Abstract
Background
Soft tissue injuries (including muscle damage after unaccustomed exercise) are common and are often associated with athletic activity. Hyperbaric oxygen therapy (HBOT) is the therapeutic administration of 100% oxygen at environmental pressures greater than one atmosphere.
Objectives
To assess the benefits and harms of HBOT for treating soft tissue injury, including delayed onset muscle soreness (DOMS).
Search methods
We searched The Cochrane Bone, Joint and Muscle Trauma Group Specialised Register (to February 2010), the Cochrane Central Register of Controlled Trials (The Cochrane Library (2010, Issue 1), MEDLINE (1950 to February 2010), EMBASE (1980 to 2010 Week 07), CINAHL (1982 to October 2008), an additional database developed in our hyperbaric facility and reference lists of articles. Relevant journals were handsearched and researchers in the field contacted.
Selection criteria
Randomised trials comparing the effect on closed soft tissue injury (including DOMS) of therapeutic regimens which include HBOT with those that exclude HBOT (with or without sham therapy).
Data collection and analysis
Four authors independently evaluated study quality and extracted data. Most of the data presented in the review were extracted from graphs in the trial reports.
Main results
Nine small trials involving 219 participants were included. Two trials compared HBOT versus sham therapy on acute closed soft tissue injuries (ankle sprain and medial collateral knee ligament injury respectively). The other seven trials examined the effect of HBOT on DOMS following eccentric exercise in unconditioned volunteers.
All 32 participants of the ankle sprain trial returned to their normal activities. There were no significant differences between the two groups in time to recovery, functional outcomes, pain, or swelling. There was no difference between the two groups in knee function scores in the second acute injury trial; however, intention‐to‐treat analysis was not possible for this trial.
Pooling of data from the seven DOMS trials showed significantly and consistently higher pain at 48 and 72 hours in the HBOT group (mean difference in pain score at 48 hours [0 to 10 worst pain] 0.88, 95% CI 0.09 to 1.67, P = 0.03) in trials where HBOT was started immediately. There were no differences between the two groups in longer‐term pain scores or in any measures of swelling or muscle strength.
No trial reported complications of HBOT but careful selection of participants was evident in most trials.
Authors' conclusions
There was insufficient evidence from comparisons tested within randomised controlled trials to establish the effects of HBOT on ankle sprain or acute knee ligament injury, or on experimentally induced DOMS. There was some evidence that HBOT may increase interim pain in DOMS. Any future use of HBOT for these injuries would need to have been preceded by carefully conducted randomised controlled trials which have demonstrated effectiveness.
Plain language summary
Hyperbaric oxygen therapy for delayed onset muscle soreness and closed soft tissue injury
Soft tissue injuries are very common. Hyperbaric oxygen therapy (HBOT) involves people breathing pure oxygen in a specially designed chamber. It is sometimes used to increase the supply of oxygen to the injured area in an attempt to speed recovery. Our review included nine small trials, involving a total of 219 participants. Two trials compared HBOT versus sham therapy on ankle sprain and knee sprain respectively. Neither trial provided sufficient evidence to determine if HBOT helped people with these injuries. The other seven trials examined the effect of HBOT on muscle injury following unaccustomed exercise. There was no evidence that HBOT helped people with muscle injury following unaccustomed exercise, but some evidence that people given HBOT had slightly more pain. Further research on HBOT is not a high priority given the variety of other treatment interventions available.
Background
Description of the condition
Soft tissue injuries are common and range from minor abrasions and bruising to major disruption of tendons, ligaments and muscles. It is difficult to obtain accurate estimates of the impact on society of soft‐tissue injuries taken in isolation, but injuries in general result in tens of millions of emergency room visits and cost hundreds of billions of healthcare dollars per annum in the USA alone (Finnegan 2003). Soft tissue injuries are commonly associated with athletic activity, and occur in both elite and recreational athletes. In both these groups, soft tissue injuries may be associated with considerable loss of work and health costs (Van Mechelen 1997). The causes of soft tissue injuries are diverse and may involve acute traumatic impact, repetitive strain and overuse, or muscle injury induced by unaccustomed exercise (Babul 2000a; Leach 1998). This review is restricted to acute closed injuries involving muscle, ligament and tendon only, and where the mechanism is unaccustomed use, trauma from a direct blow, strain or overuse injury.
Of particular interest in this review is the phenomenon of delayed onset muscle soreness (DOMS). Familiar to most individuals at some time, this is the name given to the syndrome of pain, swelling and stiffness in muscles in the days following a bout of unaccustomed activity in that muscle group. DOMS can exhibit as anything from minor muscle soreness to debilitating pain and swelling, but is most commonly described as causing a reduction in joint range of motion, shock attenuation and peak torque. A recent review confirms that the mechanisms, treatment strategies, and impact on athletic performance remain uncertain (Cheung 2003). Putative mechanisms include lactic acid accumulation, muscle spasms, connective tissue damage, inflammation and enzyme efflux secondary to muscle cell damage. DOMS is frequently used as an experimental soft tissue injury in human research because it is both self‐limiting and reliably reproduced in individuals unaccustomed to exercise.
Description of the intervention
Accepted treatment methods vary greatly with the specific injury. They may, however, be classified broadly as rest, local measures to reduce oedema (e.g. massage, cryotherapy, elevation), drug therapy (typically non‐steroidal anti‐inflammatory agents), stretching or further exercise (particularly for delayed onset muscle soreness), surgical, and rehabilitative (Cheung 2003; Kader 2002; Perryman 2002). The ultimate aim of treatment is to restore pain free function and enable the return to activity in the shortest time compatible with a low risk of re‐injury.
Hyperbaric oxygen therapy (HBOT) is the therapeutic administration of 100% oxygen at environmental pressures greater than one atmosphere absolute (ATA). Administration of HBOT involves placing the injured individual in an airtight vessel, increasing the pressure within that vessel, and administering 100% oxygen for respiration. In this way, it is possible to deliver a greatly increased partial pressure (supply) of oxygen to the tissues. Typically, treatments involve pressurisation to between 1.5 and 3.0 ATA for periods between 60 and 120 minutes once or twice daily.
HBOT is associated with some risk of adverse effects including damage to the ears, sinuses and lungs from the effects of pressure, temporary worsening of short‐sightedness, claustrophobia and oxygen poisoning. Although serious adverse events are rare, HBOT cannot be regarded as benign.
How the intervention might work
It has been suggested since 1982 that HBOT might accelerate injury recovery (Oriani 1982). HBOT has been shown in a number of injury models to reduce oedema and preserve microcirculation through vasoconstriction with enhanced oxygen delivery, a direct osmotic effect and the inactivation of white cell adhesion (Hills 1999; Nylander 1985; Staples 1995; Thom 1994). The first clinical report, which appeared in 1993, described a 55% reduction in days lost to injury by Scottish soccer players suffering from a variety of injuries following the application of HBOT (James 1993). Since then, a number of anecdotal reports in the non‐medical media suggest that the use of HBOT has become commonplace in some elite sporting clubs. In addition, some comparative human trials have been published.
Why it is important to do this review
Given the increasing use of HBOT for soft tissue injury, and the uncertainty about the benefit and risks of this therapy, it is important to carry out a systematic review of the evidence.
Objectives
The aim of this review is to assess the evidence for the use of HBOT for the treatment of soft tissue injuries including DOMS. Specifically, we wish to address, does HBOT safely improve and speed‐up functional outcome after injury?
Methods
Criteria for considering studies for this review
Types of studies
We considered any randomised or quasi‐randomised (use of a method of allocating participants to a treatment that is not strictly random; e.g. by date of birth or hospital record number) clinical trials that compared HBOT with no HBOT (no treatment or sham). We considered both trials that employed standard alternative therapies as the comparator, and those that compared HBOT to no treatment or sham alone.
Types of participants
Patients with DOMS following exercise or patients with closed injuries to tendon, ligament or muscle tissue, including repetitive strain injuries. No restrictions on age or gender were made.
Types of interventions
We accepted studies that compared treatment regimens including HBOT with similar regimens that excluded HBOT. Where co‐interventions differed significantly between studies this was clearly stated and the implications discussed.
We accepted any standard HBOT regimen used for promoting recovery from soft tissue injury. Generally, a standard regimen involves HBOT administered in a compression chamber between pressures of 1.5 ATA and 3.0 ATA and treatment times between 30 minutes and 120 minutes on at least one occasion.
Types of outcome measures
Studies were eligible for inclusion if they reported any of the following outcome measures:
Primary outcomes
(1) Recovery defined as return to pre‐injury level of activity (sports/work). (2) Rate of recovery (e.g. time to return to previous athletic activity). (3) Persistent pain (long‐term).
Secondary outcomes
(4) Patient functional assessment measures. (5) Pain or swelling. (6) Objective measures of muscle strength, joint stability or similar. (7) Complications (including re‐injury) and adverse effects of HBOT (visual disturbance; barotrauma ‐ aural, sinus, pulmonary ‐ and oxygen toxicity). Other recorded adverse effects as reported in the trials.
In addition, note was taken of reports of service utilisation or resource use; for instance, length of hospital stay and costs of HBOT.
Search methods for identification of studies
Electronic searches
We searched The Cochrane Bone, Joint and Muscle Trauma Group Specialised Register (to February 2010), the Cochrane Central Register of Controlled Trials (The Cochrane Library 2010, Issue 1), MEDLINE (1950 to February Week 3 2010), EMBASE (1980 to 2010 Week 07), CINAHL (1982 to October Week 4 2008), an additional database developed in our hyperbaric facility, The Database of Randomised Trials in Hyperbaric Medicine (Bennett 2003), and reference lists of articles (both February 2010). No language restrictions were applied.
A sensitive subject search strategy was combined with all three phases of the Cochrane optimal trial search strategy (Higgins 2006) for use in MEDLINE (OVID WEB) and modified for use in other databases (seeAppendix 1).
