Abstract
PCR was used to amplify a 537-bp region of an Ehrlichia ewingii gene encoding a homologue of the 28-kDa major antigenic protein (P28) of Ehrlichia chaffeensis. The E. ewingii p28 gene homologue was amplified from DNA extracted from whole blood obtained from four humans and one canine with confirmed cases of infection. Sequencing of the PCR products (505 bp) revealed a partial gene with homology to outer membrane protein genes from Ehrlichia and Cowdria spp.: p30 of Ehrlichia canis (≤71.3%), p28 of E. chaffeensis (≤68.3%), and map1 of Cowdria ruminantium (67.3%). The peptide sequence of the E. ewingii partial gene product was deduced (168 amino acids) and the antigenicity profile was analyzed, revealing a hydrophilic protein with ≤69.1% identity to P28 of E. chaffeensis, ≤67.3% identity to P30 of E. canis, and ≤63.1% identity to MAP1 of C. ruminantium. Primers were selected from the E. ewingii p28 sequence and used to develop a species-specific PCR diagnostic assay. The p28 PCR assay amplified the expected 215-bp product from DNA that was extracted from EDTA-treated blood from each of the confirmed E. ewingii infections that were available. The assay did not produce PCR products with DNA extracted from E. chaffeensis-, E. canis-, or E. phagocytophila-infected samples, confirming the specificity of the p28 assay for E. ewingii. The sensitivity of the E. ewingii-specific PCR assay was evaluated and determined to detect as few as 38 copies of the p28 gene.
Human ehrlichioses are emerging tick-borne infections that were first described in 1987 (12). Prior to 1999, the two known etiologic agents of human ehrlichiosis in the United States were Ehrlichia chaffeensis (1) and Ehrlichia phagocytophila (commonly referred to as the agent of human granulocytic ehrlichiosis) (7). In 1999, Ehrlichia ewingii, a pathogen originally recognized to cause granulocytic ehrlichiosis in canines (2), was implicated in causing several cases of granulocytic ehrlichiosis in humans (4). Since the earliest case, retrospectively identified from 1996, approximately 10 confirmed cases of granulocytic ehrlichiosis caused by E. ewingii have been identified from Missouri (4) and Oklahoma (C. D. Paddock, personal communication). Patients infected with E. ewingii experience symptoms similar to those of other human ehrlichioses, such as fever, headache, and myalgia, and usually report recent exposure to ticks. Many of the humans infected by E. ewingii were receiving immunosuppressive therapy at the time of infection (4).
Based on the 16S rRNA sequence, E. ewingii is most closely related to E. chaffeensis (98.1% homology) and Ehrlichia canis (98.0% homology), the agent of canine monocytic ehrlichiosis (2). Of these three, E. chaffeensis has been studied in the most detail. DNA sequences are available for numerous E. chaffeensis genes, including those encoding 16S rRNA, groESL, p120 antigen, the variable-length PCR target, and the p28 gene family (1, 17–19, 22, 23, 27, 28). The p28 gene family represents a series of 21 homologous genes (20 to 83% amino acid identity) that are arranged in tandem in the E. chaffeensis genome (28). The p28 genes encode major antigenic proteins of E. chaffeensis as determined by Western blotting (5, 6, 20). Recombinant forms of the p28 genes and the corresponding homologous p30 genes of E. canis have been expressed and shown to be useful for the serodiagnosis of these agents by use of Western and dot blot immunoassays (16, 25, 26). It has been suggested that these genes may play a role in immune response evasion through differential expression of the multigene locus (14, 18, 19). At least 6 of the 21 E. chaffeensis p28 genes and five of five E. canis p30 genes were transcriptionally active when examined in cell culture (15, 28). Two homologues of the p28 gene family have also been described for Ehrlichia muris, another member of the E. canis genogroup (GenBank accession numbers AF165813 and AF165814).
