Abstract
Venovenous extracorporeal membrane oxygenation (VV-ECMO) is increasingly used in managing challenging airway and thoracic cases with complex airway manipulations. We present a case of a complex tracheal resection needing prolonged apnea times for which VV-ECMO was electively planned. Intraoperatively, the team was faced with continued oxygen desaturations during periods of apnea. With an algorithmic approach to troubleshooting hypoxemia, several factors were taken into consideration. Apneic oxygenation was applied to the open tracheal segment. Despite an open airway, the applied apneic oxygenation facilitated oxygenation to the portion of the cardiac output that was being shunted through the lungs as opposed to the VV-ECMO circuit, enabling uninterrupted completion of the surgical resection and reanastomosis.
Keywords: Airway surgery, extracorporeal membrane oxygenation, tracheal surgery
Introduction
Venovenous extracorporeal membrane oxygenation (VV-ECMO) is widely used in caring for patients with respiratory failure in the intensive care unit (ICU). A secondary use for VV-ECMO acts as a bridge during complex airway surgery.[1] We present a unique case of elective VV-ECMO use in combination with apneic oxygenation during tracheal resection complicated by refractory hypoxemia. By use of apneic oxygenation in tandem with VV-ECMO, tracheal resection surgery was ably performed uninterrupted with adequate oxygenation throughout.
Case
A 38-year-old man presented for tracheal resection due to severe subglottic tracheal stenosis secondary to a previous prolonged intubation after a gastrointestinal bleed. See Figure 1 for tracheal imaging. Given the length of the stenosis and concerns for a difficult airway (neck circumference of 107 cm, body mass index of 34 kg/m2), the anesthesia team planned for pre-induction VV-ECMO cannulation.
Figure 1.
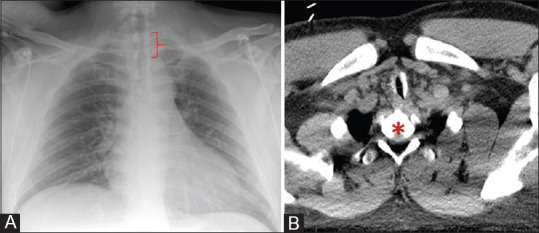
Panel A – Upright chest x-ray demonstrating stenotic tracheal segment measuring 7.5 mm diameter by 3.3 cm length (bracket). Panel B - Computed tomography demonstrating tracheal stenosis narrowest transverse section of 7.5 mm (arrow) at the level of first thoracic vertebral level (asterisk)
Under minimal sedation, ECMO cannulation in the bifemoral configuration was performed with a 27Fr drainage cannula and a 25Fr return cannula. Transthoracic echocardiography (TTE) with a subcostal view of the inferior vena cava (IVC) and right atrial junction was performed to visualize the correct position of ECMO guidewires in the IVC–right atrial junction. VV-ECMO flows of up to 6 L/min were established without cavitation (chatter) on the circuit. The sweep was kept at 8 L/min in anticipation of anesthesia induction and a post-induction 5.0 cuffed microlaryngoscopy endotracheal tube (MLT) was placed using video laryngoscopy. The patient was ventilated with tidal volumes of 300 ml, I: E of 1:2.5, respiratory rate of 12, and positive end-expiratory pressure (PEEP) of 5 cmH2O. Adequate gas exchange with pO2 of 90–120 mmHg and pCO2 of 35–45 mmHg were established along with adjuvant ECMO flows of 5 L/min and sweep of 4- L/min. Cardiac output (CO) monitoring with a Flotrac (Edwards Lifesciences, Irvine, CA) device was performed.
Despite ECMO flows of 5.5 L/min and having matched ECMO flows to 70% of patient's CO, there were multiple episodes of oxygen desaturations to 80% during periods of apnea lasting greater than 40 seconds for purposes of assessment of the length and extent of tracheal stenosis via bronchoscopy. Recirculation of returned oxygenated blood was considered and cannula positions were interrogated with TTE and adjusted to target low venous saturations (Svo2 68%) from the access cannula. Oxygenator failure was ruled out and attempts to decrease CO (<9 L/min) as a means to mitigate recirculation were made repeatedly using esmolol and propofol. Though used in an open airway, administration of apneic oxygenation via the MLT (positioned proximal to the tracheal segment to be resected) led to an immediate improvement in hypoxia and permitted apnea periods to be extended up to 7 minutes. This intervention facilitated the surgeons’ ability to manipulate the distal tracheal segments in an uninterrupted fashion. Upon anastomosis completion, the MLT was changed to a standard 6.0 endotracheal tube (ETT) positioned proximal to the anastomotic line. Emergence and extubation occurred uneventfully in the operating room. ECMO sweep gas was weaned and followed by decannulation an hour later in the ICU.
Discussion
The presented case highlights a number of key factors involved in the successful conduct of tracheal resection surgery facilitated by VV-ECMO in conjunction with the heretofore-unreported use of intraprocedural apneic oxygenation as a means to provide adequate intraoperative oxygenation.
