Abstract
Objective.
Evaluate a community-based navigator intervention to increase breast cancer patients’ and survivors’ access to information about health research participation opportunities.
Methods.
In the context of a Community Based Participatory Research collaboration, we conducted a prospective randomized controlled trial of the Health Research Engagement Intervention with pre- and post-intervention surveys (n=133). The primary outcome was health research information-seeking behavior. Secondary outcomes were health research knowledge, willingness to participate in health research, and health empowerment. Qualitative interviews (n=11) elucidated participant perspectives on the intervention.
Results.
There was no statistically significant difference between intervention and control groups’ information-seeking behavior. Knowledge that not all health research studies are about drugs or treatments increased significantly from pre- to post-test among intervention group participants (32% to 48%, p=0.012), but not in the control group (43% to 30%, p=0.059); the difference between arms was statistically significant (p=0.0012). Although survey responses indicated willingness to participate, qualitative interviews identified competing priorities that limited participants’ motivation to seek enrollment information.
Conclusions and Practice Implications.
Community-based navigators are a trusted, and therefore promising link between health research and low-income underserved communities. However, systemic barriers in health research infrastructures need to be addressed to include low income, LEP and immigrant populations.
Keywords: Cancer, Patient Navigation, Clinical Trials, Disparities, information-seeking behavior, LEP, Limited English Proficient, community based participatory research, CBPR
1. Introduction
The underrepresentation of minority groups in clinical and behavioral research is a critical issue when considering how to eliminate disparities across the cancer continuum. Recruitment to and retention in research is necessary to ensure generalizability, as well as the fit and adequacy of treatments and interventions for various subgroups. Despite efforts to address the many documented patient, provider, and systems barriers to information about and participation in health research in the years since the 1993 NIH mandate requiring inclusion of minority groups in federally funded clinical research [1–5], people of color and of low socioeconomic status remain underrepresented [6–8]. The reasons for the underrepresentation are varied, but include many structural access barriers, (e.g. eligibility criteria, access to institutions where trials are open, information about trials, language, literacy, provider invitation [9–15]. Research suggests that members of minority groups are willing to participate [16], although mistrust of clinicians, the research enterprise and medical institutions remain significant barriers [10,17].
Shanti’s Margot Murphy Women’s Cancer Program (Shanti)—a trusted community organization providing health navigation services to diverse low-income breast cancer patients and survivors—initiated this Community Based Participatory Research (CBPR) study with BreastCancerTrials.org (BCT), an online clinical trials matching resource, and University of California, San Francisco (UCSF) researchers. Shanti’s annual client survey found that the vast majority of clients neither participated in health research nor were invited to participate by their providers, a result consistent with research showing that clinical research studies are often not available where underrepresented patients seek care and providers often do not invite low income patients to participate [12,13]. Given our prior findings that San Francisco Bay Area breast cancer patients lacked knowledge of and access to clinical research [11–14], our study aimed to increase Shanti clients’ familiarity with health research and alternate ways to find information about open studies, and to empower them to seek participation opportunities in studies of interest to them through a trusted source.
Patient navigation is a patient-centered approach to care coordination, emotional support and education for patients that has long been enlisted to reduce cancer health disparities [18–22]. Navigators conduct both instrumental tasks and relationship interventions, such as facilitating access to benefits, providing language translation at medical appointments, and attending to clients’ emotional and practical concerns. A majority of navigator interventions focus on cancer screening and diagnostic resolution [23], but interventions to reduce barriers to participation in cancer clinical trials have increased [24–26] in community and clinical settings, using a range of navigator types from volunteer lay health workers to highly trained nurse navigators. Many have focused on enrollment [27–29] or retention in specific studies [30], while others have addressed awareness of and education about clinical research [31–34].
To address the health research information gap experienced by Shanti clients, and the larger problem of low participation/inclusion rates among low income, LEP and ethnically diverse patients in cancer research, this partnership developed the Health Research Engagement Intervention (HREI). We employed a collaborative iterative design process, that included formative research with Shanti clients and navigators, and sought to develop an intervention that aligned with the Shanti model of care. This design process and our pilot test of the HREI are described fully in Nickell et al [35]. The resulting HREI was tested for efficacy in the RCT reported here.
1. Methods
Overview
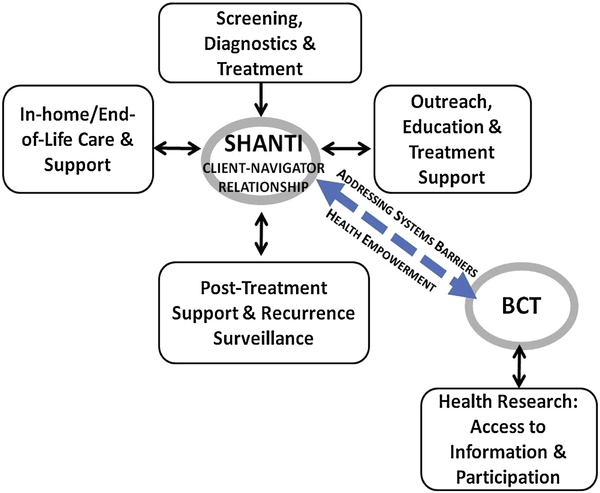
This CBPR study consisted of a prospective randomized trial of the HREI with pre- and post-surveys and qualitative interviews with a subset of trial participants to elucidate their perspectives on the intervention. The critical role of the SCN at every stage of the iterative HREI development process [35] and study implementation (testing of the survey instruments and their translations; the delivery of the intervention), reflects CBPR principles of collaboration and equity in research with marginalized communities [36]. The study was guided by a conceptual framework derived from systems theory [37,38] and the constructs of ‘relational culture’ [39] and ‘health empowerment’ [40,41]. (Figure 1.) All procedures were approved by the UCSF Institutional Review Board.
Figure 1.
Conceptual Framework
Community Based Research Partners
Shanti’s navigation program was established in 2001 and currently provides tailored care navigation and wellness programming to over 600 clients annually. Shanti Care Navigators (SCN) provide services primarily in non-clinical settings, (e.g. client’s home) and speak with clients in English, Spanish, Cantonese and Mandarin. Shanti’s breast cancer client population is 19% white, 46% Asian, 22% Latina, 10% African-American, 1% Native American, and 2% other race/ethnicity; 87% live at or below 200% of the poverty level; 40% are of limited English proficiency (LEP) and receive Shanti services in Spanish, Chinese or Russian. SCN are trained in the Shanti Model of Peer Support™, a non-directive, client-centered mode of communication that is grounded in the skills of active-listening, harm-reduction and compassionate presence.
BreastCancerTrials.org (BCT) is an online resource dedicated to helping breast cancer patients and survivors match to health research studies based on their health history, interests and other personalized factors. BCT lists over 600 studies nationwide including observational studies and clinical trials for treatment, screening, and supportive care. Launched in 2008, the service has over 5000 subscribers; users are primarily highly educated (72% have a college education or higher) and white (88%). To increase BCT’s accessibility to the study participants, we implemented a multilingual helpline with outgoing messages recorded in Spanish, Cantonese, and English, and worked with BCT staff to reduce the literacy demand of its website content.
