ABSTRACT
Antibiotic therapy of infections caused by the emerging pathogen Mycobacterium abscessus is challenging due to the organism’s inherent resistance to clinically available antimicrobials. The low bactericidal potency of currently available treatment regimens is of concern and testifies to the poor therapeutic outcomes for pulmonary M. abscessus infections. Mechanistically, we demonstrate here that the acetyltransferase Eis2 is responsible for the lack of bactericidal activity of amikacin, the standard aminoglycoside used in combination treatment. In contrast, the aminoglycoside apramycin, with a distinct structure, is not modified by any of the pathogen’s innate aminoglycoside resistance mechanisms and is not affected by the multidrug resistance regulator WhiB7. As a consequence, apramycin uniquely shows potent bactericidal activity against M. abscessus. This favorable feature of apramycin is reflected in a mouse model of pulmonary M. abscessus infection, which demonstrates superior activity, compared with amikacin. These findings encourage the development of apramycin for the treatment of M. abscessus infections and suggest that M. abscessus eradication in pulmonary disease may be within therapeutic reach.
KEYWORDS: apramycin, bactericidal activity, aminoglycoside, Mycobacterium abscessus, cystic fibrosis, pulmonary disease, drug resistance, antibiotic treatment
INTRODUCTION
Nontuberculous mycobacteria (NTM) are ubiquitous environmental organisms comprising numerous pathogens that cause chronic pulmonary infections, particularly among patients with preexisting pulmonary diseases, such as cystic fibrosis (CF), bronchiectasis, and chronic obstructive pulmonary diseases (1–4). Of the rapidly growing members of NTM species, Mycobacterium abscessus complex (MABSC) has evolved as a major respiratory pathogen in individuals with CF, where it leads to accelerated decline in pulmonary function and can compromise the success of lung transplantation (5–7). Pulmonary infections with M. abscessus have become more common, and studies from several countries worldwide have all reported significant increases in the prevalence of M. abscessus infections over the past decade (3, 8). It is estimated that 5% to 15% of individuals with CF in Europe and the United States are infected with M. abscessus (9–11).
MABSC consists of three subspecies, i.e., M. abscessus subsp. abscessus, M. abscessus subsp. bolletii, and M. abscessus subsp. massiliense (2). Its main threat as a pathogen is not least due to its high innate resistance to antibacterial agents, which affects a broad range of commonly used antibiotics (12). Consequently, limited treatment options for MABSC infections exist, and current recommendations suggest that patients with M. abscessus pulmonary disease should receive a multidrug regimen that includes at least three drugs (13–16). Amikacin is considered a cornerstone in the treatment of MABSC infections, particularly infections involving strains that exhibit inducible [erm(41)-dependent] macrolide resistance, as most clinical isolates of M. abscessus do (17, 18). Antibiotic treatment for a full 12 months after culture conversion is recommended; however, culture conversion is the exception rather than the rule. Clinical studies of therapeutic outcomes are sparse and, to date, no standardized antibiotic regimens leading to cure rates of >30% to 50% have been reported, with some variation among the subspecies (19–21). In addition to lengthy courses of antimicrobial chemotherapy, surgery may be required to decrease the burden of disease (19).
In general, antibacterial compounds are categorized as bacteriostatic or bactericidal antimicrobials. The bactericidal activity of an antibiotic is particularly relevant for treatment of chronic infections, such as endocarditis, because bacteriostatic activity alone rarely results in resolution of the infection (22). The poor treatment outcomes in pulmonary infections with M. abscessus are of concern and may be related to the limited bactericidal activity of available treatment regimens. None of the antibacterials used currently in treatment, not even the most potent drug classes such as aminoglycosides and fluoroquinolones, exhibit bactericidal activity (minimal bactericidal concentration [MBC]/MIC of ≤4) against MABSC (23–25).
RESULTS AND DISCUSSION
We determined the dose- and time-dependent kill curves for amikacin against a panel of clinical isolates representing the three MABSC subspecies (Table 1 and Fig. 1 and 2). MICs for amikacin were in the range of 1 to 4 mg/L, and dose- and time-dependent reductions in CFU were found at drug concentrations above the MIC. However, a bactericidal effect, defined as 99.9% reduction of the inoculum CFU counts, was not observed even at the highest amikacin concentration tested (32 mg/L). A concentration of 32 mg/L amikacin, however, is already 3-fold higher than the tissue, epithelial lining fluid, and sputum concentrations of about 10 mg/L achieved after intravenous infusion with therapeutic dosing (26, 27).
TABLE 1.
