Abstract
Objective:
To evaluate the influence of pH levels on interarch elastics with regard to force decay and cytotoxicity.
Materials and Methods:
One nonlatex (NLAO) group and one latex (LAO) group were tested (n = 10). Elastics were stretched to 25 mm and were held for 1, 6, 12, and 24 hours in artificial saliva solutions with pH levels of 5.0, 6.0, and 7.5. Force magnitudes were measured at 25 mm of activation. The cytotoxicity assay was performed using cell cultures (L929 mouse fibroblast cell line), which were subjected to the cell viability test with neutral red (“dye-uptake”). Force decay and cytotoxicity were assessed using analysis of variance, the Sidak method, and a Tukey's test.
Results:
The interactions between group, pH, and time showed no statistically significant differences (P = .29). When pH per time (P = .032) and group per time (P = .0009) were considered, these interactions showed statistically significant differences (P < .05). The pH did not interfere directly in the degradation results of the tested elastics. The cytotoxicity test showed that group LAO presented lower cell viability when compared with group NLAO over the course of the entire experiment. There was a gradual reduction in cell viability from 1 hour to 24 hours. A significant difference (P < .05) was found between the interactions group pH and the control group of cells, except between group NLAO at the time point of 1 hour at different pH values and at the time points of 6 and 12 hours with pH 5 (P > .05).
Conclusions:
No significant correlation between pH, force decay, and cytotoxicity was observed.
Keywords: Elastics, pH, Force decay, Cytotoxicity
INTRODUCTION
Latex elastic has been extensively used in orthodontics since the advent of the specialty. However, latex is not in the category of materials known to be entirely inoffensive.1–5 As a result of latex allergies, nonlatex orthodontic elastics are becoming increasingly popular.6 Several properties of latex and nonlatex elastics have been evaluated,5,7,8 some involving saliva or simulated saliva solutions.9–12 Few studies have investigated the effects of salivary pH levels on viscoelastic force relaxation of nonlatex interarch elastics.13 Great individual pH variability is noted within the oral cavity, and this can fluctuate with diet.14
One study15 assessed the mechanical properties of latex and nonlatex orthodontic elastics. Although the cited study compared only one brand of elastics, the authors reported that the former had greater breaking strength than the latter. A 2006 study11 indicated that nonlatex elastics become more “deformed” with use than do those made of latex. However, both show loss of force as treatment time increases, and no significant differences were noted between the two within 24 hours.
Another study16 by members of the same group concluded that there are significant differences between latex and latex-free elastics, but latex-free elastics can replace latex products if they are changed more frequently.
The term force degradation10 has been used in the orthodontic literature; however, stress relaxation is the technically correct engineering term.8 Relaxation, however, can be a result of degradation.14 Because force is being measured, force decay is the term used herein to describe this viscoelastic behavior. On the other hand, studies13,14 involving the effects of Ph levels did not consider whether the force decay:pH ratio would have an influence on the biologic properties of this material. The purpose of the present study was to test the influence of pH levels on latex and nonlatex interarch elastics with regard to force decay and cytotoxicity.
MATERIALS AND METHODS
Degradation Test Mechanical and pH
This study was designed to observe the effects of pH levels in artificial saliva on force decay and cytotoxicity in elastics. Four jig boards, each with 20 pairs of pins set 15 mm apart, were used to test 20 sets of elastics at a time.14
Two groups of 3/16-inch (4.76-mm), 6-ounce (184-g) interarch elastics (group nonlatex [NLAO; American Orthodontics, Sheboygan, Sheboygan, Wisc] and group latex [LAO; American Orthodontics]) were tested at three pH levels over four time points, with a sample size of 10 in each treatment combination. Based on a previous study,14,17 the elastics were stretched to 25 mm for force measurement.
Artificial saliva solutions set at prescribed pH levels of 5.0, 6.0, and 7.5 were provided by the pharmacy school of the Federal University of Rio de Janeiro (Rio de Janeiro, Brazil). pH levels were measured every hour using a calibrated pH/ion meter (model 300, Analyser, São Paulo, SP, Brazil) and were adjusted accordingly with 1 M citric acid or 1 M sodium hydroxide. Solutions were incubated at approximately 37°C. The tubs of artificial saliva solution were placed on a rocker (Model TS-8, Meditry, Shanghai, China) oscillating between 25 and 50 rpm during the experiment to help maintain a uniform pH.
