Abstract
Background
Component selection and placement in reverse total shoulder arthroplasty (RTSA) is still being debated. Recently, scapulothoracic orientation and posture have emerged as relevant factors when planning an RTSA. However, the degree to which those parameters may influence ROM and whether modifiable elements of implant configuration may be helpful in improving ROM among patients with different postures have not been thoroughly studied, and modeling them may be instructive.
Questions/purposes
Using a dedicated expansion of a conventional preoperative planning software, we asked: (1) How is patient posture likely to influence simulated ROM after virtual RTSA implantation? (2) Do changes in implant configuration, such as humeral component inclination and retrotorsion, or glenoid component size and centricity improve the simulated ROM after virtual RTSA implantation in patients with different posture types?
Methods
In a computer laboratory study, available whole-torso CT scans of 30 patients (20 males and 10 females with a mean age of 65 ± 17 years) were analyzed to determine the posture type (Type A, upright posture, retracted scapulae; Type B, intermediate; Type C, kyphotic posture with protracted scapulae) based on the measured scapula internal rotation as previously described. The measurement of scapular internal rotation, which defines these posture types, was found to have a high intraclass correlation coefficient (0.87) in a previous study, suggesting reliability of the employed classification. Three shoulder surgeons each independently virtually implanted a short, curved, metaphyseal impaction stem RTSA in each patient using three-dimensional (3D) preoperative surgical planning software. Modifications based on the original component positioning were automatically generated, including different humeral component retrotorsion (0°, 20°, and 40° of anatomic and scapular internal rotation) and neck-shaft angle (135°, 145°, and 155°) as well as glenoid component configuration (36-mm concentric, 36-mm eccentric, and 42-mm concentric), resulting in 3720 different RTSA configurations. For each configuration, the maximum potential ROM in different planes was determined by the software, and the effect of different posture types was analyzed by comparing subgroups.
Results
Irrespective of the RTSA implant configuration, the posture types had a strong effect on the calculated ROM in all planes of motion, except for flexion. In particular, simulated ROM in patients with Type C compared with Type A posture demonstrated inferior adduction (median 5° [interquartile range -7° to 20°] versus 15° [IQR 7° to 22°]; p < 0.01), abduction (63° [IQR 48° to 78°] versus 72° [IQR 63° to 82°]; p < 0.01), extension (4° [IQR -8° to 12°] versus 19° [IQR 8° to 27°]; p < 0.01), and external rotation (7° [IQR -5° to 22°] versus 28° [IQR 13° to 39°]; p < 0.01). Lower retrotorsion and a higher neck-shaft angle of the humeral component as well as a small concentric glenosphere resulted in worse overall ROM in patients with Type C posture, with severe restriction of motion in adduction, extension, and external rotation to below 0°.
Conclusion
Different posture types affect the ROM after simulated RTSA implantation, regardless of implant configuration. An individualized choice of component configuration based on scapulothoracic orientation seems to attenuate the negative effects of posture Type B and C. Future studies on ROM after RTSA should consider patient posture and scapulothoracic orientation.
Clinical Relevance
In patients with Type C posture, higher retrotorsion, a lower neck-shaft angle, and a larger or inferior eccentric glenosphere seem to be advantageous.
Introduction
Although one of the main goals in anatomic shoulder arthroplasty is to mimic the native anatomy as close as possible, the configuration in reverse total shoulder arthroplasty (RTSA) that is most likely to maximize ROM is still to be determined. Various implant design and positioning parameters in RTSA can influence the achievable ROM, including glenoid component size, lateral offset, inferior overhang, and the humeral component’s neck-shaft angle, torsion, and offset [26]. Recently, we found in previous research that body posture and subsequent scapulothoracic orientation affect rotational balance after RTSA [19]. In that study, we defined different posture types ranging from Type A (upright posture with a retracted scapula) to C (kyphotic posture with a protracted scapula), and showed that with increasing internal scapular rotation (Type C posture) (Fig. 1), more humeral component retrotorsion is required to balance external and internal rotation.
Fig. 1.

A-B (A) Illustrations and (B) three-dimensional CT images of patients with Types A, B, and C posture show increasing scapular internal rotation, anterior tilt, protraction, and drooping as well as kyphosis and a barrel-shaped chest according to the current study’s results and a previous study’s results [19].
Currently, RTSA planning focuses mainly on glenohumeral anatomy and does not usually consider the patient’s posture. Although the abovementioned theory of adapting the humeral component retrotorsion to patients’ scapulothoracic orientation offers a glimpse at the theoretical importance of considering posture when planning an RTSA, a more thorough investigation is needed to expand the horizon of RTSA planning beyond the confines of the glenohumeral joint. Specifically, the degree to which a patient’s posture may influence ROM after RTSA and whether modifiable elements of implant configuration may be helpful in improving ROM among patients with different postures is unknown. We believe that modeling them may be instructive. Although conventional planning software references its coordinate system based on the scapula, in the present study, we created a model that references its coordinate system on the axes of the body, and thus allowed us to determine scapulothoracic orientation and posture type, as well as their effect on simulated ROM.
By investigating a high number of different implant configurations in patients with different posture types and scapulothoracic orientation, we asked: (1) How is patient posture likely to influence simulated ROM after virtual RTSA implantation? (2) Do changes in implant configuration, such as humeral component inclination and retrotorsion, or glenoid component size and centricity improve simulated ROM after virtual RTSA implantation in patients with different posture types?