Searching other resources
In addition we made a systematic search for relevant controlled trials in specific hyperbaric literature sources by: (1) contacting experts in the field and leading hyperbaric therapy centres (as identified by personal communication and searching the Internet) to ask for additional relevant data in terms of published or unpublished randomised trials; (2) handsearching relevant hyperbaric textbooks (Jain 2009; Kindwall 2008; Oriani 1996; Neuman 2008), journals (Undersea and Hyperbaric Medicine, Hyperbaric Medicine Review, South Pacific Underwater Medicine Society (SPUMS) Journal, European Journal of Hyperbaric Medicine and Aviation, Space and Environmental Medicine Journal) and conference proceedings (Undersea and Hyperbaric Medical Society, SPUMS, European Undersea and Baromedical Society, International Congress of Hyperbaric Medicine) published since 1980; and (3) contacting authors of relevant studies to request details of unpublished or ongoing investigations.
Data collection and analysis
Selection of studies
One author (MB) was responsible for hand searching and identification of eligible studies. Three authors (MB, JT and ML) examined the electronic search results and identified potentially eligible studies. Full reports of these studies were retrieved and reviewed independently for inclusion by four authors, two (MB, ML) of whom have content expertise with HBOT and two (JT, JB) with content expertise in orthopaedics. In addition, one of the authors (MB) has expertise in clinical epidemiology. Any differences were resolved by discussion. None of the authors were allocated to consider papers they had participated in as an author or where the paper was written by authors at the same institution. We recorded data using the data extraction form developed for this review. All languages were considered. We contacted authors for clarification of trial methods and data when required. Individual patient data for calculation of means and standard deviations were not available.
Data extraction and management
Review authors extracted data and trial details using a pre‐piloted data extraction form developed for this review. Primary authors of the included trials were contacted to provide information for missing data and trial information. We also sought individual patient data to enable comparisons of mean values across studies. To perform intention‐to‐treat analyses, all data extracted reflected the original allocation groups where possible. Losses to follow up were identified where this information was given. Any differences were settled by consensus.
For the majority of trials, we estimated means and standard deviations from graphs presented without tabulated data in the trial reports. During editorial processing of the review, data extraction from graphs was repeated by Helen Handoll, acting in an editorial capacity and not as an author, and a final data set agreed with MB.
Assessment of risk of bias in included studies
In this review, risk of bias is implicitly assessed in terms of methodological quality.
Study quality was assessed using an adaptation of the method outlined in Schulz 1995. The results of the quality assessment are presented in a descriptive manner. We assessed adequacy of randomisation, adequacy of allocation concealment, completeness of outcome data and the level of masking. Details of the assessment categories are shown in Table 1.
1. Quality assessment system for included studies.
| Randomisation | Allocation concealment | Completeness of outcome data | Masking |
| A ‐ Adequate sequence generation recorded using random number tables, computer random number generation, coin toss or shuffling | A ‐ Adequate method of allocation concealment such as central randomisation, serial numbered opaque envelopes, or other method where allocation is convincingly concealed | A ‐ Trials where intention‐to‐treat analysis is possible and losses to follow up are few | A ‐ Double or triple blind |
| B ‐ Did not specify one of the methods above, but mentions randomisation method | B ‐ Unclear allocation concealment or no mention of any attempt to conceal allocation listed in A | B ‐ Trials which report exclusions at <10% | B ‐ Single blind |
| C ‐ Other method of allocation that appears unbiased | C ‐ Inadequate allocation concealment such as medical record number or alteration methods | C ‐ No mention of exclusions, exclusions 10% or above or widely differing between arms of the trial | C ‐ No blinding |
Data synthesis
Analyses were performed using the RevMan 4.2.3 software. We conducted intention‐to‐treat analyses wherever possible. Risk ratios and 95% confidence intervals (CI) were calculated for dichotomous outcomes, and mean differences and 95% confidence intervals calculated for continuous outcomes. Results of comparable groups of trials were pooled using the fixed‐effect model. We analysed different injury categories separately (tendon/ligament injury, DOMS). Heterogeneity between comparable trials was estimated using the I² statistic and consideration given to the appropriateness of pooling. Where there was an indication of significant heterogeneity, we stipulated analysis using a random‐effects model.
Subgroup analysis and investigation of heterogeneity
Where appropriate data were available, we considered subgroup analysis based on the following. (1) Injury entry grade or severity using an established specific injury classification system where the authors have employed such a system. (2) Type of injury including anatomical location. (3) Dose of oxygen received (pressure, time and length of treatment course). (4) Nature of the comparative treatment modalities, including no specific therapy. (5) Age (adults versus children). (6) Nature of the activity undertaken.
Tests of interaction were calculated to determine if the results for subgroups were significantly different. Statistical heterogeneity was assumed to be significant if the I² analysis suggested more than 30% of the variability in an analysis was due to differences between trials. Consideration was then given to the appropriateness of pooling and meta‐analysis.
Sensitivity analysis
Where appropriate, we planned to perform sensitivity analyses investigating the effects of study quality based on the Schulz quality score (Schulz 1995) and missing data. For the latter we planned best and worst case analyses. The best‐case scenario assumes that none of the originally enrolled participants missing from the primary analysis in the treatment group had the negative outcome of interest whilst all those missing from the control group did. The worst‐case scenario is the reverse.
Results
Description of studies
Results of the search
The original search in July 2004 identified 24 publications apparently dealing with the use of HBOT for the treatment of soft tissue injuries including DOMS. Initial examination confirmed four were reviews without new data, four did not involve the application of HBOT, two were case reports or case series, one was not a clinical study and one was an animal study. These reports were excluded, leaving 13 publications of possible randomised comparative trials. Repeat searching up to February 2010 of the same databases and the latest editions of textbooks and journals identified above identified no further studies. An additional reference for an already included trial (Babul 2003) was identified.
After appraisal of the full reports we included nine trials, two (Staples 1999a; Staples 1999b) of which were reported in the same paper and represent 'phase 1' and 'phase 2' of a two stage study with different comparisons in each phase. Three papers were abstracts (Babul 2000b; Mekjavic 1996; Soolsma 1997) of included trials (Babul 2003; Mekjavic 2000; Soolsma 1996). The newly identified report for Babul 2003 was a thesis (Babul 2001). We excluded one non‐randomised comparative trial (Todorovic 1996) (seeCharacteristics of included studies).
Included studies
The nine included trials were published between 1996 and 2003, and from a limited number of centres in Canada (Babul 2003; Germain 2003; Soolsma 1996; Staples 1999a; Webster 2002), the USA (Borromeo 1997; Harrison 2001) and Europe (Mekjavic 2000). The authors are unaware of any ongoing RCTs in the area. In total, these trials recruited 219 participants but presented results for only 197 participants (90%). The number of participants in each trial ranged from 12 (Webster 2002) to 49 (Staples 1999a). Further details of the trials are presented in the Characteristics of included studies.
Two trials evaluated HBOT for treating acute soft tissue injury: Borromeo 1997 enrolled individuals with acute ankle sprains presenting within 72 hours to an orthopaedic surgeon, while Soolsma 1996 enrolled individuals with grade II medial collateral ligament injuries in one knee who similarly presented within 72 hours. The other seven trials included young adult unconditioned volunteers who underwent exercise designed to produce DOMS under controlled conditions. Exercise intensity and duration varied across these studies, but all except Webster 2002 involved multiple repetitions of resistance to lengthening of the target muscle group (eccentric exercise). Four trials exercised the quadriceps. Three of these (Babul 2003; Staples 1999a; Staples 1999b) specified the non‐dominant leg and used the same protocol; the other used a similar protocol involving up to 150 repetitions (Germain 2003). Two studies exercised the forearm flexors, involving maximal resistance to elbow extension for 60 and 72 repetitions respectively (Harrison 2001; Mekjavic 2000). The remaining study (Webster 2002) involved bilateral calf muscles raises against an 80% of maximal load ‐ five repetitions to failure. None of the trials reported a failure to produce DOMS in any study participant, and all trials indicated the use of these exercise protocols in previous studies.
Both the dose of oxygen per treatment session and for the total course of treatment varied between studies. The lowest dose administered was 2.0 atmosphere absolute (ATA) for 60 minutes on three occasions (intervention groups in Staples 1999a and Staples 1999b), while the highest single treatment dose was 2.5 ATA for 100 minutes for five sessions over three days (Germain 2003; Harrison 2001). The longest course was in Soolsma 1996 who applied 60 minute treatments for 10 days. All authors therefore used between 2.0 ATA and 2.5 ATA as a maximum oxygen pressure and the total number of individual treatment sessions varied from three to 10. The mean time between injury and compression was 33 hours in Borromeo 1997 and 74 hours in Soolsma 1996. Most DOMS trials administered oxygen or sham therapy immediately (up to four hours) after the exercise session, the exceptions being one of the two HBOT groups in both Harrison 2001 and Staples 1999a, who received the first treatment approximately 24 hours after exercise ('delayed treatment').
Active HBOT was compared to a sham hyperbaric oxygen exposure breathing air at a trivial pressure in six trials (Babul 2003; Borromeo 1997; Soolsma 1996; Staples 1999a; Staples 1999b; Webster 2002). The same type of sham exposure was used for the delayed HBOT groups in Harrison 2001 and Staples 1999a, while Mekjavic 2000 employed sham exposure at pressure by administering an 8% oxygen mixture to keep inspired oxygen tension equal to that at one atmosphere. No sham was used in the control groups of Germain 2003 and Harrison 2001, and one of the two control groups of Staples 1999a.
The follow‐up period varied between trials, ranging from day three following exercise (Babul 2003) to six weeks (Soolsma 1996). Borromeo 1997 followed participants to full functional recovery. All included studies reported at least one outcome of interest. Of the outcomes identified above, the trials reported data on two primary outcomes (time to full functional recovery and proportion returning to full function) and three secondary outcomes of interest (functional assessments, pain and swelling, and muscle strength).
Other outcomes reported (including non‐clinical) include: active and passive range of motion (Borromeo 1997; Soolsma 1996), average power at varying degrees of joint position (Germain 2003), serum creatine kinase (Babul 2003; Germain 2003; Harrison 2001), serum malondialdehyde (Babul 2003), magnetic resonance imagery (Babul 2003; Harrison 2001; Soolsma 1996; Webster 2002), magnetic resonance spectroscopy (Webster 2002), ratio of figure of eight to straight running ability (Soolsma 1996) and transcutaneous oxygen measurement (Harrison 2001; Mekjavic 2000).
