40% of HCV-1b isolates in Japan but rarely found in Europe and the United States. According to our sequence data published previously (12) (DDBJ accession no. D13729 to D13731, D13734, and D13735), two (40%) of five Indonesian HCV-1b isolates could be classified into the J group, with the remaining three (60%) being classified into an as-yet-undefined fourth group. This observation may also support the hypothesis that the ISDR sequence can be used to predict IFN responsiveness of some, if not all, Indonesian patients infected with HCV.
It is noteworthy that an HCV-1b isolate (isolate 378 [Fig. 2A]) had five mutations in the ISDR and a total of 11 mutations in the PKR-binding region and yet showed a high virus titer. It was recently reported that envelope glycoprotein E2 of HCV contains a sequence identical with phosphorylation sites of PKR and eIF-2α and that E2 inhibited the kinase activity of PKR, which might be one of the mechanisms counteracting antiviral activity of IFN (33). It is possible that the sequence, designated the PKR-eIF-2α phosphorylation homology domain (PePHD), of this particular HCV-1b isolate (isolate 378) is involved in maintaining the high level of viremia while apparently lacking the IFN-inhibiting function of the ISDR. On the other hand, we found a few isolates of HCV-2a with low virus titers, which had low numbers of ISDR mutations. It would be interesting to see whether or not those isolates had high numbers of mutations in PePHD of E2, as reported recently (29). Another possibility should also be taken into consideration, i.e., that there exists another viral mechanism regulating IFN sensitivity and viral load, such as quasispecies complexity of the HCV genome (10, 25) and an as-yet-undetermined viral factor(s) (21, 34). Further study is needed to elucidate the issue.
Previous study suggested a possible correlation between the presence of the arginine residue at position 2218 and sensitivity to IFN therapy in patients infected with HCV-1b (8). In the present study, however, no significant correlation was observed for HCV-1b or HCV-1c (Fig. 2). Consistent with our observation, the lack of such a correlation was reported by other investigators (22).
It was recently demonstrated that, upon adaptation to the Huh-7 human hepatoma cell line, NS5A underwent mutations at positions 2163, 2177, 2189, 2196, 2197, 2199, and 2204, which were clustered in a defined region just upstream of the ISDR, suggesting that those mutations were responsible for the higher degrees of HCV RNA replication in Huh-7 cells (1, 17). In the present study, however, irrespective of HCV viremia titers, all of those residues as well as the serine residue at position 2201 were completely conserved among the HCV isolates of each subtype tested (data not shown). It should also be noted that all but one (position 2163) are completely conserved even across different subtypes, including subtypes other than HCV-1b, -1c, and -2a. In this connection, Pawlotsky et al. (25) reported that serine residues at positions 2197, 2201, and 2204 showed remarkable conservation, suggesting the importance of NS5A phosphorylation at those residues. Taken together, these results imply the possibility that the defined region of NS5A just upstream of the ISDR, with certain serine residues being phosphorylated, plays a crucial role in HCV replication and that the viral adaptation mechanism differs in continuously growing Huh-7 cells and nongrowing, mature hepatocytes in the human liver.
In conclusion, we have demonstrated that virus load in patients infected with HCV-1b, HCV-1c, or HCV-2a correlates inversely with the number of mutations in the ISDR sequence of NS5A of HCV in patients in Indonesia. Our results imply the possibility that the ISDR sequence plays an important role in determining the levels of HCV viremia, through differentially inhibiting antiviral activity of endogenous IFN and/or through another mechanism(s) regulating HCV replication.
ACKNOWLEDGMENTS
We are grateful to Budi Arifah, Red Cross Blood Transfusion Center, Surabaya, Indonesia, for providing serum samples obtained from blood donors.
This work was carried out during the large-scale cooperative study between Southeast Asian countries and Japan conducted by the Japan Society for the Promotion of Science. This work was also supported in part by a grant-in-aid from the Ministry of Education, Science, Sports, and Culture, Japan, and a research grant from Mitsui Life Social Welfare Foundation.
