Abstract
Background
Differential diagnosis of recurrent syncope is a routine procedure in clinical practice. Most of these syncopes are benign reflex syncopes but identifying patients with cardiac syncope is crucial to prevent fatal outcomes.
Case summary
In this case report, we present the case of a young athlete with recurrent unexplained syncope referred to us for a second opinion. Despite normal resting-electrocardiography and echocardiography, he developed frequent polymorphic and bidirectional premature ventricular contractions during exercise. Genetic testing confirmed a mutation in the RyR2-gene and the diagnosis of catecholaminergic polymorphic ventricular tachycardia was made. A medical therapy with betablockers was initiated but poorly tolerated, so that an implantable cardioverter-defibrillator was implanted. Furthermore, family screening revealed his mother and his sister to be genetic carriers as well. Implantable cardioverter-defibrillator implantation was performed in both family members. The patient did not experience any syncope or arrhythmic episodes during the follow-up period.
Discussion
This case report highlights the importance of thorough diagnostic and potential pitfalls in patients with unexplained syncope. Sometimes, the diagnostic steps need to be extended or repeated to detect rare or potential malignant causes of syncope.
Keywords: Sudden cardiac death, TLOC, Unexplained syncope, CPVT, Inherited arrhythmias, Case report
For the podcast associated with this article, please visit https://academic.oup.com/ehjcr/pages/podcast
Learning points.
Unexplained syncope is a common phenomenon in clinical practice but requires thorough anamnesis and diagnostics to identify a potential harmful cardiac origin.
If a cardiac origin is suggested, the investigation should be expanded until a definite diagnosis can be made.
Especially in young patients with unexplained syncope and normal cardiac status, it is helpful to carry out the diagnostics several times to unmask temporary changes of cardiac repolarization and ventricular ectopy.
Introduction
With a lifetime cumulative incidence of 35%, syncope is one of the most common conditions being evaluated in the cardiology department.1 Syncope is defined as total loss of consciousness (TLOC) due to cerebral hypoperfusion with sudden onset and quick recovery.2 The age distribution is characterized by two peaks, one in adolescents and one in the elderly.3 The majority of syncope in the young refers to a benign reflex mechanism,4 however, the diagnostic workup should help to differentiate between the distinct forms of syncope to guide further therapy. A thorough history taking, if necessary via third-party, is the cornerstone of the diagnostics and should guide further examinations. The sequence of diagnostic steps also depends on the mode of presentation, i.e. a patient who presents at the emergency department with acute symptoms probably requires a different diagnostic sequence than the patient who presents himself at the outpatient clinic in a stable condition. The latter is the more common situation. If the syncope occurs during exertion or supine or is accompanied by palpitations right before TLOC, a cardiac origin is highly likely. A thorough medical history should be raised and the family should be checked for sudden cardiac death (SCD). A comprehensive cardiac examination including resting-electrocardiography (ECG), cardiac imaging, Holter-, and exercise-ECG should follow. If all examinations are normal but a cardiac origin is still suspected, an implantable loop recorder (ILR) is a useful tool to further investigate an arrhythmic cause.5 However, even if some cases remain unclear despite extensive evaluation at the beginning, the diagnostic effort should not stop at that point. Sometimes it can be helpful to repeat certain examinations, especially stress tests.
In this report, we present the case of a young athlete who was referred to us for a second opinion due to unexplained syncope during physical activity. Despite normal resting-ECG and echocardiography, he developed bidirectional and polymorphic premature ventricular contractions (PVCs) during exercise-testing. He further had a family history of SCD and syncope. Taking into account a mutation in the RyR2-gene, he was diagnosed with catecholaminergic polymorphic ventricular tachycardia (CPVT).
