Abstract
Objective
A key component for precision medicine is a good prediction algorithm for patients’ response to treatments. We aim to implement machine learning (ML) algorithms into the response-adaptive randomization (RAR) design and improve the treatment outcomes.
Materials and Methods
We incorporated 9 ML algorithms to model the relationship of patient responses and biomarkers in clinical trial design. Such a model predicted the response rate of each treatment for each new patient and provide guidance for treatment assignment. Realizing that no single method may fit all trials well, we also built an ensemble of these 9 methods. We evaluated their performance through quantifying the benefits for trial participants, such as the overall response rate and the percentage of patients who receive their optimal treatments.
Results
Simulation studies showed that the adoption of ML methods resulted in more personalized optimal treatment assignments and higher overall response rates among trial participants. Compared with each individual ML method, the ensemble approach achieved the highest response rate and assigned the largest percentage of patients to their optimal treatments. For the real-world study, we successfully showed the potential improvements if the proposed design had been implemented in the study.
Conclusion
In summary, the ML-based RAR design is a promising approach for assigning more patients to their personalized effective treatments, which makes the clinical trial more ethical and appealing. These features are especially desirable for late-stage cancer patients who have failed all the Food and Drug Administration (FDA)-approved treatment options and only can get new treatments through clinical trials.
Keywords: clinical trial, adaptive design, machine learning, precision medicine
Lay Summary
In a typical controlled clinical trial, patients are equally randomized to receive different treatments. However, it is possible that one treatment demonstrates advantages over others during the trial. Utilizing that information can benefit subsequent patients. This is why response-adaptive randomization (RAR), which allows uneven treatment assignment probabilities based on existing knowledge, has become popular recently. A key component of RAR is a good prediction algorithm for patients’ response to treatments. Previous works have explored using machine learning (ML) to predict treatment response, but few incorporated ML methods into RAR. This study implements 9 commonly used ML methods into RAR trial designs. We further present an ML-ensemble RAR design that builds upon the majority consensus of the 9 ML methods’ predictions. Extensive simulation studies and real-world applications show that using ML methods in RAR leads to the assignment of more patients to their optimal treatments, increasing the overall response rate. The proposed method will become a useful tool for future clinical trial design in the era of precision medicine.
INTRODUCTION
It is known that patients respond differently to the same treatments.1 The demand for selecting the optimal treatment for each and every patient has resulted in a rapidly developing field called precision medicine, also known as personalized medicine.2 This field aims to provide guidance to select the most effective treatment based on distinctive patient biomarkers. As clinical trials also evolve in the age of precision medicine, there is a substantial need for novel trial designs to deliver more ethical and precise care. Compared with classical nonadaptive trials, adaptive trials have become popular among clinicians as they integrate accumulating patient data to modify the parameters of the trial protocol, provide personalized treatment assignment, and ultimately optimize patients’ outcomes. For example, the adaptive designs in phase 2/3 clinical trials take advantage of the interim treatment response data during the course of the trial and allocate more patients to the presumably more effective treatments.3
Among different adaptive designs, one common adaptation is response-adaptive randomization (RAR). It refers to the adjustments of treatment allocations based on intermediate patient responses and new patients’ characteristics collected during the clinical trials. This RAR design is useful when the interaction between biomarkers and treatments are only putative or not known at the beginning of a trial, and it is also practical when there are multiple treatments to be considered. Its ultimate objective is to provide more patients with their personalized optimal therapies according to their biomarker profiles. The starting point of RAR can be traced back to Thompson,4 who proposed employing a posterior probability estimated from the interim data to assign patients to the more effective treatment. Following his idea, the application of Bayesian methods with an inherent adaptive nature has boomed in area of RAR designs.5–13
Currently, there are several major successes in applying Bayesian RAR concepts in clinical trials, from protocol development through legitimate registration. The BATTLE-1 trial (Biomarker-integrated Approaches of Targeted Therapy for Lung Cancer Elimination) for patients with advanced non–small cell lung cancer (NSCLC) and the I-SPY 2 trial (Investigation of Serial Studies to Predict Your Therapeutic Response with Imaging and Molecular Analysis) for patients with breast cancer in the setting of neoadjuvant chemotherapy are 2 biomarker-based, Bayesian RAR clinical trials.14,15 However, Bayesian RAR designs have a number of challenges and limitations. Due to the modeling restrictions, Bayesian RAR methods usually consider only a very small number of biomarkers. With complex diseases or symptoms, hundreds or even thousands of biomarkers may need to be considered at the same time for treatment assignment.16 Also, some Bayesian RAR methods adjust the design by separating the cohort based on the existence of biomarker(s), and thus these methods rely heavily on how well the biomarker(s) interact(s) with the treatments. If the biomarker is chosen incorrectly, it is possible to make wrong adjustments afterwards.1,17
As the development of modern sequencing technology, clinicians have faced a massive volume of high dimensional data with a complex, nonlinear structure. How to build an effective and scalable algorithm for randomization becomes a fundamental question for the research of RAR trial designs. Machine learning (ML) methods have been applied to solve many real-world problems and have successfully demonstrated their strengths in processing large data sets, as well as capturing nonlinear data structures. With the expectations and resources to analyze this large amount of complex healthcare data, ML methods have established their supremacy in disease prediction,18 disease classification,19 imaging diagnosis,20 drug manufacturing,21 medication assignment,22 and genomic feature identification tasks.23
Although several supervised ML approaches have been applied to drug response prediction,10,24–34 little of the work has explored incorporating ML methods into RAR trial designs. In this study, we implemented 9 ML algorithms into RAR designs and further presented an ML-ensemble RAR design combining these 9 ML algorithms. Specifically, ML methods help to match patient biomarker profiles with prediction of treatment outcomes and, in turn, have determined treatment allocation for future patients. These ML methods are able to address large data and complex structures. We have successfully demonstrated, in both simulation study and a real-world example, that ML-based RAR designs have higher response rates as there are more patients receiving effective treatments. The ensemble method outperformed all other single ML methods.