Materials and Methods
Study Design, Setting, and Overview
In this study, we used whole-torso CT scans of 30 patients to investigate the influence of patient posture on simulated ROM after virtual RTSA implantation. Raw CT data were loaded into modified surgical planning software, with a novel coordinate system based on the body axes. Patients were then divided into three groups according to their internal scapula rotation (Type A, upright posture, retracted scapulae; Type B, intermediate; Type C, kyphotic posture with protracted scapulae) based on the measured scapula internal rotation as previously described [19]. The simulated ROM with different prosthetic components and configurations then was analyzed (Fig. 2).
Fig. 2.
Overview of study methods. Whole-torso CT scans were loaded into the modified RTSA planning system, using body axes as a coordinate system. Patients were grouped into three different posture types based on their scapulothoracic orientation (Types A, B, and C), and their respective simulated ROM after virtual RTSA implantation with different components and configurations was analyzed.
Patient Cohort
Beginning in January 2020, we retrospectively searched our institution’s radiology database to find patients who had undergone whole-torso CT for non-shoulder-related reasons until we identified 30 shoulders in 30 patients. The following inclusion criteria were used: age at least 18 years; CT images taken with the patient in the supine position, with the arms at the side and elbows resting on the examination table; CT images with complete visualization of the trunk from the occiput to the ischial tuberosity, including a complete depiction of at least one of the humeri; and CT scans performed with the same scanner (Discovery MI, GE Healthcare) and imaging parameters (minimal slice thickness of 1.25 mm) as used for conventional preoperative shoulder arthroplasty planning. Patients with visual findings of the thorax, spine, or upper extremities that can alter measurements of scapular orientation, scapulothoracic dimensions, and humeral torsion (such as fractures, prostheses, or dysplasia) were excluded. Patients with shoulder osteoarthritis were not excluded. All whole-torso CT scans were initially performed for trauma, infection, or malignancy workup.
Participant Characteristics
The resulting study cohort consisted of 20 males and 10 females with a mean age of 65 ± 17 years. Based on the complete visualization of the entire humerus, the right shoulder was analyzed in 21 patients and the left in nine. Of the 30 shoulders included, 16 were nonarthritic, and 14 showed mild to advanced signs of osteoarthritis.
Creation of Models and Anatomic Measurements
The CT images of all 30 patients were loaded into preoperative planning software (Glenosys version 10.5.1, Imascap). The following steps were taken to obtain the ROM for each patient: CT scan selection (manual) and loading (automated); selection of multiple landmarks (manual) in the middle of the spine and sternum (Fig. 3A), the acromion, and elbow epicondyles; three-dimensional (3D) reconstruction of the joint (automated), including the scapula, humerus, and elbow; anatomic measure computation (automated); preoperative planning (manual) of the scapula and humerus; and ROM computation (automated). The initial humerus position for computing ROM was set at 10° of abduction, with the transepicondylar axis of the elbow (selected at Step 2) perpendicular to the sagittal plane (best-fit plane on the sternum and spine points selected at Step 2), which aligns the humeral rotation with the sagittal plane (0° of rotation).
Fig. 3.

A-D (A) This figure shows the manual selection of two of the multiple landmarks in the middle of the spine and sternum in a patient with Type C posture to define the sagittal plane of the body, which is used to align the humeral rotation to 0° in the simulation. Scapulothoracic orientation is determined as follows: (B) The scapula’s internal rotation is the angle between the scapula’s transverse axis (red line) projected onto the transverse plane and the transverse axis (red arrow). (C) The scapula’s upward rotation is the angle between the scapula’s transverse axis (red line) projected onto the coronal plane and the transverse axis (red arrow). (D) The scapular tilt is the angle between an orthogonal axis to the scapular plane (red line) projected onto the sagittal plane and anterior axis (green arrow).
The patient reference coordinate system was defined as follows: The vertical axis is the CT scan axis projected onto the sagittal plane (best-fit plane on the sternum and spine points), the transverse axis is orthogonal to the sagittal plane (best-fit plane on the sternum and spine points), and the anterior axis is the cross-product of the superior axis and transverse axis. Using this reference coordinate system, we defined the following reference planes: sagittal plane, orthogonal to the transverse axis; coronal plane, orthogonal to the anterior axis; and transverse plane, orthogonal to the vertical axis. The three scapular angles were then measured as follows: The scapular internal rotation is the angle between the scapula’s transverse axis projected onto the transverse plane and the transverse axis, the scapular upward rotation is the angle between the scapula’s transverse axis projected onto the coronal plane and transverse axis, and the scapular tilt is the angle between an orthogonal axis to the scapular plane projected onto the sagittal plane and the anterior axis (Fig. 3B-D).
Based on the measured scapular internal rotation, the patients were separated into three different posture types for further subgroup analysis (Type A—upright posture, retracted scapulae; Type B—intermediate; Type C—kyphotic posture with protracted scapulae) as previously described [19]. The following published threshold values were used for classification purposes: Type A ≤ 36°, Type B > 36° to 46°, and Type C ≥ 47°. Measurement of scapular internal rotation has shown a high intraclass correlation coefficient (0.87) in our previous research [19], thus suggesting reliability of this measurement parameter.
As scapulothoracic orientation seems to be affected by thorax shape and kyphosis, the thoracic index was calculated by dividing the transverse thorax diameter at the greatest thoracic expansion by the AP diameter. Global thoracic kyphosis was measured as the Cobb angle between the upper baseplate of the T2 vertebral body and lower baseplate of T12 in the sagittal plane [19].