The use of 10 elastics per treatment combination allowed the groups to be tested simultaneously at the same pH level at 1, 6, 12, and 24 hours. The force was recorded off a horizontally secured and calibrated digital force gauge (Imada DS2-11, accuracy ±0.2%, Imada Inc, Northbrook, Ill). A consistent reading was established, usually within 4 to 5 seconds. All elastics had recent manufacturing dates and were randomly selected from different packs of the same type/brand and were appropriately distributed. The tester was blinded with regard to the type of elastic that was on each dowel pin.
Cytotoxicity Test
After the degradation test mechanical, the elastics were submitted to a cytotoxicity test. Previously, the elastics were superficially washed with deionized water (Millipore, Bedford, Mass) for 5 seconds and sterilized on both sides with ultraviolet light (Labconco, Kansas City, Mo) for 30 minutes.3–5,18
To verify the cell response to extreme situations, another three groups were included in the study: group cell control (CC), consisting of cells not exposed to any material; group positive control (C+), consisting of Tween 80; and group (negative control (C−), consisting of phosphate-buffered saline (PBS) solution in contact with the cells.
Cell culture containing L-929 line cells (mouse fibroblast; American Type Culture Collection, ATCC, Rockville, Md) was maintained in Eagles' minimum essential medium (MEM; Cultilab, Campinas, São Paulo, Brazil) by adding 0.03 mg/mL of glutamine (Sigma, St Louis, Mo), 50 µg/mL of garamicine (Schering Plough, Kenilworth, NJ), 2.5 mg/mL of fungizone (Bristol-Myers-Squibb, New York, NY), 0.25% sodium bicarbonate solution (Merck, Darmstadt, Germany), 10 mM of HEPES (Sigma), and 10% bovine fetal serum (Cultilab, Campinas) for the growth medium or no bovine fetal serum for the maintenance medium only. After this, the cell culture medium was incubated at 37°C for 48 hours.
The method for evaluating the cytotoxicity was the “dye-uptake” test.19 This method is based on neutral red dye incorporated into live cells. It was used in this experiment only for the following periods of evaluation: 1, 6, 12, and 24 hours. These periods represent the time intervals of 1, 6, 12, and 24 hours during which the elastic remained in the cell culture medium before being removed.
Dye Uptake
Volumes of 100 µL of L-929 line cells were distributed into 96-well microplates. After 48 hours, the growth medium was replaced with 100 µL of Eagles' MEM obtained after incubation in the different types of elastics and positive and negative control at 1, 6, 12, and 24 hours. Positive and negative control groups consisted of culture medium placed in contact with 100 µL of Tween 80 and 100 µL of PBS solution, respectively. After 24-hour incubation, 100 µL of 0.01% neutral red dye (Sigma) was added to the culture medium in the 96-well microplates, which were incubated again for 3 hours at 37°C so that the red dye could penetrate the live cells. After this period of time, 100 µL of 4% formaldehyde solution (Vetec, Rio de Janeiro, Brazil) in PBS (130 mM of NaCl; 2 mM of KCl; 6 mM of Na2HPO4 2 H2O; 1 mM of K2HPO4 1 mM; pH 7.2) were added in order to promote cell attachment to the plate. After 5 minutes, 100 µL of 1% acetic acid (Vetec) and 50% methanol (Vetec) were added in order to remove the dye. After 20 minutes, a spectrophotometer (BioTek, Winooski, Vt) at 492-nm wavelength (λ = 492 nm) was used to read the data.
Statistical Analysis
The standard deviation of the load measurements was estimated to be 0.11 N based on the study by Beattie and Monaghan.17 With a sample size of 10 elastics per treatment combination (total sample size of 2 × 3 × 4 × 10 = 240 elastics), the study was designed to have at least 80% power to detect a difference of 0.2 N (20 g) between any two treatment combinations, assuming two-sided tests at a 5% significance level for each set of comparisons among treatment combinations. The effects of material, pH, and time on measured loads were assessed using three-way analysis of variance (ANOVA). Pair-wise comparisons between treatment combinations were adjusted for multiple comparisons using the Sidak method. Because of nonnormal distribution of the loads, analyses were performed using the ranks of the measurements. The cytotoxicity test data presented normal distribution and were compared by ANOVA, and the Tukey multiple comparison test was used for identifying differences between the groups. The level of significance was set at P < .05.
