ABSTRACT
Although intra-articular vancomycin powder (VP) is sometimes applied before the closure of the incision to prevent periprosthetic joint infection (PJI) after joint replacement, the dosage, efficacy, and safety remain controversial. This study aimed to explore the dosage, efficacy, and safety of intra-articular VP in the prophylaxis of infection after total knee arthroplasty (TKA) in a rat model. Sixty male rats were randomly divided into five groups after receiving TKA: control (no antibiotics); systemic vancomycin (SV) (intraperitoneal injection, 88 mg/kg of body weight, equal to 1 g in a patient weighing 70 kg); and VP0.5, VP1.0, and VP2.0 (44 mg/kg, 88 mg/kg and 176 mg/kg, respectively; intra-articular). All animals were inoculated in the knee with methicillin-resistant S. aureus (MRSA). General status, serum biomarkers, radiology, microbiological assay, and histopathological tests were assessed within 14 days postoperation. Compared with the control and SV groups, bacterial counts, knee width, tissue inflammation, and osteolysis were reduced in the VP0.5, VP1.0, and VP2.0 groups, without notable body weight loss and incision complications. Among all the VP groups, VP1.0 and VP2.0 groups presented superior outcomes with regard to knee width and tissue inflammation than the VP0.5 group. Microbial culture indicated that no MRSA survived in the knee of VP1.0 and VP2.0 groups, while bacteria growth was observed in the VP0.5 group. No obvious changes in the structure and functional biomarkers of liver and kidney were observed in either the SV or VP groups. Therefore, intra-articular vancomycin powder at a dosage from 88 mg/kg to 176 mg/kg may be effective and safe in preventing PJI induced by methicillin-resistant S. aureus in the rat TKA model.
KEYWORDS: infection prophylaxis, intra-articular, periprosthetic joint infection, rat model, total knee arthroplasty, vancomycin powder
INTRODUCTION
Periprosthetic joint infection (PJI) is a serious complication after artificial joint replacement, occurring in 1% to 2% of primary arthroplasties (1), which not only severely damages the joint function of the patients but also entails a heavy medical burden. Staphylococcus aureus is one of the most common pathogens in all the PJI cases (2), while approximately 47% of S. aureus strains clinically isolated in the United States are methicillin-resistant S. aureus (MRSA) (3). Although preoperative systemic antibiotics play an important role in infection prophylaxis, PJI continues to occur. Surgeons sometimes attribute such infections to the poor antimicrobial activity of prophylactic antibiotics like the first- or second-generation cephalosporins (4). Therefore, local application of antibiotics such as vancomycin in increasingly attracting the attention of surgeons. Some clinical studies demonstrated that the use of vancomycin powder in the joint replacement surgery wound could significantly reduce the rate of PJI without severe complications (5, 6). However, several prospective and retrospective studies showed that intra-articular vancomycin did not alter the infection rate but increased the incidence of complications in the sterile wounds (7–11). All these observations show that the efficacy and safety of intra-articular VP in PJI prophylaxis are still controversial.
Theoretically, the effectiveness of antibiotics mainly depends on dosage, antibiotic spectrum, and drug half-life. The dose of intrawound VP in most of the previous studies was 1 g or 2 g, which was based on the surgeons’ experience or habits. The optimal prophylactic dosage of intra-articular VP was not evaluated in any of the aforementioned studies. We searched the literature and found that no standard protocol or guideline concerning the local application of VP in the arthroplasty surgery has been established to date. This study was intended to explore the dosage, efficacy, and safety of intra-articular VP in infection prophylaxis after total knee arthroplasty in a rat model, in order to provide evidence for clinical strategies of PJI prophylaxis.
RESULTS
General status of the animals.
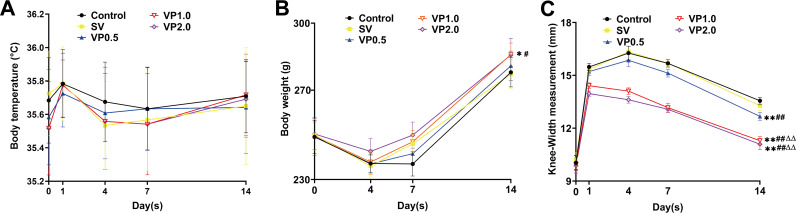
Sixty male rats were randomly divided into five groups after undergoing total knee arthroplasty (TKA): control (no antibiotics); systemic vancomycin (SV) (intraperitoneal injection, 88 mg/kg of body weight, equal to 1 g in a patient weighing 70 kg); and vancomycin powder at 44 mg/kg, 88 mg/kg, and 176 mg/kg (VP0.5, VP1.0, and VP2.0, respectively; intra-articular). No significant differences in body temperature were observed among the five groups throughout the experiment (Fig. 1A) (P > 0.05). All animals showed a trend of weight loss on day 4 after surgery. No significant differences in body weight were detected among the control, SV, and VP0.5 groups on postsurgical day 14 (Fig. 1B) (P > 0.05), whereas the body weights of the rats in the VP1.0 and VP2.0 groups were greater than those in the control, SV, and VP0.5 groups (Fig. 1B) (P < 0.05). No significant differences were observed in knee width among the five groups preoperatively (Fig. 1C) (P > 0.05). After bacterial inoculation, the surgical knee was swollen and the maximal medial-lateral knee width increased. On day 14, the average knee widths ± the standard errors of the means in control, SV, VP0.5, VP1.0, and VP2.0 groups were 13.5 ± 0.2 mm, 13.3 ± 0.3 mm, 12.7 ± 0.2 mm, 11.3 ± 0.2 mm, and 11.1 ± 0.3 mm, respectively. No significant difference between the control and SV groups was observed (Fig. 1C) (P > 0.05), whereas the knee widths of rats in the VP0.5, VP1.0, and VP2.0 groups were less than those of the control and SV groups, and rats in the VP1.0 and VP2.0 groups showed smaller knee widths than those in the VP0.5 group (Fig. 1C) (P < 0.05).
FIG 1.
Changes in general status of the rats. (A) Changes in body temperature throughout the experiment. An electronic thermometer for animals and an infrared thermometer were used to measure the anal and rectal temperatures of the rats. The body temperature of rats in each group was measured preoperatively and on postoperative days 1, 4, 7, and 14. (B) The weights of rats in each group were measured preoperatively and on postoperative days 4, 7, and 14. (C) The maximal medial-lateral knee width of rats in each group was measured preoperatively and on postoperative days 1, 4, 7, and 14. Control, no antibiotics; SV, systemic vancomycin (88 mg/kg, intraperitoneal injection, 30 min preoperation); VP0.5: vancomycin powder, 44 mg/kg, once intra-articularly before closure of the knee capsule in the TKA surgery; VP1.0: intra-articular vancomycin powder, 88 mg/kg; VP2.0: intra-articular vancomycin powder, 176 mg/kg. Data were compared by two-way ANOVA. n = 12. *, P < 0.05, and **, P < 0.01 (compared with the control group); #, P < 0.05, and ##, P < 0.01 (compared with the SV group), ΔΔ, P < 0.01 (compared with the VP0.5 group).
Radiological evaluation.