Risk of bias in included studies
Details of the quality assessment are given in the Characteristics of included studies. In general, study quality was assessed as fair to high with regard to methodology. The significance of variations in quality detailed below is unclear. Given that few analyses could be pooled, study quality was not used as a basis for sensitivity analysis.
Randomisation
Randomisation procedures were described for Babul 2003 by personal correspondence with the author (random number table (Babul 2005)) and Borromeo 1997 (random‐number table), but not in the other seven studies. Allocation concealment was adequately described only by Babul 2003. For none of the remaining studies is there a clear indication that the investigators were unable to predict the prospective group to which a participant would be allocated.
Participant baseline characteristics
Participants entered into Borromeo 1997 had all suffered acute lateral ankle sprains and had received no specific treatment other than ice, elevation, crutches and elastic bandaging, while for Soolsma 1996 participants were enrolled with grade II medial collateral ligament injuries. In both trials, participants had presented to an orthopaedic surgeon within 72 hours (Borromeo 1997: mean 33 hours to compression; Soolsma 1996: mean 74 hours to compression). Participants entered into all other trials were young healthy volunteers who were not conditioned athletes and who had not exercised vigorously for three months prior to entry into the studies (Harrison 2001 did not specify a time period). Babul 2003 enrolled females only; Harrison 2001, Mekjavic 2000, Staples 1999a, Staples 1999b and Webster 2002 enrolled males only; and Borromeo 1997, Germain 2003 and Soolsma 1996 enrolled both males and females. In total, 42 trial participants (19%) were female.
Blinding
Seven trials utilised a sham therapy in order to mask participants to HBOT (Babul 2003; Borromeo 1997; Mekjavic 2000; Soolsma 1996; Staples 1999a; Staples 1999b; Webster 2002). One study (Harrison 2001) only provided a sham session for the group receiving delayed HBOT 24 hours after injury, while another (Germain 2003) did not report any blinding of participants or investigators to therapy. No author formally tested the success of their blinding strategy.
Participants lost to follow up
Five trials did not report any losses to follow up or any violation of the study protocol (Babul 2003; Borromeo 1997; Mekjavic 2000; Staples 1999b; Webster 2002). One person with an exercise‐related complication was excluded before therapy in Germain 2003. Harrison 2001 lost two control participants and one immediate‐HBOT group participant; all three participants were excluded from the analyses. Staples 1999a was difficult to interpret in this regard, but ultimately did not report on 13 participants, nine of whom did not complete the study (allocation unknown), and four of whom were rejected due to displaying increased strength after the eccentric exercise protocol (one each lost from control, sham, immediate HBOT and delayed HBOT). Soolsma 1996 enrolled 19 participants, of whom only 14 finished the clinical assessment and only nine completed the MRI investigation. Numbers allocated to each arm in this study were not stated. Sensitivity analysis using best and worst case scenarios have not been performed as there were no dichotomous outcomes involving those studies with losses to follow up.
Intention‐to‐treat analysis
None of the included trials specifically indicated an intention‐to‐treat approach; however five trials (see above) reported full follow up and did not report any protocol violation.
Effects of interventions
We first present the results of the two trials assessing HBOT for acute injuries, respectively acute ankle sprains (Borromeo 1997) and acute injury to the medial collateral ligament of the knee (Soolsma 1996). We then present the results of the seven trials that tested HBOT for young adult unconditioned volunteers who underwent exercise designed to produce DOMS.
HBOT for acute ligament injury
Primary outcomes
Proportion returning to pre‐injury activity
Only Borromeo 1997 reported this outcome and involved 32 participants with ankle sprains, 16 allocated to HBOT and 16 to sham HBOT. All participants returned to full activity.
Time to reach full function following injury
Again, only Borromeo 1997 reported this outcome. There was no statistical significance in the mean time to recovery of full function (see Analysis 1.1 mean difference (MD) 0.60 days, 95% confidence interval (CI) ‐12.91 to 14.11 days).
1.1. Analysis.

Comparison 1 Acute ligament injury: HBOT versus sham HBOT, Outcome 1 Time to return to prior function (days).
Persisting pain following injury
No trial reported this outcome.
Secondary outcomes
Functional assessment scores
Both trials reported functional assessment scores. Based on an unvalidated seven‐point scale, where successive scores represented an increasingly difficult level of functional activity, Borromeo 1997 found no significant difference between groups in the functional scores attained at the end of the final treatment session (seeAnalysis 1.2: mean difference (MD) 1.00, 95% CI ‐0.41 to 2.41). However, Borromeo 1997 noted a significantly greater improvement in scores in the HBOT group compared with those in control group (seeAnalysis 1.2: MD 1.40, 95% CI 0.15 to 2.65; P = 0.03). Analysis 1.3 shows the subjective recovery of knee function results (presumed here to be on a 0 to 100 scale where 100 equals full recovery) at three follow‐up times for the 14 participants clinically followed up in Soolsma 1996. In presenting the results for this trial, we have assumed that there were seven participants in each group. None of the very small differences between the two groups in functional scores at two, four and six weeks were statistically significant (scores at 6 weeks: MD 0.30, 95% CI ‐2.72 to 3.32).
1.2. Analysis.
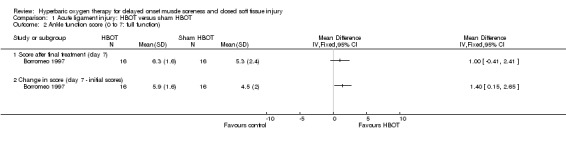
Comparison 1 Acute ligament injury: HBOT versus sham HBOT, Outcome 2 Ankle function score (0 to 7: full function).
1.3. Analysis.
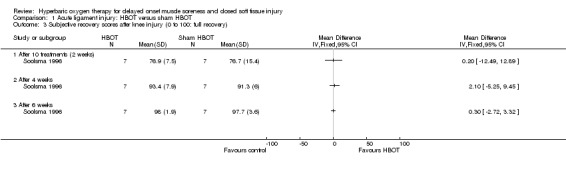
Comparison 1 Acute ligament injury: HBOT versus sham HBOT, Outcome 3 Subjective recovery scores after knee injury (0 to 100: full recovery).
Pain and swelling
Subjective pain scores, reported for both trials, decreased over time in participants of both groups. Pain scores were higher for the HBOT group of Borromeo 1997 at each measurement time. Borromeo 1997 found there was no significant difference between the HBOT and control group in pain scores after the third and final treatment session (seeAnalysis 1.4: MD 5.00, 95% CI ‐2.07 to 12.07) or in the decrease in pain from the initial scores (data not shown). Again assuming there were seven participants in each group of Soolsma 1996, the mean pain scores were statistically significantly better in the HBOT group after 10 treatments (two weeks), but not after five treatments (one week) or at four weeks follow up (seeAnalysis 1.4: MD ‐1.20, 95% CI ‐8.36 to 5.96). The two‐week result is no longer statistically significant when eight participants are assumed to be in the HBOT group and six in the control group (MD ‐12.00, 95% ‐24.31 to 0.31: Analysis not shown).
1.4. Analysis.
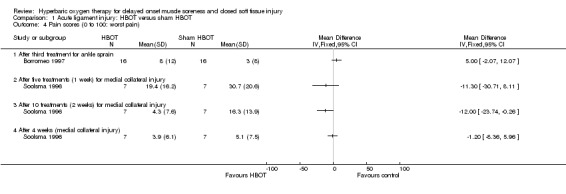
Comparison 1 Acute ligament injury: HBOT versus sham HBOT, Outcome 4 Pain scores (0 to 100: worst pain).
Swelling of the affected joint was measured in different ways in the two trials. Borromeo 1997 reported no significant difference between the two groups in the reduction of the foot and ankle volume, determined by water displacement, after each of the three treatments or overall (26 ml with HBOT versus 32 ml with sham HBOT). Soolsma 1996 found the mean difference in knee girth between the normal and injured sides, assessed by tape measure at four weeks, was less in the HBOT group (0.11 cm versus 0.43 cm). This difference was not statistically significant (seeAnalysis 1.5: MD 0.32 cm smaller with HBOT, 95% CI 0.66 cm smaller to 0.02 cm bigger).
1.5. Analysis.

Comparison 1 Acute ligament injury: HBOT versus sham HBOT, Outcome 5 Change in knee girth at 4 weeks post medial collateral ligament injury (cm).
Strength
Soolsma 1996 found no significant difference between the two groups in the mean difference between the non‐injured and injured side in the one‐legged jump distance at end of therapy (MD ‐4.30 cm, 95% CI ‐17.68 to 9.08 cm), or at four weeks (MD ‐5.30 cm, 95% CI ‐12.76 to 2.16 cm): seeAnalysis 1.6.
1.6. Analysis.
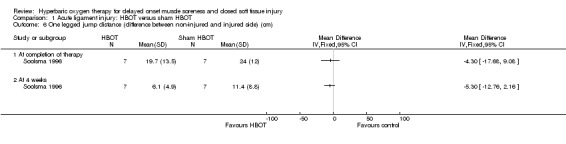
Comparison 1 Acute ligament injury: HBOT versus sham HBOT, Outcome 6 One legged jump distance (difference between non‐injured and injured side) (cm).
Complications of therapy
Borromeo 1997 reported there was no adverse effects in either treatment group, however the participants in this trial were highly selected and excluded those with an upper respiratory tract infection or a past history of claustrophobia.
HBOT for experimentally induced DOMS
Primary outcomes
Proportion returning to pre‐injury activity
None of the seven trials in this category reported these outcomes. This reflects the experimental nature of these trials (Babul 2003; Germain 2003; Harrison 2001; Mekjavic 2000; Staples 1999a; Staples 1999b; Webster 2002), none of which tested the effect of HBOT on DOMS arising from sports activity.
Time to reach full function following injury
No trial reported this outcome.
Persisting pain following injury
No trial reported this outcome.
Secondary outcomes
No data were available for pooling from Babul 2003, a small trial of 16 female participants. This trial assessed pain, swelling and strength at multiple times up to 72 hours following exercise, however only the differences between control and HBOT groups were reported rather than outcomes in each group, and we were unable to obtain suitable data for meta‐analysis. Only limited data were available from Germain 2003; even where continuous data were available, the numbers in the two treatment groups were not; the treatment group of the participant lost from follow up was also not reported. It should be noted that for most of the outcomes presented here, data were estimated from graphs in the trial reports.
