SUMMARY
Dietary restriction (DR) has long been viewed as the most robust nongenetic means to extend lifespan and healthspan. Many aging-associated mechanisms are nutrient responsive, but despite the ubiquitous functions of these pathways, the benefits of DR often vary among individuals and even among tissues within an individual, challenging the aging research field. Furthermore, it is often assumed that lifespan interventions like DR will also extend healthspan, which is thus often ignored in aging studies. In this review, we provide an overview of DR as an intervention and discuss the mechanisms by which it affects lifespan and various healthspan measures. We also review studies that demonstrate exceptions to the standing paradigm of DR being beneficial, thus raising new questions that future studies must address. We detail critical factors for the proposed field of precision nutrigeroscience, which would utilize individualized treatments and predict outcomes using biomarkers based on genotype, sex, tissue, and age.
INTRODUCTION
In 1935, Dr. Clive McCay showed that dietary restriction (DR) in the form of calorie reduction (CR) without malnutrition prolongs mean and maximal lifespan in rats compared with those allowed to eat freely. Subsequent experiments in mice showed that ~40% of normal calorie consumption results in ~50% extension in lifespan (McCay, 1939). The potential of CR to extend lifespan has created a large subfield of aging research that promises a simple but elegant method to enhance lifespan and healthspan, the period of life in which an individual is healthy, with minimal side effects. CR is now known to extend lifespan in nearly thirty species, including nonhuman primates, and remains the most robust way to extend lifespan in the laboratory. In humans, a two-year clinical study on the effects of chronic, moderate CR showed a slowing in metabolism and decreased production of free radicals (Redman et al., 2018). Though this study indicated that changes induced by CR reduced markers of aging, the long-term effects of CR in humans remain unproven.
Distinct from CR, DR encompasses all dietary interventions that restrict intake of specific nutritional components. Thus, DR incorporates CR as well as restriction of macronutrients such as protein or amino acid residues (e.g., methionine and tryptophan) or glucose, and may also include varying fasting intervals (Table 1). Despite varying DR protocols, almost all dietary interventions have been reported to influence lifespan in at least one model organism (Table 2) and largely influence similar cellular pathways. However, differences in methodologies used to induce DR within and between species have led to disparities in cross-study comparisons. Given the variation across populations, individuals, and tissues, it is reasonable to expect an array of responses to different regimens. Further, the means of assessing aging vary greatly across studies, adding to difficulties in assessing how DR benefits longevity and health. Despite these variations in methodologies and responses, the knowledge of pathways that mediate nutrient signaling remains critical to help translate findings to humans. Precision nutrition has recently been proposed as a means to mediate health by creating personalized dietary plans (Rodgers and Collins, 2020), and a multitude of companies have aimed to maximize lifespan by precision nutrition but have not incorporated omics-based signatures to maximize individual effects. We propose that developing an individualized plan, which analyzes specific age-related biomarkers of an individual to optimize diet-based therapeutics to slow age-related biological processes, will give rise to a new form of precision-nutrition medicine, which we term “precision nutrigeroscience.” This field of study will incorporate diet-based therapeutics that are specific to an individual based on a panel of genotype-, diet-, age-, sex-, and health-specific biomarkers and will elucidate specific, individualized regimens to maximize lifespan and healthspan. Here, we detail the conserved mechanisms of DR but then delve into the complexities that influence individual responses to DR, which necessitates us to develop a framework for precision nutrigeroscience.
Table 1.
Types of dietary restrictions
| Dietary intervention | Description |
|---|---|
| Caloric restriction | Reduced caloric intake (20%–30% below average) without undergoing malnutrition during the entire period of dietary intervention. |
| Intermittent fasting | The alternate pattern of ad libitum food-intake-encompassing regimes that may include alternate-day fasting (ADF), modified ADF (limited calories supplied during fasting day), 5:2 diet (days of caloric restriction per week), or daily time-restricted feeding. |
| Fasting-mimicking diet | Four days of diet that mimics fasting (FMD) consisting of very low calorie/low protein. The ad libitum diet is fed between the period of FMD cycles. |
| Glucose and carbohydrate restriction | Carbohydrate consumption is restricted relative to the average diet and is replaced by food containing a higher percentage of fat and protein. aGlucose restriction refers to specific restriction of glucose intake instead of other forms of complex carbohydrates. |
| Protein restriction | Reduction of dietary protein intake without changing the average caloric intake. |
| Amino acid restriction | Specific restriction of amino acids that commonly include threonine, histidine, lysine, methionine, and branched-chain amino acids (BCAAs). BCAAs have an aliphatic side chain, which is a carbon atom bound to at least two other carbon atoms.The three most common BCAAs are leucine, isoleucine, and valine. |
| Micronutrient restriction | Reduced intake of vitamins and minerals.Common micronutrients whose levels have been reduced by chemical chelation or inhibiting import are calcium, iron, zinc, phosphorus, and potassium. |
| Metabolite restriction | Reduction or inhibiting biosynthesis of specific reaction intermediates or end products of physiological metabolism. Most commonly: N-acylethanolamines, folate metabolism intermediates, polyunsaturated fatty acid metabolism intermediates, tricarboxylic cycle intermediates, and co-enzyme Q. |
Very-low-carbohydrate diet (<10%), low-carbohydrate diet (<26%), moderate-carbohydrate diet (<26%–44%); values based on human studies (Feinman et al., 2015)
Table 2.
Summary of dietary restriction (DR) regimens that promote lifespan
| Intervention | Species | Effects | Specified pathways | References |
|---|---|---|---|---|
| Total caloric restriction | yeast | ↑ chronological lifespan, ↑ stress resistance | SCH9, Msn2/Msn4 | Fabrizio et al., 2001 |
| ↑ replicative lifespan | TOR, SCH9 | Kaeberlein et al., 2005 | ||
| ↑ replicative lifespan | SIR2, NAD | Lin et al., 2000 | ||
| ↑ mitochondrial respiration, ↑ autophagy, ↓ translation | – | Kapahi et al., 2017 | ||
| worm | ↑ autophagy | PHA-4, TOR | Hansen et al., 2008; Kapahi et al., 2017 | |
| ↑ ER-UPR | – | Matai et al., 2019 | ||
| ↑ NMD and alternative splicing | – | Heintz et al., 2017; Rollins et al., 2019; Tabrez et al., 2017 | ||
| ↑ PUFA and cellular detoxification | MAPK | Chamoli et al., 2014, 2020; Heestand et al., 2013 | ||
| ↑ mitochondrial network and peroxisome remodeling | AMPK | Weir et al., 2017 | ||
| ↑ innate immunity | MAPK | Wu et al., 2019 | ||
| ↑ lifespan, ↓ ER stress | HIF1α | Chen et al., 2009 | ||
| fly | ↓ protein synthesis, ↑ stress resistance, ↑ lifespan | TOR, SIR2, FOXO | Kapahi et al., 2017 | |
| ↑ lifespan, ↑ fat metabolism | circadian clocks, timeless (tim) and period (per) | Katewa et al., 2016 | ||
| ↑ lifespan | olfaction pathway | Libert et al., 2007 | ||
| ↑ lifespan, ↑ mitochondrial function | 4E-BP | Zid et al., 2009 | ||
| mouse | ↑ lifespan (40% DR) | – | Blackwell et al., 1995 | |
| ↑ lifespan (30% DR) | neuropeptide Y | Chiba et al., 2014 | ||
| ↑ lifespan (40% DR) | SIRT1 | Mercken et al., 2014 | ||
| ↑ lifespan (30% DR) | BMAL1 and IGF1 | Patel et al., 2016a | ||
| ↑ lifespan (30% DR) | FOXO3 | Shimokawa et al., 2015 | ||
| ↑ lifespan (average and maximum) | – | Swindell, 2012; Weindruch and Sohal, 1997 | ||
| IF, EOD, PF, FMD | yeast | PF: ↑ median and maximum chronological lifespan | oxidative stress resistance | Brandhorst et al., 2015 |
| worm | IF: ↑ lifespan | RHEB-1, IGF, DAF-16 | Honjoh et al., 2009 | |
| fly | ↑ improved gut health | TOR independent | Catterson et al., 2018 | |
| mouse | EOD: ↑ lifespan by delay in life-limiting neoplastic disorders | – | Xie et al., 2017 | |
| periodic FMD: ↑ median lifespan, ↑ rejuvenated immune system, ↓ visceral fat, ↓ cancer incidence, ↓ skin lesions, ↓ bone mineral density loss | IGF-1, PKA | Brandhorst et al., 2015 | ||
| daily fasting: ↑ health, ↑ lifespan | – | Mitchell et al., 2019 | ||
| Glucose and carbohydrate restriction | yeast | ↑ replicative lifespan | NAD, SIR2 | Lin et al., 2000 |
| ↑ replicative lifespan, ↓ transcription and translation of methionine metabolism | – | Zou et al., 2020 | ||
| ↓ protein synthesis, ↑ replicative lifespan | GCN4 | Mittal et al., 2017 | ||
| ↑ replicative lifespan | 60S ribosomal subunit, GCN4 | Steffen et al., 2008 | ||
| worm | ↑ mitochondrial respiration | aak-2 | Schulz et al., 2007 | |
| fly | ↑ maximum lifespan, no change in mean lifespan | – | Troen et al., 2007 | |
| asparagine and glutamate | ||||
| restriction: ↑ chronological lifespan | Msn2, Msn4 | Powers et al., 2006 | ||
| methionine restriction and low amino acid | ||||
| status: ↑ replicative lifespan | TOR | Lee et al., 2014 | ||
| methionine restriction: ↑ autophagy-dependent vacuolar | ||||
| acidification, ↑ chronological lifespan | – | Ruckenstuhl et al., 2014 | ||
| methionine restriction and glutamic acid | ||||
| yeast | addition: ↑ chronological lifespan | – | Wu et al., 2013 | |
| worm | ↓ protein synthesis | TOR | Pan et al., 2007 | |
| fly | low-protein/high-carbohydrate diet (1:16), with constant calorie: ↑ lifespan | TOR | Jensen et al., 2015 | |
| ↑ lifespan, ↑ fatty acid synthesis and breakdown | AKH | Katewa et al., 2012 | ||
| low-protein/high-carbohydrate diet (1:16): ↑ lifespan | – | Lee et al., 2008 | ||
| developmental yeast restriction: ↑ lifespan | – | Stefana et al., 2017 | ||
| low-protein/high-carbohydrate diet (1:21), with constant calorie: ↑ lifespan | – | Fanson et al., 2009 | ||
| Protein/yeast restriction | mouse | low-protein/high-carbohydrate diet: ↑ hepatic mTORC1 | BCAA and glucose metabolism | Solon-Biet et al., 2014 |
| EAA or BCAA restriction | worm | dietary sulfur amino acid restriction: ↑ DICER in intestine | – | Guerra et al., 2019 |
| fly | BCAAs/threonine, histidine, and lysine (THK) diet: ↑ lifespan, ↓ age-related intestinal pathology | – | Juricic et al., 2020 | |
| balanced EAA level diet: ↑ lifespan | – | Grandison et al., 2009a | ||
| ↑ trans-sulfuration pathway | – | Kabil et al., 2011 | ||
| methionine restriction and low amino acid status: ↑ lifespan | TOR | Lee et al., 2014 | ||
| ↑ lifespan, ↑ regulation of ISCs and gut health, ↓ age-related gut pathology | SESTRIN | Lu et al., 2021 | ||
| mouse | methionine restriction: ↑ liver mRNA for macrophage migration inhibition factor, no change in lifespan in GH-deficient mice, ↓ serum IGF-I, insulin, glucose, and thyroid hormone | – | Miller et al., 2005 | |
| methionine restriction (0.16%): ↑ lifespan | GH | Brown-Borg et al., 2014 | ||
| lifelong restriction of BCAAs: ↑ lifespan in males, ↑ healthspan, ↓ frailty | TOR signaling in liver | Richardson et al., 2021 | ||
| EAA/non-EAA balance: ↑ lifespan, ↓ DR-induced muscle weakness, ↑ renoprotection | – | Yoshida et al., 2018 | ||
| rat | methionine restriction: ↑ mean and maximum lifespan | – | Zimmerman et al., 2003 | |
| tryptophan restriction: ↑ maximum lifespan | – | Ooka et al., 1988 | ||
| methionine restriction: ↑ lifespan of male | – | Orentreich et al., 1993 | ||
| methionine restriction: ↑ mean and maximum lifespan | GSH | Richie et al., 1994 | ||
| methionine restriction: ↑ maximum lifespan, ↓ mitochondrial ROS, ↓ oxidative damage to mitochondrial DNA and protein | – | Sanz et al., 2006 | ||
| Micronutrient/metabolite restriction | yeast | ↑ lifespan, ↓ K levels | vacuolar acidity | Sasikumar et al., 2019 |
| worm | ↑ lifespan, ↓ Fe levels | proteostasis | Klang et al., 2014 | |
| ↑ lifespan, ↓ Zn levels | DAF-16 | Kumar et al., 2016 | ||
| ↑ lifespan, ↓ co-enzyme Q | – | Larsen and Clarke, 2002 | ||
| ↑ lifespan, ↓ N-acylethano-lamine levels | PHA-4 | Lucanic et al., 2011 | ||
| ↑ lifespan, ↓ folate in HT115 3-dehydroquinate dehydratase | – | Virk et al., 2016 | ||
| fly | ↓ adenosine nucleotide biosynthesis, ↑ lifespan | AMPK | Stenesen et al., 2013 | |
| phosphate restriction: ↑ lifespan | – | Bergwitz, 2012 |
4E-BP, eukaryotic translation initiation factor 4E-binding protein (4E-BP1 ortholog); aak-2, AMP-activated kinase 2 (AMPK ortholog); AKH, adipokinetic hormone; AMPK, adenosine monophosphate-activated protein kinase; BCAA, branched-chain amino acid; BMAL, brain and muscle ARNT-like 1; CR, caloric restriction; DAF-2, abnormal dauer formation 2 (IR/IGFR ortholog); DAF-16, abnormal dauer formation 16 (FOXO ortholog); EAA, essential amino acid; EOD, every-other-day fasting; ER, endoplasmic restriction; Fe, iron; FMD, fasting-mimicking diet; FOXO3, forkhead box O3; GCN4, general control nondepressible 4; GH, growth hormone; GSH, glutathione; HIF1α, hypoxia inducible factor 1 alpha; IF, intermittent fasting; IGF-1, insulin-like growth factor 1; IIS, insulin/IGF-1 signaling; ISCs, intestinal stem cells; JNK, c-Jun N-terminal kinase; K, potassium; MAPK, mitogen-activated protein kinase; Msn2/4, multiple suppressor of SNF1 mutation 2/4; NAD, nicotinamide adenine dinucleotide; NPY, neuropeptide Y; PF, periodic fasting; PHA-4, defective pharynx development 4 (FOXA ortholog); PKA, protein kinase A; RHEB-1, Ras homolog enriched in brain (RHEB ortholog); ROS, reactive oxygen species; Sch9, serine/threonine protein kinase (S6 kinase ortholog); SIR2, silent information regulator 2 (SIRT1 ortholog); SIRT1, sirtuin 1; TOR, target of rapamycin (mTOR ortholog); UPR, unfolded protein response; Zn, zinc.
MOLECULAR MECHANISMS UNDERLYING DR
The conserved mechanisms that are generally considered universally responsible for the effects of DR on lifespan can generally be grouped into three categories (Table 2; Figure 1): (1) nutrient-sensing mechanisms, including sensory pathways that help assess an organism’s energy status; (2) DR-response regulators, including metabolic reprogramming mechanisms, which facilitate maintenance of alternative physiological states; and (3) effector mechanisms that mediate the downstream effects of DR to improve homeostasis.
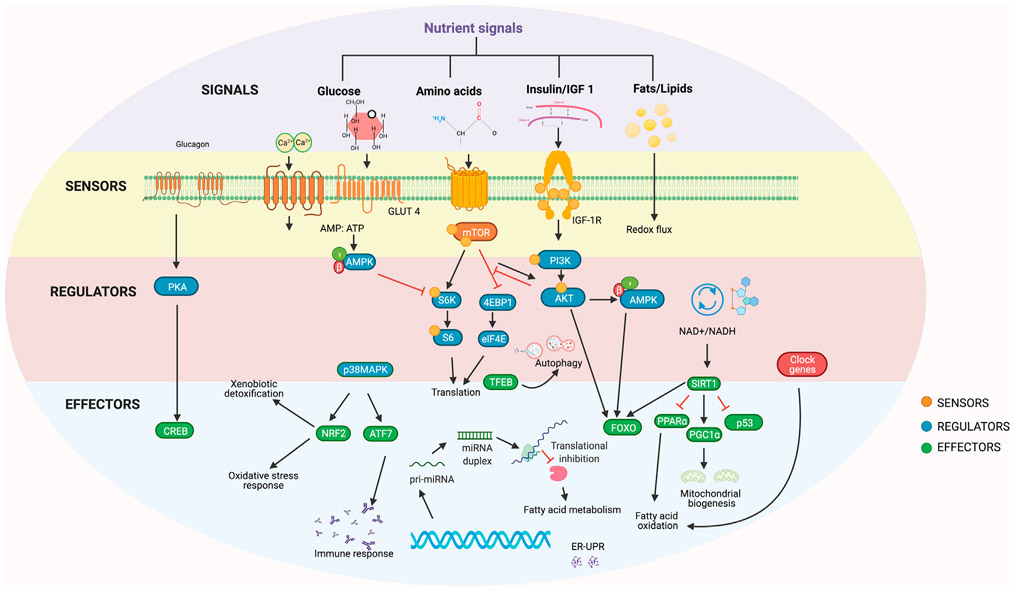
Figure 1. Biochemical mechanisms of dietary restriction.
Nutrient signals (purple) are received by sensors at the cell membrane. These sensors (yellow) activate regulators (red) of diet-responsive mechanisms, which in turn regulate the effectors (blue) of stress response, cell growth, and metabolism.
Nutrient-sensing mechanisms
IGF-1-like signaling, TOR, and AMPK
Nutrients are central to an organism’s growth and reproduction. The evolutionary theory of aging suggests that when nutrient signaling is reduced, cellular growth and reproduction are halted and organisms switch to reducing damage and enhancing repair, which increases lifespan (Holliday, 1989); this theory has been experimentally demonstrated (Chen et al., 2007; Fanson et al., 2009). It is hypothesized that response to DR evolved as an adaptation to promote survival under nutrient-limiting conditions; however, a multitude of other hypotheses have also been proposed, such as that DR causes substantial biological remodeling which coincidentally extends lifespan and healthspan (Speakman, 2020), that DR is simply a laboratory artifact (Adler and Bonduriansky, 2014), or that the biological processes regulated by DR are too complex to point to specific mechanisms or pathways (Regan et al., 2020). These ideas are consistent with the finding that the inhibition of three major nutrient-sensing pathways (insulin/IGF-1-like signaling [IIS], target of rapamycin kinase [TOR], and AMP-kinase signaling) extends lifespan in multiple species ranging from yeast to mammals (Fontana and Partridge, 2015). In vertebrates, the IIS pathway responds to glucose with insulin secretion, which in turn binds to IIS receptors and regulates glucose levels by stimulating glucose uptake into muscle and fat and gluconeogenesis in the liver (Wilcox, 2005). The mTOR kinases comprise two distinct complexes, mTORC1 and mTORC2, which respond to extracellular and intracellular nutrient signals including amino acids, growth factors, stress signals, and oxygen to regulate growth and survival as well as protein and lipid synthesis (Papadopoli et al., 2019). Reduced mTOR activity extends lifespan in flies, worms, and yeast, which is not further extended by DR (Kapahi et al., 2004, 2010; Kauwe et al., 2016; Nicks et al., 2014). However, neither genetic modification of the TOR pathway nor treatment of rapamycin in Ercc1Δ/− mice is capable of granting lifespan extension similarly to DR in mice, suggesting that additional DR-mediated pathways are involved in this extension by DR (Birkisdóttir et al., 2021). AMP-activated protein kinase (AMPK) is a nutrient sensor molecule that is activated in response to a high AMP:ATP ratio, which reflects a cell’s energy status (Hardie et al., 2012). AMPK activation extends lifespan in multiple species (Fontana and Partridge, 2015; Greer et al., 2009; Kapahi et al., 2017). As an immediate response to low cellular energy levels, AMPK turns on catabolic pathways like glycolysis and fatty acid oxidation to replenish ATP levels (Kapahi et al., 2017; Lin and Hardie, 2018; Saxton and Sabatini, 2017). The complexity of this conserved nutrient-response pathway and the interplay between its components provide specific targets necessary to consider for precision nutrigeroscience.
Metabolic reprogramming regulators
Forkhead box O transcription factor (FOXO)
FOXO is a downstream effector of the IIS pathway that regulates metabolic homeostasis, stress response, cellular proliferation, and development (Calissi et al., 2021). The differential regulation of FOXO target genes and the pathways in response to varying environmental stimuli makes it a critical regulator of aging, but its role in DR is surprisingly not observed ubiquitously. In C. elegans, daf-16/FOXO is essential for some (but not all) DR protocols, including DR on solid-media growth plates and peptone dilutions (Greer and Brunet, 2009). Although not essential for DR-mediated lifespan, overexpression of dFOXO in the adult fat body in flies modulates DR response (Giannakou et al., 2008). In yeast, DR fails to extend the lifespan of FKH1 and FKH2 (FOXO orthologs) mutants (Postnikoff et al., 2012). In mammals, FOXO1’s mRNA levels and targets are elevated in skeletal muscles and the liver, while FOXO4 is upregulated in adipose tissue and skeletal muscles in mice subjected to DR (Furuyama et al., 2002; Kim et al., 2014; Miyauchi et al., 2019; Yamaza et al., 2010). Foxo3+/− and Foxo3−/− mice fail to show lifespan extension upon DR (Shimokawa et al., 2015). As such, some of DR’s beneficial effects on health and diseases were shown to be dependent on FOXO (Miyauchi et al., 2019; Yamaza et al., 2010). FOXO is also modulated by AMPK, sirtuins, and mTOR status (Greer et al., 2009). AMPK activation also directly phosphorylates and regulates FOXO3 transcriptional activity in mammalian cell cultures without affecting its nuclear localization (Greer et al., 2007b). Although the requirement of FOXO in regulating DR-mediated lifespan extension is well established, we still lack understanding of how FOXO regulates the gene-expression program essential for survival during DR. More importantly, identifying factors like post-translational modifications and protein partners, which regulate how FOXO signaling integrates signals originating from different environmental stimuli in vivo will be critical for understanding its role in DR. This necessitates more context-specific analysis of the role of FOXO.
Sirtuins and NAD
Sirtuins function as protein deacylases and are dependent on the levels of cellular co-enzyme nicotinamide adenine dinucleotide (NAD+), a key currency of metabolism (Choudhary et al., 2014). Sir2 has been shown to modulate the DR-mediated lifespan extension in yeast (Kennedy et al., 1995; Lin et al., 2000), but in other species its role remains a matter of debate (Kaeberlein and Powers, 2007). However, Sir2 overexpression confers health benefits in other organisms including worms, flies, and mice (Chen et al., 2005; Lin et al., 2000; Tissenbaum and Guarente, 2001; Wood et al., 2004). In both worms and mammalian cells, Sir2 directly regulates transcriptional activity of DAF-16/FOXO by deacetylation, which activates FOXO-dependent transcription of stress-responsive genes and extends lifespan (Berdichevsky et al., 2006; Brunet et al., 2004). In hepatocytes, sirtuin activation increases nuclear translocation of FOXO1, leading to enhanced expression of FOXO1 target genes, including those relating to gluconeogenesis and glucose release (Frescas et al., 2005). In mice, deacetylation by hepatic Sir2/SIRT1 also regulates activity of transcription factor co-activator PGC-1α, which has emerged as a master regulator of mitochondrial biogenesis and function (Austin and St-Pierre, 2012). PGC-1α is prone to deacetylation and activation by SIRT1 when it is phosphorylated by AMPK at Thr177 and Ser538 (Cantó et al., 2009). However, other nuclear receptors and transcriptions also interact with and may recruit PGC-1α to the promoters of target genes that execute its metabolic effects, such as mitochondrial function and lipid oxidation in response to changes in nutrient fluctuation (Austin and St-Pierre, 2012).
NAD serves as a critical co-enzyme for redox reactions and as a co-factor for nonredox NAD+-dependent enzymes, including sirtuins, poly(ADP-ribose) polymerases (PARPs), and CD38 (Covarrubias et al., 2021). NAD+ levels decrease with age and multiple disorders, including metabolic diseases (Covarrubias et al., 2021). Thus, interventions boosting NAD+ levels have promising therapeutic potential in aging and age-related disease (Katsyuba et al., 2020). In this regard, DR is particularly beneficial as it increases NAD+ levels (Ramsey et al., 2009).
Circadian clock regulators
The circadian clock is an internal time-keeping system that helps maintain a 24-h rhythmic oscillation in behavior, physiology, and metabolism and is present across animals of different taxa (Green et al., 2008). The rhythmic activity of clock molecules regulates the cyclic transcription of many genes, which in turn synchronizes different cellular processes to environmental stimuli such as light. Deregulation of circadian rhythms is a major factor in aging and disease (Mattis and Sehgal, 2016). In addition to light, clock genes can derive cues from diet. DR enhances the amplitude of clock-gene mRNA expression in mice and fly models (Katewa et al., 2016; Patel et al., 2016b). Evidently, flies lacking core clock genes timeless (tim) and period (per) fail to show lifespan extension upon DR (Katewa et al., 2016). In mice, the clock transcription factor BMAL1 helps reduce blood IGF-1 levels during DR, and its absence prevents DR-mediated lifespan extension (Patel et al., 2016b). The role of clock genes in DR is an emerging area; however, how they couple with nutrient-response pathways remains to be understood. Mediators of DR, including SIRT1 and AMPK, also interact with circadian factors including BMAL1 (Lee and Kim, 2013). Studying the regulation of circadian clock genes in response to nutrient signals is challenging and requires extensive experimental analysis. Although few studies in plasma, muscle biopsies, and subcutaneous adipose tissue have been conducted (Grant et al., 2019), detailed sequential circadian transcriptomic and metabolomic analyses in several other human tissues are necessary to identify signatures of circadian metabolic function. Identifying signals and pathways that govern the variations in rhythmic molecules within individuals and how they impact health is essential for translational application of individualized circadian biology.