MICs and MBCs of M. abscessus strains
| Strain | MIC or MBC (mg/L)a |
|||||
|---|---|---|---|---|---|---|
| Amikacin |
Apramycin |
|||||
| MIC | MBC18 | MBC36 | MIC | MBC18 | MBC36 | |
| Clinical isolates | ||||||
| M. abscessus subsp. abscessus 500043/08 | 1 | >32 | >32 | 0.5 | 2 | 1 |
| M. abscessus subsp. abscessus 500042/08 | 1 | >32 | >32 | 0.5 | 4 | 2 |
| M. abscessus subsp. massiliense 500044/09 | 1 | >32 | >32 | 0.5 | 2 | 1 |
| M. abscessus subsp. massiliense 500446/19 | 1 | >32 | >32 | 0.5 | 4 | 2 |
| M. abscessus subsp. bolletii 179709/08 | 1 | >32 | >32 | 0.5 | 4 | 2 |
| M. abscessus subsp. bolletii 181739/08 | 4 | >32 | >32 | 0.5 | 4 | 2 |
| ATCC type strain and isogenic mutants | ||||||
| M. abscessus ATCC 19977 | 1 | >32 | >32 | 0.5 | 2 | 1 |
| M. abscessus Δeis2 | 0.25 | 1 | 1 | 0.5 | 2 | 1 |
| M. abscessus Δaac(2′) | 1 | >32 | >32 | 0.5 | 4 | 2 |
| M. abscessus Δaac(2′) Δeis2 | 0.25 | 2 | 1 | 0.5 | 4 | 2 |
| M. abscessus ΔwhiB7 | 0.25 | ND | ND | 0.5 | ND | ND |
a MBC18, MBC at 18 h of drug exposure; MBC36, MBC at 36 h of drug exposure; ND, not done.
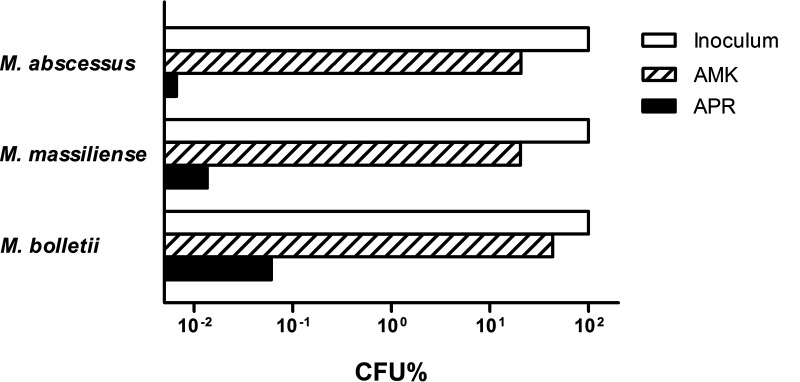
FIG 1.
Bactericidal activity of amikacin (AMK) and apramycin (APR) on clinical isolates. Values indicate the percentage of input CFU (x = 100%) following 18 h of incubation in the presence of 4 mg/L amikacin or 4 mg/L apramycin. For details, see Fig. 2.
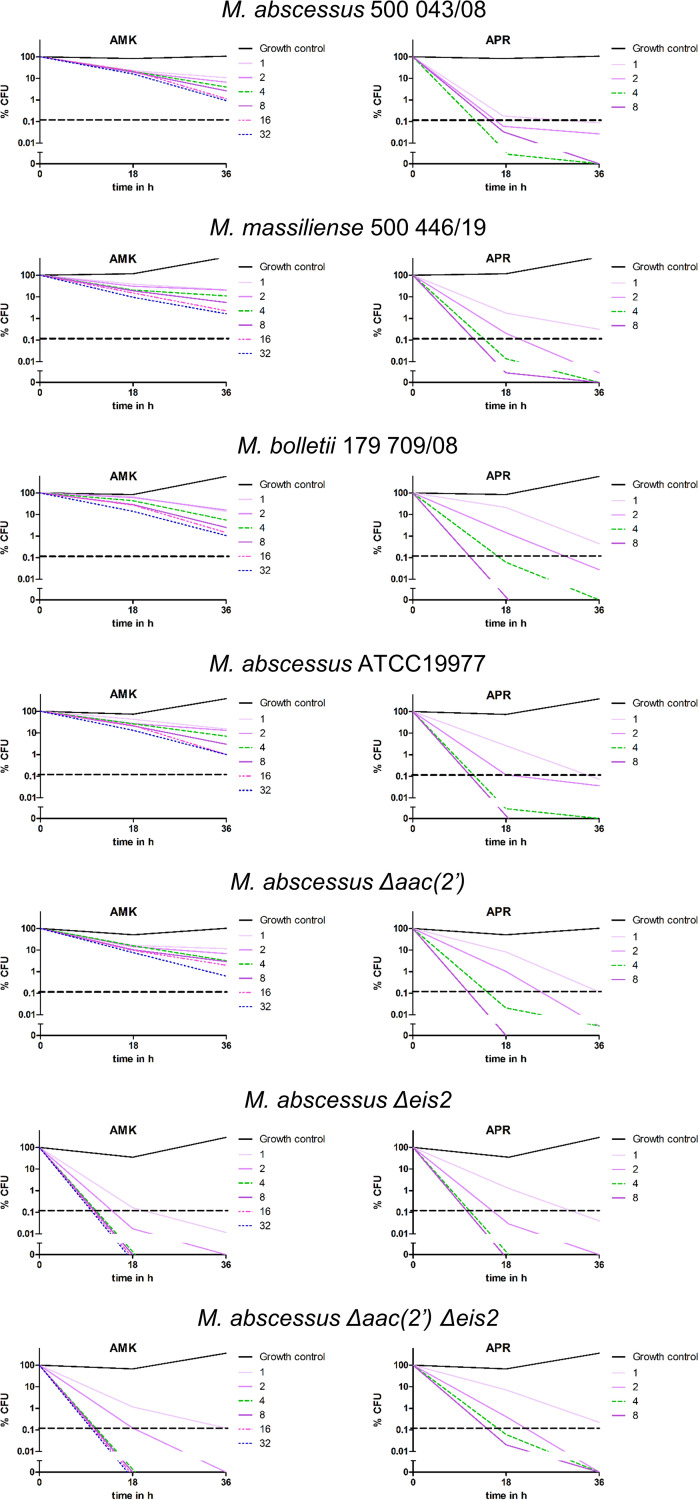
FIG 2.