Posture Types A, B, and C were characterized by an increase in scapular internal rotation and scapular anterior tilt, as well as a decrease in the thoracic index and decreased scapular upward rotation (Table 1).
Table 1.
Comparison of scapulothoracic orientation and thoracic characteristics between different posture types
| Parameter | Type A | Type B | Type C | p value |
| Scapular internal rotation, ° | 32 ± 6 | 42 ± 3 | 53 ± 5 | < 0.001 |
| Scapular upward rotation, ° | -3 ± 6 | -12 ± 7 | -15 ± 13 | 0.07 |
| Scapular anterior tilt, ° | 23 ± 11 | 24 ± 8 | 33 ± 7 | 0.03 |
| Thoracic index (transverse diameter/AP diameter) | 2.5 ± 0.4 | 2.2 ± 0.4 | 1.9 ± 0.3 | 0.41 |
| Thoracic kyphosis, ° | 36 ± 7 | 45 ± 13 | 44 ± 14 | 0.02 |
Data are presented as the mean ± SD; posture Type A = upright posture with retracted scapulae, Type B = intermediate, and Type C = kyphotic posture with protracted scapulae.
Simulated Arthroplasty Configurations
Three experienced shoulder surgeons from different centers (PM, PR, JDW) who were blinded to the posture types assigned to each patient were instructed to virtually plan a short, curved, metaphyseal impaction stem RTSA (Ascend Flex/Perform, Wright Medical Inc.) to the best of their ability in each of the 30 patients. This implant was chosen because it was the one in common use in those surgeons’ practices during the study period, and so they were most familiar with it. As they performed this templating, they were asked to adhere to the following rules: the glenoid baseplate had to be placed within 0° to 10° of retroversion and 0° to 10° of inferior inclination. Although the use of wedges or bone grafts was allowed to correct possible glenoid bone loss and restore the native joint line as represented by the paleoglenoid (the unworn anterior surface of the glenoid), no metallic or bony increased offset baseplate for further lateralization of the native joint line was allowed to be planned. Minimum baseplate seating of 80% had to be achieved, and offset of the humeral component was not allowed.
After we finalized implant position, the following configuration modifications were automatically generated based on the original positioning of the components: humeral component retrotorsion of 0°, 20°, and 40°, as well as individualized retrotorsion based on anatomic retrotorsion of the humeral head and the scapula’s internal rotation; neck-shaft angle of 135°, 145°, and 155°; and 36-mm concentric, 36-mm eccentric, and 42-mm concentric glenosphere. We chose the term retrotorsion instead of the more commonly used term retroversion because it is more suitable to describe the actual geometric variable (Fig. 4). The humeral component’s retrotorsion based on anatomic retrotorsion of the humeral head ranged from 9° to 57° (mean 34°) and internal rotation of the scapula ranged from 20° to 64° (mean 44°).
Fig. 4.
This illustration shows the different changes in humeral component alignment to the proximal humeral metaphysis evaluated in this study, including torsion and inclination. Torsion is rotation of the component around its own axis, and inclination and version are tilt of the component tray or cup in relation to the component’s shaft axis, 90° perpendicular to each other.
Based on the templating performed by the three surgeons in the 30 CT scans, 4050 different RTSA conditions, including different humeral retrotorsion and inclination as well as glenoid component size and eccentricity, were simulated. Three hundred thirty configurations were excluded because of limitations of component combinations in the planning software, leaving 3720 configurations for analysis.
Of the 3720 simulated RTSA configurations, 660 were attributed to five patients with Type A posture, 1650 to 13 patients with Type B posture, and 1410 to 12 patients with Type C posture.
Determination of Simulated ROM
Simulated ROM was automatically computed by the preoperative planning software. Considering the implants that were selected and positioned during the manual planning step, we initially positioned the humerus in 10° of abduction and with the transepicondylar axis of the elbow aligned perpendicular to the sagittal plane. The ROM coordinate system was positioned so that the flexion and extension rotation planes were parallel to the sagittal plane. ROM was simulated in each direction by rotating the humerus, and no impingement was detected between the scapula and humerus (including their respective implants). When there was impingement in the initial humerus position, we reported negative ROM values for adduction, extension, and external rotation. Negative ROM values corresponded to the opposite movement needed to avoid the initial impingement (for example, if 10° of abduction was needed to avoid the initial impingement for adduction, then a -10° adduction value was reported).
For each condition, the maximum potential ROM was exported to a spreadsheet for further analysis. The evaluated planes of motion included adduction, abduction, flexion, extension, internal rotation, and external rotation, as well as hand to the contralateral side, combing hair, and hand to the back pocket motions. The resting position with the arms at the side in the CT scanner was defined as the starting position of the humerus for all planes of measurement. The starting rotation of the humerus for all planes of measurement was adjusted to the body axes to correspond to 0° of arm rotation.
To better display the overall simulated ROM achievable via varying RTSA configurations in the three different posture types, we assigned a combined motion score by summing up the median degrees of motion for all examined planes.
Ethical Approval
This study was approved by the institutional review board at Charité - Universitätsmedizin Berlin, corporate member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, Germany (EA4/119/19).
Statistical Analysis
To analyze the data, we used SPSS Statistics Version 26.0 (IBM). All outcome variables were tested for normal distribution using the Kolmogorov-Smirnov test. All ROM-related outcome variables were not normally distributed. Therefore, results are displayed in terms of medians and interquartile ranges in the text and in the bar charts, and a Kruskal-Wallis test with a Bonferroni correction was used to compare distributions between groups. For better illustration purposes, bar charts were chosen over box plots even if that meant that some significant distribution differences were not clearly visualized. To compare scapular orientation measurements among groups, we used a one-way ANOVA with a Tukey honestly significant difference post hoc test because all three parameters were normally distributed. The level of statistical significance was set to an alpha of 0.05, and all tests were two-sided.