RESULTS
Degradation Test Mechanical and pH
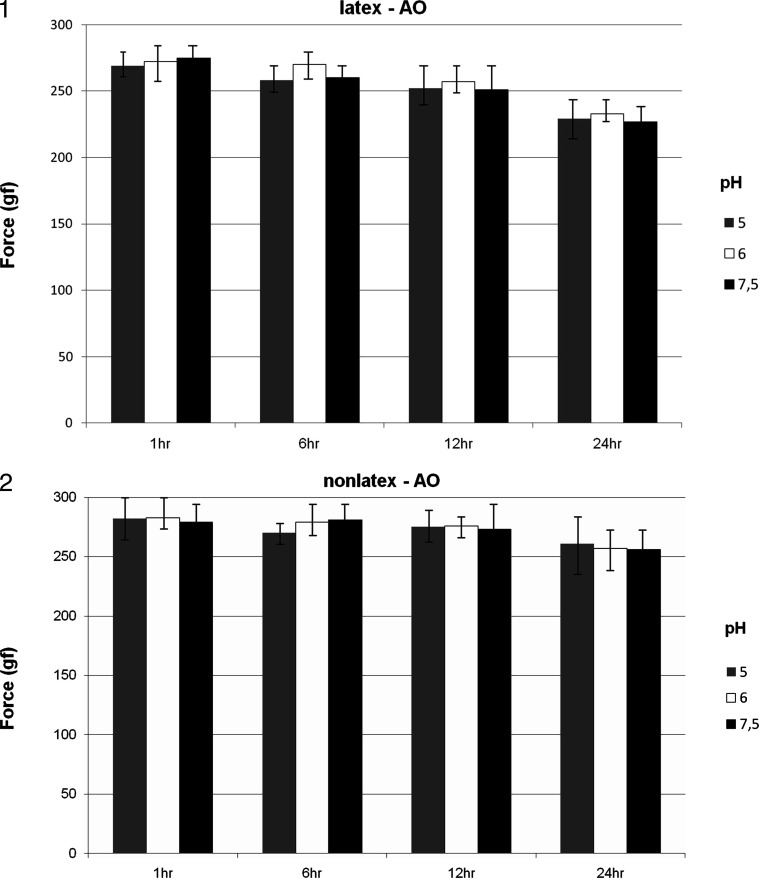
The interactions among group, pH, and time showed no statistically significant differences (P = .29) (Figures 1 and 2). The interactions between groups considering the different pH values were not significant (P = .52). However, when pH per time (P = .032) and group per time (P = .0009) were considered, these interactions showed statistically significant differences (P < .05) (Figures 1 and 2). Force degradation was directly proportional to the increase in evaluation time. Nonlatex elastics showed better performance (Figure 2) with higher force release and lower degradation loss when compared with latex elastics (Figure 1) over the course of the entire experiment. The pH did not interfere directly in the degradation results of the elastics tested.
Figure 1 and 2.
Elastic force decay (mean and standard deviation) of the latex (group LAO) and nonlatex (group NLAO) elastics of American Orthodontics for the different pH levels and times evaluated.