X-ray imaging showed that the prostheses were still in position on postoperative day 14 (Fig. 2A). Mild to moderate osteolysis was observed in rats from the VP0.5, SV, and control groups by micro-computed tomography (micro-CT) (Fig. 2B); bone volumes (BV, in cubic millimeters) of the distal femur, proximal tibia, and total bone in the VP1.0 and VP2.0 groups were greater than those of the control, SV, and VP0.5 groups (Fig. 2C to E) (P < 0.05). No significant differences in BV of the distal femur, proximal tibia, and total bone were detected between the VP1.0 and VP2.0 groups (Fig. 2C to E) (P = 0.075, 0.602, and 0.076).
FIG 2.
Radiological evaluation of the knee joint and prostheses on postoperation day 14. (A) Representative X-ray of each group on day 14 (n = 6). (B) Representative CT scan of distal femurs and proximal tibias on days 14 (n = 5). (C) Bone volume analysis of the distal femur. (D) Bone volume analysis of the proximal tibia. (E) Total bone volume analysis of the distal femur and proximal tibia. Control, no antibiotics; SV, systemic vancomycin (88 mg/kg, intraperitoneal injection, 30 min preoperation); VP0.5, vancomycin powder, 44 mg/kg, once intra-articularly before closure of the knee capsule in TKA; VP1.0, intra-articular vancomycin powder, 88 mg/kg; VP2.0, intra-articular vancomycin powder, 176 mg/kg.
Local infection in the surgical knee.
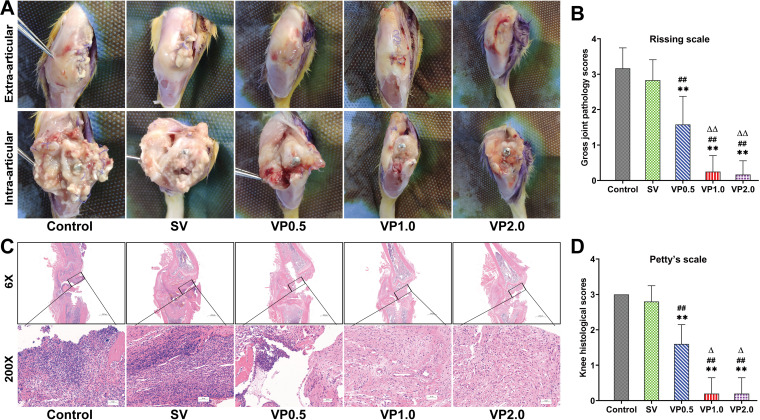
Distorted soft tissue, ulceration, purulent intra-articular material, eroded periprosthetic bone, and prosthesis loosening were observed in the control and SV groups by macroscopic examination of the surgical knee, while such changes were all improved in the VP0.5, VP1.0, and VP2.0 groups, especially in the VP1.0 and VP2.0 groups (Fig. 3A). Mean modified Rissing scale scores in the VP1.0 and VP2.0 groups were significantly lower than those in the control, SV, and VP0.5 groups at 14 days postoperation (P<0.01) (Fig. 3B). Acute osteomyelitis was observed in the control and SV groups, with intramedullary abscess and infiltrations of lymphocytes and leukocytes (Fig. 3C). Such changes were largely attenuated in the VP groups, and the smallest inflammatory changes were observed in rats in the VP1.0 and VP2.0 groups, with almost no lymphocyte or leukocyte infiltration in the articular cavity. Mean modified Petty’s scale scores in the VP1.0 and VP2.0 groups were significantly lower than those in the control, SV, and VP0.5 groups (P<0.01) (Fig. 3D).
FIG 3.
Macroscopic examination and histopathological assessment of the surgical knee. (A) Representative macroscopic examination of extra-articular and intra-articular knee joints on postoperative day 14 in each treatment group. (B) The gross joint pathology scores based on the criteria of modified Rissing scale on postoperative day 14 in each treatment group. (C) Representative pathological H&E staining of knee joints in each group on postoperative day 14 in each treatment group. Control, no antibiotics; SV, systemic vancomycin (88 mg/kg, 30 min preoperation); VP0.5, vancomycin powder, 44 mg/kg, once intra-articularly before closure of the knee capsule in the TKA surgery; VP1.0, intra-articular vancomycin powder, 88 mg/kg; VP2.0: intra-articular vancomycin powder, 176 mg/kg. n = 5. The black box represents the area of distal femur osteotomy. (D) Knee histological scores based on the criteria of modified Petty’s scale on postoperative day 14 in each treatment group.
Counts of bacteria in the surgical knee.
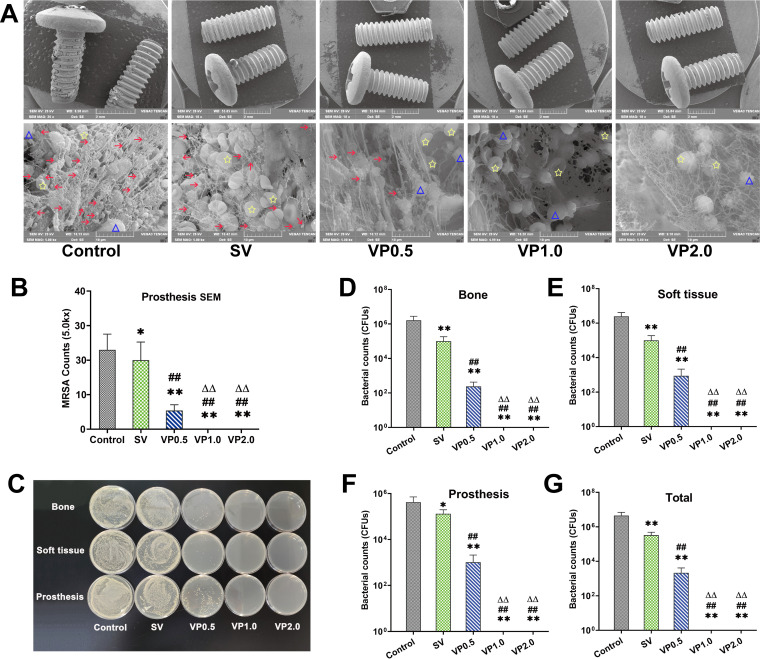
Greater quantities of MRSA particles were observed on the prostheses in the control and SV groups using scanning electron microscopy (SEM), surrounded by host leukocytes or/and erythrocytes, with no other microbial contamination found in any field of the view (Fig. 4A). No bacteria were detected on the prostheses from the VP1.0 and VP2.0 groups. Few microorganisms remained in the VP0.5 group, with fewer MRSA particles detected in the VP0.5 group than in the control and SV groups. (Fig. 4A and B). The numbers of bacterial colonies of each specimen and the whole animal in each treatment group are shown in Table 1, and typical microbial cultures on LB agar are shown in Fig. 4C. The average numbers of CFU of each specimen and whole animals in the VP0.5 group were significantly lower than those in the control and SV groups but higher than those in the VP1.0 and VP2.0 groups (no bacterial residue in any specimen) (Fig. 4D to G) (P < 0.01).
FIG 4.