The diagnostic assays currently available for the identification of infection by E. ewingii have limitations. The most reliable test for identifying an ehrlichial infection is isolation of the agent in tissue culture (8). However, E. ewingii has not yet been cultured in vitro. Indirect fluorescence antibody assays are commonly used for the diagnosis of E. chaffeensis and E. canis infections, and while either of these may serve as a surrogate antigen to screen for suspected E. ewingii infections, an indirect fluorescence antibody assay using surrogate antigens cannot be confirmatory (9, 12). Laboratory confirmation of E. ewingii infection has ultimately been determined by molecular methods, particularly PCR. In contrast to the numerous genes that have been identified and sequenced from the E. chaffeensis genome, only two genes, the 16S rRNA and groE genes, have been characterized and may currently serve as PCR targets for E. ewingii (3, 4, 24). This study describes the identification and characterization of a gene in E. ewingii that is homologous to the major antigenic proteins of E. chaffeensis (p28) and E. canis (p30). The unique regions of the E. ewingii p28 homologue were used to design primers for a species-specific PCR diagnostic assay. The ability of the p28 gene PCR assay to identify and differentiate infection by E. ewingii is also evaluated in this study.
MATERIALS AND METHODS
Samples and DNA extraction.
DNA was extracted from each sample in this study using the QIAamp blood kit (Qiagen Inc., Valencia, Calif.). Control samples included DNA extracted from EDTA-treated human blood specimens that were previously confirmed at the Centers for Disease Control and Prevention as E. chaffeensis infected, and DNA was extracted from tissue cultures infected with either E. canis strain Arkansas (DH82 cells) or E. phagocytophila strain USG3 (HL60 cells).
PCR amplification.
PCR amplifications were done in a 9600 thermal cycler (Perkin-Elmer, Applied Biosystems, Foster City, Calif.) using the Taq Master PCR kit (Qiagen). The final volume for each reaction was 25 μl, with reagent concentrations of 0.5 μM for each primer, 10 mM Tris-HCl, 50 mM KCl, 1.5 mM MgCl2, 200 μM for each deoxynucleoside triphosphate, 1.5 U of Taq DNA polymerase, and 2.5 μl of template. All PCRs used the following conditions: 95°C (2 min) followed by 40 cycles of 94°C (30 s), variable annealing temperature (30 s), and 72°C (1 min), followed by an extension period (72°C, 5 min).
Primers FECH2 (5′-ACATCAGTGGAAAATACATG) and REC1 (5′-ACCTAACTTTCCTTGGTAAG) were derived from primers used in a previous study (19) to amplify the p28 of E. chaffeensis. These primers were initially used to amplify the p28 gene from the human and canine E. ewingii-infected samples, using an annealing temperature of 45.7°C. The PCR diagnostic assay targeting the p28 gene of E. ewingii used primers EEM2F (5′-GGAGCTAAAATAGAAGATAATC) and EEM1R (5′-GTGCCAAAAGGT AATACAT) with an annealing temperature of 55°C.
The annealing temperatures for each PCR assay were optimized by use of a temperature gradient thermal cycler (Mastercycler gradient; Eppendorf Scientific, Westbury, N.Y.). Results of the PCRs were assessed by electrophoresis of 6 μl of each product in a 2% agarose gel containing ethidium bromide.
Cloning of pMAP.
Amplified product from the FECH2-REC1 PCR of E. ewingii OK-1 DNA was cloned into vector pCR 2.1 using the Original TA cloning kit (Invitrogen, Carlsbad, Calif.). The presence of the insert was confirmed by DNA sequencing. This plasmid, named pMAP, was subsequently used as a positive control for evaluation of the p28 assay.
Sensitivity assessment.
The sensitivity of the assay was determined, using the vector pMAP, through a previously developed method (13). In brief, 10-fold serial dilutions of a known quantity of pMAP were added to aliquots of DNA extracted from uninfected EDTA-treated human blood. The assay was done on the dilution series, and the limit of detection was determined by the final dilution at which a PCR product was still visible. The dilution series was quantitated using an MBA 2000 spectrophotometer (Perkin-Elmer, Norwalk, Conn.).
Nucleotide sequencing.
PCR products were purified using a Wizard PCR Preps DNA Purification Kit (Promega, Madison, Wis.). The purified PCR products were sequenced with forward and reverse primers using the Prism Ready Reaction DyeDeoxy Cycle Sequencing kit in a 9600 Perkin-Elmer thermal cycler (Perkin-Elmer, Applied Biosystems). Unincorporated fluorescence-labeled dideoxynucleoside triphosphates were removed using the Dye-Ex Spin kit (Qiagen). Sequencing reaction products were separated and sequence data collected using a 377 ABI automated sequencer (Perkin-Elmer, Applied Biosystems).
Data analysis.
