Abstract
As is widely recognized, CD8+ cytotoxic T lymphocytes (CTLs) play a crucial role in hepatitis C virus (HCV) infection, both in pathogenesis of liver injury and in clearing the virus. CTL studies with HCV-infected patients have been difficult because of the relatively low frequency of CTL precursors in the peripheral blood and because the targeted epitopes vary depending on the human leukocyte antigen (HLA) types of the individuals. This study attempts to overcome these problems by assessing the feasibility of using autologous peripheral blood mononuclear cells (PBMCs) expressing viral antigens as stimulators or targets in order to monitor the CTL responses. Primary PBMCs were transduced using a retroviral vector pseudotyped with a vesicular stomatitis virus G glycoprotein expressing the HCV core gene. Additionally, the vector-transduced PBMCs were used as targets of CTL assays to measure the HCV core-specific CTL activities from the liver-infiltrating lymphocytes of six different HLA-type patients with chronic HCV infection. The core-expressing PBMCs also served as stimulators, allowing us to measure core-specific CD8+ T-cell responses by intracellular gamma interferon staining of the peripheral blood of hepatitis C patients who had received treatment with alpha interferon plus ribavirin. This approach provides an efficient means of measuring antigen-specific CTL responses without HLA constraints.
Hepatitis C virus (HCV) infection often persists despite the generation of virus-specific antibodies and T-cell responses (5, 11, 14, 24). Mounting evidence suggests that T-cell responses are vital in determining the outcome of viral infection (22). Particularly, cytotoxic T lymphocytes (CTLs) may be crucial to the injury of liver cells (3) as well as clearance of the virus (30). In chronically HCV-infected patients, CTLs have been recovered from both the liver (22, 23, 40) and the peripheral blood (5, 14, 38). The frequency of HCV-specific CTL precursors in the peripheral blood appears to be low, making it extremely difficult to measure CTL activity by conventional 51Cr release assays. Although HCV-specific CTLs are more frequent in the liver (22, 23, 40), the limited number of intrahepatic lymphocytes recovered from liver biopsies precludes the possibility of cytolysis functional assays of the CTLs without in vitro expansion of the reactivated T cells (31, 40).
Peripheral blood mononuclear cells (PBMCs) are predominantly resting, but they can be activated in vitro by various methods. Activation of PBMCs with phytohemagglutinin A (PHA) and recombinant interleukin-2 (rIL-2) leads to a cell population comprised mostly of T cells (9). T cells could be promising candidates for serving as autologous stimulators and/or targets of CTLs, since they express high levels of major histocompatibility complex (MHC) class I molecules on the cell surface, as long as proper costimulation signals are provided.
The development of vectors for transducing genes into primary PBMCs, e.g., murine amphotropic retroviral vectors (10, 15, 27, 36), adeno-associated viral vectors (29), and nonviral vectors (20, 39), has received considerable interest. However, PBMCs appear to be refractory to most of these viral or nonviral gene transfer methods, except for retroviral vectors. Although murine amphotropic retroviral vectors can infect human cells, the transduction efficiency in primary PBMCs is low owing to the low viral titer. Pesudotyping the Molony murine leukemia virus (MoMLV)-based retroviral vector with vesicular stomatitis virus G protein (VSV-G) (12) gives a much broader host range than the conventional retrovirus and is more stable, thus permitting concentration by ultracentrifugation without loss of infectivity (4, 16). Research has demonstrated that retroviruses pseudotyped with VSV-G can achieve efficient gene transfer in human T lymphocytes (17, 35).
In this study, concentrated VSV-G-pseudotyped retroviruses expressing the HCV core protein, or a control green fluorescence protein (GFP), were used to infect human primary PBMCs. Analysis of the results indicated that with the gene-transduced PBMCs as autologous targets, core-specific CTL activities could be detected in the liver-infiltrating lymphocytes (LILs) of six HCV-infected patients. Additionally, a 6-h stimulation with autologous PBMCs expressing the HCV core antigen activated core-specific CD8+ T cells from the peripheral blood of HCV-infected patients treated with alpha interferon (IFN-α) plus ribavirin, as indicated by the production of IFN-γ. The effectiveness of this strategy for monitoring CD8+ T-cell responses from the peripheral blood of HCV-infected patients irrespective of their HLA types allows us to elucidate the roles of CTLs in the pathogenesis and clearance of the virus during chronic HCV infection.
MATERIALS AND METHODS
Cell lines.
GP+AM12, an amphotropic packaging cell line (26), was maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal calf serum (FCS). The prepackaging cell line for VSV-G-pseudotyped retrovirus, PtG-S2, was a derivative of human fibrosarcoma cell line HT1080, the genome of which carries the gag and pol genes from MoMLV and a VSV-G gene that is silent before a Cre recombinase is introduced (2). The cell line was maintained in DMEM supplemented with 10% FCS in the presence of 4 μg of blasticidin S per ml and 1 μg of G418 (Sigma) per ml. All of these cells were kept at 37°C in a 5% CO2 incubator.
Preparation and production of VSV-G-pseudotyped retroviruses.
The cDNA fragment encoding GFP or the HCV core antigen (C191) was cloned at the multiple cloning sites of a bicistronic retroviral vector, S2 (Fig. 1A) (19). Twenty micrograms of the resulting constructs (GFP/S2 and C191/S2) or the control vector (S2) was transfected into the GP+AM12 cells using the calcium phosphate precipitation method. Stable clones producing the amphotropic retroviruses were generated by selection with 0.8 mg of G418 per ml.
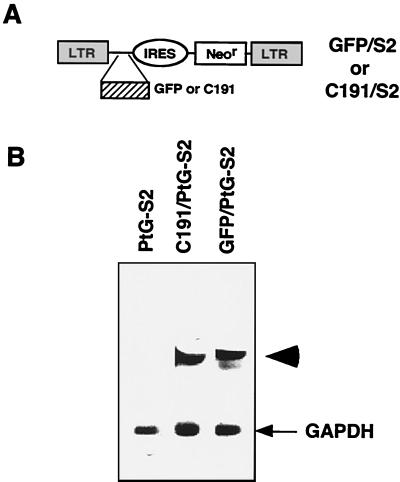
FIG. 1.