Non-coding RNAs
A significant proportion (~70%) of the human genome encodes non-coding RNAs (ncRNAs), which lack protein-coding potential but regulate transcription, stability, or translation of protein-coding genes (Bartel, 2009). MicroRNAs (miRNAs) are the most well studied of these ncRNAs and play central roles in metabolic pathways involved in nutrient sensing and aging (Victoria et al., 2017). Several miRNA families have been implicated in targeting mRNAs that code for components of the major nutrient-sensing pathways linked to aging, including insulin-IGF (Mariño et al., 2010; Zhu et al., 2011), mTOR (Dubinsky et al., 2014; Rubie et al., 2016), AMPK (Godlewski et al., 2010), and sirtuins (Lee et al., 2010; Menghini et al., 2009; Sharma et al., 2013). However, the relevance of the miRNA-mediated networks in promoting lifespan has not been evaluated at an organismal level for many of these miRNAs. Inappropriate expression of miRNAs and their processing factors has also been linked to many late-onset diseases and age-related functional decline (Chawla et al., 2016; Dimmeler and Nicotera, 2013; Liu et al., 2012). Additionally, the ability of miRNAs to circulate in body fluids (e.g., blood plasma, urine, tears, saliva, cerebrospinal fluid) as RNAse-resistant entities, either in extracellular vesicles or in vesicle-free form, highlights the utility of these biomolecules as noninvasive biomarkers for diagnosis and monitoring of aging and age-related diseases (Dhahbi, 2014; Turchinovich et al., 2012). In addition to specific miRNA biogenesis factors, factors that alter the access to the target site, sequence variants and single nucleotide polymorphisms in miRNAs or complementary recognition sequence may lead to creation, destruction, or alteration in the affinity of the miRNA-mRNA interaction (Sethupathy and Collins, 2008; Zorc et al., 2012). Hence, a better understanding of the role of miR-polymorphisms in response to different dietary interventions may aid in the development of population-specific or personalized nutrition-based strategies that are potentially safer. Several profiling studies have identified miRNA signatures that can be extensively correlated with lifespan interventions (Csiszar et al., 2014; Dhahbi, 2014; Masternak et al., 2004; Mercken et al., 2013; Mori et al., 2014; Pandit et al., 2014; Victoria et al., 2015) (Table 4). Emerging evidence also suggests that modulating miRNAs and their relevant targets influences lifespan (Pandey et al., 2021; Vora et al., 2013).
Table 4.
Summary of molecular biomarkers of aging that have been used in dietary restriction studies
| Biomarker | Model | Biological process | Biomarker criteria met |
Trend with DR |
Cells/tissues | References |
|---|---|---|---|---|---|---|
| DNA methylation | m, rm, h | epigenetic clocks | I, II, III, IV | ↑ | whole blood | Maegawa et al., 2017 |
| γH2A.X | h | genomic integrity | I, II, IV | ↓ | PBMCs | de Groot et al., 2015 |
| immunohistochemistry | m | lung | Li et al., 2020 | |||
| F2-isoprostanes | h | oxidative stress | I, II, III | ↓ | urine | Il’yasova et al., 2018 |
| miRNAs | r | post-transcriptional regulation of gene expression | I, II | ↑ | brain | Wood et al., 2015 |
| rm | I, II, III | ↑↓ | plasma | Schneider et al., 2017 | ||
| rm | I, II | ↑↓ | skeletal muscle | Mercken et al., 2013 | ||
| m | I, II | ↑ | adipose tissue | Mori et al., 2012 | ||
| m | I, II | ↓ | brain | Khanna et al., 2011 | ||
| M | I, II | ↑ | liver | Makwana et al., 2017; Zhang et al., 2019 | ||
| m | I, II | ↑↓ | serum | Dhahbi et al., 2013 | ||
| Dm | I, II | ↑ | brain and fat tissue | Pandey et al., 2021 | ||
| Ce | I, II | ↓ | intestine | Vora et al., 2013 | ||
| Cortisol (glucocorticoid) | h | inflammation | I, II, III | ↑ | serum | Yang et al., 2016 |
| mTOR | r | nutrient sensing | I, II | ↓ | skeletal muscle | Chen et al., 2019 |
| SIRT1 | m | nutrient sensing | I, II, IV | ↑ | brain | Wahl et al., 2018 |
| h | muscle | Civitarese et al., 2007 | ||||
| IL-10 | m, h | immune signaling | I, II, IV | ↑ | hippocampus | Wahl et al., 2018 |
| h | serum | Jung et al., 2008 | ||||
| p16INK4A | m, r | cell senescence | I, II | ↓ | kidney | Krishnamurthy et al., 2004 |
| h | WI-38, IMR-90, and MRC-5 | Li and Tollefsbol, 2011 | ||||
| IL-6 | h | inflammation | I, II, III | ↓ | serum | Lettieri-Barbato et al., 2016; Reed et al., 2010 |
| Adiponectin/leptin | h | lipid metabolism | I, II, III | ↑ | serum | Lettieri-Barbato et al., 2016 |
| Dicer | m | miRNA processing | I, II | ↑ | preadipocytes | Mori et al., 2012 |
| c | intestine | Guerra et al., 2019 | ||||
| Protein carbonyls | h | oxidative stress | I, II, III, IV | ↓ | plasma | Meydani et al., 2011 |
| Protein carbonyls, ROS | m | I, II, IV | cerebral hemispheres | Dkhar and Sharma, 2014 | ||
| Protein carbonyls | brain | Forster et al., 2000 | ||||
| Insulin | h | insulin signaling | I, II, III, IV | ↓ | serum | Heilbronn et al., 2006 |
| h | plasma | Fontana et al., 2004 | ||||
| Insulin/IGF-I | h | serum | Lettieri-Barbato et al., 2016 | |||
| m | plasma | Sonntag et al., 1999 | ||||
| 8OHdG | m | oxidative stress | I, II | ↓ | skeletal muscle, brain, heart, kidney, liver | Donati et al., 2013; Ke et al., 2020; Sohal et al., 1994 |
| 8OHdG | r | oxidative stress | I, II, III | ↓ | urine | Donati et al., 2013 |
| DNA-PK | m | genomic integrity | I, II | ↑ | skin, brain | Ke et al., 2020 |
| LC3, HSP70, Beclin | h | autophagy, stress response | I, II, IV | ↑ | skeletal muscle | Yang et al., 2016 |
| LC3 | m | autophagy | SVF cells | Ghosh et al., 2016 | ||
| SA-β-gal | r | cellular senescence | I, II | ↓ | colon, heart | Fontana et al., 2018a, 2018b; Shinmura et al., 2011 |
| SA-β-gal | m, r | kidney | Krishnamurthy et al., 2004 | |||
| NF-κB | h | inflammation | I, II, IV | ↓ | skeletal muscle | Yang et al., 2016 |
| p-NF-κB | m | kidney | Mulrooney et al., 2011 | |||
| NF-κB | r | kidney | Xu et al., 2015b; Zhang et al., 2016a |
m, mouse; r, rat; rm, rhesus monkey; h, human; dm, Drosophila melanogaster; ce, C. elegans criterion I, an aging biomarker must predict the rate of aging; criterion II, an aging biomarker must monitor a basic process that underlies the aging process; criterion III, an aging biomarker must be able to be tested repeatedly without harming the person; criterion IV, an aging biomarker must be something that works in humans and in laboratory animals; PBMCs, peripheral blood mononuclear cells; mTOR, mechanistic target of rapamycin; SIRT1, sirtuin (silent mating type information regulation 2 homolog) 1; IL-10, interleukin 10; IL-6, interleukin 6; ROS, reactive oxygen species; 8OHdG, 8-hydroxy-2-deoxyguanosine; DNA-PK, DNA-dependent protein kinase; LC3, microtubule-associated proteins 1A/1B light chain 3B; HSP70, 70-kDa heat shock protein; SVF, adipose-tissue-derived stromal vascular fraction cells; SA-β-gal, senescence-associated beta galactosidase; NF-κB, nuclear factor kappa B; p-NF-κB, phosphorylated nuclear factor kappa B.
While the modulation of nutrient-responsive pathways and their regulators by nutrient restriction is well established, future research efforts focused on identification and validation of functional polymorphisms will be required for translating these findings into the development of personalized therapeutic strategies that could improve healthspan and treat late-onset diseases.
Effector mechanisms that mediate DR
Fat and lipid metabolism
Fat tissue is argued to have evolved to serve as an energy store, helping organisms counteract periods of starvation. Thus, fat metabolism is likely linked to DR. Increased fat deposition is a characteristic of aging in many organisms. Although increased fat deposit is correlated with insulin resistance, enhanced reliance on fat metabolism is often observed in long-lived mutants in the IIS and mTOR pathways (Caron et al., 2015; Hardy et al., 2012). Work in Sirt1+/− mice and in cell culture reveals that Sirt1 promotes fat mobilization in white adipocytes by repressing genes controlled by the fat regulator PPARγ (Picard et al., 2004). AMPK also regulates lipid metabolism through the phosphorylation of acetyl-CoA carboxylase 2 (ACC2) (Merrill et al., 1997). One important caveat of triglyceride measurements is that steady-state triglyceride levels are often reported, but not the flux analysis, which is more reflective of physiological function. Importantly, studies in nutrient restriction in flies and mice using tracers show an increase in both fat synthesis and breakdown, suggesting a switch toward triglyceride utilization (Bruss et al., 2010; Katewa et al., 2012). Inhibition of triglyceride synthesis by inhibition of acetyl-CoA carboxylase or mitochondrial beta-oxidation results in a loss of DR-dependent lifespan extension (Katewa et al., 2012). In flies, enhanced expression of clock genes drives fatty acid oxidation and breakdown in peripheral tissues (Katewa et al., 2016). In worms, the inhibition of genes regulating fatty acid oxidation, synthesis, and desaturation, such as nuclear hormone receptors NHR-49/PPAR-α and NHR-80/HNF4, is essential for DR-mediated longevity and ameliorates proteotoxic effects in polyQ Huntington’s models (Marcellino et al., 2018; Van Gilst et al., 2005). The increased abundance of desaturated mono- and polyunsaturated fatty acids during DR is an essential component of cell membrane structure and has been shown to upregulate prosurvival mechanisms such as cellular detoxification (Chamoli et al., 2020). These studies suggest that enhanced rates of fatty acid synthesis and breakdown are both important regulators of DR-mediated longevity, and caution should be taken when measuring the effects of DR on fatty acid metabolism. Techniques that measure lipid turnover using radiolabeled nucleotides from nuclear testing have helped measure the fat-turnover rate in humans, showing that low levels of lipid turnover are associated with unhealthy metabolic outcomes (Arner et al., 2011). Together, this suggests the importance of measuring flux analysis rather than steady-state fat levels as well as the role of genotype in assessing the contribution of fat metabolism in mediating the protective effects of DR.
Proteostasis and autophagy
Many proteins become insoluble and accumulate with age, which contributes to disease (Kaushik and Cuervo, 2015). Several of these proteins are determinants of lifespan, as their inhibition increases lifespan in C. elegans (Reis-Rodrigues et al., 2012). Whether interventions like DR could delay such global changes with accumulation of age-related insoluble proteins is yet to be determined, but evidence suggests that DR improves protein homeostasis. Work in C. elegans suggests that DR extends lifespan by inducing ER hormesis and maintaining proteostasis (Matai et al., 2019). A critical component of proteostasis is the timely degradation of toxic protein buildups, which largely requires autophagy (Chen et al., 2011), which is activated in response to nutrient scarcity (Russell et al., 2014). Many genes related to autophagic machinery are shown from yeast to humans to be essential for longevity and healthspan benefits with DR (Hansen et al., 2008; Most et al., 2017). For example, eat-2 mutants in C. elegans, a genetic model of DR, show enlarged acidic lysosomal compartments and enhanced autophagic flux, which is important for maintaining intestinal barrier function and extending lifespan (Gelino et al., 2016). Similarly, livers from mice undergoing 40% caloric restriction for 4 months have shown increased autophagic flux, measured by LC3-II/LC3-I ratio (Luévano-Martínez et al., 2017). In humans undergoing 30% CR from 3 to 15 years, autophagy genes such as LC3 and beclin are increased in skeletal-muscle biopsy samples (Yang et al., 2016). Autophagy is also important for mediating coordinated metabolic response to DR and food availability by mobilizing stored lipids via lipophagy (Singh and Cuervo, 2012). The autophagic response to DR is also, in part, mediated by inhibition of mTOR activity and upregulation of AMPK (Meijer et al., 2015) and SIRT1 activity (Farina et al., 2015). There is an intricate relationship between DR and autophagy, which ought to be better elucidated in different tissues and under different forms of DR.
Mitochondrial function
Mitochondrial function is critical for many biological processes. As such, defective mitochondrial function underlies aging and many age-related diseases (López-Otín et al., 2013). Many interventions that extend healthspan or lifespan, including DR, do so by altering mitochondrial function and quality-control mechanisms (Quiles and Gustafsson, 2020). However, there is no clear consensus as to how DR alters mitochondrial function to improve mitochondrial homeostasis and extend lifespan. In addition to variation in the experimental procedures, such as in the study design, timing of experimental readouts, and approaches used to study mitochondrial function, genetic variation could also explain these discrepancies. Several studies claim that reduced mitochondrial activity generates low ROS and thus there is less ROS-induced damage during DR (Pamplona and Barja, 2006). For example, mitochondria of the primary hepatocytes isolated from ad libitum (AL) and 40% CR rats (3–12 months) show reduced oxygen consumption, reduced membrane potential, and generation of less ROS while maintaining their critical ATP production, suggesting increased mitochondrial efficiency under CR (López-Lluch et al., 2006). The techniques used to produce these findings were unique, however, using cultured serum from animals undergoing CR, which could thus be a cause for these interesting findings (López-Lluch et al., 2006). Short-term CR in humans induces the formation of “efficient mitochondria” in skeletal muscle by inducing biogenesis of mitochondria that consume less oxygen, thus inducing reduced oxidative stress, though no enzymes typical of mitochondrial function were found to be increased in these tissues (Civitarese et al., 2007). Despite these differences in oxygen consumption rate, DR has been reported to show consistent effects on transcriptional upregulation of mitochondrial genes in multiple studies and models (López-Lluch et al., 2006; Nisoli et al., 2005; Pamplona and Barja, 2006; Zid et al., 2009).
The beneficial effects of increased mitochondrial oxygen consumption rate are well supported by the concept of mitohormesis. The initial transient upregulation of mitochondrial ROS during DR may lead to increased mitochondrial activity, which explains sustained reduced ROS under DR conditions (Sohal and Forster, 2014). This transient ROS induction upregulates cellular defense mechanisms, thus extending lifespan in C. elegans (Schulz et al., 2007). Similar to this, mitochondrial stress caused by the imbalance of mitonuclear protein, proteotoxicity, and loss in mitochondrial fitness may trigger the mitochondrial unfolded protein response (mtUPR) or other mitochondrial homeostasis mechanisms (Houtkooper et al., 2013; Naresh and Haynes, 2019). In worms, mtUPR contributes to extended longevity caused by an alteration in electron-transport chain components (Durieux et al., 2011). Growing evidence also demonstrates that DR may reduce damage caused to mitochondrial DNA (mtDNA) (Lam and McKeague, 2019). In mammals, DR induces expression of several mitophagy-related markers, such as PINK1, Parkin, ubiquitin, and p62/LC3-II (Mehrabani et al., 2020), suggesting enhanced turnover of damaged mitochondria.
We postulate that under DR there is a switch in substrate utilization toward fatty acids that reduce ROS production through a variety of mechanisms. Due to differential entry points into the mitochondrial electron-transport system (ETS), for example, the reducing equivalents from fatty acids in beta-oxidation enter the ETS via electron transfer flavoprotein: ubiquinone oxidoreductase (ETF:QO; oxidizing FADH2) and the electrons are deposited to ubiquinone, thereby bypassing complex I, a major site of ROS production. On the other hand, under a rich diet, reducing equivalents from carbohydrates directly enter complex I, producing more ROS (Guarente, 2008). Altogether, evidence suggests that mitochondrial metabolism plays an essential role in DR-mediated longevity. However, studies that account for variation (genetic, age, sex) within and across species are required to understand the extent to which nutrient limitation impacts mitochondrial function in different tissues in the context of aging.
Reduction of cellular senescence and toxic molecules that enhance inflammation
DR also reduces proinflammatory molecules that build up with age by reducing senescent cell markers and associated inflammation in mouse and human tissues (Fontana et al., 2018a; Wang et al., 2010). Cells can become senescent in response to a wide range of stresses, such as genotoxic or oxidative stress, metabolic dysfunction, mitochondrial stress, epigenetic changes, oncogene induction, or loss of tumor-suppressor genes (McHugh and Gil, 2018). DR has been shown to reverse or prevent the negative impacts of many of these stresses and thus reduce the accumulation of senescent cells. These mechanisms include enhanced clearance of damaged mitochondria by autophagy (Ruetenik and Barrientos, 2015), reduced oxidative stress and ROS production (Walsh et al., 2014), and enhanced activity of DNA repair mechanisms (Cabelof et al., 2003) by DR.
Healthy, nonobese human adults undergoing 25% CR for up to 24 months also show reduced proinflammatory markers (C reactive protein, TNF-α, leptin, ICAM1) (Meydani et al., 2016). Among the multiple mechanisms proposed, reduced visceral adiposity associated with IL-6 (Fontana et al., 2007) and changes in adipokines (leptin and adiponectin) influence DR-mediated pleiotropic effects on metabolism and systematic inflammation (Carbone et al., 2012). DR reduces lipotoxic molecules, including acetyl carnitines that accumulate due to reduced beta-oxidation in nonadipose tissues, which drives insulin resistance (Yechoor et al., 2002). Recent advances in the field have recognized prominent roles for the gut microbiome and the metabolites it secretes in shaping immune health (Zhang et al., 2021). In this context, DR is likely to have a huge impact, as the gut microbiome is significantly affected by diet (von Schwartzenberg et al., 2021).
Neurogenesis
CR is reported to delay age-related cognitive decline in complex mammals including humans (Witte et al., 2009). Although detailed mechanisms of how DR prevents cognitive decline are yet to be discovered, one possible mechanism is linked to enhanced neurogenesis and neuron survival (Bondolfi et al., 2004). Age-related structural changes in neurogenesis sites in the dentate gyrus subventricular zone (SVZ) and subgranular layer (SGL) are associated with fewer neuroblasts and progenitor cells and less cell proliferation (Luo et al., 2006). Interestingly, 3 months of alternate-day DR in 8-week-old mice increased cell survival in the dentate gyrus compared with normally fed mice (Lee et al., 2002). DR animals were also found to have increased brain-derived neurotrophic factor (BDNF) and neurotrophin-3 (NT-3) (Lee et al., 2002). Alternate-day DR enhanced survival of newly generated dentate gyrus cells in 3-month-old rats, suggesting enhanced neurogenesis (Lee et al., 2000). Similarly, 40% CR in mice initiated at 16 weeks of age prevented age-related loss in neurogenesis. Only young mice (6–7 months old) undergoing CR showed increased proliferation of neural stem cells and progenitor cells, whereas aged mice (12–18 months) undergoing CR showed reduced proliferation despite having neurogenesis equivalent to that of young mice. This contrast has been attributed to either increased survival of newborn neurons or a switch in cell fate to astrocytes, a mechanism seen in the hippocampus (Encinas et al., 2011). These studies further demonstrate how context-specific measurements, which take into consideration age and form of DR, are necessary to determine how it influences neurogenesis with age.
COMPLEXITIES OF MEASURING AGING IN RESPONSE TO DR
Most of the studies delineating the protective effects of DR on aging use lifespan as their metric to measure aging. However, to best assess how dietary interventions can be optimized to improve longevity, it is essential to determine the appropriate means to assess aging. The gold-standard measurement in studies that examine DR is the survival rate of a population over time. However, these lifelong measurements are often reduced to a single metric, such as mean, median, or maximum lifespan, and a change in these values is used to assess the impact of treatments (Grandison et al., 2009b). These approaches do not consider the heterogeneity in how individuals in a population with identical genotypes age and also do not give insight into when or how DR alters mortality. Determining the impact of DR on an organism’s mortality risk is an alternative approach that has been used to help elucidate its effects (Cheng et al., 2019; Good and Tatar, 2001; Mair et al., 2003).
Mortality curves, which can be represented by initial mortality (α) and rate of aging (β), have been extensively utilized to quantify aging in DR studies of many organisms (Colman et al., 2009; Good and Tatar, 2001; Gordon-Dseagu et al., 2017; Mair et al., 2003; Simons et al., 2013; Zhang et al., 2016b; Zhao et al., 2017b). The species- and genotype-specific impacts of DR previously mentioned have similar variability in their impact on these parameters. In flies, for example, DR decreases mortality rate in a genotype-specific manner, as shown by the varying response across 11 Drosophila Genetic Reference Panel (DGRP) lines (McCracken et al., 2020). A study measuring the role of dietary sugar modulation in Ceratitis capitata has shown mixed results on mortality, with no significant effects of diet on “active life expectancy,” or the fraction of a fly’s life before becoming supine, despite a large impact on total lifespan (Papadopoulos et al., 2011). However, a more recent study showed significant β changes upon dietary alterations in the same species (Papanastasiou et al., 2019). Importantly, the impact of DR on mortality is conserved in mammals, though results have been variable. For example, while a meta-analysis in rodents found that only β was significantly decreased on DR (Simons et al., 2013), studies in mice revealed a decreased β and an increase in α upon DR (Garratt et al., 2016). Another recent study found a slight decrease in β for mice on DR compared with ad libitum feeding and further showed that a late-onset diet change had stronger initial effects on mortality going from DR to ad libitum than the reciprocal, though the difference disappeared over time (Hahn et al., 2019). In humans, the role of dietary macronutrients in mortality is complex; mortality risk follows a U-shaped association with dietary carbohydrate composition (Seidelmann et al., 2018), and fat consumption leads to either higher (animal fats) or lower (plant-based fats) mortality (Seidelmann et al., 2018). These differences highlight the variability in genetic response to DR, and the ability of mortality analyses to give more granular insight into the species- and genotype-specific nature of DR. The independent nature of each data point in a mortality curve allows for more flexibility and creativity when designing DR studies (Cheng et al., 2019). Incorporating mortality-rate analysis will shed light on dissecting the aging measures impacted by DR-related longevity mechanisms to better assess how individualized treatments can be applied.
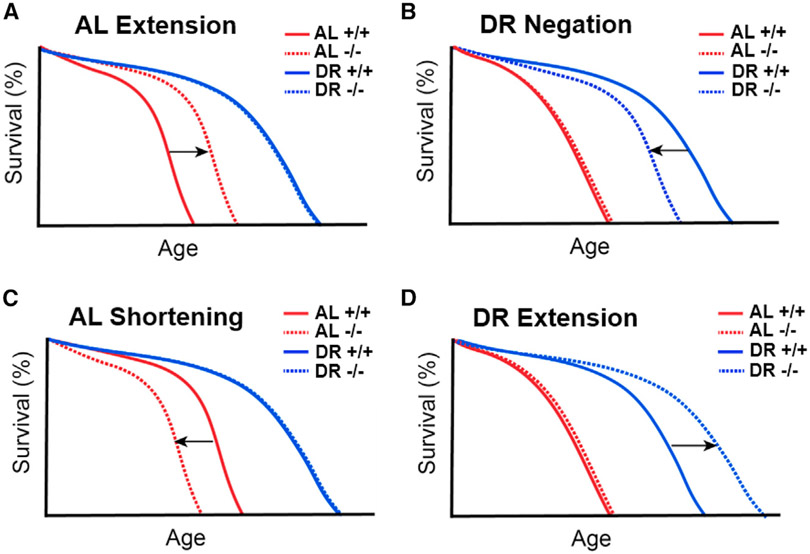
Different classes of diet-responsive genes using mortality analysis to decipher mechanisms of DR
Deciphering the genetic mechanisms of DR responses has classically relied on using mortality curves as an output in response to both dietary and genetic perturbations. Four phenotypic classes have been described to identify genetic perturbations which mediate lifespan changes under ad libitum or DR. The first class is lifespan extension in mean or median lifespan upon ad libitum conditions, but not DR (Figure 2A), as has been seen upon downregulation of genes in the mTOR and insulin/IGF-1 pathways in flies (Kapahi et al., 2017). In the second class, lifespan is shortened specifically upon DR but not under ad libitum conditions (Figure 2B), as has been shown with loss of SIR2 in yeast, AMPK in worms, or 4E-BP or IRE1 in flies (Greer et al., 2007a; Lin et al., 2000; Luis et al., 2016; Zid et al., 2009). These proteins likely regulate the genes that mediate the protective effects of DR. In a third, less examined class, gene knockout shortens lifespan specifically on a rich diet but not on DR (Figure 2C). This is seen upon loss of the urate oxidase gene in flies, which leads to the accumulation of purines (Lang et al., 2019). These genes are likely to regulate pathways that impact the buildup of toxic products on a rich diet but not on DR. Such genes have the potential to limit lifespan and enhance age-related diseases, especially on a rich diet (Lang et al., 2019). Another example of this is that mice deficient in the DNA excision-repair gene 1 Ercc-1 show a tripling of lifespan under CR, suggesting that the buildup of specific DNA damage is detrimental to lifespan in a diet-dependent manner (Vermeij et al., 2016). Additionally, the glyoxalase gene knockout results in accumulation of advanced glycation endproducts (AGEs) in C. elegans (Chaudhuri et al., 2016). This shortens lifespan, which is rescued under a fasted state. In the fourth class, a gene modulation extends lifespan upon DR, but not a rich diet (Figure 2D). One example is observed in yeast where, paradoxically, loss of Sir2 in combination with CR enhances survival under stress and starvation and has opposite effects on chronological lifespan and replicative lifespan (Fabrizio et al., 2005). These cases signify the complex relationship between DR and the genes it regulates, suggesting that further analysis is necessary to determine how modifying a specific molecular mechanism might influence longevity in different dietary contexts. Future genotype-diet interaction studies that take into account all four possibilities and their combinations would reveal further insights into how aging is modulated by diet-responsive pathways. Furthermore, in addition to using mean and median changes in lifespan, accounting for the mortality parameters α and β throughout lifespan will be instrumental in understanding which genetic pathways influence mortality parameters and age-related decline in biological function.