MATERIALS AND METHODS
Adaptive design: response-adaptive randomization
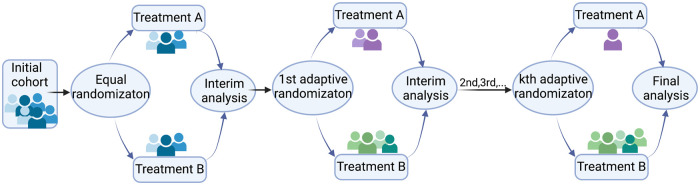
In clinical trial design, adaptive design means making changes to the trial protocol after the trial has started and some data have been collected. These changes are based on the information from the collected data, including (1) the total sample size, (2) interim analyses, (3) patient allocation to different treatment arms, and more.35 For (3), it refers to the RAR design in which the treatment allocation probability varies in order to favor the treatment estimated to be more effective and to increase the response rate in patients. The initial concept can be traced back to Thompson4 and Robbins,36 and led to others. Some famous RAR trials include the extracorporeal membrane oxygenation (ECMO) trial, which tested the efficacy of ECMO in patients with severe acute respiratory distress syndrome (ARDS),37 and the first large-scale double-blind, placebo-controlled study which tested the superiority of fluoxetine over placebo in children and adolescents with depression.38 A general scheme of the RAR designs is shown in Figure 1.
Figure 1.
Response-adaptive randomization (RAR) design. The number of adaptive randomization is adjustable per application.
Benchmark design: equal randomization
Randomization as a standard means for addressing the selection bias in treatment assignments has been extensively used in clinical trials.35 It helps to achieve balance among treatment groups and accounts for the genuine uncertainty about which treatment is better at the beginning of the trial. Randomly assigning patients to treatment arms on a 1:1 basis is known as equal randomization (ER). Friedman et al39 (p. 41) presented that equal allocation in principle maximizes statistical power and is consistent with the concept of equipoise that should exist before the trial starts. Here, we used the ER design as a benchmark randomization design to evaluate the performance of ML-based RAR designs.
Allocation rule
The key of the proposed method is to model the relationship of patient responses and biomarkers. Such a model will then predict the response rate of each treatment for each new patient and provide guidance for treatment assignment. In detail, current enrolled patients’ biomarker profiles and treatment response data were used to train ML models, which later were used to predict future patients’ treatment responses based on their biomarker profiles. Given treatment A and B, the probability of allocating each treatment for patient i is shown as below:
where and , respectively, denote the response probability of treatment and for patient predicted by the ML model.
ML algorithms and a ML ensemble
We selected 9 mainstream ML algorithms and implemented them in the RAR design to predict treatment response. The prediction models were built using the best-fitting parameters for each model, which were obtained by the grid search method with a 10-fold cross-validation.40,41 Grid search is a standard method which allows us to try a variety of tuning parameter combinations for the model within a reasonable amount of time. The 10-fold cross-validation performs the fitting process for a total of 10 times with randomly selected nine-tenths of the data (90%) to train the model in each fit and the rest of the data to validate. By doing this, we avoid bias from using a random single split. The selected model will generalize better to all of the samples in the dataset. Combining grid search with cross-validation, we evaluate the performance of each parameter combination and select the best parameters for each ML model. Here we conducted this hyperparameter tuning procedure in R using the “Caret” package42; similar techniques are available in the scikit-learn Python ML library.43 These selected ML algorithms can be roughly divided into 2 categories:
Parametric models: logistic regression,44 LASSO regression,45 and Ridge regression.46
Nonparametric models: gradient boosting machine (GBM),47 random forest (RF),48 support vector machine (SVM),49 Naive Bayes,50 k-nearest neighbors (KNN),51 and artificial neural networks (NNs).52
For logistic regression, Ridge regression and Lasso regression, they are all considered parametric models. In detail, logistic regression assumes the linearity of independent variables and log odds. It is a particular form of GLM.24 Ridge regression and LASSO regression assume that there is a linear relationship between the “dependent” variable and the explanatory variables. They are 2 regularization methods of GLM to prevent an over-fitting issue by adding penalties on the predictor variables that are less significant.46,53
KNN, which classifies data points based on the points that are most similar to it, is a typical nonparametric model such that there is no assumption for underlying data distribution, and the number of parameters grows with the size of the data.51 With NNs, however, there has been some debate regarding whether they belong to parametric or nonparametric methods. NNs typically consist of 3 layers: input layer, hidden layer, and output layer. Here we classify NNs as a nonparametric method, as the network architecture grows adaptively to match the complexity of given data.52
Both GBM and RF are nonparametric methods that consist of sets of decision trees. Specifically, GBM builds one tree at a time and each new tree helps to correct errors made by previously trained tree by adding weights to the observations with the worst prediction from the previous iteration; RF trains each tree independently using a random sample of the data, and the results are aggregated in the end.47,48
NB and SVM can be either parametric or nonparametric depending on whether they use kernel tricks. For the NB classifier, it becomes nonparametric if using a kernel density estimation (KDE) to obtain a more realistic estimate of the probability of an observation belonging to a class.50 And for SVM, the basic idea is finding a hyperplane that best divides a dataset into 2 classes. It is considered a nonparametric when using the kernel trick to find this hyperplane. This is because the kernel is constructed by computing the pair-wise distances between the training points, and the complexity of the model grows with the size of the dataset.49
Combing these 9 models, an ML ensemble method was built and implemented in the RAR design to obtain a better treatment allocation rule. We defined the treatment allocation probability function for patient i as follows:
where is the number of agreed models, is the total number of models, is the threshold number of agreed models, and is the threshold treatment allocation probability. Here we chose and for ; these threshold values can be adjusted accordingly for different application purposes. To further understand the impacts of selecting different parameter values, we did simulations using different threshold number of agreed models and different threshold allocation probabilities . The results are shown in Supplementary Figures S1S4. In Supplementary Figures S1 and S3 right-sided figures, when the treatment main effect is high, as the threshold parameter increases, the response rate of the ensemble method increases and the individual loss decreases. This intuitively makes sense because when the consensus method reaches the correct decision, increasing will increase the probability of patients receiving their optimal treatments (Supplementary Figure S2). When the treatment main effect is low, the increments of the response rate are not very significant (Supplementary Figure S1, left), but we still observe obvious differences regarding the optimal treatment percentage and the individual loss (Supplementary Figures S2 and S3, left). Meanwhile, it is still desirable to maintain some randomness of treatment assignment in a clinical trial and thus an allocation probability of 1 for the optimal treatment is not recommended. For Supplementary Figure S4, we show the results of the response rate and the optimal treatment percentage, and we can see that the difference of using different threshold values of agreed ML models is minor.