Time-kill curves for amikacin and apramycin against M. abscessus strains. M. abscessus clinical isolates of the three subspecies, i.e., M. abscessus subsp. abscessus, M. abscessus subsp. massiliense, and M. abscessus subsp. bolletii, as well as M. abscessus ATCC 19977 and its genetically engineered deletion mutants M. abscessus Δeis2, M. abscessus Δaac(2′), and M. abscessus Δaac(2′) Δeis2, were exposed for 18 h or 36 h to various concentrations (0 and 0.125 to 32 mg/L) of amikacin (AMK) and apramycin (APR). Serial dilutions were spotted and incubated at 37°C for 96 h. Bacteria were counted, and the relative number of CFU, compared to time zero, was plotted. The dashed horizontal lines indicate the 99.9% killing threshold that defines bactericidal activity.
The genome of M. abscessus encodes several drug-modifying enzymes (12, 18). We recently showed that M. abscessus aminoglycoside susceptibility is affected by chromosomally encoded aminoglycoside-modifying acetyltransferases (Mabs_4532c and Mabs_4395) (28). Mabs_4532c encodes the promiscuous multiacetyltransferase Eis2 and affects susceptibility to, for example, amikacin and the peptide antibiotic capreomycin, while Mabs_4395 encodes an aminoglycoside 2′-N-acetyltransferase [AAC(2′)] that specifically reduces susceptibility to 2′-NH2-aminoglycosides such as kanamycin B, tobramycin, and gentamicin but spares the 2′-OH aminoglycoside amikacin (28). We hypothesized that eis2 may prevent the bactericidal activity of amikacin. We determined amikacin MIC and time-kill curves for M. abscessus Δeis2 and M. abscessus Δaac(2′) Δeis2 strains. As controls, we used a wild-type (wt) strain and a genetically engineered M. abscessus Δaac(2′) strain. As expected, genetic deletion of aac(2′) did not affect amikacin MICs, while the amikacin MICs in the Δeis2 and Δaac(2′) Δeis2 deletion mutants were 4-fold lower than the amikacin MICs in the isogenic parental strain (28) (Table 1). Similarly, amikacin susceptibility in M. abscessus was affected by whiB7. A strain with whiB7 deleted showed a 4-fold decreased amikacin MIC (Table 1). The whiB7 gene encodes a conserved stress response transcription factor that confers broad-range drug resistance in M. abscessus by acting through various effector mechanisms, e.g., by regulating genes involved in drug modification (eis2), target-modifying genes [erm(41)], and drug efflux pumps (29–31). No bactericidal activity of amikacin was observed for the wt strain or the Δaac(2′) deletion mutant. In contrast, a potent bactericidal effect at low drug concentrations (MBC of 1.0 mg/L) was found for the Δeis2 mutant and the Δaac(2′) Δeis2 double deletion mutant. No difference in the bactericidal activity of amikacin was found between the Δeis2 and Δaac(2′) Δeis2 deletion strains (Table 1 and Fig. 2). These data demonstrate that eis2 is necessary and sufficient to specifically abolish the bactericidal activity of amikacin in M. abscessus.
Apramycin is an aminoglycoside of unique structure that shows potent MIC activity against M. abscessus and exhibits minimal cross-resistance to other aminoglycosides, combined with therapeutic lung exposure and a low toxicity profile (32–35). We determined time- and dose-dependent apramycin kill curves for various MABSC strains, as listed in Table 1. In addition to the genetically engineered deletion mutants of M. abscessus, this panel of strains includes clinical isolates representative of the three subspecies, M. abscessus subsp. abscessus, M. abscessus subsp. bolletii, and M. abscessus subsp. massiliense. The apramycin MICs for all strains were 0.5 mg/L, independent of the subspecies or the presence or absence of eis2, aac(2′), and whiB7. These findings indicate that apramycin is neither a substrate for acetylation by Eis2 or Aac(2′) nor a target for any of the numerous whiB7-dependent drug resistance mechanisms. Consequently, the efficacy of apramycin is unlikely to be affected by antagonistic drug interactions, as observed in clarithromycin-amikacin combination treatment due to macrolide-induced upregulation of WhiB7 (31). Most importantly, at doses as low as 1 to 2 mg/L, apramycin exhibited potent bactericidal activity for all strains tested, irrespective of the presence of eis2 or aac(2′) (Table 1 and Fig. 1 and 2). These findings demonstrate that apramycin overcomes the inherent lack of aminoglycoside bactericidal activity in M. abscessus.