Figure 2. Possible gene-specific lifespan responses to dietary interventions.
Loss of a gene can lead to one of four general responses:
(A) Lifespan extension on a nutrient-rich diet, but no effect under DR.
(B) Negation of typical DR-mediated lifespan extension, but no effect on a nutrient-rich diet.
(C) Lifespan shortening under nutrient-rich conditions, but no effect under DR.
(D) DR-specific lifespan extension, but no effect under nutrient-rich conditions.
Genotype-dependent variation in responses to DR
Genetic variations in APOE and FOXO3 are known to influence the risk of age-related disease and overall mortality in humans (Broer et al., 2015; Carr et al., 2018); however, human variants for response to DR have not been identified. These genetic variations strongly influence responsiveness to DR, and thus are of paramount importance in studying precision nutrigeroscience. Studies using mice have found that not all strains tested had increased lifespan with one specific level of CR (Liao et al., 2010) and that fertility with CR also varied greatly between strains (Rikke et al., 2010). Although one form of CR is not necessarily expected to be beneficial for all genetic backgrounds and though only ~10 mice were tested for each diet, it was surprising that some strains performed poorly upon CR. We previously demonstrated that genotype influences DR responses such as starvation resistance, body mass, triglyceride levels, protein levels, and glucose levels by using the DGRP fly strains (Nelson et al., 2016). Following characterization of these strains, we used genome-wide association studies (GWAS) for the above physiological traits and found that genes associated with ceramide synthase activity, the fly huntingtin gene, and a previously uncharacterized gene, heavyweight, influenced body mass under ad libitum conditions and starvation resistance upon DR (Nelson et al., 2016). Further work with the DGRP revealed how DR impacts longevity and climbing ability. While decline in climbing ability with age was slowed by DR in nearly all strains, lifespan was not ubiquitously improved, with ~20% of strains having shorter lives under DR. We also showed that strains of flies prone to longer life under ad libitum conditions performed worse under DR (Wilson et al., 2020). Additional work utilizing the DGRP highlights how genotype-diet interactions can lead to metabolomic variability (Jin et al., 2020). While these studies uncovered new diet-responsive genes that influence lifespan (such as decima), healthspan (such as daedalus), and the metabolome (such as CCHa2-R), these findings were limited by only using measures for mean or median lifespan. Future studies to determine the effects of DR in various genotypes will require methods that capture its effects over the entire lifespan, such as mortality rates, to best determine how and when to apply diet-based therapeutic strategies.
Sexual dimorphism in the lifespan response to DR
Sex-specific differences in lifespan have been reported in many species, including humans (Gordon et al., 2017). Similarly, aging interventions such as DR also have disparate effects based on sex (Wu et al., 2020). In humans, reproductive rates have been linked to nutrient availability, and reproductive activity is shown to be negatively correlated with lifespan in some cases (Jasienska et al., 2017). Women with reduced reproduction trend toward a longer lifespan, whereas this effect is not seen in men (Bolund et al., 2016). Females display a stronger response to this reproduction-longevity trade-off largely owing to the differences in reproductive burden (Travers et al., 2015). Reduced dietary protein relative to carbohydrates can also maximize lifespan, but this is not optimal for reproduction, a response that is variable by genotype and sex (Jensen et al., 2015). It is noted that mice that are prone to lifespan extension with DR are also prone to increased litters (Rikke et al., 2010). This group further recognized genotype and sex-genotype interactive differences in lifespan and body fat (Liao et al., 2010,2011). Others have linked nonreproductive reasons to sexual disparity in diet response, such as differences in intestinal strength under DR in sheep, and noted that inducing femalelike pathology in the male gut intestine is capable of extending life (Regan et al., 2016). In rhesus macaques undergoing CR, females showed higher early-life mortality than males noted to be due to endometriosis, whereas males showed reduced serum cholesterol with age compared with females (Mattison et al., 2012). Overall, the outcomes and optimal dosage of DR differ by sex and by strain (Mitchell et al., 2016), which necessitates study into the best means to treat different sexes with DR-based treatments.
Healthspan versus lifespan debate
Though it is often assumed that lifespan extension is accompanied by healthspan extension, recent work has called this relationship into question. This debate has created two schools of thought: one which focuses on lifespan extension as the most relevant metric for slowing aging and another which uses functional changes in aging measured by healthspan metrics. One challenge in determining healthspan, however, is how to best assess this in model organisms. In humans, walking speed is often used for this (Freedman et al., 2002), though its role as a predictor of mortality is questionable (Studenski et al., 2011) because most aged humans have multiple disabilities, imposing frailty by different means (Clegg et al., 2013). Nonetheless, the period of overall functionality is used as a measure for determining overall healthspan in model organisms, particularly in response to DR. Work in C. elegans focusing on known DR-related genes such as eat-2, which restricts dietary intake in worms, and daf-2, which inhibits IGF signaling, demonstrated that lifespan extension is not necessarily accompanied by similar healthspan extension, as assessed by oxidative stress, heat resistance, and overall movement (Bansal et al., 2015), suggesting an uncoupling of lifespan and healthspan. Others, however, have analyzed functionality measures differently, such as recording movement decline relative to maximum velocity, to suggest a correlation between lifespan and healthspan in the same daf-2 mutant used in the previous study (Hahm et al., 2015). Still others continue to find no evidence of correlation between health and longevity in multiple species (Podshivalova et al., 2017). In flies, we found no correlation between measures of lifespan and healthspan in response to DR across 160 wild strains, and DR extended healthspan more consistently than lifespan (Wilson et al., 2020). We further identified genes that regulated lifespan specifically (decima) or climbing ability specifically (daedalus) in response to diet. We found that DR alters body mass and other metabolic traits (Nelson et al., 2016), but these also did not correlate with lifespan (Wilson et al., 2020). The potential separation between lifespan and health factors has also been observed in more complex mammalian systems. Work in mice has suggested aging interventions that improve healthspan or lifespan but not both conjunctively, notably with the result that downregulation of mTOR activity extended lifespan but did not affect hepatic function or insulin sensitivity (Lamming et al., 2012). Healthspan may be improved without extending lifespan with dietary optimization to incorporate nicotinamide (Mitchell et al., 2018). More recently, knockout of the stress-response gene Nrf2 resulted in reduced stress-response activation, but there was no effect on lifespan or physical performance in mice undergoing CR, further suggesting a range of potential health-related responses to CR (Pomatto et al., 2020). CR primate models also demonstrate a potential uncoupling of healthspan and lifespan in response to dietary modulation across studies; one study demonstrated benefits in healthspan measures such as triglyceride levels and disease onset as well as lifespan (Mattison et al., 2017), whereas another using a different CR regimen showed that there is an improvement in the same health measures, but not lifespan (Mattison et al., 2012), suggesting compression of morbidity but not extended lifespan. However, measurements of body mass also indicated no change between CR and control primates, suggesting that the implementation of CR in these animals may have contributed to the lack of change in lifespan (Mattison et al., 2012). These studies also emphasize the importance of diet composition when considering how DR might influence longevity and healthspan. Humans consuming reduced red meat and trans fats showed increased longevity and healthspan on average, but there was a notable compression of morbidity later in life (McCullough et al., 2002). These findings have led to the recommendation that both healthspan and lifespan traits be measured in all age-related studies to better report which interventions improve healthspan and which improve lifespan (Richardson et al., 2016). Furthermore, genotype- and environment-specific factors may also provide critical context as to how aging is driven and how age-related diseases develop. Given the heterogeneity in aging across tissues and genotypes, perhaps observing specific individuals to understand how they age is more effective than using generalized means to determine how aging influences the individual.
DR in tissue aging and disease
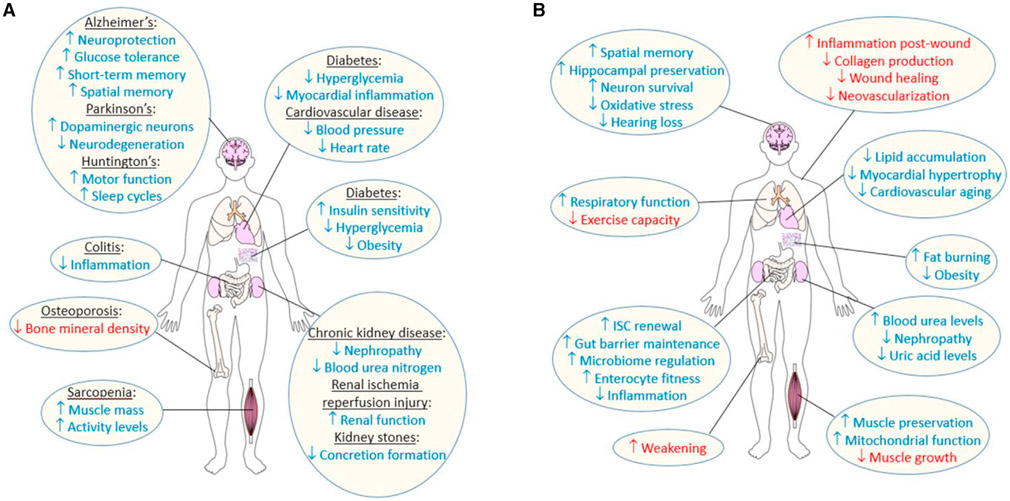
Considering that diet-responsive molecular pathways are present in all cells, one might assume that DR-derived benefits would be beneficial in all tissues of a single organism. However, DR impacts tissue aging in multiple ways and to different degrees (Figure 3) and has various effects on DR-treated disease models (Table 3), complicating how DR can be used to broadly treat age-related decline. Here, we detail how DR impacts tissues and age-related diseases, which must be considered when investigating individualized DR-based treatments.
Figure 3. Reported effects of DR on human tissues in disease and normal aging.
(A) Reported benefits and detriments of DR under different disease states in the brain, intestine, bone, muscle, heart, fat stores, and kidneys.
(B) Benefits and detriments of DR under normal tissue aging. Benefits are shown in blue, detriments in red. See text for details.
Table 3.
Effects of DR on disease models
| Disease | DR regimen |
Species | Model | Effect | Reference |
|---|---|---|---|---|---|
| Diabetes | MR | mouse | NZO | ↑ insulin sensitivity, ↑ energy expenditure | Castaño-Martinez et al., 2019 |
| PR | mouse | NZO | ↓ hyperglycemia, ↓ β cell loss | Laeger et al., 2018 | |
| CR | mouse | db/db | ↑ insulin sensitivity, ↑ β cell mass, ↑ utilization of fatty acids, ↓ apoptosis, ↓ oxidative stress, ↓ myocardial inflammation, ↓ fibrosis, ↓ inflammation | Cohen et al., 2017; Kanda et al., 2015; Waldman et al., 2018 | |
| CR and IF | mouse | NZO | ↑ protection against hyperglycemia, ↓ diacylglycerol in liver | Baumeier et al., 2015 | |
| Cardiovascular disease | CR | rat | SHROB | ↓ blood pressure, ↓ heart rate, ↓ weight | Ernsberger et al., 1994 |
| CR | mouse | db/db | ↑ iron homeostasis, ↓ myocyte hypertrophy, ↓ inflammation, ↓ fibrosis, ↓ oxidative stress | An et al., 2020 | |
| CR | mouse | ob/ob | ↑ iron homeostasis, ↓ myocyte hypertrophy, ↓ inflammation, ↓ fibrosis, ↓ oxidative stress | An et al., 2020 | |
| Colitis | MR | mouse | colitis by DSS treatment | ↑ ROS response, ↓ inflammation | Liu et al., 2017 |
| CR | mouse | colitis by trinitrobenzene sulfonic acid | ↑ balanced immunoregulation, ↓ colitis | Shibolet et al., 2002 | |
| Renal ischemia reperfusion injury | CR and PR | mouse | bilateral induction of renal ischemia reperfusion | ↑ protection from injury post-reperfusion | Robertson et al., 2015 |
| CR and STF | mouse | bilateral induction of renal ischemia reperfusion | ↑ renal function, ↑ protection against injury post-reperfusion, ↓ inflammation | Shushimita et al., 2015 | |
| Kidney stones | PR and PuR | fly | hyperuricemia by urate oxidase RNAi | ↓ concretions in malpighian tubule | Lang et al., 2019 |
| Alzheimer’s disease | PR | fly | arctic mutant Aβ42 and 4R tau overexpression | ↑ lifespan, no neuronal benefits | Kerr et al., 2011 |
| IF | rat | ovariectomized β-amyloid-infused | ↑ glucose tolerance, ↑ fat loss, ↑ short-term and spatial memory | Shin et al., 2018 | |
| IF | mouse | App NL-G-F | ↓ neuronal hyperexcitability, ↓ hippocampal synaptic plasticity deficits | Liu et al., 2019 | |
| IF | mouse | 5xFAD | ↑ brain inflammation, ↑ neuronal injury | Lazic et al., 2020 | |
| CR | yeast | expression of human α-synuclein | ↓ α-synuclein-related toxicity | Sampaio-Marques et al., 2018 | |
| CR | mouse | APPswe/ind | ↓ amyloid-β accumulation, ↓ astrocyte activation | Patel et al., 2005 | |
| CR | mouse | APPswe+PS1M146L | ↓ amyloid-β accumulation, ↓ astrocyte activation | Patel et al., 2005 | |
| CR | mouse | Tg2576 | ↓ neurite plaques, ↓ amyloid-related pathology, ↓ cognitive deficits | Schafer et al., 2015; Wang et al., 2005 | |
| CR | mouse | dtg APP/PS1 | ↓ neuropathy | Mouton et al., 2009 | |
| CR | mouse | Tg4510 | ↓ memory deficits | Brownlow et al., 2014 | |
| CR | mouse | APOE knockout | ↑ neuroprotection | Rühlmann et al., 2016 | |
| CR | mouse | hTau | ↑ tauopathy | Gratuze et al., 2017 | |
| CR | mouse | PDAPP-J20 | ↓ cognitive deficits, ↓ amyloid-β-related pathology | Gregosa et al., 2019 | |
| IF and CR | mouse | 3xTgAD | ↑ spatial memory, ↑ exploratory behavior | Halagappa et al., 2007 | |
| Parkinson’s disease | IF | rat | 6-OHDA-induced | no effects | Armentero et al., 2008 |
| CR via bacteria dilution | worm | 6-OHDA-induced | ↓ dopaminergic neuron degeneration, ↓ microglial activation | Jadiya et al., 2011 | |
| CR | monkey | MPTP-induced | ↑ striatal dopamine | Maswood et al., 2004 | |
| CR | mouse | MPTP-induced | ↑ dopaminergic neuron protection, ↓ dopaminergic neuron degeneration | Bayliss et al., 2016; Duan and Mattson, 1999 | |
| Huntington’s disease | EODF | mouse | N171-Q82 | ↑ BDNF in striatum and cortex, ↑ hsp70 in striatum and cortex | Duan et al., 2003 |
| CR | mouse | YAC128 | ↑ improved body weight, ↑ improved blood glucose, ↑ motor function, ↑ Hdac2 regulation | Moreno et al., 2016 | |
| TRF | mouse | Q175 | ↑ locomotor activity, ↑ sleep cycles, ↓ heart rate variability | Wang et al., 2018 | |
| Cancer | MR | mouse | Yoshida sarcoma transplantation | ↓ metastasis | Hoffman and Stern, 2019 |
| MR | mouse | breast carcinoma transplantation | ↑ efficacy of cisplatin | Hoffman and Stern, 2019 | |
| MR | rat | Yoshida sarcoma transplantation | ↑ efficacy of doxorubicin, ↑ efficacy of vincristine | Hoffman and Stern, 2019 | |
| PR | mouse | GL26 glioma implantation | no effects on tumor growth | Brandhorst et al., 2013 | |
| PR | mouse | LuCaP23.1 prostate cancer xenograft model | ↓ tumor growth | Fontana et al., 2013 | |
| PR | mouse | WHIM16 breast cancer xenograft model | ↓ tumor growth | Fontana et al., 2013 | |
| PR | mouse | C57B/6 with RP-B6-Myc prostate tumor transplantation | ↑ response to immunotherapies, ↑ proinflamma-tory phenotypes, ↓ tumor growth | Orillion et al., 2018 | |
| PR | mouse | C57B/6 with RENCA tumor transplantation | ↑ response to immunotherapies, ↑ proinflammatory phenotype, ↓ tumor growth | Orillion et al., 2018 | |
| EODF | mouse | p53-het/Sirt1-tg | ↑ survival, ↓ tumor onset | Herranz et al., 2011 | |
| EODF | mouse | p53 deficiency | ↑ survival, ↓ tumor onset | Herranz et al., 2011 | |
| short-term intermittent CR | mouse | BalB/C mice with 4T1 breast cancer cell transplantation | no effect on chemotherapy efficacy | Brandhorst et al., 2013 | |
| CR | mouse | Rb+/− | no benefit in delaying growth or progression of neuroendocrine tumors | Sharp et al., 2003 | |
| CR | mouse | Foxo3 deficiency | ↑ lifespan, ↓ tumors | Shimokawa et al., 2015 | |
| CR | mouse | high-fat-diet-induced Lewis lung carcinoma | ↓ proinflammatory cytokines, ↓ angiogenic factors, ↓ insulin, ↓ adiposity, ↓ tumor metastasis | Sundaram and Yan, 2016 | |
| CR | mouse | Balb/c with breast cancer cell transplantation | ↑ survival, ↓ metastasis, ↓ IGF-1R, ↓ inflammatory cytokines with cisplatin/doce-taxol treatment | Simone et al., 2018 |
6-OHDA, 6-hydroxydopamine; APOE, apolipoprotein E; BDNF, brain-derived neurotrophic factor; CR, caloric restriction; DSS, dextran sulfate sodium; EODF, every-other-day feeding; Hdac2, histone deacetylase 2; hsp70, heat shock protein 70; IF, intermittent fasting; IGF-1R, insulin growth factor 1 receptor; MPTP, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; MR, methionine restriction; NZO, New Zealand Obese; PR, protein restriction; PuR, purine restriction; Q85/Q175, poly-glutamine repeats in the huntingtin gene; Rb, retinoblastoma; RENCA, renal carcinoma; SHROB, Spontaneously Hypertensive Obese; STF, short-term fasting; TRF, time-restricted feeding.
Obesity and diabetes
Overnutrition contributes to tissue decline and accelerated disease progression, most notably with obesity (Must et al., 1999). DR increases leptin receptors, a satiety signal in the brain, which reduces free leptin in plasma (Yamada et al., 2019). However, leptin signaling has also been linked to improved fatty acid beta-oxidation and improved cardiovascular health. In diet-induced obese mice, restriction of branched-chain amino acids (BCAAs) induces rapid fat loss and improved cardiovascular health (Cummings et al., 2018). BCAA restriction is capable of improving health in nonobese animals as well as in humans (Fontana et al., 2016). However, these benefits do not always correlate with extended longevity (Nelson et al., 2016; Wilson et al., 2020), suggesting that DR can alter physiology without affecting lifespan. In flies, protein restriction increases triglyceride levels but also turnover, thus increasing triglyceride utilization (Katewa et al., 2012; Nelson et al., 2016). This suggests that metabolic shifts in DR can help allow for more effective utilization of fat storage.
Obesity can potentially give rise to a myriad of diseases, including diabetes, cardiovascular disease (CVD), and cancer (Low Wang et al., 2016; Massetti et al., 2017), though studies have investigated the incidence of “healthy obese” individuals who do not suffer from disease despite being obese (Stefan et al., 2013). Obesity’s targets are widespread and oftentimes lead to comorbidities within the same individual. Since dietary intake greatly contributes to obesity, DR is routinely prescribed to combat these disorders. Specifically, carbohydrate restriction improves metabolic syndrome in patients, as determined by a panel of fat-related readouts (Hyde et al., 2019). Extensive work in mouse models of type 2 diabetes has shown extensive benefits of DR (Table 3). Time-restricted feeding can also improve cardiometabolic disorders (Melkani and Panda, 2017). Additionally, dietary modulation is proposed to delay or treat liver diseases in patients (Romero-Gómez et al., 2017) such as nonalcoholic fatty liver disease, which is more prevalent with obesity (Hannah and Harrison, 2016). Protein malnutrition is a common symptom of liver cirrhosis, and thus protein restriction proves detrimental in cirrhosis patients (Toshikuni et al., 2014). Thus, while the link between dietary intake and induction of metabolic disorders is clear, in specific cases like liver cirrhosis, DR may be detrimental. This evidences the need for a case-by-case analysis for how diet can be utilized therapeutically for specific obesity-related disorders.
Cardiovascular aging and heart disease
Cardiovascular aging is closely linked to obesity, and CVD is the leading cause of death in the United States, particularly in individuals with type 2 diabetes (Jin, 2018). Diet also contributes to the onset of cardiovascular decline and CVD (Micha et al., 2017). Because of this link, research on cardiovascular health has often utilized dietary interventions. Obese ob/ob mice demonstrate rapid cardiovascular decline (Raju et al., 2006), and CR reduces lipid accumulation and reverses myocardial hypertrophy (Sloan et al., 2011; Verreth et al., 2004). CR also reduces oxidative stress, inflammation, and fibrosis in these mice (An et al., 2020). In wild-type mice fed a high-fat diet, risk of cardiovascular aging is elevated but is reversed through fasting (Tanner et al., 2010). CR also benefits cardiovascular health in primates (Colman et al., 2009, 2014; Mattison et al., 2012). Forms of DR are often utilized for individuals at risk for CVD (Brandhorst and Longo, 2019a; Mirzaei et al., 2016), as well as to slow age-related decline in cardiovascular health (Fontana, 2018; Wei et al., 2017). Rat strains such as the spontaneously hypertensive obese model have demonstrated how cardiovascular decline occurs (Ernsberger et al., 1999) and how CR can prevent this decline (Table 3) (Ernsberger et al., 1994). Though the genetics behind CVD are not well elucidated, studies in primates have helped identify novel loci associated with its onset in response to elevated nutrient load (Karere et al., 2013; Rainwater et al., 2003). Studies in CVD patients further demonstrate the benefits of DR, such as reduced low-density lipoprotein and triglyceride levels following carbohydrate restriction (Bhanpuri et al., 2018) or CR (García-Prieto and Fernandez-Alfonso, 2016). In all, complications relating to cardiovascular aging can largely be mediated, in part, through dietary interventions.
Digestive tissue and intestinal dysfunction
DR has profound effects on the intestinal tract. Intestinal homeostasis is regulated in response to diet through altered expression of transcription factors such as Myc (Akagi et al., 2018) and XBP1 in flies (Luis et al., 2016), upregulated autophagy in worms (Gelino et al., 2016), and prevention of inflammation due to intestinal microbiome leakage in flies, pigs, and humans (Fan et al., 2017; Rera et al., 2012; Santoro et al., 2018). This suggests that DR can be used to regulate intestinal health and aging (Keenan et al., 2015) as well as intestinal disorders (Tuck and Vanner, 2018). mTORC1 also plays an important role in regulating healthy intestinal stem cell function to preserve intestinal homeostasis, and continued overactivation of mTORC1 such as through overnutrition has been shown to induce age-related stem cell decline in flies (Haller et al., 2017). However, mTORC1 is also shown to be upregulated during CR to induce expansion of the intestinal stem cell (ISC) population, which ensures gut homeostasis (Igarashi and Guarente, 2016) and ISC self-renewal in mice (Yilmaz et al., 2012). These conflicting results provide a complicated interaction for maintaining health.
Patients with digestive disorders such as inflammatory bowel diseases, including Crohn’s disease and colitis, are regularly prescribed dietary interventions, particularly those low in fat or carbohydrates, to improve symptoms (Prince et al., 2016). In mouse models, methionine restriction (MR) (Liu et al., 2017) or CR (Shibolet et al., 2002) have been useful for combating the worsening of digestive disorders (Table 3). Overall, DR appears useful to maintain proper intestinal barrier function and metabolism (Lian et al., 2018), but the biochemical mechanisms involved must be closely regulated.
Excretory system and renal diseases
Renal system aging is also largely improved with DR, whereas its decline is exacerbated by overnutrition. Studies in murine models have demonstrated improved renal stress resistance under short-term DR, an effect that is, in part, regulated by IIS (Jongbloed et al., 2017), and other studies have shown altered ammonia metabolism following DR (Lee et al., 2015). Reduction of dietary AGEs alters gut-microbiome composition specifically in patients undergoing peritoneal dialysis, and these effects are suggested to potentially have a positive effect on the incidence of CVD (Yacoub et al., 2017). A meta-analysis of CR studies in rodents for chronic kidney disease identified robustly positive effects through measures such as blood urea nitrogen and degree of nephropathy (Xu et al., 2015a). Studies to determine how diet might influence renal ischemia reperfusion also show positive effects with DR (Table 3), suggesting that DR has therapeutic potential. Additionally, in a fly model for hyperuricemia, DR reduces the incidence of uric-acid concretions in the malpighian tubule, which is analogous to the human kidney, and this is regulated by IIS activity (Lang et al., 2019). In all, data demonstrate that renal dysfunction can largely be alleviated by DR.
Nervous system and neurodegenerative diseases
DR is a rapidly expanding area of study due to hope of finding potential means to promote neuroprotection (Gräff et al., 2013). The low-carbohydrate ketogenic diet (Newman et al., 2017) and CR improve late-life memory in mice (Rojic-Becker et al., 2019). DR in rats has been shown to improve proteasome activity (Shruthi et al., 2016) and overall neuronal protection (Contestabile et al., 2004). Mechanisms of neuronal decline are also delayed by CR in C. elegans (Steinkraus et al., 2008), rodents (Gräff et al., 2013), and primates (Bendlin et al., 2011). DR also improves neurotrophic factors and stress response in mice (Duan et al., 2003) as well as DNA maintenance in mice with DNA repair deficiencies (Ercc1Δ/− and Ercc5−/−) (Vermeij et al., 2016). In all, DR contributes to the fine-tuning of nervous system function for the promotion of healthier aging. As a result, DR has also been used to study diet’s effects on disease states in models (Table 3). Specifically, DR has been shown to improve symptoms of Parkinson’s disease through reduction of oxidative stress and protection of mouse dopaminergic neurons (Duan and Mattson, 1999) and the substantia nigra (Bayliss et al., 2016), though other studies in rats have not seen benefits (Armentero et al., 2008). DR also provides benefits in a multitude of Alzheimer’s disease (AD) models (Table 3), in part due to metabolic improvement, as shown in rats (Shin et al., 2018). In fact, the pathologies of AD and diabetes are often intertwined; many AD patients demonstrate diabetic complications (Vieira et al., 2018), and similar mechanisms such as oxidative stress have been implicated for this co-morbidity (Rosales-Corral et al., 2015). This crosstalk has led many to call AD “type 3 diabetes” (Kandimalla et al., 2017). As such, dietary intervention is often prescribed to AD patients, and studies have demonstrated improvements in patients who implement dietary changes during neurodegenerative treatments (Bredesen et al., 2016). Early-life DR implementation has also been shown to delay the effect of AD pathologies, owing to blood-brain barrier preservation, which typically declines with age in rat models (Tomi et al., 2013), but studies in flies have not necessarily seen benefits to neuronal health (Kerr et al., 2011). A potential, underexplored area of study is how DR impacts individuals of different APOE genotypes. The harms of APOE deficiency, which induces neurodegeneration, are improved by DR (Rühlmann et al., 2016), though the three human alleles need to be explored further. Huntington’s disease (HD) is also characterized by protein aggregates in the brain, and DR in HD mice shows positive effects relating to histone acetylation (Moreno et al., 2016). Time-restricted feeding in the HD Q175 model improved motor function as well as circadian rhythms (Wang et al., 2018). It is clear that diet can have a profound effect on neurological aging and continues to be a promising field for future work.