Apart from comparing with the ER “benchmark” design, the current study also examined whether the ML ensemble could assign more patients to the best available treatment beyond other ML methods in adaptive design with the same assessment of individuals.
Inverse probability of treatment weighting
Similar to the observational study in which certain outcomes are measured without attempting to change the outcome, the treatment selection of future patients in RAR trials is often influenced by individual characteristics of the initial block of patients.25 As a result, when estimating the impact of treatment on responses, systemic variations in baseline characteristics between differently treated individuals must be taken into account. Here we applied the inverse probability of treatment weighted (IPTW) method to decrease or remove the effects of confounding when using the observational data to estimate the treatment effects. The idea of IPTW is to use weights based on the propensity score to create a synthetic sample in which the distribution of baseline characteristics is independent of treatment.54 The propensity score refers to the probability of treatment allocation tied to the observed individual characteristics. And the weight based on it is defined as follows:
where is the treatment indicator and is the propensity score for the th subject. Different estimators for treatment effects based on IPTW have been developed; here we used an estimator of the average treatment effect (ATE), which is defined as where is the effect of treatment.53 Incorporated with the IPTW idea, the ATE estimator is defined as follows:
where denotes the response variable of the th subject, denotes the total number of subjects, and still denotes the propensity score.55
Evaluation metrics
Two commonly used criterion in the field of precision medicine, namely the overall response rate and the percentage of individuals receiving optimal treatments, are our primary evaluation metrics. The formulas of the response rate and the optimal treatment percentage are as follows:
The power and the average treatment effect (ATE) adjusted by the IPTW method were also reported to thoroughly evaluate each methods’ performance. The power of a clinical trial refers to the probability of detecting a difference between different treatment groups when it truly exists; ATE was defined in the previous section. Additionally, we proposed a new criterion, the individual loss to quantify the loss for each patient due to receiving suboptimal treatments. For the individual loss, we first define a match for the enrolled patients. A match occurs when the patient’s actual treatment received is the same as the best treatment from the true model. For an enrolled patient with signature let denote the probability of responding to the received treatment . Let denote the probability of responding to the optimal treatment determined by the true model. Then we define the personalized loss function as follows:
A low individual loss value suggests that the majority of patients have received the treatment and will respond at least as well as the real model’s optimal therapy.
RESULTS
Simulation
We used simulation studies to evaluate the proposed methods.
Setting
We generated the patient’s response from a logistic regression model with 10 biomarkers:
where is the treatment indicator (either treatment 0 or treatment 1), is the treatment main effect coefficient, is the biomarker for patient , and incorporates some polynomial and step function terms. is the biomarker-treatment interaction coefficient and were assumed to interact with the treatment. A random noise for each subject is denoted as . In detail, each biomarker was assumed to follow a normal distribution with a mean of 0 and a standard deviation of 1. Among these 10 biomarkers, contributed to the true model as third-degree polynomials, while contributed to the true model as step functions:
Seven scenarios of different treatment main effects (=0, 0.5, 0.7, 1, 1.3, 1.5, 1.7) and a fixed treatment-biomarker interaction () were considered. We conducted 1000 Monte Carlo simulations for each scenario and compared the results obtained by the ML-based and ML-ensemble RAR designs with the results from the ER design.
Response rate and optimal treatment percentage
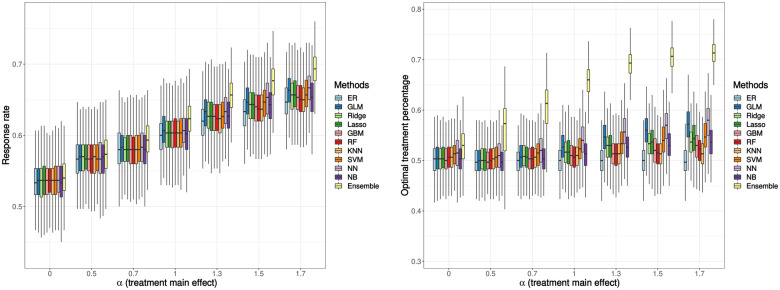
The response rate results and the percentage of receiving the optimal treatment are shown in Figure 2. Overall, the performance of ML-based RAR designs is better than the performance of the ER design. When the treatment main effect is zero, the differences for both response rate and the optimal treatment percentage between ML-based RAR designs and the ER design are not significant. As the treatment effect increases, these differences become more obvious. Among these 9 ML algorithms, the neural network has the highest response rate and the highest proportion of patients receiving their optimal treatments. Additionally, the ensemble method combining these 9 ML methods outperforms all other methods and achieves an approximate 5% higher response rate and a more than 20% larger optimal treatment percentage compared to the ER design.