Results
Influence of Posture on Simulated ROM
Irrespective of the RTSA implant configuration, posture type had a strong effect on the calculated ROM in all planes of motion except for flexion. In particular, simulated ROM in patients with Type C compared with Type A posture demonstrated inferior adduction (median 5° [interquartile range -7° to 20°] versus 15° [IQR 7° to 22°]; p < 0.01), abduction (63° [IQR 48° to 78°] versus 72° [IQR 63° to 82°]; p < 0.01), extension (4° [IQR -8° to 12°] versus 19° [IQR 8° to 27°]; p < 0.01), and external rotation (7° [IQR -5° to 22°] versus 28° [IQR 13° to 39°]; p < 0.01), while having only an advantage in internal rotation (104° [ IQR 92° to 121°] versus 92° [IQR 80° to 102°]; p < 0.01) and combing hair motion (36° [IQR 27° to 36°] versus 27° [IQR 27° to 36°]; p < 0.01) (Fig. 5).
Fig. 5.

This graph shows the median simulated ROM and interquartile range in different planes of motion depending on the posture type, irrespective of the component configuration in RTSA. Significant differences are marked by ap < 0.01.
Influence of Implant Configuration on Simulated ROM in Different Posture Types
Lower retrotorsion and a higher neck-shaft angle of the humeral component as well as a small concentric glenosphere led to worse overall ROM in patients with Type C posture compared with Type A, with severe restriction of motion in adduction, extension, and external rotation as described below.
Humeral Component Retrotorsion
In patients with Type A posture, increasing retrotorsion of the humeral component led to gradual improvement in adduction, abduction, external rotation, and hand to the back pocket motion; retrotorsion in flexion, internal rotation, and the combing hair motion gradually diminished. Choosing an individualized humeral component retrotorsion based on anatomic retrotorsion of the humeral head or on the scapular internal rotation produced a more balanced ROM, similar to a general choice of 20° of retrotorsion (Fig. 6A). Patients with Types B and C posture similarly showed improved adduction, abduction, extension, external rotation, hand to contralateral side motion, and hand to the back pocket motion with increasing retrotorsion. Flexion, internal rotation, and the combing hair motion gradually diminished. However, low humeral component retrotorsion in patients with Type B posture and more prominent Type C posture led to severe restrictions in adduction, extension, and external rotation to negative values (Fig. 6B-C).
Fig. 6.
A-D These graphs show the median simulated ROM and interquartile range in different planes of motion depending on the humeral component’s retrotorsion, analyzed by (A) Type A posture, (B) Type B posture, and (C) Type C posture. (D) This graph shows the combined motion score (points = the sum of all median ROM values) for each posture type depending on the humeral component’s retrotorsion. Significant differences are marked by ap < 0.01. IR = internal rotation.
Overall, in patients with Types A and B posture, the humeral component’s retrotorsion had an effect on ROM, but a move in either direction created both advantages and disadvantages. In patients with Type C posture, higher amounts of retrotorsion and individualized retrotorsion based on anatomic retrotorsion of the humeral head and the scapula’s internal rotation led to a better overall ROM (Fig. 6D).
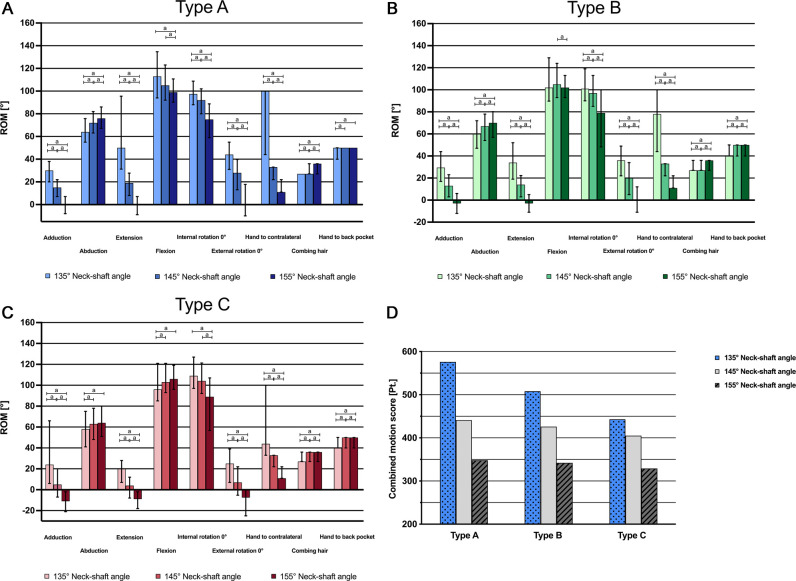
Humeral Component Inclination
In patients with Type A posture, a neck-shaft angle of 135° led to improved adduction, extension, flexion, internal rotation, external rotation, and hand to the contralateral side motion, with disadvantages in abduction and the combing hair and hand to the back pocket motions. The 155° configuration improved in abduction, the combing hair motion, and hand to the back pocket motion (Fig. 7A). In patients with Type B posture, the patterns remained the same except for flexion, for which the advantage of the 135° neck-shaft angle was lost (Fig. 7B). The former advantage in flexion even reversed in patients with Type C posture, while the other advantages persisted. The 155° configuration in patients with Type C posture resulted in severe restriction in adduction, extension, and external rotation (Fig. 7C). Generally, in patients with Type C posture, lower degrees of overall ROM for all three neck-shaft angle configurations were observed. Although the overall ROM advantages of a neck-shaft angle of 135° diminished from Type A posture to Type B to Type C, some of the overall advantage was still evident in patients with Type C posture (Fig. 7D).