The potent bactericidal activity of apramycin prompted us to test its activity in an in vivo M. abscessus infection model. SCID mice were infected with 106 CFU of M. abscessus and rested for 1 day. Then, groups of mice were treated for 8 consecutive days with either a high dose of amikacin (150 mg/kg), three different doses of apramycin (150, 50, and 15 mg/kg), or vehicle control. CFU counts in lung homogenates were determined at day 1 (before the start of treatment) to confirm manifestation and 1 day after administration of the last antibiotic dose (Fig. 3). An amikacin dose of 150 mg/kg reduced CFU counts in the lungs by approximately 1 log unit. A 10-fold lower apramycin dose of 15 mg/kg resulted in a similar CFU reduction. Upon application of higher doses of apramycin, a dose-dependent CFU reduction of up to 2 log units was observed. Thus, apramycin exceeded the efficacy of amikacin by 1 order of magnitude when the drugs were administered at equivalent doses.
FIG 3.
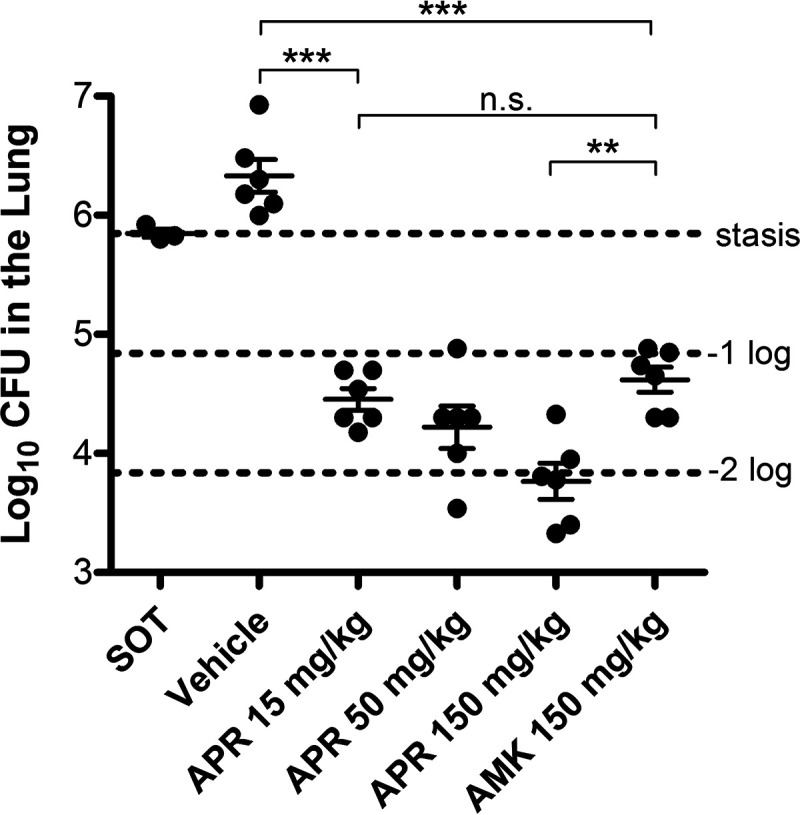
M. abscessus CFU counts in the lungs of mice. SCID mice were intravenously infected with M. abscessus. One day later, once-daily treatment with either amikacin or different doses of apramycin for 8 consecutive days was started. Mice were euthanized 1 day after receiving the last antibiotic dose. Lungs were homogenized, and extracts were plated on 7H11 agar. A group of three mice was used to calculate the bacterial load at the start of treatment (SOT). One control group (n = 6) did not receive antibiotics (vehicle). Statistical analysis was performed using GraphPad Prism version 5 (GraphPad Software). P values were calculated using one-way ANOVA, and Tukey’s multiple-comparison test. n.s., not significant; **, P < 0.01; ***, P < 0.001.
In summary, we demonstrate here that apramycin is an antibiotic with bactericidal activity against M. abscessus. Its activity is affected neither by M. abscessus acetyltransferases nor by the multidrug resistance regulator WhiB7. Our in vitro findings translate into potent pathogen reduction in an in vivo pulmonary infection model, where apramycin is significantly more potent than amikacin, a drug considered a cornerstone in the treatment of M. abscessus infections. These findings warrant the consideration of apramycin for treatment of infections with M. abscessus and suggest that apramycin may offer the promising prospect of M. abscessus eradication in pulmonary disease. In particular, chronically infected CF patients may benefit from the potent bactericidal activity of this drug candidate.
MATERIALS AND METHODS
Mycobacterial strains and culture conditions.
Mycobacterium abscessus strains were grown in cation-adjusted Mueller-Hinton (CAMH) broth. Clinical isolates were obtained from the Institute of Medical Microbiology, University of Zurich, and the National Reference Laboratory for Mycobacteria (Zurich, Switzerland). Strains were identified by rrs (16S rRNA) gene sequencing and typed to the subspecies level by rpoB and erm(41) sequencing (36–38). Genetically engineered derivatives of M. abscessus ATCC 19977 with gene deletions in eis2, aac(2′), or aac(2′) eis2 have been described previously (28). A targeted deletion mutant of M. abscessus deficient in whiB7 (MAB_ 3508c) was constructed by electroporation of competent cells with plasmid pKH-ΔwhiB7, following the procedure described by Rominski et al. (39). In brief, plasmid pKH-whiB7 is a suicide vector containing approximately 1.5 kbp of the upstream and downstream regions of the target gene, facilitating homologous recombination. The upstream-downstream region is cloned adjacent to an aac(3)IV resistance cassette and a DsRed2 marker gene for positive selection and the M. tuberculosis katG gene for negative selection (isoniazid susceptibility) (40). Transformants were selected on apramycin-containing plates and identified by red fluorescence. Single crossover transformants were identified by Southern blotting and subjected to isoniazid counterselection. Single colonies were purified, and deletion of the whiB7 locus was confirmed by Southern blotting.