Figure 2.
Simulation result: response rate (left), percentage of patients receiving their optimal treatments (right). The treatment-biomarker interaction, is fixed at 0.5. Boxplots display the median (middle line), the interquartile range (hinges), and 1.5 times the interquartile range (lower and upper whiskers) based on 1000 times simulation. The mean (over 1000 simulations) response rate ranges from 0.53 to 0.69, and the mean of optimal treatment percentages ranges from 0.50 to 0.71.
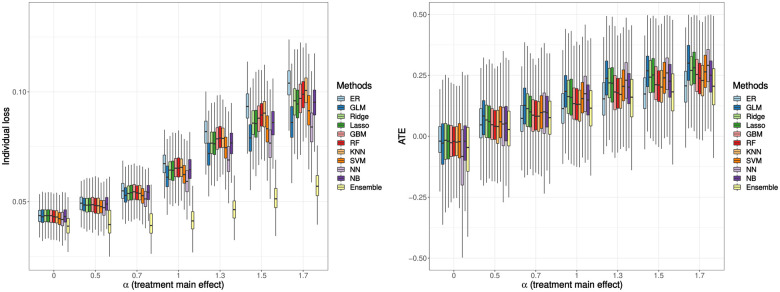
Individual loss, ATE, and power
The individual loss and the ATE results are shown in Figure 3. The interpretation of the individual loss results coincides with the previous response rate results and the optimal treatment percentage results such that the ML-ensemble RAR design has the lowest individual loss value among all scenarios, which is preferred in the trial. The ATE has been adjusted by the IPTW method to account for confounding effect of using observational data. The logistic regression method now has the highest ATE, followed by the NN method. The ensemble method has a relatively low ATE, but it is higher than the ER method when the treatment main effect becomes larger. This shows that the average effect of changing the entire population from untreated to treated using RAR designs is better than that of using the ER design.
Figure 3.
Simulation result: individual loss (left), average treatment effect (ATE, right). The treatment-biomarker interaction, is fixed at 0.5. Boxplots display the median (middle line), the interquartile range (hinges), and 1.5 times the interquartile range (lower and upper whiskers) based on 1000 times simulation. The mean (over 1000 simulations) individual loss ranges from 0.04 to 0.10, and the mean ATE ranges from -0.12 to 0.30.
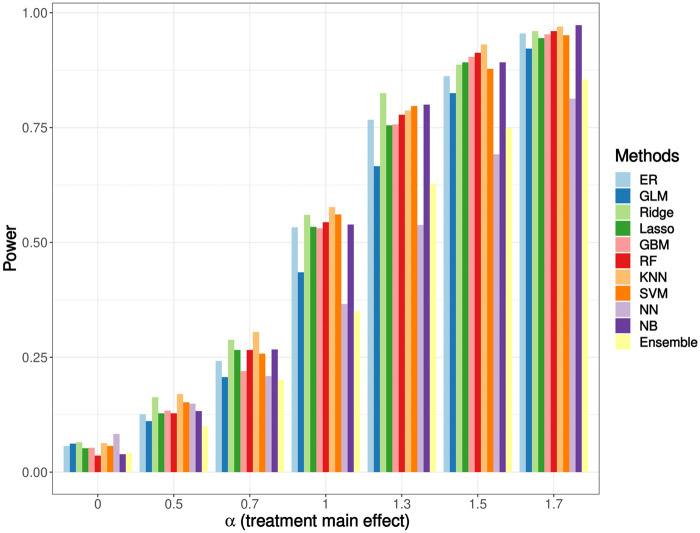
Power
The power results are shown in Figure 4. The power is also weighted by the IPTW method to address potential bias. For the power analysis, the Type I error is controlled at 0.05. Several papers have shown in their simulation studies that the correlation among treatment assignments was inevitable when performing inference on the data from RAR design-implemented studies.56,57 This correlation can increase the binomial variability and lower the power. In our simulation, the RAR design using the NN method has the lowest power, followed by using the logistic regression method. However, other ML-based RAR designs have comparable or even higher power than that of the ER design. The ensemble method has a relatively low power, but it is still better than the NN method.
Figure 4.
Simulation result: power. The treatment-biomarker interaction, is fixed at 0.5. The Type I error is controlled at 0.05. The power ranges from 0.04 to 0.97.
Real-world example
We analyzed a publicly available acute myeloid leukemia (AML) dataset from Kornblau et al58 where most of the clinical biomarkers are expression levels of cellular proteins. Kornblau et al sequenced protein expressions in leukemia-enriched cells from 256 newly diagnosed AML patients with a primary goal of eventually establishing a proteomic-based categorization of AML. The treatment and the response variables were carefully adjusted to binary variables. Specifically, the treatments were binarized to high-dose ara-C (HDAC)–based treatments and non-HDAC treatments; the responses were binarized to complete response (CR) and non-CR.