Fig. 7.
A-D These graphs show the median simulated ROM and interquartile range in different planes of motion depending on the humeral component’s inclination, analyzed by (A) Type A posture, (B) Type B posture, and (C) Type C posture. (D) This graph shows the combined motion score (points = sum of all median ROM values) in each posture type depending on the humeral component’s inclination. Significant differences are marked by ap < 0.01.
Glenoid Component Configuration
When analyzing the effect of different glenoid configurations based on Type A posture, we found that a larger glenoid size of 42 mm or an inferior eccentric glenosphere of 36 mm achieved a better ROM than a 36-mm glenosphere in adduction, extension, internal rotation, external rotation, and hand to the contralateral side motion. The larger glenosphere showed better extension and the inferior eccentric glenosphere had better abduction (Fig. 8A). In patients with Type B posture, the larger glenosphere showed advantages in adduction, extension, internal rotation, external rotation, and hand to the contralateral side and combing hair motions compared with the smaller concentric and eccentric glenospheres, which only prevailed in abduction (Fig. 8B). Finally, in patients with Type C posture, the larger glenosphere and eccentric smaller glenosphere showed no differences in ROM, but both were superior to the concentric smaller glenosphere in all planes of motion except for abduction and flexion. Notably, adduction, extension, and external rotation were severely restricted with the concentric 36-mm glenosphere (Fig. 8C). Overall, with worsening posture types from A to B to C, the ROM diminished for all glenosphere configurations. The larger and smaller eccentric glenospheres were superior to the smaller concentric glenospheres in all posture types. Although the larger glenosphere showed the best overall ROM in patients with Type B posture and in those with Type A posture, the eccentric smaller glenosphere achieved a similar combined motion score in those with Type C posture (Fig. 8D).
Fig. 8.
A-D These graphs show the median simulated ROM and interquartile range in different planes of motion depending on the glenoid component’s configuration, analyzed by (A) Type A posture, (B) Type B posture, and (C) Type C posture. (D) This graph shows the combined motion score (points = sum of all median ROM values) in each posture type depending on the glenoid component’s configuration. Significant differences are marked by ap < 0.01.
Discussion
Currently, preoperative planning software for shoulder arthroplasty considers only glenohumeral anatomy. However, our own recent findings suggest the importance of body posture and scapulothoracic orientation to obtain rotational balance when planning an RTSA [19]. In the present study, we looked beyond the confines of the glenohumeral joint by adding an analysis of scapulothoracic orientation to conventional shoulder arthroplasty planning software. The goal was to investigate whether patient posture influences simulated ROM after virtual RTSA implantation and which implant configurations provide the best simulated ROM in different posture types. We found that different posture types indeed have a strong effect on the simulated ROM in various planes after RTSA, regardless of implant configuration. An individualized choice of component configuration based on scapulothoracic orientation seems to attenuate the negative effects of posture Types B and C. In patients with Type C posture, who had severe restriction especially in extension and external rotation, we found higher retrotorsion, a lower neck-shaft angle, and a larger or inferior eccentric glenosphere to be advantageous.
Limitations
This study has limitations. For the purpose of this study, we retrospectively searched the institution’s database to find patients who had undergone whole-torso CTs for non-shoulder-related reasons, as shoulder replacement patients only receive shoulder CT scans as standard of care. Nonetheless, we believe that our findings are equally applicable to shoulder arthroplasty patients who have their CT scans performed in the same position and with the same imaging parameters. In the future, it would be interesting to analyze the frequency of different posture types in shoulder arthroplasty patients, and also to evaluate age and sex differences along with posture. We note that CT scans were performed with the patient in the supine position, which affects scapulothoracic orientation. However, in general, only small differences of scapulothoracic orientation have been observed between the supine and standing position for the same participants in a prior comparative CT study (supine: 32° ± 5° internal rotation, 12° ± 5° anterior tilt, 16° ± 4° upward rotation; standing: 30° ± 6° internal rotation, 8° ± 5°anterior tilt, 10° ± 5° upward rotation) [17]. Regardless of the effect of the supine or standing position on posture, the current study’s results suggest that scapulothoracic orientation appears to influence ROM after RTSA. However, future addition of scapulothoracic motion in the analysis seems warranted because this motion contributes to the global ROM in patients who undergo RTSA [29].
Even though only one specific implant was investigated, and the findings might be altered by different implant designs, the general trend of lower neck-shaft angle, more humeral retrotorsion, and more inferior clearance space of the glenosphere deemed beneficial for posture Type C patients in this study is likely to translate to other implant designs in similar ways. Although we considered important component parameters such as humeral inclination and retrotorsion, glenoid component size, and inferior offset, not all possible parameters could be investigated in this study because the resulting number of possible combinations would have multiplied the already considerable number of configurations (n = 3720). To limit the potential confounding effect of glenoid component version and inclination, these parameters were restricted to a range between 0° and 10°. The choice to have three different surgeons perform all virtual implantations was made to limit the influence of personal surgical preferences and improve generalizability of the results.