Preparation of prepackaging cell line for generating VSV-G-pseudotyped retroviruses. (A) MoMLV-based retroviral vector. The bicistronic retroviral vector, S2 (19), contains an internal ribosome entry site (IRES) derived from the swine vesicular disease virus, thereby allowing the Neor gene to be translated internally. The cDNA of GFP or HCV core antigen (C191) was cloned at the first cistron in front of the IRES, yielding GFP/S2 or C191/S2, respectively. (B) Expression of vector RNA in the prepackaging cell line. The GFP/S2 or C191/S2 viral vector was transduced into the PtG-S2 cell line via an amphotropic retroviral infection (see Materials and Methods). A single clone that expressed the highest levels of vector RNA was selected as indicated by Northern blot analysis. The data show the RNAs from the clones selected, which were hybridized with Neor and glyceraldehyde phosphate dehydrogenase (GAPDH) probes. The arrowhead indicates the vector RNA; the arrow indicates the GAPDH RNA.
Amphotropic retroviruses were used to infect PtG-S2 cells in the presence of 8 μg of Polybrene per ml for 18 h. The cells were infected four times over 2 weeks, at 3-day intervals. The retrovirus-infected PtG-S2 cells were seeded on plates at a low cell density to allow isolated colonies to form. Each selected clone was examined for the expression of GFP or core gene by Northern blot analysis, and the clone producing the highest levels of vector RNA was used as the virus-producing clone. To prepare VSV-G-pseudotyped retroviruses (S2/VSV-G, GFP/VSV-G, or C191/VSV-G), the S2 vector-, GFP-, or core-expressing PtG-S2 cells were infected with an adenovirus containing a Cre gene (AxCANCre) (2) at a multiplicity of infection (MOI) of 10 for 18 h and then washed twice with phosphate-buffered saline (PBS) and refed with DMEM. Culture supernatant was collected daily from day 2 to day 5 after AxCANCre infection, and the titers were determined on NIH 3T3 cells. Titers of 1 × 106 to 2 × 106 CFU/ml for days 2 and 3 and of 5 × 105 to 1 × 106 CFU/ml for days 4 and 5 could generally be obtained from the culture supernatant (data not shown).
The PtG-S2 cells harboring GFP/S2 or C191/S2 vector were seeded in 30 T-162 flasks (107 cells per flask) to produce large quantities of VSV-G-pseudotyped retrovirus. Cells were infected with the AxCANCre adenovirus at an MOI of 10 for 18 h. From day 2 to day 5 after adenovirus infection, the culture supernatant was collected daily, pooled, and then centrifuged in an SW28 rotor (Beckman) at 20,000 rpm for 2 h at 4°C. The supernatant was removed by decantation, and viral particles were resuspended in a small volume of serum-free DMEM. Viral titers were determined on NIH 3T3 cells, and the virus stock was stored at −80°C until use.
Patients.
Blood and liver specimens were obtained from patients with chronic HCV infection who attended the outpatient clinics of National Taiwan University Hospital. Liver tissues used to prepare LILs were obtained by needle aspiration from six hepatitis C patients and one hepatitis B patient. Their PBMCs were concurrently isolated and prepared for CTL assays. PBMCs were isolated from 16 HCV-infected patients receiving treatment with IFN-α plus ribavirin and from five HBV-positive, HCV-negative control patients. These PBMCs were then used to analyze functional CD8+ T-cell responses by intracellular IFN-γ assays. All specimens were collected with the informed consent of the patients.
Preparation of PBMCs and LILs.
The PBMCs were separated from whole blood using Ficoll-Hypaque density gradient centrifugation. Cells were resuspended in RPMI 1640 supplemented with 10% FCS (complete medium). To prepare autologous stimulators or target cells, PBMCs were first grown in a complete medium containing 0.5 μg of PHA (Sigma) per ml and 20 ng of rIL-2 per ml for 2 days. The PHA-stimulated PBMCs either were frozen at −80°C until use or were infected immediately with cell-free VSV-G-pseudotyped retroviruses at the MOI indicated in the presence of 8 μg of Polybrene per ml for 18 h. The infected cells were washed twice with PBS and then incubated in complete RPMI 1640 medium supplemented with rIL-2 (20 ng/ml) for another 4 days. The virus-infected PBMCs could serve as autologous stimulators or target cells. Freshly isolated PBMCs were used in parallel as effectors and were cocultured with the aforementioned antigen-expressing autologous stimulators for intracellular IFN-γ assays (see below).
The LILs were isolated from liver biopsy cores (2- to 4-mm-diameter fragments). The tissue fragments were washed three times with RPMI 1640 medium, cut into small pieces, and then incubated in RPMI 1640 supplemented with PHA (2 μg/ml) and rIL-2 (20 ng/ml) for 2 days to release LILs from the liver tissue. The PHA-stimulated LILs were then recovered from dishes, cultured in fresh RPMI 1640 medium containing only rIL-2 (20 ng/ml) for another 4 days, and used as effector cells in the CTL assays (see below).
Cytotoxicity assays.
The GFP/VSV-G or C191/VSV-G retrovirus-infected PBMCs (GFP/PBMCs or C191/PBMCs, respectively) were labeled with 51Cr for 1 h. The PHA-stimulated LILs (effectors) were incubated with 5 × 103 51Cr-labeled GFP/PBMCs or C191/PBMCs (targets) in 96-well round-bottom microtiter plates at an effector/target ratio of 1, 5, or 10. The cytotoxicity was measured using a standard 4-h 51Cr release assay (8).
Intracellular core or INF-γ staining.