Data from comparisons in two trials (Harrison 2001; Staples 1999a) testing the effect of delayed HBOT are presented separately from those testing immediately applied HBOT in the following.
Functional assessment scores
No trial reported data on this outcome
Pain and swelling
1. Pain scores (10 = worst pain) with immediate HBOT
Pooled data from four trials (Harrison 2001; Mekjavic 2000; Staples 1999a; Staples 1999b) for pain scores at 24, 48 and 72 hours, and from between four and seven days are shown in Analysis 2.1. These show no significant differences between the two groups at 24 hours or later on (at end of treatment), but statistically significant differences in favour of the control group at 48 hours (MD 0.88, 95% CI 0.09 to 1.67) and 72 hours (MD 0.72, 95% CI 0.06 to 1.37). Heterogeneity was assessed as low for each comparison (I² = 0%). Subgroup analysis by muscle group exercised showed a similar effect with regard to arm and leg muscles (seeAnalysis 2.2). Participants were pain free by 15 days in Harrison 2001 and were nearly pain free at 10 days in Mekjavic 2000.
2.1. Analysis.
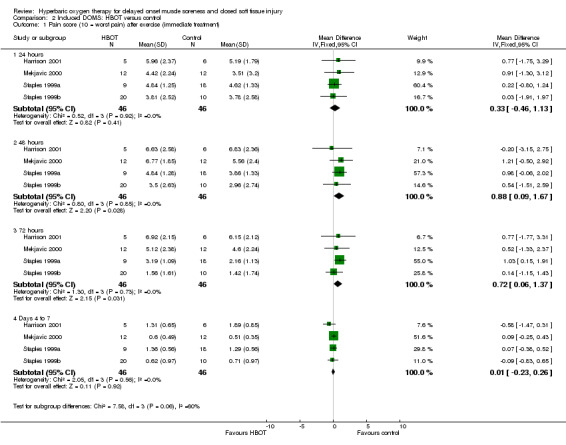
Comparison 2 Induced DOMS: HBOT versus control, Outcome 1 Pain score (10 = worst pain) after exercise (immediate treatment).
2.2. Analysis.
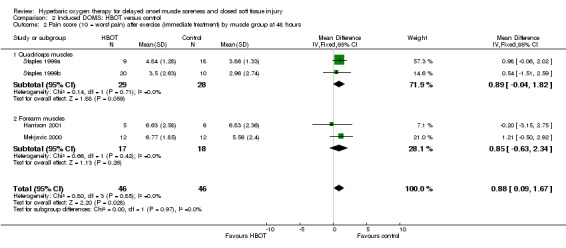
Comparison 2 Induced DOMS: HBOT versus control, Outcome 2 Pain score (10 = worst pain) after exercise (immediate treatment) by muscle group at 48 hours.
Two trials did not report standard deviations (Babul 2003; Germain 2003) for pain data. Babul 2003 reported there was no difference in perceived muscle soreness between the two groups at 24, 48 or 72 hours after exercise (mean difference in visual analogue scale score (0‐10) at 72 hours between control and HBOT: MD 1.35, 95% CI 0.07 to 2.64; this was reported as being no longer statistically significant after applying a Bonferroni correction for multiplicity of outcomes). Though Germain 2003 reported muscle soreness had returned to baseline levels at day three for the HBOT group while still being elevated for the control group, there were apparently no significant differences in the overall results over time. The presentation of the pain results of Webster 2002 was unclear and incomplete and could not be used to confirm the reported enhanced recovery for pain sensation in the HBOT group.
2. Pain scores (10 = worst pain) with delayed HBOT
Pooled data from the two trials (Harrison 2001; Staples 1999a) examining delayed HBOT for pain scores at 24, 48 and 72 hours, and at four and seven days respectively (seeAnalysis 2.3). These show no significant differences between the two groups at 24 or 48 hours or later on (at end of treatment), but statistically significant differences in favour of the control group at 72 hours (MD 0.85, 95% CI 0.06 to 1.64). However, the results for the two trials were significantly heterogeneous for this follow‐up time (I² = 62%).
2.3. Analysis.
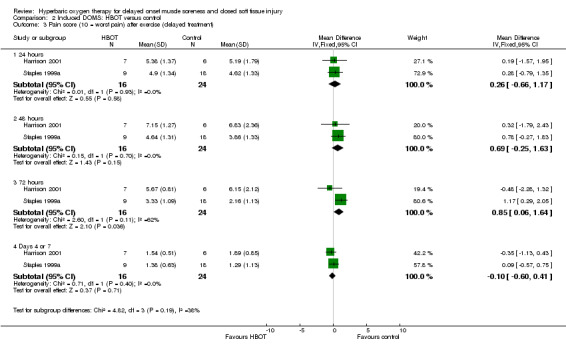
Comparison 2 Induced DOMS: HBOT versus control, Outcome 3 Pain score (10 = worst pain) after exercise (delayed treatment).
3. Swelling
Various physical measures were used to represent swelling. Three trials (Babul 2003; Germain 2003; Harrison 2001) reported on limb circumference; and two trials (Harrison 2001; Webster 2002) reported respectively on the actual values and percentage change from baseline for the cross‐sectional area of different muscles. Babul 2003 and Germain 2003 did not report mean difference or standard deviations. Babul 2003 reported there was no difference in quadriceps circumference, measured at 10 cm and 20 cm above the patella, between the two groups at 24, 48 or 72 hours after exercise (mean difference in quadriceps circumference (10 cm level) at 72 hours between control and HBOT: MD ‐0.66 cm, 95% CI ‐6.52 to 5.19 cm). Germain 2003 reported there were no significant differences between the two groups or across time. Analysis 2.4 shows the results at different times for the other three trials testing immediate HBOT; there were no statistically significant differences between the HBOT and control groups for any trial. This was also the case for the results of delayed treatment in Harrison 2001 (seeAnalysis 2.5).
2.4. Analysis.
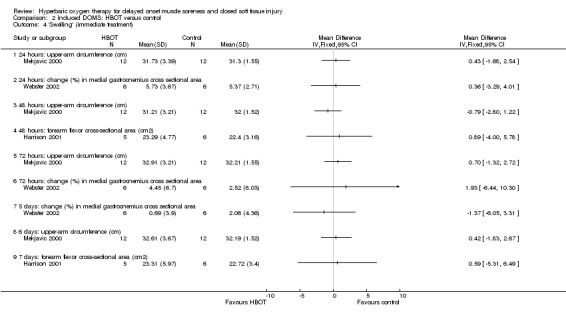
Comparison 2 Induced DOMS: HBOT versus control, Outcome 4 'Swelling' (immediate treatment).
2.5. Analysis.
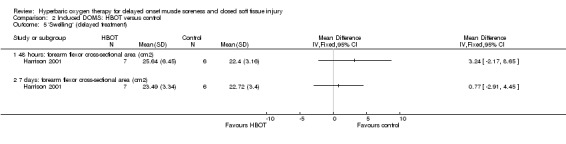
Comparison 2 Induced DOMS: HBOT versus control, Outcome 5 'Swelling' (delayed treatment).
Strength
The strength of the various muscles specifically exercised in the seven trials was measured and reported in several ways. Four trials (Babul 2003; Germain 2003; Staples 1999a; Staples 1999b) reported maximal eccentric quadriceps muscle torque in Newton metres (Nm). However, Babul 2003 did not report mean values or standard deviations in either group and therefore did not contribute to the pooled result. Germain 2003 did not report the numbers of participants in each group. Sensitivity analyses allowing for seven participants in the HBOT group and eight in the control group, or vice versa, in Germain 2003 showed non‐significant results between the two groups in muscle torque. Harrison 2001 reported the percentage of the initial isometric strength of forearm flexors, while Mekjavic 2000 reported the maximal isometric strength of elbow flexor muscles in kilopascals. Webster 2002 reported the percentage reduction of peak torque calf muscles.
Analysis 2.6 shows the pooled results at different times for five of the seven trials testing immediate HBOT: there were no statistically significant differences in the various measures between the HBOT and control groups for any trial. This was also the case for the results of delayed treatment in Harrison 2001 (seeAnalysis 2.7). The variety of measures used means that the actual pooled results in both these graphs are difficult to interpret. The main message is the lack of statistically significant difference as well as the low heterogeneity (I² = 0%) at all times for the two comparisons, with the one exception being the 96 hours results for the delayed HBOT versus control comparison (I² = 64%). It is also notable that recovery of muscle strength (88% versus 82%) was incomplete in both groups of Harrison 2001 by day 15. In contrast, the results of both groups of Webster 2002 exceeded their initial values (101.5% versus 103.9%) by day five. Babul 2003 reported there was no difference in maximal eccentric torque of the quadriceps muscle between the two groups at 24, 48 or 72 hours after exercise (mean difference in maximal eccentric torque at 72 hours between control and HBOT: MD ‐28.25 Nm, 95% CI ‐58.21 to 1.71 Nm).
2.6. Analysis.
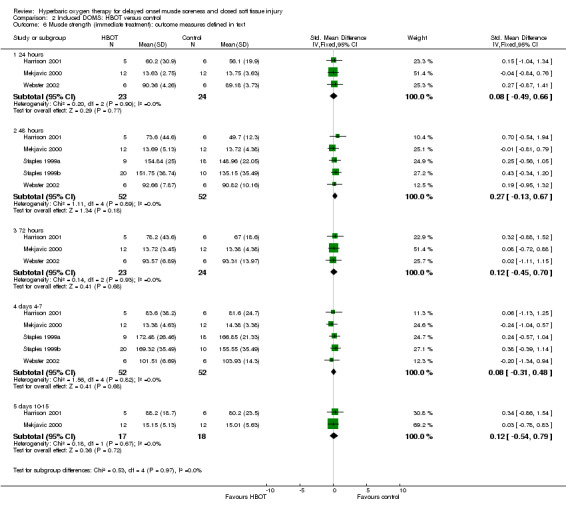
Comparison 2 Induced DOMS: HBOT versus control, Outcome 6 Muscle strength (immediate treatment): outcome measures defined in text.