Tracheal resection surgery involving a long subglottic tracheal segment, typically defined as a stenotic length >40 mm, is an uncommon procedure despite it being the definitive treatment for complex acquired laryngotracheal stenosis.[2] Typically, these cases are completed with in-field ventilation utilizing a conventional ETT. If surgical complexity is such that an unobstructed field is required, alternative airway management strategies may be required. Aside from VV-ECMO, other strategies may include apneic oxygenation, jet ventilation, a surgically placed bronchial tube, or cardiopulmonary bypass (CPB).[3] In-field methods of ventilation, typically a first choice of airway management in thoracic surgery, are limited by the physical space that an ETT occupies.[4] One option is passive oxygenation with intermittent placement and removal of an ETT, or via conventional ventilation with a stationary ETT with intermittent apnea. These methods are frequently insufficient in terms of oxygenation when used in isolation and may also lead to excess hypercapnia. Tracheal surgeries requiring both an unobstructed surgical field and prolonged apnea require other strategies such as CPB, which has specific and high-demand prerequisites. Hence, CPB is often precluded or is reserved as a last-line intervention. Evolving from CPB, ECMO during tracheal resection surgery has increased over the past decade and has been shown to both reduce the requirements for anticoagulation and reduce facility demands.[5]
VV-ECMO use as a means of oxygenation and ventilation in the surgical population has been reported primarily in complex airway surgery, mediastinal tumor resections, bullae surgery, as well before and during lung transplantation.[6] These cases all highlight VV-ECMO as an oxygenation option for major tracheal bronchial surgery and single lung procedures when in-field ventilation is not possible.[7] First described in 2009, preinduction VV-ECMO in tracheal surgery was performed without sedation and was used specifically to facilitate laryngoscopy and excision of intratracheal papillomas.[8] In contrast to the presented case requiring prolonged apnea intervals, this report involved a less invasive surgery and required less apneic time with no further alteration to the initial VV-ECMO flow rates. As our case demonstrates, inadequate oxygenation despite optimal VV-ECMO flows necessitates the consideration of alternate oxygenation options. In our case, the anesthesiology team utilized apneic oxygenation via continuous oxygen flow via an orally placed ETT to supplement VV-ECMO flow. Additionally, apneic oxygenation acted as an oxygenation supplement during tracheal anastomosis without compromising the surgical field. The case (patient) had additional factors such as a high BMI and extensive requirement for apneic periods; both of these issues differentiated him from previous case reports and previous approaches applied in similar cases.[9] We hypothesized that a phenomenon akin to apneic oxygenation and/or airway dead-space washout with 100% oxygen facilitated maintaining oxygen saturations >92%, thereby significantly extending apnea that facilitated the swift surgical resection and anastomosis.
Outside of the surgical arena, several reports describe the benefits of passive oxygenation to aid in de-nitrogenation beyond standard means of hyperventilation and hyperoxygenation. 'NODESAT,’ an acronym by Levitan et al.,[10] described the use of nasal cannula as a benefit to have “no desaturation” while instrumenting the airway. Successfully used in a variety of complex airway procedures, 'Transnasal Humidified Rapid-Insufflation Ventilatory Exchange’ (THRIVE) is a similar more aggressive means of increasing apneic time using high-flow oxygen resulting in a flow-dependent dead-space flush-out of CO2 and nitrogen.[11] VV-ECMO is typically a titratable oxygenation adjunct to airway surgery allowing for prolonged periods of apnea during airway instrumentation. VV-ECMO rarely needs supplementation beyond ventilator changes such as increases in FiO2 or respiratory rate to improve oxygenation. However, systemic oxygenation is a poorly understood complex interplay between intrapulmonic shunts, inspired oxygen concentration, and oxygen consumption among other factors. As demonstrated in our case, the improvement in oxygenation by apneic oxygenation provided enough oxygen that the VV-ECMO circuit was able to amplify the exchange rate during high circuit flows, which are normally not seen in any appreciable amount with non-supported respirations. In our case, apneic oxygenation used during the open airway portion of the surgery provided not only tracheal washout but also facilitated turbulent non-laminar flow to the distal airways explaining the substantial increase in oxygenation and prolongation of ventilatory arrest. Our presented case demonstrates also provides a paradigm for troubleshooting parameters to institute along with common VV-ECMO hypoxemia issues.