Muscle aging and sarcopenia
Aging induces a reduction in overall muscle function, typically through muscle degradation or improper ability to build new muscle, as has been detailed in flies (Haller et al., 2017). A prominent mechanism for muscle growth with DR is through mTORC1 regulation, of which CR has been shown to reduce activity in rats (Margolis et al., 2016). Understanding the proper activity of mTOR creates one of the greater challenges in studying muscle function, as mTOR activity is naturally elevated in humans with resistance training (Fujita et al., 2007a), which can reverse the effects of muscle aging (Melov et al., 2007). Exercise is also capable of reducing age-related insulin resistance in the muscle through mTOR-related mechanisms (Fujita et al., 2007b). Despite the importance of mTOR activity in muscle building, it has also been shown that DR preserves muscle structure late in life (Mattison et al., 2012), despite the finding that mTOR is downregulated by DR (Kapahi et al., 2004). Thus, tissue-specific regulation of mTOR activity, and, to that extent, dietary interventions, must be optimized for preserving muscle function. Additional targets for DR have been identified as providing muscular benefits, including improved mitochondrial function and autophagy in murine response to CR (Gutiérrez-Casado et al., 2019). Reduced oxidative stress in muscle tissue in response to DR has even been observed in rodent models lacking antioxidant enzymes, suggesting persistence of benefits from DR (Jang et al., 2012). CR also improves muscle health in rodent models by optimizing the proteasome (Hepple et al., 2008). Additionally, factors such as IL-15 are upregulated in CR rats, which in turn prevents muscular apoptosis (Marzetti et al., 2009a). Because of these benefits, DR is frequently studied in muscle disease to determine its efficacy. CR preserves age-related muscle mass in early stages of sarcopenia in rhesus monkeys (McKiernan et al., 2011) as well as in sarcopenic mice (van Norren et al., 2015). In patients with sarcopenia, however, CR and its mimetics are not effective (Burks and Cohn, 2011). Sarcolemmal rat models demonstrate prevention of age-related effects in response to CR (Hord et al., 2016). The multitude of mechanistic means by which DR improves muscle health have led to the investigation into how it influences human health (Marzetti et al., 2009b), though results in patients thus far have not been promising and will need to be more heavily investigated before it can be considered for treatment.
Skeletal tissue and osteoporosis
Bone-density deterioration is prevalent with age. However, many studies in humans and model organisms have found increased risk of bone weakening and osteoporosis following CR or intermittent fasting, potentially due to reduced dietary mineral intake (Hattori et al., 2014; Ouattara et al., 2016; Villareal et al., 2016), but others have suggested that this can be improved if CR is paired with exercise (Cao, 2018) or if the form of DR is mild (Huang and Ables, 2016). DR in rats also shows reduced bonehealing ability following fracture (Botega et al., 2019). This detriment has also been seen in osteoporotic rat models (Bodnar et al., 2012). Differential results have also been observed in aged-mouse models undergoing CR depending on the bones being observed (Behrendt et al., 2016). In total, DR appears to have negative effects on bone health that ought to be monitored closely (Figure 3).
Integumentary system and wound healing
Both the structural integrity of the integumentary system (Moura et al., 2019) and wound healing (Holcomb et al., 2009) decline with age and are exacerbated by overeating and obesity-related disease. As such, researchers have investigated how DR might influence skin aging, particularly with respect to wound healing. Unfortunately, results have been mixed. Some studies suggest that DR can delay wound healing as well as immune function (Brandhorst and Longo, 2019b), while other studies in rodents and primates suggest no influence from DR (Roth et al., 1997), including with regard to operative integumentary wounds. Preoperative protein or MR in mice (Trocha et al., 2019) and postoperative CR in rats (Emery and Sanderson, 1995) have demonstrated no wound-healing benefits. However, it has also been observed in rats that protein restriction can be detrimental, as evidenced by delayed healing, poorly deposited extracellular matrix, reduced neovascularization, and increased inflammatory cells (Otranto et al., 2009), as well as reduced collagen production (Reiser et al., 1995). These effects are observed even in young rats, suggesting the importance of diet in healing at any age (Hunt et al., 2012). This study in rats also demonstrated that the degree of restriction influences the severity of these phenotypes, suggesting that close regulation of DR could mediate these negative effects (Otranto et al., 2009). However, variability in the rate of aging between individuals has been observed in fibroblast samples and mice, further complicating how diet and aging can influence integumentary structure and wound healing between individuals (Mahmoudi et al., 2019). Nonetheless, the potential of delaying age-related effects on skin and wound healing has led to the investigation of anti-aging and DR-mimetic therapeutics such as metformin, resveratrol, and rapamycin for their effects. These studies have found that aged skin in rat models has suppressed AMPK activity, which can be reversed by these therapeutic options (Zhao et al., 2017a). However, only metformin promoted wound healing, suggesting that further complication of the mechanisms by these DR mimetics might influence integumentary systems (Zhao et al., 2017a). In total, skin aging and particularly wound healing does not appear to benefit from DR and must be closely considered when an individual is at risk following wounding (Figure 3).
Endocrine signaling
The endocrine system regulates several age-related processes, including many that can contribute to metabolism, development, and reproduction. Dietary intake influences hormonal signaling, but hormonal signaling also can regulate the mechanisms of DR. Thus, the relationship between DR and endocrine signaling is remarkably complex and not always positive. The pituitary and hypothalamic transcriptome are heavily influenced by aging as well as by DR (Bédard et al., 2013). These changes signal to several different signaling systems. In the adrenal glands, glucocorticoids have been implicated in neuroprotective properties, an effect that is amplified in the case of DR in rats (Qiu et al., 2012). Earlier in life, CR has been found to inhibit ovulation and mammary-gland development in female mice, suggesting a perturbation in signaling between the pituitary gland and ovaries (Koizumi et al., 1989). In men, testosterone levels, which are typically reduced in obese individuals, are elevated by CR (Schulte et al., 2014). DR has been utilized in the attempts to delay the decline in reproductive ability in aged mice. Although endocrine signals were altered, this decline was not attenuated (May et al., 1992). In addition to the role of androgens in reproduction, age-related androgen decline has also been linked to mild cognitive impairment (Cherrier et al., 2015) and dementia in humans (Białek et al., 2004). Metabolism-related endocrine signaling and DR are intricately linked. Linking ghrelin, the “hunger hormone,” to reproduction, knockout of ghrelin O-acyltransferase proved fatal in pregnant mice undergoing CR due to hypoglycemia, which was not observed in nonpregnant mice (Trivedi et al., 2017). These results were due to regulation of gluconeogenesis by acylated ghrelin in response to CR. CR is also linked to hepatic thyroid-hormone signaling, which is altered and thus suggested to play a role in changes seen in nonthyroidal illness syndrome in rats (de Vries et al., 2015). In individuals with comorbidities, such as in elders, this syndrome can prove fatal (Wang et al., 2019). Growth-related hormonal signaling is reduced in rats undergoing DR (Molon-Noblot et al., 2003). Somatotropes, which produce growth hormone, are preserved in response to DR due to increased turnover in late-life rats (Shimokawa et al., 1997). Growth-hormone receptors are also closely regulated in aged rats following long-term CR (Girard et al., 1998). In total, the different glands of the endocrine system provide a complex structure of how DR influences signaling, and these signals are incredibly important in determining the proper DR-based treatment for human aging.
Respiratory system
The respiratory system is also impaired with age, especially in obese individuals (Dixon and Peters, 2018). One study in humans using the ketogenic diet found that individuals had reduced storage of carbon dioxide in the lungs, which was suggested to potentially preserve respiratory function (Rubini et al., 2015). However, chronic low-carbohydrate diets such as the ketogenic diet have also been shown to impair exercise performance in middle-aged individuals who were considered healthy (Pilis et al., 2018) (Figure 3). CR has been used to boost immune response in the lungs (Palma et al., 2021). In chronic obstructive pulmonary disease (COPD), which is not strictly age-related but becomes more prevalent with age (Cosio et al., 2014), using DR to treat obesity was capable of easing symptoms such as exercise ability (McDonald et al., 2016). Depending on the form of DR, there is no consensus, and research on how DR affects respiratory function is limited. The initial results, however, suggest that forms of DR can improve immune function and disease in the lungs, but that it can also result in worsened exercise performance.
Cancer
Given that cell growth and replication are primary hallmarks of cancer (Hanahan and Weinberg, 2011), DR as a preventative and treatment option has been explored (Brandhorst and Longo, 2016). Work in mice has demonstrated that even 5% CR can result in reduced metastasis of lung carcinomas (Sundaram and Yan, 2016). IGF-1 signaling is linked to tumor growth and cancer development, and work in humans and mice has shown that reduction of IGF-1 by CR and fasting-mimicking diets is capable of delaying cancer risk (Wei et al., 2017). The p53 tumor-suppressor protein plays roles in DNA repair, cell-cycle arrest, and apoptosis as a means for preventing the onset or progression of cancer. DR has been shown to upregulate p53 in xenograft mouse models (Ma et al., 2018), and MR helps restrict cancerous tumor growth in cell cultures (Hoffman, 2019; Hoffman and Stern, 2019). Mutant forms of p53 have been shown in cell culture to promote tumor development, and glucose restriction can downregulate mutant p53 as it becomes deacetylated and is routed for autophagy (Rodriguez et al., 2012). In mice that are deficient for p53, every-other-day fasting is capable of extending life through the role of sirtuins in limiting cancer (Herranz et al., 2011). Similarly, CR has been found to increase SIRT1 levels while reducing p53 in human fibroblasts (de Cabo et al., 2015). An additional mechanism found to be associated with DR and cancer is that of FOXO3, which is necessary in mice for DR-dependent lifespan extension and tumor suppression in mice (Shimokawa et al., 2015); mTOR has also been proposed as a target for DR-mediated tumor suppression (Di Malta et al., 2017). Along these lines, mice with overactive PI3K signaling are resistant to DR-mediated cancer suppression (Kalaany and Sabatini, 2009). This effect was counteracted by activating the PI3K inhibitor PTEN, resulting in restored responsiveness to DR in cells and mice (Kalaany and Sabatini, 2009). Mice with tumor grafts have also shown reduced tumor growth with protein restriction (Fontana et al., 2013), though this effect has not always been observed (Brandhorst et al., 2013). As such, DR mimetics such as rapamycin and metformin have been proposed as potential treatments for cancer (Meynet and Ricci, 2014).
DR also influences responsiveness to cancer treatments underway. General reviews of patients undergoing DR while receiving treatment have been positive (Shingler et al., 2019), and nutritional regulation of autophagy is one cellular component implicated in this response (Antunes et al., 2018). MR in mice has proven useful with chemotherapy, as tumors became suppressed (Hoffman and Stern, 2019). Specific stresses due to cancer-related therapies have largely been eased in response to DR. For example, mouse models of breast cancer have shown reduced inflammation caused by chemotherapy following CR (Simone et al., 2018). Similarly, short-term DR may ease the stresses of chemotherapy as well as surgical stress (Brandhorst et al., 2017). Immunotherapy in particular is enhanced with protein restriction in animal models in in vivo and ex vivo samples (Orillion et al., 2018). Due to all of this, DR and DR mimetics have been tested in preclinical trials and have largely shown promising results (O’Flanagan et al., 2017). However, cases do remain where treatment efficacy is unaffected (Brandhorst et al., 2013).
Unfortunately, DR has not always proven efficacious for cancer treatments (Lv et al., 2014). Moderate forms of CR appear ineffective against the onset of pituitary tumors, though stronger forms were more effective (Molon-Noblot et al., 2003). In a retinoblastoma-susceptible mouse model, CR was unable to delay or halt neuroendocrine tumor formation (Sharp et al., 2003). As with many other disease states, the results of DR appear to be variable depending on the type of cancer, individual, and how the disease takes form.
PROBING DR NETWORKS WITH BIOMARKERS AND SYSTEMS BIOLOGY APPROACHES
Biomarkers of aging are quantitative measures that can be utilized reproducibly for predicting adverse outcomes in late life and for monitoring response to interventions that are designed to reduce them (Xia et al., 2017). The three main criteria for a biomarker of aging, as proposed by the American Federation for Aging Research, are as follows: (1) it must be able to predict mortality better than chronological age, (2) it should allow noninvasive tracking in animal models and humans longitudinally, and (3) it should measure a biological process underlying aging and not the effects of a disease. Ideally, a biomarker or a set of biomarkers should be able to capture the variability of the rate of aging in individuals of the same chronological age, thus enabling the design of appropriate interventions for people at risk (Guerville et al., 2020). Based on the key hallmarks of aging, several markers have been proposed, but none have fulfilled all of the criteria. Some of these include DNA damage indicators, DNA methylation, ncRNAs, IGF-1, triglyceride levels, oxidative stress markers, and cell senescence markers (Table 4). Despite the documentation of several biomarkers, studies that indicate a high degree of correlation between markers are limited (Belsky et al., 2018). In contrast, the availability of “omics”-based approaches including methylomics, metabolomics, proteomics, transcriptomics, and ionomics has helped identify several candidates that can serve as age indicators and be utilized to assess aging rate (Cheung et al., 2018). These approaches provide a more in-depth view of the complex aging changes that are occurring within the whole organism (Avanesov et al., 2014; Laye et al., 2015). Systems biology uses omics data to understand the interplay between aging and nutritional interventions and to uncover novel molecular and genetic networks that underlie DR (Lacroix et al., 2015). This approach utilizes network analysis, molecular signature identification, and integration of data from multi-omics platforms to predict how a biological system would behave under different perturbations or DR (Hou et al., 2016). The evolution of omic approaches has provided a strong framework to understand and precisely predict how an individual would respond to DR and to provide a more holistic view of how DR influences health. Though undeveloped, the omics revolution has paved the way for personalized DR-based research in model organisms and even humans (van Ommen et al., 2017). In the coming years, one can envisage that systems-based approaches that quantify and consider the inherent variation in the genetic makeup of an individual and response to environmental factors will lead the way to personalized DR recommendations that can prevent and cure many late-onset diseases. This development will provide the foundation for the application of precision nutrigeroscience, allowing for improved DR-based therapeutic treatments personalized to individual and tissue-specific health status.
SUMMARY AND THE NEED FOR PRECISION NUTRIGEROSCIENCE
Broadly, nutrient restriction is an effective strategy to promote health and counteract age-related morbidities. The inverse relationship between dietary intake and lifespan in multiple species highlights the importance of the metabolic reprogramming induced by DR in lifespan and healthspan extension; however, since chronic DR is hard to sustain over long periods, alternative therapeutic interventions that partially mimic the physiological and molecular benefits of DR are of great interest to researchers in the field of aging biology. Given the difficulty to restrict nutrients in humans, efforts are needed to identify compounds or behavioral interventions that aid or function as DR inducers and mimetics. It is also becoming clear that the protective effects of DR are not exerted by a single mechanism and are unlikely to be universally beneficial to all individuals. Furthermore, the effects of DR on different tissues and healthspan measures are likely to involve different molecular mechanisms (Figure 4). A viable and seemingly promising approach is to optimize strategies that combine moderate DR with therapeutic and behavioral interventions such as exercise to obtain synergistic effects that promote health and longevity at the tissue and organismal level. The optimal diet or related intervention for a particular individual at a given age is also difficult to determine. Given that DR can have both beneficial and adverse effects, a careful design of therapies and an assessment of the mechanisms involved in mediating all effects of DR need to be thoroughly investigated. Thus, we propose the need for precision nutrigeroscience to develop biomarker panels for measuring biological age and determining how biomarkers are altered by dietary interventions within specific individuals and then optimize these interventions accordingly to optimize age-related health outcomes. Continued advances in systems biology, artificial-intelligence-based tools, and large-scale data analysis together with key mechanistic findings in the fields of aging are necessary to pave the way for the development of reliable biomarkers that can help predict outcomes for sets of individuals rather than populations to develop a framework for precision medicine approaches toward individualized healthy aging.
Figure 4. A framework for precision nutrigeroscience based on optimizing diet/pharmacology-based interventions to optimize health and longevity to accommodate factors that lead to individual variation.
We propose the concept of precision nutrigeroscience, the study of which will aim to utilize nutrient interventions and compounds that influence nutrient-signaling pathways to optimize lifespan, healthspan, and tissue-specific decline. However, these interventions need to be optimized based on factors such as sex, genetics, epigenetics, and age to optimize the desired outcomes. Furthermore, the use of biomarkers like circulating factors in the blood from transcriptomic, proteomic, or metabolomic changes associated with health outcomes could be used to appropriately change the nutrient and pharmacological interventions to optimize health outcomes. Together, the combination of these approaches could help achieve the goals of precision nutrigeroscience.
ACKNOWLEDGMENTS
This work was funded by grants from the American Federation of Aging Research (P.K.), NIH grants R01AG038688 and R01AG061165 (P.K.), and the Larry L. Hillblom Foundation. This work was supported by the DBT/Well-come Trust India Alliance Fellowship/grant number IA/I(S)/17/1/503085 awarded to G.C. K.A.W. is supported by T32AG000266-22. M.C. is supported by a postdoctoral fellowship from the Larry L. Hillblom Foundation. Thanks to Dr. Malene Hansen, Dr. Daniel Promislow, Dr. Lisa Ellerby, and the members of the Kapahi lab for their helpful comments.
Footnotes
DECLARATION OF INTERESTS
P.K. is founder and a member of the Scientific Advisory Board at Juvify Bio.