Furthermore, of the 4050 theoretically possible configurations, only 3720 could be simulated. The remaining 330 configurations would have resulted in component mismatches that are not approved by the implant company because of lack of compatibility, and therefore are not available in the planning software. Finally, we were only able to analyze the virtual ROM that can be achieved with a given RTSA configuration in the bony confinements of the patient’s anatomy. Although many different configurations were analyzed, this model did not include all of the complex factors that can influence postoperative ROM after RTSA, especially that of the soft tissues.
Influence of Posture on Simulated ROM
Posture type had a strong effect on the calculated ROM. Specifically, patients with kyphotic posture with protracted scapulae (Type C) demonstrated generally worse ROM than did patients with upright posture and retracted scapulae (Type A) (Fig. 1). The main explanation for this observation is that patients with Type C posture have more internally rotated and anteriorly tilted scapulae, influencing the simulated ROM after RTSA because of alteration in the starting position between the scapula and humerus. Although scapular upward rotation did not show statistically relevant differences between the groups (p = 0.07), there was a clear trend toward more downward rotation in patients with Types B and C posture, which can also influence the scapulohumeral relation and thus potentially the achievable ROM in different planes. According to the present study’s results, these differences in scapulothoracic orientation between posture types might be a function of change in thoracic dimensions because patients with Type C posture are more likely to have a barrel-shaped thorax. In a previous study, there was an association between Type C posture and increased thoracic kyphosis [19].
Influence of Implant Configuration on Simulated ROM in Different Posture Types
Several adjustments of the implant configuration based on the posture types may help to improve shoulder ROM after RTSA. Especially in patients with posture Type C, the choice of a higher retrotorsion and lower neck-shaft angle of the humeral component, as well as a larger or inferior eccentric glenosphere, seems to be advantageous.
Humeral Component Retrotorsion
Humeral component retrotorsion is being debated regarding its effect on notching and ROM in RTSA [10, 27]. Although a computer-modeling study using 3D models has suggested that less retrotorsion is recommended to facilitate internal rotation after RTSA [10], a biomechanical study indicated that more retrotorsion leads to better external rotation [27]. Although recommendations regarding the ideal humeral component retrotorsion differ, the findings in those two studies were essentially the same; these studies showed that depending on the degree of retrotorsion, there is a tradeoff between external and internal rotation with the arm in adduction. With increasing abduction, this rotational restriction in either direction is reduced [10, 27]. A more recent computer simulation study assessing global ROM found that higher degrees of retrotorsion allowed for improved overall ROM, while less retrotorsion might be better for activities of daily living because these often involve tasks requiring internal rotation [14]. Two clinical studies that compared patients who underwent RTSA and had less humeral component retrotorsion with patients who had higher retrotorsion found no differences in ROM [1, 24]. However, research from Oh et al. [22] showed better ROM with individualized humeral component retrotorsion based on the native humeral head retrotorsion than with a standardized retrotorsion of 20°. The results of the present study confirm the findings of a previous study stating that to achieve balanced ROM, the humeral component’s retrotorsion must be individually adapted to the scapulothoracic orientation and thus the patient’s posture. In patients with Types A and B posture, there are advantages and disadvantages to different degrees of humeral component retrotorsion regarding ROM in different planes. However, in patients with Type C posture, a higher degree of retrotorsion of the humeral component or individualized retrotorsion based on anatomic retrotorsion of the humeral head or scapular internal rotation seems to be advantageous for overall ROM (Fig. 9).
Fig. 9.
A-B These images show a patient with Type C posture and high scapular internal rotation. (A) A virtual implantation of an RTSA implant with the humeral component in 0° of retrotorsion. With the arm in neutral rotation, unbalanced opposition of the humeral and glenoid component can be observed. (B) A virtual implantation of an RTSA implant with the humeral component retrotorsion matching the scapula’s internal rotation. With the arm in neutral rotation, balanced opposition of the components is visible.
Humeral Component Inclination
Humeral component inclination often is set at 155°, based on the Grammont principle [5]. Newer designs of RTSA feature more-anatomic neck-shaft angles as low as 135°. In computer model analyses, a lower neck-shaft angle leads to increased adduction, extension, and external rotation with the arm at the side, with only slightly reduced abduction [11, 15, 30]. In a systematic review of clinical outcomes data, no differences in elevation and abduction were noted, but there was better external rotation with a lower inclination angle of the humeral component [6]. However, a recent randomized trial did not show a difference in ROM or functional outcome after implantation of the same RTSA type in 155° or 135° of humeral component inclination [8]. We found worsening overall ROM from Type A to Type B to Type C, regardless of the humeral component’s inclination. A lower neck-shaft angle of 135° seems to render the best overall ROM in all posture types, although the advantage diminishes from Type A to Type B to Type C posture. Furthermore, in Type C posture, we observed severe restriction in adduction, extension, and external rotation with humeral components with a neck-shaft angle of 155°. The results of our modeling study found a lower neck-shaft angle to be advantageous, especially in patients with Type C posture, and clinicians might consider this during prosthesis selection and templating.