The Health Research Engagement Intervention (HREI)
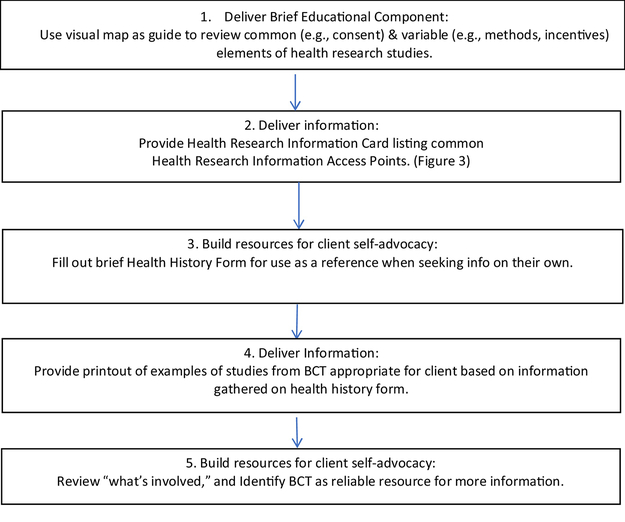
The HREI utilized SCN to provide (1) general education about the spectrum of breast cancer research in a neutral manner not tied to enrollment in a specific trial, and (2) resources to independently find health research participation opportunities [35]. The term “health research” addresses our formative research finding that SCN and their clients understood “clinical trials” as limited to treatment trials and had negative associations with the term [35]. “Health research” includes behavioral, epidemiological, lifestyle intervention, and qualitative research studies as well as treatment trials. The HREI consists of five navigator-delivered components (Figure 2). The Health Research Information Card provided in component 2 (Figure 3) includes contact information in English, Spanish or Chinese for organizations that offer information about clinical trials and other health research participation opportunities.
Figure 2.
Health Research Engagement Intervention (HREI) Components
Figure 3.
Health Research Information Card listing health research information access points
Eligibility, Recruitment and Trial Design
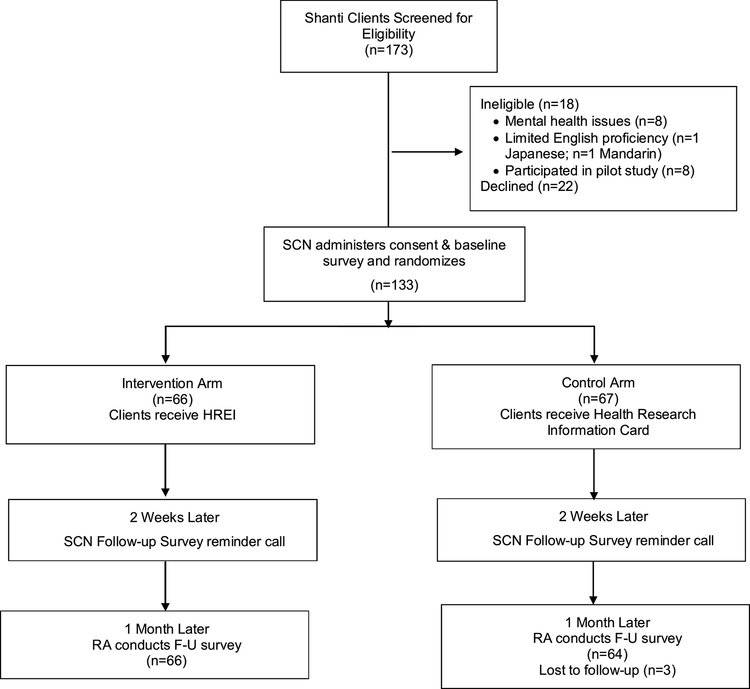
Women breast cancer patients and survivors who were Shanti clients, spoke English, Cantonese or Spanish, and had “low care navigation needs” were eligible to participate. These were clients who had completed active treatment or clients who were involved in Wellness Program activities. 173 clients were screened and 133 recruited in one of three ways: (1) during a face-to-face meeting with a SCN at the time of transition from intensive care navigation to the Wellness Program (n=95); (2) via flier in a bi-annual mailing of the Wellness Program calendar (n=27); or (3) via SCN phone invitation to clients identified as having “low care navigation needs” but who did not respond to the flyer (n=11).
Participants were randomized 1-to-1 to intervention or control in blocks of 4 stratified by language using a computer-generated random number sequence; randomization assignments were prepared by the study statistician and placed in sealed envelopes. At the enrollment visit, a language-concordant SCN administered consent and the baseline survey, randomized the participant, and then either administered the HREI (intervention arm) or provided the Health Research Information Card (Card) without the HREI education (control arm), with both groups receiving a thank you letter (Figure 4). On average, SCN spent 30 minutes administering the baseline surveys, and 20 additional minutes administering the HREI with participants in the intervention arm. Two weeks later, the SCN called the participant to remind her of the upcoming follow-up survey, leaving a message if they could not reach her directly. Two weeks after this call (one month after the enrollment visit), a language-concordant UCSF Research Associate called to schedule and then administer the follow-up survey by phone. Participants received a $25 gift card for each survey, and were reimbursed for transportation to and from the enrollment visit.
Figure 4.
Trial Design
Survey Development
We developed the baseline and follow-up surveys based on our conceptual framework (Figure 1), review of the literature, and development of new items needed to evaluate the effect of the HREI (e.g. whether they had talked to anyone, called a number, or visited a website to get information about health research studies). New measures were tested for reliability and validity. Validated measures in other domains included: health information-seeking efficacy and behavior [11]; knowledge and attitudes toward health research [42]; trust in medical care [43,44]; socio-demographics; intervention related behaviors [35]; and relationship with the Shanti care navigator [22].
The surveys were professionally translated into Spanish and Chinese and then pre-tested with English-, Spanish- and Cantonese-speakers. Research staff conducted two rounds of cognitive interviews with three Shanti clients and one SCN in each language in each round using standard cognitive interview techniques to ensure that questions were understood and consistency of meaning across languages [45,46]. After the first round, surveys were revised and the second round of cognitive interviews was conducted. Measures are shown in Table 1.
Table 1.