Microorganisms in specimens from rats in each treatment group. (A) SEM scanning of the prostheses. On day 14, the microbes on the surface of prostheses in each group were observed by SEM at low magnification (×20) and high magnification (×5,000). Red arrows indicate MRSA, blue triangles indicate leukocytes, and yellow stars indicate erythrocytes. (B) Bacterial counting by SEM. Five visual fields were randomly selected from the prostheses for observation under high magnification (×5,000) of SEM and bacterial counting. (C) Representative image of LB agar petri dishes of microbial culture of prostheses, bone, and soft tissue from each treatment group of animals. (D) Bacterial counts of knee joint bones in each treatment group. (E) Bacterial counts of all soft tissues around the knee joints in each treatment group. (F) Bacterial counts of prostheses in each treatment group. (G) Bacterial counts of the whole knee in each treatment group. Control, no antibiotics; SV, systemic vancomycin (88 mg/kg, intraperitoneal injection, 30 min preoperation); VP0.5, vancomycin powder, 44 mg/kg, once intra-articularly before closure of the knee capsule in TKA; VP1.0, intra-articular vancomycin powder, 88 mg/kg; VP2.0, intra-articular vancomycin powder, 176 mg/kg. Data were compared by an unpaired 1-tailed Mann-Whitney test. n = 7. *, P < 0.05, and **, P < 0.01 (compared with the control group); ##, P < 0.01 (compared with the SV group); ΔΔ, P < 0.01 (compared with the VP0.5 group).
TABLE 1.
| Groupa | No. of CFU (mean ± standard errors of the means)b |
|||
|---|---|---|---|---|
| Bone | Prosthesis | Soft tissue | Total | |
| Control | 1.62 × 106 ± 1.19 × 106 | 4.24 × 105 ± 2.90 × 105 | 2.49 × 106 ± 1.64 × 106 | 4.28 × 106 ± 2.75 × 106 |
| SV | 1.01 × 105 ± 8.22 × 104** | 1.32 × 105 ± 6.53 × 104* | 1.00 × 105 ± 8.89 × 104** | 3.32 × 105 ± 1.53 × 105** |
| VP0.5 | 239 ± 184**# | 1,035 ± 1,078**# | 871 ± 1,277**# | 2,145 ± 1,996**# |
| VP1.0 | 0**#Δ | 0**#Δ | 0**#Δ | 0**#Δ |
| VP2.0 | 0**#Δ | 0**#Δ | 0**#Δ | 0**#Δ |
Control, no antibiotics; SV, systemic vancomycin (88 mg/kg, intraperitoneal injection, 30 min preoperation); VP0.5, vancomycin powder, 44 mg/kg, once intra-articularly before closure of the knee capsule in TKA; VP1.0, intra-articular vancomycin powder, 88 mg/kg; VP2.0: intra-articular vancomycin powder, 176 mg/kg.
Data were compared by one-way ANOVA. n = 7. *, P < 0.05, and **, P < 0.01 (compared with the control group); #, P < 0.01 (compared with the SV group); Δ, P < 0.01 (compared with the VP0.5 group).
Safety of intra-articular VP after TKA surgery.
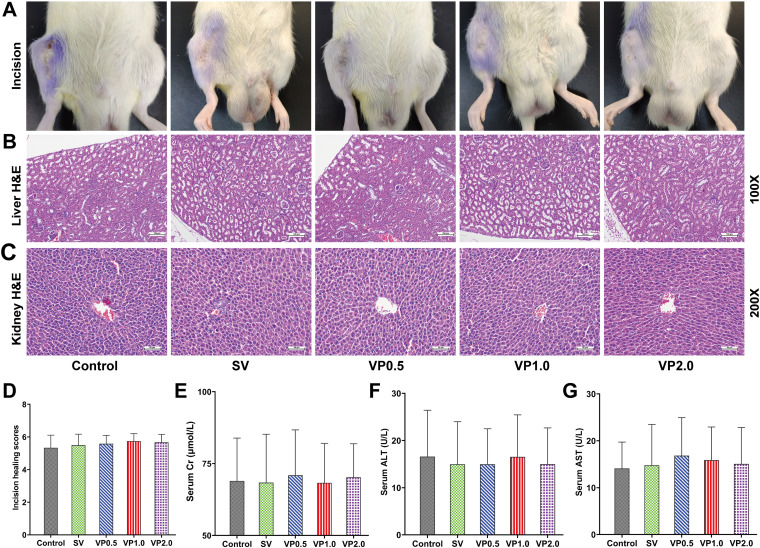
All the incisions were completely healed on day 14 in each treatment group without wound rupture or exudation; the scores for incision healing were greater than 4 (Fig. 5A and D). No obvious pathological changes in the liver and kidney, such as degeneration and necrosis, were observed in any animal (Fig. 5B and C); the scores for renal and liver injury were 0. No significant differences were observed among the control and vancomycin treatment groups in serum creatinine (Cr), alanine aminotransferase (ALT), and aspartate aminotransferase (AST) (Fig. 5E to G) (P > 0.05).
FIG 5.
Drug safety assessments. (A) Representative photographs of incision healing in rats in each treatment group. (B) Representative pathological H&E staining of the kidney (×100) in each treatment group. (C) Representative pathological H&E staining of the liver (×200) in each treatment group. (D) Incision healing scores of each treatment group on postoperative day 14. (E) Serum Cr on postoperative day 14 in each treatment group. (F) Serum ALT on postoperative day 14 in each treatment group. (G) Serum AST on postoperative day 14 in each treatment group. Control, no antibiotics; SV, system vancomycin (88 mg/kg, intraperitoneal injection, 30 min preoperation); VP0.5, vancomycin powder, 44 mg/kg, once intra-articularly before closure of the knee capsule in TKA; VP1.0, intra-articular vancomycin powder, 88 mg/kg; VP2.0, intra-articular vancomycin powder, 176 mg/kg. Data were compared by two-way ANOVA. n = 12.
DISCUSSION
Several studies have demonstrated that the use of VP in the incision of joint replacement surgery could reduce the rate of PJI. Patel et al. reviewed 460 total joint arthroplasty procedures performed by a single surgeon and found that intrawound vancomycin decreased both the overall infection rate (0.57% versus 2.7%; P = 0.031) and the PJI rate (0.29% versus 2.7%; P = 0.009) compared with the untreated group (5). A report by Smith et al. also indicated that the use of vancomycin as the perioperative prophylactic antibiotic for primary total joint arthroplasties appeared to be effective in decreasing the rate of PJI and, when such infections do occur, may result in infections with less virulent organisms (12). Whiteside also reported that intra-articular vancomycin significantly reduced the prevalence of PJI within the first 3 months from 4.1% to 0% (13). A meta-analysis concerning the local application of VP for the prevention of surgical site infections (SSIs) in primary total joint arthroplasty (TJA) suggested that VP could significantly decrease the rate of SSIs and PJI in primary TJA without modifying the spectrum of bacteria involved (14). However, almost all these studies were retrospective studies, and the optimal and safe prophylactic dosage of intra-articular VP was not evaluated in any of them. Our rat-based study used a simplified model that mimics the application of VP in clinical TKA, which might provide supplemental evidence concerning the ideal dosage of VP in preventing infection after TJA surgery. Our animal study data showed that more than 88 mg/kg of vancomycin intra-articular powder (equal to 1.0 g in patients weighing 70 kg) could eliminate MRSA in the joint tissues and on the surface of the prostheses and resulted in less body weight loss, less bone osteolysis around the prostheses, and milder inflammatory reactions in the joint tissues than in the control group and the groups receiving 88 mg/kg of systematic vancomycin and 44 mg/kg of intra-articular vancomycin powder (equal to 0.5 g in a patient). All this evidence suggested that proper intra-articular vancomycin powder before closure of the incision might be effective in preventing PJI after total joint replacement.