Nucleotide sequences were edited and assembled using the STADEN sequence analysis package (10). Sequence homology comparisons and multiple sequence alignments were made with the GAP and PILEUP programs, respectively, of the Genetics Computer Group (Madison, Wis.) package (11). Nucleotide sequence homology searches were made through the National Center for Biotechnology Information BLAST network service. Phylogenetic analysis was done by use of the PAUP program (version 4.0.0d64) on a Power Macintosh 9500/132.
RESULTS
Analysis of an E. ewingii p28 homologue.
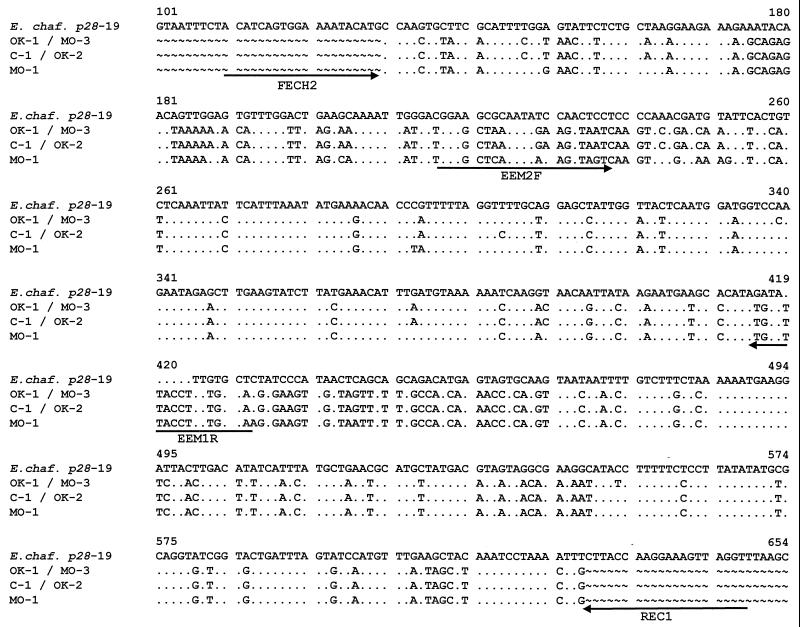
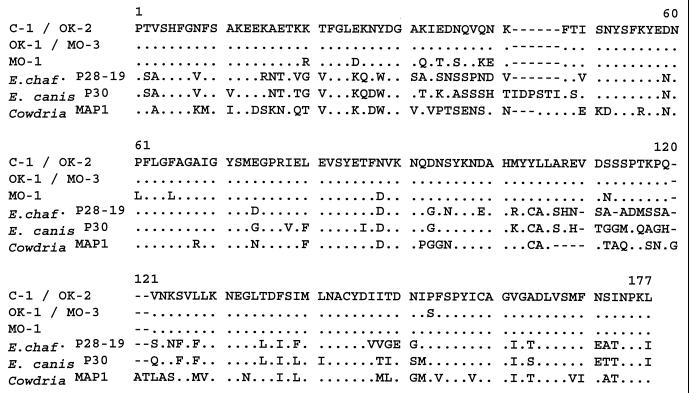
Four human samples and one canine sample infected with E. ewingii were obtained (Table 1) that had been confirmed infected at the Centers for Disease Control and Prevention by PCR amplification of the 16S rRNA gene and/or the groESL gene. PCR products were obtained from each of the five E. ewingii-infected samples using primers specific for a partial gene fragment of a p28 of E. chaffeensis, the Arkansas strain (19). The sample designation (C-1, OK-1, OK-2, MO-1, or MO-3), sample source, location of collection, and GenBank accession number that was assigned to each E. ewingii p28 gene sequence are indicated in Table 1. Sequence analysis of each product revealed a 505-bp region (Fig. 1) from which a 168-amino-acid peptide could be deduced (Fig. 2). The sequence was highly conserved among the five samples; C-1 and OK-2 nucleotide and peptide sequences for this region were identical. The OK-1 and MO-3 sequences were also identical; however, they differed by two nucleotides when compared with the C-1 and OK-2 sequences. This nucleotide difference results in a peptide difference of only one amino acid (position 153 in Fig. 2). The MO-1 sequence for this region was the most divergent, differing by 19 nucleotides when compared to the C-1 and OK-1 sequences, resulting in a protein coding difference of 11 amino acids. Database sequence homology searches of the 505-bp region revealed nucleotide and peptide similarity of ≤70% to major outer membrane protein homologues of species closely related to E. ewingii, namely, the p28 genes of E. chaffeensis, the p30 genes of E. canis, and the map1 gene of Cowdria ruminantium. The nucleotide and peptide sequence homologies of the E. ewingii gene to the closest p28 homologues are shown in Table 2.