Measures
| Measure | Description | Pre-test | Post-test |
|---|---|---|---|
| Socio-demographic characteristics | Race/ethnicity, birthplace, years in U.S. (if foreign born), age, marital status, education, employment, income | X | |
| Health-related characteristics | Primary doctor, health status | X | |
| Health Information-seeking variables [11] | How often uses the internet, sources of health information (internet, helplines), thinks she can find information about health that she trusts (yes, no, maybe) | X | |
| Trust in medical care [43,44] | Corbie-Smith distrust index: summary score of 7 agree/disagree, yes/no items: If your doctors wanted you to participate in a research study, they would fully explain it to you; your doctors will honestly answer any questions you ask; your doctors would not ask you to participate in research if they thought it would harm you; doctors have ever given you treatment as part of an experiment without your permission; people might be used as guinea pigs without their consent; doctors ever prescribe medication experimenting on people without their knowledge or consent; in deciding what treatments you will get, your doctors always try to protect you from unnecessary risk | X | |
| Health empowerment [22,41] | A single Likert-scale item on level of confidence in finding information about health research studies | X | X |
| Knowledge of health research [35,42] | 5 true/false questions; knowledge score was computed by adding up the number of correct responses | X | X |
| Willingness to participate health research [42] | 9 yes/no questions about studies with different topics and methods of assessment | X | X |
| Seeking information about health research [28] | Measured in two ways: (1) face value: information-seeking took place if the respondent answered “yes” to one or more questions asking whether she had spoken to someone about the information that the navigator provided her, called one of the numbers or visited one of the websites listed on the Card; (2) confirmed: information-seeking took place if participant confirmed in the openended follow-up questions that what happened and what was discussed when the participant spoke to someone, called the number or accessed the website, was in fact related to the | X | |
| health research information provided in the study. | |||
| Relationship with the navigator [15,22]. | Overall rating of their navigator’s qualities on a Likert scale from poor to excellent, and agree/disagree items about navigator qualities: was compassionate, respectful, friendly, helped me to feel less afraid and anxious, helped me to find community services I needed, helped me to find information about health I needed, and was available when I needed her help | X |
Statistical Methods
Participants’ characteristics were summarized overall and by language using descriptive statistics (mean and standard deviation) for numeric variables and frequencies and percentages for categorical variables (Table 2). These characteristics were then compared by language and by study arm using ANOVA or t-tests for numeric variables and chi-square tests for categorical variables. We compared the study arms with respect to our primary outcome, seeking information about health research between pre- and post-test, using a chi-square test. We assessed pre-post changes in knowledge and willingness to participate in health research in each study arm (Table 3) using McNemar’s tests for proportions and a paired t-test for mean knowledge score. We then compared the study arms with respect to pre-post changes in proportions using z-tests, and in knowledge score using ANCOVA to adjust for pre-test knowledge. We also used logistic regression to determine factors associated with seeking information about health research (confirmed) between pre- and post-test. The full model included study arm, language, and characteristics potentially related to information-seeking according to our conceptual framework, including secondary outcomes with statistically significant intervention effects in bivariate comparisons: age, employment status, prior use of the internet or telephone helplines to obtain health information, distrust score, pre-post change in knowledge score, and pre-test knowledge score. Backward elimination was used to obtain a parsimonious model including effects with p<0.05, as well as study arm and language; adjusted odds ratios with 95% confidence intervals were estimated from the parsimonious model (Table 4). Frequencies of responses were computed for items on navigator qualities, and the study arms were compared with respect to the overall navigator rating (excellent vs. not excellent) using a chi-square test. Statistical significance was assessed at the 0.05 level (2-sided).
Table 2:
Health Research Engagement RCT Participant Characteristics by Language, San Francisco CA 2015-2017
| English (n=59) n (%) | Spanish (n=26) n (%) | Chinese (n=48) n (%) | Total (n=133) n (%) | p-value | |
|---|---|---|---|---|---|
| Race/Ethnicity | <.0001 | ||||
| Latina | 5 (9) | 26 (100) | 0 (0) | 31 (24) | |
| Chinese | 5 (9) | 0 (0) | 45 (94) | 50 (38) | |
| Other Asian | 10 (18) | 0 (0) | 2 (4) | 12 (9) | |
| Black | 8 (14) | 0 (0) | 0 (0) | 8 (6) | |
| White | 27 (47) | 0 (0) | 0 (0) | 27 (21) | |
| Multi-race | 2 (4) | 0 (0) | 1 (2) | 3 (2) | |
|
| |||||
| Birthplace | <.0001 | ||||
| U.S. | 35 (60) | 0 (0) | 0 (0) | 35 (27) | |
| Other | 23 (40) | 26 (100) | 0 (0) | 48 (100) | 97 (73) |
|
| |||||
| Years in U.S.* | 0.78 | ||||
| ≤ 10 | 2 (9) | 5 (19) | 7 (15) | 14 (14) | |
| 11–20 | 7 (30) | 6 (23) | 16 (33) | 29 (30) | |
| > 20 | 14 (61) | 15 (58) | 25 (52) | 54 (56) | |
| mean (SD) | 29.5 (16.5) | 25.2 (15.3) | 23.2 (12.6) | 25.2 (14.4) | 0.22 |
|
| |||||
| Age | 0.32 | ||||
| < 50 | 13 (23) | 6 (23) | 4 (8) | 23 (18) | |
| 50–59 | 18 (32) | 9 (35) | 16 (33) | 43 (33) | |
| 60–69 | 18 (32) | 10 (38) | 19 (40) | 47 (36) | |
| ≥ 70 | 8 (14) | 1 (4) | 9 (19) | 18 (14) | |
| mean (SD) | 58.9 (12.1) | 56.6 (9.1) | 61.3 (9.7) | 59.3 (10.8) | 0.19 |
|
| |||||
| Marital Status | 0.0004 | ||||
| Married/living together | 12 (21) | 11 (42) | 29 (60) | 52 (40) | |
| Formerly married | 23 (40) | 11 (42) | 13 (27) | 47 (36) | |
| Never married | 22 (39) | 4 (15) | 6 (13) | 32 (24) | |
|
| |||||
| Education | <.0001 | ||||
| < High school graduate | 2 (3) | 15 (58) | 18 (38) | 35 (26) | |
| High school graduate | 6 (10) | 3 (12) | 21 (44) | 30 (23) | |
| Some college | 19 (32) | 5 (19) | 6 (13) | 30 (23) | |
| College graduate | 32 (54) | 3 (12) | 3 (6) | 38 (29) | |
|
| |||||
| Employed | 19 (33) | 8 (31) | 12 (25) | 39 (30) | 0.68 |
|
| |||||
| Monthly Income | 0.3 | ||||
| < $2000 | 33 (59) | 19 (76) | 32 (68) | 84 (66) | |
| ≥ $2000 | 23 (41) | 6 (24) | 15 (32) | 44 (34) | |
|
| |||||
| Has Primary Doctor | 51 (86) | 23 (88) | 48 (100) | 122 (92) | 0.032 |
|
| |||||
| Health Status | <.0001 | ||||
| Excellent/very good | 10 (17) | 3 (12) | 1 (2) | 14 (11) | |
| Good | 27 (46) | 12 (46) | 6 (13) | 45 (34) | |
| Fair | 16 (27) | 9 (35) | 32 (67) | 57 (43) | |
| Poor | 6 (10) | 2 (8) | 9 (19) | 17 (13) | |
|
| |||||
| Corbie-Smith Distrust Index | 1.9 (2.0) | 2.2 (2.2) | 1.3 (1.7) | 1.7 (2.0) | 0.11 |
|
| |||||
| How often uses the Internet? | 0.0003 | ||||
| Every day | 46 (78) | 9 (35) | 17 (35) | 72 (54) | |
| Once/twice a week | 3 (5) | 2 (8) | 6 (13) | 11 (8) | |
| Less often | 2 (3) | 4 (15) | 5 (10) | 11 (8) | |
| Never | 8 (14) | 10 (38) | 20 (42) | 38 (29) | |
| DK | 0 (0) | 1 (4) | 0 (0) | 1 (1) | |
|
| |||||
| Sources of health information | |||||
| Internet** | 49(100) | 7 (54) | 19 (83) | 75 (88) | <.0001 |
| Telephone helplines | 12 (20) | 2 (8) | 1 (2) | 15 (11) | 0.0099 |
|
| |||||
| Overall, do you think you can find information about health that you trust? | 0.18 | ||||
| Yes | 42 (71) | 19 (73) | 30 (63) | 91 (68) | |
| No | 1 (2) | 3 (12) | 6 (13) | 10 (8) | |
| Maybe | 16 (27) | 4 (15) | 12 (25) | 32 (24) | |
If not born in U.S.