Dial et al. retrospectively reviewed 265 consecutive patients undergoing TJA, including 128 patients who did not receive VP and 137 patients who received VP at the time of wound closure, and found that the sterile wound complication rate was 4.4% in the VP group and 0% in the control group (7). Ishida et al. suggested that local use of VP mixed with the bone graft may have detrimental effects on spinal fusion outcomes, especially at a supraphysiological dosage (15). This finding may cause surgeons to vacillate about the application of VP in the incision for total joint replacement, as there has not been a sufficient safety evaluation of VP. Here, we determined the serum concentration of VP application in the incision after TKA surgery and found the serum levels of vancomycin in the SV, VP1.0, and VP2.0 groups were all higher than the MIC for MRSA (ATCC 43300; 2 μg/mL) within 2 h after TKA surgery but lower than the serum level that could cause nephrotoxicity (15 to 20 μg/mL) (16–19) (Table 2). Moreover, no obvious toxicity was observed in the structure and function of the liver and kidney, as well as any severe incision complications in rats receiving VP in the articular cavity after TKA. BV analysis by micro-CT also showed that at the same level of MRSA infection, local application of VP from 88 mg/kg to 176 mg/kg had no significantly adverse effects on the bone retention after TKA in rats. Taken together, our data indicated that intra-articular use of VP might be a safe prophylaxis for PJI after TKA caused by MRSA in a rat model.
TABLE 2.
Serum levels of vancomycin after the use of vancomycin in TKA in each group
| Groupa | Serum level (μg/mL) atb: |
|||
|---|---|---|---|---|
| 0.5 h | 2 h | 4 h | 8 h | |
| Control | 0 | 0 | 0 | 0 |
| SV | 10.09 ± 1.25 | 3.79 ± 0.31 | 0.49 ± 0.04 | — |
| VP0.5 | 1.48 ± 0.27 | 0.53 ± 0.07 | 0.40 ± 0.06 | 0.35 ± 0.02 |
| VP1.0 | 3.02 ± 0.33 | 0.69 ± 0.10 | 0.53 ± 0.04 | 0.38 ± 0.03 |
| VP2.0 | 5.31 ± 0.78 | 1.42 ± 0.18 | 0.74 ± 0.07 | 0.44 ± 0.03 |
Control, no antibiotics; SV, systemic vancomycin (88 mg/kg, intraperitoneal injection, 30 min preoperation); VP0.5, vancomycin powder, 44 mg/kg, once intra-articularly before closure of the knee capsule in TKA; VP1.0, intra-articular vancomycin powder, 88 mg/kg; VP2.0: intra-articular vancomycin powder, 176 mg/kg.
Data are presented as the means ± standard errors of the means. n = 6. —, below the limit of detection (0.1 μg/mL).
However, this study has some limitations. First, the absorption, distribution, metabolism, and excretion of drugs in rats are not the same as in humans, the biodynamics and biomechanics of the rat knee are also not the same as those of the human knee, and the prostheses were screwed into the tibia and femur without any fixation by cement, which means that the current rat-based study could not exactly mimic the PJI that occurs in patients after TKA. Second, S. aureus and Staphylococcus epidermidis are the most common pathogens in PJI cases after primary joint arthroplasty (2), while only methicillin-resistant S. aureus was used in the current study. Meanwhile, 105 CFU of MRSA, a higher bacterial load than is found in clinical PJI cases, was applied in this study, although the bacterial load was determined based on previous animal studies that indicated a repeatable and stable infection in rat model (20–27). Third, our study focused only on early local infection after TKA, rather than late chronic infection or blood-borne infection. Thus, further studies are needed to evaluate the application of intra-articular VP in preventing late chronic infection and blood-borne infection, as well as infections caused by other pathogens. Fourth, although some available literature suggested that diluted povidone-iodine with/without vancomycin powder can affect the PJI microorganism profiles (28, 29), our study aimed to compare the prophylactic effect of different vancomycin administration approaches based on uniform TKA surgical procedures. Last, the 14-day endpoint may have been too early for observation the long-term toxicity of vancomycin, third-body wear at the prostheses interface, late infection, and bone healing; further studies are needed.
In conclusion, intra-articular vancomycin powder at dosages from 88 mg/kg to 176 mg/kg (equal to 1.0 g to 2.0 g in a patient) may be effective and safe in preventing PJI induced by methicillin-resistant S. aureus in the rat TKA model.
MATERIALS AND METHODS
Animals and reagents.
Specific-pathogen-free (SPF) Wistar rats (male, aged 10 weeks, weighing 250 g ± 7 g) were used. All animal experimental procedures were performed following the Guidelines for the Care and Use of Laboratory Animals of the Animal Welfare Committee. Clinical-grade vancomycin hydrochloride for injection was obtained from Lilly (Japan).
Bacteria.
Individual colonies of methicillin-resistant S. aureus (MRSA; ATCC 43300) were grown in Luria-Bertani (LB) broth. When log-phase growth was achieved, bacterial suspension was centrifuged and the supernatant was discarded. Bacteria were resuspended with phosphate-buffered saline (PBS) solution to achieve a concentration of approximately 1.66 × 107 CFU/mL as confirmed by serial dilution and plating on agar plates. In a pilot study, we established that inoculation of 40 μl of 1.66 × 107 CFU/mL ATCC 43300 was sufficient to produce a stable PJI in a rat model after TKA surgery in 2 weeks.
Study design.
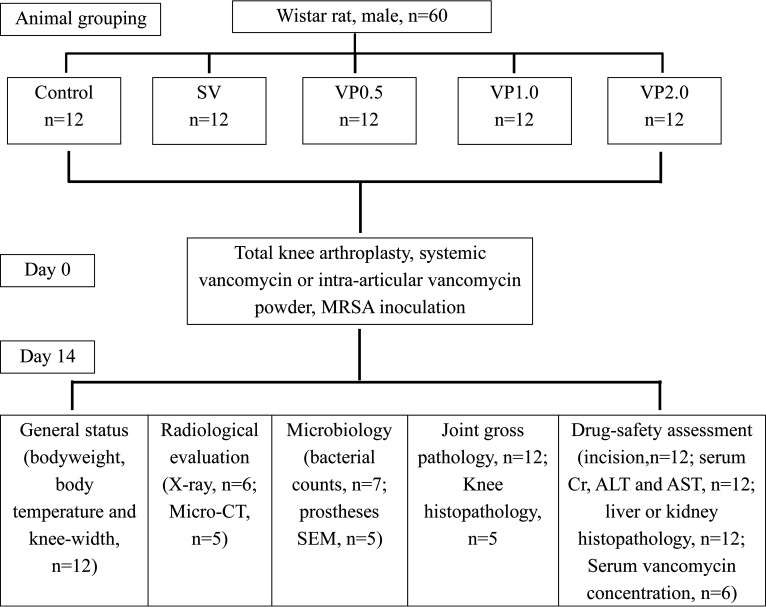
On the basis of previously described murine PJI models (30–32), we chose stainless steel metal and polyethylene to mimic clinical TKA surgery. Sixty male Wistar rats were randomly assigned to 5 groups consisting of 12 rats each: (i) control (no antibiotics), (ii) SV (systemic vancomycin; 88 mg/kg per rat via intraperitoneal injection 30 min preoperation, equal to 1 g in a patient weighing 70 kg), (iii) VP0.5 (intra-articular vancomycin powder, 44 mg/kg per rat once intra-articularly before closure of the knee capsule in TKA, equal to 0.5 g in a patient weighing 70 kg), (iv) VP1.0 (intra-articular vancomycin powder, 88 mg/kg per rat, equal to 1 g in a patient weighing 70 kg), and (v) VP2.0 (intra-articular vancomycin powder, 176 mg/kg per rat, equal to 2 g in a patient weighing 70 kg). Doses for the weight-based systemic vancomycin and local vancomycin administrations were based on routine prophylactic antibiotic doses used for skeletal infections in humans (33–35) and correspond to vancomycin doses used in a prior rat model (27, 36, 37). Table 3 reports the allocation of animals per group and the analyses performed.
