Abstract
A). Purpose of Review:
Accurate imaging of the aortic root during valve implantation is crucial for proper prosthesis positioning during TAVR. The purpose of this review was to determine if routine use of the cusp-overlap view should be adopted for self-expanding valves.
B). Recent Findings:
Use of the cusp-overlap view with the Evolut, Portico, ACURATE neo/neo2, and JenaValve systems is associated with lower post-procedural new permanent pacemaker implantation rates when compared to the standard 3-cusp view, presumably due to more precise valve implantation relative to the conduction system by the non-coronary cusp.
C). Summary:
By elongating the left ventricular outflow tract and accentuating the right-non commissure in the center of the fluoroscopic view, the cusp-overlap technique allows operators to more precisely control the prosthesis implant depth during self-expanding valve deployment. While the early experience with this approach in Evolut TAVR has been promising, the results of larger studies with longer follow-up across multiple self-expanding systems are warranted.
Keywords: Aortic Stenosis, Transcatheter Aortic Valve Replacement, Cusp-Overlap, Self-Expanding, Permanent Pacemaker Implantation, Commissural Alignment
Introduction
Transcatheter aortic valve replacement (TAVR) has now been approved for patients with native severe symptomatic aortic stenosis across the entire surgical risk spectrum [1], and recently surpassed surgical aortic valve replacement (SAVR) in annual volume of cases in the United States [2]. Over the years, technological advancements have produced transcatheter heart valve (THV) systems that continue to push the boundaries of technical performance and efficiency. The procedural conduct of TAVR has concurrently evolved and has undergone various refinements to maximize patient safety, minimize risk and complications, and achieve optimal clinical efficacy [3, 4]. Despite these advancements, paravalvular leak (PVL) and the need for new permanent pacemaker (PPM) implantation remain among the most frequent limitations. While THV type and implantation depth have historically been the most common procedural factors associated with PVL and conduction disturbances post-TAVR, device positioning has now also emerged as an important determinant of these adverse outcomes. This is especially true for self-expanding valves, with higher device implantation resulting in lower rates of post-procedural conduction abnormalities [5, 6]. Since a few millimeters in implantation depth can make a big difference in PPM rates, accurate imaging of the aortic root during valve implantation is imperative for proper THV positioning. However, this is often limited by parallax of the valve frame during deployment. Recently, a “cusp-overlap” approach for deployment of self-expanding valves was suggested that is based on overlapping the right (RCC) and left coronary cusps (LCC), thus isolating the non-coronary cusp (NCC) [7]. Given its numerous advantages over the standard coplanar 3-cusp view and its successful application in Evolut R and PRO(+) (Medtronic, Minneapolis, MN), Portico (Abbott Structural Heart, Santa Clara, CA), and ACURATE neo/neo2 (Boston Scientific, Marlborough, MA) deployment, the cusp-overlap technique should become the standard implantation strategy for self-expanding THVs.
Optimal Fluoroscopic Projection for Self-Expanding TAVR
While the procedural steps of balloon-expandable THV deployment have remained similar for years, the deployment of self-expanding THV systems continues to evolve. A crucial step in self-expanding TAVR is the identification of the ideal fluoroscopic projection that allows operators to achieve accurate prosthesis deployment precisely at a depth beneath the annular plane. In this angiographic view, there is ideally no foreshortening of the patient’s anatomy or of the delivery system. This has important consequences for peri-procedural complications since implanting the valve too high or too low, within a margin of millimeters, can lead to significant PVL and atrioventricular conduction abnormalities, respectively [8].
Over the last two decades, various strategies for determining the optimal selection of fluoroscopic projections of the aortic valve for self-expanding TAVR have been proposed, including use of various imaging modalities such as multidetector computed tomography (MDCT), repeated aortic root angiograms from different angles, and three-dimensional angiographic reconstructions (3DA) of the aortic root captured from rotational C-arm fluoroscopic images [9–12]. Many centers rely on pre-procedural MDCT to predict optimal fluoroscopic projections that achieve a perspective orthogonal to the plane of the aortic annulus. However, this commits the operator to numerous intraprocedural adjustments to correct for parallax of the THV prosthesis as it is positioned across the annulus [13]. Thus, a fluoroscopic view that eliminates the parallax of the THV system and simultaneously maintains the true aortic annular plane is essential for accurate device placement [14].