2.7. Analysis.
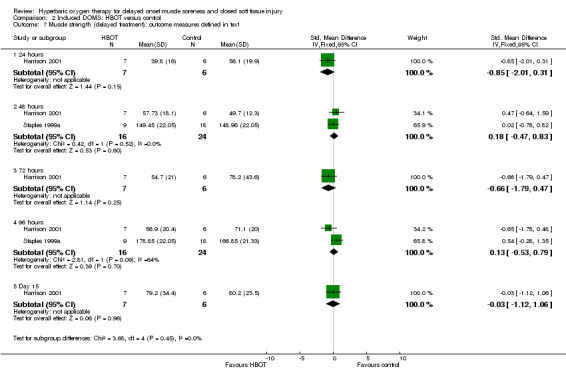
Comparison 2 Induced DOMS: HBOT versus control, Outcome 7 Muscle strength (delayed treatment): outcome measures defined in text.
Adverse effects of therapy
No trial reported complications of HBOT. The exclusion of one participant from Germain 2003 due to quadriceps muscle compartment syndrome occurred prior to treatment and is likely to have resulted from the exercise protocol.
Discussion
This review has included data from nine trials and we believe these represent all the randomised controlled clinical trials in this area, both published and unpublished, at the time of searching the databases. While we have made every effort to locate further unpublished data, it remains possible that this review is subject to a positive publication bias, with generally favourable trials more likely to achieve reporting. Given this possibility, the general lack of benefit detected for HBOT for the two trials of closed soft tissue injuries (ankle and knee sprains) or the seven trials of experimentally induced DOMS is notable. Another limitation is the lack of data on long‐term effects or on quality of life outcomes.
We located only nine trials with 219 participants in total, and there were substantially fewer participants in the data available for pooling. Two trials (Germain 2003; Soolsma 1996) failed to provide the numbers of each group included in their results: intention‐to‐treat analysis was not possible for these. Most of the data presented in the review graphs were estimated from graphs published in the trial reports. While clearly unsatisfactory, this was the best we could do in the absence of information from the trial investigators. The general scarcity of data precluded most subgroup analyses but also it is notable that, with few exceptions, the results of trials testing HBOT for DOMS were homogeneous. Other problems for this review were the failure to report on primary functional outcomes in many studies, poor reporting of means and standard deviations, the variable methods used for reporting similar outcomes across studies and the lack of data regarding the treatment of uncontrolled muscle injury. In particular, the concentration of these studies on a short‐term, self‐limiting injury with a 100% recovery rate (DOMS) demands a cautious interpretation of the results.
For ankle sprains, Borromeo 1997 reported no significant benefits in the return to previous activities, in time to recovery, in functional outcomes, pain or swelling. Though the authors found a statistically significant improvement for the HBOT group in the change scores for ankle function, the practical significance of this finding should be interpreted cautiously as this scale has not been validated and the clinical impact is difficult to assess.
The trial of HBOT for grade II medial collateral ligament injury conducted by Soolsma 1996 (only reported to date in an unpublished Master of Science thesis) has several features that remain unclear. Importantly, the number of participants allocated to each arm and the scales on which a number of the outcomes are measured are unknown. Our inability to conduct intention‐to‐treat analyses for this trial and the difficulties in assessing clinical significance severely restricts the usefulness of this small trial.
We found no evidence that HBOT improves the speed of recovery from delayed onset muscle soreness (DOMS) following eccentric exercise designed to cause this problem. There was some indication from the analysis of pooled data from four trials (Harrison 2001; Mekjavic 2000; Staples 1999a; Staples 1999b) that HBOT might actually hinder recovery from muscle soreness, (e.g. at 48 hours after injury there was a statistically significant difference in pain scores between the groups of 0.88 points on a 0 to 10 scale), while there was no evidence of improvement in swelling or the return of muscle strength. These results were relatively uniform, and subgroup analysis by the site of muscle injury did not suggest a differential effect of HBOT for different muscle groups. The clinical significance of the modest differences in reported pain is unclear. There were no differences between the two groups in any of the physical measures of swelling or muscle strength. Similarly, the response to treatment when HBOT was delayed for 24 hours did not suggest important effects in the relevant groups from Harrison 2001 and Staples 1999a.
None of these reviewed trials reported adverse effects with HBOT or control therapies, so we are unable to assess any negative impact of HBOT on the outcome of these patients other than the outcomes discussed above. HBOT is generally regarded as a relatively benign intervention. There are few major adverse effects (pulmonary barotrauma, drug reactions, injuries or death related to chamber fire). There are a number of more minor complications that may occur commonly. Visual disturbance, usually reduction in visual acuity secondary to conformational changes in the lens, is very commonly reported ‐ perhaps as many as 50% of those having a course of 30 treatments (Khan 2003). While the great majority of patients recover spontaneously over a period of days to weeks, a small proportion of patients continue to require correction to restore sight to pre‐treatment levels. The second most common adverse effect associated with HBOT is barotrauma. Barotrauma can affect any air‐filled cavity in the body (including the middle ear, lungs and respiratory sinuses) and occurs as a direct result of compression. Aural barotrauma is by far the most common as the middle ear air space is small, largely surrounded by bone and the sensitive tympanic membrane, and usually requires active effort by the patient in order to inflate the middle ear through the eustachian tube on each side. Barotrauma is thus not a consequence of HBOT directly, but rather of the physical conditions required to administer it. Most episodes of barotrauma are mild, easily treated or recover spontaneously and do not require the therapy to be abandoned. Less commonly, HBOT may be associated with acute neurological toxicity manifesting as seizure. While there was no report of adverse effects of HBOT in any of the included trials, a careful selection of participants was evident in most.
Authors' conclusions
Implications for practice.
There was insufficient evidence from comparisons tested within randomised controlled trials to establish the effects of HBOT on ankle sprain or acute knee ligament injury, or on experimentally induced DOMS. There was some evidence that HBOT may increase pain in DOMS. Thus, the use of HBOT in these patients cannot be justified by this review.
Implications for research.
Given the findings of this review, the self‐limiting nature of the injury and the availability of other interventions, there is little case for further investigation of HBOT as a possible therapy for DOMS. While more information may be useful on a range of real clinical injuries, subsets of injury severity and time of presentation, any further investigations would need to be carefully justified. The effect of differing oxygen dosage and effect of other therapies administered simultaneously is not known. Any future trials would need to consider in particular:
appropriate sample sizes with power to detect expected differences;
careful definition and selection of target patients;
appropriate range of oxygen doses per treatment session (pressure and time);
appropriate and carefully defined comparator therapy;
use of an effective sham therapy;
effective and explicit blinding of outcome assessors;
appropriate outcome measures including all those listed in this review;
careful elucidation of any adverse effects;
the cost‐utility of the therapy;
appropriate and full reporting.
What's new
| Date | Event | Description |
|---|---|---|
| 26 April 2010 | New search has been performed | For this update, published in Issue 6, 2010, the search was updated to February 2010. No new evidence was discovered. |
History
Protocol first published: Issue 2, 2004 Review first published: Issue 4, 2005
| Date | Event | Description |
|---|---|---|
| 9 September 2008 | Amended | Converted to new review format. |
Acknowledgements
The authors acknowledge Dr Michael Lepawsky for his assistance as co‐author on the original version of the review. They also acknowledge the support and suggestions of Kate Rowntree and the editors of the Cochrane Bone, Joint and Muscle Trauma Group for their assistance in the preparation of this review. In particular we acknowledge the help of Lesley Gillespie, Joanne Elliott and Lindsey Elstub for their tireless assistance and for developing and running the search strategies used. Helen Handoll greatly improved the quality of this review by repeating and modifying both data extraction and analysis, along with advice on interpretation during the appraisal stages of this review.