MIC determinations.
Amikacin and apramycin were purchased from Sigma-Aldrich. Antibiotics were dissolved in water according to the manufacturer’s recommendations, filter sterilized, aliquoted into stock solutions, and stored at −20°C. MIC determinations were performed according to CLSI guideline M24 (41) and as described (39). Antibiotic stock solutions were prepared in CAMH broth to a concentration of 64 mg/L, and 2-fold serial dilutions in CAMH broth were prepared using sterile 96-well microtiter plates (Greiner Bio-One, Switzerland). A positive growth control lacking antibiotic and a sterile negative control containing CAMH broth only were included in each 96-well microtiter plate. For preparation of the inoculum, three or four colonies from a bacterial strain grown on LB agar were transferred, using a sterile cotton swab, into a tube containing 2 mL of NaCl. In order to achieve a final inoculum titer of 1 × 105 to 5 × 105 CFU/mL for MIC and 1 × 106 to 5 × 106 CFU/mL for MBC (see below), respectively, all bacterial suspensions were adjusted to turbidity equivalent to that of a 0.50 McFarland standard and subsequently diluted in CAMH broth. The final test volume in each well of the microtiter plate was 0.1 mL. The correct titer of each inoculum was checked by assessing CFU counts on LB agar plates. The microdilution plates were capped with adhesive sealing covers and incubated at 37°C for 3 days before the MIC values were assessed by visual inspection. All MIC assays were conducted in triplicate. The MIC was defined as the lowest antibiotic concentration that prevented visible bacterial growth.
Time-kill curves and MBC determinations.
At the start of the experiment, the bacterial inoculum was determined by spotting 10-fold serial dilutions of the bacterial suspension from the no-drug control on agar plates and counting CFU. After 18 h and 36 h of incubation, bacterial cells from the MIC plates were resuspended by pipetting prior to spotting of 5-μl aliquots of 10-fold serial dilution series on LB agar plates. The agar plates were incubated for 96 h at 37°C, and CFU were counted. The relative CFU counts were adjusted to the inoculum at time zero. The MBC was defined as the lowest antibiotic concentration that reduced the CFU of the inoculum by ≥99.9%.
In vivo infection experiments.
Female SCID mice (Charles River Laboratories), 7 to 9 weeks of age, were infected by intravenous tail vein injection with 1 × 106 CFU/mouse of M. abscessus (strain 103, a clinical isolate from a CF patient) (42). Three mice were sacrificed at day 1 postinfection to determine bacterial manifestation prior to the start of treatment. Once-daily antibiotic treatment by subcutaneous injection was started 1 day after infection and continued for 8 consecutive days. The following doses were applied: amikacin, 150 mg/kg/day; apramycin, 150 mg/kg/day, 50 mg/kg/day, or 15 mg/kg/day. Saline served as a vehicle control. Treated mice were sacrificed at day 10 postinfection (including 8 days of antibiotic treatment). Whole lungs were extracted, homogenized in 4.5 mL of 1× phosphate-buffered saline (PBS), and plated in 10-fold serial dilutions on Middlebrook 7H11 agar. Plates were incubated for 7 days at 37°C prior to CFU counting.
The Colorado State University (CSU) animal care program follows the recommendations of the National Research Council Guide for the Care and Use of Laboratory Animals (43), the requirements of the Public Health Service (PHS) grants administration manual, and the Animal Welfare Act as amended. CSU files assurances with the DHHS Office of Extramural Research, Office of Laboratory Animal Welfare (OLAW), PHS, and adheres to NIH standards and practices for grantees. The CSU animal welfare assurance number is A3572-01.
Statistical analysis.
Bacterial burdens in the untreated control and drug-treated animal organs were analyzed with GraphPad Prism version 5 (GraphPad Software, San Diego, CA). P values were calculated using one-way analysis of variance (ANOVA) and Tukey’s multiple-comparison test. Data are presented using the mean values (n = 6) ± the standard errors of the mean (SEMs). Significance was considered for P values of <0.05.
ACKNOWLEDGMENTS
Work in the laboratory of P. Sander is supported by the Swiss National Science Foundation (grant 310030_197699) and the Cystic Fibrosis Foundation Switzerland (CFCH). Several of the more recent in vivo approaches were developed using funding from NIH grants AI070456 and AI08959 and a New Innovative Award to D. J. Ordway. This study was also supported by the University of Zurich, Institute of Medical Microbiology.
We thank Susanna Salas for expert secretarial assistance. We thank the Mycobacteria Research Laboratory at CSU for their contributions to the development of the animal model described here.
E. C. Böttger and P. Sander conceived the study. D. J. Ordway, S. N. Hobbie, E. C. Böttger, and P. Sander designed the experiments. P. Selchow and A. Petrig conducted in vitro experiments. D. Verma and N. Whittel conducted animal experiments. E. C. Böttger and P. Sander wrote the manuscript, with input from all coauthors. The current version of the manuscript was approved by all authors.