A potential solution was first proposed by Piazza and colleagues [15]. They showed that the aortic annulus remained in-plane in a range of views with different relative cusp positions, described by an S-curve, and that the device plane could similarly be imaged from different angiographic angles, described by a second S-curve. These two S-curves intersect in a right anterior oblique/caudal (RAO/CAU) view in most cases. This view represents the unique projection in which both the prosthesis and annulus are in-plane, and allows the true device depth below the annulus to be assessed [16]. Dedicated computed tomography (CT) imaging software, such as 3mensio (Pie Medical Imaging BV, Bilthoven, NL) is used to determine the S-curve of the annulus at the time of pre-procedural planning. However, determining the S-curve of the device requires intraprocedural image analysis. As expected, this is not always routinely available, and the S-curve technique has consequently not been widely adopted [17].
In 2018, Tang et al. introduced the “cusp-overlap” approach to simplify fluoroscopy-guided implantation of the CoreValve/Evolut THV system [7]. This contemporary method for achieving the optimal projection for deployment is based on the fact that self-expanding THVs engage the aortic valve from the outer aortic curve and are deployed from the NCC towards the LCC. In contrast, balloon-expandable valves are commonly centered and deployed in a perpendicular view of the aortic valve annulus, with the RCC projected between the NCC and LCC. In the cusp-overlap technique, preprocedural MDCT is first used to determine the plane of the aortic annulus and the cusp-overlap fluoroscopic angle. Multiplanar reconstructions along the S-curve of the aortic valve are then used to generate a view in which the designated LCC and RCC hinge points appear overlapped, thereby isolating the NCC on the opposite side. The corresponding fluoroscopic angulation is defined as the cusp-overlap angulation [17].
The cusp-overlap approach offers several advantages over the standard coplanar projection with three cusps (Figure 1). First, use of a caudal/cranial view eliminates parallax of the delivery catheter. Furthermore, the delivery catheter is naturally positioned towards the outer curve of the aortic root and is therefore more centered across the aortic valve. The cusp-overlap technique also allows THV deployment in the true coplanar view and eliminates parallax of the delivery system across the annulus, thereby simplifying the deployment process. When using the Evolut THV system, annular contact occurs from the NCC to the LCC, which visually occurs over a shorter distance during valve flowering and deployment. In addition, the en-face view of the NCC enables the prosthesis implantation at a higher depth with little concern for device “pop-out” upon release. This is especially true when dealing with large annuli with minimal oversizing (or undersizing) [7]. Finally, the cusp-overlap approach allows the operator to visually elongate the left ventricular outflow tract (LVOT) and localize the membranous septum just beneath the RCC-NCC (R-N) commissure. On fluoroscopy, the R-N commissure is generally isolated to the leftward-most portion of the aortic annulus. As mentioned before, the NCC is usually engaged first when deploying self-expanding valves, and thus, the cusp-overlap view is particularly advantageous in estimating the depth of prosthesis implantation [8].
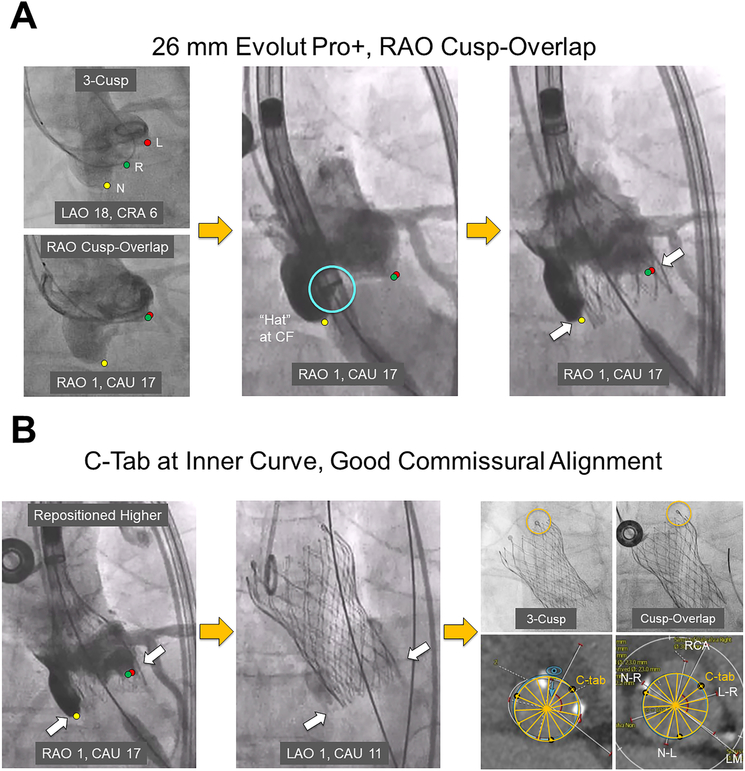
Figure 1. Deployment of the Evolut PRO+ Transcatheter Aortic Valve System Using the Cusp-Overlap Technique with Commissural Alignment: Step-by-Step.