Appendices
Appendix 1. Search strategies
Cochrane CENTRAL Register of Controlled Trials (Wiley InterScience)
#1 MeSH descriptor Athletic Injuries, this term only #2 MeSH descriptor Soft Tissue Injuries, this term only #3 (arm* or leg* or muscle* or tendon* or ligament*):ti,ab,kw #4 (injur* or trauma* or lesion* or damage* or wound* or destruction* or oedema* or edema* or contusion* or concus* or commotion* or pressur* or soreness):ti,ab,kw #5 (#3 AND #4) #6 (#1 OR #2 OR #5) #7 MeSH descriptor Hyperbaric Oxygenation, this term only #8 (high* adj3 (pressure or tension*)):ti,ab,kw #9 (hyperbaric*):ti,ab,kw #10 (#8 OR #9) #11 (oxygen*):ti,ab,kw #12 (#10 AND #11) #13 (HBO or HBOT):ti,ab,kw #14 ((monoplace or multiplace) adj chamber*):ti,ab,kw #15 (#7 OR #12 OR #13 OR #14) #16 (#6 AND #15)
MEDLINE (OVID WEB)
1 Athletic Injuries/ 2 Soft Tissue Injuries/ 3 (arm$ or leg$ or muscle$ or tendon$ or ligament$).tw. 4 (injur$ or trauma$ or lesion$ or damage$ or wound$ or destruction$ or oedema$ or edema$ or contusion$ or concus$ or commotion$ or pressur$ or soreness).tw. 5 and/3‐4 6 or/1‐2,5 7 Hyperbaric Oxygenation/ 8 (high$ adj3 (pressure or tension$)).tw. 9 hyperbaric$.tw. 10 or/8‐9 11 oxygen$.tw. 12 and/10‐11 13 (HBO or HBOT).tw. 14 ((monoplace or multiplace) adj chamber$).tw. 15 or/7,12‐14 16 and/6,15 17 Randomized controlled trial.pt. 18 Controlled clinical trial.pt. 19 Randomized Controlled Trials/ 20 Random Allocation/ 21 Double Blind Method/ 22 Single Blind Method/ 23 or/17‐22 24 Animals/ not Humans/ 25 23 not 24 26 Clinical trial.pt. 27 exp Clinical Trials as topic/ 28 (clinic$ adj25 trial$).tw. 29 ((singl$ or doubl$ or trebl$ or tripl$) adj25 (blind$ or mask$)).tw. 30 Placebos/ 31 placebo$.tw. 32 random$.tw. 33 Research Design/ 34 or/26‐33 35 34 not 24 36 35 not 25 37 Comparative Study.pt. 38 Evaluation Studies.pt. 39 Follow Up Studies/ 40 Prospective Studies/ 41 (control$ or prospectiv$ or volunteer$).tw. 42 or/37‐41 43 42 not 24 44 43 not (25 or 36) 45 Or/25,36,44 46 And/16,45
EMBASE (OVID WEB)
1 Sport Injury/ 2 Soft Tissue Injury/ 3 (arm$ or leg$ or muscle$ or tendon$ or ligament$).tw. 4 (injur$ or trauma$ or lesion$ or damage$ or wound$ or destruction$ or oedema$ or edema$ or contusion$ or concus$ or commotion$ or pressur$ or soreness).tw. 5 and/3‐4 6 or/1‐2,5 7 Hyperbaric Oxygen/ 8 (high$ adj3 (pressure or tension$)).tw. 9 hyperbaric$.tw. 10 or/8‐9 11 oxygen$.tw. 12 and/10‐11 13 (HBO or HBOT).tw. 14 ((monoplace or multiplace) adj chamber$).tw. 15 or/7,12‐14 16 and/6,15 17 exp Randomized Controlled trial/ 18 exp Double Blind Procedure/ 19 exp Single Blind Procedure/ 20 exp Crossover Procedure/ 21 Controlled Study/ 22 or/17‐21 23 ((clinical or controlled or comparative or placebo or prospective$ or randomi#ed) adj3 (trial or study)).tw. 24 (random$ adj7 (allocat$ or allot$ or assign$ or basis$ or divid$ or order$)).tw. 25 ((singl$ or doubl$ or trebl$ or tripl$) adj7 (blind$ or mask$)).tw. 26 (cross?over$ or (cross adj1 over$)).tw. 27 ((allocat$ or allot$ or assign$ or divid$) adj3 (condition$ or experiment$ or intervention$ or treatment$ or therap$ or control$ or group$)).tw. 28 or/23‐27 29 or/22,28 30 limit 29 to Human 31 and/16,30
CINAHL (OVID WEB)
1 Athletic Injuries/ 2 Soft Tissue Injuries/ 3 (arm$ or leg$ or muscle$ or tendon$ or ligament$).tw. 4 (injur$ or trauma$ or lesion$ or damage$ or wound$ or destruction$ or oedema$ or edema$ or contusion$ or concus$ or commotion$ or pressur$ or soreness).tw. 5 and/3‐4 6 or/1‐2,5 7 Hyperbaric Oxygenation/ 8 (high$ adj3 (pressure or tension$)).tw. 9 hyperbaric$.tw. 10 or/8‐9 11 oxygen$.tw. 12 and/10‐11 13 (HBO or HBOT).tw. 14 ((monoplace or multiplace) adj chamber$).tw. 15 or/7,12‐14 16 and/6,15 17 exp Clinical Trials/ 18 exp Evaluation Research/ 19 exp Comparative Studies/ 20 exp Crossover Design/ 21 clinical trial.pt. 22 or/17‐21 23 ((clinical or controlled or comparative or placebo or prospective or randomi#ed) adj3 (trial or study)).tw. 24 (random$ adj7 (allocat$ or allot$ or assign$ or basis$ or divid$ or order$)).tw. 25 ((singl$ or doubl$ or trebl$ or tripl$) adj7 (blind$ or mask$)).tw. 26 (cross?over$ or (cross adj1 over$)).tw. 27 ((allocat$ or allot$ or assign$ or divid$) adj3 (condition$ or experiment$ or intervention$ or treatment$ or therap$ or control$ or group$)).tw. 28 or/23‐27 29 or/22,28 30 16 and 29
Data and analyses
Comparison 1. Acute ligament injury: HBOT versus sham HBOT.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Time to return to prior function (days) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Ankle function score (0 to 7: full function) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Score after final treatment (day 7) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Change in score (day 7 ‐ initial scores) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Subjective recovery scores after knee injury (0 to 100: full recovery) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 After 10 treatments (2 weeks) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 After 4 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 After 6 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Pain scores (0 to 100: worst pain) | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 After third treatment for ankle sprain | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 After five treatments (1 week) for medial collateral injury | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 After 10 treatments (2 weeks) for medial collateral injury | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.4 After 4 weeks (medial collateral injury) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Change in knee girth at 4 weeks post medial collateral ligament injury (cm) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6 One legged jump distance (difference between non‐injured and injured side) (cm) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.1 At completion of therapy | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 At 4 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 2. Induced DOMS: HBOT versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain score (10 = worst pain) after exercise (immediate treatment) | 4 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 24 hours | 4 | 92 | Mean Difference (IV, Fixed, 95% CI) | 0.33 [‐0.46, 1.13] |
| 1.2 48 hours | 4 | 92 | Mean Difference (IV, Fixed, 95% CI) | 0.88 [0.09, 1.67] |
| 1.3 72 hours | 4 | 92 | Mean Difference (IV, Fixed, 95% CI) | 0.72 [0.06, 1.37] |
| 1.4 Days 4 to 7 | 4 | 92 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.23, 0.26] |
| 2 Pain score (10 = worst pain) after exercise (immediate treatment) by muscle group at 48 hours | 4 | 92 | Mean Difference (IV, Fixed, 95% CI) | 0.88 [0.09, 1.67] |
| 2.1 Quadriceps muscles | 2 | 57 | Mean Difference (IV, Fixed, 95% CI) | 0.89 [‐0.04, 1.82] |
| 2.2 Forearm muscles | 2 | 35 | Mean Difference (IV, Fixed, 95% CI) | 0.85 [‐0.63, 2.34] |
| 3 Pain score (10 = worst pain) after exercise (delayed treatment) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 24 hours | 2 | 40 | Mean Difference (IV, Fixed, 95% CI) | 0.26 [‐0.66, 1.17] |
| 3.2 48 hours | 2 | 40 | Mean Difference (IV, Fixed, 95% CI) | 0.69 [‐0.25, 1.63] |
| 3.3 72 hours | 2 | 40 | Mean Difference (IV, Fixed, 95% CI) | 0.85 [0.06, 1.64] |
| 3.4 Days 4 or 7 | 2 | 40 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.60, 0.41] |
| 4 'Swelling' (immediate treatment) | 3 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 24 hours: upper‐arm circumference (cm) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 24 hours: change (%) in medial gastrocnemius cross sectional area | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 48 hours: upper‐arm circumference (cm) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.4 48 hours: forearm flexor cross‐sectional area (cm2) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.5 72 hours: upper‐arm circumference (cm) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.6 72 hours: change (%) in medial gastrocnemius cross sectional area | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.7 5 days: change (%) in medial gastrocnemius cross sectional area | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.8 6 days: upper‐arm circumference (cm) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.9 7 days: forearm flexor cross‐sectional area (cm2) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 'Swelling' (delayed treatment) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 48 hours: forearm flexor cross‐sectional area (cm2) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 7 days: forearm flexor cross‐sectional area (cm2) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Muscle strength (immediate treatment): outcome measures defined in text | 5 | Std. Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1 24 hours | 3 | 47 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.08 [‐0.49, 0.66] |
| 6.2 48 hours | 5 | 104 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.27 [‐0.13, 0.67] |
| 6.3 72 hours | 3 | 47 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.12 [‐0.45, 0.70] |
| 6.4 days 4‐7 | 5 | 104 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.08 [‐0.31, 0.48] |
| 6.5 days 10‐15 | 2 | 35 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.12 [‐0.54, 0.79] |
| 7 Muscle strength (delayed treatment): outcome measures defined in text | 2 | Std. Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.1 24 hours | 1 | 13 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.85 [‐2.01, 0.31] |
| 7.2 48 hours | 2 | 40 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.18 [‐0.47, 0.83] |
| 7.3 72 hours | 1 | 13 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.66 [‐1.79, 0.47] |
| 7.4 96 hours | 2 | 40 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.13 [‐0.53, 0.79] |
| 7.5 Day 15 | 1 | 13 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.03 [‐1.12, 1.06] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Babul 2003.
| Methods | Randomised (random‐numbers table), concealed allocation and patient and assessor blind. No report of intention‐to‐treat analysis or of exclusions. | |
| Participants | 16 healthy female volunteers. Underwent deliberately provocative exercise of non‐dominant quadriceps muscle. | |
| Interventions | HBOT
100% oxygen at 2.0 ATA for 60 minutes at 4, 24, 48 and 72 hours post‐injury. CONTROL Sham HBOT at 1.2 ATA on air on the same schedule. |
|
| Outcomes | PAIN SCORE
Visual analogue scale (0 to 10) STRENGTH Change from baseline expressed as maximum eccentric torque in Nm. SWELLING Tape measurement by blinded researcher at a standard point. expressed relative to pre‐injury value. |
|
| Notes | Schulz rating:
Randomisation ‐ A
Allocation concealment ‐ A
Completeness of outcome data ‐ A
Double‐blinding ‐ A
Very small study with multiple outcomes. Complex experimental design with 2 distinct phases with somewhat different therapy arms. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Borromeo 1997.
| Methods | Randomised, patient and assessor blind. Intention‐to‐treat analysis with 100% follow up. | |
| Participants | 32 adults (11 females) with lateral ankle sprain within 72 hours (mean 33 hours). Only rest, ice and elevation prior to enrolment. Excluded if fractured or specific contra‐indication to HBOT. | |
| Interventions | BOTH GROUPS
Posterior splint, crutches, NSAID, active ROM exercises, ankle stirrup. HBOT HBOT at 2.0 ATA on 100% oxygen for 90 minutes, 3 sessions over 7 days. CONTROL Sham HBOT exposure to 1.1 ATA breathing air for 90 minutes, 3 sessions over 7 days. |
|
| Outcomes | HEALED AT FINAL FOLLOW UP TIME TO NO FURTHER SYMPTOMS FUNCTIONAL SCORE (1) Time to reach maximum on 7 point scale. Recorded by blinded researcher. (2) Highest level attained on a 7 point functional scale. PAIN SCORE Visual analogue scale (0 to 10). SWELLING Assessed using a water displacement volumeter. |
|
| Notes | Schulz rating: Randomisation ‐ A
Allocation concealment ‐ B
Completeness of outcome data ‐ A
Double‐blinding ‐ A
Relatively long delay to treatment and small number of compressions. The researcher measuring outcomes underwent a comparison with three sports physicians using 10 other cases of ankle sprain. There were high inter‐observer regression coefficients for all physical measures on patient examination. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Germain 2003.