To detect HCV core antigen expression, C191/VSV-G retrovirus-infected PBMCs were washed twice with PBS containing 0.5% (wt/vol) bovine serum albumin (Sigma) and fixed with PBS containing 4% (vol/vol) paraformaldehyde (Sigma) at 4°C overnight. After being washed with PBS, cells were permeabilized with PBS containing 0.5% (wt/vol) saponin (Sigma) and 2% bovine serum albumin for 10 min at room temperature and incubated with mouse anti-HCV core antibodies for 30 min at 4°C. After being washed with a permeabilizing buffer, the cells were further incubated with fluorescein isothiocyanate-conjugated rat anti-mouse immunoglobulin G (PharMingen) for 30 min at 4°C and then washed as described above and analyzed with a FACScan (Becton Dickinson).
Intracellular IFN-γ detection was performed as previously described (32). Fresh PBMCs (105) were incubated with either mitogen (20 ng of PHA per ml and 1 μM ionomycin) or 105 irradiated (3,000 rads) VSV-G-pseudotyped retrovirus-infected PBMCs (GFP/PBMCs or C191/PBMCs) at 37°C for 6 h in the presence of 2 μM monesin (Sigma) and 1 μg of anti-human CD28 antibody (PharMingen) per ml. The PBMCs were washed twice with PBS and then stained with fluorescein isothiocyanate-conjugated mouse anti-human CD8 antibodies (PharMingen) for 30 min at 4°C. The PBMCs were then fixed and permeabilized as described for intracellular core staining. The PBMCs were incubated with 0.25 μg of phycoerythrin-conjugated mouse anti-human IFN-γ antibodies (PharMingen) for 30 min at 4°C. Cells were washed twice with PBS and analyzed with a FACScan. The percentage of functional CD8+ cells was defined as [(IFN-γ+ CD8+)/ (IFN-γ+ CD8+) + (IFN-γ− CD8+)] × 100%. The percentage of core-specific functional T cells was defined as (PC191/PBMCs − PGFP/PBMCs), where PC191/PBMCs and PGFP/PBMCs represent the percentages of functional CD8+ T cells stimulated by C191/PBMCs and GFP/PBMCs, respectively.
RESULTS
Preparation of VSV-G-pseudotyped retroviruses.
The VSV-G-pseudotyped retroviruses were prepared from a stable VSV-G-prepackaging cell line, PtG-S2, which constitutively expresses the gag-pol genes of MoMLV and contains an inducible expression unit for VSV-G. From this unit, infection of PtG-S2 by an adenovirus containing a Cre gene (AxCANCre) could induce the expression of VSV-G (21). To produce higher titers of VSV-G-pseudotyped retroviruses from the prepackaging cell line, the MoMLV-based retroviral vectors S2, GFP/S2, and C191/S2 (Fig. 1A) were transfected into the GP+AM12 cells to generate amphotropic retroviruses. The resulting amphotropic retroviruses were in turn used to infect PtG-S2 cells to generate S2/VSV-G-, GFP/VSV-G-, or C191/VSV-G-pseudotyped retroviruses. Multiple cycles of infection of PtG-S2 cells with amphotropic retroviruses were performed to increase the copy numbers of transduced genes in the prepackaging cell line. A single clone that expressed the highest levels of vector RNA was selected as indicated by Northern blot analysis (Fig. 1B) and then expanded and infected with the AxCANCre virus. The VSV-G-pseudotyped retroviruses were collected from culture supernatant from day 2 to day 5 after AxCANCre infection and concentrated by ultracentrifugation to make high-titer stocks (usually in excess of 108 CFU/ml on NIH 3T3 cells) which were stable when stored at −80°C.
Optimization of VSV-G-pseudotyped retroviral vector-mediated gene transfer to PBMCs.
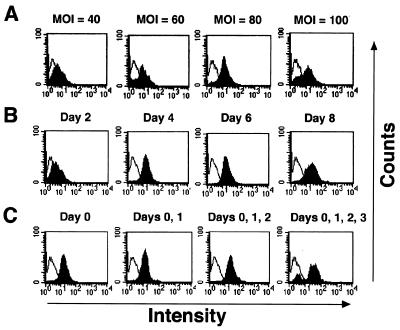
Productive MoMLV infection requires the division of target cells. Isolated PBMCs were therefore in vitro activated with PHA and rIL-2 for 48 h to induce proliferation. The optimal MOI was determined in order to increase the infection efficiency on the PBMCs. The PHA-activated PBMCs were infected with GFP/VSV-G retroviruses at different MOIs. Expression of GFP was examined 4 days after infection. The mean fluorescence intensity increased with MOI and stabilized at an MOI of 100 (Fig. 2A), at which point cell viability was severely compromised (fewer than 65% of the cells survived [data not shown]). Since cell viability might affect the readout of CTL assays later on, we chose an MOI of 80 for PBMC infection.
FIG. 2.
Optimizing the VSV-G-pseudotyped retroviral infection on primary PBMCs. A GFP/VSV-G retrovirus was used to determine the optimal conditions for infecting PBMCs. (A) Optimal MOI. Following isolation, PBMCs were stimulated with PHA and rIL-2 for 2 days and then infected with GFP/VSV-G retroviruses at different MOIs. Expression of GFP was analyzed on day 4 after retroviral infection with a FACScan. (B) Expression period. PBMCs were infected with GFP/VSV-G retroviruses at an MOI of 80, and GFP expression was measured at different time points after retroviral infection. (C) Infection frequency. The cells were infected with GFP/VSV-G retroviruses at an MOI of 80 for 1, 2, 3, or 4 consecutive days starting from day 0, and GFP expression was measured on day 4. The white peaks are the PBMCs infected with S2/VSV-G control retroviruses; the black peaks are the PBMCs infected with GFP/VSV-G retroviruses.
The expression of a transduced gene in PBMCs was monitored at different time points following retroviral infection. Although the expression levels remained steady on days 4, 6, and 8 after retroviral infection (Fig. 2B), cell viability markedly decreased on day 6. Therefore, PBMCs were harvested for functional analysis on day 4 after infection.
Multiple cycles of infection were performed with PBMCs for 1, 2, 3, or 4 consecutive days to increase gene expression by increasing the gene dosages. The viability of PBMCs dramatically decreased with the increase of infection frequency, however, although with increased GFP expression (Fig. 2C). Therefore, infecting PBMCs only once at such high MOIs would be preferred.