S. N. Hobbie and E. C. Böttger are cofounders of and equity holders in Juvabis AG. The other authors have nothing to declare.
REFERENCES
- 1.Iseman MD, Marras TK. 2008. The importance of nontuberculous mycobacterial lung disease. Am J Respir Crit Care Med 178:999–1000. 10.1164/rccm.200808-1258ED. [DOI] [PubMed] [Google Scholar]
- 2.Tortoli E, Fedrizzi T, Meehan CJ, Trovato A, Grottola A, Giacobazzi E, Serpini GF, Tagliazucchi S, Fabio A, Bettua C, Bertorelli R, Frascaro F, De Sanctis V, Pecorari M, Jousson O, Segata N, Cirillo DM. 2017. The new phylogeny of the genus Mycobacterium: the old and the news. Infect Genet Evol 56:19–25. 10.1016/j.meegid.2017.10.013. [DOI] [PubMed] [Google Scholar]
- 3.Gardner AI, McClenaghan E, Saint G, McNamara PS, Brodlie M, Thomas MF. 2019. Epidemiology of nontuberculous mycobacteria infection in children and young people with cystic fibrosis: analysis of UK Cystic Fibrosis Registry. Clin Infect Dis 68:731–737. 10.1093/cid/ciy531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.To K, Cao R, Yegiazaryan A, Owens J, Venketaraman V. 2020. General overview of nontuberculous mycobacteria opportunistic pathogens: Mycobacterium avium and Mycobacterium abscessus. J Clin Med 9:2541. 10.3390/jcm9082541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Esther CR, Jr, Esserman DA, Gilligan P, Kerr A, Noone PG. 2010. Chronic Mycobacterium abscessus infection and lung function decline in cystic fibrosis. J Cyst Fibros 9:117–123. 10.1016/j.jcf.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Orens JB, Estenne M, Arcasoy S, Conte JV, Corris P, Egan JJ, Egan T, Keshavjee S, Knoop C, Kotloff R, Martinez FJ, Nathan S, Palmer S, Patterson A, Singer L, Snell G, Studer S, Vachiery JL, Glanville AR, Pulmonary Scientific Council of the International Society for Heart and Lung Transplantation. 2006. International guidelines for the selection of lung transplant candidates: 2006 update–a consensus report from the Pulmonary Scientific Council of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant 25:745–755. 10.1016/j.healun.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 7.Qvist T, Taylor-Robinson D, Waldmann E, Olesen HV, Hansen CR, Mathiesen IH, Høiby N, Katzenstein TL, Smyth RL, Diggle PJ, Pressler T. 2016. Comparing the harmful effects of nontuberculous mycobacteria and Gram negative bacteria on lung function in patients with cystic fibrosis. J Cyst Fibros 15:380–385. 10.1016/j.jcf.2015.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bryant JM, Grogono DM, Greaves D, Foweraker J, Roddick I, Inns T, Reacher M, Haworth CS, Curran MD, Harris SR, Peacock SJ, Parkhill J, Floto RA. 2013. Whole-genome sequencing to identify transmission of Mycobacterium abscessus between patients with cystic fibrosis: a retrospective cohort study. Lancet 381:1551–1560. 10.1016/S0140-6736(13)60632-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seddon P, Fidler K, Raman S, Wyatt H, Ruiz G, Elston C, Perrin F, Gyi K, Bilton D, Drobniewski F, Newport M. 2013. Prevalence of nontuberculous mycobacteria in cystic fibrosis clinics, United Kingdom, 2009. Emerg Infect Dis 19:1128–1130. 10.3201/eid1907.120615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cystic Fibrosis Foundation. 2012. Patient registry: annual data report 2011. Cystic Fibrosis Foundation, Bethesda, MD. [Google Scholar]
- 11.Low D, Wilson DA, Flume PA. 2020. Screening practices for nontuberculous mycobacteria at US cystic fibrosis centers. J Cyst Fibros 19:569–574. 10.1016/j.jcf.2020.02.013. [DOI] [PubMed] [Google Scholar]
- 12.Nessar R, Cambau E, Reyrat JM, Murray A, Gicquel B. 2012. Mycobacterium abscessus: a new antibiotic nightmare. J Antimicrob Chemother 67:810–818. 10.1093/jac/dkr578. [DOI] [PubMed] [Google Scholar]
- 13.Daley CL, Iaccarino JM, Lange C, Cambau E, Wallace RJ, Andrejak C, Böttger EC, Brozek J, Griffith DE, Guglielmetti L, Huitt GA, Knight SL, Leitman P, Marras TK, Olivier KN, Santin M, Stout JE, Tortoli E, van Ingen J, Wagner D, Winthrop KL. 2020. Treatment of nontuberculous mycobacterial pulmonary disease: an official ATS/ERS/ESCMID/IDSA clinical practice guideline. Eur Respir J 56:2000535. 10.1183/13993003.00535-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haworth CS, Banks J, Capstick T, Fisher AJ, Gorsuch T, Laurenson IF, Leitch A, Loebinger MR, Milburn HJ, Nightingale M, Ormerod P, Shingadia D, Smith D, Whitehead N, Wilson R, Floto RA. 2017. British Thoracic Society guideline for the management of non-tuberculous mycobacterial pulmonary disease (NTM-PD). BMJ Open Respir Res 4:e000242. 10.1136/bmjresp-2017-000242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lange C, Böttger EC, Cambau E, Griffith DE, Guglielmetti L, van Ingen J,Knight SL, Marras TK, Oliver KN, Santin M, Stout JE, Tortoli E, Wagner D, Winthrop K, Daley CL, on behalf of the Expert Panel Group for Management Recommendations in NTM-PD. Consensus management recommendations for non-tuberculous mycobacterial pulmonary diseases. Lancet Infect Dis, in press. [DOI] [PubMed] [Google Scholar]