Cytotoxicity Test
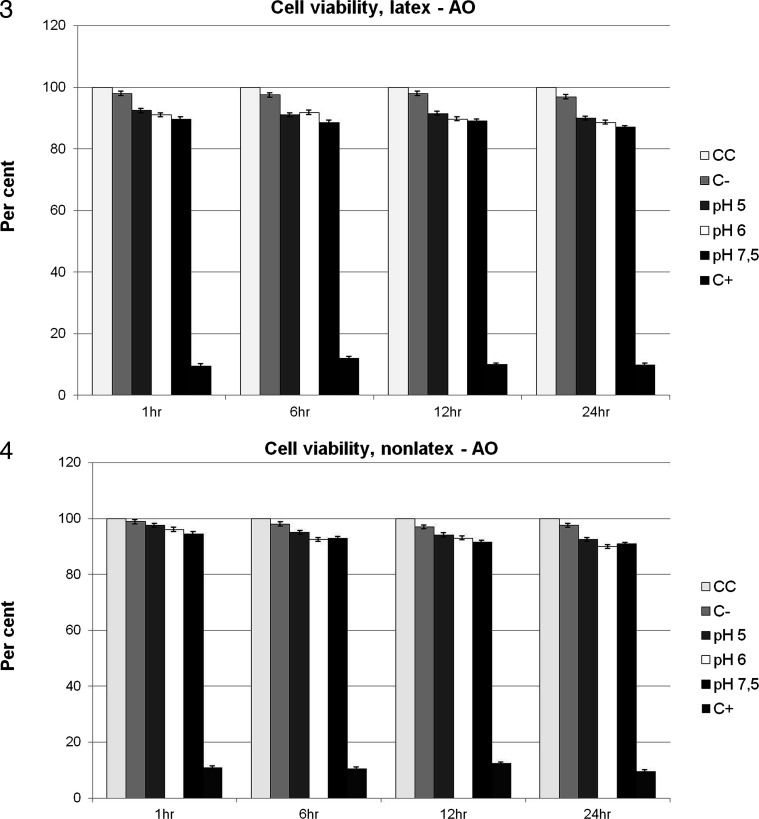
Viability was established by comparison with the viability of control cells, which was arbitrarily set at 100%. Group LAO showed lower cell viability when compared with group NLAO during the entire experiment. There was gradual reduction in cell viability from 1 hour to 24 hours (Figures 3 and 4). Cell viability ranged from 97.5% (±1.76%) to 90% (±1.09%) in group NLAO and from 92.5% (±1.61%) to 87% (±1.17%) in group LAO, in comparison with control cells (Figures 3 and 4). A significant difference (P < .05) was found between the interactions group-pH and control group of cells, except between group NLAO at the time point of 1 hour at the different pH values and at the time points of 6 and 12 hours at pH 5 (P > .05).
Figure 3 and 4.
Cell viability (in percent) of latex (group LAO) and nonlatex (group NLAO) elastics of American Orthodontics for the different pH levels and times evaluated and for the control group: group CC = cell control; group C− = PBS solution; and group C+ = Tween 80.
DISCUSSION
Various studies5,7,9,10,12,13,16 have attempted to establish the mechanical and environmental factors that contribute to the force decay of interact elastics. However, studies13,14 involving the effects of Ph levels did not consider whether the force decay:pH ratio would have an influence on the biologic properties of this material.
In the present study, pH did not contribute significantly to force decay. Over the 24-hour span of the experiment, time appeared to be influential, with latex elastics exhibiting a marked decrease in force in comparison with the nonlatex type, regardless of pH, although a nonsignificant decrease was found in both groups. The rapid decrease in force is consistent with the results of studies14,17 in the literature.
During the experiment, factors such as the artificial saliva temperature, time in solution, and deformations during handling on the jig board were kept as consistent as possible.14 Nonetheless, as in other studies,9,12,14 a high level of variability was noted, leading to questions about uniformity in composition and dimension of these materials. Indeed, unmagnified examination of the tested elastics revealed obvious quality control issues in terms of thickness and uniformity,14 which the authors believe contributed greatly to the variability in force measurements.
With regard to the cytotoxicity test, the monolayer cell culture model was used in the present study.4,20 This model was used together with the dye-uptake technique19 because the cytotoxicity of materials can be determined by spectrophotometry.
Spectrophotometric assay allows rapid and reliable evidence of cell viability to be obtained, based on the use of vital stain incorporated into viable cells. In this study, neutral red dye was used because it is widely used for identification of L-929 cell viability.3,4 Dead or damaged cells cannot incorporate vital stain and are thus not recognized on optical reading. Therefore, spectrophotometry does not allow dead cells to be distinguished from the damaged ones.3 The choice of L-929 mouse fibroblasts resulted from the fact that they show results comparable with those of primary human gingival fibroblasts,21,22 but one cannot interpret the cell culture results as a human response.
Allergy to natural latex occurs because of the presence of many types of proteins, and the powder covering the orthodontic elastics works as a transporter of these proteins.3 Therefore, the development of nonlatex elastics has become increasingly important for clinical use.
In the present study, the American Orthodontics nonlatex elastics had fewer cytotoxic effects than did the latex elastics. As the powder covering the elastomeric ligatures was removed before performing the in vitro studies, it was not possible to know whether this powder would have any effect. The powder was removed in order to standardize the samples, as the composition and quantity of powder present in the elastics could interfere with the results.