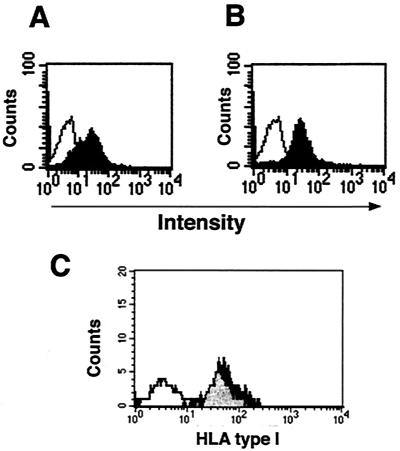
Under optimized conditions, almost all of the PBMCs from individuals with different HLA types were infected efficiently by the GFP/VSV-G retroviruses, with an infection efficiency rate of around 70% (data not shown). The C191/VSV-G retroviruses also had a similar infection efficiency rate on PBMCs as determined by intracellular staining of the core protein (Fig. 3A). Not only could the freshly activated PBMCs be infected by VSV-G-pseudotyped retroviruses, but the activated PBMCs also were still susceptible to VSV-G-pseudotyped retroviral infection after thawing from frozen storage, with a comparable infection efficiency and cell viability (Fig. 3B and data not shown). This finding confirmed the feasibility of preparing multiple batches of autologous stimulators and targets from a single isolation of PBMCs. Infection of PBMCs with VSV-G-pseudotyped retroviruses did not alter the expression of MHC class I molecules on the cell surface (Fig. 3C) and did not appear to interfere with their role of serving as the targets of CTL assays or as the stimulators of antigen-specific CD8+ T cells.
FIG. 3.
Infection of PBMCs with C191/VSV-G retroviruses. (A and B) After activation with PHA and rIL-2, the fresh PBMCs were immediately infected with C191/VSV-G retroviruses at an MOI of 80 (A) or stored in liquid nitrogen for a week and then thawed and infected (B). Expression of core antigen was detected by intracellular staining and analyzed with a FACScan. The black peaks show the PBMCs infected with C191/VSV-G retroviruses; the white peaks show the cells infected with S2/VSV-G retroviruses. (C) No changes of HLA type I molecules on PBMCs after retroviral infection. The levels of surface HLA type I molecules on PBMCs with or without retroviral infection were examined with mouse anti-human HLA-A, -B, and -C antibodies. The black peak shows the PBMCs infected with C191/VSV-G retroviruses, the gray peak shows the cells without infection, and the white peak shows the cells stained with the isotype control antibody.
Use of gene-transduced autologous PBMCs as targets of CTL assays.
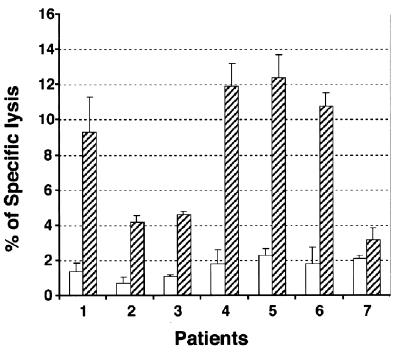
The feasibility of using the core gene-transduced PBMCs as autologous targets of CTL assays was evaluated. PBMCs were isolated from six HCV-positive patients with different HLA types and one HBV-positive, HCV-negative patient, in vitro activated with PHA and rIL-2 for 2 days, and then infected with GFP/VSV-G or C191/VSV-G retroviruses at an MOI of 80. The cells were 51Cr labeled on the fourth day after retroviral infection and served as targets. LILs were isolated concurrently from the liver biopsies of corresponding patients, in vitro stimulated with nonspecific mitogen (PHA-rIL-2) for 48 h, and then exposed to the 51Cr-labeled autologous, gene-transduced PBMCs. Significantly (P < 0.001) higher levels of core-specific CTL responses were observed in the LILs of the six HCV-positive patients than in those of the single HBV-positive patient (Fig. 4). Notably, the background levels of cytolysis using autologous GFP/PBMCs as targets were extremely low. However, when using this protocol CTL activity from the peripheral blood of chronic hepatitis C patients could not be detected, probably due to the low frequency of CTLs in the PBMCs (data not shown).
FIG. 4.
Measurement of CTL activities in LILs using core-expressing autologous PBMCs as target cells. The LILs and PBMCs from six HCV-positive patients (no. 1 to 6) and one HBV-positive, HCV-negative patient (no. 7) were prepared for 4-h 51Cr release assays. The data shown are at an effector/target cell ratio of 10. The white bars show CTL activity measured using GFP/PBMCs as targets; the hatched bars show activity with C191/PBMCs as targets. A generalized estimating equation was used to calculate the correlated measurements of each individual. The differences between HCV-and HBV-infected patients were statistically significant (P < 0.001). Error bars indicate standard deviations.
Use of gene-transduced PBMCs to stimulate core-specific CD8+ T cells in vitro.