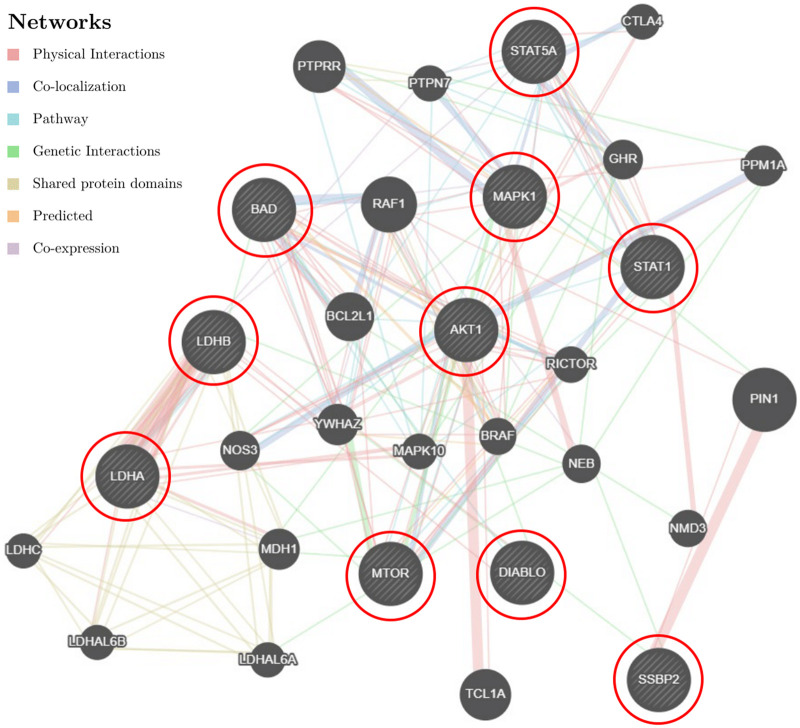
We first performed a feature selection to decide what interaction terms should be included in the model. We used each protein-treatment interaction term to build the generalized linear model (GLM) model and reported the p-value for each interaction to assess whether it has strong correlation with the dependent variable/the treatment response. The top 10 proteins whose interaction variables have the smallest P-values were selected. We then performed a gene network analysis on the genes that code for these proteins using GeneMANIA (http://genemania.org).26 This analysis helps to illustrate the hidden interaction and network of the corresponding genes. Additionally, it shows other genes that have been reported to associate with the input 10 genes, using extensive existing knowledge such as protein and genetic interactions, pathways, co-expression, co-localization, and protein domain similarity. The results are presented in Figure 5. The top 10 genes corresponding to the biomarkers identified in our study are highlighted with red circles.
Figure 5.
AML data: the gene network analysis. The input 10 genes, namely the genes coding for top 10 proteins that significantly interacted with the treatment, were highlighted using red circles. Other genes that were presumably involved in AML were returned by GeneMANIA.
Using a cut-off P-value of 0.1 among 71 proteins, the expression levels of 3 of them were found to have the most significant interactions with the treatment, that is, the strongest correlation with the treatment outcomes: phosphothreonine 308 of Akt (Thr 308 p-Akt), the mechanistic target of rapamycin (mTOR), and signal transducer and activator of transcription 1 (STAT1). Studies have shown that these 3 proteins play critical roles in human AML. The level of Thr 308 p-Akt is associated with high-risk cytogenetics and predicts poor overall survival for AML patients.27 In AML, the mTOR signaling pathway is deregulated and activated as a consequence of genetic and cytogenetic abnormalities. The mTOR inhibitors are often used to target aberrant mTOR activation and signaling.59,60 The STAT1 transcription factor is constitutively activated in human AML cell lines and might contribute to the autonomous proliferation of AML blasts. The inhibition of this pathway can be of great interest for AML treatments.61,62 Hence, we chose these 3 proteins to build ML models in our proposal.
The whole dataset (256 observations) was randomly shuffled and divided into 2 equal-sized blocks: block 1 and block 2. Each block was taken in turn as either the training set or the testing set. The results were aggregated after 100 repetitions. Since this clinical trial is already completed and it is not possible to get actual treatment responses using our methods, we separated the enrolled patients into 2 groups: a consistent group whose real treatments are the same as the treatments using the ML-based RAR designs and an inconsistent group whose real treatments are different from the treatments using the ML-based RAR designs. We compared the response rates in these 2 groups to elucidate the potential gain if the proposed RAR had been implemented. The results of each method are shown in Figure 6. In the consistent group, the response patient percentages are at least 10% higher than 50%; while the response patient percentages in the inconsistent group are all lower than 50%, that is, we observe higher response rates in the consistent group. This means that patients in the inconsistent group may likely benefit from the RAR method we developed.
Figure 6.
AML data result: the response percentage. Patients in the consistent group (left) were assigned to the same treatments using our ML-based RAR designs, while patients in the inconsistent group (right) were assigned to different treatments using our ML-based RAR designs. The 50% response percentage is marked with a black dashed line.
DISCUSSION
Patients are accrued in groups sequentially. RAR designs determine the treatment allocation for new groups of patients based on the accrued information of how previous groups of patients responded to their treatments. The number of RAR implementations, , should be predefined. The choice of may depend on the total sample size, trial length, and other logistics and practical considerations. Our simulation study used for a total sample size of . In the real data analysis with a smaller sample size of 256 subjects, we used .
We developed novel methods for RAR designs by incorporating 9 ML methods to predict treatment response and assign treatments accordingly. We showed that our ML-based RAR designs can effectively improve treatment response rates among patients. We further proposed an ensemble approach based on the consensus of the 9 ML methods to improve the prediction and decision making. Our proposed ML-ensemble RAR design builds on the predictive ability of 9 ML methods and can further improve predication accuracy and patient outcome. Specifically, suppose out of 9 models indicate that treatment is better than treatment for patient , then we let for , let for , and for . For , we keep the assignment probability as a constant of 0.85 because we still want to reserve some randomness in the trial. These settings can be tuned based on prior knowledge of the treatment selections.