REFERENCES
- 1.Blight K J, Kolykhalov A A, Rice C M. Efficient initiation of HCV RNA replication in cell culture. Science. 2000;290:1972–1974. doi: 10.1126/science.290.5498.1972. [DOI] [PubMed] [Google Scholar]
- 2.Chayama K, Tsubota A, Kobayashi M, Okamoto K, Hashimoto M, Miyano Y, Koike H, Kobayashi M, Koida I, Arase Y, Saitoh S, Suzuki Y, Murashima N, Ikeda K, Kumada H. Pretreatment virus load and multiple amino acid substitutions in the interferon sensitivity-determining region predict the outcome of interferon treatment in patients with chronic genotype 1b hepatitis C virus infection. Hepatology. 1997;25:745–749. doi: 10.1002/hep.510250342. [DOI] [PubMed] [Google Scholar]
- 3.Chayama K, Suzuki F, Tsubota A, Kobayashi M, Arase Y, Saitoh S, Suzuki Y, Murashima N, Ikeda K, Takahashi N, Kinoshita M, Kumada H. Association of amino acid sequence in the PKR-eIF2 phosphorylation homology domain and response to interferon therapy. Hepatology. 2000;32:1138–1144. doi: 10.1053/jhep.2000.19364. [DOI] [PubMed] [Google Scholar]
- 4.Doi H, Apichartpiyakul C, Ohba K, Mizokami M, Hotta H. Hepatitis C virus (HCV) subtype prevalence in Chiang Mai, Thailand, and identification of novel subtypes of HCV major type 6. J Clin Microbiol. 1996;34:569–574. doi: 10.1128/jcm.34.3.569-574.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duverlie G, Khorsi H, Castelain S, Jaillon O, Izopet J, Lunel F, Eb F, Penin F, Wychowski C. Sequence analysis of the NS5A protein of European hepatitis C virus 1b isolates and relation to interferon sensitivity. J Gen Virol. 1998;79:1373–1381. doi: 10.1099/0022-1317-79-6-1373. [DOI] [PubMed] [Google Scholar]
- 6.Enomoto N, Sakuma I, Asahina Y, Kurosaki M, Murakami T, Yamamoto C, Ogura Y, Izumi N, Marumo F, Sato C. Mutations in the nonstructural protein 5A gene and response to interferon in patients with chronic hepatitis C virus 1b infection. N Engl J Med. 1996;334:77–81. doi: 10.1056/NEJM199601113340203. [DOI] [PubMed] [Google Scholar]
- 7.Enomoto N, Sakuma I, Asahina Y, Kurosaki M, Murakami T, Yamamoto C, Izumi N, Marumo F, Sato C. Comparison of full-length sequences of interferon-sensitive and resistant hepatitis C virus 1b. Sensitivity to interferon is conferred by amino acid substitutions in the NS5A region. J Clin Investig. 1995;96:224–230. doi: 10.1172/JCI118025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frangeul L, Cresta P, Perrin M, Lunel F, Opolon P, Agut H, Huraux J-M. Mutations in NS5A region of hepatitis C virus genome correlate with presence of NS5A antibodies and response to interferon therapy for most common European hepatitis C virus genotypes. Hepatology. 1998;28:1674–1679. doi: 10.1002/hep.510280630. [DOI] [PubMed] [Google Scholar]
- 9.Gale M, Jr, Blakely C M, Kwieciszewski B, Tan S L, Dosset M, Tang N M, Korth M J, Polyak S J, Gretch D R, Katze M G. Control of PKR protein kinase by hepatitis C virus nonstructural protein 5A: molecular mechanisms of kinase regulation. Mol Cell Biol. 1998;18:5208–5218. doi: 10.1128/mcb.18.9.5208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gerotto M, Sullivan D G, Polyak S J, Chemello L, Cavalletto L, Pontisso P, Alberti A, Gretch D R. Effect of retreatment with interferon alone or interferon plus ribavirin on hepatitis C virus quasispecies diversification in nonresponder patients with chronic hepatitis C. J Virol. 1999;73:7241–7247. doi: 10.1128/jvi.73.9.7241-7247.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hofgärtner W T, Polyak S J, Sullivan D G, Carithers R L, Jr, Gretch D R. Mutations in the NS5A gene of hepatitis C virus in North American patients infected with HCV genotype 1a or 1b. J Med Virol. 1997;53:118–126. doi: 10.1002/(sici)1096-9071(199710)53:2<118::aid-jmv3>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 12.Hotta H, Doi H, Hayashi T, Purwanta M, Soemarto W, Mizokami M, Ohba K, Homma M. Analysis of the core and E1 envelope region sequences of a novel variant of hepatitis C virus obtained in Indonesia. Arch Virol. 1994;136:53–62. doi: 10.1007/BF01538816. [DOI] [PubMed] [Google Scholar]