Timeline
| 2013 | Syncope with traumatic brain injury |
| 2014 | Near-drowning with resuscitation during lifeguard training |
| February 2015 | First contact with cardiologist |
| Normal electrocardiography (ECG) | |
| Normal echocardiography | |
| Normal cardiac magnetic resonance imaging | |
| Implantation of an implantable loop recorder (ILR) | |
| March 2016 | ILR explantation at request of the patient |
| January 2019 | Frequent palpitations and arrhythmias |
| June 2019 | First contact with our clinic |
| Pathological exercise-ECG and suspected catecholaminergic polymorphic ventricular tachycardia | |
| November 2019 | Genetic testing revealing a mutation in RyR2-gene |
| March 2020 | Implantation of an one-chamber implantable cardioverter-defibrillator |
Case presentation
A 24-year-old white male was referred to us in January 2019 for a second opinion due to recurrent syncope, related to physical activity. The patient was an amateur athlete, performing marathons and triathlons on a high-performance level and was trained in martial arts.
In 2013, he suffered a traumatic brain injury caused by a syncope during physical activity. At this point, the syncope was explained by the high temperatures at that day and no further examinations were carried out.
A year later, near drowning occurred during life guard training requiring short-term resuscitation by his colleagues. Following this incidence, the patient presented himself at a cardiologist for the first time. Routine diagnostic work-up including 12-lead ECG, echocardiography, and cardiac magnetic resonance imaging revealed no evidence of a structural heart disease or channelopathy. A genetic testing was not performed due to the patients’ reluctant attitude towards diagnostics. Due to the recurrent unexplained syncope, an ILR was inserted in the following. Since then, no syncope occurred, however, the ILR regularly recorded frequent PVC and one high-rate episode at 250 b.p.m., which was interpreted as supraventricular tachycardia. Despite recommendations to the contrary, he continued the ambitious sporting activity. In 2016, the patient requested the explantation of the ILR against the doctors advise because he reported abnormal sensations from it and he did not expect any further findings from it.
Between 2016 and the first contact at our outpatient clinic, he was asymptomatic most of the time. At the time of his first presentation, he reported frequent palpitations. This was also the reason for his presentation at our clinic. A detailed family history revealed that his mother had been suffering from physical and emotional stress associated syncope for years. Furthermore, his maternal grandfather died of SCD at the age of 28, his two great uncles died at a young age. One died suddenly at the age of 17 while playing soccer, whereas the other one died of unknown cause in a mine.
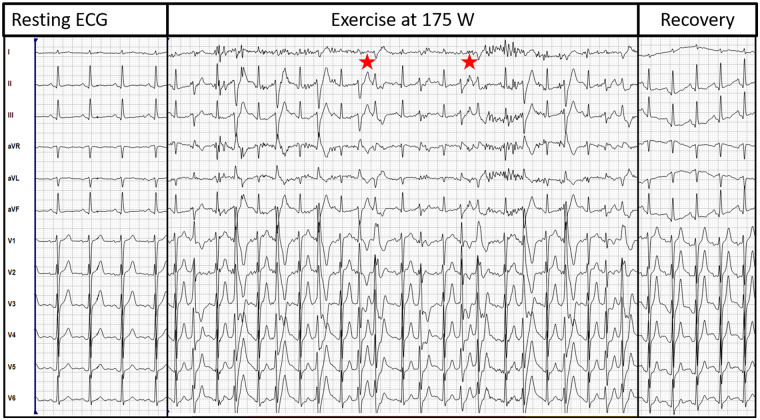
He presented himself in a very well-trained condition. Physical examination showed no abnormalities, the ECG showed a normal axis with regular intervals without any signs of pre-excitation or early repolarization (Figure 1). Besides a mild mitral insufficiency, the echocardiography was inconspicuous. During exercise-ECG, he developed frequent PVCs at a resistance of 175 Watts (W), these PVC were predominantly polymorphic but also bidirectional. At a resistance of 250 W a significant drop of blood pressure (>20 mmHg) was noted and the patient reported dizziness, while he showed bidirectional bigemini and trigemini, but no sustained ventricular tachycardia (VT) (see Figure 1). The arrhythmias stopped immediately during recovery time. A genetic testing was performed which revealed a mutation in the cardiac RyR2-gene. Based on the clinical findings, the genetic blood test and the family history, the diagnosis CPVT was made. A therapy with betablockade was initiated but the patient tolerated only the minimal dosage due to significant sinus bradycardia. Furthermore, he planned to continue with physical activity on a high-performance level despite advice to the contrary. Therefore, we suggested to implant an implantable cardioverter-defibrillator (ICD) based on the history of recurrent syncope. The patient agreed to a single-chamber ICD implantation, which was performed in March 2020. The betablocker therapy was continued on the minimum dosage. Since then, no further episode of syncope or VT/ventricular fibrillation (VF) occurred. The RyR2 mutation was also detected in his mother and his sister. Since the sister also experienced exercise-related syncope in the meantime, ICD implantation was performed in both family members as well. Figure 2 depicts the family tree including the definite and suspected mutation carriers.