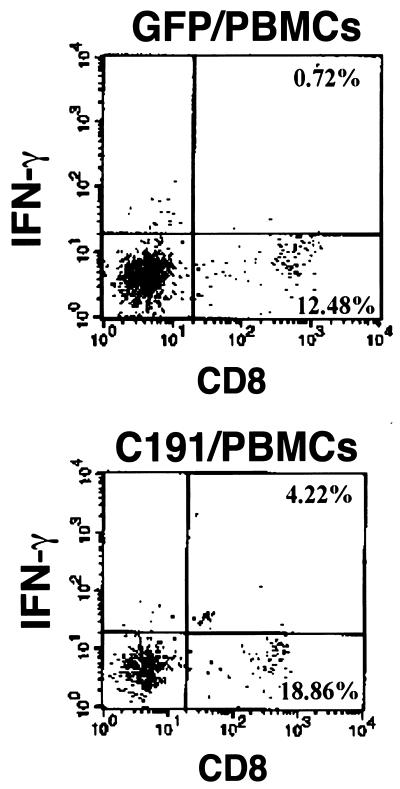
Since the precursor frequency of core-specific CD8+ T cells in the peripheral blood of chronic hepatitis C patients might be too low for these cells to be expanded for conventional CTL assays, this study also examined whether core-specific T-cell responses could be monitored by intracellular IFN-γ assays using the VSV-G-pseudotyped retrovirus-transduced PBMCs as autologous stimulators. The PBMCs were activated and infected as before and then irradiated at 3,000 rads. They were cocultured with freshly isolated PBMCs of the respective patients for 6 h in the presence of anti-CD28, monesin, and rIL-2 (32). The mixtures were then subjected to surface CD8 staining followed by intracellular IFN-γ staining. Figure 5 summarizes the data from one representative patient. The GFP/PBMCs induced only 5.45% [i.e., 0.72%/(0.72% + 12.48%); see definition in Materials and Methods] of the CD8+ T cells to express IFN-γ, whereas the C191/PBMCs induced 18.28% [i.e., 4.22%/(4.22% + 18.86%)] of the CD8+ T cells to express IFN-γ. The percentage of core-specific CD8+ T cells was therefore 12.83% (i.e., 18.28% − 5.45%). The percentages of IFN-γ+ CD8+ T cells significantly increased in some cases if the anti-CD28 antibody was included in the 6-h stimulation (data not shown). The production of IFN-γ indicates the possibility of using autologous PBMCs transduced with C191/VSV-G retroviruses to activate core-specific CD8+ T cells from the peripheral blood of HCV-infected patients.
FIG. 5.
Detection of core-specific CD8+ T-cell responses from the peripheral blood of an HCV-positive patient by intracellular IFN-γ staining. PBMCs from one representative HCV-positive patient were prepared for in vitro stimulation and intracellular IFN-γ staining. The total number of cells subjected to FACScan analysis was 104. The numbers shown in the corners indicate the percentages of IFN-γ+ CD8+ (upper right quadrant) and IFN-γ− CD8+ (lower right quadrant) cells in the fresh PBMCs stimulated with GFP/PBMCs (upper panel) or C191/PBMCs (lower panel).
Detection of core-specific CD8+ T cells in the peripheral blood of HCV-infected patients.
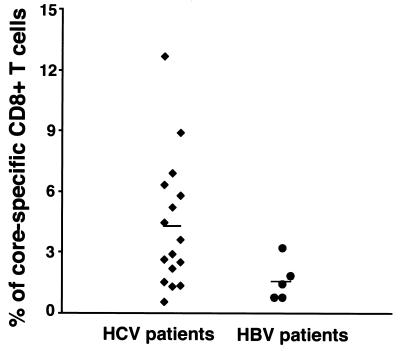
Measurements were taken of the core-specific CD8+ T-cell responses of 16 HCV-positive patients undergoing IFN-α–ribavirin combination therapy and of 5 HBV-positive, HCV-negative patients, without prior knowledge of their HLA types. Significantly (P = 0.002) higher percentages of CD8+ T cells from the HCV-positive patients than from the HBV-positive patients were induced to express IFN-γ upon stimulation with their autologous PBMCs expressing the core antigen (Fig. 6). These results indicate that core-specific CD8+ T cells in the peripheral blood of the patients receiving the IFN-α–ribavirin treatment could be readily activated by their autologous C191/PBMCs.
FIG. 6.
Core-specific CD8+ T-cell responses in the peripheral blood of HCV-positive patients undergoing treatment with IFN-α plus ribavirin. PBMCs from 16 HCV-positive patients treated with interferon-α plus ribavirin and from 5 HBV-positive, HCV-negative patients were isolated, and intracellular IFN-γ assays were performed to determine the percentage of core-specific T cells in the peripheral blood. The mean value for HCV patients was 4.328 ± 3.23, and that for HBV patients was 1.626 ± 1.00 (P = 0.002). A generalized estimating equation was used to evaluate the difference.
DISCUSSION
This study has developed a quick and efficient means of measuring antigen-specific functional CD8+ T cells from patients irrespective of their HLA types. Qualitatively and quantitatively measuring antigen-specific CD8+ T cells is crucial in monitoring the immune status during disease and in assessing treatment efficacy. Conventional assays have analyzed the bulk population of T cells for cytotoxicity by 51Cr release assays. This method requires the expansion of the antigen-specific T-cell population in vitro. Researchers have used primary B-lymphoblastoid cells transformed with an Epstein-Barr virus as the autologous stimulators and/or targets, which are either pulsed with specific peptides (37) or infected with a recombinant vaccinia virus encoding the antigens (23, 30, 41). The procedures are complicated, and the results usually cannot be compared quantitatively.
Some studies used HLA-matched allogenic cell lines to express a specific antigen as the stimulator and/or target of CTLs (6, 7, 33, 34). Although this method is more convenient, its use is limited to patients with specific HLA type, so screening of patients before the CTL assays is necessary. The strong alloreaction also often influences the levels of CTL activities, and the results are difficult to analyze.
A novel method was recently developed, using synthetic HLA-peptide tetrameric complexes to directly quantitate antigen-specific CTLs (1). This method can provide quantitative readouts, since it enumerates antigen-specific T cells without a lengthy in vitro restimulation. The tetramer technique, however, can only identify T cells with single peptide-MHC specificity (18, 25, 28). The patients suitable for study are limited, and numerous peptides corresponding to various predicted epitope motifs have to be synthesized. Also, the tetramers measure antigen specificity without regard to function. Since some T cells in vivo may represent anergic populations, the use of a functional assay may still be necessary. The enzyme-linked immunospot assay (ELISPOT) and intracellular cytokine assays can also be used to enumerate antigen-specific T cells without lengthy in vitro expansion. However, in contrast to tetramers, both methods measure a functional readout (cytokine [e.g., IFN-γ] production). A major advantage of intracellular cytokine assays over ELISPOT is the ability to concurrently analyze multiple parameters, e.g., CD4, CD8, and activation markers, from every single cell.