If uses the internet at least once a week
Note: SD=standard deviation; p-value from ANOVA (mean age, years in US, distrust score) or chi-square test (all other variables)
Table 3:
Health Research Engagement RCT: Pre-Post Changes in Knowledge and Willingness to Participate in Health Research San Francisco CA 2015–2017 (n=130)
| Intervention | Control | |||||
|---|---|---|---|---|---|---|
| Pre n (%) | Post n (%) | p-value | Pre n (%) | Post n (%) | pvalue | |
| Health Empowerment | ||||||
| Very confident can find information about health research studies | 14 (21) | 13 (20) | 0.76 | 16 (25) | 16(25) | 1 |
|
| ||||||
| Knowledge about health research | ||||||
| All health research studies are about drugs or treatments: False* | 21 (32) | 32 (48) | 0.012 | 27 (43) | 19 (30) | 0.059 |
| Must give consent to be able to participate in a study: True | 65 (98) | 62 (94) | 0.083 | 59 (92) | 60 (94) | 0.71 |
| Researchers must tell participants about risks and potential benefits: True | 64 (97) | 61 (92) | 0.18 | 58 (91) | 58 (91) | 1 |
| People have the right to withdraw from a study at any time: True | 64 (97) | 63 (95) | 0.65 | 58 (92) | 56 (89) | 0.53 |
| Anyone who wants to participate in a study will be allowed to do so: False | 25 (38) | 29 (44) | 0.32 | 24 (38) | 20 (32) | 0.29 |
| Knowledge Score†: Mean (SD) | 3.62 (0.92) | 3.74 (1.07) | 0.31 | 3.53(1.05) | 3.34 (1.06) | 0.13 |
|
| ||||||
| Willingness to participate in health research: Would consider a study involving | ||||||
| Testing a new drug | 22 (33) | 22 (33) | 1 | 17 (27) | 21 (33) | 0.32 |
| Blood draws | 48 (74) | 48 (74) | 1 | 43 (67) | 40 (63) | 0.47 |
| Exercise | 59 (89) | 60 (91) | 0.65 | 55 (87) | 54 (86) | 0.74 |
| Food and nutrition | 61 (92) | 61 (92) | 1 | 61 (95) | 55 (86) | 0.034 |
| Questionnaires | 63 (97) | 58 (89) | 0.025 | 59 (92) | 52 (81) | 0.052 |
| Interviews | 63 (95) | 60 (91) | 0.32 | 61 (95) | 54 (84) | 0.035 |
| Physical exams | 53 (80) | 50 (76) | 0.41 | 48 (75) | 47 (73) | 0.78 |
| Tests like mammograms | 51 (80) | 50 (78) | 0.76 | 49 (77) | 47 (73) | 0.53 |
| A clinical trial | 33 (52) | 36 (57) | 0.44 | 29 (45) | 24 (38) | 0.25 |
Note: SD=standard deviation, I=Intervention, C=Control; pre-post comparison p-values by study arm from paired t-tests (knowledge score) or McNemar’s tests (all other variables)
Difference between study arms in pre-post change: p=0.0012 (z-test)
Difference between study arms in pre-post change, adjusted for pre-test knowledge: p=0.028 (ANCOVA)
Table 4:
Health Research Engagement RCT: Factors associated with seeking information about health research, San Francisco CA 2015-2017 (n=128)
| Sought Information | ||
|---|---|---|
| OR* | 95% CI* | |
| Study Arm | ||
| Intervention | 1.27 | 0.38 – 4.27 |
| Control | 1.00 | |
| Language | ||
| Chinese | 3.96 | 0.87 – 18.0 |
| Spanish | 3.53 | 0.41 – 30.6 |
| English | 1.00 | |
| Distrust (per item) | 0.35 | 0.16 – 0.77 |
| Knowledge (per item) | ||
| Pre-intervention | 5.56 | 1.94 – 16.0 |
| Pre to post change | 2.99 | 1.30 – 6.87 |
Adjusted for all tabulated variables
Note: OR=odds ratio, CI=confidence interval
Qualitative Interviews
We conducted in-person qualitative interviews (n=11) with a subset of trial participants in the intervention arm (4 Spanish, 4 English and 3 Chinese). Written informed consent was obtained immediately prior to each interview, and participants received taxi vouchers and a $25 gift card. Interview questions addressed participants’ health research information-seeking, perceptions of the HREI, and relationships with SCN. Interviews were audio recorded and transcribed/translated verbatim. Coding and data analysis were conducted using Atlas-ti 8.0. Two members of the team read and coded the first few transcripts independently and then met to reconcile coding discrepancies and establish a codebook. They coded the remaining transcripts using the codebook, meeting to discuss and reconcile any discrepancies. Coded text was subsequently re-read by the coders who wrote memos to describe emerging themes. These memos were then discussed at monthly team meetings where investigators finalized the themes described below.
2. Results
2.1. Participants
Table 2 shows participants’ characteristics by language. The majority of participants were foreign born, including 40% of English speakers and all Spanish and Chinese speakers. Foreign-born participants tended to be long-time US residents (more than 20 years on average). Mean distrust scores did not differ significantly by language, and the majority of participants (68%) thought they could find information about health that they trust. However, there were substantial differences by language in sources of health information. English speakers were much more likely to use the internet daily (78%) compared to Spanish or Chinese speakers (35% each, p=0.0003), and among those who used it at least once a week, the internet was a source of health information for all English speakers vs. 54% of Spanish and 83% of Chinese speakers (p<.0001). In addition, English speakers were more likely than Spanish or Chinese speakers to get health information from telephone helplines (20% vs. 8% and 2%, respectively, p=0.0099). There were no statistically significant differences between the study arms in participant characteristics.