A) A 3-cusp coplanar fluoroscopic view, followed by the RAO-CAU cusp-overlap view, is obtained. This can be achieved using the CT-derived fluoroscopic view or by placing a wire and pigtail respectively at the right- and left-coronary cusps and superimposing the two to get the cusp-overlap view. The Evolut PRO+ delivery system, once positioned across the aortic annulus, can be seen with the “Hat” marker facing center front (light blue circle) without parallax, thus simplifying the deployment process. At 80% deployment, if there is no parallax seen at the same cusp-overlap view, one does not need to rotate the C-arm to a more LAO projection to visualize the implant depth (solid white arrows) relative to the left cusp.
B) As in this case, to avoid new conduction abnormalities, recapture and repositioning of the Evolut PRO+ valve higher relative to the non-coronary cusp (solid white arrows) may be required. Again, given that no parallax is seen at the inflow of the valve, there is no need to rotate the C-arm to a more LAO projection to visualize the implant depth relative to the left cusp. After release, the Evolut PRO+ valve is seen positioned at the optimal depth (solid white arrows). A cine image without contrast injection is obtained of the valve using both the 3-cusp and cusp-overlap views, thus confirming that the C-tab (orange circle) is at the inner curve of the ascending aorta. Using the fluoro-CT co-registration technique, good commissural alignment of the Evolut PRO+ valve with the native commissures can be visualized, potentially facilitating post-TAVR coronary reaccess.
CAU = caudal, CRA = cranial, CT = computed tomography, L = left, LAO = left anterior oblique, L-R = left-right, LM = left main, N = non, N-L = non-left, N-R = non-right, R = right, RAO = right anterior oblique, RCA = right coronary artery.
In the majority of cases, the cusp-overlap view occurs in a RAO/CAU projection, which usually represents a three-chamber view and allows operators to visualize the left atrium, left ventricle, and LVOT and aortic root with perpendicular separation of the aortic and mitral valves [18]. This is sometimes more desirable than the left anterior oblique (LAO)-cranial projections of the left heart provided by the standard 3-cusp view [15]. Whereas the latter images the heart in a four-chamber view with a drastically foreshortened LVOT, the former has several theoretical advantages. For one, in the cusp-overlap view, the NCC marks the annular border at the most inferior hinge point on two-dimensional fluoroscopy. This is generally not true for LAO views. In addition, RAO/CAU projections display the minor axis of the aortic annulus, which allows TAVR operators to confirm the earliest contact between the THV prosthesis and annulus during deployment. Depiction of the short axis of the aortic annulus also permits more complete evaluation of valve expansion when compared to LAO views that typically represent the long axis of the aortic annulus. Furthermore, RAO/CAU projections expose operators to lower radiation doses [8, 19].
Technique of Valve Implantation Using the Cusp-Overlap View
The technical principles of valve implantation using the cusp-overlap approach are generally similar across the various self-expanding platforms. During preprocedural planning, a coplanar view is identified that isolates the NCC and fully overlaps the LCC/RCC. As mentioned previously, this is a RAO/CAU view in most patients that elongates the LVOT and extends the visual distance from the base of the non-coronary cusp to the conduction system in the muscular septum. In the case of the Evolut system, a stiff, double-curved Lunderquist wire (Cook Medical, Bloomington, IN) is recommended to maintain wire position in the R-N commissure and also to begin valve deployment in the posterior aspect of the annular plane. The stiffer wire results in more symmetrical deployment and is especially useful when deploying larger-sized THVs. THV deployment is also begun with the ring marker in the mid-portion of the pigtail to obtain a final implantation depth of approximately 3–5 mm below the NCC. Note that using the cusp-overlap view to maintain a reference to the plane of the aortic annulus may lead to the marker band on the THV delivery catheter losing parallax when approaching the annular plane. This is often a result of the delivery catheter following the stiff left ventricular wire that is generally positioned in the R-N commissure. Adequate pacing during the deployment is employed to minimize cardiac output and the occurrence of premature ventricular contractions. When the prosthesis has been 80% deployed, parallax is removed from the THV in a LAO view that allows for depth assessment at the level of the LCC. Finally, after verifying that the inflow is not supra-annular, the valve is released from the delivery system [20].
Contemporary Outcomes
Limited data are available on the outcomes of self-expanding THV deployment with the cusp-overlap approach given its relatively recent introduction to the TAVR arsenal. Nevertheless, several of the more commonly utilized THV systems are discussed below.
Evolut R & Evolut PRO(+)
The vast majority of the cusp-overlap experience with self-expanding valves has been with CoreValve THVs. For instance, Fraser and colleagues reported their cusp-overlap experience with 93 CoreValve cases (Evolut R, n = 50; Evolut PRO, n = 43) from 2013–2019. The optimal annular plane projection was routinely predicted from MDCT images. An implant depth of 3–5 mm below the annulus was achieved for all patients. At 30 days post-procedure, 4 of 87 CoreValve recipients (4.6%, 3 Evolut R, 1 Evolut PRO) required a PPM. Two CoreValve recipients (2.3%) had moderate aortic regurgitation (AR) on echocardiography, and there were no cases of valve embolization [21].