TABLE 1.
Data for E. ewingii samples used for p28 gene amplification and sequencing
FIG. 1.
Comparison of E. chaffeensis (E. chaf.) p28-19 gene (Arkansas strain; accession no. U72291) to the E. ewingii p28 genes (C-1, OK-1, OK-2, MO-1, and MO-3). Dots represent sequence agreement, letters represent nucleotide differences, and the tilde symbol indicates that the sequence was not available. All primers used in this study are indicated by arrows for orientation.
FIG. 2.
Deduced amino acid sequences of E. ewingii P28 (C-1, OK-1, OK-2, MO-1, and MO-3) compared with the amino acid sequences of the most-homologous copies of E. chaffeensis (E. chaf.) P28 (Arkansas P28-19; accession no. U72291), E. canis P30 (Oklahoma; accession no. AF078553), and C. ruminantium MAP1 (Nyatsanga; accession no. U50834).
TABLE 2.
p28 gene nucleotide sequence and deduced amino acid residue homologya
| Sample | Homology
|
|||||
|---|---|---|---|---|---|---|
| C-1 and OK-2 | OK-1 and MO-3 | MO-1 | E. chaffeensis p28-19 | E. canis p30 | C. ruminantium map1 | |
| C-1 and OK-2 | 0.9960 | 0.9624 | 0.6752 | 0.7109 | 0.6733 | |
| OK-1 and MO-3 | 0.9940 | 0.9584 | 0.6713 | 0.7069 | 0.6693 | |
| MO-1 | 0.9464 | 0.9405 | 0.6832 | 0.7129 | 0.6693 | |
| E. chaffeensis P28-19b | 0.6905 | 0.6845 | 0.6845 | 0.7566 | 0.6763 | |
| E. canis P30b | 0.6726 | 0.6667 | 0.6667 | 0.7790 | 0.6817 | |
| C. ruminantium MAP1b | 0.6310 | 0.6250 | 0.6190 | 0.6848 | 0.6851 | |
Sequences were aligned using the Genetics Computer Group Pileup program, and percent identity was determined by the Olddistances program using “Length of shorter sequence without gaps” as the denominator. Data in roman type are for p28 gene nucleotide sequence; data in italic type are for deduced amino acid residues.
Based on the relative size of the complete gene sequences of the E. chaffeensis and E. canis major outer membrane protein homologues, the 505-bp region of the p28 of E. ewingii is predicted to encode approximately 60% of the total protein. This partial protein sequence possesses numerous differences that are unique to E. ewingii when compared with its closest P28/P30 homologues, with two particularly notable regions with extensive differences (Fig. 2). As shown in Fig. 2, there is a 6-amino-acid deletion present in the E. chaffeensis P28 protein, relative to the E. canis P30 protein, that may contribute to the apparent difference in the molecular mass between the E. chaffeensis (28-kDa) and E. canis (30-kDa) homologues. This deletion is also found in the E. ewingii sequence, suggesting that the mature E. ewingii protein may be similar in size to the E. chaffeensis 28-kDa homologue. The amino acid composition of the E. ewingii partial protein revealed a primarily hydrophilic protein, consistent with P28 being a strongly immunogenic protein (data not shown).
A phylogenetic analysis was also used to compare the amino acid sequence deduced from the copy of the E. ewingii p28 gene that was amplified from each of the four human and one canine samples to each other and to the 21 copies of the P28 protein encoded in the E chaffeensis genome (data not shown). This analysis confirmed that the E. ewingii-encoded P28 proteins comprise a very closely related group that is distinct from the E. chaffeensis P28 proteins. Among the E. chaffeensis P28 proteins, those encoded by the p28 genes p28-15 through p28-19 represented the closest homologues to the E. ewingii P28 proteins.
Development and evaluation of the E. ewingii-specific PCR assay.