2.2. Health research information-seeking behavior
There was no statistically significant difference between study arms in information-seeking behavior. Almost one-third of participants in both arms reported having talked to someone about health research or having called a telephone number or visited a website listed on the Card (30% vs. 30%, p=0.94); a smaller proportion of participants confirmed that their information-seeking was related to health research vs. health in general (17% vs. 9%, p=0.22). Spanish and Cantonese speakers were no less likely to seek health research information than English speakers.
2.3. Secondary Outcomes
Table 3 shows pre-post changes in knowledge and willingness to participate in health research by study arm. At pre-test, most participants knew that one must give consent to participate in a study, that researchers must tell participants about potential risks and benefits, and that participants have the right to withdraw at any time; however, the majority incorrectly believed that anyone who wants to participate would be allowed to do so. This did not change at post-test for either arm. There was a significant increase from pre- to post-test in the proportion of intervention group participants who knew that not all health research studies are about drugs or treatments (32% to 48%, p=0.012), whereas there was no increase in the control group (43% to 30%, p=0.059). The difference between the study arms was statistically significant (p=0.0012). On average the change from pre- to post-test in knowledge score, adjusted for pre-test knowledge, was greater in the intervention group than in the control group (p=0.028). However, the proportion of participants who were very confident that they could find health research information (had health empowerment) remained essentially unchanged in both study arms (intervention: 20% post vs. 21% pre, p=0.76; control: 25% post vs. 25% pre, p=1.00). Women in both arms were much more likely to be willing to participate in research involving food and nutrition or exercise than in clinical or drug trials.
3.4. Multivariable model of information-seeking
Table 4 shows factors associated with seeking information about health research. Women were more likely to seek information if they had higher pre-test knowledge scores (odds ratio [OR]=5.6 per item, 95% confidence interval [CI] 1.9–16) or a greater increase in knowledge from pre- to post-test (OR=3.0 per item, 95% CI 1.3–6.9), and less likely to seek information if they had greater distrust in doctors (OR=0.3 per item, 95% CI 0.2–0.8); there was no association between information-seeking and study arm (OR=1.3, 95% CI 0.4–4.3).
3.5. Relationship with navigators
Almost all participants (>92%) agreed that their navigators had each of the listed qualities (see Measures section). The majority of participants in both study arms rated their navigators as excellent (intervention: 80%, control: 67%, p=0.089).
3.6. Qualitative interviews
Several themes emerged from the qualitative interviews that elucidate the survey results. The themes described below and interview quotes in Table 5 illustrate that although survey responses indicated willingness to participate in health research, in practice, participants had multiple competing priorities that limited their motivation to seek information about enrollment opportunities.
Table 5.
Qualitative Interview excerpts by theme
| Reasons for not seeking health research information |
Not wanting to think about cancer anymore At home, I just cannot bring myself to do it at home, … on the weekend, and you know, my mom is just, recently lost my dad, just a year ago. … Every weekend I go to my mom and it just takes a long time, &My sister and I, we, we just change days. She goes one day. I go another day.‘Cause she’s still, still adjusting. & And then, I have my chores, regular chores also, you know, the cleaning, (laughs). … So, i’s basically if I have to, to choose between okay, here’s my free time—do I want to do my study search or do I want to go and, you know, watch a movie, (laughs)? I go with the easiest way, (laughs). …I’s not what I should be doing. I really should be doing the study, but I’m, I’m just, I guess, just lazy is what it is. (EN-152) I just don’ want even to talk about it [cancer] if I don’ have it. & I was just like pushing it out & away from me as if, if I don’ talk about it, then it doesn’ exist. (EN-152) Language Barriers I just know that if I dial that number, I’ll be unable to communicate with them due to language issues. (CHI-149) Confusion about the Information Card Interviewer: …So what do you think the purpose of calling one of the numbers was? Participant: …The American Cancer Association, they help you, they help you navigate through hard times, you know, like if you need someone to talk to or of you need someone & If you need a taxi voucher, because, you know that & You may not have someone to bring you back and forth from your chemo or your radiation, they’re the person, they’re the go-to-person you can go to. Interviewer: Mm-hmm (affirmative). And have you ever talked to them? Participant: Um, yes, uh-huh. When I used to live in [name of county] I used them a lot. The American Cancer Society. (EN_142) ------- Participant: Susan, Susan [Komen] helps you, um& when, when you are diagnosed with cancer. Interviewer: Uh-huh. Participant: She helps you financially. Interviewer: Did you call any of these numbers? Participant: […] Mm& I would talk with Susan. Interviewer: Did they give you information? Did they give you financial help when you first got diagnosed? Participant: In the beginning, yes. Yes, they helped me out. (SP-133) |
| Positive attitudes toward participation and barriers to enrollment in research | We don’ participate very much because, as a Latino person, uh, you get to this country, you don’ have time. You have two jobs, you have a family. You don’ have, you don’ have the time, or the means to come and go one, two, three hours, to participate in the study. No, I think that limits us, as Latinos. And we can barely get by with that. And we’re living daily with such big financial problems, with family problems, so the, the rest, we say, i’s, i’s already enough with what we already have, to go get involved in something else. I think that is a big part of it. (SP-66) I: What would need to be different in your life to increase the possibility of learning about health research at another time? For example, you talked about using skype, and your availability. SP: Convenience. The timing, if I have this health issue that comes up. If you have an exercise research, then I will be interested and have the need. …It has to interest me. It has to match what I need. I: Is there anything else? What kind of changes do you think the health research needs? SP: The health research is not invasive. I: invasive, what do you mean? SP: Like when you tell me to take medication…or implanting, that’s invasive. (CH-153) CG: What made you participate in this study? SP: & What made me participate was, like I’ve said, there are different kinds of diseases. And someone else may find herself with something similar to me&And maybe something that I might have put down could help that person. (SP-133) [She is referring to some of the answers she gave during the survey] |
| Trust in Shanti at the core of motivation for participation | SP: They were- they were thorough, they had everything together. Yeah, it was like, “Yeah, I want to participate in this.” Because I think this is going to go somewhere, i’s going to get to somebody, i’s gonna reach somebody. Whereas, if i’s somebody outside, and you don’ know them, you’re like,‘Ehh, no.’‘Cause you’re not sure wha’s going to happen with that information. (ENG-109) I: So, what made you decide to call breastcancertrials.org? SP: Um, I felt like when I talked with [my navigator] I was making a commitment & to follow through on some of the information that she had given me. (ENG-135) I: Mm-hm. It was your Shanti navigator who invited you to this study. Is there any difference if, for example, someone calls you on the phone and i’s a person you don’ know on the other end of the line, and that person invites you to participate in a study like this one? In other words, would you participate if a stranger invited you to participate in a study like this one? SP: Well, no. I: Why? SP: Because I don’ know the person and I don’ know if i’s really true. I know them and tha’s why& (LAUGHS) (SP-133) |
| Confusion between health research and health care/education/promotion | I: under what circumstances, if there are any, do you think you’d be interested in participating in a health research study? What would make it worth your time? SP: Well, something that I knew would help me with my& with my treatment, (EN/SP-161) |
Reasons for not seeking health research information
Participants cited limited time, the desire to move on from the cancer diagnosis, and the expectation of language barriers on the part of LEP women as reasons for not seeking health research information independently. Several reported that they were too busy. One woman described herself as “lazy” for prioritizing self-care over searching for research opportunities during her “free” time. Others said they were less motivated to seek information about research opportunities because their treatment was over, and they worried that doing so would rouse painful emotions associated with their cancer diagnosis. LEP women were reluctant to call or visit the websites because they expected to have to communicate in English by phone or to navigate English language websites, despite the fact that the resources were listed on the Card in Spanish and Chinese, and only organizations with capacity in those languages were listed. Despite the intervention’s effort to convey otherwise, a number of women were confused about the purpose of the information on the Card; they recognized two organizations listed (ACS and Susan Love’s Army of Women, which some confused with Susan G. Komen Foundation) as resources they had previously engaged for social services and general information about cancer, but not as health research resources.