- 13.Hotta H, Handajani R, Lusida M I, Soemarto W, Doi H, Miyajima H, Homma M. Subtype analysis of hepatitis C virus in Indonesia on the basis of NS5b region sequences. J Clin Microbiol. 1994;32:3049–3051. doi: 10.1128/jcm.32.12.3049-3051.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Houghton M. Hepatitis C viruses. In: Fields B N, Knipe D M, Howley P M, editors. Virology. 3rd ed. Vol. 1. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 1035–1058. [Google Scholar]
- 15.Kato N, Hijikata M, Ootsuyama Y, Nakagawa M, Ohkoshi S, Sugimura T, Shimotohno K. Molecular cloning of the human hepatitis C virus genome from Japanese patients with non-A, non-B hepatitis. Proc Natl Acad Sci USA. 1990;87:9524–9528. doi: 10.1073/pnas.87.24.9524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kurosaki M, Enomoto N, Murakami T, Sakuma I, Asahina Y, Yamamoto C, Ikeda T, Tozuka S, Izumi N, Marumo F, Sato C. Analysis of genotypes and amino acid residues 2209 to 2248 of the NS5A region of hepatitis C virus in relation to the response to interferon-β therapy. Hepatology. 1997;25:750–753. doi: 10.1002/hep.510250343. [DOI] [PubMed] [Google Scholar]
- 17.Lohmann V, Körner F, Dobierzewska A, Bartenschlager R. Mutations in hepatitis C virus RNAs conferring cell culture adaptation. J Virol. 2001;75:1437–1449. doi: 10.1128/JVI.75.3.1437-1449.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lusida M I, Soetjipto, Handajani R, Nidom C A, Soemarto, Darmadi S, Adi P, Fujii M, Fujita T, Ishido S, Hotta H. Viral load in Indonesian patients with chronic liver disease and in blood donors infected with different subtypes of hepatitis C virus. Jpn J Infect Dis. 2000;53:67–69. [PubMed] [Google Scholar]
- 19.Mellor J, Hawkins A, Simmonds P. Genotype dependence of hepatitis C virus load measurement in commercially available quantitative assays. J Clin Microbiol. 1999;37:2525–2532. doi: 10.1128/jcm.37.8.2525-2532.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murakami T, Enomoto N, Kurosaki M, Izumi N, Marumo F, Sato C. Mutations in nonstructural protein 5A gene and response to interferon in hepatitis C virus genotype 2 infection. Hepatology. 1999;30:1045–1053. doi: 10.1002/hep.510300405. [DOI] [PubMed] [Google Scholar]
- 21.Nakano I, Fukuda Y, Katano Y, Nakano S, Kumada T, Hayakawa T. Why is the interferon sensitivity-determining region (ISDR) system useful in Japan? J Hepatol. 1999;30:1014–1022. doi: 10.1016/s0168-8278(99)80254-2. [DOI] [PubMed] [Google Scholar]
- 22.Nousbaum J-B, Polyak S J, Ray S C, Sullivan D G, Larson A M, Carithers R L, Jr, Gretch D R. Prospective characterization of full-length hepatitis C virus NS5A quasispecies during induction and combination antiviral therapy. J Virol. 2000;74:9028–9038. doi: 10.1128/jvi.74.19.9028-9038.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Okamoto H, Kojima M, Sakamoto M, Iizuka H, Hadiwandowo S, Suwignyo S, Miyakawa Y, Mayumi M. The entire nucleotide sequence and classification of a hepatitis C virus isolate of a novel genotype from an Indonesian patient with chronic liver disease. J Gen Virol. 1994;75:629–635. doi: 10.1099/0022-1317-75-3-629. [DOI] [PubMed] [Google Scholar]