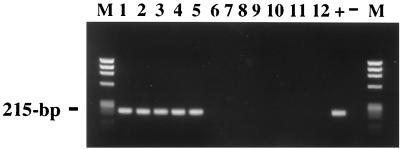
The nucleotide differences between the p28 gene of E. ewingii and the E. chaffeensis and E. canis homologues were considered significant enough to utilize the gene sequence as a target for a species-specific PCR assay. Primers were chosen in regions of the gene fragment unique to E. ewingii. After testing numerous primer sets, primers EEM2F and EEM1R (shown in Fig. 1) amplified the p28 gene of E. ewingii most efficiently and with minimal background. The assay using these primers was tested on DNA extracted from the five PCR-positive E. ewingii-infected samples and from samples containing E. chaffeensis, E. canis, and E. phagocytophila (Fig. 3). The assay amplified a 215-bp region of the p28 gene from DNA from each of the E. ewingii-infected samples (Fig. 3, lanes 1 to 5). Sequencing of the PCR products confirmed amplification of a p28 gene of E. ewingii. No products were obtained when the template for the PCR assay was representative DNA from E. chaffeensis (Fig. 3, lanes 6 to 10), E. canis (Fig. 3, lane 11), and E. phagocytophila (Fig. 3, lane 12).
FIG. 3.
Species-specific p28 PCR assay results for E. ewingii with primers EEM2F and EEM1R. The first five lanes show PCR products obtained with the E. ewingii-infected template DNAs (lane 1, C-1; lane 2, OK-1; lane 3, OK-2; lane 4, MO-1; and lane 5, MO-3). The remaining lanes show PCR products amplified from DNAs from samples containing E. chaffeensis (lanes 6 to 10), E. canis (lane 11), and E. phagocytophila (lane 12) as well as positive (+) and negative (−) controls. The expected size (215 bp) is indicated. Lanes M show size standards (HaeIII digest of phage φX174 DNA).
Sensitivity of the E. ewingii-specific PCR assay.
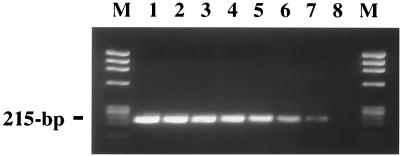
The sensitivity of the E. ewingii assay was determined by performing a PCR amplification using a dilution series of a known amount of a plasmid containing the E. ewingii p28 gene (pMAP) in the presence of DNA extracted from uninfected EDTA-treated human blood. Results of the sensitivity testing are shown in Fig. 4. The assay detected as few as 38 copies of the p28 gene (Fig. 4, lane 7).
FIG. 4.
Sensitivity of the species-specific p28 PCR assay for E. ewingii with primers EEM2F and EEM1R. A 10-fold dilution series of pMAP template amplified by the p28 PCR assay is shown (lane 1, 3.84 × 107 copies of pMAP, through lane 8, 3.84 copies of pMAP). The expected size (215 bp) is noted. Lanes M show size standards (HaeIII digest of phage φX174 DNA).
DISCUSSION
Molecular and serologic evidence suggests that E. ewingii is closely related to two other members of the Ehrlichia genus, E. chaffeensis and E. canis. Whereas multigene families encoding homologous 28- to 30-kDa immunodominant outer membrane proteins have been described for E. chaffeensis and E. canis, no such homologue had been identified previously for E. ewingii. Previous studies using sera from E. ewingii-infected human and canine samples either did not react to native and recombinant 28-kDa (P28) and 30-kDa (P30) protein antigens of E. chaffeensis and E. canis (4, 25) or showed weak reactivity (20). This suggested that if E. ewingii does encode P28/P30 homologues these proteins would exhibit significantly different antigenic properties compared to the homologues in E. chaffeensis and E. canis.
In this report we describe the PCR amplification and cloning of a partial open reading frame from E. ewingii that encodes a member of the p28/p30 gene family. The fact that the E. ewingii P28 protein shows only 70% homology to the corresponding antigens of E. chaffeensis and E. canis may explain the absence of seroreactivity to this range of antigens in patients infected by E. ewingii. The p28 gene described in this report is likely to be the first of numerous homologous genes to be described in E. ewingii. Loci encoding multiple copies of the homologous p28, p30, and map1 genes have been described for E. chaffeensis, E. canis, and C. ruminantium, respectively, and similar multigene loci are likely to be found in the E. ewingii genome (14–19, 28). Two partial copies of p28 genes of E muris were deposited recently in GenBank (accession numbers AF165813 and AF165814). Like the E. chaffeensis and E. canis homologues, the E. muris genes showed 72 to 76% identity over a 302-bp region to the E. ewingii p28 genes (data not shown). Of the p28 copies that have been described in E. chaffeensis, the E. ewingii p28 gene identified in this paper has the highest nucleotide homology to p28-18, previously referred to as omp-1F (17) and orf-4 (19), and p28-19, previously referred to as p28 (17) and orf-5 (19). Both p28-18 and p28-19 have been shown to be transcriptionally active (19, 28). Phylogenetic analysis of the amino acid sequences predicted from the E. ewingii p28 genes shows them to be most closely related to the P28 proteins encoded within the p28-15 to p28-19 gene cluster (28). On the basis of the relatively high homology of these five genes to the E. canis and E. muris p28/p30 homologues, Yu et al. (28) have suggested that these genes represent the precursors of the current 21 copies that comprise the E. chaffeensis p28 gene family, and our findings support this hypothesis (17, 28). Of interest, the P23 antigen that is expressed in E. chaffeensis is believed to be derived from the p28-18 open reading frame following posttranslational cleavage (17). Although we have no evidence that the E. ewingii p28 gene is expressed, on the basis of the antigenicity profile of the protein, it is likely that it will be an immunodominant antigen and a suitable candidate for serodiagnosis. These questions will be more readily answered once the organism is cultured and expression studies can be performed.