- 16.Haworth CS, Floto RA. 2017. Introducing the new BTS Guideline: management of non-tuberculous mycobacterial pulmonary disease (NTM-PD). Thorax 72:969–970. 10.1136/thoraxjnl-2017-210929. [DOI] [PubMed] [Google Scholar]
- 17.Johansen MD, Herrmann JL, Kremer L. 2020. Non-tuberculous mycobacteria and the rise of Mycobacterium abscessus. Nat Rev Microbiol 18:392–407. 10.1038/s41579-020-0331-1. [DOI] [PubMed] [Google Scholar]
- 18.Luthra S, Rominski A, Sander P. 2018. The role of antibiotic-target-modifying and antibiotic-modifying enzymes in Mycobacterium abscessus drug resistance. Front Microbiol 9:2179. 10.3389/fmicb.2018.02179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jarand J, Levin A, Zhang LN, Huitt G, Mitchell JD, Daley CL. 2011. Clinical and microbiologic outcomes in patients receiving treatment for Mycobacterium abscessus pulmonary disease. Clin Infect Dis 52:565–571. 10.1093/cid/ciq237. [DOI] [PubMed] [Google Scholar]
- 20.Jeon K, Kwon OJ, Lee NY, Kim B-J, Kook Y-H, Lee S-H, Park YK, Kim CK, Koh W-J. 2009. Antibiotic treatment of Mycobacterium abscessus lung disease: a retrospective analysis of 65 patients. Am J Respir Crit Care Med 180:896–902. 10.1164/rccm.200905-0704OC. [DOI] [PubMed] [Google Scholar]
- 21.Kwak N, Dalcolmo MP, Daley CL, Eather G, Gayoso R, Hasegawa N, Jhun BW, Koh W-J, Namkoong H, Park J, Thomson R, van Ingen J, Zweijpfenning SMH, Yim J-J. 2019. Mycobacterium abscessus pulmonary disease: individual patient data meta-analysis. Eur Respir J 54:1801991. 10.1183/13993003.01991-2018. [DOI] [PubMed] [Google Scholar]
- 22.Habib G, Hoen B, Tornos P, Thuny F, Prendergast B, Vilacosta I, Moreillon P, de Jesus Antunes M, Thilen U, Lekakis J, Lengyel M, Müller L, Naber CK, Nihoyannopoulos P, Moritz A, Zamorano JL, ESC Committee for Practice Guidelines. 2009. Guidelines on the prevention, diagnosis, and treatment of infective endocarditis (new version 2009). Eur Heart J 30:2369–2413. 10.1093/eurheartj/ehp285. [DOI] [PubMed] [Google Scholar]
- 23.Maurer FP, Bruderer VL, Ritter C, Castelberg C, Bloemberg GV, Bottger EC. 2014. Lack of antimicrobial bactericidal activity in Mycobacterium abscessus. Antimicrob Agents Chemother 58:3828–3836. 10.1128/AAC.02448-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee J, Ammerman N, Agarwal A, Naji M, Li SY, Nuermberger E. 2021. Differential in vitro activities of individual drugs and bedaquiline-rifabutin combinations against actively multiplying and nutrient-starved Mycobacterium abscessus. Antimicrob Agents Chemother 65:e02179-20. 10.1128/AAC.02179-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yam YK, Alvarez N, Go ML, Dick T. 2020. Extreme drug tolerance of Mycobacterium abscessus “persisters.” Front Microbiol 11:359. 10.3389/fmicb.2020.00359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Canis F, Husson MO, Turck D, Vic P, Launay V, Ategbo S, Vincent A, Courcol RJ. 1997. Pharmacokinetics and bronchial diffusion of single daily dose amikacin in cystic fibrosis patients. J Antimicrob Chemother 39:431–433. 10.1093/jac/39.3.431. [DOI] [PubMed] [Google Scholar]
- 27.Cruciani M, Gatti G, Cazzadori A, Concia E. 1996. Pharmacokinetics of antimicrobial agents in the respiratory tract. Zentralbl Bakteriol 284:1–31. 10.1016/S0934-8840(96)80150-2. [DOI] [PubMed] [Google Scholar]
- 28.Rominski A, Selchow P, Becker K, Brulle JK, Dal Molin M, Sander P. 2017. Elucidation of Mycobacterium abscessus aminoglycoside and capreomycin resistance by targeted deletion of three putative resistance genes. J Antimicrob Chemother 72:2191–2200. 10.1093/jac/dkx125. [DOI] [PubMed] [Google Scholar]