This study took advantage of intracellular IFN-γ assays and successfully measured core-specific CD8+ T-cell responses from the peripheral blood of chronically HCV-infected patients undergoing treatment with IFN-α plus ribavirin, by simply using the gene-transduced, autologous PBMCs as stimulators. However, attempts to use the same stimulators to expand core-specific T cells from the PBMCs for standard CTL assays have been less successful (data not shown). The results indicate that inducing peripheral T-cell precursors to proliferate and enriching them to certain levels are technically harder, and often take longer, than directly detecting IFN-γ expression from antigen-activated T cells. Since IFN-γ staining assays analyzed all of the CD8+ T cells that recognized the endogenous peptides presented by various types of MHC class I molecules, the percentages of antigen-specific T cells were expected to be higher than those measured from one specific MHC-peptide. Moreover, the antiviral treatments may also contribute to the higher rate of T cell responses in these patients (13). Using the autologous PBMCs as targets of CTL assays, our data demonstrated that the HCV core-specific CTLs could be detected by non-antigen-specific stimulation from the liver (Fig. 4) but not from the peripheral blood (data not shown). Although the percentages of specific lysis are not particularly high, the low background cytolysis when using autologous targets renders the killing activities on specific targets significantly different from those on nonspecific targets (P < 0.001). The results are consistent with previous findings (22, 23, 40) indicating a higher frequency of antigen-specific CTLs in the HCV-infected liver than in the peripheral blood.
Recently, Wong et al. (41) reported the detection of CTLs from the peripheral blood of chronically HCV-infected patients using Epstein-Barr virus-transformed B-lymphoblastoid cells as autologous stimulators, which were infected with a recombinant vaccinia virus encoding the entire translated proteins of HCV. The method they used also eliminates the need for HLA typing. The advantage of this method is its ability to detect a wider scope of, and probably stronger, T-cell responses against various HCV antigens, which thus enabled those authors to easily identify CTLs in the PBMCs from seven of nine patients. However, since standard 51Cr release assays were used in that study, a lengthy in vitro stimulation was unavoidable. The CTL responses measured might reflect only the antigen-specific cells that survived during the incubation period rather than the true magnitude of the immune responses present in the PBMCs. In this regard, intracellular IFN-γ staining assays provide faster, more sensitive, and less biased measurements of the CD8+ T-cell responses in the PBMCs of HCV-infected patients. A potential problem with the system described here is that to assess the immune responses against the entire proteins of HCV, several VSV-G-pseudotyped retroviral vectors need to be constructed.
The immune responses against the HCV core antigens of different individuals receiving treatment with IFN-α plus ribavirin treatment varied to a great extent (Fig. 6). This study initially evaluated the feasibility of measuring functional immune responses with the use of autologous PBMCs, so the CD8+ responses were measured at random time points during the treatment of patients. Whether the heterogeneity in CD8+ T-cell responses represents a prognosis indicator is therefore unclear. To draw a conclusive answer regarding the CTL responses and treatment efficacy, it would be better to longitudinally monitor individual patients receiving the treatments. A new study has been initiated which will recruit more patients and systematically monitor their CTL responses before, during, and after the treatments. The possibility that the less reactive T-cell responses might come from patients infected with different genotypes of HCV cannot be excluded. Since the sequences of HCV core antigen are quite conserved among different genotypes of HCV, however, this is unlikely.
The CTL responses to HCV infection are multispecific. This study investigated only the core-specific CD8+ T-cell responses. It could certainly be extended to examine other HCV antigens or other virus infections, for measuring the CTL responses in individuals of any HLA types. The method also provides a valuable reference for the study of cancer immunology if the tumor-associated antigens are known. The direct quantitation of HCV-specific CD8+ T cells in different patients in different clinical settings, in conjunction with their virological, biochemical, and histological analysis, should provide further insight into viral pathogenesis and clarify the mechanisms of clearance or persistence of HCV.
ACKNOWLEDGMENTS
We thank Wen-Yi Shau for statistical analysis and Pei-Jer Chen and Ted Knoy for critical reviews of the manuscript.
This work was supported by grant NSC 89-2315-B-002-014-MH from the National Science Council, Executive Yuan, Taiwan.
REFERENCES
- 1.Altman J D, Moss P A, Goulder P J, Barouch D H, McHeyzer-Williams M G, Bell J I, McMichael A J, Davis M M. Phenotypic analysis of antigen-specific T lymphocytes. Science. 1996;274:94–96. [PubMed] [Google Scholar]