Whereas the P28 protein sequences for four of the E. ewingii samples (C-1, OK-1, OK-2, and MO-3) were highly homologous (>99.4% identity), the P28 sequence from sample MO-1 showed 94.0 to 94.6% identity to each of the others. There are several possible explanations for the difference between the MO-1 P28 sequences and the others. It is possible that a different copy of the p28 gene was amplified from the MO-1 sample than was amplified from each of the other samples. Alternatively, it may be that the MO-1 sample represents a different strain of E. ewingii, although the 16S rRNA gene sequences were identical for MO-1 and the other samples. The latter hypothesis is supported by the low homology noted for the E. chaffeensis p28 gene family, for which each copy of the P28 protein shows <83% identity to the other copies (28). Similarly low P28 protein homology has been shown among the multiple genes identified in E. canis (five copies; <74% identity), E. muris (two copies; <81% identity), and C. ruminantium (two copies; <47% identity) (15, 21). Although these data suggest that we have amplified the same copy of the E. ewingii p28 gene from each of the five samples tested, the examination of additional genes, particularly single-copy genes that are more variable than the 16S rRNA, are needed to determine whether MO-1 represents a variant strain.
The proper diagnosis of infection by E. ewingii is extremely important in determining the incidence and prevalence of infection and, in particular, whether these numbers are increasing. However, the limited number of genes that have been identified for E. ewingii and the close antigenic relationship of E. ewingii to E. chaffeensis and E. canis have restricted the molecular and serologic tools available for the diagnosis of human E. ewingii infections. There currently are no serologic assays designed to specifically identify infection by E. ewingii. In fact, previous serologic assays may have incorrectly identified E. ewingii infections as infections with E. chaffeensis due to the serologic cross-reactivity of these two agents (4). Therefore, molecular-based assays provide the most specific and sensitive means of identifying E. ewingii infections. Alignment of the E. ewingii P28 protein with the P28/P30 homologues from other species of Ehrlichia revealed two variable regions with numerous amino acid differences from these proteins (Fig. 2). These two regions were previously described as being hypervariable for the p28/p30 genes of E. chaffeensis and E. canis and the map1 genes of C. ruminantium (15, 17, 19, 21, 28). These hypervariable regions were used to develop a PCR assay that targets the p28 of E. ewingii and provides a useful species-specific diagnostic tool for molecular confirmation of infection by E. ewingii. The assay successfully identified each of five cases of confirmed infection by E. ewingii and did not amplify DNA from closely related species (E. chaffeensis, E. canis, and E. phagocytophila). In addition, the sensitivity of the assay was shown by the detection of as few as 38 copies of the gene.
Identification of the p28 gene in E. ewingii presents several possibilities for utilizing the protein in serologic diagnostic assays. Serologic assays have a wider applicability due to the more frequent availability of patient sera than whole blood and to the lack of expensive equipment and expertise needed to perform PCR in most clinical facilities. Because the protein has only a 70% similarity to its nearest homologue, the P28 of E. ewingii may prove useful in species-specific identification in serologic assays. On the other hand, knowledge of the conserved antigenic regions between the P28 and P30 homologues may lead to a broad-spectrum serologic assay for detection of ehrlichiosis caused by E. chaffeensis, E. canis, and E. ewingii.
ACKNOWLEDGMENTS
We are grateful to Chris Paddock for his contributions throughout the study and to the CDC Biotechnology Core Facility for the synthesis of oligonucleotides. We thank Greg Dasch and Jamie Childs for review of the manuscript and useful suggestions.