| Methods | Randomised, not blinded. Intention‐to‐treat status unknown with 1 missing participant not accounted for. | |
| Participants | 16 healthy volunteers (10 females). Underwent deliberately provocative exercise of quadriceps muscle. | |
| Interventions | HBOT
95% oxygen at 2.5 ATA for 100 minutes at 1 and 6 hours post injury, then 1 treatment the next day and 2 treatments on the next day separated by 6 hours. CONTROL Nil specific therapy. |
|
| Outcomes | PAIN SCORE
Visual analogue scale (0 to 100). STRENGTH Change from baseline measured as maximum torque measured in Nm. SWELLING Tape measure at standard point by blinded observer. |
|
| Notes | Schulz rating: Randomisation ‐ B Allocation concealment ‐ B Completeness of outcome data ‐ C Double‐blinding ‐ C Very small study with multiple outcomes. We do not know how many in each arm or which arm the missing volunteer was lost from. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Harrison 2001.
| Methods | Randomised, patient partial blinding. No intention‐to‐treat analysis but exclusions are accounted for. Complex experimental design with 2 active treatment groups. | |
| Participants | 21 healthy male volunteers. Underwent deliberately provocative exercise of elbow flexors. | |
| Interventions | HBOT (2 groups)
(1) Immediate HBOT: 100% oxygen at 2.5 ATA for 100 minutes. Treatments immediately post‐injury and 24, 48, 72 and 96 hours.
(2) Delayed HBOT. Immediate sham (on air at minimal pressure), then the same HBOT schedule as group 1. CONTROL No specific therapy. |
|
| Outcomes | PAIN SCORE
Verbally anchored 10 point scale (estimated from graphical representation). STRENGTH Change from baseline as maximum strength measured in kilograms. SWELLING Cross‐sectional area estimated from MRI in mm². |
|
| Notes | Schulz rating: Randomisation ‐ C Allocation concealment ‐ B Completeness of outcome data ‐ C Double‐blinding ‐ B Personal communication confirms partial blinding and random allocation. Pain scores were given only with SEM and have been converted to SD. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Mekjavic 2000.
| Methods | Randomised, patient and statistician blind. Intention‐to‐treat analysis with 100% follow up. | |
| Participants | 24 healthy male volunteers. Underwent deliberately provocative exercise of elbow flexors. | |
| Interventions | HBOT
Standard exercise protocol followed by 7 sessions in 100% oxygen for 60 minutes daily at 2.5 ATA. CONTROL Standard exercise protocol followed by 10 sessions in a sham hyperbaric treatment (2.5 ATA, 8% oxygen for 60 min), once daily. |
|
| Outcomes | PAIN SCORE
Visual analogue scale (0 to 10) (estimated from graphical representation). STRENGTH Change from baseline as maximal isometric strength in kilopascals before and for 10 days following exercise. Measured by blinded researcher. (Estimated from graphical representation.) SWELLING Arm circumference (cm) |
|
| Notes | Schulz rating:
Randomisation ‐ B
Allocation concealment ‐ B
Completeness of outcome data ‐ A
Double‐blinding ‐ A
Small study with multiple outcomes. Most point estimates are derived from graphs. The number of treatment sessions was 10 in the extended abstract report of this trial. We have taken the number (7) in the peer‐reviewed publication. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Soolsma 1996.
| Methods | Randomised, method not specified. Participant and assessor blind. No intention‐to‐treat analysis. | |
| Participants | 19 participants (5 females) with grade II injury to the medial collateral ligament of the knee suffered during sporting activity and confirmed by magnetic resonance imaging. All were adults, without history of similar injury or surgery to the knee and who presented to an orthopaedic surgeon within 72 hours of injury. 14 participants finished the clinical stage and 9 had final MRI. | |
| Interventions | BOTH GROUPS
Regular icing, stretching and strengthening exercise rehabilitation program.
Within 96 hours of injury participants received either: HBOT At 2.0 ATA on 100% oxygen for 60 minutes, 10 sessions over 2 weeks CONTROL Sham HBOT exposure to 1.2 ATA breathing air on the same schedule. |
|
| Outcomes | SUBJECTIVE RECOVERY INDEX
participants self‐completed a questionnaire. PAIN SCORE Visual analogue pain scale (0 to 10) (estimated from graphical representation). RANGE OF MOTION ONE‐LEGGED HOP TEST SWELLING Changes in girth measured with a tape and volume performed by blinded researcher from magnetic resonance imaging: pre‐ versus post‐treatment volumetric analysis with two blinded radiologists reaching consensus. FIGURE OF EIGHT PERFORMANCE TEST Time taken to complete a standard course, measured by a blinded researcher. |
|
| Notes | Schulz rating: Randomisation ‐ B Allocation concealment ‐ B Completeness of outcome data ‐ C Double‐blinding ‐ A Relatively long delay to treatment . | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Staples 1999a.
| Methods | Randomised, participant and probably assessor blind. No intention‐to‐treat analysis but exclusions were reported (see next section). | |
| Participants | 49 healthy male volunteers. Underwent deliberately provocative exercise of non‐dominant quadriceps muscle. Nine individuals were randomised but did not complete the study (recent respiratory track infection or confinement anxiety). There was no indication to which group these participants were allocated. Four participants, one from each group, excluded because of abnormal response to exercises: increased muscle torque, | |
| Interventions | Phase 1 (see Notes) HBOT (2 groups) (1) 100% oxygen at 2.0 ATA for 1 hour at 0, 24 and 48 hours after exercise, followed by two sham treatments at 72 and 96 hours. (2) Sham at 0 and 24 hours, followed by HBOT at 48, 72 and 96 hours. CONTROL (2 groups) (1) No specific intervention. (2) Sham HBOT by exposure to 1.2 ATA breathing air at 0, 24, 48, 72 and 96 hours for one hour on each occasion. |
|
| Outcomes | PAIN SCORE
Visual analogue (0 to 10) (estimated from graphical representation). STRENGTH Change from baseline as maximal eccentric torque measured in Nm (estimated from graphical representation). |
|
| Notes | Schulz rating:
Randomisation ‐ B
Allocation concealment ‐ B
Completeness of outcome data ‐ C
Double‐blinding ‐ A
Complex protocol makes interpretation difficult. Most point estimates are derived from graphs with means and SEM.
Where results have been given with standard errors, these have been converted to standard deviations. Complex experimental design with 2 distinct phases: see Staples 1999b for phase 2. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Staples 1999b.
| Methods | Randomised, participant and probably assessor blind. Intention‐to‐treat analysis and no exclusions. | |
| Participants | 30 healthy male volunteers. Underwent deliberately provocative exercise of non‐dominant quadriceps muscle. | |
| Interventions | Phase 2 (see Notes) HBOT (2 groups) (1) 100% oxygen at 2.0 ATA for 1 hour at 0, 24 and 48 hours after exercise, followed by two sham treatments at 72 and 96 hours. (2) same HBOT on five occasions at 0, 24, 48, 72 and 96 hours. CONTROL Sham HBOT by exposure to 1.2 ATA breathing air at 0, 24, 48, 72 and 96 hours for one hour on each occasion. |
|
| Outcomes | PAIN SCORE
Visual analogue (0 to 10) (estimated from graphical representation). STRENGTH Change from baseline as maximal eccentric torque measured in Nm (estimated from graphical representation). |
|
| Notes | Schulz rating:
Randomisation ‐ B
Allocation concealment ‐ B
Completeness of outcome data ‐ C
Double‐blinding ‐ A
Complex protocol makes interpretation difficult. Most point estimates are derived from graphs with means and SEM.
Where results have been given with standard errors, these have been converted to standard deviations. Complex experimental design with 2 distinct phases: see Staples 1999a for phase 1. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Webster 2002.
| Methods | Randomised, patient and assessor blind. Intention‐to‐treat analysis with 100% follow up. | |
| Participants | 12 healthy young male volunteers. Underwent deliberately provocative exercise of gastrocnemius muscle. | |
| Interventions | HBOT
100% oxygen at 2.5 ATA for 60 minutes at 3, 24 and 48 hours post‐injury. CONTROL Sham HBOT at 1.3 ATA on air on the same schedule. |
|
| Outcomes | PAIN SCORE
Descriptor differential scale expressed as percentage changes compared to maximal pain. (Data not used due to difficulties in interpretation.) STRENGTH Change from baseline as maximal eccentric torque expressed as percentage (estimated from graphical representation). SWELLING Per cent change in cross‐sectional area of medial gastrocnemius (estimated from graphical representation). |
|
| Notes | Schulz rating: Randomisation ‐ B Allocation concealment ‐ B Completeness of outcome data ‐ A Double‐blinding ‐ A Very small study with multiple outcomes. Most point estimates are derived from graphs: those for pain were not used ‐ see preceding section. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
ATA: atmospheres absolute HBOT: hyperbaric oxygen therapy MRI: magnetic resonance imaging Nm: Newton metres NSAID: non‐steroidal anti‐inflammatory drug ROM: range of movement SEM: standard error of the mean SD: standard deviation
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Todorovic 1996 | Poorly reported trial with mention of a control group, but no indication of allocation method. Heterogeneous injuries and no standard number of HBO exposures. Author contact attempted but not successful. |
HBO: hyperbaric oxygen
Contributions of authors
Bennett: Original conception and principal author. Handsearching and study identification, critical appraisal and data extraction. Compilation and completion of the first draft and subsequent versions of the review. Content expert in hyperbaric medicine and clinical epidemiology.
Babul‐Weller: Co‐author background and editorial input to discussion. Content expert in sports medical research.
Best: Co‐author background, results and discussion, and content expert in sports medicine.
Taunton: Co‐author background, critical appraisal and data extraction. Content expert in sports medicine.