In one of the largest single-center experiences, Gada et al. reported on 169 consecutive patients undergoing 34-mm Evolut R TAVR between 2016 and 2019. The cusp-overlap technique was successfully performed in 88% of patients, with the remainder having near-complete overlap or no overlap due to steep gantry angles. 134 patients had no prior PPM, with a 30-day PPM rate of 5.2%. The in-hospital rate of new left bundle branch block (LBBB) post-TAVR was 10.9%. The implant depth below the NCC was 3.8 ± 2.6 mm in patients requiring a new PPM versus 2.4 mm in those who did not (p = 0.23). A pre-existing right bundle branch block (RBBB) was the only independent predictor of new PPM implantation (OR 10.6; 95% CI, 1.7–66.2, p = 0.01). The authors concluded that the rates of PPM implantation and new LBBB following 34-mm Evolut R TAVR were lower with the cusp-overlap approach due to shallower implant depths relative to the conduction system [22].
Given that the cusp-overlap approach is a relatively new technique, the authors of the previous study initiated a focused didactic experience involving several low-intermediate volume centers in Latin America and Europe, and retrospectively compared 257 consecutive patients undergoing TAVR with self-expandable valves using either the conventional 3-cusp view (n = 101) or the cusp-overlap view (n = 156). The 30-day incidence of new-onset LBBB (12.9% vs. 5.8%; p = 0.05) and PPM implantation rate (17.8% vs. 6.4%; p = 0.004) was significantly lower when using the cusp-overlap approach. There were no differences between the two groups with regards to 30-day incidence of death (4.9% vs. 2.6%), any stroke (0% vs. 0.6%), and the need for surgical aortic valve replacement (0% for both groups). The authors concluded that the cusp-overlap view can be used to significantly reduce the incidence of post-procedural conduction abnormalities in comparison with the traditional 3-cusp view, without compromising TAVR outcomes, when using self-expandable prostheses. This study also validated the successful adoption of this approach with formal didactics and limited case observation [23].
Several comparisons of the cusp-overlap approach with other standard techniques have also been made. As an example, Aljabbary et al. retrospectively compared the rates of postprocedure PPM implantation among 127 patients who underwent Evolut TAVR using the standard 3-cusp view (2016–2017) with 393 patients who received Evolut THVs using the cusp-overlap view (2018–2020). The authors found that 16.5% of the standard 3-cusp view patients required a new PPM at 30 days, compared to 7.2% of the cusp-overlap patients (p = 0.002) [24]. Similarly, Mendiz et al. analyzed their experience with self-expanding TAVR and compared the conventional 3-cusp view (n = 382) to the cusp-overlap technique (n = 61). Pre-procedure, 16.7% vs. 26.2% (p = non-significant, or NS) of patients had atrial fibrillation, and 4.1% vs. 6.5% (p = NS) had RBBB. 93.1% and 86.9% of patients in the standard 3-cusp view and cusp-overlap view groups, respectively, received Evolut R/Evolut PRO THVs. Major adverse cardiac events at 30 days included death (3.1% vs. 4.9%, p = NS), acute myocardial infarction (0.3% vs. 0%, p = NS), major stroke (0.78% vs. 0%, p = NS), minor stroke (0.3% vs. 0%, p = NS), SAVR (0.3% vs. 0%, p = NS), and new PPM implantation (30.9% vs. 6.5%, p = 0.0001). The authors inferred that the cusp-overlap technique decreases the 30-day PPM implantation rate without any significant differences in major adverse cardiac events when compared to the standard 3-cusp view for self-expanding TAVR [25]. Finally, in their comparison of 50 conventional implant patients with 15 cusp-overlap patients (all Evolut R/Evolut PRO between 2017 and 2019), Sztejfman et al. reported that the 30-day rate of PPM implantation was 24.9% versus 0% (p = 0.041). The cusp-overlap cohort also had a reduced risk of major complications (6.67% vs. 42%, p = 0.011). There were no significant differences in the time of fluoroscopy, volume of contrast used, renal outcomes, or mortality [26].
The cusp-overlap technique may also be applied to valve-in-valve TAVR in degenerated surgical bioprostheses. In their experience with the cusp-overlap view in 13 consecutive valve-in-valve procedures using the Evolut THV, Kitamura et al. reported optimal commissural alignment (using post-procedural CT) in 37 of 39 commissures (95%). Furthermore, severe overlap between the coronary ostium and neocommissure (defined as <20°) was observed in one right coronary artery (7.7%) and in no left coronary artery (0%) [27].