This study was supported in part by the APHL-CDC Emerging Infectious Diseases Fellowship Program.
REFERENCES
- 1.Anderson B E, Dawson J E, Jones D C, Wilson K H. Ehrlichia chaffeensis, a new species associated with human ehrlichiosis. J Clin Microbiol. 1991;29:2838–2842. doi: 10.1128/jcm.29.12.2838-2842.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson B E, Greene C E, Jones D C, Dawson J E. Ehrlichia ewingii sp. nov., the etiologic agent of canine granulocytic ehrlichiosis. Int J Syst Bacteriol. 1992;42:299–302. doi: 10.1099/00207713-42-2-299. [DOI] [PubMed] [Google Scholar]
- 3.Breitschwerdt E B, Hegarty B C, Hancock S I. Sequential evaluation of dogs naturally infected with Ehrlichia canis, Ehrlichia chaffeensis, Ehrlichia equi, Ehrlichia ewingii, or Bartonella vinsonii. J Clin Microbiol. 1998;36:2645–2651. doi: 10.1128/jcm.36.9.2645-2651.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buller R S, Arens M, Hmiel S P, Paddock C D, Sumner J W, Rikihisa Y, Unver A, Gaudreault-Keener M, Manian F A, Liddell A M, Schmulewitz N, Storch G A. Ehrlichia ewingii, a newly recognized agent of human ehrlichiosis. N Engl J Med. 1999;341:148–155. doi: 10.1056/NEJM199907153410303. [DOI] [PubMed] [Google Scholar]
- 5.Chen S M, Cullman L C, Walker D H. Western immunoblotting analysis of the antibody responses of patients with human monocytotropic ehrlichiosis to different strains of Ehrlichia chaffeensis and E. canis. Clin Diagn Lab Immunol. 1997;4:731–735. doi: 10.1128/cdli.4.6.731-735.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen S-M, Dumler J S, Feng H-M, Walker D H. Identification of the antigenic constituents of Ehrlichia chaffeensis. Am J Trop Med Hyg. 1994;50:52–58. [PubMed] [Google Scholar]
- 7.Chen S-M, Dumler J S, Bakken J S, Walker D H. Identification of a granulocytotropic Ehrlichia species as the etiologic agent of human disease. J Clin Microbiol. 1994;32:589–595. doi: 10.1128/jcm.32.3.589-595.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Childs J E, Sumner J W, Nicholson W L, Massung R F, Standaert S M, Paddock C D. Outcome of diagnostic tests using samples from patients with culture-proven human monocytic ehrlichiosis: implications for surveillance. J Clin Microbiol. 1999;37:2997–3000. doi: 10.1128/jcm.37.9.2997-3000.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dawson J E, Rikihisa Y, Ewing S A, Fishbein D B. Serologic diagnosis of human ehrlichiosis using two E. canis isolates. J Infect Dis. 1991;163:564–567. doi: 10.1093/infdis/163.3.564. [DOI] [PubMed] [Google Scholar]
- 10.Dear S, Staden R. A sequence assembly and editing program for efficient management of large projects. Nucleic Acids Res. 1991;19:3907–3911. doi: 10.1093/nar/19.14.3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Devereux J, Haeberli P, Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984;12:387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maeda K, Markowitz N, Hawley R C, Ristic M, Cox D, McDade J E. Human infection with Ehrlichia canis, a leukocytic rickettsia. N Engl J Med. 1987;316:853–856. doi: 10.1056/NEJM198704023161406. [DOI] [PubMed] [Google Scholar]
- 13.Massung R F, Slater K, Owens J H, Nicholson W L, Mather T N, Solberg V B, Olson J G. Nested PCR assay for detection of granulocytic ehrlichiae. J Clin Microbiol. 1998;36:1090–1095. doi: 10.1128/jcm.36.4.1090-1095.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McBride J W, Yu X, Walker D H. Molecular cloning of the gene for a conserved major immunoreactive 28-kilodalton protein of Ehrlichia canis: a potential serodiagnostic antigen. Clin Diagn Lab Immunol. 1999;6:392–399. doi: 10.1128/cdli.6.3.392-399.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McBride J W, Yu X, Walker D H. A conserved, transcriptionally active p28 multigene locus of Ehrlichia canis. Gene. 2000;254:245–252. doi: 10.1016/s0378-1119(00)00256-0. [DOI] [PubMed] [Google Scholar]