Apneic oxygenation as an adjunctive measure in the situation described may therefore function in several fashions. Foremost, it was able to sustain oxygen levels by augmenting FiO2. It is unclear as to whether the flow provided was enough to augment functional residual capacity or was simply a function of tracheal washout amplified by VV-ECMO. Secondly, the improvement in oxygenation was during ventilatory arrest. During this time interval, cardiogenic oscillations may have been enough to potentiate oxygen exchange with apneic oxygenation when amplified by VV-ECMO. It is believed that mass airflow during hypoventilation can facilitate mass flow of oxygen into the alveolus when coupled with airflow alterations caused by cardiac contractions.[12] Moreover, as the intrapulmonary shunt (blood bypassing ECMO circuit) increases due to higher CO, there is greater diffusion of CO2 into the alveoli thereby reducing the effective pO2 in it. Additionally, the role of the location of flow is yet to be determined and perhaps the dead-space airways have a more substantial role in de-nitrogenation than previously thought when exposed to high flow rates. In the ICU, maximal ventilatory settings are typically established before VV-ECMO is considered and as such it is used as a treatment option after conventional mechanical ventilation fails. In the operative setting, VV-ECMO was established pre-induction when the patient did not meet these criteria. Then the patient developed subsequent refractory hypoxemia and ventilatory augmentation was achieved to enhance oxygenation. When intraoperative hypoxemia developed, the authors were able to correct it via apneic oxygenation. This alternate augmentation in oxygenation with the airway in discontinuity has not been previously reported.
Although VV-ECMO has become a widely used tool in the ICU and is used increasingly in lung transplant cases, its use during airway surgery still remains limited. VV-ECMO is a useful tool to facilitate an unobstructed surgical field during complex airway surgery but as highlighted in our case, may require adjunctive measures to insure adequate intraprocedural oxygenation and ventilation. Hence, consideration should be given to the use of apneic oxygenation as an adjunctive oxygenation technique when VV-ECMO is used during airway surgery.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.de Perrot M, Fadel E, Mussot S, de Palma A, Chapelier A, Dartevelle P. Resection of locally advanced (T4) non-small cell lung cancer with cardiopulmonary bypass. Ann Thorac Surg. 2005;79:1691–7. doi: 10.1016/j.athoracsur.2004.10.028. [DOI] [PubMed] [Google Scholar]
- 2.Carpenter PS, Pierce JL, Smith ME. Outcomes after cricotracheal resection for idiopathic subglottic stenosis. Laryngoscope. 2018;128:2268–72. doi: 10.1002/lary.27263. [DOI] [PubMed] [Google Scholar]
- 3.Jiménez MJ, Sadurní M, Tió M, Rovira I, Fita G, Martínez E, et al. Apnoeic oxygenation in complex tracheal surgery: O-58. Eur J Anaesthesiol. 2006;23:20. [Google Scholar]
- 4.Fuzaylov G, Cauley BD. Spontaneous ventilation via facemask and laryngeal mask airway as bridge to extracorporeal membrane oxygenation during long-segment tracheal stenosis repair. Pediatr Anesth. 2012;22:1226–8. doi: 10.1111/pan.12044. [DOI] [PubMed] [Google Scholar]
- 5.Cheng QH, Zhang JL, Wang HW, Zhang RJ, Yue Y, Li L. Effect of acute hypercapnia on outcomes and predictive risk factors for complications among patients receiving bronchoscopic interventions under general anesthesia. PLoS One. 2015;10:e0130771. doi: 10.1371/journal.pone.0130771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pillow JJ. High-frequency oscillatory ventilation: Mechanisms of gas exchange and lung mechanics. Crit Care Med. 2005;33(3 Suppl):S135–41. doi: 10.1097/01.ccm.0000155789.52984.b7. [DOI] [PubMed] [Google Scholar]
- 7.Vaporciyan AA, Rice D, Correa AM, Walsh G, Putnam JB, Swisher S, et al. Resection of advanced thoracic malignancies requiring cardiopulmonary bypass. Eur J Cardiothorac Surg. 2002;22:47–52. doi: 10.1016/s1010-7940(02)00204-x. [DOI] [PubMed] [Google Scholar]
- 8.Smith IJ, Sidebotham DA, McGeorge AD, Dorman EB, Wilsher ML, Kolbe J. Use of extracorporeal membrane oxygenation during resection of tracheal papillomatosis. Anesthesiology. 2009;110:427–9. doi: 10.1097/ALN.0b013e3181943288. [DOI] [PubMed] [Google Scholar]
- 9.Patel B, Arcaro M, Chatterjee S. Bedside troubleshooting during venovenous extracorporeal membrane oxygenation (ECMO) J Thorac Dis. 2019;11(Suppl 14):S1698–S1707. doi: 10.21037/jtd.2019.04.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levitan R. No desat! nasal oxygen during efforts securing a tube. [Last accessed 2021 Aug 31]. Available at: http://epmonthly.com/article/no-desat .
- 11.Gleason JM, Christian BR, Barton ED. Nasal cannula apneic oxygenation prevents desaturation during endotracheal intubation: An integrative literature review. West J Emerg Med. 2018;19:403–11. doi: 10.5811/westjem.2017.12.34699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.West JB, Hugh-Jones P. Pulsatile gas flow in bronchi caused by the heart beat. J Appl Physiol. 1961;16:697–702. doi: 10.1152/jappl.1961.16.4.697. [DOI] [PubMed] [Google Scholar]