Positive attitudes toward participation and barriers to seeking information about research
We found that women’s willingness to participate in research came with a constellation of limitations and pre-conditions to accommodate physically demanding jobs and work schedules, long commutes, family caregiving, treatment side effects and other comorbidities (e.g., anxiety, depression, joint pain). The emotional and physical toll of their cancer diagnosis—and, for women now cancer free, a fear of recurrence—amplified stress and contributed to a general feeling that they had little time to participate in research. Among possible types of research, women expressed interest in topics related to their experiences and current conditions, (e.g., cooling helmets to prevent hair loss or herbal remedies to mitigate side effects of chemotherapy). They emphasized the need for protocols that they perceived to be helpful to themselves or others without being onerous (e.g. without long commutes or invasive procedures).
Trust in Shanti at the core of motivation for participation in our study
In the context of few participants’ seeking information about research on their own, we asked why they had been willing to enroll in our study. The common factor in their responses was the role of SCN in recruiting them and conducting the study. Participants perceived Shanti staff as competent and dedicated service connectors and advocates. In addition, the minimal time commitment, accessible location, and reimbursement for transportation costs facilitated their participation. One woman explicitly ascribed her decision to call one of the organizations listed on the Card to her relationship with her SCN.
4. Discussion and Conclusion
4.1. Discussion
Our study tested the HREI, an intervention that utilized community-based navigators to provide general education about the spectrum of breast cancer research in a neutral manner not tied to enrollment in a specific trial, and resources to independently find health research participation opportunities. Given that clinical trials and other studies are often not available where underserved patients seek care, and providers often do not invite low income patients to participate in research [12,13], offering alternate ways to access participation opportunities could help reduce information and enrollment disparities.
While the HREI did not increase health research information-seeking behavior, our qualitative interviews revealed that participants did not view research as a priority in the context of their busy and often difficult daily lives, and thus did not see health research as a “vital resource” like the others that SCN typically help clients access, e.g., food, emergency financial assistance, and emotional support. This finding adds nuance to prior research suggesting that competing demands related to time and financial costs of participation can limit motivation to participate [10]. Although clients were not motivated by the intervention to seek health research information independently, they did report willingness to participate in a variety of study types and topics, if the study protocols were convenient and addressed relevant issues, such as quality of life, improvements to their health (e.g. nutrition and exercise), or opportunities to help others by sharing their experiences. This finding is consistent with prior research [16]. The qualitative data also showed that the timing of discussions about health research was crucial. Although we aimed to reach women after the crisis of diagnosis and initial treatment, some participants who were in remission or finishing their treatments preferred not to think about cancer more than necessary at that point.
Furthermore, the HREI did not increase health empowerment (confidence in finding health research information). This outcome may be partially explained by how we asked participants to seek information (by phone or website), compared with their usual practice; few participants from any language group reported using telephone helplines to obtain health information, while Spanish and Chinese speakers reported lower usage of the internet for health information compared with English speakers. Our data coincides with other research that shows that Latino and Chinese immigrants use the internet to look for health information at relatively low rates [47–49].
Importantly, we found that participants with greater knowledge (at either pre- or post-test) were more likely to seek health research information independently. The long-term goal of our intervention was to build a foundation for increased enrollment of underrepresented patients and survivors in all types of research (behavioral, clinical, genetic) by increasing familiarity with relevant elements of health research; in the event of being approached for research participation, participants would have baseline knowledge needed for appropriate decision-making. As Ford and colleagues outlined in the conceptual framework for their 2008 systematic review, awareness and knowledge are necessary precursors to deciding whether or not to participate in research [9]. Given our goal, increased knowledge among intervention arm participants and correlation with health information seeking behavior is promising [9,50]. Nevertheless, our study also shows that factors other than knowledge – other priorities, anxieties, and concerns – may influence decisions to participate or not in clinical trials as much as or more than education.
Mistrust is a well-documented barrier to participation in research [10,51–53]. We designed the HREI to address mistrust in researchers and research by having trusted community-based navigators provide the intervention. Yet our multivariable model found that women were less likely to seek information about health research if they had greater distrust in doctors. Trusted SCN were able to recruit participants to our own study and some participants sought health research information due to a sense of obligation or loyalty to the SCN who delivered the intervention; however, this trust in SCN/Shanti did not appear to translate to trust in doctors who conduct research. The trust in SCN/Shanti supports the trend of engaging navigators as a promising link between low income and LEP communities and researchers [24–26,54], however, to effectively leverage this trust, the distrust of doctors and the research enterprise must be directly addressed.
Limitations
Underserved populations are willing to participate in clinical research [1–5]; however, a variety of conditions or circumstances must first be addressed, such as establishing trust, ensuring transportation and identifying other common logistical and systems barriers to participation. These well-known systems barriers [11,13,14] were not fully addressed in our study; we attempted to address them in a limited way by bringing together disconnected resources in the health care system (BCT and Shanti) and collaborating with BCT to improve its literacy and language accessibility. Nevertheless, some participants’ expectations of encountering language barriers prevented them from seeking information, and their expectations were largely correct. While BCT was able to provide information by telephone through professional healthcare interpreters, the study information on breastcancertrials.org remains in English. Furthermore, observational studies typically had surveys available only in English, and few studies listed on BCT focused on the wellness topics of most interest to our participants. As a result, the HREI could not provide participants with specific information about studies that were available to them, and aligned with their interests, literacy level and language capacity.