REFERENCES
- Adler MI, and Bonduriansky R (2014). Why do the well-fed appear to die young?: A new evolutionary hypothesis for the effect of dietary restriction on lifespan. BioEssays 36, 439–450. [DOI] [PubMed] [Google Scholar]
- Akagi K, Wilson KA, Katewa SD, Ortega M, Simons J, Hilsabeck TA, Kapuria S, Sharma A, Jasper H, and Kapahi P (2018). Dietary restriction improves intestinal cellular fitness to enhance gut barrier function and lifespan in D. melanogaster. PLoS Genet. 14, e1007777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An HS, Lee JY, Choi EB, Jeong EA, Shin HJ, Kim KE, Park KA, Jin Z, Lee JE, Koh JS, et al. (2020). Caloric restriction reverses left ventricular hypertrophy through the regulation of cardiac iron homeostasis in impaired leptin signaling mice. Sci. Rep 10, 7176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antunes F, Erustes AG, Costa AJ, Nascimento AC, Bincoletto C, Ureshino RP, Pereira GJS, and Smaili SS (2018). Autophagy and intermittent fasting: the connection for cancer therapy? Clinics (Sao Paulo) 73, e814s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armentero MT, Levandis G, Bramanti P, Nappi G, and Blandini F (2008). Dietary restriction does not prevent nigrostriatal degeneration in the 6-hydroxydopamine model of Parkinson’s disease. Exp. Neurol 212, 548–551. [DOI] [PubMed] [Google Scholar]
- Arner P, Bernard S, Salehpour M, Possnert G, Liebl J, Steier P, Buchholz BA, Eriksson M, Arner E, Hauner H, et al. (2011). Dynamics of human adipose lipid turnover in health and metabolic disease. Nature 478, 110–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin S, and St-Pierre J (2012). PGC1alpha and mitochondrial metabolism–emerging concepts and relevance in ageing and neurodegenerative disorders. J. Cell Sci 125, 4963–4971. [DOI] [PubMed] [Google Scholar]
- Avanesov AS, Ma S, Pierce KA, Yim SH, Lee BC, Clish CB, and Gladyshev VN (2014). Age- and diet-associated metabolome remodeling characterizes the aging process driven by damage accumulation. eLife 3, e02077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansal A, Zhu LJ, Yen K, and Tissenbaum HA (2015). Uncoupling lifespan and healthspan in Caenorhabditis elegans longevity mutants. Proc. Natl. Acad. Sci. USA 112, E277–E286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartel DP (2009). MicroRNAs: target recognition and regulatory functions. Cell 136, 215–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumeier C, Kaiser D, Heeren J, Scheja L, John C, Weise C, Eravci M, Lagerpusch M, Schulze G, Joost H-G, et al. (2015). Caloric restriction and intermittent fasting alter hepatic lipid droplet proteome and diacylglycerol species and prevent diabetes in NZO mice. Biochim. Biophys. Acta 1851, 566–576. [DOI] [PubMed] [Google Scholar]
- Bayliss JA, Lemus MB, Stark R, Santos VV, Thompson A, Rees DJ, Galic S, Elsworth JD, Kemp BE, Davies JS, and Andrews ZB (2016). Ghrelin-AMPK signaling mediates the neuroprotective effects of calorie restriction in Parkinson’s disease. J. Neurosci 36, 3049–3063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bédard K, Bédard J, Rocheleau G, Ferland G, and Gaudreau P (2013). Aging and diets regulate the rat anterior pituitary and hypothalamic transcriptome. Neuroendocrinology 97, 146–159. [DOI] [PubMed] [Google Scholar]
- Behrendt AK, Kuhla A, Osterberg A, Polley C, Herlyn P, Fischer DC, Scotland M, Wree A, Histing T, Menger MD, et al. (2016). Dietary restriction-induced alterations in bone phenotype: effects of lifelong versus short-term caloric restriction on femoral and vertebral bone in C57BL/6 mice. J. Bone Miner. Res 31, 852–863. [DOI] [PubMed] [Google Scholar]
- Belsky DW, Moffitt TE, Cohen AA, Corcoran DL, Levine ME, Prinz JA, Schaefer J, Sugden K, Williams B, Poulton R, and Caspi A (2018). Eleven telomere, epigenetic clock, and biomarker-composite quantifications of biological aging: do they measure the same thing? Am. J. Epidemiol 187, 1220–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendlin BB, Canu E, Willette A, Kastman EK, McLaren DG, Kosmatka KJ, Xu G, Field AS, Colman RJ, Coe CL, et al. (2011). Effects of aging and calorie restriction on white matter in rhesus macaques. Neurobiol. Aging 32, 2319.e1–2319.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berdichevsky A, Viswanathan M, Horvitz HR, and Guarente L (2006). C. elegans SIR-2.1 interacts with 14–3-3 proteins to activate DAF-16 and extend life span. Cell 125, 1165–1177. [DOI] [PubMed] [Google Scholar]
- Bergwitz C (2012). Dietary phosphate modifies lifespan in Drosophila. Nephrol. Dial. Transplant 27, 3399–3406. [DOI] [PubMed] [Google Scholar]
- Bhanpuri NH, Hallberg SJ, Williams PT, McKenzie AL, Ballard KD, Campbell WW, McCarter JP, Phinney SD, and Volek JS (2018). Cardiovascular disease risk factor responses to a type 2 diabetes care model including nutritional ketosis induced by sustained carbohydrate restriction at 1 year: an open label, non-randomized, controlled study. Cardiovasc. Diabetol 17, 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Białek M, Zaremba P, Borowicz KK, and Czuczwar SJ (2004). Neuroprotective role of testosterone in the nervous system. Pol. J. Pharmacol 56, 509–518. [PubMed] [Google Scholar]
- Birkisdóttir MB, Jaarsma D, Brandt RMC, Barnhoorn S, van Vliet N, Imholz S, van Oostrom CT, Nagarajah B, Portilla Fernández E, Roks AJM, et al. (2021). Unlike dietary restriction, rapamycin fails to extend lifespan and reduce transcription stress in progeroid DNA repair-deficient mice. Aging Cell 20, e13302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell BN, Bucci TJ, Hart RW, and Turturro A (1995). Longevity, body weight, and neoplasia in ad libitum-fed and diet-restricted C57BL6 mice fed NIH-31 open formula diet. Toxicol. Pathol 23, 570–582. [DOI] [PubMed] [Google Scholar]
- Bodnar M, Skalicky M, Viidik A, and Erben RG (2012). Interaction between exercise, dietary restriction and age-related bone loss in a rodent model of male senile osteoporosis. Gerontology 58, 139–149. [DOI] [PubMed] [Google Scholar]
- Bolund E, Lummaa V, Smith KR, Hanson HA, and Maklakov AA (2016). Reduced costs of reproduction in females mediate a shift from a male-biased to a female-biased lifespan in humans. Sci. Rep 6, 24672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondolfi L, Ermini F, Long JM, Ingram DK, and Jucker M (2004). Impact of age and caloric restriction on neurogenesis in the dentate gyrus of C57BL/6 mice. Neurobiol. Aging 25, 333–340 [DOI] [PubMed] [Google Scholar]
- Botega II, Zamarioli A, Guedes PMSG, Silva RABD, Issa JPM, Butezloff MM, Sousa YTCS, Ximenez JPB, and Volpon JB (2019). Bone callus formation is highly disrupted by dietary restriction in growing rats sustaining a femoral fracture1. Acta Cir. Bras 34, e20190010000002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandhorst S, Choi IY, Wei M, Cheng CW, Sedrakyan S, Navarrete G, Dubeau L, Yap LP, Park R, Vinciguerra M, et al. (2015). A periodic diet that mimics fasting promotes multi-system regeneration, enhanced cognitive performance, and healthspan. Cell Metab 22, 86–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandhorst S, Harputlugil E, Mitchell JR, and Longo VD (2017). Protective effects of short-term dietary restriction in surgical stress and chemotherapy. Ageing Res. Rev 39, 68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandhorst S, and Longo VD (2016). Fasting and caloric restriction in cancer prevention and treatment. Recent Results Cancer Res 207, 241–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandhorst S, and Longo VD (2019a). Dietary restrictions and nutrition in the prevention and treatment of cardiovascular disease. Circ. Res 124, 952–965. [DOI] [PubMed] [Google Scholar]
- Brandhorst S, and Longo VD (2019b). Protein quantity and source, fasting-mimicking diets, and longevity. Adv. Nutr 10, S340–S350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandhorst S, Wei M, Hwang S, Morgan TE, and Longo VD (2013). Short-term calorie and protein restriction provide partial protection from chemotoxicity but do not delay glioma progression. Exp. Gerontol 48, 1120–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredesen DE, Amos EC, Canick J, Ackerley M, Raji C, Fiala M, and Ahdidan J (2016). Reversal of cognitive decline in Alzheimer’s disease. Aging (Albany, NY) 8, 1250–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broer L, Buchman AS, Deelen J, Evans DS, Faul JD, Lunetta KL, Sebastiani P, Smith JA, Smith AV, Tanaka T, et al. (2015). GWAS of longevity in CHARGE Consortium confirms APOE and FOXO3 candidacy. J. Gerontol. A Biol. Sci. Med. Sci 70, 110–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown-Borg HM, Rakoczy SG, Wonderlich JA, Rojanathammanee L, Kopchick JJ, Armstrong V, and Raasakka D (2014). Growth hormone signaling is necessary for lifespan extension by dietary methionine. Aging Cell 13, 1019–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownlow ML, Joly-Amado A, Azam S, Elza M, Selenica ML, Pappas C, Small B, Engelman R, Gordon MN, and Morgan D (2014). Partial rescue of memory deficits induced by calorie restriction in a mouse model of tau deposition. Behav. Brain Res 271, 79–88. [DOI] [PubMed] [Google Scholar]
- Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer PL, Lin Y, Tran H, Ross SE, Mostoslavsky R, Cohen HY, et al. (2004). Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science 303, 2011–2015. [DOI] [PubMed] [Google Scholar]
- Bruss MD, Khambatta CF, Ruby MA, Aggarwal I, and Hellerstein MK (2010). Calorie restriction increases fatty acid synthesis and whole body fat oxidation rates. Am. J. Physiol. Endocrinol. Metab 298, E108–E116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burks TN, and Cohn RD (2011). One size may not fit all: anti-aging therapies and sarcopenia. Aging (Albany, NY) 3, 1142–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabelof DC, Yanamadala S, Raffoul JJ, Guo Z, Soofi A, and Heydari AR (2003). Caloric restriction promotes genomic stability by induction of base excision repair and reversal of its age-related decline. DNA Repair (Amst) 2, 295–307. [DOI] [PubMed] [Google Scholar]
- Calissi G, Lam EW, and Link W (2021). Therapeutic strategies targeting FOXO transcription factors. Nat. Rev. Drug Discov 20, 21–38. [DOI] [PubMed] [Google Scholar]
- Cantó C, Gerhart-Hines Z, Feige JN, Lagouge M, Noriega L, Milne JC, Elliott PJ, Puigserver P, and Auwerx J (2009). AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature 458, 1056–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao JJ (2018). Caloric restriction combined with exercise is effective in reducing adiposity and mitigating bone structural deterioration in obese rats. Ann. N. Y. Acad. Sci 1433, 41–52. [DOI] [PubMed] [Google Scholar]
- Carbone F, La Rocca C, and Matarese G (2012). Immunological functions of leptin and adiponectin. Biochimie 94, 2082–2088. [DOI] [PubMed] [Google Scholar]
- Caron A, Richard D, and Laplante M (2015). The roles of mTOR complexes in lipid metabolism. Annu. Rev. Nutr 35, 321–348. [DOI] [PubMed] [Google Scholar]
- Carr JS, Bonham LW, Morgans AK, Ryan CJ, Yokoyama JS, and Geier EG; Alzheimer’s Disease Neuroimaging Initiative (2018). Genetic variation in the androgen receptor and measures of plasma testosterone levels suggest androgen dysfunction in Alzheimer’s disease. Front. Neurosci 12, 529. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Castaño-Martinez T, Schumacher F, Schumacher S, Kochlik B, Weber D, Grune T, Biemann R, McCann A, Abraham K, Weikert C, et al. (2019). Methionine restriction prevents onset of type 2 diabetes in NZO mice. FASEB J 33, 7092–7102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterson JH, Khericha M, Dyson MC,Vincent AJ, Callard R, Haveron SM, Rajasingam A, Ahmad M, and Partridge L (2018). Short-term, intermittent fasting induces long-lasting gut health and TOR-independent lifespan extension. Curr. Biol 28, 1714–1724.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamoli M, Singh A, Malik Y, and Mukhopadhyay A (2014).A novel kinase regulates dietary restriction-mediated longevity in Caenorhabditis elegans. Aging Cell 13, 641–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamoli M, Goyala A, Tabrez SS, Siddiqui AA, Singh A, Antebi A, Lithgow GJ, Watts JL, and Mukhopadhyay A (2020). Polyunsaturated fatty acids and p38-MAPK link metabolic reprogramming to cytoprotective gene expression during dietary restriction. Nat. Commun 11, 4865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhuri J, Bose N, Gong J, Hall D, Rifkind A, Bhaumik D, Peiris TH, Chamoli M, Le CH, Liu J, et al. (2016). A Caenorhabditis elegans model elucidates a conserved role for TRPA1-Nrf signaling in reactive alpha-dicarbonyl detoxification. Curr. Biol 26, 3014–3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chawla G, Deosthale P, Childress S, Wu YC, and Sokol NS (2016). A let-7-to-miR-125 microRNA switch regulates neuronal integrity and lifespan in Drosophila. PLoS Genet 12, e1006247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D, Steele AD, Lindquist S, and Guarente L (2005). Increase in activity during calorie restriction requires Sirt1. Science 310, 1641. [DOI] [PubMed] [Google Scholar]
- Chen D, Pan KZ, Palter JE, and Kapahi P (2007). Longevity determined by developmental arrest genes in Caenorhabditis elegans. Aging Cell 6, 525–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D,Thomas EL, and Kapahi P (2009). HIF-1 modulates dietary restriction-mediated lifespan extension via IRE-1 in Caenorhabditis elegans. PLoS Genetics 5, e1000486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B, Retzlaff M, Roos T, and Frydman J (2011). Cellular strategies of protein quality control. Cold Spring Harb. Perspect. Biol 3, a004374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CN, Liao YH, Tsai SC, and Thompson LV (2019). Age-dependent effects of caloric restriction on mTOR and ubiquitin-proteasome pathways in skeletal muscles. GeroScience 41, 871–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng CJ, Gelfond JAL, Strong R, and Nelson JF (2019). Genetically heterogeneous mice exhibit a female survival advantage that is age- and site-specific: results from a large multi-site study. Aging Cell 18, e12905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherrier MM, Anderson K, Shofer J, Millard S, and Matsumoto AM (2015). Testosterone treatment of men with mild cognitive impairment and low testosterone levels. Am. J. Alzheimers Dis. Other Demen 30, 421–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung P, Vallania F, Warsinske HC, Donato M, Schaffert S, Chang SE, Dvorak M, Dekker CL, Davis MM, Utz PJ, et al. (2018). Singlecell chromatin modification profiling reveals increased epigenetic variations with aging. Cell 173, 1385–1397.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba T, Tamashiro Y, Park D, Kusudo T, Fujie R, Komatsu T, Kim SE, Park S, Hayashi H, Mori R, et al. (2014). A key role for neuropeptide Y in lifespan extension and cancer suppression via dietary restriction. Sci. Rep 4, 4517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhary C, Weinert BT, Nishida Y, Verdin E, and Mann M (2014). The growing landscape of lysine acetylation links metabolism and cell signalling. Nat. Rev. Mol. Cell Biol 15, 536–550. [DOI] [PubMed] [Google Scholar]
- Civitarese AE, Carling S, Heilbronn LK, Hulver MH, Ukropcova B, Deutsch WA, Smith SR, and Ravussin E; CALERIE Pennington Team (2007). Calorie restriction increases muscle mitochondrial biogenesis in healthy humans. PLoS Med 4, e76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clegg A, Young J, Iliffe S, Rikkert MO, and Rockwood K (2013). Frailty in elderly people. Lancet 381, 752–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen K, Waldman M, Abraham NG, Laniado-Schwartzman M, Gurfield D, Aravot D, Arad M, and Hochhauser E (2017). Caloric restriction ameliorates cardiomyopathy in animal model of diabetes. Exp. Cell Res 350, 147–153. [DOI] [PubMed] [Google Scholar]
- Colman RJ, Anderson RM, Johnson SC, Kastman EK, Kosmatka KJ, Beasley TM, Allison DB, Cruzen C, Simmons HA, Kemnitz JW, and Weindruch R (2009). Caloric restriction delays disease onset and mortality in rhesus monkeys. Science 325, 201–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman RJ, Beasley TM, Kemnitz JW, Johnson SC, Weindruch R, and Anderson RM (2014). Caloric restriction reduces age-related and allcause mortality in rhesus monkeys. Nat. Commun 5, 3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contestabile A, Ciani E, and Contestabile A (2004). Dietary restriction differentially protects from neurodegeneration in animal models of excitotoxicity. Brain Res 1002, 162–166. [DOI] [PubMed] [Google Scholar]
- Cosio MG, Cazzuffi R, and Saetta M (2014). Is chronic obstructive pulmonary disease a disease of aging? Respiration 87, 508–512. [DOI] [PubMed] [Google Scholar]
- Covarrubias AJ, Perrone R, Grozio A, and Verdin E (2021). NAD+ metabolism and its roles in cellular processes during ageing. Nat. Rev. Mol. Cell Biol 22, 119–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csiszar A, Gautam T, Sosnowska D, Tarantini S, Banki E, Tucsek Z, Toth P, Losonczy G, Koller A, Reglodi D, et al. (2014). Caloric restriction confers persistent anti-oxidative, pro-angiogenic, and anti-inflammatory effects and promotes anti-aging miRNA expression profile in cerebromicrovascular endothelial cells of aged rats. Am. J. Physiol. Heart Circ. Physiol 307, H292–H306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings NE, Williams EM, Kasza I, Konon EN, Schaid MD, Schmidt BA, Poudel C, Sherman DS, Yu D, Arriola Apelo SI, et al. (2018). Restoration of metabolic health by decreased consumption of branched-chain amino acids. J. Physiol 596, 623–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Cabo R, Liu L, Ali A, Price N, Zhang J, Wang M, Lakatta E, and Irusta PM (2015). Serum from calorie-restricted animals delays senescence and extends the lifespan of normal human fibroblasts in vitro. Aging (Albany, NY) 7, 152–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groot S, Vreeswijk MP, Welters MJ, Gravesteijn G, Boei JJ, Jochems A, Houtsma D, Putter H, van der Hoeven JJ, Nortier JW, et al. (2015). The effects of short-term fasting on tolerance to (neo) adjuvant chemotherapy in HER2-negative breast cancer patients: a randomized pilot study. BMC Cancer 15, 652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries EM, van Beeren HC, Ackermans MT, Kalsbeek A, Fliers E, and Boelen A (2015). Differential effects of fasting vs food restriction on liver thyroid hormone metabolism in male rats. J. Endocrinol 224, 25–35. [DOI] [PubMed] [Google Scholar]
- Dhahbi JM (2014). Circulating small noncoding RNAs as biomarkers of aging. Ageing Res. Rev 17, 86–98. [DOI] [PubMed] [Google Scholar]
- Dhahbi JM, Spindler SR, Atamna H, Yamakawa A, Guerrero N, Boffelli D, Mote P, and Martin DI (2013). Deep sequencing identifies circulating mouse miRNAs that are functionally implicated in manifestations of aging and responsive to calorie restriction. Aging (Albany, NY) 5, 130–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Malta C, Siciliano D, Calcagni A, Monfregola J, Punzi S, Pastore N, Eastes AN, Davis O, De Cegli R, Zampelli A, et al. (2017). Transcriptional activation of RagD GTPase controls mTORC1 and promotes cancer growth. Science 356, 1188–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimmeler S, and Nicotera P (2013). MicroRNAs in age-related diseases. EMBO Mol. Med 5, 180–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon AE, and Peters U (2018). The effect of obesity on lung function. Expert Rev. Respir. Med 12, 755–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dkhar P, and Sharma R (2014). Late-onset dietary restriction modulates protein carbonylation and catalase in cerebral hemispheres of aged mice. Cell. Mol. Neurobiol 34, 307–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donati A, Cavallini G, and Bergamini E (2013). Effects of aging, antiaging calorie restriction and in vivo stimulation of autophagy on the urinary excretion of 8OHdG in male Sprague-Dawley rats. Age (Dordr) 35, 261–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan W, Guo Z, Jiang H, Ware M, Li XJ, and Mattson MP (2003). Dietary restriction normalizes glucose metabolism and BDNF levels, slows disease progression, and increases survival in huntingtin mutant mice. Proc. Natl. Acad. Sci. USA 100, 2911–2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan W, and Mattson MP (1999). Dietary restriction and 2-deoxyglucose administration improve behavioral outcome and reduce degeneration of dopaminergic neurons in models of Parkinson’s disease. J. Neurosci. Res 57, 195–206. [DOI] [PubMed] [Google Scholar]
- Dubinsky AN, Dastidar SG, Hsu CL, Zahra R, Djakovic SN, Duarte S, Esau CC, Spencer B, Ashe TD, Fischer KM, et al. (2014). Let-7 coordinately suppresses components of the amino acid sensing pathway to repress mTORC1 and induce autophagy. Cell Metab 20, 626–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durieux J, Wolff S, and Dillin A (2011). The cell-non-autonomous nature of electron transport chain-mediated longevity. Cell 144, 79–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery PW, and Sanderson P (1995). Effect of dietary restriction on protein synthesis and wound healing after surgery in the rat. Clin. Sci. (Lond.) 89, 383–388. [DOI] [PubMed] [Google Scholar]
- Encinas JM, Michurina TV, Peunova N, Park JH, Tordo J, Peterson DA, Fishell G, Koulakov A, and Enikolopov G (2011). Division-coupled astrocytic differentiation and age-related depletion of neural stem cells in the adult hippocampus. Cell Stem Cell 8, 566–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernsberger P, Koletsky RJ, Baskin JS, and Foley M (1994). Refeeding hypertension in obese spontaneously hypertensive rats. Hypertension 24, 699–705. [DOI] [PubMed] [Google Scholar]
- Ernsberger P, Koletsky RJ, and Friedman JE (1999). Molecular pathology in the obese spontaneous hypertensive Koletsky rat: a model of syndrome X. Ann. N. Y. Acad. Sci 892, 272–288. [DOI] [PubMed] [Google Scholar]
- Fabrizio P, Pozza F, Pletcher SD, Gendron CM, and Longo VD (2001). Regulation of longevity and stress resistance by Sch9 in yeast. Science 292, 288–290. [DOI] [PubMed] [Google Scholar]
- Fabrizio P, Li L, and Longo VD (2005). Analysis of gene expression profile in yeast aging chronologically. Mech. Ageing Dev 126, 11–16. [DOI] [PubMed] [Google Scholar]
- Fan P, Liu P, Song P, Chen X, and Ma X (2017). Moderate dietary protein restriction alters the composition of gut microbiota and improves ileal barrier function in adult pig model. Sci. Rep 7, 43412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanson BG, Weldon CW, Pérez-Staples D, Simpson SJ, and Taylor PW (2009). Nutrients, not caloric restriction, extend lifespan in Queensland fruit flies (Bactrocera tryoni). Aging Cell 8, 514–523. [DOI] [PubMed] [Google Scholar]
- Farina AR, Cappabianca L, Ruggeri P, Gneo L, Maccarone R, and Mackay AR (2015). Retrograde TrkAIII transport from ERGIC to ER: a re-localisation mechanism for oncogenic activity. Oncotarget 6, 35636–35651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinman RD, Pogozelski WK, Astrup A, Bernstein RK, Fine EJ, Westman EC, Accurso A, Frassetto L, Gower BA, McFarlane SI, et al. (2015). Dietary carbohydrate restriction as the first approach in diabetes management: critical review and evidence base. Nutrition 31, 1–13. [DOI] [PubMed] [Google Scholar]
- Fontana L (2018). Interventions to promote cardiometabolic health and slow cardiovascular ageing. Nat. Rev. Cardiol 15, 566–577. [DOI] [PubMed] [Google Scholar]
- Fontana L, and Partridge L (2015). Promoting health and longevity through diet: from model organisms to humans. Cell 161, 106–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana L, Meyer TE, Klein S, and Holloszy JO (2004). Long-term calorie restriction is highly effective in reducing the risk for atherosclerosis in humans. Proc. Natl. Acad. Sci. USA 101, 6659–6663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana L, Eagon JC, Trujillo ME, Scherer PE, and Klein S (2007). Visceral fat adipokine secretion is associated with systemic inflammation in obese humans. Diabetes 56, 1010–1013. [DOI] [PubMed] [Google Scholar]
- Fontana L, Adelaiye RM, Rastelli AL, Miles KM, Ciamporcero E, Longo VD, Nguyen H, Vessella R, and Pili R (2013). Dietary protein restriction inhibits tumor growth in human xenograft models. Oncotarget 4, 2451–2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana L, Cummings NE, Arriola Apelo SI, Neuman JC, Kasza I, Schmidt BA, Cava E, Spelta F, Tosti V, Syed FA, et al. (2016). Decreased consumption of branched-chain amino acids improves metabolic health. Cell Rep 16, 520–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana L, Mitchell SE, Wang B, Tosti V, van Vliet T, Veronese N, Bertozzi B, Early DS, Maissan P, Speakman JR, and Demaria M (2018a). The effects of graded caloric restriction: XII. Comparison of mouse to human impact on cellular senescence in the colon. Aging Cell 17, e12746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana L, Nehme J, and Demaria M (2018b). Caloric restriction and cellular senescence. Mech. Ageing Dev 176, 19–23. [DOI] [PubMed] [Google Scholar]
- Forster MJ, Sohal BH, and Sohal RS (2000). Reversible effects of long-term caloric restriction on protein oxidative damage. J. Gerontol. A Biol. Sci. Med. Sci 55, B522–B529. [DOI] [PubMed] [Google Scholar]
- Freedman VA, Martin LG, and Schoeni RF (2002). Recent trends in disability and functioning among older adults in the United States: a systematic review. JAMA 288, 3137–3146. [DOI] [PubMed] [Google Scholar]
- Frescas D, Valenti L, and Accili D (2005). Nuclear trapping of the forkhead transcription factor FoxO1 via Sirt-dependent deacetylation promotes expression of glucogenetic genes. J. Biol. Chem 280, 20589–20595. [DOI] [PubMed] [Google Scholar]
- Fujita S, Abe T, Drummond MJ, Cadenas JG, Dreyer HC, Sato Y, Volpi E, and Rasmussen BB (2007a). Blood flow restriction during low-intensity resistance exercise increases S6K1 phosphorylation and muscle protein synthesis. J. Appl. Physiol. (1985) 103, 903–910. [DOI] [PubMed] [Google Scholar]
- Fujita S, Rasmussen BB, Cadenas JG, Drummond MJ, Glynn EL, Sattler FR, and Volpi E (2007b). Aerobic exercise overcomes the age-related insulin resistance of muscle protein metabolism by improving endothelial function and Akt/mammalian target of rapamycin signaling. Diabetes 56, 1615–1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuyama T, Yamashita H, Kitayama K, Higami Y, Shimokawa I, and Mori N (2002). Effects of aging and caloric restriction on the gene expression of FoxO1, 3, and 4 (FKHR, FKHRL1, and AFX) in the rat skeletal muscles. Microsc. Res. Tech 59, 331–334. [DOI] [PubMed] [Google Scholar]
- García-Prieto CF, and Fernández-Alfonso MS (2016). Caloric restriction as a strategy to improve vascular dysfunction in metabolic disorders. Nutrients 8, 370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garratt M, Nakagawa S, and Simons MJ (2016). Comparative idiosyncrasies in life extension by reduced mTOR signalling and its distinctiveness from dietary restriction. Aging Cell 15, 737–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelino S, Chang JT, Kumsta C, She X, Davis A, Nguyen C, Panowski S,and Hansen M (2016). Intestinal autophagy improves healthspan and longevity in C. elegans during dietary restriction. PLoS Genet 12, e1006135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh AK, Mau T, O’Brien M, Garg S, and Yung R (2016). Impaired autophagy activity is linked to elevated ER-stress and inflammation in aging adipose tissue. Aging (Albany, NY) 8, 2525–2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannakou ME, Goss M, and Partridge L (2008). Role of dFOXO in lifespan extension by dietary restriction in Drosophila melanogaster: not required, but its activity modulates the response. Aging Cell 7, 187–198. [DOI] [PubMed] [Google Scholar]