- 16.Ohashi N, Unver A, Zhi N, Rikihisa Y. Cloning and characterization of multigenes encoding the immunodominant 30-kilodalton major outer membrane proteins of Ehrlichia canis and application of the recombinant protein for serodiagnosis. J Clin Microbiol. 1998;36:2671–2680. doi: 10.1128/jcm.36.9.2671-2680.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ohashi N, Zhi N, Zhang Y, Rikihisa Y. Immunodominant major outer membrane proteins of Ehrlichia chaffeensis are encoded by a polymorphic multigene family. Infect Immun. 1998;66:132–139. doi: 10.1128/iai.66.1.132-139.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reddy G R, Streck C P. Variability in the 28-kDa surface antigen protein multigene locus of isolates of the emerging disease agent Ehrlichia chaffeensis suggests that it plays a role in immune evasion. Mol Cell Biol Res Commun. 1999;1:167–175. doi: 10.1006/mcbr.1999.0133. [DOI] [PubMed] [Google Scholar]
- 19.Reddy G R, Sulsona C R, Barbet A F, Mahan S M, Burridge M J, Alleman A R. Molecular characterization of a 28 kDa surface antigen gene family of the tribe Ehrlichiae. Biochem Biophys Res Commun. 1998;247:636–643. doi: 10.1006/bbrc.1998.8844. [DOI] [PubMed] [Google Scholar]
- 20.Rikihisa Y, Ewing S A, Fox J C. Western immunoblot of Ehrlichia chaffeensis, E. canis, or E. ewingii infections in dogs and humans. J Clin Microbiol. 1994;32:2107–2112. doi: 10.1128/jcm.32.9.2107-2112.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sulsona C R, Mahan S M, Barbet A F. The map1 gene of Cowdria ruminantium is a member of a multigene family containing both conserved and variable genes. Biochem Biophys Res Commun. 1999;257:300–305. doi: 10.1006/bbrc.1999.0459. [DOI] [PubMed] [Google Scholar]
- 22.Sumner J W, Childs J E, Paddock C D. Molecular cloning and characterization of the Ehrlichia chaffeensis variable-length PCR target: an antigen-expressing gene that exhibits interstrain variation. J Clin Microbiol. 1999;37:1447–1453. doi: 10.1128/jcm.37.5.1447-1453.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sumner J W, Sims K G, Jones D C, Anderson B E. Ehrlichia chaffeensis expresses an immunoreactive protein homologous to the Escherichia coli GroEL protein. Infect Immun. 1993;61:3536–3539. doi: 10.1128/iai.61.8.3536-3539.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sumner J W, Storch G A, Buller R S, Liddell A M, Stockham S L, Rikihisa Y, Messenger S, Paddock C D. PCR amplification and phylogenetic analysis of groESL operon sequences from Ehrlichia ewingii and Ehrlichia muris. J Clin Microbiol. 2000;38:2746–2749. doi: 10.1128/jcm.38.7.2746-2749.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Unver A, Rikihisa Y, Ohashi N, Cullman L C, Buller R, Storch G. Western and dot blotting analyses of Ehrlichia chaffeensis indirect fluorescent-antibody assay-positive and -negative human sera by using native and recombinant E. chaffeensis and E. canis antigens. J Clin Microbiol. 1999;37:3888–3895. doi: 10.1128/jcm.37.12.3888-3895.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu X-J, Crocquet-Valdes P, Cullman L C, Popov V L, Walker D H. Comparison of Ehrlichia chaffeensis recombinant proteins for serologic diagnosis of human monocytotropic ehrlichiosis. J Clin Microbiol. 1999;37:2568–2575. doi: 10.1128/jcm.37.8.2568-2575.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu X-J, Crocquet-Valdes P, Cullman L C, Walker D H. Cloning and sequencing of the gene for a 120-kDa immunodominant protein of Ehrlichia chaffeensis. Gene. 1997;184:149–154. doi: 10.1016/s0378-1119(96)00586-0. [DOI] [PubMed] [Google Scholar]
- 28.Yu X-J, McBride J W, Zhang X-F, Walker D H. Characterization of the complete transcriptionally active Ehrlichia chaffeensis 28 kDa outer membrane protein multigene family. Gene. 2000;248:59–68. doi: 10.1016/s0378-1119(00)00147-5. [DOI] [PubMed] [Google Scholar]