We also tried the combination of NN and GLM algorithms as another binary-combination method and conducted additional simulations. Since these 2 models may not always be in consensus regarding optimal treatment selection for each individual, we took the average of the treatment assigning probabilities of the NN and GLM methods. Similar as we have done previously for the 9 ML methods and the ensemble approach, we evaluated its performance through the overall response rate in simulated trials, the percentage of patients receiving their individually optimal treatment, and the average individual loss for all trial participants. We have provided the results in the Supplementary Figure S5. Although the performance of this combination is slightly better than using either NN or GLM alone, it is still substantially worse than that of the ensemble using 9 ML algorithms, especially when the treatment main effect is high.
While we only considered settings of 2 treatment options in this work, ML-based RAR design can extend to multiple targeted treatments. Given treatments, the treatment allocating probability of patient is shown as , where denotes the response probability of treatment for patient predicted by the ML algorithm. For example, NN can naturally adapt to a multiclass classification problem by replacing the binary cross-entropy loss to a categorical cross-entropy loss.63
Although our work can effectively improve the treatment outcomes in the clinical trial, there are a few limitations that we would like to point out as directions for further research. First, equal weight was given to each of the 9 ML algorithms in the ensemble method. However, it is likely that different ML methods have distinct prediction accuracy at different scenarios. Incorporating such information by attaching different weights for different ML algorithms in the ensemble method could potentially lead to better adaptation to the data and may provide more precise treatment suggestions for personalized medicine. Second, although our method has been extensively evaluated using simulated and real data, we did not consider the setting with high-dimensional data, for example, the data from omics experiments. With the development of modern sequencing technology, more clinical trials seek to include such information in clinical decision making and trial design. With high-dimensional data, there are more challenges, such as adding appropriate feature selection steps, etc. Moreover, our current model did not consider the situation when complex interactions between treatment and individualized biomarkers exist in the dataset. When this problem is of interest, we might resort to other models that are specifically designed to address the heterogeneous treatment effect caused by these interactions, such as the honest causal forest model,64 that are specifically designed to address the heterogeneous treatment effect caused by these interactions.
CONCLUSION
ML methods have successfully demonstrated their superior prediction performance in many applications, but have not been applied to conduct RAR in clinical trials. In this study, we developed novel methods for RAR designs by incorporating ML algorithms to predict treatment response and assign treatments accordingly. We showed that the ML-based RAR designs have better performance than that of the traditional ER design. And the ensemble approach demonstrated better results than the ER design at the greatest extent. As the ML field is getting mature and abundant packages are available on different programming software, our method is easy to implement in current clinical trial systems.
FUNDING
The research of XH was partially supported by the US National Institutes of Health grants U54CA096300, U01CA253911, and 5P50CA100632, and the Dr. Mien-Chie Hung and Mrs. Kinglan Hung Endowed Professorship.
AUTHOR CONTRIBUTIONS
XH, ZL, and YW conceived the concept of the study and designed the method. YW implemented the method, performed the experiments, and drafted the initial manuscript. BC interpreted the real-word data for the work. All authors edited and approved the final manuscript.
SUPPLEMENTARY MATERIAL
Supplementary material is available at JAMIA Open online.
CONFLICT OF INTEREST STATEMENT
None declared.
DATA AVAILABILITY
Simulation data can be reproduced by the R script that has been deposited in the online Dryad repositor.65 The AML dataset used for real-world illustration can be downloaded from https://bioinformatics.mdanderson.org/public-datasets/supplements/ under “RPPA Data in AML”.58
Supplementary Material
REFERENCES
- 1.Antoniou M, Kolamunnage-Dona R, Jorgensen AL.. Biomarker-guided non-adaptive trial designs in phase II and Phase III: a methodological review. JPM 2017; 7 (1): 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ginsburg GS, Phillips KA.. Precision medicine: from science to value. Health Aff (Millwood) 2018; 37 (5): 694–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maca J, Bhattacharya S, Dragalin V, Gallo P, Krams M.. Adaptive seamless phase II/III designs—background, operational aspects, and examples. Drug Information J 2006; 40 (4): 463–73. [Google Scholar]
- 4.Thompson WR. On the likelihood that one unknown probability exceeds another in view of the evidence of two samples. Biometrika 1933; 25 (3-4): 285–94. [Google Scholar]
- 5.Bello GA, Sabo RT.. Outcome-adaptive allocation with natural lead-in for three-group trials with binary outcomes. J Stat Comput Simul 2016; 86 (12): 2441–9. [Google Scholar]
- 6.Zhou X, Liu S, Kim ES, Herbst RS, Lee JJ.. Bayesian adaptive design for targeted therapy development in lung cancer—a step toward personalized medicine. Clin Trials 2008; 5 (3): 181–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Williamson SF, Jacko P, Villar SS, Jaki T.. A Bayesian adaptive design for clinical trials in rare diseases. Comput Stat Data Anal 2017; 113: 136–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trippa L, Lee EQ, Wen PY, et al. Bayesian adaptive randomized trial design for patients with recurrent glioblastoma. J Clin Oncol 2012; 30 (26): 3258–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brannath W, Zuber E, Branson M, et al. Confirmatory adaptive designs with Bayesian decision tools for a targeted therapy in oncology. Stat Med 2009; 28 (10): 1445–63. [DOI] [PubMed] [Google Scholar]
- 10.Lee JJ, Xuemin G, Suyu L.. Bayesian adaptive randomization designs for targeted agent development. Clin Trials 2010; 7 (5): 584–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee JJ, Chu CT.. Bayesian clinical trials in action. Stat Med 2012; 31 (25): 2955–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stallard N, Whitehead J, Cleall S.. Decision-making in a phase II clinical trial: a new approach combining Bayesian and frequentist concepts. Pharm Stat 2005; 4 (2): 119–28. [Google Scholar]
- 13.Dong G, Shih WJ, Moore D, Quan H, Marcella S.. A Bayesian-frequentist two-stage single-arm phase II clinical trial design. Stat Med 2012; 31 (19): 2055–67. [DOI] [PubMed] [Google Scholar]
- 14.Kim ES, Herbst RS, Wistuba II, et al. The BATTLE trial: personalizing therapy for lung cancer. Cancer Discov 2011; 1 (1): 44–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barker AD, Sigman CC, Kelloff GJ, Hylton NM, Berry DA, Esserman LJ.. I-SPY 2: an adaptive breast cancer trial design in the setting of neoadjuvant chemotherapy. Clin Pharmacol Ther 2009; 86 (1): 97–100. [DOI] [PubMed] [Google Scholar]
- 16.Nevozhay D, Adams RM, Murphy KF, Josi K, Balzsi G.. Negative autoregulation linearizes the dose-response and suppresses the heterogeneity of gene expression. Proc Natl Acad Sci U S A 2009; 106 (13): 5123–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee JJ, Liu S, Gu X.. Bayesian adaptive randomization designs for targeted agent development. Clin Cancer Res 2008; 14 (19 Suppl): PL06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ma F, Gao J, Suo Q, You Q, Zhou J, Zhang A. Risk prediction on electronic health records with prior medical knowledge. In: Proceedings of the 24th ACM SIGKDD International Conference on Knowledge Discovery & Data Mining. London, UK: Association for Computing Machinery; 2018: 1910–9.