The Optimize PRO study (NCT04091048), a prospective, non-randomized study of the safety and efficacy of Evolut PRO and Evolut PRO+ (Medtronic, Minneapolis, MN) TAVR using the cusp-overlap approach, is currently underway. Preliminary data from an interim analysis of 71 roll-in and 100 main cohort patients showed excellent 30-day outcomes with no deaths or disabling strokes, and an 8.8% PPM implantation rate [28].
Portico, ACURATE neo/neo2, & JenaValve
In the aforementioned study by Mendiz et al., among 382 patients who underwent self-expanding TAVR using the standard 3-cusp view, THVs implanted included the following systems: ACURATE neo 4.7%, Portico 1.3%, and JenaValve (JenaValve Technology, Inc., Irvine, CA) 0.8%. The corresponding numbers for the cusp-overlap cohort were: Portico 8.2% and ACURATE neo 4.9%. In this series, which was comprised largely of Evolut patients, the risk of PPM implantation at 30 days was significantly higher with the 3-cusp view technique as compared to the cusp-overlap view approach (30.9% vs. 6.5%, p = 0.0001) [25].
Recently, Wong et al. demonstrated that the cusp-overlap view can be successfully used to implant the ACURATE neo2 valve. The authors further noted that with the cusp-overlap projection, optimal neocommissural alignment can be achieved if the commissural posts are properly aligned in relation to the aortic cusps [29]. This finding has been confirmed by the Denmark group with post-TAVR CT evaluation (personal communication, Lars Sondargaard, 2021).
Tagliari et al. have also reported the use of the cusp-overlap technique to optimize commissural alignment with the Portico THV system (Abbott Structural Heart, Santa Clara, CA) on a bench model [30]. Given the growing need for coronary reaccess after TAVR and utility of commissural alignment in facilitating this, the cusp-overlap technique and its ability to identify the commissural tabs during valve deployment may prove advantageous over the conventional fluoroscopic view.
Limitations of the Cusp-Overlap Technique
There are a few important drawbacks of the cusp-overlap approach. Perhaps most importantly, shallow implantation of THVs may lead to a higher rate of valve embolism, or “pop-outs”. However, this has not been commonly seen in reported series thus far. In practice, the cusp-overlap technique facilitates a more thorough understanding of the true depth of the prosthesis relative to the NCC. Since the LVOT is elongated in the cusp-overlap view, depth assessment is likely to be more accurate than if the 3-cusp view is used. A target 3–5 mm implantation depth with respect to the NCC is recommended for use across the various self-expanding platforms. This implantation depth also provides a buffer for potentiation shortening of the prosthesis during post-dilatation, when indicated [31].
Higher implantation of THVs could also impede future coronary artery catheterizations. This has important implications for younger patients who have an increased lifetime risk of complications of coronary artery disease. Coronary reaccess is complicated not just by the obstructive prosthesis stent frame, but also by an in situ barrier formed by the native aortic leaflets. In such cases, preprocedural CT planning is crucial in evaluating the anatomy of the aortic root in relation to the valve stent frame [32, 33]. When using the Evolut THV, commissural alignment to promote easier coronary reaccess can also be enhanced by starting with the flush port at 3 o’clock during insertion of the delivery catheter into the femoral artery. This approach has been validated in the ALIGN-TAVR [34] study and the Evolut Low Risk LTI sub-study [35].
The cusp-overlap technique is also limited by the disadvantages of the RAO/CAU view. Here, the amount of tension on the delivery catheter and its position along the inner or outer curve of the aorta cannot be easily assessed. To circumvent this, an LAO projection on the S-curve can be used to determine the position of the delivery catheter following depth assessment in the cusp-overlap view and valve deployment prior to the point of no re-capture [36]. A combination of two orthogonal views may also be utilized to better control the implant depth and the tension observed in the catheter before final release, and is actually preferred by some operators as it provides a more complete evaluation of the position of the prosthesis across the annulus and within the aortic root [8]. The RAO/CAU view may also reduce fluoroscopic image quality in obese patients. Here, the standard 3-cusp view or a LAO/cranial view that overlaps the right and non-coronary cusps may be advantageous [7].
Conclusions
The cusp-overlap technique, performed by overlapping the right and left coronary cusps along the basal annular plane, and simultaneously isolating the non-coronary cusp, facilitates precise valve positioning during self-expanding TAVR. This is of paramount importance since the depth at which a prosthesis is implanted in the LVOT has consistently been associated with the requirement for a new permanent pacemaker. The cusp-overlap approach elongates the LVOT and accentuates the right-non commissure in the center of the fluoroscopic view, thus allowing operators more precise control of the prosthesis implant depth. While the early experience with this technique in Evolut TAVR has been promising, the results of larger studies with longer follow-up across multiple self-expanding valve systems are eagerly awaited.