According to Schmalz,23 the great danger is that potentially cytotoxic intraoral elastics could release substances that might be ingested by the patient over time, thus causing diseases that result from a cumulative effect. It is known that latex is not entirely biocompatible, as it may interact with foods2 and medications.24
The elastomeric ligatures evaluated in this study showed over 87% cell viability in all experimental periods. Hanson and Lobner25 evaluated 3/16-inch interior lumen (medium) latex and nonlatex elastics and found cell lysis to be 50% higher for latex elastics compared with the nonlatex type. However, the authors considered both types of elastics appropriate for orthodontic use. Therefore, it is suggested that elastics showing cell viability of less than 50% should be avoided in order to prevent the cumulative effects of the cytotoxic components released from these elastics into the body.23
This study showed that both elastics presented great cell viability, and the influence of pH on the degradation of elastic strength and cytotoxicity was not confirmed, indicating an appropriate process of industrialization and/or the presence of stabilizing substances noncytotoxic in the composition these elastics. However, the nonlatex elastics showed better performance with regard to mechanical and biological properties, although further studies are suggested to examine the manufacturing challenges and lack of consistency of shape among interarch elastics.14
CONCLUSIONS
Within the limits of this in vitro study, no significant correlation among pH, force decay, and cytotoxicity was observed. The time of use and imperfections in the shape of elastics had more influence and contributed to the variability in results.
REFERENCES
- 1.Palosuo T, Alenius H, Turjanmaa K. Quantitation of latex allergens. Methods. 2002;27:52–58. doi: 10.1016/S1046-2023(02)00051-8. [DOI] [PubMed] [Google Scholar]
- 2.Turjanmaa K, Alenius H, Makinen-Kiljunen S, Reunala T, Palosuo T. Natural rubber latex allergy. Allergy. 1996;51:593–602. doi: 10.1111/j.1398-9995.1996.tb04678.x. [DOI] [PubMed] [Google Scholar]
- 3.Santos R. L, Pithon M. M, Martins F. O, Romanos M. T, Ruellas A. C. Cytotoxicity of latex and non-latex orthodontic elastomeric ligatures on L929 mouse fibroblasts. Braz Dent J. 2010;21:205–210. doi: 10.1590/s0103-64402010000300005. [DOI] [PubMed] [Google Scholar]
- 4.dos Santos R. L, Pithon M. M, Martins F. O, Romanos M. T, de Oliveira Ruellas A. C. Evaluation of the cytotoxicity of latex and non-latex orthodontic separating elastics. Orthod Craniofac Res. 2010;13:28–33. doi: 10.1111/j.1601-6343.2009.01469.x. [DOI] [PubMed] [Google Scholar]
- 5.Dos Santos R. L, Pithon M. M, Da Silva Mendes G, Romanos M. T, De Oliveira Ruellas A. C. Cytotoxicity of intermaxillary orthodontic elastics of different colors: an in vitro study. J Appl Oral Sci. 2009;17:326–329. doi: 10.1590/S1678-77572009000400010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Snyder H. A, Settle S. The rise in latex allergy: implications for the dentist. J Am Dent Assoc. 1994;125:1089–1097. doi: 10.14219/jada.archive.1994.0129. [DOI] [PubMed] [Google Scholar]
- 7.Paige S. Z, Tran A. M, English J. D, Powers J. M. The effect of temperature on latex and non-latex orthodontic elastics. Tex Dent J. 2008;125:244–249. [PubMed] [Google Scholar]
- 8.Meyers M. A, Chawla K. K. Mechanical Behavior of Materials 1st ed. Upper Saddle River, NJ: Prentice-Hall; 1999. [Google Scholar]
- 9.Tran A. M, English J. D, Paige S. Z, Powers J. M, Bussa H. I, Lee R. P. Force relaxation between latex and non-latex orthodontic elastics in simulated saliva solution. Tex Dent J. 2009;126:981–985. [PubMed] [Google Scholar]