- Girard N, Ferland G, Boulanger L, and Gaudreau P (1998). Long-term calorie restriction protects rat pituitary growth hormone-releasing hormone binding sites from age-related alterations. Neuroendocrinology 68, 21–29. [DOI] [PubMed] [Google Scholar]
- Godlewski J, Nowicki MO, Bronisz A, Nuovo G, Palatini J, De Lay M, Van Brocklyn J, Ostrowski MC, Chiocca EA, and Lawler SE (2010). MicroRNA-451 regulates LKB1/AMPK signaling and allows adaptation to metabolic stress in glioma cells. Mol. Cell 37, 620–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good TP, and Tatar M (2001). Age-specific mortality and reproduction respond to adult dietary restriction in Drosophila melanogaster. J. Insect Physiol 47, 1467–1473. [DOI] [PubMed] [Google Scholar]
- Gordon EH, Peel NM, Samanta M, Theou O, Howlett SE, and Hubbard RE (2017). Sex differences in frailty: a systematic review and meta-analysis. Exp. Gerontol 89, 30–40. [DOI] [PubMed] [Google Scholar]
- Gordon-Dseagu VLZ, Thompson FE, Subar AF, Ruder EH, Thiébaut ACM, Potischman N, and Stolzenberg-Solomon R (2017). A cohort study of adolescent and midlife diet and pancreatic cancer risk in the NIH-AARP diet and health study. Am. J. Epidemiol 186, 305–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gräff J, Kahn M, Samiei A, Gao J, Ota KT, Rei D, and Tsai LH (2013). A dietary regimen of caloric restriction or pharmacological activation of SIRT1 to delay the onset of neurodegeneration. J. Neurosci 33, 8951–8960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandison RC, Piper MD, and Partridge L (2009a). Amino-acid imbalance explains extension of lifespan by dietary restriction in Drosophila. Nature 462, 1061–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandison RC, Wong R, Bass TM, Partridge L, and Piper MD (2009b). Effect of a standardised dietary restriction protocol on multiple laboratory strains of Drosophila melanogaster. PLoS One 4, e4067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant LK, Ftouni S, Nijagal B, De Souza DP, Tull D, McConville MJ, Rajaratnam SMW, Lockley SW, and Anderson C (2019). Circadian and wake-dependent changes in human plasma polar metabolites during prolonged wakefulness: a preliminary analysis. Sci. Rep 9, 4428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratuze M, Julien J, Morin F, Marette A, and Planel E (2017). Differential effects of voluntary treadmill exercise and caloric restriction on tau pathogenesis in a mouse model of Alzheimer’s disease-like tau pathology fed with Western diet. Prog. Neuropsychopharmacol. Biol. Psychiatry 79, 452–461. [DOI] [PubMed] [Google Scholar]
- Green CB, Takahashi JS, and Bass J (2008). The meter of metabolism. Cell 134, 728–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer EL, and Brunet A (2009). Different dietary restriction regimens extend lifespan by both independent and overlapping genetic pathways in C. elegans. Aging Cell 8, 113–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer EL, Dowlatshahi D, Banko MR, Villen J, Hoang K, Blanchard D, Gygi SP, and Brunet A (2007a). An AMPK-FOXO pathway mediates longevity induced by a novel method of dietary restriction in C. elegans. Curr. Biol 17, 1646–1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer EL, Oskoui PR, Banko MR, Maniar JM, Gygi MP, Gygi SP, and Brunet A (2007b). The energy sensor AMP-activated protein kinase directly regulates the mammalian FOXO3 transcription factor. J. Biol. Chem 282, 30107–30119. [DOI] [PubMed] [Google Scholar]
- Greer EL, Banko MR, and Brunet A (2009). AMP-activated protein kinase and FoxO transcription factors in dietary restriction-induced longevity. Ann. N. Y. Acad. Sci 1170, 688–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregosa Á, Vinuesa A, Todero MF, Pomilio C, Rossi SP, Bentivegna M, Presa J, Wenker S, Saravia F, and Beauquis J (2019). Periodic dietary restriction ameliorates amyloid pathology and cognitive impairment in PDAPP-J20 mice: potential implication of glial autophagy. Neurobiol. Dis 132, 104542. [DOI] [PubMed] [Google Scholar]
- Guarente L (2008). Mitochondria-a nexus for aging, calorie restriction, and sirtuins? Cell 132, 171–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra BA, Brandão BB, Pinto SS, Salgueiro WG, De-Souza EA, Reis FCG, Batista TM, Cavalcante-Silva V, D’Almeida V, Castilho BA, et al. (2019). Dietary sulfur amino acid restriction upregulates DICER to confer beneficial effects. Mol. Metab 29, 124–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerville F, De Souto Barreto P, Ader I, Andrieu S, Casteilla L, Dray C, Fazilleau N, Guyonnet S, Langin D, Liblau R, et al. (2020). Revisiting the hallmarks of aging to identify markers of biological age. J. Prev. Alzheimers Dis 7, 56–64. [DOI] [PubMed] [Google Scholar]
- Gutiérrez-Casado E, Khraiwesh H, López-Domínguez JA, Montero-Guisado J, López-Lluch G, Navas P, de Cabo R, Ramsey JJ, González-Reyes JA, and Villalba JM (2019). The impact of aging, calorie restriction and dietary fat on autophagy markers and mitochondrial ultrastructure and dynamics in mouse skeletal muscle. J. Gerontol. A Biol. Sci. Med. Sci 74, 760–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahm JH, Kim S, DiLoreto R, Shi C, Lee SJ, Murphy CT, and Nam HG (2015). C. elegans maximum velocity correlates with healthspan and is maintained in worms with an insulin receptor mutation. Nat. Commun 6, 8919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn O, Drews LF, Nguyen A, Tatsuta T, Gkioni L, Hendrich O, Zhang Q, Langer T, Pletcher S, Wakelam MJO, et al. (2019). A nutritional memory effect counteracts benefits of dietary restriction in old mice. Nat. Metab 1, 1059–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halagappa VK, Guo Z, Pearson M, Matsuoka Y, Cutler RG, Laferla FM, and Mattson MP (2007). Intermittent fasting and caloric restriction ameliorate age-related behavioral deficits in the triple-transgenic mouse model of Alzheimer’s disease. Neurobiol. Dis 26, 212–220. [DOI] [PubMed] [Google Scholar]
- Haller S, Kapuria S, Riley RR, O’Leary MN, Schreiber KH, Andersen JK, Melov S, Que J, Rando TA, Rock J, et al. (2017). mTORC1 activation during repeated regeneration impairs somatic stem cell maintenance. Cell Stem Cell 21, 806–818.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D, and Weinberg RA (2011). Hallmarks of cancer: the next generation. Cell 144, 646–674. [DOI] [PubMed] [Google Scholar]
- Hannah WN Jr., and Harrison SA (2016). Effect of weight loss, diet, exercise, and bariatric surgery on nonalcoholic fatty liver disease. Clin. Liver Dis 20, 339–350. [DOI] [PubMed] [Google Scholar]
- Hansen M, Chandra A, Mitic LL, Onken B, Driscoll M, and Kenyon C (2008). A role for autophagy in the extension of lifespan by dietary restriction in C. elegans. PLoS Genet 4, e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie DG, Ross FA, and Hawley SA (2012). AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat. Rev. Mol. Cell Biol 13, 251–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy OT, Czech MP, and Corvera S (2012). What causes the insulin resistance underlying obesity? Curr. Opin. Endocrinol. Diabetes Obes 19, 81–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori S, Park JH, Agata U, Oda M, Higano M, Aikawa Y, Akimoto T, Nabekura Y, Yamato H, Ezawa I, and Omi N (2014). Food restriction causes low bone strength and microarchitectural deterioration in exercised growing male rats. J. Nutr. Sci. Vitaminol. (Tokyo) 60, 35–42. [DOI] [PubMed] [Google Scholar]
- Heestand BN, Shen Y, Liu W, Magner DB, Storm N, Meharg C, Habermann B, and Antebi A (2013). Dietary restriction induced longevity is mediated by nuclear receptor NHR-62 in Caenorhabditis elegans. PLoS Genet 9, e1003651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilbronn LK, de Jonge L, Frisard MI, DeLany JP, Larson-Meyer DE, Rood J, Nguyen T, Martin CK, Volaufova J, Most MM, et al. (2006). Effect of 6-month calorie restriction on biomarkers of longevity, metabolic adaptation, and oxidative stress in overweight individuals: a randomized controlled trial. JAMA 295, 1539–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heintz C, Doktor TK, Lanjuin A, Escoubas C, Zhang Y, Weir HJ, Dutta S, Silva-García CG, Bruun GH, Morantte I, et al. (2017). Splicing factor 1 modulates dietary restriction and TORC1 pathway longevity in C. elegans. Nature 541, 102–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepple RT, Qin M, Nakamoto H, and Goto S (2008). Caloric restriction optimizes the proteasome pathway with aging in rat plantaris muscle: implications for sarcopenia. Am. J. Physiol. Regul. Integr. Comp. Physiol 295, R1231–R1237. [DOI] [PubMed] [Google Scholar]
- Herranz D, Iglesias G, Muñoz-Martín M, and Serrano M (2011). Limited role of Sirt1 in cancer protection by dietary restriction. Cell Cycle 10, 2215–2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman RM (2019). Methionine restriction and life-span extension. Methods Mol. Biol 1866, 263–266. [DOI] [PubMed] [Google Scholar]
- Hoffman RM, and Stern PH (2019). Dietary methionine restriction-based cancer chemotherapy in rodents. Methods Mol. Biol 1866, 83–94. [DOI] [PubMed] [Google Scholar]
- Holcomb VB, Keck VA, Barrett J, Hong J, Libutti SK, and Nunez NP (2009). Obesity impairs wound healing in ovariectomized female mice. In Vivo 23, 515–518. [PubMed] [Google Scholar]
- Holliday R (1989). Food, reproduction and longevity: is the extended lifespan of calorie-restricted animals an evolutionary adaptation? BioEssays 10, 125–127. [DOI] [PubMed] [Google Scholar]
- Honjoh S, Yamamoto T, Uno M, and Nishida E (2009). Signalling through RHEB-1 mediates intermittent fasting-induced longevity in C. elegans. Nature 457, 726–730. [DOI] [PubMed] [Google Scholar]
- Hord JM, Botchlett R, and Lawler JM (2016). Age-related alterations in the sarcolemmal environment are attenuated by lifelong caloric restriction and voluntary exercise. Exp. Gerontol 83, 148–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou L, Wang D, Chen D, Liu Y, Zhang Y, Cheng H, Xu C, Sun N, McDermott J, Mair WB, and Han J-DJ (2016). A systems approach to reverse engineer lifespan extension by dietary restriction. Cell Metab 23, 529–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houtkooper RH, Mouchiroud L, Ryu D, Moullan N, Katsyuba E, Knott G, Williams RW, and Auwerx J (2013). Mitonuclear protein imbalance as a conserved longevity mechanism. Nature 497, 451–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang TH, and Ables GP (2016). Dietary restrictions, bone density, and bone quality. Ann. N. Y. Acad. Sci 1363, 26–39. [DOI] [PubMed] [Google Scholar]
- Hunt ND, Li GD, Zhu M, Miller M, Levette A, Chachich ME, Spangler EL, Allard JS, Hyun DH, Ingram DK, et al. (2012). Effect of calorie restriction and refeeding on skin wound healing in the rat. Age (Dordr) 34, 1453–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde PN, Sapper TN, Crabtree CD, LaFountain RA, Bowling ML, Buga A, Fell B, McSwiney FT, Dickerson RM, Miller VJ, et al. (2019). Dietary carbohydrate restriction improves metabolic syndrome independent of weight loss. JCI Insight 4, e128308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igarashi M, and Guarente L (2016). mTORC1 and SIRT1 cooperate to foster expansion of gut adult stem cells during calorie restriction. Cell 166, 436–450. [DOI] [PubMed] [Google Scholar]
- Il’yasova D, Fontana L, Bhapkar M, Pieper CF, Spasojevic I, Redman LM, Das SK, Huffman KM, and Kraus WE; CALeRiE Study Investigators (2018). Effects of 2 years of caloric restriction on oxidative status assessed by urinary F2-isoprostanes: the CALERIE 2 randomized clinical trial. Aging Cell 17, e12719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jadiya P, Chatterjee M, Sammi SR, Kaur S, Palit G, and Nazir A (2011). Sir-2.1 modulates ‘calorie-restriction-mediated’ prevention of neurodegeneration in Caenorhabditis elegans: implications for Parkinson’s disease. Biochem. Biophys. Res. Commun 413, 306–310. [DOI] [PubMed] [Google Scholar]
- Jang YC, Liu Y, Hayworth CR, Bhattacharya A, Lustgarten MS, Muller FL, Chaudhuri A, Qi W, Li Y, Huang J-Y, et al. (2012). Dietary restriction attenuates age-associated muscle atrophy by lowering oxidative stress in mice even in complete absence of CuZnSOD. Aging Cell 11, 770–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasienska G, Bribiescas RG, Furberg AS, Helle S, and Núñez-de la Mora A (2017). Human reproduction and health: an evolutionary perspective. Lancet 390, 510–520. [DOI] [PubMed] [Google Scholar]
- Jensen K, McClure C, Priest NK, and Hunt J (2015). Sex-specific effects of protein and carbohydrate intake on reproduction but not lifespan in Drosophila melanogaster. Aging Cell 14, 605–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin J (2018). Risk assessment for cardiovascular disease with nontraditional risk factors. JAMA 320, 316. [DOI] [PubMed] [Google Scholar]
- Jin K, Wilson KA, Beck JN, Nelson CS, Brownridge GW 3rd, Harrison BR, Djukovic D, Raftery D, Brem RB, Yu S, et al. (2020). Genetic and metabolomic architecture of variation in diet restriction-mediated lifespan extension in Drosophila. PLoS Genet 16, e1008835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jongbloed F, Saat TC, Verweij M, Payan-Gomez C, Hoeijmakers JH, van den Engel S, van Oostrom CT, Ambagtsheer G, Imholz S, Pennings JL, et al. (2017). A signature of renal stress resistance induced by short-term dietary restriction, fasting, and protein restriction. Sci. Rep 7, 40901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung SH, Park HS, Kim KS, Choi WH, Ahn CW, Kim BT, Kim SM, Lee SY, Ahn SM, Kim YK, et al. (2008). Effect of weight loss on some serum cytokines in human obesity: increase in IL-10 after weight loss. J. Nutr. Biochem 19, 371–375. [DOI] [PubMed] [Google Scholar]
- Juricic P, Grönke S, and Partridge L (2020). Branched-chain amino acids have equivalent effects to other essential amino acids on lifespan and aging-related traits in Drosophila. J. Gerontol. A Biol. Sci. Med. Sci 75, 24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabil H, Kabil O, Banerjee R, Harshman LG, and Pletcher SD (2011). Increased transsulfuration mediates longevity and dietary restriction in Drosophila. Proc. Natl. Acad. Sci. USA 108, 16831–16836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaeberlein M, and Powers RW 3rd. (2007). Sir2 and calorie restriction in yeast: a skeptical perspective. Ageing Res. Rev 6, 128–140. [DOI] [PubMed] [Google Scholar]
- Kaeberlein M, Powers RW 3rd, Steffen KK, Westman EA, Hu D, Dang N, Kerr EO, Kirkland KT, Fields S, and Kennedy BK (2005). Regulation of yeast replicative life span by TOR and Sch9 in response to nutrients. Science 310, 1193–1196. [DOI] [PubMed] [Google Scholar]
- Kalaany NY, and Sabatini DM (2009). Tumours with PI3K activation are resistant to dietary restriction. Nature 458, 725–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanda Y, Hashiramoto M, Shimoda M, Hamamoto S, Tawaramoto K, Kimura T, Hirukawa H, Nakashima K, and Kaku K (2015). Dietary restriction preserves the mass and function of pancreatic beta cells via cell kinetic regulation and suppression of oxidative/ER stress in diabetic mice. J. Nutr. Biochem 26, 219–226. [DOI] [PubMed] [Google Scholar]
- Kandimalla R, Thirumala V, and Reddy PH (2017). Is Alzheimer’s disease a type 3 diabetes? A critical appraisal. Biochim. Biophys. Acta Mol. Basis Dis 1863, 1078–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapahi P, Zid BM, Harper T, Koslover D, Sapin V, and Benzer S (2004). Regulation of lifespan in Drosophila by modulation of genes in the TOR signaling pathway. Curr. Biol 14, 885–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapahi P, Chen D, Rogers AN, Katewa SD, Li PW, Thomas EL, and Kockel L (2010). With TOR, less is more: a key role for the conserved nutrient-sensing TOR pathway in aging. Cell Metab 11, 453–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapahi P, Kaeberlein M, and Hansen M (2017). Dietary restriction and lifespan: lessons from invertebrate models. Ageing Res. Rev 39, 3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karere GM, Glenn JP, Birnbaum S, Hafizi S, Rainwater DL, Mahaney MC, VandeBerg JL, and Cox LA (2013). Identification of candidate genes encoding an LDL-C QTL in baboons. J. Lipid Res 54, 1776–1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katewa SD, Demontis F, Kolipinski M, Hubbard A, Gill MS, Perrimon N, Melov S, and Kapahi P (2012). Intramyocellular fatty-acid metabolism plays a critical role in mediating responses to dietary restriction in Drosophila melanogaster. Cell Metab 16, 97–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katewa SD, Akagi K, Bose N, Rakshit K, Camarella T, Zheng X, Hall D, Davis S, Nelson CS, Brem RB, et al. (2016). Peripheral circadian clocks mediate dietary restriction-dependent changes in lifespan and fat metabolism in Drosophila. Cell Metab 23, 143–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsyuba E, Romani M, Hofer D, and Auwerx J (2020). NAD+ homeostasis in health and disease. Nat. Metab 2, 9–31. [DOI] [PubMed] [Google Scholar]
- Kaushik S, and Cuervo AM (2015). Proteostasis and aging. Nat. Med 21, 1406–1415. [DOI] [PubMed] [Google Scholar]
- Kauwe G, Tsurudome K, Penney J, Mori M, Gray L, Calderon MR, Elazouzzi F, Chicoine N, Sonenberg N, and Haghighi AP (2016). Acute fasting regulates retrograde synaptic enhancement through a 4E-BP-dependent mechanism. Neuron 92, 1204–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke Z, Firsanov D, Spencer B, Seluanov A, and Gorbunova V (2020). Short-term calorie restriction enhances DNA repair by non-homologous end joining in mice. NPJ Aging Mech. Dis 6, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keenan MJ, Marco ML, Ingram DK, and Martin RJ (2015). Improving healthspan via changes in gut microbiota and fermentation. Age (Dordr) 37, 98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy BK, Austriaco NR, Zhang J, and Guarente L (1995). Mutation in the silencing gene SIR4 can delay aging in S. cerevisiae. Cell 80, 485–496. [DOI] [PubMed] [Google Scholar]
- Kerr F, Augustin H, Piper MD, Gandy C, Allen MJ, Lovestone S, and Partridge L (2011). Dietary restriction delays aging, but not neuronal dysfunction, in Drosophila models of Alzheimer’s disease. Neurobiol. Aging 32, 1977–1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna A, Muthusamy S, Liang R, Sarojini H, and Wang E (2011). Gain of survival signaling by down-regulation of three key miRNAs in brain of calorie-restricted mice. Aging (Albany, NY) 3, 223–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DH, Park MH, Chung KW, Kim MJ, Jung YR, Bae HR, Jang EJ, Lee JS, Im DS, Yu BP, and Chung HY (2014). The essential role of FoxO6 phosphorylation in aging and calorie restriction. Age (Dordr) 36, 9679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klang IM, Schilling B, Sorensen DJ, Sahu AK, Kapahi P, Andersen JK, Swoboda P, Killilea DW, Gibson BW, and Lithgow GJ (2014). Iron promotes protein insolubility and aging in C. elegans. Aging (Albany, NY) 6, 975–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koizumi A, Masuda H, Wada Y, Tsukada M, Kawamura K, Kamiyama S, and Walford RL (1989). Caloric restriction perturbs the pituitary-ovarian axis and inhibits mouse mammary tumor virus production in a high-spontaneous-mammary-tumor-incidence mouse strain (C3H/SHN). Mech. Ageing Dev 49, 93–104. [DOI] [PubMed] [Google Scholar]
- Krishnamurthy J, Torrice C, Ramsey MR, Kovalev GI, Al-Regaiey K, Su L, and Sharpless NE (2004). Ink4a/Arf expression is a biomarker of aging. J. Clin. Invest 114, 1299–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar J, Barhydt T, Awasthi A, Lithgow GJ, Killilea DW, and Kapahi P (2016). Zinc levels modulate lifespan through multiple longevity pathways in Caenorhabditis elegans. PLoS One 11, e0153513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacroix S, Lauria M, Scott-Boyer MP, Marchetti L, Priami C, and Caberlotto L (2015). Systems biology approaches to study the molecular effects of caloric restriction and polyphenols on aging processes. Genes Nutr 10, 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laeger T, Castaño-Martinez T, Werno MW, Japtok L, Baumeier C, Jonas W, Kleuser B, and Schürmann A (2018). Dietary carbohydrates impair the protective effect of protein restriction against diabetes in NZO mice used as a model of type 2 diabetes. Diabetologia 61, 1459–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam J, and McKeague M (2019). Dietary modulation of mitochondrial DNA damage: implications in aging and associated diseases. J. Nutr. Biochem 63, 1–10. [DOI] [PubMed] [Google Scholar]
- Lamming DW, Ye L, Katajisto P, Goncalves MD, Saitoh M, Stevens DM, Davis JG, Salmon AB, Richardson A, Ahima RS, et al. (2012). Rapamycin-induced insulin resistance is mediated by mTORC2 loss and uncoupled from longevity. Science 335, 1638–1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang S, Hilsabeck TA, Wilson KA, Sharma A, Bose N, Brackman DJ, Beck JN, Chen L, Watson MA, Killilea DW, et al. (2019). A conserved role of the insulin-like signaling pathway in diet-dependent uric acid pathologies in Drosophila melanogaster. PLoS Genet 15, e1008318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen PL, and Clarke CF (2002). Extension of life-span in Caenorhabditis elegans by a diet lacking coenzyme Q. Science 295, 120–123. [DOI] [PubMed] [Google Scholar]
- Laye MJ, Tran V, Jones DP, Kapahi P, and Promislow DE (2015). The effects of age and dietary restriction on the tissue-specific metabolome of Drosophila. Aging Cell 14, 797–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazic D, Tesic V, Jovanovic M, Brkic M, Milanovic D, Zlokovic BV, Kanazir S, and Perovic M (2020). Every-other-day feeding exacerbates inflammation and neuronal deficits in 5XFAD mouse model of Alzheimer’s disease. Neurobiol. Dis 136, 104745. [DOI] [PubMed] [Google Scholar]
- Lee Y, and Kim EK (2013). AMP-activated protein kinase as a key molecular link between metabolism and clockwork. Exp. Mol. Med 45, e33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Duan W, Long JM, Ingram DK, and Mattson MP (2000). Dietary restriction increases the number of newly generated neural cells, and induces BDNF expression, in the dentate gyrus of rats. J. Mol. Neurosci 15, 99–108. [DOI] [PubMed] [Google Scholar]
- Lee J, Seroogy KB, and Mattson MP (2002). Dietary restriction enhances neurotrophin expression and neurogenesis in the hippocampus of adult mice. J. Neurochem 80, 539–547. [DOI] [PubMed] [Google Scholar]
- Lee KP, Simpson SJ, Clissold FJ, Brooks R, Ballard JW, Taylor PW, Soran N, and Raubenheimer D (2008). Lifespan and reproduction in Drosophila: new insights from nutritional geometry. Proc. Natl. Acad. Sci. USA 105, 2498–2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Padhye A, Sharma A, Song G, Miao J, Mo YY, Wang L, and Kemper JK (2010). A pathway involving farnesoid X receptor and small heterodimer partner positively regulates hepatic sirtuin 1 levels via microRNA-34a inhibition. J. Biol. Chem 285, 12604–12611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BC, Kaya A, Ma S, Kim G, Gerashchenko MV, Yim SH, Hu Z, Harshman LG, and Gladyshev VN (2014). Methionine restriction extends lifespan of Drosophila melanogaster under conditions of low amino-acid status. Nat. Commun 5, 3592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HW, Osis G, Handlogten ME, Guo H, Verlander JW, and Weiner ID (2015). Effect of dietary protein restriction on renal ammonia metabolism. Am. J. Physiol. Renal Physiol 308, F1463–F1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lettieri-Barbato D, Giovannetti E, and Aquilano K (2016). Effects of dietary restriction on adipose mass and biomarkers of healthy aging in human. Aging (Albany, NY) 8, 3341–3355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, and Tollefsbol TO (2011). p16(INK4a) suppression by glucose restriction contributes to human cellular lifespan extension through SIRT1-mediated epigenetic and genetic mechanisms. PLoS One 6, e17421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Chen S, Li Q, Chen L, Zhang H, Li H, Yu D, Zhang R, Niu Y, Lu S, et al. (2020). Caloric restriction attenuates C57BL/6 J mouse lung injury and extra-pulmonary toxicity induced by real ambient particulate matter exposure. Part. Fibre Toxicol 17, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian T, Wu Q, Hodge BA, Wilson KA, Yu G, and Yang M (2018). Drosophila gut-A nexus between dietary restriction and lifespan. Int. J. Mol. Sci 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao CY, Rikke BA, Johnson TE, Diaz V, and Nelson JF (2010). Genetic variation in the murine lifespan response to dietary restriction: from life extension to life shortening. Aging Cell 9, 92–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao CY, Rikke BA, Johnson TE, Gelfond JA, Diaz V, and Nelson JF (2011). Fat maintenance is a predictor of the murine lifespan response to dietary restriction. Aging Cell 10, 629–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libert S, Zwiener J, Chu X,Vanvoorhies W, Roman G, and Pletcher SD (2007). Regulation of Drosophila life span by olfaction and food-derived odors. Science 315, 1133–1137. [DOI] [PubMed] [Google Scholar]
- Lin SC, and Hardie DG (2018). AMPK: sensing glucose as well as cellular energy status. Cell Metab 27, 299–313. [DOI] [PubMed] [Google Scholar]
- Lin SJ, Defossez PA, and Guarente L (2000). Requirement of NAD and SIR2 for life-span extension by calorie restriction in Saccharomyces cerevisiae. Science 289, 2126–2128. [DOI] [PubMed] [Google Scholar]
- Liu G, Yu L, Fang J, Hu C-AA, Yin J, Ni H, Ren W, Duraipandiyan V, Chen S, Al-Dhabi NA, and Yin Y (2017). Methionine restriction on oxidative stress and immune response in dss-induced colitis mice. Oncotarget 8, 44511–44520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu N, Landreh M, Cao K, Abe M, Hendriks GJ, Kennerdell JR, Zhu Y, Wang LS, and Bonini NM (2012). The microRNA miR-34 modulates ageing and neurodegeneration in Drosophila. Nature 482, 519–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Cheng A, Li YJ, Yang Y, Kishimoto Y, Zhang S, Wang Y, Wan R, Raefsky SM, Lu D, et al. (2019). SIRT3 mediates hippocampal synaptic adaptations to intermittent fasting and ameliorates deficits in APP mutant mice. Nat. Commun 10, 1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Lluch G, Hunt N, Jones B, Zhu M, Jamieson H, Hilmer S, Cascajo MV, Allard J, Ingram DK, Navas P, and de Cabo R (2006). Calorie restriction induces mitochondrial biogenesis and bioenergetic efficiency. Proc. Natl. Acad. Sci. USA 103, 1768–1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Otín C, Blasco MA, Partridge L, Serrano M, and Kroemer G (2013). The hallmarks of aging. Cell 153, 1194–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low Wang CC, Hess CN, Hiatt WR, and Goldfine AB (2016). Clinical update: cardiovascular disease in diabetes mellitus: atherosclerotic cardiovascular disease and heart failure in type 2 diabetes mellitus - mechanisms, management, and clinical considerations. Circulation 133, 2459–2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Temp U, Müller-Hartmann A, Esser J, Grönke S, and Partridge L (2021). Sestrin is a key regulator of stem cell function and lifespan in response to dietary amino acids. Nat. Aging 1, 60–72. [DOI] [PubMed] [Google Scholar]