Footnotes
Compliance with Ethical Standards
Conflict of Interest
Dr. Khera is a consultant for Abbott Structural Heart, Medtronic, and Boston Scientific, and has received speakers’ honoraria from Medtronic. Dr. Dangas is on the advisory board and is a consultant for Boston Scientific, and has common stock with Medtronic that is fully divested. Dr. Sharma has served on the Speakers Bureau for Abbott Vascular, Boston Scientific, TriReme, and Cardiovascular Systems. Dr. Tang has served as a physician proctor for Medtronic, and is a consultant for Medtronic and Abbott Structural Heart. The other authors have no conflicts of interest to declare.
Human and Animal Rights and Informed Consent
All reported studies/experiments with human or animal subjects performed by the authors have been previously published and complied with all applicable ethical standards (including the Helsinki declaration and its amendments, institutional/national research committee standards, and international/national/institutional guidelines).
References:
- 1.Otto CM, Nishimura RA, Bonow RO, Carabello BA, Erwin JP 3rd, Gentile F et al. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2021;143(5):e72–e227. doi: 10.1161/CIR.0000000000000923. [DOI] [PubMed] [Google Scholar]
- 2.Carroll JD, Mack MJ, Vemulapalli S, Herrmann HC, Gleason TG, Hanzel G et al. STS-ACC TVT Registry of Transcatheter Aortic Valve Replacement. J Am Coll Cardiol. 2020;76(21):2492–516. doi: 10.1016/j.jacc.2020.09.595. [DOI] [PubMed] [Google Scholar]
- 3.Tchetche D, Van Mieghem NM. New-generation TAVI devices: description and specifications. Eurointervention. 2014;10 Suppl U:U90–U100. doi: 10.4244/EIJV10SUA13. [DOI] [PubMed] [Google Scholar]
- 4.Tchetche D, de Biase C, Brochado B, Mastrokostopoulos A. How to Make the TAVl Pathway More Efficient. Interv Cardiol 2019;14(1):31–3. doi: 10.15420/icr.2018.28.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jilaihawi H, Zhao Z, Du R, Staniloae C, Saric M, Neuburger PJ et al. Minimizing Permanent Pacemaker Following Repositionable Self-Expanding Transcatheter Aortic Valve Replacement. JACC Cardiovasc Interv 2019;12(18):1796–807. doi: 10.1016/j.jcin.2019.05.056. [DOI] [PubMed] [Google Scholar]
- 6.Zaid S, Sengupta A, Okoli K, Tsoi M, Khan A, Ahmad H et al. Novel Anatomic Predictors of New Persistent Left Bundle Branch Block After Evolut Transcatheter Aortic Valve Implantation. Am J Cardiol 2020;125(8):1222–9. doi: 10.1016/j.amjcard.2020.01.008. [DOI] [PubMed] [Google Scholar]
- 7. Tang GHL, Zaid S, Michev I, Ahmad H, Kaple R, Undemir C et al. "Cusp-Overlap" View Simplifies Fluoroscopy-Guided Implantation of Self-Expanding Valve in Transcatheter Aortic Valve Replacement. JACC Cardiovasc Interv 2018;11(16):1663–5. doi:10.1016/j.jcin.2018.03.018.30139479 * This study was one of the first to conceptualize and popularize the concepts behind the cusp-overlap approach.