- 24.Okamoto H, Okada S, Sugiyama Y, Kurai K, Iizuka H, Machida A, Miyakawa Y, Mayumi M. Nucleotide sequence of the genomic RNA of hepatitis C virus isolated from a human carrier: comparison with reported isolates for conserved and divergent regions. J Gen Virol. 1991;72:2697–2704. doi: 10.1099/0022-1317-72-11-2697. [DOI] [PubMed] [Google Scholar]
- 25.Pawlotsky J-M, Germanidis G, Neumann A U, Pellerin M, Frainais P-O, Dhumeaux D. Interferon resistance of hepatitis C virus genotype 1b: relationship to nonstructural 5A gene quasispecies mutations. J Virol. 1998;72:2795–2805. doi: 10.1128/jvi.72.4.2795-2805.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Robertson B, Myers G, Howard C, Brettin T, Bukh J, Gaschen B, Gojobori T, Maertens G, Mizokami M, Nainan O, Netesov S, Nishioka K, Shin-i T, Simmonds P, Smith D, Stuyver L, Weiner A. Classification, nomenclature, and database development for hepatitis C virus (HCV) and related viruses: proposals for standardization. Arch Virol. 1998;143:2493–2503. doi: 10.1007/s007050050479. [DOI] [PubMed] [Google Scholar]
- 27.Sáiz J-C, López-Labrador F-X, Ampurdanés S, Dopazo J, Forns X, Sánchez-Tapias J-M, Rodés J. The prognostic relevance of the nonstructural 5A gene interferon sensitivity determining region is different in infections with genotype 1b and 3a isolates of hepatitis C virus. J Infect Dis. 1998;177:839–847. doi: 10.1086/515243. [DOI] [PubMed] [Google Scholar]
- 28.Sarrazin C, Berg T, Lee J-H, Teuber G, Dietrich C F, Roth W K, Zeuzem S. Improved correlation between multiple mutations within the NS5A region and virological response in European patients chronically infected with hepatitis C virus type 1b undergoing combination therapy. J Hepatol. 1999;30:1004–1013. doi: 10.1016/s0168-8278(99)80253-0. [DOI] [PubMed] [Google Scholar]
- 29.Sarrazin C, Kornetzky I, Ruster B, Lee J-H, Kronenberger B, Bruch K, Roth W K, Zeuzem S. Mutations within the E2 and NS5A protein in patients infected with hepatitis C virus type 3a and correlation with treatment response. Hepatology. 2000;31:1360–1370. doi: 10.1053/jhep.2000.7987. [DOI] [PubMed] [Google Scholar]
- 30.Simmonds P, Holmes E C, Cha T-A, Chan S-W, McOmish F, Irvine B, Beall E, Yap P L, Kolberg J, Urdea M S. Classification of hepatitis C virus into six major genotypes and a series of subtypes by phylogenetic analysis of the NS-5 region. J Gen Virol. 1993;74:2391–2399. doi: 10.1099/0022-1317-74-11-2391. [DOI] [PubMed] [Google Scholar]
- 31.Soetjipto, Handajani R, Lusida M I, Darmadi S, Adi P, Soemarto, Ishido S, Katayama Y, Hotta H. Differential prevalence of hepatitis C virus subtypes in healthy blood donors, patients on maintenance hemodialysis, and patients with hepatocellular carcinoma in Surabaya, Indonesia. J Clin Microbiol. 1996;34:2875–2880. doi: 10.1128/jcm.34.12.2875-2880.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Squadrito G, Orlando M E, Cacciola I, Rumi M G, Artini M, Picciotto A, Loiacono O, Siciliano R, Levrero M, Raimondo G. Long-term response to interferon alpha is unrelated to “interferon sensitivity determining region” variability in patients with chronic hepatitis C virus-1b infection. J Hepatol. 1999;30:1023–1027. doi: 10.1016/s0168-8278(99)80255-4. [DOI] [PubMed] [Google Scholar]
- 33.Taylor D R, Shi S T, Romano P R, Barber G N, Lai M M C. Inhibition of the interferon-inducible protein kinase PKR by HCV E2 protein. Science. 1999;285:107–110. doi: 10.1126/science.285.5424.107. [DOI] [PubMed] [Google Scholar]
- 34.Terazawa Y, Yoshioka K, Kobayashi M, Watanabe K, Ishigami M, Yano M, Takagi K, Kakumu S. Mutations in interferon sensitivity-determining region of hepatitis C virus: its relation to change in viral load. Am J Gastroenterol. 2000;95:1781–1787. doi: 10.1111/j.1572-0241.2000.02176.x. [DOI] [PubMed] [Google Scholar]