Figure 1.
Exercise-electrocardiogram of the patient. The resting-electrocardiogram is without any pathological findings. During exercise at 175 Watts, the patient develops frequent premature ventricular contractions, which are completely gone during recovery. The asterisks highlight bidirectional premature ventricular contractions.
Figure 2.
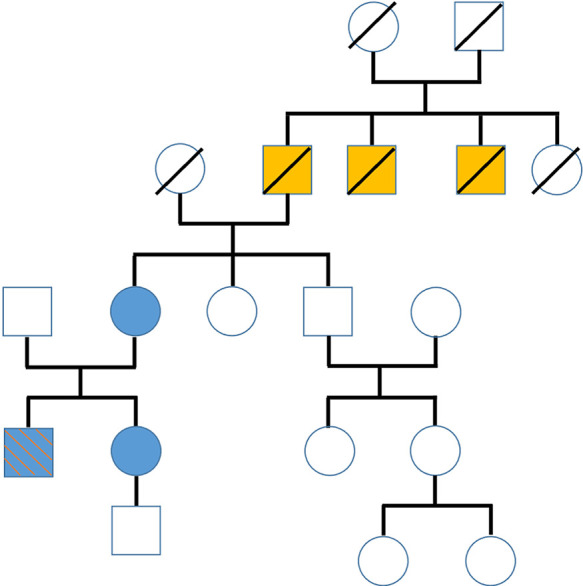
Family tree of the patient. Family members with definite diagnosis of catecholaminergic polymorphic ventricular tachycardia (the patient, his sister and his mother) are marked in blue. The blue box with the red stripes indicates the patient. The orange boxes indicate members with sudden cardiac death at a young age and suspected catecholaminergic polymorphic ventricular tachycardia (crossed boxes = deceased).
Discussion
Catecholaminergic polymorphic ventricular tachycardia belongs to the group of inherited arrhythmias. It is defined as an adrenergic driven VT or PVC with a bidirectional or polymorphic QRS morphology in the absence of a structural heart disease.6 Its clinical presentation features physical or emotional stress related syncope or cardiac arrest due to VT/VF.7 Two types of CPVT have been defined according to genetic findings: an autosomal dominant form with a mutation of the cardiac ryanodine receptor gene (RyR2) and a recessive form linked to mutations in the calsequestrin gene (CASQ2).8 With an estimated prevalence of 1:10 000 it belongs to the rare forms of inherited arrhythmias.9 Therapy includes medical betablockade and the implantation of an ICD. It is crucial to keep this arrhythmia in mind during the routine diagnostic workup in young patients presenting with syncope. This might explain the significant delay between symptom onset and definite diagnosis in the majority of patients.7
In this case, the time period between symptom onset and subsequent diagnosis was 5 years; although our patients’ history and symptoms were typical for CPVT. The diagnosis is based on ECG findings during exercise, maximal effort during exercise is a prerequisite for the diagnosis because the arrhythmias typically appear around the maximum exercise capacity.10 If the maximum capacity is not reached, it is sometimes useful to switch the type of exercise from bicycle to treadmill testing which is more suitable for untrained persons to reach the maximum capacity.11 Importantly, programmed ventricular stimulation is not valuable for risk stratification in CPVT.9
Also, genetic testing can be helpful in finding the correct diagnosis; however, is prevalent in only half the patients with CPVT.12,13 Still, genetic testing and screening of family members should be part of the diagnostic workup in patients in whom an inherited arrhythmia is suspected. In our case, a genetic background was obvious based on the accumulation of SCD in the mothers’ family and the mothers’ history of palpitations and syncope.