- Lucanic M, Held JM, Vantipalli MC, Klang IM, Graham JB, Gibson BW, Lithgow GJ, and Gill MS (2011). N-acylethanolamine signalling mediates the effect of diet on lifespan in Caenorhabditis elegans. Nature 473, 226–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luévano-Martínez LA, Forni MF, Peloggia J, Watanabe IS, and Kowaltowski AJ (2017). Calorie restriction promotes cardiolipin biosynthesis and distribution between mitochondrial membranes. Mech. Ageing Dev 162, 9–17. [DOI] [PubMed] [Google Scholar]
- Luis NM, Wang L, Ortega M, Deng H, Katewa SD, Li PW, Karpac J, Jasper H, and Kapahi P (2016). Intestinal IRE1 is required for increased triglyceride metabolism and longer lifespan under dietary restriction. Cell Rep 17, 1207–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J, Daniels SB, Lennington JB, Notti RQ, and Conover JC (2006). The aging neurogenic subventricular zone. Aging Cell 5, 139–152. [DOI] [PubMed] [Google Scholar]
- Lv M, Zhu X, Wang H, Wang F, and Guan W (2014). Roles of caloric restriction, ketogenic diet and intermittent fasting during initiation, progression and metastasis of cancer in animal models: a systematic review and meta-analysis. PLoS One 9, e115147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma D, Chen X, Zhang PY, Zhang H, Wei LJ, Hu S, Tang JZ, Zhou MT, Xie C, Ou R, et al. (2018). Upregulation of the ALDOA/DNA-PK/p53 pathway by dietary restriction suppresses tumor growth. Oncogene 37, 1041–1048. [DOI] [PubMed] [Google Scholar]
- Maegawa S, Lu Y, Tahara T, Lee JT, Madzo J, Liang S, Jelinek J, Colman RJ, and Issa JJ (2017). Caloric restriction delays age-related methylation drift. Nat. Commun 8, 539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoudi S, Mancini E, Xu L, Moore A, Jahanbani F, Hebestreit K, Srinivasan R, Li X, Devarajan K, Prélot L, et al. (2019). Heterogeneity in old fibroblasts is linked to variability in reprogramming and wound healing. Nature 574, 553–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mair W, Goymer P, Pletcher SD, and Partridge L (2003). Demography of dietary restriction and death in Drosophila. Science 301, 1731–1733. [DOI] [PubMed] [Google Scholar]
- Makwana K, Patel SA, Velingkaar N, Ebron JS, Shukla GC, and Kondratov RVKV (2017). Aging and calorie restriction regulate the expression of miR-125a-5p and its target genes Stat3, Casp2 and Stard13. Aging (Albany, NY) 9, 1825–1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcellino BK, Ekasumara N, and Mobbs CV (2018). Dietary restriction and glycolytic inhibition reduce proteotoxicity and extend lifespan via NHR-49. Curr. Neurobiol 9, 1–7. [PMC free article] [PubMed] [Google Scholar]
- Margolis LM, Rivas DA, Berrone M, Ezzyat Y, Young AJ, McClung JP, Fielding RA, and Pasiakos SM (2016). Prolonged calorie restriction downregulates skeletal muscle mTORC1 signaling independent of dietary protein intake and associated microRNA expression. Front. Physiol 7, 445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariño G, Ugalde AP, Fernández AF, Osorio FG, Fueyo A, Freije JM, and López-Otín C (2010). Insulin-like growth factor 1 treatment extends longevity in a mouse model of human premature aging by restoring somatotroph axis function. Proc. Natl. Acad. Sci. USA 107, 16268–16273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzetti E, Carter CS, Wohlgemuth SE, Lees HA, Giovannini S, Anderson B, Quinn LS, and Leeuwenburgh C (2009a). Changes in IL-15 expression and death-receptor apoptotic signaling in rat gastrocnemius muscle with aging and life-long calorie restriction. Mech. Ageing Dev 130, 272–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzetti E, Lees HA, Wohlgemuth SE, and Leeuwenburgh C (2009b). Sarcopenia of aging: underlying cellular mechanisms and protection by calorie restriction. BioFactors 35, 28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massetti GM, Dietz WH, and Richardson LC (2017). Excessive weight gain, obesity, and cancer: opportunities for clinical intervention. JAMA 318, 1975–1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masternak MM, Al-Regaiey K, Bonkowski MS, Panici J, Sun L, Wang J, Przybylski GK, and Bartke A (2004). Divergent effects of caloric restriction on gene expression in normal and long-lived mice. J. Gerontol. A Biol. Sci. Med. Sci 59, 784–788. [DOI] [PubMed] [Google Scholar]
- Maswood N, Young J, Tilmont E, Zhang Z, Gash DM, Gerhardt GA, Grondin R, Roth GS, Mattison J, Lane MA, et al. (2004). Caloric restriction increases neurotrophic factor levels and attenuates neurochemical and behavioral deficits in a primate model of Parkinson’s disease. Proc. Natl. Acad. Sci. USA 101, 18171–18176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matai L, Sarkar GC, Chamoli M, Malik Y, Kumar SS, Rautela U, Jana NR, Chakraborty K, and Mukhopadhyay A (2019). Dietary restriction improves proteostasis and increases life span through endoplasmic reticulum hormesis. Proc. Natl. Acad. Sci. USA 116, 17383–17392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattis J, and Sehgal A (2016). Circadian rhythms, sleep, and disorders of aging. Trends Endocrinol. Metab 27, 192–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattison JA, Roth GS, Beasley TM, Tilmont EM, Handy AM, Herbert RL, Longo DL, Allison DB, Young JE, Bryant M, et al. (2012). Impact of caloric restriction on health and survival in rhesus monkeys from the NIA study. Nature 489, 318–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattison JA, Colman RJ, Beasley TM, Allison DB, Kemnitz JW, Roth GS, Ingram DK, Weindruch R, de Cabo R, and Anderson RM (2017). Caloric restriction improves health and survival of rhesus monkeys. Nat. Commun 8, 14063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PC, Telford N, Salo D, Anderson C, Kohama SG, Finch CE, Walford RL, and Weindruch R (1992). Failure of dietary restriction to retard age-related neurochemical changes in mice. Neurobiol. Aging 13, 787–791. [DOI] [PubMed] [Google Scholar]
- McCay CM, Maynard LA, Sperling G, and Barnes LL (1939). Retarded growth, life span, ultimate body size and age changes in the albino rat after feeding diets restricted in calories. J. Nutr 73, 1–13. [DOI] [PubMed] [Google Scholar]
- McCracken AW, Adams G, Hartshorne L, Tatar M, and Simons MJP (2020). The hidden costs of dietary restriction: implications for its evolutionary and mechanistic origins. Sci. Adv 6, eaay3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullough ML, Feskanich D, Stampfer MJ, Giovannucci EL, Rimm EB, Hu FB, Spiegelman D, Hunter DJ, Colditz GA, and Willett WC (2002). Diet quality and major chronic disease risk in men and women: moving toward improved dietary guidance. Am. J. Clin. Nutr 76, 1261–1271. [DOI] [PubMed] [Google Scholar]
- McDonald VM, Gibson PG, Scott HA, Baines PJ, Hensley MJ, Pretto JJ, and Wood LG (2016). Should we treat obesity in COPD? The effects of diet and resistance exercise training. Respirology 21, 875–882. [DOI] [PubMed] [Google Scholar]
- McHugh D, and Gil J (2018). Senescence and aging: causes, consequences, and therapeutic avenues. J. Cell Biol 217, 65–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKiernan SH, Colman RJ, Lopez M, Beasley TM, Aiken JM, Anderson RM, and Weindruch R (2011). Caloric restriction delays aging-induced cellular phenotypes in rhesus monkey skeletal muscle. Exp. Gerontol 46, 23–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehrabani S, Bagherniya M, Askari G, Read MI, and Sahebkar A (2020). The effect of fasting or calorie restriction on mitophagy induction: a literature review. J. Cachexia Sarcopenia Muscle 11, 1447–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer AJ, Lorin S, Blommaart EF, and Codogno P (2015). Regulation of autophagy by amino acids and MTOR-dependent signal transduction. Amino Acids 47, 2037–2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melkani GC, and Panda S (2017). Time-restricted feeding for prevention and treatment of cardiometabolic disorders. J. Physiol 595, 3691–3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melov S, Tarnopolsky MA, Beckman K, Felkey K, and Hubbard A (2007). Resistance exercise reverses aging in human skeletal muscle. PLoS One 2, e465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menghini R, Casagrande V, Cardellini M, Martelli E, Terrinoni A, Amati F, Vasa-Nicotera M, Ippoliti A, Novelli G, Melino G, et al. (2009). MicroRNA 217 modulates endothelial cell senescence via silent information regulator 1. Circulation 120, 1524–1532. [DOI] [PubMed] [Google Scholar]
- Mercken EM, Majounie E, Ding J, Guo R, Kim J, Bernier M, Mattison J, Cookson MR, Gorospe M, de Cabo R, and Abdelmohsen K (2013). Age-associated miRNA alterations in skeletal muscle from rhesus monkeys reversed by caloric restriction. Aging (Albany, NY) 5, 692–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercken EM, Hu J, Krzysik-Walker S, Wei M, Li Y, McBurney MW, de Cabo R, and Longo VD (2014). SIRT1 but not its increased expression is essential for lifespan extension in caloric-restricted mice. Aging Cell 13, 193–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill GF, Kurth EJ, Hardie DG, and Winder WW (1997). AICA riboside increases AMP-activated protein kinase, fatty acid oxidation, and glucose uptake in rat muscle. Am. J. Physiol 273, E1107–E1112. [DOI] [PubMed] [Google Scholar]
- Meydani M, Das S, Band M, Epstein S, and Roberts S (2011). The effect of caloric restriction and glycemic load on measures of oxidative stress and antioxidants in humans: results from the CALERIE trial of human caloric restriction. J. Nutr. Health Aging 15, 456–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meydani SN, Das SK, Pieper CF, Lewis MR, Klein S, Dixit VD, Gupta AK, Villareal DT, Bhapkar M, Huang M, et al. (2016). Long-term moderate calorie restriction inhibits inflammation without impairing cell-mediated immunity: a randomized controlled trial in non-obese humans. Aging (Albany, NY) 8, 1416–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meynet O, and Ricci JE (2014). Caloric restriction and cancer: molecular mechanisms and clinical implications. Trends Mol. Med 20, 419–427. [DOI] [PubMed] [Google Scholar]
- Micha R, Peñalvo JL, Cudhea F, Imamura F, Rehm CD, and Mozaffarian D (2017). Association between dietary factors and mortality from heart disease, stroke, and type 2 diabetes in the United States. JAMA 317, 912–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RA, Buehner G, Chang Y, Harper JM, Sigler R, and Smith-Wheelock M (2005). Methionine-deficient diet extends mouse lifespan, slows immune and lens aging, alters glucose, T4, IGF-I and insulin levels, and increases hepatocyte MIF levels and stress resistance. Aging Cell 4, 119–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirzaei H, Di Biase S, and Longo VD (2016). Dietary interventions, cardiovascular aging, and disease: animal models and human studies. Circ. Res 118, 1612–1625. [DOI] [PubMed] [Google Scholar]
- Mitchell SJ, Madrigal-Matute J, Scheibye-Knudsen M, Fang E, Aon M, González-Reyes JA, Cortassa S, Kaushik S, Gonzalez-Freire M, Patel B, et al. (2016). Effects of sex, strain, and energy intake on hallmarks of aging in mice. Cell Metab 23, 1093–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell SJ, Bernier M, Aon MA, Cortassa S, Kim EY, Fang EF, Palacios HH, Ali A, Navas-Enamorado I, Di Francesco A, et al. (2018). Nicotinamide improves aspects of healthspan, but not lifespan, in mice. Cell Metab. 27, 667–676.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell SJ, Bernier M, Mattison JA, Aon MA, Kaiser TA, Anson RM, Ikeno Y, Anderson RM, Ingram DK, and de Cabo R (2019). Daily fasting improves health and survival in male mice independent of diet composition and calories. Cell Metab 29, 221–228.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal N, Guimaraes JC, Gross T, Schmidt A, Vina-Vilaseca A, Nedialkova DD, Aeschimann F, Leidel SA, Spang A, and Zavolan M (2017). The Gcn4 transcription factor reduces protein synthesis capacity and extends yeast lifespan. Nat. Commun 8, 457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyauchi T, Uchida Y, Kadono K, Hirao H, Kawasoe J, Watanabe T, Ueda S, Okajima H, Terajima H, and Uemoto S (2019). Up-regulation of FOXO1 and reduced inflammation by beta-hydroxybutyric acid are essential diet restriction benefits against liver injury. Proc. Natl. Acad. Sci. USA 116, 13533–13542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molon-Noblot S, Laroque P, Coleman JB, Hoe CM, and Keenan KP (2003). The effects of ad libitum overfeeding and moderate and marked dietary restriction on age-related spontaneous pituitary gland pathology in Sprague-Dawley rats. Toxicol. Pathol 31, 310–320. [DOI] [PubMed] [Google Scholar]
- Moreno CL, Ehrlich ME, and Mobbs CV (2016). Protection by dietary restriction in the YAC128 mouse model of Huntington’s disease: relation to genes regulating histone acetylation and HTT. Neurobiol. Dis 85, 25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori MA, Raghavan P, Thomou T, Boucher J, Robida-Stubbs S, Macotela Y, Russell SJ, Kirkland JL, Blackwell TK, and Kahn CR (2012). Role of microRNA processing in adipose tissue in stress defense and longevity. Cell Metab 16, 336–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori MA, Thomou T, Boucher J, Lee KY, Lallukka S, Kim JK, Torriani M, Yki-Järvinen H, Grinspoon SK, Cypess AM, and Kahn CR (2014). Altered miRNA processing disrupts brown/white adipocyte determination and associates with lipodystrophy. J. Clin. Invest 124, 3339–3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Most J, Tosti V, Redman LM, and Fontana L (2017). Calorie restriction in humans: an update. Ageing Res. Rev 39, 36–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moura J, Madureira P, Leal EC, Fonseca AC, and Carvalho E (2019). Immune aging in diabetes and its implications in wound healing. Clin. Immunol 200, 43–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouton PR, Chachich ME, Quigley C, Spangler E, and Ingram DK (2009). Caloric restriction attenuates amyloid deposition in middle-aged dtg APP/PS1 mice. Neurosci. Lett 464, 184–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulrooney TJ, Marsh J, Urits I, Seyfried TN, and Mukherjee P (2011). Influence of caloric restriction on constitutive expression of NF-κB in an experimental mouse astrocytoma. PLoS One 6, e18085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Must A, Spadano J, Coakley EH, Field AE, Colditz G, and Dietz WH (1999). The disease burden associated with overweight and obesity. JAMA 282, 1523–1529. [DOI] [PubMed] [Google Scholar]
- Naresh NU, and Haynes CM (2019). Signaling and regulation of the mitochondrial unfolded protein response. Cold Spring Harb. Perspect. Biol 11, a033944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson CS, Beck JN, Wilson KA, Pilcher ER, Kapahi P, and Brem RB (2016). Cross-phenotype association tests uncover genes mediating nutrient response in Drosophila. BMC Genomics 17, 867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman JC, Covarrubias AJ, Zhao M, Yu X, Gut P, Ng CP, Huang Y, Haldar S, and Verdin E (2017). Ketogenic diet reduces midlife mortality and improves memory in aging mice. Cell Metab 26, 547–557.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicks J, Lee S, Harris A, Falk DJ, Todd AG, Arredondo K, Dunn WA Jr., and Notterpek L (2014). Rapamycin improves peripheral nerve myelination while it fails to benefit neuromuscular performance in neuropathic mice. Neurobiol. Dis 70, 224–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisoli E, Tonello C, Cardile A, Cozzi V, Bracale R, Tedesco L, Falcone S, Valerio A, Cantoni O, Clementi E, et al. (2005). Calorie restriction promotes mitochondrial biogenesis by inducing the expression of eNOS. Science 310, 314–317. [DOI] [PubMed] [Google Scholar]
- O’Flanagan CH, Smith LA, McDonell SB, and Hursting SD (2017). When less may be more: calorie restriction and response to cancer therapy. BMC Med 15, 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooka H, Segall PE, and Timiras PS (1988). Histology and survival in age-delayed low-tryptophan-fed rats. Mech. Ageing Dev 43, 79–98. [DOI] [PubMed] [Google Scholar]
- Orentreich N, Matias JR, DeFelice A, and Zimmerman JA (1993). Low methionine ingestion by rats extends life span. J. Nutr 123, 269–274. [DOI] [PubMed] [Google Scholar]
- Orillion A, Damayanti NP, Shen L, Adelaiye-Ogala R, Affronti H, Elbanna M, Chintala S, Ciesielski M, Fontana L, Kao C, et al. (2018). Dietary protein restriction reprograms tumor-associated macrophages and enhances immunotherapy. Clin. Cancer Res 24, 6383–6395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otranto M, Souza-Netto I, Aguila MB, and Monte-Alto-Costa A (2009). Male and female rats with severe protein restriction present delayed wound healing. Appl. Physiol. Nutr. Metab 34, 1023–1031. [DOI] [PubMed] [Google Scholar]
- Ouattara A, Cooke D, Gopalakrishnan R, Huang TH, and Ables GP (2016). Methionine restriction alters bone morphology and affects osteoblast differentiation. Bone Rep 5, 33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palma C, La Rocca C, Gigantino V, Aquino G, Piccaro G, Di Silvestre D, Brambilla F, Rossi R, Bonacina F, Lepore MT, et al. (2021). Caloric restriction promotes immunometabolic reprogramming leading to protection from tuberculosis. Cell Metab 33, 300–318.e12. [DOI] [PubMed] [Google Scholar]
- Pamplona R, and Barja G (2006). Mitochondrial oxidative stress, aging and caloric restriction: the protein and methionine connection. Biochim. Biophys. Acta 1757, 496–508. [DOI] [PubMed] [Google Scholar]
- Pan KZ, Palter JE, Rogers AN, Olsen A, Chen D, Lithgow GJ, and Kapahi P (2007). Inhibition of mRNA translation extends lifespan in Caenorhabditis elegans. Aging Cell 6, 111–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey M, Bansal S, Bar S, Yadav AK, Sokol NS, Tennessen JM, Kapahi P, and Chawla G (2021). miR-125-chinmo pathway regulates dietary restriction-dependent enhancement of lifespan in Drosophila. eLife 10, e62621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandit A, Jain V, Kumar N, and Mukhopadhyay A (2014). PHA-4/FOXA-regulated microRNA feed forward loops during Caenorhabditis elegans dietary restriction. Aging (Albany, NY) 6, 835–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoli D, Boulay K, Kazak L, Pollak M, Mallette F, Topisirovic I, and Hulea L (2019). mTOR as a central regulator of lifespan and aging. F1000Res 8, F1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulos NT, Papanastasiou S, Müller HG, Wang JL, Yang W, and Carey JR (2011). Dietary effects on sex-specific health dynamics of medfly: support for the dynamic equilibrium model of aging. Exp. Gerontol 46, 1026–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papanastasiou SA, Carey JR, and Papadopoulos NT (2019). Effects of early-life protein starvation on longevity and sexual performance of male medfly. PLoS One 14, e0219518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel NV, Gordon MN, Connor KE, Good RA, Engelman RW, Mason J, Morgan DG, Morgan TE, and Finch CE (2005). Caloric restriction attenuates Abeta-deposition in Alzheimer transgenic models. Neurobiol. Aging 26, 995–1000. [DOI] [PubMed] [Google Scholar]
- Patel SA, Chaudhari A, Gupta R, Velingkaar N, and Kondratov RV (2016a). Circadian clocks govern calorie restriction-mediated life span extension through BMAL1- and IGF-1-dependent mechanisms. FASEB J 30, 1634–1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel SA, Velingkaar N, Makwana K, Chaudhari A, and Kondratov R (2016b). Calorie restriction regulates circadian clock gene expression through BMAL1 dependent and independent mechanisms. Sci. Rep 6, 25970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard F, Kurtev M, Chung N, Topark-Ngarm A, Senawong T, Machado De Oliveira R, Leid M, McBurney MW, and Guarente L (2004). Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR-gamma. Nature 429, 771–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilis K, Pilis A, Stec K, Pilis W, Langfort J, Letkiewicz S, Michalski C, Czuba M, Zych M, and Chalimoniuk M (2018). Three-year chronic consumption of low-carbohydrate diet impairs exercise performance and has a small unfavorable effect on lipid profile in middle-aged men. Nutrients 10, 1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podshivalova K, Kerr RA, and Kenyon C (2017). How a mutation that slows aging can also disproportionately extend end-of-life decrepitude. Cell Rep 19, 441–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomatto LCD, Dill T, Carboneau B, Levan S, Kato J, Mercken EM, Pearson KJ, Bernier M, and de Cabo R (2020). Deletion of Nrf2 shortens lifespan in C57BL6/J male mice but does not alter the health and survival benefits of caloric restriction. Free Radic. Biol. Med 152, 650–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postnikoff SD, Malo ME, Wong B, and Harkness TA (2012). The yeast forkhead transcription factors fkh1 and fkh2 regulate lifespan and stress response together with the anaphase-promoting complex. PLoS Genet 8, e1002583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers RW 3rd, Kaeberlein M, Caldwell SD, Kennedy BK, and Fields S (2006). Extension of chronological life span in yeast by decreased TOR pathway signaling. Genes Dev 20, 174–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince AC, Myers CE, Joyce T, Irving P, Lomer M, and Whelan K (2016). Fermentable carbohydrate restriction (low FODMAP diet) in clinical practice improves functional gastrointestinal symptoms in patients with inflammatory bowel disease. Inflamm. Bowel Dis 22, 1129–1136. [DOI] [PubMed] [Google Scholar]
- Qiu G, Spangler EL, Wan R, Miller M, Mattson MP, So KF, de Cabo R, Zou S, and Ingram DK (2012). Neuroprotection provided by dietary restriction in rats is further enhanced by reducing glucocortocoids. Neurobiol. Aging 33, 2398–2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiles JM, and Gustafsson ÅB (2020). Mitochondrial quality control and cellular proteostasis: two sides of the same coin. Front. Physiol 11, 515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainwater DL, Kammerer CM, Mahaney MC, Rogers J, Cox LA, Schneider JL, and VandeBerg JL (2003). Localization of genes that control LDL size fractions in baboons. Atherosclerosis 168, 15–22. [DOI] [PubMed] [Google Scholar]
- Raju SV, Zheng M, Schuleri KH, Phan AC, Bedja D, Saraiva RM, Yiginer O, Vandegaer K, Gabrielson KL, O’Donnell CP, et al. (2006). Activation of the cardiac ciliary neurotrophic factor receptor reverses left ventricular hypertrophy in leptin-deficient and leptin-resistant obesity. Proc. Natl. Acad. Sci. USA 103, 4222–4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey KM, Yoshino J, Brace CS, Abrassart D, Kobayashi Y, Marcheva B, Hong HK, Chong JL, Buhr ED, Lee C, et al. (2009). Circadian clock feedback cycle through NAMPT-mediated NAD+ biosynthesis. Science 324, 651–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redman LM, Smith SR, Burton JH, Martin CK, Il’yasova D, and Ravussin E (2018). Metabolic slowing and reduced oxidative damage with sustained caloric restriction support the rate of living and oxidative damage theories of aging. Cell Metab 27, 805–815.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed JL, De Souza MJ, and Williams NI (2010). Effects of exercise combined with caloric restriction on inflammatory cytokines. Appl. Physiol. Nutr. Metab 35, 573–582. [DOI] [PubMed] [Google Scholar]
- Regan CE, Pilkington JG, Pemberton JM, and Crawley MJ (2016). Sex differences in relationships between habitat use and reproductive performance in Soay sheep (Ovis aries). Ecol. Lett 19, 171–179. [DOI] [PubMed] [Google Scholar]
- Regan JC, Froy H, Walling CA, Moatt JP, and Nussey DH (2020). Dietary restriction and insulin-like signalling pathways as adaptive plasticity: a synthesis and re-evaluation. Funct. Ecol 34, 107–128. [Google Scholar]
- Reiser K, McGee C, Rucker R, and McDonald R (1995). Effects of aging and caloric restriction on extracellular matrix biosynthesis in a model of injury repair in rats. J. Gerontol. A Biol. Sci. Med. Sci 50A, B40–B47. [DOI] [PubMed] [Google Scholar]
- Reis-Rodrigues P, Czerwieniec G, Peters TW, Evani US, Alavez S, Gaman EA, Vantipalli M, Mooney SD, Gibson BW, Lithgow GJ, and Hughes RE (2012). Proteomic analysis of age-dependent changes in protein solubility identifies genes that modulate lifespan. Aging Cell 11, 120–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rera M, Clark RI, and Walker DW (2012). Intestinal barrier dysfunction links metabolic and inflammatory markers of aging to death in Drosophila. Proc. Natl. Acad. Sci. USA 109, 21528–21533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson A, Fischer KE, Speakman JR, de Cabo R, Mitchell SJ, Peterson CA, Rabinovitch P, Chiao YA, Taffet G, Miller RA, et al. (2016). Measures of healthspan as indices of aging in mice-a recommendation. J. Gerontol. A Biol. Sci. Med. Sci 71, 427–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson NE, Konon EN, Schuster HS, Mitchell AT, Boyle C, Rodgers AC, Finke M, Haider LR, Yu D, Flores V, et al. (2021). Lifelong restriction of dietary branched-chain amino acids has sex-specific benefits for frailty and life span in mice. Nat. Aging 1, 73–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richie JP Jr., Leutzinger Y, Parthasarathy S, Malloy V, Orentreich N, and Zimmerman JA (1994). Methionine restriction increases blood glutathione and longevity in F344 rats. FASEB J 8, 1302–1307. [DOI] [PubMed] [Google Scholar]
- Rikke BA, Liao CY, McQueen MB, Nelson JF, and Johnson TE (2010). Genetic dissection of dietary restriction in mice supports the metabolic efficiency model of life extension. Exp. Gerontol 45, 691–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson LT, Treviño-Villarreal JH, Mejia P, Grondin Y, Harputlugil E, Hine C, Vargas D, Zheng H, Ozaki CK, Kristal BS, et al. (2015). Protein and calorie restriction contribute additively to protection from renal ischemia reperfusion injury partly via leptin reduction in male mice. J. Nutr 145, 1717–1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers GP, and Collins FS (2020). Precision nutrition-the answer to "what to eat to stay healthy". JAMA 324, 735–736. [DOI] [PubMed] [Google Scholar]
- Rodriguez OC, Choudhury S, Kolukula V, Vietsch EE, Catania J, Preet A, Reynoso K, Bargonetti J, Wellstein A, Albanese C, and Avantaggiati ML (2012). Dietary downregulation of mutant p53 levels via glucose restriction: mechanisms and implications for tumor therapy. Cell Cycle 11, 4436–4446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojic-Becker D, Portero-Tresserra M, Martí-Nicolovius M, Vale-Martínez A, and Guillazo-Blanch G (2019). Caloric restriction modulates the monoaminergic and glutamatergic systems in the hippocampus, and attenuates age-dependent spatial memory decline. Neurobiol. Learn. Mem 166, 107107. [DOI] [PubMed] [Google Scholar]
- Rollins JA, Shaffer D, Snow SS, Kapahi P, and Rogers AN (2019). Dietary restriction induces posttranscriptional regulation of longevity genes. Life Sci. Alliance 2, e201800281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero-Gómez M, Zelber-Sagi S, and Trenell M (2017). Treatment of NAFLD with diet, physical activity and exercise. J. Hepatol 67, 829–846. [DOI] [PubMed] [Google Scholar]