- 29.Hurst-Hess K, Rudra P, Ghosh P. 2017. Mycobacterium abscessus WhiB7 regulates a species-specific repertoire of genes to confer extreme antibiotic resistance. Antimicrob Agents Chemother 61:e01347-17. 10.1128/AAC.01347-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Richard M, Gutierrez AV, Kremera L. 2020. Dissecting erm(41)-mediated macrolide-inducible resistance in Mycobacterium abscessus. Antimicrob Agents Chemother 64:e01879-19. 10.1128/AAC.01879-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pryjma M, Burian J, Kuchinski K, Thompson CJ. 2017. Antagonism between front-line antibiotics clarithromycin and amikacin in the treatment of Mycobacterium abscessus infections is mediated by the whiB7 gene. Antimicrob Agents Chemother 61:e01353-17. 10.1128/AAC.01353-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ishikawa M, García-Mateo N, Čusak A, López-Hernández I, Fernández-Martínez M, Müller M, Rüttiger L, Singer W, Löwenheim H, Kosec G, Fujs Š, Martínez-Martínez L, Schimmang T, Petković H, Knipper M, Durán-Alonso MB. 2019. Lower ototoxicity and absence of hidden hearing loss point to gentamicin C1a and apramycin as promising antibiotics for clinical use. Sci Rep 9:2410. 10.1038/s41598-019-38634-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Juhas M, Widlake E, Teo J, Huseby DL, Tyrrell JM, Polikanov YS, Ercan O, Petersson A, Cao S, Aboklaish AF, Rominski A, Crich D, Böttger EC, Walsh TR, Hughes D, Hobbie SN. 2019. In vitro activity of apramycin against multidrug-, carbapenem- and aminoglycoside-resistant Enterobacteriaceae and Acinetobacter baumannii. J Antimicrob Chemother 74:944–952. 10.1093/jac/dky546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matt T, Ng CL, Lang K, Sha S-H, Akbergenov R, Shcherbakov D, Meyer M, Duscha S, Xie J, Dubbaka SR, Perez-Fernandez D, Vasella A, Ramakrishnan V, Schacht J, Böttger EC. 2012. Dissociation of antibacterial activity and aminoglycoside ototoxicity in the 4-monosubstituted 2-deoxystreptamine apramycin. Proc Natl Acad Sci USA 109:10984–10989. 10.1073/pnas.1204073109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Becker K, Aranzana-Climent V, Cao S, Nilsson A, Shariatgorji R, Haldimann K, Platzack B, Hughes D, Andrén PE, Böttger EC, Friberg LE, Hobbie SN, ENABLE Consortium. 2021. Efficacy of EBL-1003 (apramycin) against Acinetobacter baumannii lung infections in mice. Clin Microbiol Infect 27:1315–1321. 10.1016/j.cmi.2020.12.004. [DOI] [PubMed] [Google Scholar]
- 36.Kim H-Y, Kim BJ, Kook Y, Yun Y-J, Shin JH, Kim B-J, Kook Y-H. 2010. Mycobacterium massiliense is differentiated from Mycobacterium abscessus and Mycobacterium bolletii by erythromycin ribosome methyltransferase gene (erm) and clarithromycin susceptibility patterns. Microbiol Immunol 54:347–353. 10.1111/j.1348-0421.2010.00221.x. [DOI] [PubMed] [Google Scholar]
- 37.Adékambi T, Colson P, Drancourt M. 2003. rpoB-based identification of nonpigmented and late-pigmenting rapidly growing mycobacteria. J Clin Microbiol 41:5699–5708. 10.1128/JCM.41.12.5699-5708.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Springer B, Stockman L, Teschner K, Roberts GD, Bottger EC. 1996. Two-laboratory collaborative study on identification of mycobacteria: molecular versus phenotypic methods. J Clin Microbiol 34:296–303. 10.1128/jcm.34.2.296-303.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rominski A, Roditscheff A, Selchow P, Bottger EC, Sander P. 2017. Intrinsic rifamycin resistance of Mycobacterium abscessus is mediated by ADP-ribosyltransferase MAB_0591. J Antimicrob Chemother 72:376–384. 10.1093/jac/dkw466. [DOI] [PubMed] [Google Scholar]
- 40.Gagliardi A, Selchow P, Luthra S, Schafle D, Schulthess B, Sander P. 2020. KatG as counterselection marker for nontuberculous mycobacteria. Antimicrob Agents Chemother 64:e02508-19. 10.1128/AAC.02508-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Clinical and Laboratory Standards Institute. 2018. Susceptibility testing of mycobacteria, Nocardia spp., and other aerobic actinomycetes, 3rd ed. CLSI document M24. Clinical and Laboratory Standards Institute, Wayne, PA. [PubMed] [Google Scholar]
- 42.Obregón-Henao A, Arnett KA, Henao-Tamayo M, Massoudi L, Creissen E, Andries K, Lenaerts AJ, Ordway DJ. 2015. Susceptibility of Mycobacterium abscessus to antimycobacterial drugs in preclinical models. Antimicrob Agents Chemother 59:6904–6912. 10.1128/AAC.00459-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.National Research Council. 2011. Guide for the care and use of laboratory animals, 8th ed. National Academies Press, Washington, DC. [Google Scholar]