- 2.Arai T, Matsumoto K, Saitoh K, Ui M, Ito T, Murakami M, Kanegae Y, Saito I, Cosset F L, Takeuchi Y, Iba H. A new system for stringent, high-titer vesicular stomatitis virus G protein-pseudotyped retrovirus vector induction by introduction of Cre recombinase into stable prepackaging cell lines. J Virol. 1998;72:1115–1121. doi: 10.1128/jvi.72.2.1115-1121.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ballardini G, Groff P, Pontisso P, Giostra F, Francesconi R, Lenzi M, Zauli D, Alberti A, Bianchi F B. Hepatitis C virus (HCV) genotype, tissue HCV antigens, hepatocellular expression of HLA-A,B,C, and intercellular adhesion-1 molecules. Clues to pathogenesis of hepatocellular damage and response to interferon treatment in patients with chronic hepatitis C. J Clin Invest. 1995;95:2067–2075. doi: 10.1172/JCI117893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burns J C, Friedmann T, Driever W, Burrascano M, Yee J K. Vesicular stomatitis virus G glycoprotein pseudotyped retroviral vectors: concentration to very high titer and efficient gene transfer into mammalian and nonmammalian cells. Proc Natl Acad Sci USA. 1993;90:8033–8037. doi: 10.1073/pnas.90.17.8033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cerny A, McHutchison J G, Pasquinelli C, Brown M E, Brothers M A, Grabscheid B, Fowler P, Houghton M, Chisari F V. Cytotoxic T lymphocyte response to hepatitis C virus-derived peptides containing the HLA A2.1 binding motif. J Clin Invest. 1995;95:521–530. doi: 10.1172/JCI117694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang K M, Gruener N H, Southwood S, Sidney J, Pape G R, Chisari F V, Sette A. Identification of HLA-A3 and -B7-restricted CTL response to hepatitis C virus in patients with acute and chronic hepatitis C. J Immunol. 1999;162:1156–1164. [PubMed] [Google Scholar]
- 7.Chang K M, Rehermann B, McHutchison J G, Pasquinelli C, Southwood S, Sette A, Chisari F V. Immunological significance of cytotoxic T lymphocyte epitope variants in patients chronically infected by the hepatitis C virus. J Clin Invest. 1997;100:2376–2385. doi: 10.1172/JCI119778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coligan J E, Kruisbeek A M, Margulies D H, Shevach E M, Strober W. Current protocols in immunology. New York, N.Y: John Wiley & Sons, Inc.; 1991. pp. 3–11. [Google Scholar]
- 9.Costello E, Munoz M, Buetti E, Meylan P R, Diggelmann H, Thali M. Gene transfer into stimulated and unstimulated T lymphocytes by HIV-1-derived lentiviral vectors. Gene Ther. 2000;7:596–604. doi: 10.1038/sj.gt.3301135. [DOI] [PubMed] [Google Scholar]
- 10.Culver K, Cornetta K, Morgan R, Morecki S, Aebersold P, Kasid A, Lotze M, Rosenberg S A, Anderson W F, Blaese R M. Lymphocytes as cellular vehicles for gene therapy in mouse and man. Proc Natl Acad Sci USA. 1991;88:3155–3159. doi: 10.1073/pnas.88.8.3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diepolder H M, Zachoval R, Hoffmann R M, Wierenga E A, Santantonio T, Jung M C, Eichenlaub D, Pape G R. Possible mechanism involving T-lymphocyte response to non-structural protein 3 in viral clearance in acute hepatitis C virus infection. Lancet. 1995;346:1006–1007. doi: 10.1016/s0140-6736(95)91691-1. [DOI] [PubMed] [Google Scholar]
- 12.Emi N, Friedmann T, Yee J K. Pseudotype formation of murine leukemia virus with the G protein of vesicular stomatitis virus. J Virol. 1991;65:1202–1207. doi: 10.1128/jvi.65.3.1202-1207.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fang S H, Hwang L H, Chen D S, Chiang B L. Ribavirin enhancement of hepatitis C virus core antigen-specific type 1 T helper cell response correlates with the increased IL-12 level. J Hepatol. 2000;33:791–798. doi: 10.1016/s0168-8278(00)80312-8. [DOI] [PubMed] [Google Scholar]
- 14.Farci P, Bukh J, Purcell R H. The quasispecies of hepatitis C virus and the host immune response. Springer Semin Immunopathol. 1997;19:5–26. doi: 10.1007/BF00945022. [DOI] [PubMed] [Google Scholar]
- 15.Fauser A A. Long-term expression of gene introduction into normal human T-lymphocytes by retroviral-mediated gene transfer. J Cell Biochem. 1991;45:353–358. doi: 10.1002/jcb.240450408. [DOI] [PubMed] [Google Scholar]
- 16.Friedmann T, Yee J K. Pseudotyped retroviral vectors for studies of human gene therapy. Nat Med. 1995;1:275–277. doi: 10.1038/nm0395-275. [DOI] [PubMed] [Google Scholar]
- 17.Gallardo H F, Tan C, Ory D, Sadelain M. Recombinant retroviruses pseudotyped with the vesicular stomatitis virus G glycoprotein mediate both stable gene transfer and pseudotransduction in human peripheral blood lymphocytes. Blood. 1997;90:952–957. [PubMed] [Google Scholar]
- 18.He X S, Rehermann B, Lopez-Labrador F X, Boisvert J, Cheung R, Mumm J, Wedemeyer H, Berenguer M, Wright T L, Davis M M, Greenberg H B. Quantitative analysis of hepatitis C virus-specific CD8(+) T cells in peripheral blood and liver using peptide-MHC tetramers. Proc Natl Acad Sci USA. 1999;96:5692–5697. doi: 10.1073/pnas.96.10.5692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hsieh C L, Chen B F, Wang C C, Liu H H, Chen D S, Hwang L H. Improved gene expression by a modified bicistronic retroviral vector. Biochem Biophys Res Commun. 1995;214:910–917. doi: 10.1006/bbrc.1995.2373. [DOI] [PubMed] [Google Scholar]
- 20.Hui K M, Sabapathy T K, Oei A A, Chia T F. Generation of allo-reactive cytotoxic T lymphocytes by particle bombardment-mediated gene transfer. J Immunol Methods. 1994;171:147–155. doi: 10.1016/0022-1759(94)90035-3. [DOI] [PubMed] [Google Scholar]
- 21.Kanegae Y, Takamori K, Sato Y, Lee G, Nakai M, Saito I. Efficient gene activation system on mammalian cell chromosomes using recombinant adenovirus producing Cre recombinase. Gene. 1996;181:207–212. doi: 10.1016/s0378-1119(96)00516-1. [DOI] [PubMed] [Google Scholar]
- 22.Koziel M J. The role of immune responses in the pathogenesis of hepatitis C virus infection. J Viral Hepat. 1997;4:31–41. doi: 10.1111/j.1365-2893.1997.tb00178.x. [DOI] [PubMed] [Google Scholar]