- 10.Wang T, Zhou G, Tan X, Dong Y. Evaluation of force degradation characteristics of orthodontic latex elastics in vitro and in vivo. Angle Orthod. 2007;77:688–693. doi: 10.2319/022306-76. [DOI] [PubMed] [Google Scholar]
- 11.Bertoncini C, Cioni E, Grampi B, Gandini P. In vitro properties' changes of latex and non-latex orthodontic elastics. Prog Orthod. 2006;7:76–84. [PubMed] [Google Scholar]
- 12.Kersey M. L, Glover K, Heo G, Raboud D, Major P. W. An in vitro comparison of 4 brands of nonlatex orthodontic elastics. Am J Orthod Dentofacial Orthop. 2003;123:401–407. doi: 10.1067/mod.2003.22. [DOI] [PubMed] [Google Scholar]
- 13.Ferriter J. P, Meyers C. E, Jr, Lorton L. The effect of hydrogen ion concentration on the force-degradation rate of orthodontic polyurethane chain elastics. Am J Orthod Dentofacial Orthop. 1990;98:404–410. doi: 10.1016/S0889-5406(05)81648-8. [DOI] [PubMed] [Google Scholar]
- 14.Sauget P. S, Stewart K. T, Katona T. R. The effect of pH levels on nonlatex vs latex interarch elastics. Angle Orthod. 2011;81:1070–1074. doi: 10.2319/011811-34.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Russell K. A, Milne A. D, Khanna R. A, Lee J. M. In vitro assessment of the mechanical properties of latex and non-latex orthodontic elastics. Am J Orthod Dentofacial Orthop. 2001;120:36–44. doi: 10.1067/mod.2001.114642. [DOI] [PubMed] [Google Scholar]
- 16.Gandini P, Gennai R, Bertoncini C, Massironi S. Experimental evaluation of latex-free orthodontic elastics' behaviour in dynamics. Prog Orthod. 2007;8:88–99. [PubMed] [Google Scholar]
- 17.Beattie S, Monaghan P. An in vitro study simulating effects of daily diet and patient elastic band change compliance on orthodontic latex elastics. Angle Orthod. 2004;74:234–239. doi: 10.1043/0003-3219(2004)074<0234:AIVSSE>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 18.Santos R. L, Pithon M. M, Oliveira M. V, Mendes G. S, Romanos M. T. V, Ruellas A. C. O. Cytotoxicity of intraoral orthodontic elastics. Braz J Oral Sci. 2008;24:1520–1525. [Google Scholar]
- 19.Neyndorff H. C, Bartel D. L, Tufaro F, Levy J. G. Development of a model to demonstrate photosensitizer-mediated viral inactivation in blood. Transfusion. 1990;30:485–490. doi: 10.1046/j.1537-2995.1990.30690333476.x. [DOI] [PubMed] [Google Scholar]
- 20.Tomakidi P, Koke U, Kern R, et al. Assessment of acute cyto- and genotoxicity of corrosion eluates obtained from orthodontic materials using monolayer cultures of immortalized human gingival keratinocytes. J Orofac Orthop. 2000;61:2–19. doi: 10.1007/BF02340928. [DOI] [PubMed] [Google Scholar]
- 21.Schedle A, Samorapoompichit P, Rausch-Fan X. H, et al. Response of L-929 fibroblasts, human gingival fibroblasts, and human tissue mast cells to various metal cations. J Dent Res. 1995;74:1513–1520. doi: 10.1177/00220345950740081301. [DOI] [PubMed] [Google Scholar]
- 22.Franz A, Konig F, Skolka A, et al. Cytotoxicity of resin composites as a function of interface area. Dent Mater. 2007;23:1438–1446. doi: 10.1016/j.dental.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 23.Schmalz G. Use of cell cultures for toxicity testing of dental materials—advantages and limitations. J Dent. 1994;22(suppl 2):S6–S11. doi: 10.1016/0300-5712(94)90032-9. [DOI] [PubMed] [Google Scholar]
- 24.Towse A, O'Brien M, Twarog F. J, Braimon J, Moses A. C. Local reaction secondary to insulin injection. A potential role for latex antigens in insulin vials and syringes. Diabetes Care. 1995;18:1195–1197. doi: 10.2337/diacare.18.8.1195. [DOI] [PubMed] [Google Scholar]
- 25.Hanson M, Lobner D. In vitro neuronal cytotoxicity of latex and nonlatex orthodontic elastics. Am J Orthod Dentofacial Orthop. 2004;126:65–70. doi: 10.1016/j.ajodo.2003.07.006. [DOI] [PubMed] [Google Scholar]