- Rosales-Corral S, Tan DX, Manchester L, and Reiter RJ (2015). Diabetes and Alzheimer disease, two overlapping pathologies with the same background: oxidative stress. Oxid. Med. Cell. Longev 2015, 985845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth GS, Kowatch MA, Hengemihle J, Ingram DK, Spangler EL, Johnson LK, and Lane MA (1997). Effect of age and caloric restriction on cutaneous wound closure in rats and monkeys. J. Gerontol. A Biol. Sci. Med. Sci 52, B98–B102. [DOI] [PubMed] [Google Scholar]
- Rubie C, Kölsch K, Halajda B, Eichler H, Wagenpfeil S, Roemer K, and Glanemann M (2016). microRNA-496 - a new, potentially aging-relevant regulator of mTOR. Cell Cycle 15, 1108–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubini A, Bosco G, Lodi A, Cenci L, Parmagnani A, Grimaldi K, Zhongjin Y, and Paoli A (2015). Effects of twenty days of the ketogenic diet on metabolic and respiratory parameters in healthy subjects. Lung 193, 939–945. [DOI] [PubMed] [Google Scholar]
- Ruckenstuhl C, Netzberger C, Entfellner I, Carmona-Gutierrez D, Kickenweiz T, Stekovic S, Gleixner C, Schmid C, Klug L, Sorgo AG, et al. (2014). Lifespan extension by methionine restriction requires autophagy-dependent vacuolar acidification. PLoS Genet 10, e1004347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruetenik A, and Barrientos A (2015). Dietary restriction, mitochondrial function and aging: from yeast to humans. Biochim. Biophys. Acta 1847, 1434–1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rühlmann C, Wölk T, Blümel T, Stahn L, Vollmar B, and Kuhla A (2016). Long-term caloric restriction in ApoE-deficient mice results in neuroprotection via FGF21-induced AMPK/mTOR pathway. Aging (Albany, NY) 8, 2777–2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell RC, Yuan HX, and Guan KL (2014). Autophagy regulation by nutrient signaling. Cell Res 24, 42–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampaio-Marques B, Pereira H, Santos AR, Teixeira A, and Ludovico P (2018). Caloric restriction rescues yeast cells from alpha-synuclein toxicity through autophagic control of proteostasis. Aging (Albany, NY) 10, 3821–3833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoro A, Ostan R, Candela M, Biagi E, Brigidi P, Capri M, and Franceschi C (2018). Gut microbiota changes in the extreme decades of human life: a focus on centenarians. Cell. Mol. Life Sci 75, 129–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz A, Caro P, Ayala V, Portero-Otin M, Pamplona R, and Barja G (2006). Methionine restriction decreases mitochondrial oxygen radical generation and leak as well as oxidative damage to mitochondrial DNA and proteins. FASEB J 20, 1064–1073. [DOI] [PubMed] [Google Scholar]
- Sasikumar AN, Killilea DW, Kennedy BK, and Brem RB (2019). Potassium restriction boosts vacuolar acidity and extends lifespan in yeast. Exp. Gerontol 120, 101–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxton RA, and Sabatini DM (2017). mTOR signaling in growth, metabolism, and disease. Cell 168, 960–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer MJ, Alldred MJ, Lee SH, Calhoun ME, Petkova E, Mathews PM, and Ginsberg SD (2015). Reduction of beta-amyloid and gamma-secretase by calorie restriction in female Tg2576 mice. Neurobiol. Aging 36, 1293–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider A, Dhahbi JM, Atamna H, Clark JP, Colman RJ, and Anderson RM (2017). Caloric restriction impacts plasma microRNAs in rhesus monkeys. Aging Cell 16, 1200–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte DM, Hahn M, Oberhäuser F, Malchau G, Schubert M, Heppner C, Müller N, Güdelhöfer H, Faust M, Krone W, and Laudes M (2014). Caloric restriction increases serum testosterone concentrations in obese male subjects by two distinct mechanisms. Horm. Metab. Res 46, 283–286. [DOI] [PubMed] [Google Scholar]
- Schulz TJ, Zarse K, Voigt A, Urban N, Birringer M, and Ristow M (2007). Glucose restriction extends Caenorhabditis elegans life span by inducing mitochondrial respiration and increasing oxidative stress. Cell Metab 6, 280–293. [DOI] [PubMed] [Google Scholar]
- Seidelmann SB, Claggett B, Cheng S, Henglin M, Shah A, Steffen LM, Folsom AR, Rimm EB, Willett WC, and Solomon SD (2018). Dietary carbohydrate intake and mortality: a prospective cohort study and meta-analysis. Lancet Public Health 3, e419–e428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethupathy P, and Collins FS (2008). MicroRNA target site polymorphisms and human disease. Trends Genet 24, 489–497. [DOI] [PubMed] [Google Scholar]
- Sharma A, Diecke S, Zhang WY, Lan F, He C, Mordwinkin NM, Chua KF, and Wu JC (2013). The role of SIRT6 protein in aging and reprogramming of human induced pluripotent stem cells. J. Biol. Chem 288, 18439–18447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp ZD, Lee WH, Nikitin AY, Flesken-Nikitin A, Ikeno Y, Reddick R, Richardson AG, and Nelson JF (2003). Minimal effects of dietary restriction on neuroendocrine carcinogenesis in Rb+/− mice. Carcinogenesis 24, 179–183. [DOI] [PubMed] [Google Scholar]
- Shibolet O, Alper R, Avraham Y, Berry EM, and Ilan Y (2002). Immunomodulation of experimental colitis via caloric restriction: role of Nk1.1 + T cells. Clin. Immunol 105, 48–56. [DOI] [PubMed] [Google Scholar]
- Shimokawa I, Tomita M, Higami Y, Okimoto T, Kawahara T, and Ikeda T (1997). Dietary restriction maintains the basal rate of somatotrope renewal in later life in male rats. Age (Omaha) 20, 169–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimokawa I, Komatsu T, Hayashi N, Kim SE, Kawata T, Park S, Hayashi H, Yamaza H, Chiba T, and Mori R (2015). The life-extending effect of dietary restriction requires Foxo3 in mice. Aging Cell 14, 707–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin BK, Kang S, Kim DS, and Park S (2018). Intermittent fasting protects against the deterioration of cognitive function, energy metabolism and dyslipidemia in Alzheimer’s disease-induced estrogen deficient rats. Exp. Biol. Med. (Maywood) 243, 334–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shingler E, Perry R, Mitchell A, England C, Perks C, Herbert G, Ness A, and Atkinson C (2019). Dietary restriction during the treatment of cancer: results of a systematic scoping review. BMC Cancer 19, 811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinmura K, Tamaki K, Sano M, Murata M, Yamakawa H, Ishida H, and Fukuda K (2011). Impact of long-term caloric restriction on cardiac senescence: caloric restriction ameliorates cardiac diastolic dysfunction associated with aging. J. Mol. Cell. Cardiol 50, 117–127. [DOI] [PubMed] [Google Scholar]
- Shruthi K, Reddy SS, Reddy PY, Shivalingam P, Harishankar N, and Reddy GB (2016). Amelioration of neuronal cell death in a spontaneous obese rat model by dietary restriction through modulation of ubiquitin proteasome system. J. Nutr. Biochem 33, 73–81. [DOI] [PubMed] [Google Scholar]
- Shushimita S, van der Pol P, W F de Bruin R, N M Ijzermans J, van Kooten C, and Dor FJ (2015). Mannan-binding lectin is involved in the protection against renal ischemia/reperfusion injury by dietary restriction. PLoS One 10, e0137795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simone BA, Palagani A, Strickland K, Ko K, Jin L, Lim MK, Dan TD, Sarich M, Monti DA, Cristofanilli M, and Simone NL (2018). Caloric restriction counteracts chemotherapy-induced inflammation and increases response to therapy in a triple negative breast cancer model. Cell Cycle 17, 1536–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons MJ, Koch W, and Verhulst S (2013). Dietary restriction of rodents decreases aging rate without affecting initial mortality rate – a meta-analysis. Aging Cell 12, 410–414. [DOI] [PubMed] [Google Scholar]
- Singh R, and Cuervo AM (2012). Lipophagy: connecting autophagy and lipid metabolism. Int. J. Cell Biol 2012, 282041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan C, Tuinei J, Nemetz K, Frandsen J, Soto J, Wride N, Sempokuya T, Alegria L, Bugger H, and Abel ED (2011). Central leptin signaling is required to normalize myocardial fatty acid oxidation rates in caloric-restricted ob/ob mice. Diabetes 60, 1424–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohal RS, and Forster MJ (2014). Caloric restriction and the aging process: a critique. Free Radic. Biol. Med 73, 366–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohal RS, Agarwal S, Candas M, Forster MJ, and Lal H (1994). Effect of age and caloric restriction on DNA oxidative damage in different tissues of C57BL/6 mice. Mech. Ageing Dev 76, 215–224. [DOI] [PubMed] [Google Scholar]
- Solon-Biet SM, McMahon AC, Ballard JW, Ruohonen K, Wu LE, Cogger VC, Warren A, Huang X, Pichaud N, Melvin RG, et al. (2014). The ratio of macronutrients, not caloric intake, dictates cardiometabolic health, aging, and longevity in ad libitum-fed mice. Cell Metab 19, 418–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonntag WE, Lynch CD, Cefalu WT, Ingram RL, Bennett SA, Thornton PL, and Khan AS (1999). Pleiotropic effects of growth hormone and insulin-like growth factor (IGF)-1 on biological aging: inferences from moderate caloric-restricted animals. J. Gerontol. A Biol. Sci. Med. Sci 54, B521–B538. [DOI] [PubMed] [Google Scholar]
- Speakman JR (2020). Why does caloric restriction increase life and health-span? The ‘clean cupboards’ hypothesis. Natl. Sci. Rev 7, 1153–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefan N, Häring HU, Hu FB, and Schulze MB (2013). Metabolically healthy obesity: epidemiology, mechanisms, and clinical implications. Lancet Diabetes Endocrinol 1, 152–162. [DOI] [PubMed] [Google Scholar]
- Stefana MI, Driscoll PC, Obata F, Pengelly AR, Newell CL, MacRae JI, and Gould AP (2017). Developmental diet regulates Drosophila lifespan via lipid autotoxins. Nat. Commun 8, 1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffen KK, MacKay VL, Kerr EO, Tsuchiya M, Hu D, Fox LA, Dang N, Johnston ED, Oakes JA, Tchao BN, et al. (2008). Yeast life span extension by depletion of 60s ribosomal subunits is mediated by Gcn4. Cell 133, 292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinkraus KA, Smith ED, Davis C, Carr D, Pendergrass WR, Sutphin GL, Kennedy BK, and Kaeberlein M (2008). Dietary restriction suppresses proteotoxicity and enhances longevity by an hsf-1-dependent mechanism in Caenorhabditis elegans. Aging Cell 7, 394–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenesen D, Suh JM, Seo J, Yu K, Lee KS, Kim JS, Min KJ, and Graff JM (2013). Adenosine nucleotide biosynthesis and AMPK regulate adult life span and mediate the longevity benefit of caloric restriction in flies. Cell Metab 17, 101–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studenski S, Perera S, Patel K, Rosano C, Faulkner K, Inzitari M, Brach J, Chandler J, Cawthon P, Connor EB, et al. (2011). Gait speed and survival in older adults. JAMA 305, 50–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundaram S, and Yan L (2016). Dietary energy restriction reduces high-fat diet-enhanced metastasis of Lewis lung carcinoma in mice. Oncotarget 7, 65669–65675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swindell WR (2012). Dietary restriction in rats and mice: a meta-analysis and review of the evidence for genotype-dependent effects on lifespan. Ageing Res. Rev 11, 254–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabrez SS, Sharma RD, Jain V, Siddiqui AA, and Mukhopadhyay A (2017). Differential alternative splicing coupled to nonsense-mediated decay of mRNA ensures dietary restriction-induced longevity. Nat. Commun 8, 306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner JM, Kearns DT, Kim BJ, Sloan C, Jia Z, Yang T, Abel ED, and Symons JD (2010). Fasting-induced reductions in cardiovascular and metabolic variables occur sooner in obese versus lean mice. Exp. Biol. Med. (Maywood) 235, 1489–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tissenbaum HA, and Guarente L (2001). Increased dosage of a sir-2 gene extends lifespan in Caenorhabditis elegans. Nature 410, 227–230. [DOI] [PubMed] [Google Scholar]
- Tomi M, Zhao Y, Thamotharan S, Shin BC, and Devaskar SU (2013). Early life nutrient restriction impairs blood-brain metabolic profile and neurobehavior predisposing to Alzheimer’s disease with aging. Brain Res 1495, 61–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toshikuni N, Arisawa T, and Tsutsumi M (2014). Nutrition and exercise in the management of liver cirrhosis. World J. Gastroenterol 20, 7286–7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travers LM, Garcia-Gonzalez F, and Simmons LW (2015). Live fast die young life history in females: evolutionary trade-off between early life mating and lifespan in female Drosophila melanogaster. Sci. Rep 5, 15469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi A, Babic S, Heiman M, Gibson WT, and Chanoine JP (2017). Ghrelin, ghrelin O-acyltransferase, and carbohydrate metabolism during pregnancy in calorie-restricted mice. Horm. Metab. Res 49, 64–72. [DOI] [PubMed] [Google Scholar]
- Trocha K, Kip P, MacArthur MR, Mitchell SJ, Longchamp A, Treviño-Villarreal JH, Tao M, Bredella MA, De Amorim Bernstein K, Mitchell JR, et al. (2019). Preoperative protein or methionine restriction preserves wound healing and reduces hyperglycemia. J. Surg. Res 235, 216–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troen AM, French EE, Roberts JF, Selhub J, Ordovas JM, Parnell LD, and Lai C (2007). Lifespan modification by glucose and methionine in Drosophila melanogaster fed a chemically defined diet. Age (Dordr.) 29, 29–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuck CJ, and Vanner SJ (2018). Dietary therapies for functional bowel symptoms: recent advances, challenges, and future directions. Neurogastroenterol. Motil 30. [DOI] [PubMed] [Google Scholar]
- Turchinovich A, Weiz L, and Burwinkel B (2012). Extracellular miRNAs: the mystery of their origin and function. Trends Biochem. Sci 37, 460–465. [DOI] [PubMed] [Google Scholar]
- Van Gilst MR, Hadjivassiliou H, Jolly A, and Yamamoto KR (2005). Nuclear hormone receptor NHR-49 controls fat consumption and fatty acid composition in C. elegans. PLoS Biol 3, e53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Norren K, Rusli F, van Dijk M, Lute C, Nagel J, Dijk FJ, Dwarkasing J, Boekschoten MV, Luiking Y, Witkamp RF, et al. (2015). Behavioural changes are a major contributing factor in the reduction of sarcopenia in caloric-restricted ageing mice. J. Cachexia Sarcopenia Muscle 6, 253–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ommen B, van den Broek T, de Hoogh I, van Erk M, van Someren E, Rouhani-Rankouhi T, Anthony JC, Hogenelst K, Pasman W, Boorsma A, and Wopereis S (2017). Systems biology of personalized nutrition. Nutr. Rev 75, 579–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeij WP, Dollé ME, Reiling E, Jaarsma D, Payan-Gomez C, Bombardieri CR, Wu H, Roks AJ, Botter SM, van der Eerden BC, et al. (2016). Restricted diet delays accelerated ageing and genomic stress in DNA-repair-deficient mice. Nature 537, 427–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verreth W, De Keyzer D, Pelat M, Verhamme P, Ganame J, Bielicki JK, Mertens A, Quarck R, Benhabilès N, Marguerie G, et al. (2004). Weight-loss-associated induction of peroxisome proliferator-activated receptor-alpha and peroxisome proliferator-activated receptor-gamma correlate with reduced atherosclerosis and improved cardiovascular function in obese insulin-resistant mice. Circulation 110, 3259–3269. [DOI] [PubMed] [Google Scholar]
- Victoria B, Dhahbi JM, Nunez Lopez YO, Spinel L, Atamna H, Spindler SR, and Masternak MM (2015). Circulating microRNA signature of genotype-by-age interactions in the long-lived Ames dwarf mouse. Aging Cell 14, 1055–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victoria B, Nunez Lopez YO, and Masternak MM (2017). MicroRNAs and the metabolic hallmarks of aging. Mol. Cell. Endocrinol 455, 131–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira MNN, Lima-Filho RAS, and De Felice FG (2018). Connecting Alzheimer’s disease to diabetes: underlying mechanisms and potential therapeutic targets. Neuropharmacology 136, 160–171. [DOI] [PubMed] [Google Scholar]
- Villareal DT, Fontana L, Das SK, Redman L, Smith SR, Saltzman E, Bales C, Rochon J, Pieper C, Huang M, et al. (2016). Effect of two-year caloric restriction on bone metabolism and bone mineral density in non-obese younger adults: a randomized clinical trial. J. Bone Miner. Res 31, 40–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virk B, Jia J, Maynard CA, Raimundo A, Lefebvre J, Richards SA, Chetina N, Liang Y, Helliwell N, Cipinska M, and Weinkove D (2016). Folate acts in E. coli to accelerate C. elegans aging independently of bacterial biosynthesis. Cell Rep 14, 1611–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Schwartzenberg RJ, Bisanz JE, Lyalina S, Spanogiannopoulos P, Ang QY, Cai J, Dickmann S, Friedrich M, Liu S-Y, Collins SL, et al. (2021). Caloric restriction disrupts the microbiota and colonization resistance. Nature 595, 272–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vora M, Shah M, Ostafi S, Onken B, Xue J, Ni JZ, Gu S, and Driscoll M (2013). Deletion of microRNA-80 activates dietary restriction to extend C. elegans healthspan and lifespan. PLoS Genet 9, e1003737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl D, Solon-Biet SM, Wang QP, Wali JA, Pulpitel T, Clark X, Raubenheimer D, Senior AM, Sinclair DA, Cooney GJ, et al. (2018). Comparing the effects of low-protein and high-carbohydrate diets and caloric restriction on brain aging in mice. Cell Rep 25, 2234–2243.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldman M, Cohen K, Yadin D, Nudelman V, Gorfil D, Laniado-Schwartzman M, Kornwoski R, Aravot D, Abraham NG, Arad M, and Hochhauser E (2018). Regulation of diabetic cardiomyopathy by caloric restriction is mediated by intracellular signaling pathways involving ‘SIRT1 and PGC-1alpha’. Cardiovasc. Diabetol 17, 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh ME, Shi Y, and Van Remmen H (2014). The effects of dietary restriction on oxidative stress in rodents. Free Radic. Biol. Med 66, 88–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Ho L, Qin W, Rocher AB, Seror I, Humala N, Maniar K, Dolios G, Wang R, Hof PR, and Pasinetti GM (2005). Caloric restriction attenuates beta-amyloid neuropathology in a mouse model of Alzheimer’s disease. FASEB J 19, 659–661. [DOI] [PubMed] [Google Scholar]
- Wang C, Maddick M, Miwa S, Jurk D, Czapiewski R, Saretzki G, Langie SA, Godschalk RW, Cameron K, and von Zglinicki T (2010). Adult-onset, short-term dietary restriction reduces cell senescence in mice. Aging (Albany, NY) 2, 555–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HB, Loh DH, Whittaker DS, Cutler T, Howland D, and Colwell CS (2018). Time-restricted feeding improves circadian dysfunction as well as motor symptoms in the Q175 mouse model of Huntington’s disease. eNeuro 5, ENEURO.0431–17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JW, Ren Y, Lu ZG, Gao J, Zhao CC, Li LX, and Wei M (2019). The combination of nonthyroidal illness syndrome and renal dysfunction further increases mortality risk in patients with acute myocardial infarction: a prospective cohort study. BMC Cardiovasc. Disord 19, 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei M, Brandhorst S, Shelehchi M, Mirzaei H, Cheng CW, Budniak J, Groshen S, Mack WJ, Guen E, Di Biase S, et al. (2017). Fasting-mimicking diet and markers/risk factors for aging, diabetes, cancer, and cardiovascular disease. Sci. Transl. Med 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weindruch R, and Sohal RS (1997). Seminars in medicine of the Beth Israel Deaconess Medical Center. Caloric intake and aging. N. Engl. J. Med 337, 986–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir HJ, Yao P, Huynh FK, Escoubas CC, Goncalves RL, Burkewitz K, Laboy R, Hirschey MD, and Mair WB (2017). Dietary restriction and AMPK increase lifespan via mitochondrial network and peroxisome remodeling. Cell Metab 26, 884–896.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox G (2005). Insulin and insulin resistance. Clin. Biochem. Rev 26, 19–39. [PMC free article] [PubMed] [Google Scholar]
- Wilson KA, Beck JN, Nelson CS, Hilsabeck TA, Promislow D, Brem RB, and Kapahi P (2020). GWAS for lifespan and decline in climbing ability in flies upon dietary restriction reveal decima as a mediator of insulin-like peptide production. Curr. Biol 30, 2749–2760.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte AV, Fobker M, Gellner R, Knecht S, and Flöel A (2009). Caloric restriction improves memory in elderly humans. Proc. Natl. Acad. Sci. USA 106, 1255–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood JG, Rogina B, Lavu S, Howitz K, Helfand SL, Tatar M, and Sinclair D (2004). Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature 430, 686–689. [DOI] [PubMed] [Google Scholar]
- Wood SH, van Dam S, Craig T, Tacutu R, O’Toole A, Merry BJ, and de Magalhaes JP (2015). Transcriptome analysis in calorie-restricted rats implicates epigenetic and post-translational mechanisms in neuroprotection and aging. Genome Biol 16, 285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Song L, Liu SQ, and Huang D (2013). Independent and additive effects of glutamic acid and methionine on yeast longevity. PLoS One 8, e79319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Isik M, Moroz N, Steinbaugh MJ, Zhang P, and Blackwell TK (2019). Dietary restriction extends lifespan through metabolic regulation of innate immunity. Cell Metab 29, 1192–1205.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Yu G, Cheng X, Gao Y, Fan X, Yang D, Xie M, Wang T, Piper MDW, and Yang M (2020). Sexual dimorphism in the nutritional requirement for adult lifespan in Drosophila melanogaster. Aging Cell 19, e13120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia X, Chen W, McDermott J, and Han JJ (2017). Molecular and phenotypic biomarkers of aging. F1000Res 6, 860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie K, Neff F, Markert A, Rozman J, Aguilar-Pimentel JA, Amarie OV, Becker L, Brommage R, Garrett L, Henzel KS, et al. (2017). Every-other-day feeding extends lifespan but fails to delay many symptoms of aging in mice. Nat. Commun 8, 155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu XM, Cai GY, Bu R, Wang WJ, Bai XY, Sun XF, and Chen XM (2015a). Beneficial effects of caloric restriction on chronic kidney disease in rodent models: a meta-analysis and systematic review. PLoS One 10, e0144442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu XM, Ning YC, Wang WJ, Liu JQ, Bai XY, Sun XF, Cai GY, and Chen XM (2015b). Anti-inflamm-aging effects of long-term caloric restriction via overexpression of SIGIRR to inhibit NF-κB signaling pathway. Cell. Physiol. Biochem 37, 1257–1270. [DOI] [PubMed] [Google Scholar]
- Yacoub R, Nugent M, Cai W, Nadkarni GN, Chaves LD, Abyad S, Honan AM, Thomas SA, Zheng W, Valiyaparambil SA, et al. (2017). Advanced glycation end products dietary restriction effects on bacterial gut microbiota in peritoneal dialysis patients; a randomized open label controlled trial. PLoS One 12, e0184789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada R, Odamaki S, Araki M, Watanabe T, Matsuo K, Uchida K, Kato T, Ozaki-Masuzawa Y, and Takenaka A (2019). Dietary protein restriction increases hepatic leptin receptor mRNA and plasma soluble leptin receptor in male rodents. PLoS One 14, e0219603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaza H, Komatsu T, Wakita S, Kijogi C, Park S, Hayashi H, Chiba T, Mori R, Furuyama T, Mori N, and Shimokawa I (2010). FoxO1 is involved in the antineoplastic effect of calorie restriction. Aging Cell 9, 372–382. [DOI] [PubMed] [Google Scholar]
- Yang L, Licastro D, Cava E, Veronese N, Spelta F, Rizza W, Bertozzi B, Villareal DT, Hotamisligil GS, Holloszy JO, and Fontana L (2016). Long-term calorie restriction enhances cellular quality-control processes in human skeletal muscle. Cell Rep 14, 422–428. [DOI] [PubMed] [Google Scholar]
- Yechoor VK, Patti ME, Saccone R, and Kahn CR (2002). Coordinated patterns of gene expression for substrate and energy metabolism in skeletal muscle of diabetic mice. Proc. Natl. Acad. Sci. USA 99, 10587–10592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilmaz ÖH, Katajisto P, Lamming DW, Gültekin Y, Bauer-Rowe KE, SenGupta S, Birsoy K, Dursun A, Yilmaz VO, Selig M, et al. (2012). mTORC1 in the Paneth cell niche couples intestinal stem-cell function to calorie intake. Nature 486, 490–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S, Yamahara K, Kume S, Koya D, Yasuda-Yamahara M, Takeda N, Osawa N, Chin-Kanasaki M, Adachi Y, Nagao K, et al. (2018). Role of dietary amino acid balance in diet restriction-mediated lifespan extension, renoprotection, and muscle weakness in aged mice. Aging Cell 17, e12796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N, Li Z, Mu W, Li L, Liang Y, Lu M, Wang Z, Qiu Y, and Wang Z (2016a). Calorie restriction-induced SIRT6 activation delays aging by suppressing NF-κB signaling. Cell Cycle 15, 1009–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang WB, Sinha DB, Pittman WE, Hvatum E, Stroustrup N, and Pincus Z (2016b). Extended twilight among isogenic C. elegans causes a disproportionate scaling between lifespan and health. Cell Syst 3, 333–345.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Wang X, Qu J-H, Liu B, Zhang P, Zhang T, Fan P-C, Wang X-M, Xiao G-Y, Su Y, et al. (2019). Caloric restriction induces microRNAs to improve mitochondrial proteostasis. iScience 17, 155–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Yang Y, Su J, Zheng X, Wang C, Chen S, Liu J, Lv Y, Fan S, Zhao A, et al. (2021). Age-related compositional changes and correlations of gut microbiome, serum metabolome, and immune factor in rats. GeroScience 43, 709–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao P, Sui B-D, Liu N, Lv Y-J, Zheng C-X, Lu Y-B, Huang W-T, Zhou C-H, Chen J, Pang D-L, et al. (2017a). Anti-aging pharmacology in cutaneous wound healing: effects of metformin, resveratrol, and rapamycin by local application. Aging Cell 16, 1083–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Gilliat AF, Ziehm M, Turmaine M, Wang H, Ezcurra M, Yang C, Phillips G, McBay D, Zhang WB, et al. (2017b). Two forms of death in ageing Caenorhabditis elegans. Nat. Commun 8, 15458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, Shyh-Chang N, Segrè AV, Shinoda G, Shah SP, Einhorn WS, Takeuchi A, Engreitz JM, Hagan JP, Kharas MG, et al. (2011). The Lin28/let-7 axis regulates glucose metabolism. Cell 147, 81–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zid BM, Rogers AN, Katewa SD, Vargas MA, Kolipinski MC, Lu TA, Benzer S, and Kapahi P (2009). 4E-BP extends lifespan upon dietary restriction by enhancing mitochondrial activity in Drosophila. Cell 139, 149–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman JA, Malloy V, Krajcik R, and Orentreich N (2003). Nutritional control of aging. Exp. Gerontol 38, 47–52. [DOI] [PubMed] [Google Scholar]
- Zorc M, Skok DJ, Godnic I, Calin GA, Horvat S, Jiang Z, Dovc P, and Kunej T (2012). Catalog of microRNA seed polymorphisms in vertebrates. PLoS One 7, e30737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou K, Rouskin S, Dervishi K, McCormick MA, Sasikumar A, Deng C, Chen Z, Kaeberlein M, Brem RB, Polymenis M, et al. (2020). Life span extension by glucose restriction is abrogated by methionine supplementation: cross-talk between glucose and methionine and implication of methionine as a key regulator of life span. Sci. Adv 6, eaba1306. [DOI] [PMC free article] [PubMed] [Google Scholar]