4.2. Conclusions and Practice Implications
Our study sheds light on populations often left out of clinical trials and other health research: low income, LEP and immigrant populations. Our study provides further evidence that community-based navigators are a trusted and therefore promising link between health research and these communities. However, finding an efficient and effective mode of engaging navigators and their clients at the right time in studies for which they would be eligible and interested remains a barrier to fully engaging these populations. Our findings suggest that participants are likely to respond to specific study information from a trusted source like their navigator, rather than general information about health research and how to seek further information. Low-income, LEP women need more than information about health research in order to consider enrolling in a study. In light of our findings, we contend that rather than placing the burden on potential participants to seek out additional information and enrollment opportunities, interventions should facilitate outreach and inclusion on the part of researchers to medically underserved populations. Systems barriers, such as English-only research staff and materials, the high literacy level of verbal and written communication about research, and the failure to invite low-income and minority patients and survivors to participate, continue to restrict participation in research. One-on-one interventions like the HREI may be too time intensive to be sustainable for community-based organizations like Shanti, but group interventions, such as those that mirror the support groups where cancer patients often get information, should be explored.
Highlights.
Low-income and LEP breast cancer survivors are willing to participate in research
Empowering individuals to seek information is not enough to engage them in research
Efforts to change the health research system are needed to facilitate inclusion
Studies must be timely, LEP accessible, and aligned with survivors’ interests
Acknowledgements.
We gratefully acknowledge the Shanti Care Navigators and breastcancertrials.org staff whose commitment to underserved breast cancer patients and survivors made this study possible. We are extremely grateful to the Shanti clients who participated in the study.
Funding: This work was supported by the California Breast Cancer Research Fund, #20BB-2100 (PIs Joseph and Nickell).
Footnotes
“I confirm all patient/personal identifiers have been removed or disguised so the patient/person(s) described are not identifiable and cannot be identified through the details of the story.”
Conflict of interest statement. The authors have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- [1].Moffitt K, Brogan F, Brown C, Kasper M, Rosenblatt J, Smallridge R, Sullivan D, Kromrey J, Statewide Cancer Clinical Trial Navigation Service, J. Oncol. Pract 6 (2010) 127–132. doi: 10.1200/JOP.200006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].De Las Nueces D, Hacker K, DiGirolamo A, Hicks LS, A Systematic Review of Community-Based Participatory Research to Enhance Clinical Trials in Racial and Ethnic Minority Groups, Health Serv. Res 47 (2012) 1363–1386. doi: 10.1111/j.1475-6773.2012.01386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Lang R, Kelkar VA, Byrd JR, Edwards CL, Pericak-Vance M, Byrd GS, African American Participation in Health-Related Research Studies: Indicators for Effective Recruitment, J. Public Health Manag. Pract 19 (2013) 110–118. doi: 10.1097/PHH.0b013e31825717ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Nápoles AM, Chadiha LA, Advancing the Science of Recruitment and Retention of Ethnically Diverse Populations, The Gerontologist. 51 (2011) S142–S146. doi: 10.1093/geront/gnr019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Spiker CA, Weinberg AD, Policies to address disparities in clinical trials: the EDICT Project, J. Cancer Educ. Off. J. Am. Assoc. Cancer Educ 24 (2009) S39–49. doi: 10.1080/08858190903400500. [DOI] [PubMed] [Google Scholar]
- [6].Chen MS, Lara PN, Dang JHT, Paterniti DA, Kelly K, Twenty Years Post-NIH Revitalization Act: Renewing the Case for Enhancing Minority Participation in Cancer Clinical Trials, Cancer. 120 (2014) 1091–1096. doi: 10.1002/cncr.28575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Sharrocks K, Spicer J, Camidge DR, Papa S, The impact of socioeconomic status on access to cancer clinical trials, Br. J. Cancer. 111 (2014) 1684–1687. doi: 10.1038/bjc.2014.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Kwiatkowski K, Coe K, Bailar JC, Swanson GM, Inclusion of minorities and women in cancer clinical trials, a decade later: Have we improved?, Cancer. 119 (2013) 2956–2963. doi: 10.1002/cncr.28168. [DOI] [PubMed] [Google Scholar]
- [9].Ford JG, Howerton MW, Lai GY, Gary TL, Bolen S, Gibbons MC, Tilburt J, Baffi C, Tanpitukpongse TP, Wilson RF, Powe NR, Bass EB, Barriers to recruiting underrepresented populations to cancer clinical trials: A systematic review, Cancer. 112 (2007) 228–242. doi: 10.1002/cncr.23157. [DOI] [PubMed] [Google Scholar]
- [10].George S, Duran N, Norris K, A Systematic Review of Barriers and Facilitators to Minority Research Participation Among African Americans, Latinos, Asian Americans, and Pacific Islanders, Am. J. Public Health 104 (2013) e16–e31. doi: 10.2105/AJPH.2013.301706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Burke NJ, Luce J, Guerra C, Nibbe A, Zbaren N, Kaplan C, Pasick R, COMMUNICATING ABOUT CLINICAL TRIALS: BRINGING THE CIS TO THE UNDERSERVED, in: Ann. Behav. Med, SPRINGER; 233 SPRING ST, NEW YORK, NY 10013 USA, 2013: pp. S263–S263. [Google Scholar]
- [12].Joseph G, Dohan D, Diversity of participants in clinical trials in an academic medical center, Cancer. 115 (2009) 608–615. doi: 10.1002/cncr.24028. [DOI] [PubMed] [Google Scholar]
- [13].Joseph G, Dohan D, Recruiting minorities where they receive care: Institutional barriers to cancer clinical trials recruitment in a safety-net hospital, Contemp. Clin. Trials 30 (2009) 552–559. doi: 10.1016/j.cct.2009.06.009. [DOI] [PubMed] [Google Scholar]
- [14].Joseph G, Dohan D, Recruitment Practices and the Politics of Inclusion in Cancer Clinical Trials, Med. Anthropol. Q 26 (2012) 338–360. doi: 10.1111/j.1548-1387.2012.01222.x. [DOI] [PubMed] [Google Scholar]
- [15].Livaudais-Toman J, Burke NJ, Napoles A, Kaplan CP, Health Literate Organizations: Are Clinical Trial Sites Equipped to Recruit Minority and Limited Health Literacy Patients?, J. Health Disparities Res. Pract 7 (2014) 1–13. [PMC free article] [PubMed] [Google Scholar]