TABLE 3.
Allocation of animals per group and investigation
| Analyses | Animal (n = 12/group) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
| TKA surgery + bacterial inoculation (day 0) | X | X | X | X | X | X | X | X | X | X | X | X |
| Serum levels of vancomycin (day 0) | X | X | X | X | X | X | ||||||
| General status (days 0, 1, 4, 7, 14) | X | X | X | X | X | X | X | X | X | X | X | X |
| Incision examination (days 0, 1, 4, 7, 14) | X | X | X | X | X | X | X | X | X | X | X | X |
| X-ray (day 14) | X | X | X | X | X | X | ||||||
| Micro-CT (day 14) | X | X | X | X | X | |||||||
| Knee histology (day 14) | X | X | X | X | X | |||||||
| Prosthesis SEM (day 14) | X | X | X | X | X | |||||||
| Microbiology (day 14) | X | X | X | X | X | X | X | |||||
| Liver or kidney histology (day 14) | X | X | X | X | X | X | X | X | X | X | X | X |
| Serum ALT, AST, or Cr analysis (day 14) | X | X | X | X | X | X | X | X | X | X | X | X |
Surgical procedure.
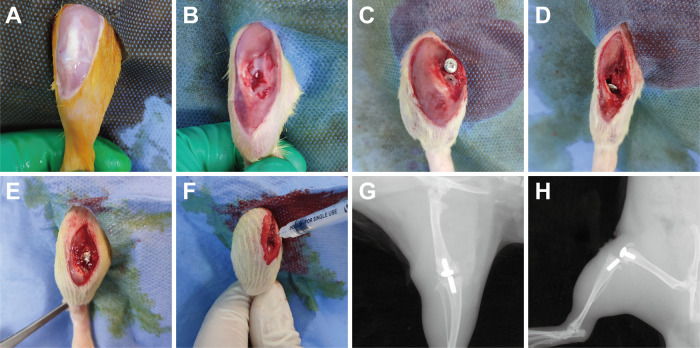
The rats (n = 12 per group) were anesthetized using 2.5% isoflurane by inhalation delivered via nose cone and were given preoperative analgesics consisting of subcutaneous buprenorphine (0.1 mg/kg) and parecoxib (2 mg/kg). After sterile draping of the surgical site, a 2-cm midline longitudinal skin incision was made over the right knee and a medial parapatellar arthrotomy was performed (Fig. 6A and B). After knee joint exposure, the anterior cruciate ligament and medial and lateral meniscus were removed. Most of the cartilage surface of the knee joint was cut off. Then, an artificial joint prosthesis (stainless steel metal and common polyethylene, ethylene oxide sterilization) was attached to the distal femur and proximal tibia after reaming (Fig. 6C and D). The vancomycin powder was then uniformly distributed into the wound (medullary cavity, articular cavity, prosthesis interface, synovial surface) in animals in the VP groups (Fig. 6E). After suturing of the joint capsule, the joint cavity was injected with 40 μL of 1.66 × 107 CFU/mL MRSA (ATCC 43300) using a 29-gauge needle (Fig. 6F). X-rays were performed immediately after surgery to confirm the position of the prosthesis (Fig. 6G and H). Pain was controlled with buprenorphine (0.1 mg/kg) for 3 days postsurgery. The affected limb was not fixed or immobilized. The rats were monitored daily for incision status, joint swelling, and any local soft tissue or systemic reaction related to vancomycin. On day 14 after surgery, all animals were sacrificed for blood collection and tissue harvest in accordance with the Institutional Animal Care and Use Committee-approved protocol (Fig. 7).
FIG 6.
Surgical procedures for total knee arthroplasty and modeling of the periprosthetic joint infection in rat. (A to D) Total knee arthroplasty (TKA) surgery procedures in a rat. (E) The vancomycin powder (VP) was placed directly into knee joint cavity and surrounding synovial tissue in each rat that assigned to the VP groups. (F) Forty microliters of 1.66 × 107 CFU/mL of methicillin-resistant S. aureus (MRSA, ATCC 43300) was inoculated intra-articularly after capsule suturing. (G and H) Anteroposterior and lateral X rays were performed postoperatively.
FIG 7.
Treatment scheme for the animals in this experiment.
General status and serum biomarkers.
Body weight, body temperature, and maximal medial-lateral knee width (as described by Miller et al. [1]) of rats in each group were measured preoperatively and on postoperative days 1, 4, 7, and 14. Serum samples were obtained by centrifugation (3,000 rpm, 4°C, 15 min). Serum Cr, ALT, and AST were measured with an enzyme-linked immunosorbent assay (ELISA) kit (Cusabio, China) on postoperative day 14. The serum concentrations of vancomycin at 0.5 h, 2 h, 4 h, and 8 h after vancomycin application were detected by high-performance liquid chromatography-mass spectrometry (HPLC-MS; Thermo TSQ Quantis, USA) (external standard; filter, SRM MS2 725.80–1307.30 m/z; mass, 1,307.30 m/z; retention time, 1.58 min; solvents, 1% formic acid water and pure acetonitrile; columns, Hypersil Gold [Thermo Fisher], 100 by 2.1 mm, 3 μm; flow rate, 0.2 mL/min; time, 6 min).
X-ray evaluation of the knee.
X-ray images (n = 6) were obtained for the right limbs of the animals on days 14 and assessed by experienced observers blind to treatment, to confirm the position of the prosthesis and the osteolysis around the prosthesis (Bruker Xtreme BI, Germany; filter, 0.4 mm; kilovoltage peak, 45 kV; exposure time, 1.2 s; bin, 1 × 1 pixel; field of view [FOV], 10 cm; F stop, 2).
Micro-computed tomographic and data analysis.
The prostheses were carefully removed from the joints, and then micro-CT analysis (n = 5) was performed to evaluate the bone volume of the distal femur and proximal tibia using a SkyScan 1276 scanner (Bruker, Germany) (voltage, 85 kV; current, 200 μA; exposure time, 384 ms/projection; scan duration, 0 h 6 min 50 s; camera pixel size, 17.420 μm; isotropic resolution, 93 μm; image pixel size, 9.0338 μm; filter, Al 0.5 mm; 180° rotation). Scan images were reconstructed and bone parameters surrounding the prostheses were assessed using CT-viewer version 2.0.4.5 software (Materialise, Belgium). We analyzed the three-dimensional reconstructed bone images with CT-An software (Bruker, Germany). After scan calibration, we created two identical box volumes of interest (VOI), with a size of 1,327.446 mm3 (x, 13.55 mm; y, 13.55 mm; z, 7.23 mm) for the distal femoral and proximal tibial metaphysis (the number of femur or tibia images inside the VOI was 800). The BV within these two volumes of interest was quantitatively measured and reported as the residual bone volume in each group.