Helen Handoll (editor of Cochrane Bone, Joint and Muscle Trauma Group): data extraction and restructuring of the results section during editorial review. [Significant contribution without authorship]
Sources of support
Internal sources
Faculty of Medicine, University of New South Wales, Australia.
External sources
No sources of support supplied
Declarations of interest
Dr Bennett is a hyperbaric physician who does not routinely treat soft‐tissue injuries or delayed onset muscle soreness. Several included trials have been conducted by authors of this review (JB, SB, JT, ML). These individuals were not involved in the selection or critical appraisal of their own studies.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Babul 2003 {published data only}
- Babul S. The effects of intermittent exposure to hyperbaric oxygen for the treatment of an acute soft tissue injury (PhD). The University of British Columbia (Canada), 2001. [DOI] [PubMed] [Google Scholar]
- Babul S. personal communication May 14 2005.
- Babul S. Hyperbaric oxygen therapy to enhance recovery from delayed onset muscle soreness [abstract]. Clinical Journal of Sport Medicine 2000;10(4):308. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Babul S, Rhodes EC, Taunton JE, Lepawsky M. Effects of intermittent exposure to hyperbaric oxygen for the treatment of an acute soft tissue injury. Clinical Journal of Sport Medicine 2003;13(3):138‐47. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Borromeo 1997 {published data only}
- Borromeo CN, Ryan JL, Marchetto PA, Peterson R, Bove AA. Hyperbaric oxygen therapy for acute ankle sprains. American Journal of Sports Medicine 1997;25(5):619‐25. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Germain 2003 {published data only}
- Germain G, Delaney J, Moore G, Lee P, Lacroix V, Montgomery D. Effect of hyperbaric oxygen therapy on exercise‐induced muscle soreness. Undersea & Hyperbaric Medicine 2003;30(2):135‐45. [MEDLINE: ] [PubMed] [Google Scholar]
Harrison 2001 {published data only}
- Harrison BC. personal communication March 21 2005.
- Harrison BC, Robinson D, Davison BJ, Foley B, Seda E, Byrnes WC. Treatment of exercise‐induced muscle injury via hyperbaric oxygen therapy. Medicine & Science in Sports and Exercise 2001;33(1):36‐42. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mekjavic 2000 {published data only}
- Mekjavic IB, Exner JA, Tesch PA, Eiken O. Hyperbaric oxygen therapy does not affect recovery from delayed onset muscle soreness. Medicine & Science in Sports & Exercise 2000;32(3):558‐63. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Mekjavic IB, Exner JA, Tesch PA, Eiken O. Recovery of exercise‐induced loss of muscle strength and muscle soreness is unaffected by HBO therapy. Proceedings of the International Joint Meeting on Hyperbaric and Underwater Medicine. Instituto Ortopedico Galeazzi Milano. Bologna: Graphica Victoria, 1996:557‐9.
Soolsma 1996 {unpublished data only}
- Soolsma SJ. The effect of intermittent hyperbaric oxygen on short term recovery from grade II medial collateral ligament injuries [thesis]. Vancouver (CA): Univ. of British Columbia, 1996. [Google Scholar]
- Soolsma SJ, Clement DB, Connell DC, McKenzie DC, Taunton JB, Staples JR, et al. The effect of intermittent hyperbaric oxygen on short term recovery from grade II medial collateral injuries [abstract]. Clinical Journal of Sport Medicine 1997;7(3):240. [Google Scholar]
Staples 1999a {published data only}
- Staples JR, Clement DB, Taunton JE, McKenzie DC. Effects of hyperbaric oxygen on a human model of injury. American Journal of Sports Medicine 1999;27(5):600‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Staples 1999b {published data only}
- Staples JR, Clement DB, Taunton JE, McKenzie DC. Effects of hyperbaric oxygen on a human model of injury. American Journal of Sports Medicine 1999;27(5):600‐5. [DOI] [PubMed] [Google Scholar]
Webster 2002 {published data only}
- Webster AL, Syrotuik DG, Bell GJ, Jones RL, Hanstock CC. Effects of hyperbaric oxygen on recovery from exercise‐induced muscle damage in humans. Clinical Journal of Sport Medicine 2002;12(3):139‐50. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Todorovic 1996 {published data only}
- Todorovic BS. Sports injuries and hyperbaric oxygen [abstract]. Proceedings of the International Joint Meeting on Hyperbaric and Underwater Medicine. Instituto Ortopedico Galeazzi Milano. Bologna: Graphica Victoria, 1996:561‐4.
Additional references
Babul 2000a
- Babul S, Rhodes EC. The role of hyperbaric oxygen therapy in sports medicine. Sports Medicine 2000;30(6):395‐403. [DOI] [PubMed] [Google Scholar]
Babul 2000b
- Babul S. Hyperbaric oxygen therapy to enhance recovery from delayed onset muscle soreness [abstract]. Clinical Journal of Sport Medicine 2000;10(4):308. [DOI] [PubMed] [Google Scholar]
Babul 2001
- Babul S. The effects of intermittent exposure to hyperbaric oxygen for the treatment of an acute soft tissue injury (PhD). The University of British Columbia (Canada), 2001. [DOI] [PubMed] [Google Scholar]
Babul 2005
- Babul S. personal communication May 14 2005.
Bennett 2003
- Bennett M. The database of randomised controlled trials in hyperbaric medicine. http://www.hboevidence.com/ (accessed 10/12/03).
Cheung 2003
- Cheung K, Hume P, Maxwell L. Delayed onset muscle soreness: treatment strategies and performance factors. Sports Medicine 2003;33(2):145‐64. [DOI] [PubMed] [Google Scholar]
Finnegan 2003
- Finnegan M. Injury prevention: Editorial comment. Clinical Orthopaedics and Related Research 2003;(409):2. [Google Scholar]
Higgins 2006
- Higgins JPT, Green S, editors. Highly sensitive search strategies for identifying reports of randomized controlled trials in MEDLINE. Cochrane Handbook for Systematic Reviews of Interventions 4.2.6 [updated September 2006]; Appendix 5b. www.cochrane.org/resources/handbook/hbook.htm (accessed 01 May 2007).
Hills 1999
- Hills BA. A role for oxygen‐induced osmosis in hyperbaric oxygen therapy. Medical Hypotheses 1999;52(3):259‐63. [DOI] [PubMed] [Google Scholar]
Jain 2009
- Jain KK. In: Jain KK editor(s). Textbook of hyperbaric medicine. 5th Edition. Seattle: Hogrefe and Huber, 2009. [MEDLINE: ] [Google Scholar]
James 1993
- James PB, Scott B, Allen MW. Hyperbaric oxygen therapy in sports injuries. Physiotherapy 1993;79(8):571‐2. [Google Scholar]
Kader 2002
- Kader D, Saxena A, Movin T, Maffulli N. Achilles tendinopathy: some aspects of basic science and clinical management. British Journal of Sports Medicine 2002;36(4):239‐49. [DOI] [PMC free article] [PubMed] [Google Scholar]
Khan 2003
- Khan B, Evans AW, Easterbrook M. Refractive changes in patients undergoing hyperbaric oxygen therapy: a prospective study. Undersea and Hyperbaric Medicine 2003;24(Suppl):9. [Google Scholar]
Kindwall 2008
- Kindwall EP, Whelan HT, editors. Hyperbaric Medicine Practice. 3rd Edition. Flagstaff, AZ: Best Publishing Company, 2008. [Google Scholar]
Leach 1998
- Leach RE. Hyperbaric oxygen therapy in sports. American Journal of Sports Medicine 1998;26(4):489‐90. [PubMed] [Google Scholar]
Mekjavic 1996
- Mekjavic IB, Exner JA, Tesch PA, Eiken O. Recovery of exercise‐induced loss of muscle strength and muscle soreness is unaffected by HBO therapy [abstract]. Proceedings of the International Joint Meeting on Hyperbaric and Underwater Medicine. Instituto Ortopedico Galeazzi Milano. Bologna: Graphica Victoria, 1996:557‐9.
Neuman 2008
- Neuman T, Thom S. Physiology and medicine of hyperbaric oxygen therapy. 1st Edition. Philadelphia: Saunders, 2008. [Google Scholar]
Nylander 1985
- Nylander G, Lewis D, Nordstrom H, Larsson J. Reduction of postischemic edema with hyperbaric oxygen. Plastic and Reconstructive Surgery 1985;76(4):596‐603. [DOI] [PubMed] [Google Scholar]
Oriani 1982
- Oriani G, Barnini C, Marroni G. Hyperbaric oxygen therapy in the treatment of various orthopedic disorders [L'ossigenoterapia iperbarica nel trattamento di alcune patologie ortopediche]. Minerva Medica 1982;73(42):2983‐8. [PubMed] [Google Scholar]
Oriani 1996
- Oriani G, Marroni A, Wattel F. In: Oriani G, Marroni A, Wattel F editor(s). Handbook on hyperbaric medicine. 1st Edition. Milan: Springer, 1996. [MEDLINE: ] [Google Scholar]
Perryman 2002
- Perryman JR, Hershman EB. The acute management of soft tissue injuries of the knee. Orthopedic Clinics of North America 2002;33(3):575‐85. [DOI] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408‐12. [DOI] [PubMed] [Google Scholar]
Soolsma 1997
- Soolsma SJ, Clement DB, Connell DC, McKenzie DC, Taunton JB, Staples JR, et al. The effect of intermittent hyperbaric oxygen on short term recovery from grade II medial collateral injuries [abstract]. Clinical Journal of Sport Medicine 1997;7(3):240. [Google Scholar]
Staples 1995
- Staples JR, Clement DB, McKenzie DC. The effects of intermittent hyperbaric oxygen on biochemical muscle metabolites of eccentrically exercised rats [abstract]. Canadian Journal of Applied Physiology 1995;20 Suppl:49. [Google Scholar]
Thom 1994
- Thom SR, Mendiguren H, Nebolon M, Campbell D, Kilpatrick L. Temporary inhibition of human neutrophil B2 intgrin function by hyperbaric oxygen [abstract]. Clinical Research 1994;42:130A. [Google Scholar]
Van Mechelen 1997
- Mechelen W. The severity of sports injuries. Sports Medicine 1997;24(3):176‐80. [DOI] [PubMed] [Google Scholar]