- 19.Fakoor R, Ladhak F, Nazi A, Huber M. Using deep learning to enhance cancer diagnosis and classification. In: Proceedings of the International Conference on Machine Learning; 2013: 3937–49.
- 20.Liu S, Liu S, Cai W, Pujol S, Kikinis R, Feng D. Early diagnosis of Alzheimer's disease with deep learning. In: 2014 IEEE 11th International Symposium on Biomedical Imaging (ISBI); 2014: 1015–8.
- 21.Pantuck A, Lee D-K, Kee T, et al. Modulating BET bromodomain inhibitor ZEN‐3694 and enzalutamide combination dosing in a metastatic prostate cancer patient using CURATE.AI, an artificial intelligence platform. Adv Ther 2018; 1 (6): 1800104. [Google Scholar]
- 22.Liang Z, Zhang G, Huang X, Hu QV. Deep learning for healthcare decision making with EMRs. IEEE Int Conf Bioinform Biomed 2014: 556–9. doi 10.1109/BIBM.2014.6999219.
- 23.Zhou J, Troyanskaya OG.. Predicting effects of noncoding variants with deep learning-based sequence model. Nat Methods 2015; 12 (10): 931–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tibshirani R, Hastie T, Friedman J.. Regularized paths for generalized linear models via coordinate descent. J Stat Soft 2010; 33 (1): 1–22. [PMC free article] [PubMed] [Google Scholar]
- 25.Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res 2011; 46 (3): 399–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Warde-Farley D, Donaldson SL, Comes O, et al. The GeneMANIA prediction server: biological network integration for gene prioritization and predicting gene function. Nucleic Acids Res 2010; 38 (Web Server issue): W214–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gallay N, Santos CD, Cuzin L, et al. The level of AKT phosphorylation on threonine 308 but not on serine 473 is associated with high-risk cytogenetics and predicts poor overall survival in acute myeloid leukemia. Blood 2008; 112 (11): 1503. [DOI] [PubMed] [Google Scholar]
- 28.ChekroudAM, , ZottiRJ, , Shehzad Z,. et al. Cross-trial prediction of treatment outcome in depression: a machine learning approach. Lancet Psychiatry 2016; 3 (3): 243–50. [DOI] [PubMed] [Google Scholar]
- 29.ShimabukuroDW, , BartonCW, , FeldmanMD, , MatarasoSJ, , Das R.. Effect of a machine learning-based severe sepsis prediction algorithm on patient survival and hospital length of stay: a randomised clinical trial. BMJ Open Respir Res 2017; 4 (1): e000234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.ZazziM, , IncardonaF, , Rosen-Zvi M,. et al. Predicting response to antiretroviral treatment by machine learning: the EuResist project. Intervirology 2012; 55 (2): 123–7. [DOI] [PubMed] [Google Scholar]
- 31.PatelMJ, , AndreescuC, , PriceJC, , EdelmanKL, , ReynoldsCF, , Aizenstein HJ.. Machine learning approaches for integrating clinical and imaging features in late-life depression classification and response prediction. Int J Geriatr Psychiatry 2015; 30 (10): 1056–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schmidt-ErfurthU, , BogunovicH, , Sadeghipour A,. et al. Machine learning to analyze the prognostic value of current imaging biomarkers in neovascular age-related macular degeneration. Ophthalmol Retina 2018; 2 (1): 24–30. [DOI] [PubMed] [Google Scholar]
- 33.RajkomarA, , DeanJ, , Kohane I.. Machine learning in medicine. N Engl J Med 2019; 380 (14): 1347–58. [DOI] [PubMed] [Google Scholar]
- 34.VamathevanJ, , ClarkD, , Czodrowski P,. et al. Applications of machine learning in drug discovery and development. Nat Rev Drug Discov 2019; 18 (6): 463–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rosenberger WF, Lachin JM.. Randomization in Clinical Trials: Theory and Practice. Hoboken, NJ: John Wiley & Sons; 2015. [Google Scholar]
- 36.Robbins H. Some aspects of the sequential design of experiments. Bull Amer Math Soc 1952; 58 (5): 527–35. [Google Scholar]
- 37.Combes A, Hajage D, Capellier G; EOLIA Trial Group, REVA, and ECMONet, et al. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. N Engl J Med 2018; 378 (21): 1965–75. [DOI] [PubMed] [Google Scholar]
- 38.Emslie G, Rush A, Weinberg W, et al. A double-blind, randomized, placebo-controlled trial of fluoxetine in children and adolescents with depression. Arch Gen Psychiatry 1997; 54 (11): 1031–7. [DOI] [PubMed] [Google Scholar]
- 39.Friedman LM, Furberg C, DeMets DL.. Fundamentals of Clinical Trials. Boston, MA: J. Wright, PSG Inc.; 1981. [Google Scholar]
- 40.Stone M. Cross-validatory choice and assessment of statistical predictions. J R Stat Soc Ser B (Methodol) 1974; 36 (2): 111–33. [Google Scholar]
- 41.Bergstra J, Bengio Y.. Random search for hyper-parameter optimization. J Mach Learn Res 2012; 13 (10): 281–305. [Google Scholar]
- 42.Kuhn M. caret: Classification and Regression Training; 2015. [Database] https://github.com/topepo/caret/ Accessed October 9, 2021.