SEM scanning of the prostheses.
The prostheses (n = 5) were carefully removed, and their surfaces were examined by a single experienced observer blind to treatment. The samples were fixed (2.5% glutaraldehyde at 4°C for 24 h, osmium acid for 2 h), dehydrated in an alcohol gradient (concentrations of 50%, 60%, 80%, 95%, and 100% for 10 min per concentration), dried in an EM CPD300 critical point dryer (Leica, Germany), coated with a conductive coating using a Q150R S Plus sputter coater (Quorumtech, England), and observed using a Zeiss Auriga field emission SEM and Gatan digital camera system (SEM voltage, 20 kV; SEM magnification, ×5,000; Zeiss, Germany). MRSA was identified as spherical structures with the following features: no surface deformities, organization in pairs or clusters, and diameters of approximately 0.8 μm to 1.0 μm (32). Five fields of view on each prosthesis were randomly selected for observation under high magnification (×5,000) and bacterial counting.
Incision healing evaluation, gross joint pathology, and histopathology.
On day 14, a modified index of incision healing was used for evaluation (38). According to the criteria of a modified Rissing scale score (39), gross joint pathology (n = 12) was assessed and scored separately by an experienced observer blind to treatment. Histopathological analyses (knee joint, n = 5) were carried out to assess the tissue morphology of inflammation and verify the presence of tissue degeneration or necrosis in liver (n = 12) and kidney (n = 12). Tissue samples were soaked and fixed in 4% paraformaldehyde for 24 h. After decalcification (0.3 M EDTA, 28 days) (bone), ethanol dehydration, clearing with xylene, and embedding in paraffin (all samples), samples were sectioned (4 μm) and stained with hematoxylin and eosin (H&E). All the slices were observed and photographed with an H550S photo imaging system (Nikon, Japan). Histopathology of each sample was assessed and scored separately by 2 pathologists blind to treatment by means of a semiquantitative criterion-based scoring method. The histological score of knee joints referred to the modified Petty’s scale (26, 40, 41), as follows: 0 (absent), absence of inflammatory cells; 1 (mild), presence of occasional polymorphic nucleated leukocytes; 2 (moderate), scattered polymorphic nucleated leukocytes and microabscesses; 3 (severe), diffuse polymorphic nucleated leukocytes with several microabscesses and large abscesses. The histological scores for renal and liver injury were assessed using renal and liver scoring systems, respectively (42, 43).
Microbiologic analyses.
The tissue specimens (n = 7), including all the muscles, synovium tissues, and bone around the knee, as well as all components of the prostheses were harvested by sterile instruments on day 14. The soft tissue or bone was added with the same amount (8 mL) of sterile PBS solution and homogenized with a tissue grinder (70HZ; 10 min; JXFSTPRP-48, Jingxin, China). Then, 100 μL of supernatant was inoculated onto LB agar petri dishes and grown for 24 h at 37°C. The prosthesis was placed in 2 mL of sterile PBS solution (containing 0.3% Tween 20) and sonicated to stimulate release of bacterial biofilm from the prosthesis (vortexed for 60 s and sonicated for 15 min in a water bath [UC125, 52 kHz; Biosonic, Switzerland] followed by an additional 60 s of vortexing) (44). A 100-μL aliquot of prosthesis supernatant was plated in the same manner as the tissue supernatants (21, 45). Bacterial colonies were quantified using the plate count method (41, 46). Colonies were confirmed to be MRSA by Gram staining, catalase testing, plasma coagulase rapid agglutination tests, and cefoxitin susceptibility disc tests.
Statistical analysis.
On the basis of previous rat PJI studies (26, 41, 45, 47, 48), we chose appropriate sample size to reduce type 1 errors using the G-power program (version 3.1; Franz, Universitat Kiel, Germany; serum vancomycin levels, n = 6; microbiological analysis of joint tissue, n = 7; micro-CT analysis of the knee joint, n = 5; SEM of the prosthesis, n = 5; histopathological analysis of the knee, n = 5). Data were analyzed using SPSS software (version 22.0; SPSS Inc., USA) and are presented as means and standard errors of the means. Data were compared by analysis of variance (ANOVA) or unpaired 1-tailed Mann-Whitney test. P values of <0.05 were considered significant.
Data availability.
All relevant data analyzed during this study are in the paper.
ACKNOWLEDGMENTS
We thank Qi Yin from the microbiology laboratory of Zhongnan Hospital of Wuhan University of China for providing methicillin-resistant S. aureus (MRSA) ATCC 43300.
This work was supported by grants from the National Natural Science Foundation of China (no. 81673490, 81673524, 81603214, and 81972036) and the Key Research and Development Project of Hubei province (no. 2020BCA071).
Jian Wei and Kai Tong designed and performed the research, analyzed the data and prepared the manuscript. Yinxian Wen and Liaobin Chen designed the research, analyzed the data, and revised the manuscript. Hui Wang also analyzed the data and revised the manuscript.
No potential conflict of interest was reported by the authors.
Contributor Information
Yinxian Wen, Email: wenyinxian202102@163.com.
Liaobin Chen, Email: lbchen@whu.edu.cn.