- 23.Koziel M J, Dudley D, Afdhal N, Choo Q L, Houghton M, Ralston R, Walker B D. Hepatitis C virus (HCV)-specific cytotoxic T lymphocytes recognize epitopes in the core and envelope proteins of HCV. J Virol. 1993;67:7522–7532. doi: 10.1128/jvi.67.12.7522-7532.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koziel M J, Dudley D, Afdhal N, Grakoui A, Rice C M, Choo Q L, Houghton M, Walker B D. HLA class I-restricted cytotoxic T lymphocytes specific for hepatitis C virus. Identification of multiple epitopes and characterization of patterns of cytokine release. J Clin Invest. 1995;96:2311–2321. doi: 10.1172/JCI118287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maini M K, Boni C, Ogg G S, King A S, Reignat S, Lee C K, Larrubia J R, Webster G J, McMichael A J, Ferrari C, Williams R, Vergani D, Bertoletti A. Direct ex vivo analysis of hepatitis B virus-specific CD8(+) T cells associated with the control of infection. Gastroenterology. 1999;117:1386–1396. doi: 10.1016/s0016-5085(99)70289-1. [DOI] [PubMed] [Google Scholar]
- 26.Markowitz D, Goff S, Bank A. Construction and use of a safe and efficient amphotropic packaging cell line. Virology. 1988;167:400–406. [PubMed] [Google Scholar]
- 27.Mavilio F, Ferrari G, Rossini S, Nobili N, Bonini C, Casorati G, Traversari C, Bordignon C. Peripheral blood lymphocytes as target cells of retroviral vector-mediated gene transfer. Blood. 1994;83:1988–1997. [PubMed] [Google Scholar]
- 28.Migueles S A, Sabbaghian M S, Shupert W L, Bettinotti M P, Marincola F M, Martino L, Hallahan C W, Selig S M, Schwartz D, Sullivan J, Connors M. HLA B∗5701 is highly associated with restriction of virus replication in a subgroup of HIV-infected long term nonprogressors. Proc Natl Acad Sci USA. 2000;97:2709–2714. doi: 10.1073/pnas.050567397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Muro-Cacho C A, Samulski R J, Kaplan D. Gene transfer in human lymphocytes using a vector based on adeno-associated virus. J Immunother. 1992;11:231–237. doi: 10.1097/00002371-199205000-00001. [DOI] [PubMed] [Google Scholar]
- 30.Nelson D R, Marousis C G, Davis G L, Rice C M, Wong J, Houghton M, Lau J Y. The role of hepatitis C virus-specific cytotoxic T lymphocytes in chronic hepatitis C. J Immunol. 1997;158:1473–1481. [PubMed] [Google Scholar]
- 31.Nelson D R, Marousis C G, Ohno T, Davis G L, Lau J Y. Intrahepatic hepatitis C virus-specific cytotoxic T lymphocyte activity and response to interferon alfa therapy in chronic hepatitis C. Hepatology. 1998;28:225–230. doi: 10.1002/hep.510280129. [DOI] [PubMed] [Google Scholar]
- 32.Prussin C, Metcalfe D D. Detection of intracytoplasmic cytokine using flow cytometry and directly conjugated anti-cytokine antibodies. J Immunol Methods. 1995;188:117–128. doi: 10.1016/0022-1759(95)00209-x. [DOI] [PubMed] [Google Scholar]
- 33.Rehermann B, Chang K M, McHutchinson J, Kokka R, Houghton M, Rice C M, Chisari F V. Differential cytotoxic T-lymphocyte responsiveness to the hepatitis B and C viruses in chronically infected patients. J Virol. 1996;70:7092–7102. doi: 10.1128/jvi.70.10.7092-7102.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scognamiglio P, Accapezzato D, Casciaro M A, Cacciani A, Artini M, Bruno G, Chircu M L, Sidney J, Southwood S, Abrignani S, Sette A, Barnaba V. Presence of effector CD8+ T cells in hepatitis C virus-exposed healthy seronegative donors. J Immunol. 1999;162:6681–6689. [PubMed] [Google Scholar]
- 35.Sharma S, Cantwell M, Kipps T J, Friedmann T. Efficient infection of a human T-cell line and of human primary peripheral blood leukocytes with a pseudotyped retrovirus vector. Proc Natl Acad Sci USA. 1996;93:11842–11847. doi: 10.1073/pnas.93.21.11842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Strair R K, Towle M, Heald P W, Smith B R. Retroviral mediated transfer and expression of exogenous genes in primary lymphoid cells: assaying for a viral transactivator activity in normal and malignant cells. Blood. 1990;76:1201–1208. [PubMed] [Google Scholar]
- 37.Tsai S L, Chen Y M, Chen M H, Huang C Y, Sheen I S, Yeh C T, Huang J H, Kuo G C, Liaw Y F. Hepatitis C virus variants circumventing cytotoxic T lymphocyte activity as a mechanism of chronicity. Gastroenterology. 1998;115:954–965. doi: 10.1016/s0016-5085(98)70268-9. [DOI] [PubMed] [Google Scholar]
- 38.Wentworth P A, Sette A, Celis E, Sidney J, Southwood S, Crimi C, Stitely S, Keogh E, Wong N C, Livingston B, Alazard D, Vitiello A, Grey H M, Chisari F V, Chesnut R W, Fikes J. Identification of A2-restricted hepatitis C virus-specific cytotoxic T lymphocyte epitopes from conserved regions of the viral genome. Int Immunol. 1996;8:651–659. doi: 10.1093/intimm/8.5.651. [DOI] [PubMed] [Google Scholar]
- 39.Woffendin C, Yang Z Y, Udaykumar, Xu L, Yang N S, Sheehy M J, Nabel G J. Nonviral and viral delivery of a human immunodeficiency virus protective gene into primary human T cells. Proc Natl Acad Sci USA. 1994;91:11581–11585. doi: 10.1073/pnas.91.24.11581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wong D K, Dudley D D, Afdhal N H, Dienstag J, Rice C M, Wang L, Houghton M, Walker B D, Koziel M J. Liver-derived CTL in hepatitis C virus infection: breadth and specificity of responses in a cohort of persons with chronic infection. J Immunol. 1998;160:1479–1488. [PubMed] [Google Scholar]
- 41.Wong D K H, Dudley D D, Dohrenwend P B, Lauer G M, Chung R T, Thomas D L, Walker B D. Detection of diverse hepatitis C virus (HCV)-specific cytotoxic T lymphocytes in peripheral blood of infected persons by screening for responses to all translated proteins of HCV. J Virol. 2001;75:1229–1235. doi: 10.1128/JVI.75.3.1229-1235.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]