Glenoid Component Configuration
The glenoid component’s configuration can also affect notching and ROM in patients who undergo RTSA. Computer simulation and cadaver studies have shown that lateralization, inferior overhang, and increasing size can improve postoperative ROM by reducing notching and bony impingement [2-4, 12, 13, 16, 30]. Nonetheless, there is disagreement on the importance of one parameter over the other or combinations. Clinical trials comparing smaller and larger glenospheres showed improved ROM and reduced notching with larger glenospheres [18, 20, 28]. However, Sabesan et al. [25] did not find that glenosphere size influenced postoperative ROM. Regarding the presumed advantage of inferior, eccentric glenospheres over concentric glenospheres used for RTSA, although a randomized clinical trial did not show a difference in ROM [23], inferior overhang greater than 3.5 mm seemed to decrease notching. In comparative clinical studies of lateralized and nonlateralized glenoid components, there was increased external rotation with lateralized glenoid components [7, 9, 21]. We found worsening overall ROM from Types A to B to C posture, regardless of the glenoid component’s configuration. The larger and smaller eccentric glenospheres provided better ROM than the smaller concentric glenospheres in all posture types. Especially in patients with Type C posture, there was severe restriction in adduction, extension, and external rotation with small, concentric glenospheres. Therefore, inferior overhang of the glenoid component through a larger glenosphere size or inferior eccentricity should especially be considered in patients with Type C posture.
Conclusion
Different posture types affect the simulated ROM after virtual RTSA implantation, regardless of implant configuration. Patients with posture Types B and C show worse overall ROM after RTSA. An individualized choice of component configuration based on scapulothoracic orientation benefits the potential ROM and could diminish the negative effects of posture Types B and C. Especially in patients with Type C posture, higher retrotorsion and lower neck-shaft angle of the humeral component and a larger or inferior eccentric glenosphere seem to be advantageous and should therefore be considered in clinical practice. Moreover, future clinical studies on ROM after RTSA might also consider patients’ posture and scapulothoracic orientation.
Footnotes
Two authors (MU, JC) are employees of Imascap, Plouzané, France.
One of the authors (MU) certifies receipt of personal payments or benefits, during the study period, in an amount of USD 10,000 to USD 100,000 from Stryker.
One of the authors (PR) certifies receipt of personal payments or benefits, during the study period, in an amount of USD 10,000 to USD 100,000 from Wright Medical Inc.
One of the authors (JDW) certifies receipt of personal payments or benefits, during the study period, in an amount of less than USD 10,000 from FH Ortho.
One of the authors (JC) certifies receipt of personal payments or benefits, during the study period, in an amount of USD 10,000 to USD 100,000 from Stryker.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research® editors and board members are on file with the publication and can be viewed on request.
Ethical approval for this study was obtained from the Charité - Universitätsmedizin Berlin, corporate member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, Germany (EA4/119/19).
This work was performed at the Charité - Universitätsmedizin, Berlin, Germany.
Contributor Information
Philipp Moroder, Email: philipp.moroder@charite.de.
Manuel Urvoy, Email: manuel.urvoy@wright.com.
Patric Raiss, Email: patric.raiss@ocm-muenchen.de.
Jean-David Werthel, Email: jdwerthel@gmail.com.
Doruk Akgün, Email: doruk.akguen@charite.de.
Jean Chaoui, Email: jean.chaoui@wright.com.
References
- 1.Aleem AW, Feeley BT, Austin LS, et al. Effect of humeral component version on outcomes in reverse shoulder arthroplasty. Orthopedics. 2017;40:179-186. [DOI] [PubMed] [Google Scholar]
- 2.Arenas-Miquelez A, Murphy RJ, Rosa A, Caironi D, Zumstein MA. Impact of humeral and glenoid component variations on range of motion in reverse geometry total shoulder arthroplasty: a standardized computer model study. J Shoulder Elbow Surg. 2021;30:763-771. [DOI] [PubMed] [Google Scholar]
- 3.Berhouet J, Garaud P, Favard L. Evaluation of the role of glenosphere design and humeral component retroversion in avoiding scapular notching during reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23:151-158. [DOI] [PubMed] [Google Scholar]
- 4.Berhouet J, Garaud P, Slimane M, et al. Effect of scapular pillar anatomy on scapular impingement in adduction and rotation after reverse shoulder arthroplasty. Orthop Traumatol Surg Res. 2014;100:495-502. [DOI] [PubMed] [Google Scholar]
- 5.Boileau P, Watkinson DJ, Hatzidakis AM, Balg F. Grammont reverse prosthesis: design, rationale, and biomechanics. J Shoulder Elbow Surg. 2005;14:147S-161S. [DOI] [PubMed] [Google Scholar]
- 6.Erickson BJ, Harris JD, Romeo AA. The effect of humeral inclination on range of motion in reverse total shoulder arthroplasty: a systematic review. Am J Orthop (Belle Mead NJ). 2016;45:E174-179. [PubMed] [Google Scholar]
- 7.Franceschetti E, Ranieri R, Giovanetti de Sanctis E, Palumbo A, Franceschi F. Clinical results of bony increased-offset reverse shoulder arthroplasty (BIO-RSA) associated with an onlay 145 degrees curved stem in patients with cuff tear arthropathy: a comparative study. J Shoulder Elbow Surg. 2020;29:58-67. [DOI] [PubMed] [Google Scholar]