REFERENCES
- 1.Miller RJ, Thompson JM, Zheng J, Marchitto MC, Archer NK, Pinsker BL, Ortines RV, Jiang X, Martin RA, Brown ID, Wang Y, Sterling RS, Mao HQ, Miller LS. 2019. In vivo bioluminescence imaging in a rabbit model of orthopaedic implant-associated infection to monitor efficacy of an antibiotic-releasing coating. J Bone Joint Surg Am 101:e12. 10.2106/JBJS.18.00425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Villa JM, Pannu TS, Theeb I, Buttaro MA, Onativia JI, Carbo L, Rienzi DH, Fregeiro JI, Kornilov NN, Bozhkova SA, Sandiford NA, Piuzzi NS, Higuera CA, Kendoff DO. 2021. International organism profile of periprosthetic total hip and knee infections. J Arthroplasty 36:274–278. 10.1016/j.arth.2020.07.020. [DOI] [PubMed] [Google Scholar]
- 3.Garvin KL, Hinrichs SH, Urban JA. 1999. Emerging antibiotic-resistant bacteria. Their treatment in total joint arthroplasty. Clin Orthop Relat Res 369:110–123. 10.1097/00003086-199912000-00012. [DOI] [PubMed] [Google Scholar]
- 4.Bratzler DW, Dellinger EP, Olsen KM, Perl TM, Auwaerter PG, Bolon MK, Fish DN, Napolitano LM, Sawyer RG, Slain D, Steinberg JP, Weinstein RA. 2013. Clinical practice guidelines for antimicrobial prophylaxis in surgery. Surg Infect (Larchmt) 14:73–156. 10.1089/sur.2013.9999. [DOI] [PubMed] [Google Scholar]
- 5.Patel NN, Guild GN, III, Kumar AR. 2018. Intrawound vancomycin in primary hip and knee arthroplasty: a safe and cost-effective means to decrease early periprosthetic joint infection. Arthroplast Today 4:479–483. 10.1016/j.artd.2018.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Winkler C, Dennison J, Wooldridge A, Larumbe E, Caroom C, Jenkins M, Brindley G. 2018. Do local antibiotics reduce periprosthetic joint infections? A retrospective review of 744 cases. J Clin Orthop Trauma 9:S34–S39. 10.1016/j.jcot.2017.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dial BL, Lampley AJ, Green CL, Hallows R. 2018. Intrawound vancomycin powder in primary total hip arthroplasty increases rate of sterile wound complications. Hip Pelvis 30:37–44. 10.5371/hp.2018.30.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yavuz IA, Oken OF, Yildirim AO, Inci F, Ceyhan E, Gurhan U. 2020. No effect of vancomycin powder to prevent infection in primary total knee arthroplasty: a retrospective review of 976 cases. Knee Surg Sports Traumatol Arthrosc 28:3055–3060. 10.1007/s00167-019-05778-8. [DOI] [PubMed] [Google Scholar]
- 9.Tubaki VR, Rajasekaran S, Shetty AP. 2013. Effects of using intravenous antibiotic only versus local intrawound vancomycin antibiotic powder application in addition to intravenous antibiotics on postoperative infection in spine surgery in 907 patients. Spine 38:2149–2155. 10.1097/BRS.0000000000000015. [DOI] [PubMed] [Google Scholar]
- 10.Horii C, Yamazaki T, Oka H, Azuma S, Ogihara S, Okazaki R, Kawamura N, Takano Y, Morii J, Takeshita Y, Maruyama T, Yamakawa K, Murakami M, Oshima Y, Tanaka S. 2018. Does intrawound vancomycin powder reduce surgical site infection after posterior instrumented spinal surgery? A propensity score-matched analysis. Spine J 18:2205–2212. 10.1016/j.spinee.2018.04.015. [DOI] [PubMed] [Google Scholar]
- 11.Hanada M, Nishikino S, Hotta K, Furuhashi H, Hoshino H, Matsuyama Y. 2019. Intrawound vancomycin powder increases post-operative wound complications and does not decrease periprosthetic joint infection in primary total and unicompartmental knee arthroplasties. Knee Surg Sports Traumatol Arthrosc 27:2322–2327. 10.1007/s00167-019-05498-z. [DOI] [PubMed] [Google Scholar]
- 12.Smith EB, Wynne R, Joshi A, Liu H, Good RP. 2012. Is it time to include vancomycin for routine perioperative antibiotic prophylaxis in total joint arthroplasty patients? J Arthroplasty 27:55–60. 10.1016/j.arth.2012.03.040. [DOI] [PubMed] [Google Scholar]
- 13.Whiteside LA. 2016. Prophylactic peri-operative local antibiotic irrigation. Bone Joint J 98-B:23–26. 10.1302/0301-620X.98B1.36357. [DOI] [PubMed] [Google Scholar]
- 14.Peng Z, Lin X, Kuang X, Teng Z, Lu S. 2021. The application of topical vancomycin powder for the prevention of surgical site infections in primary total hip and knee arthroplasty: a meta-analysis. Orthop Traumatol Surg Res 107:102741. 10.1016/j.otsr.2020.09.006. [DOI] [PubMed] [Google Scholar]
- 15.Ishida W, Perdomo-Pantoja A, Elder BD, Locke J, Holmes C, Witham TF, Lo SL. 2019. Effects of intraoperative intrawound antibiotic administration on spinal fusion: a comparison of vancomycin and tobramycin in a rat model. J Bone Joint Surg Am 101:1741–1749. 10.2106/JBJS.18.00988. [DOI] [PubMed] [Google Scholar]
- 16.Bosso JA, Nappi J, Rudisill C, Wellein M, Bookstaver PB, Swindler J, Mauldin PD. 2011. Relationship between vancomycin trough concentrations and nephrotoxicity: a prospective multicenter trial. Antimicrob Agents Chemother 55:5475–5479. 10.1128/AAC.00168-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cano EL, Haque NZ, Welch VL, Cely CM, Peyrani P, Scerpella EG, Ford KD, Zervos MJ, Ramirez JA, Kett DH. 2012. Incidence of nephrotoxicity and association with vancomycin use in intensive care unit patients with pneumonia: retrospective analysis of the IMPACT-HAP database. Clin Ther 34:149–157. 10.1016/j.clinthera.2011.12.013. [DOI] [PubMed] [Google Scholar]
- 18.Lodise TP, Patel N, Lomaestro BM, Rodvold KA, Drusano GL. 2009. Relationship between initial vancomycin concentration-time profile and nephrotoxicity among hospitalized patients. Clin Infect Dis 49:507–514. 10.1086/600884. [DOI] [PubMed] [Google Scholar]
- 19.Carreno JJ, Jaworski A, Kenney RM, Davis SL. 2013. Comparative incidence of nephrotoxicity by age group among adult patients receiving vancomycin. Infect Dis Ther 2:201–208. 10.1007/s40121-013-0022-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sweet FA, Forsthoefel CW, Sweet AR, Dahlberg RK. 2018. Local versus systemic antibiotics for surgical infection prophylaxis in a rat model. J Bone Joint Surg Am 100:e120. 10.2106/JBJS.18.00105. [DOI] [PubMed] [Google Scholar]
- 21.Zebala LP, Chuntarapas T, Kelly MP, Talcott M, Greco S, Riew KD. 2014. Intrawound vancomycin powder eradicates surgical wound contamination: an in vivo rabbit study. J Bone Joint Surg Am 96:46–51. 10.2106/JBJS.L.01257. [DOI] [PubMed] [Google Scholar]
- 22.Cavanaugh DL, Berry J, Yarboro SR, Dahners LE. 2009. Better prophylaxis against surgical site infection with local as well as systemic antibiotics. An in vivo study. J Bone Joint Surg Am 91:1907–1912. 10.2106/JBJS.G.01237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Edelstein AI, Weiner JA, Cook RW, Chun DS, Monroe E, Mitchell SM, Kannan A, Hsu WK, Stulberg SD, Hsu EL. 2017. Intra-articular vancomycin powder eliminates methicillin-resistant S. aureus in a rat model of a contaminated intra-articular implant. J Bone Joint Surg Am 99:232–238. 10.2106/JBJS.16.00127. [DOI] [PubMed] [Google Scholar]
- 24.Shimazaki T, Miyamoto H, Ando Y, Noda I, Yonekura Y, Kawano S, Miyazaki M, Mawatari M, Hotokebuchi T. 2010. In vivo antibacterial and silver-releasing properties of novel thermal sprayed silver-containing hydroxyapatite coating. J Biomed Mater Res B Appl Biomater 92:386–389. 10.1002/jbm.b.31526. [DOI] [PubMed] [Google Scholar]