The basic principle of therapy in CPVT is reducing cardiac sympathetic activation via betablockers (Class IC).8 If the patient is still symptomatic, the antiarrhythmic regimen can be expanded with flecainide (Class IIa).8 Implantable cardioverter-defibrillator implantation is recommended in patients with documented cardiac arrest, recurrent syncopes or sustained VT despite optimal therapy (Class IC).8 An ICD should be carefully considered due to the relatively young patient group. There are reasonable concerns that the ICD itself causes fatal events in these patients, e.g. by increasing sympathetic activity due to inappropriate shocks.14,15 Furthermore, a recent observational study proved, the ICD implantation was not superior to medical therapy in previously undiagnosed CPVT patients who survived SCD.16 However, in this case, we decided to perform ICD implantation because the patients’ adherence to medical therapy was low.
The patients’ education in congenital arrhythmia syndromes is crucial for a successful treatment since the diagnosis represents a turning point in the future of these young patients. Our task is to put the pieces of diagnostics into a complete picture at the beginning to ensure an early diagnosis to prevent fatal outcomes.
Lead author biography

Leonard Bergau is a cardiologist with a clinical focus on interventional electrophysiology. He graduated from medical school and finished his doctoral thesis in Göttingen and completed his training in internal medicine and cardiology at the University Clinic Göttingen and the Herz- und Diabeteszentrum Nordrhein-Westfalen in Bad Oeynhausen. His scientific focus is the risk stratification of sudden cardiac death and the interventional therapy of atrial fibrillation. He currently works at the Department for Electrophysiology at the Herz- und Diabeteszentrum Nordrhein-Westfalen.
Supplementary material
Supplementary material is available at European Heart Journal - Case Reports online.
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementary data.
Consent: The authors confirm that written consent for submission and publication of this case report including images and associated text has been obtained from the patient in line with COPE guidance.
Conflict of interest: None declared.
Funding: None declared.
Supplementary Material
References
- 1.Ganzeboom KS, Mairuhu G, Reitsma JB, Linzer M, Wieling W, van Dijk N.. Lifetime cumulative incidence of syncope in the general population: a study of 549 Dutch subjects aged 35-60 years. J Cardiovasc Electrophysiol 2006;17:1172–1176. [DOI] [PubMed] [Google Scholar]
- 2.Brignole M, Moya A, de Lange FJ, Deharo JC, Elliott PM, Fanciulli A. et al. ; ESC Scientific Document Group. 2018 ESC Guidelines for the diagnosis and management of syncope. Eur Heart J 2018;39:1883–1948. [DOI] [PubMed] [Google Scholar]
- 3.Colman N, Nahm K, Ganzeboom KS, Shen WK, Reitsma J, Linzer M. et al. Epidemiology of reflex syncope. Clin Auton Res 2004;14(Suppl 1):9–17. [DOI] [PubMed] [Google Scholar]
- 4.Chen LY, Gersh BJ, Hodge DO, Wieling W, Hammill SC, Shen WK.. Prevalence and clinical outcomes of patients with multiple potential causes of syncope. Mayo Clin Proc 2003;78:414–420. [DOI] [PubMed] [Google Scholar]
- 5.Solbiati M, Casazza G, Dipaola F, Barbic F, Caldato M, Montano N. et al. The diagnostic yield of implantable loop recorders in unexplained syncope: a systematic review and meta-analysis. Int J Cardiol 2017;231:170–176. [DOI] [PubMed] [Google Scholar]