- 43.Pedregosa F, Varoquaux G, Gramfort A, et al. Scikit-learn: machine learning in Python. J Mach Learn Res 2011; 12: 2825–30. [Google Scholar]
- 44.Cramer JS. The Origins of Logistic Regression. Tinbergen Institute Discussion Papers 02-119/4. Amsterdam, Netherlands: Tinbergen Institute; 2002. [Google Scholar]
- 45.Tibshirani R. Regression shrinkage and selection via the Lasso. J R Stat Soc Ser B (Methodol) 1996; 58 (1): 267–88. [Google Scholar]
- 46.Hoerl AE, Kennard RW.. Ridge regression: biased estimation for nonorthogonal problems. Technometrics 1970; 12 (1): 55–67. [Google Scholar]
- 47.Friedman JH. Greedy function approximation: a gradient boosting machine. Ann Statist 2001; 29 (5): 1189–232. [Google Scholar]
- 48.Ho TK. Random Decision Forests. In: Proceedings of 3rd international conference on document analysis and recognition. Vol. 1. Manhattan, NY: IEEEOxford University Press; 1995: 278–82. [Google Scholar]
- 49.Boser BE, Guyon IM, Vapnik VN.. A Training Algorithm for Optimal Margin Classifiers. In: Proceedings of the fifth annual workshop on Computational learning theory (COLT '92). New York, NY: Association for Computing Machinery (ACM); 1992: 338–45. [Google Scholar]
- 50.John GH, Langley P.. Estimating Continuous Distributions in Bayesian Classifiers. In: Proceedings of the Eleventh conference on Uncertainty in artificial intelligence (UAI'95). San Francisco, CA: Morgan Kaufmann Publishers; 1995: 338–45. [Google Scholar]
- 51.Altman NS. An introduction to Kernel and nearest-neighbor nonparametric regression. Am Statist 1992; 46 (3): 175–85. [Google Scholar]
- 52.Grossberg S. Nonlinear neural networks: principles, mechanisms, and architectures. Neural Networks 1988; 1 (1): 17–61. [Google Scholar]
- 53.Imbens G. Nonparametric estimation of average treatment effects under exogeneity: a review. Rev Econ Stat 2004; 86 (1): 4–29. [Google Scholar]
- 54.Rosenbaum PR. Model-based direct adjustment. J Am Stat Assoc 1987; 82 (398): 387–94. [Google Scholar]
- 55.Lunceford JK, Davidian M.. Stratification and weighting via the propensity score in estimation of causal treatment effects: a comparative study. Stat Med 2004; 23 (19): 2937–60. [DOI] [PubMed] [Google Scholar]
- 56.Melfi VF, Page C.. Variability in adaptive designs for estimation of success probabilities. Project Euclid 1998; 34: 106–14. [Google Scholar]
- 57.Rosenberger WF, Stallard N, Ivanova A, Harper CN, Ricks ML.. Optimal adaptive designs for binary response trials. Biometrics 2001; 57 (3): 909–13. [DOI] [PubMed] [Google Scholar]
- 58.Kornblau SM, Tibes R, Qiu YH, et al. Functional proteomic profiling of AML predicts response and survival. Blood 2009; 113 (1): 154–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Feng Y, Chen X, Cassady K, et al. The role of mTOR inhibitors in hematologic disease: from bench to bedside. Front Oncol 2020; 10: 611690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sandhöfer N, Metzeler KH, Rothenberg M, et al. Dual PI3K/mTOR inhibition shows antileukemic activity in MLL-rearranged acute myeloid leukemia. Leukemia 2015; 29 (4): 828–38. [DOI] [PubMed] [Google Scholar]
- 61.Gouilleux-Gruart V, Gouilleux F, Desaint C, et al. STAT-related transcription factors are constitutively activated in peripheral blood cells from acute leukemia patients. Blood 1996; 87 (5): 1692–97. [PubMed] [Google Scholar]
- 62.Weber-Nordt RM, Egen C, Wehinger J, et al. Constitutive activation of STAT proteins in primary lymphoid and myeloid leukemia cells and in Epstein-Barr Virus (EBV)–related lymphoma cell lines. Blood 1996; 88 (3): 809–16. [PubMed] [Google Scholar]
- 63.Goodfellow I, Bengio Y, Courville A.. Deep Learning. Cambridge, MA: MIT Press; 2016. [Google Scholar]
- 64.Athey S, Tibshirani J, Wager S. Generalized Random Forests; 2018. https://grf-labs.github.io/grf/ Accessed July 14, 2021.
- 65.Wang Y, Huang XH, Li Z.. Application of machine learning methods in clinical trials for precision medicine. Dryad 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Simulation data can be reproduced by the R script that has been deposited in the online Dryad repositor.65 The AML dataset used for real-world illustration can be downloaded from https://bioinformatics.mdanderson.org/public-datasets/supplements/ under “RPPA Data in AML”.58