- 25.Lovati AB, Bottagisio M, de Vecchi E, Gallazzi E, Drago L. 2017. Animal models of implant-related low-grade infections. A twenty-year review. Adv Exp Med Biol 971:29–50. 10.1007/5584_2016_157. [DOI] [PubMed] [Google Scholar]
- 26.Lovati AB, Romano CL, Bottagisio M, Monti L, De Vecchi E, Previdi S, Accetta R, Drago L. 2016. Modeling Staphylococcus epidermidis-induced non-unions: subclinical and clinical evidence in rats. PLoS One 11:e0147447. 10.1371/journal.pone.0147447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wei J, Wen Y, Tong K, Wang H, Chen L. 2021. Local application of vancomycin in one-stage revision of PJI caused by MRSA in a rat model. Antimicrob Agents Chemother 65:e000303-21. 10.1128/AAC.00303-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gilotra M, Nguyen T, Jaffe D, Sterling R. 2015. Dilute betadine lavage reduces implant-related bacterial burden in a rabbit knee prosthetic infection model. Am J Orthop 44:E38–E41. [PubMed] [Google Scholar]
- 29.Buchalter DB, Teo GM, Kirby DJ, Schwarzkopf R, Aggarwal VK, Long WJ. 2021. Does the organism profile of periprosthetic joint infections change with a topical vancomycin powder and dilute povidone-iodine lavage protocol? J Arthroplasty 36:S314–S319. 10.1016/j.arth.2020.12.036. [DOI] [PubMed] [Google Scholar]
- 30.Belmatoug N, Cremieux AC, Bleton R, Volk A, Saleh-Mghir A, Grossin M, Garry L, Carbon C. 1996. A new model of experimental prosthetic joint infection due to methicillin-resistant Staphylococcus aureus: a microbiologic, histopathologic, and magnetic resonance imaging characterization. J Infect Dis 174:414–417. 10.1093/infdis/174.2.414. [DOI] [PubMed] [Google Scholar]
- 31.Soe NH, Jensen NV, Nurnberg BM, Jensen AL, Koch J, Poulsen SS, Pier G, Johansen HK. 2013. A novel knee prosthesis model of implant-related osteomyelitis in rats. Acta Orthop 84:92–97. 10.3109/17453674.2013.773121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carli AV, Bhimani S, Yang X, Shirley MB, de Mesy Bentley KL, Ross FP, Bostrom MP. 2017. Quantification of peri-implant bacterial load and in vivo biofilm formation in an innovative, clinically representative mouse model of periprosthetic joint infection. J Bone Joint Surg Am 99:e25. 10.2106/JBJS.16.00815. [DOI] [PubMed] [Google Scholar]
- 33.Bratzler DW, Dellinger EP, Olsen KM, Perl TM, Auwaerter PG, Bolon MK, Fish DN, Napolitano LM, Sawyer RG, Slain D, Steinberg JP, Weinstein RA. 2013. Clinical practice guidelines for antimicrobial prophylaxis in surgery. Am J Health Syst Pharm 70:195–283. 10.2146/ajhp120568. [DOI] [PubMed] [Google Scholar]
- 34.Kang DG, Holekamp TF, Wagner SC, Lehman RA, Jr.. 2015. Intrasite vancomycin powder for the prevention of surgical site infection in spine surgery: a systematic literature review. Spine J 15:762–770. 10.1016/j.spinee.2015.01.030. [DOI] [PubMed] [Google Scholar]
- 35.Reagan-Shaw S, Nihal M, Ahmad N. 2008. Dose translation from animal to human studies revisited. FASEB J 22:659–661. 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]
- 36.O'Donnell JN, Rhodes NJ, Lodise TP, Prozialeck WC, Miglis CM, Joshi MD, Venkatesan N, Pais G, Cluff C, Lamar PC, Briyal S, Day JZ, Gulati A, Scheetz MH. 2017. 24-hour pharmacokinetic relationships for vancomycin and novel urinary biomarkers of acute kidney injury. Antimicrob Agents Chemother 61:e0046-17. 10.1128/AAC.00416-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rhodes NJ, Prozialeck WC, Lodise TP, Venkatesan N, O'Donnell JN, Pais G, Cluff C, Lamar PC, Neely MN, Gulati A, Scheetz MH. 2016. Evaluation of vancomycin exposures associated with elevations in novel urinary biomarkers of acute kidney injury in vancomycin-treated rats. Antimicrob Agents Chemother 60:5742–5751. 10.1128/AAC.00591-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gokce EH, Tuncay Tanriverdi S, Eroglu I, Tsapis N, Gokce G, Tekmen I, Fattal E, Ozer O. 2017. Wound healing effects of collagen-laminin dermal matrix impregnated with resveratrol loaded hyaluronic acid-DPPC microparticles in diabetic rats. Eur J Pharm Biopharm 119:17–27. 10.1016/j.ejpb.2017.04.027. [DOI] [PubMed] [Google Scholar]
- 39.Rissing JP, Buxton TB, Weinstein RS, Shockley RK. 1985. Model of experimental chronic osteomyelitis in rats. Infect Immun 47:581–586. 10.1128/iai.47.3.581-586.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lovati AB, Drago L, Monti L, De Vecchi E, Previdi S, Banfi G, Romano CL. 2013. Diabetic mouse model of orthopaedic implant-related Staphylococcus aureus infection. PLoS One 8:e67628. 10.1371/journal.pone.0067628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lovati AB, Bottagisio M, Maraldi S, Violatto MB, Bortolin M, De Vecchi E, Bigini P, Drago L, Romano CL. 2018. Vitamin E phosphate coating stimulates bone deposition in implant-related infections in a rat model. Clin Orthop Relat Res 476:1324–1338. 10.1097/01.blo.0000534692.41467.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aronoff GR, Sloan RS, Dinwiddie CB, Jr, Glant MD, Fineberg NS, Luft FC. 1981. Effects of vancomycin on renal function in rats. Antimicrob Agents Chemother 19:306–308. 10.1128/AAC.19.2.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brown GT, Kleiner DE. 2016. Histopathology of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Metabolism 65:1080–1086. 10.1016/j.metabol.2015.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thompson JM, Saini V, Ashbaugh AG, Miller RJ, Ordonez AA, Ortines RV, Wang Y, Sterling RS, Jain SK, Miller LS. 2017. Oral-only linezolid-rifampin is highly effective compared with other antibiotics for periprosthetic joint infection: study of a mouse model. J Bone Joint Surg Am 99:656–665. 10.2106/JBJS.16.01002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morris JL, Letson HL, Grant A, Wilkinson M, Hazratwala K, McEwen P. 2019. Experimental model of peri-prosthetic infection of the knee caused by Staphylococcus aureus using biomaterials representative of modern TKA. Biol Open 8:bio045203. 10.1242/bio.045203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Singh R, Ray P, Das A, Sharma M. 2009. Role of persisters and small-colony variants in antibiotic resistance of planktonic and biofilm-associated Staphylococcus aureus: an in vitro study. J Med Microbiol 58:1067–1073. 10.1099/jmm.0.009720-0. [DOI] [PubMed] [Google Scholar]
- 47.Alt V, Lips KS, Henkenbehrens C, Muhrer D, Oliveira Cavalcanti MC, Sommer U, Thormann U, Szalay G, Heiss C, Pavlidis T, Domann E, Schnettler R. 2011. A new animal model for implant-related infected non-unions after intramedullary fixation of the tibia in rats with fluorescent in situ hybridization of bacteria in bone infection. Bone 48:1146–1153. 10.1016/j.bone.2011.01.018. [DOI] [PubMed] [Google Scholar]
- 48.Bostian PA, Karnes JM, Cui S, Robinson LJ, Daffner SD, Witt MR, Emery SE. 2017. Novel rat tail discitis model using bioluminescent Staphylococcus aureus. J Orthop Res 35:2075–2081. 10.1002/jor.23497. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data analyzed during this study are in the paper.