- 8.Tchetche D, Siddiqui S. Optimizing Fluoroscopic Projections for TAVR: Any Difference Between the Double S-Curve and the Cusp-Overlap Technique? JACC Cardiovasc Interv 2021;14(2):195–7. doi: 10.1016/j.jcin.2020.11.020. [DOI] [PubMed] [Google Scholar]
- 9.Arnold M, Achenbach S, Pfeiffer I, Ensminger S, Marwan M, Einhaus F et al. A method to determine suitable fluoroscopic projections for transcatheter aortic valve implantation by computed tomography. J Cardiovasc Comput Tomogr. 2012;6(6):422–8. doi: 10.1016/j.jcct.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 10.Cockburn J, Trivedi U, de Belder A, Hildick-Smith D. Optimal projection for transcatheter aortic valve implantation determined from the reference projection angles. Catheter Cardiovasc Interv. 2012;80(6):973–7. doi: 10.1002/ccd.23393. [DOI] [PubMed] [Google Scholar]
- 11.Hell MM, Biburger L, Marwan M, Schuhbaeck A, Achenbach S, Lell M et al. Prediction of fluoroscopic angulations for transcatheter aortic valve implantation by CT angiography: influence on procedural parameters. Eur Heart J Cardiovasc Imaging. 2017;18(8):906–14. doi: 10.1093/ehjci/jew144. [DOI] [PubMed] [Google Scholar]
- 12.Binder RK, Leipsic J, Wood D, Moore T, Toggweiler S, Willson A et al. Prediction of optimal deployment projection for transcatheter aortic valve replacement: angiographic 3-dimensional reconstruction of the aortic root versus multidetector computed tomography. Circ Cardiovasc Interv. 2012;5(2):247–52. doi: 10.1161/CIRCINTERVENTIONS.111.966531. [DOI] [PubMed] [Google Scholar]
- 13.Tzikas A, Schultz C, Van Mieghem NM, de Jaegere PP, Serruys PW. Optimal projection estimation for transcatheter aortic valve implantation based on contrast-aortography: validation of a Prototype Software. Catheter Cardiovasc Interv. 2010;76(4):602–7. doi: 10.1002/ccd.22641. [DOI] [PubMed] [Google Scholar]
- 14.Steinvil A, Weissman G, Ertel AW, Weigold G, Rogers T, Koifman E et al. Accuracy of predicted orthogonal projection angles for valve deployment during transcatheter aortic valve replacement. J Cardiovasc Comput Tomogr. 2018;12(5):398–403. doi: 10.1016/j.jcct.2018.05.017. [DOI] [PubMed] [Google Scholar]
- 15.Piazza N, Mylotte D, Theriault Lauzier P. Fluoroscopic "heart chamber" anatomy - the case for imaging modality-independent terminology. EuroIntervention. 2016;12(Y):Y9–Y15. doi: 10.4244/EIJV12SYA3. [DOI] [PubMed] [Google Scholar]
- 16.Piazza N Understanding the Value of the FluoroCT "Double S Curve": Finding the Optimal View for TAVR. TVT; 2017; Chicago, IL. [Google Scholar]
- 17.Ben-Shoshan J, Alosaimi H, Lauzier PT, Pighi M, Talmor-Barkan Y, Overtchouk P et al. Double S-Curve Versus Cusp-Overlap Technique: Defining the Optimal Fluoroscopic Projection for TAVR With a Self-Expanding Device. JACC Cardiovasc Interv 2021; 14(2): 185–94. doi: 10.1016/j.jcin.2020.10.033. [DOI] [PubMed] [Google Scholar]
- 18.Theriault-Lauzier P, Andalib A, Martucci G, Mylotte D, Cecere R, Lange R et al. Fluoroscopic anatomy of left-sided heart structures for transcatheter interventions: insight from multislice computed tomography.JACCCardiovascInterv.2014;7(9):947–57 doi: 10.1016/j.jcin.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 19.Kuon E, Dahm JB, Empen K, Robinson DM, Reuter G, Wucherer M. Identification of less-irradiating tube angulations in invasive cardiology. J Am Coll Cardiol. 2004;44(7):1420–8. doi: 10.1016/j.jacc.2004.06.057. [DOI] [PubMed] [Google Scholar]
- 20.Vora AN, Gada H. Staying in the Shallow End: Minimizing Permanent Pacemaker Implantation With a Novel Implantation Method. Circ Cardiovasc Interv. 2021;14(1):e010330. doi: 10.1161/CIRCINTERVENTIONS.120.010330. [DOI] [PubMed] [Google Scholar]
- 21.Pisaniello AD, Makki HBE, Jahangeer S, Daniels MJ, Hasan R, Fraser DGW. Low Rates of Permanent Pacing Are Observed Following Self-Expanding Transcatheter Aortic Valve Replacement Using an Annular Plane Projection for Deployment. Circ Cardiovasc Interv. 2021;14(1):e009258. doi: 10.1161/CIRCINTERVENTIONS.120.009258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gada H, Vora A, Siddique S, Wert Y, Michev I, Piazza N et al. TCT CONNECT-457 Reduction of Rates of Permanent Pacemaker Implantation With 34-MM Evolut R Using Cusp Overlap Technique. Journal of the American College of Cardiology. 2020;76(17 Supplement S):B196–B. doi: 10.1016/j.jacc.2020.09.486. [DOI] [Google Scholar]