- 6.Leenhardt A, Lucet V, Denjoy I, Grau F, Ngoc DD, Coumel P.. Catecholaminergic polymorphic ventricular tachycardia in children. A 7-year follow-up of 21 patients. Circulation 1995;91:1512–1519. [DOI] [PubMed] [Google Scholar]
- 7.Roston TM, Vinocur JM, Maginot KR, Mohammed S, Salerno JC, Etheridge SP. et al. Catecholaminergic polymorphic ventricular tachycardia in children: analysis of therapeutic strategies and outcomes from an international multicenter registry. Circ Arrhythm Electrophysiol 2015;8:633–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Priori SG, Blomstrom-Lundqvist C, Mazzanti A, Blom N, Borggrefe M, Camm J. et al. ; ESC Scientific Document Group. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: the Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Eur Heart J 2015;36:2793–2867. [DOI] [PubMed] [Google Scholar]
- 9.Priori SG, Wilde AA, Horie M, Cho Y, Behr ER, Berul C. et al. HRS/EHRA/APHRS expert consensus statement on the diagnosis and management of patients with inherited primary arrhythmia syndromes: document endorsed by HRS, EHRA, and APHRS in May 2013 and by ACCF, AHA, PACES, and AEPC in June 2013. Heart Rhythm 2013;10:1932–1963. [DOI] [PubMed] [Google Scholar]
- 10.Blich M, Marai I, Suleiman M, Lorber A, Gepstein L, Boulous M. et al. Electrocardiographic comparison of ventricular premature complexes during exercise test in patients with CPVT and healthy subjects. Pacing Clin Electrophysiol 2015;38:398–402. [DOI] [PubMed] [Google Scholar]
- 11.Fletcher GF, Ades PA, Kligfield P, Arena R, Balady GJ, Bittner VA. et al. ; American Heart Association Exercise, Cardiac Rehabilitation, and Prevention Committee of the Council on Clinical Cardiology, Council on Nutrition, Physical Activity and Metabolism, Council on Cardiovascular and Stroke Nursing, and Council on Epidemiology and Prevention. Exercise standards for testing and training: a scientific statement from the American Heart Association. Circulation 2013;128:873–934. [DOI] [PubMed] [Google Scholar]
- 12.Leenhardt A, Denjoy I, Guicheney P.. Catecholaminergic polymorphic ventricular tachycardia. Circ Arrhythm Electrophysiol 2012;5:1044–1052. [DOI] [PubMed] [Google Scholar]
- 13.Priori SG, Napolitano C, Memmi M, Colombi B, Drago F, Gasparini M. et al. Clinical and molecular characterization of patients with catecholaminergic polymorphic ventricular tachycardia. Circulation 2002;106:69–74. [DOI] [PubMed] [Google Scholar]
- 14.Pizzale S, Gollob MH, Gow R, Birnie DH.. Sudden death in a young man with catecholaminergic polymorphic ventricular tachycardia and paroxysmal atrial fibrillation. J Cardiovasc Electrophysiol 2008;19:1319–1321. [DOI] [PubMed] [Google Scholar]
- 15.Mohamed U, Gollob MH, Gow RM, Krahn AD.. Sudden cardiac death despite an implantable cardioverter-defibrillator in a young female with catecholaminergic ventricular tachycardia. Heart Rhythm 2006;3:1486–1489. [DOI] [PubMed] [Google Scholar]
- 16.van der Werf C, Lieve KV, Bos JM, Lane CM, Denjoy I, Roses-Noguer F. et al. Implantable cardioverter-defibrillators in previously undiagnosed patients with catecholaminergic polymorphic ventricular tachycardia resuscitated from sudden cardiac arrest. Eur Heart J 2019;40:2953–2961. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.