- 8.Gobezie R, Shishani Y, Lederman E, Denard PJ. Can a functional difference be detected in reverse arthroplasty with 135 degrees versus 155 degrees prosthesis for the treatment of rotator cuff arthropathy: a prospective randomized study. J Shoulder Elbow Surg. 2019;28:813-818. [DOI] [PubMed] [Google Scholar]
- 9.Greiner S, Schmidt C, Herrmann S, Pauly S, Perka C. Clinical performance of lateralized versus non-lateralized reverse shoulder arthroplasty: a prospective randomized study. J Shoulder Elbow Surg. 2015;24:1397-1404. [DOI] [PubMed] [Google Scholar]
- 10.Gulotta LV, Choi D, Marinello P, et al. Humeral component retroversion in reverse total shoulder arthroplasty: a biomechanical study. J Shoulder Elbow Surg. 2012;21:1121-1127. [DOI] [PubMed] [Google Scholar]
- 11.Gutierrez S, Comiskey CA 4th, Luo ZP, Pupello DR, Frankle MA. Range of impingement-free abduction and adduction deficit after reverse shoulder arthroplasty. Hierarchy of surgical and implant-design-related factors. J Bone Joint Surg Am. 2008;90:2606-2615. [DOI] [PubMed] [Google Scholar]
- 12.Huish EG, Jr, Athwal GS, Neyton L, Walch G. Adjusting implant size and position can improve internal rotation after reverse total shoulder arthroplasty in a three-dimensional computational model. Clin Orthop Relat Res. 2021;479:198-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kolmodin J, Davidson IU, Jun BJ, et al. Scapular notching after reverse total shoulder arthroplasty: prediction using patient-specific osseous anatomy, implant location, and shoulder motion. J Bone Joint Surg Am. 2018;100:1095-1103. [DOI] [PubMed] [Google Scholar]
- 14.Kontaxis A, Chen X, Berhouet J, et al. Humeral version in reverse shoulder arthroplasty affects impingement in activities of daily living. J Shoulder Elbow Surg. 2017;26:1073-1082. [DOI] [PubMed] [Google Scholar]
- 15.Ladermann A, Denard PJ, Boileau P, et al. Effect of humeral stem design on humeral position and range of motion in reverse shoulder arthroplasty. Int Orthop. 2015;39:2205-2213. [DOI] [PubMed] [Google Scholar]
- 16.Ladermann A, Denard PJ, Collin P, et al. Effect of humeral stem and glenosphere designs on range of motion and muscle length in reverse shoulder arthroplasty. Int Orthop. 2020;44:519-530. [DOI] [PubMed] [Google Scholar]
- 17.Matsumura N, Yamada Y, Oki S, et al. Three-dimensional alignment changes of the shoulder girdle between the supine and standing positions. J Orthop Surg Res. 2020;15:411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mollon B, Mahure SA, Roche CP, Zuckerman JD. Impact of glenosphere size on clinical outcomes after reverse total shoulder arthroplasty: an analysis of 297 shoulders. J Shoulder Elbow Surg. 2016;25:763-771. [DOI] [PubMed] [Google Scholar]
- 19.Moroder P, Akgun D, Plachel F, Baur ADJ, Siegert P. The influence of posture and scapulothoracic orientation on the choice of humeral component retrotorsion in reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2020;29:1992-2001. [DOI] [PubMed] [Google Scholar]
- 20.Muller AM, Born M, Jung C, et al. Glenosphere size in reverse shoulder arthroplasty: is larger better for external rotation and abduction strength? J Shoulder Elbow Surg. 2018;27:44-52. [DOI] [PubMed] [Google Scholar]
- 21.Nunes B, Linhares D, Costa F, et al. Lateralized versus nonlateralized glenospheres in reverse shoulder arthroplasty: a systematic review with meta-analysis. J Shoulder Elbow Surg. 2021;30:1700-1713. [DOI] [PubMed] [Google Scholar]
- 22.Oh JH, Sharma N, Rhee SM, Park JH. Do individualized humeral retroversion and subscapularis repair affect the clinical outcomes of reverse total shoulder arthroplasty? J Shoulder Elbow Surg. 2020;29:821-829. [DOI] [PubMed] [Google Scholar]
- 23.Poon PC, Chou J, Young SW, Astley T. A comparison of concentric and eccentric glenospheres in reverse shoulder arthroplasty: a randomized controlled trial. J Bone Joint Surg Am. 2014;96:e138. [DOI] [PubMed] [Google Scholar]
- 24.Rhee YG, Cho NS, Moon SC. Effects of humeral component retroversion on functional outcomes in reverse total shoulder arthroplasty for cuff tear arthropathy. J Shoulder Elbow Surg. 2015;24:1574-1581. [DOI] [PubMed] [Google Scholar]
- 25.Sabesan VJ, Lombardo DJ, Shahriar R, Petersen-Fitts GR, Wiater JM. The effect of glenosphere size on functional outcome for reverse shoulder arthroplasty. Musculoskelet Surg. 2016;100:115-120. [DOI] [PubMed] [Google Scholar]
- 26.Sheth U, Saltzman M. Reverse total shoulder arthroplasty: implant design considerations. Curr Rev Musculoskelet Med. 2019;12:554-561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stephenson DR, Oh JH, McGarry MH, Hatch GFR, 3rd, Lee TQ. Effect of humeral component version on impingement in reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2011;20:652-658. [DOI] [PubMed] [Google Scholar]
- 28.Torrens C, Guirro P, Miquel J, Santana F. Influence of glenosphere size on the development of scapular notching: a prospective randomized study. J Shoulder Elbow Surg. 2016;25:1735-1741. [DOI] [PubMed] [Google Scholar]
- 29.Walker D, Matsuki K, Struk AM, Wright TW, Banks SA. Scapulohumeral rhythm in shoulders with reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2015;24:1129-1134. [DOI] [PubMed] [Google Scholar]
- 30.Werner BS, Chaoui J, Walch G. The influence of humeral neck shaft angle and glenoid lateralization on range of motion in reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2017;26:1726-1731. [DOI] [PubMed] [Google Scholar]