- 23.Mendiz OA, Noc M, Fava CM, Gutierrez Jaikel LA, Sztejfman M, Pleskovic A et al. Impact of Cusp-Overlap View for TAVR with Self-Expandable Valves on 30-Day Conduction Disturbances. J Interv Cardiol. 2021;2021:9991528. doi: 10.1155/2021/9991528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aljabbary T, Wijeysundera H, Radhakrishnan S. Cusp Overlap Method For Self-Expanding Transcatheter Aortic Valve Replacement. Canadian Journal of Cardiology. 2020;36(10):S32–S3. doi: 10.1016/j.cjca.2020.07.078. [DOI] [PubMed] [Google Scholar]
- 25.Mendiz O, Noc M, Fava C, Valdivieso L, Gamboa P, Lev G. TCT CONNECT-129 Cusp Overlapping Technique for TAVR Procedures With Self-Expandable Valves. Journal of the American College of Cardiology. 2020;76(17 Supplement S):B57–B. doi: 10.1016/j.jacc.2020.09.143. [DOI] [Google Scholar]
- 26.Sztejfman M, Giuliani C, Zaidel E, Peralta S, Jubany G, Murillo L et al. TCT CONNECT-480 Impact of Cusp-Overlap Technique on Pacemaker Requirement among Patients Receiving Transcatheter Aortic Valve Replacement. Journal of the American College of Cardiology. 2020;76(17 Supplement S):B205–B. doi: 10.1016/j.jacc.2020.09.509. [DOI] [Google Scholar]
- 27.Kitamura M, Wilde J, Dumpies O, Gutberlet M, Gohmann R, Shibata M et al. Patient-Specific Neocommissural Alignment of the Evolut Valve: A Pilot Study in Transcatheter Aortic Valve-in-Valve Replacement. JACC Cardiovasc Interv. 2021;14(8):934–6. doi: 10.1016/j.jcin.2020.12.002. [DOI] [PubMed] [Google Scholar]
- 28.Grubb K, editor. An Optimized TAVR Care Pathway Using Evolut PRO and PRO+: Early Results From the Optimize PRO Study. Society for Cardiovascular Angiography and Interventions’ SCAI 2021 Virtual Scientific Sessions.
- 29.Wong I, Bieliauskas G, De Backer O, Sondergaard L. Technical Considerations for Transcatheter Aortic Valve Replacement With ACURATE neo2. JACC Cardiovasc Interv. 2021;14(2):224–6. doi: 10.1016/j.jcin.2020.10.022. [DOI] [PubMed] [Google Scholar]
- 30.Tagliari AP, Vicentini L, Zimmermann JM, Miura M, Ferrari E, Perez D et al. Transcatheter Aortic Valve Neo-Commissure Alignment with the Portico System. EuroIntervention. 2020. doi: 10.4244/EIJ-D-20-01095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vora AN, Tang GHL, Reardon MJ, Deeb GM, Yakubov SJ, Huang J et al. Transcatheter Aortic Valve Implant Depth Measurements Differ by Aortography Versus Computed Tomography. JACC Cardiovasc Interv. 2021. doi: 10.1016/j.jcin.2020.12.035. [DOI] [PubMed] [Google Scholar]
- 32.Sengupta A, Alexis SL, Kovacic JC, Tang GHL. Current challenges in TAVI: neo-commissural alignment to mimic more physiologic valve implantation. Vessel Plus. 2020;4:40. doi: 10.20517/2574-1209.2020.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alexis SL, Zaid S, Sengupta A, Lerakis S, Khera S, Sharma SK et al. Transcatheter aortic valve replacement aortic root orientation: implications for future coronary access and redo transcatheter aortic valve replacement. Ann Cardiothorac Surg. 2020;9(6):502–4. doi: 10.21037/acs-2020-av-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tang GHL, Zaid S, Fuchs A, Yamabe T, Yazdchi F, Gupta E et al. Alignment of Transcatheter Aortic-Valve Neo-Commissures (ALIGN TAVR): Impact on Final Valve Orientation and Coronary Artery Overlap. JACC Cardiovasc Interv. 2020;13(9):1030–42. doi:10.1016/j.jcin.2020.02.005.32192985 ** This was the first systematic evaluation of commissural alignment in transcatheter aortic valve repalcement (TAVR). The authors showed that specific initial orientations of the Evolut transcatheter heart valve system improved alignement, with important implications for future coronary artery reaccess and valve-in-valve TAVR.
- 35.Tang G, Alexis S, Sengupta A, Zaid S, Leipsic J, Blanke P et al. TCT CONNECT-93 Commissural Alignment in Evolut TAVR: Results From the Low Risk LTI Substudy. Journal of the American College of Cardiology.2020;76(17SupplementS):B41–B. doi: 10.1016/j.jacc.2020.09.107. [DOI] [Google Scholar]
- 36.Khalique OK, Pulerwitz TC, Halliburton SS, Kodali SK, Hahn RT, Nazif TM et al. Practical considerations for optimizing cardiac computed tomography protocols for comprehensive acquisition prior to transcatheter aortic valve replacement. J Cardiovasc Comput Tomogr. 2016;10(5):364–74. doi: 10.1016/j.jcct.2016.07.007. [DOI] [PubMed] [Google Scholar]