- [16].Katz RV, Kegeles SS, Kressin NR, Green BL, Wang MQ, James SA, Russell SL, Claudio C, The Tuskegee Legacy Project: willingness of minorities to participate in biomedical research, J. Health Care Poor Underserved 17 (2006) 698–715. doi: 10.1353/hpu.2006.0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Smirnoff M, Wilets I, Ragin DF, Adams R, Holohan J, Rhodes R, Winkel G, Ricci EM, Clesca C, Richardson LD, A paradigm for understanding trust and mistrust in medical research: The Community VOICES study, AJOB Empir. Bioeth 9 (2018) 39–47. doi: 10.1080/23294515.2018.1432718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Freund KM, Battaglia TA, Calhoun E, Dudley DJ, Fiscella K, Paskett E, Raich PC, Roetzheim RG, National Cancer Institute Patient Navigation Research Program, Cancer. 113 (2008) 3391–3399. doi: 10.1002/cncr.23960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Freeman HP, Muth BJ, Kerner JF, Expanding access to cancer screening and clinical follow-up among the medically underserved, Cancer Pract. 3 (1995) 19–30. [PubMed] [Google Scholar]
- [20].Freeman HP, Poverty, Culture, and Social Injustice: Determinants of Cancer Disparities, CA. Cancer J. Clin 54 (2008) 72–77. doi: 10.3322/canjclin.54.2.72. [DOI] [PubMed] [Google Scholar]
- [21].Nelson A, Unequal treatment: confronting racial and ethnic disparities in health care., J. Natl. Med. Assoc 94 (2002) 666–668. [PMC free article] [PubMed] [Google Scholar]
- [22].Gabitova G, Burke NJ, Improving healthcare empowerment through breast cancer patient navigation: a mixed methods evaluation in a safety-net setting, BMC Health Serv. Res 14 (2014) 407. doi: 10.1186/1472-6963-14-407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Krok-Schoen JL, Oliveri JM, Paskett ED, Cancer Care Delivery and Women’s Health: The Role of Patient Navigation, Front. Oncol 6 (2016) 2. doi: 10.3389/fonc.2016.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Ghebre RG, Jones LA, Wenzel JA, Martin MY, Durant RW, Ford JG, State-of-the-science of patient navigation as a strategy for enhancing minority clinical trial accrual, Cancer. 120 (2014) 1122–1130. doi: 10.1002/cncr.28570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Heller C, Balls-Berry JE, Nery JD, Erwin PJ, Littleton D, Kim M, Kuo WP, Strategies addressing barriers to clinical trial enrollment of underrepresented populations: A systematic review, Contemp. Clin. Trials 39 (2014) 169–182. doi: 10.1016/j.cct.2014.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Wells KJ, Valverde P, Ustjanauskas AE, Calhoun EA, Risendal BC, What are patient navigators doing, for whom, and where? A national survey evaluating the types of services provided by patient navigators, Patient Educ. Couns 101 (2017) 285–294. doi: 10.1016/j.pec.2017.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Kanekar S, Petereit D, Walking Forward: A Program Designed to Lower Cancer Mortality Rates among American Indians in Western South Dakota, S. D. Med. J. S. D. State Med. Assoc 62 (2009) 151–159. [PMC free article] [PubMed] [Google Scholar]
- [28].Larkey LK, Ogden SL, Tenorio S, Ewell T, Latino recruitment to cancer prevention/screening trials in the Southwest: setting a research agenda, Appl. Nurs. Res 21 (2008) 30–39. doi: 10.1016/j.apnr.2006.09.003. [DOI] [PubMed] [Google Scholar]
- [29].Cartmell KB, Bonilha HS, Matson T, Bryant DC, Zapka J, Bentz TA, Ford ME, Hughes-Halbert C, Simpson KN, Alberg AJ, Patient participation in cancer clinical trials: A pilot test of lay navigation, Contemp. Clin. Trials Commun 3 (2016) 86–93. doi: 10.1016/j.conctc.2016.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Fouad MN, Acemgil A, Bae S, Forero A, Lisovicz N, Martin MY, Oates GR, Partridge EE, Vickers SM, Patient Navigation As a Model to Increase Participation of African Americans in Cancer Clinical Trials, J. Oncol. Pract 12 (2016) 556–563. doi: 10.1200/JOP.2015.008946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Williams LB, Tingen M, McCall A, Khleif SN, Abstract A35: Reducing lung cancer mortality in disparate populations through cancer-Community Awareness Access Research and Education (c-CARE), Cancer Epidemiol. Prev. Biomark 25 (2016) A35–A35. doi: 10.1158/1538-7755.DISP15-A35. [DOI] [Google Scholar]
- [32].Hanson H, Lendrem DW, O’Brien N, Pratt AG, Isaacs JD, Rapley T, 125. HOW TO EXPAND AWARENESS OF AND RECRUITMENT TO RESEARCH: A MIXED METHODS FEASIBILITY STUDY, Rheumatology. 56 (2017). doi: 10.1093/rheumatology/kex062.126. [DOI] [Google Scholar]
- [33].Meropol NJ, Wong Y-N, Albrecht T, Manne S, Miller SM, Flamm AL, Benson AB, Buzaglo J, Collins M, Egleston B, Fleisher L, Katz M, Kinzy TG, Liu TM, Margevicius S, Miller DM, Poole D, Roach N, Ross E, Schluchter MD, Randomized Trial of a Web-Based Intervention to Address Barriers to Clinical Trials, J. Clin. Oncol 34 (2016) 469–478. doi: 10.1200/JCO.2015.63.2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Wallington SF, Luta G, Noone A-M, Caicedo L, Lopez-Class M, Sheppard V, Spencer C, Mandelblatt J, Assessing the Awareness of and Willingness to Participate in Cancer Clinical Trials Among Immigrant Latinos, J. Community Health 37 (2012) 335–343. doi: 10.1007/s10900-011-9450-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Nickell A, Burke NJ, Cohen E, Caprio M, Joseph G, Educating Low-SES and LEP Survivors About Breast Cancer Research: Pilot Test of the Health Research Engagement Intervention, J. Cancer Educ 29 (2014) 746–752. doi: 10.1007/s13187-014-0650-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Minkler M, Wallerstein N, eds., Community-Based Participatory Research for Health, Jossey-Bass Publishers, San Francisco, 2003. [Google Scholar]
- [37].Begun JW, Zimmerman B, Dooley K, Health Care Organizations as Complex Adaptive Systems, in: Adv. Health Care Organ. Theory San Franc, San Francisco: Jossey-Bass, 2003: pp. 253–288. [Google Scholar]
- [38].Keshavarz N, Nutbeam D, Rowling L, Khavarpour F, Schools as social complex adaptive systems: A new way to understand the challenges of introducing the health promoting schools concept, Soc. Sci. Med 70 (2010) 1467–1474. doi: 10.1016/j.socscimed.2010.01.034. [DOI] [PubMed] [Google Scholar]
- [39].Pasick RJ, Barker JC, Otero-Sabogal R, Burke NJ, Joseph G, Guerra C, Intention, Subjective Norms, and Cancer Screening in the Context of Relational Culture, Health Educ. Behav. Off. Publ. Soc. Public Health Educ 36 (2009) 91S–110S. doi: 10.1177/1090198109338919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Garfield C, Kleinmaier C, Training Volunteers for Community Service: The Step-By-Step Guide of the Shanti National Training Institute (ring-binder), Jossey-Bass Publishers, San Francisco, 2000. [Google Scholar]
- [41].Crawford Shearer NB, Health Empowerment Theory as a Guide for Practice, Geriatr. Nur. (Lond.). 30 (2009) 4–10. doi: 10.1016/j.gerinurse.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Schutt RK, Schapira L, Maniates J, Santiccioli J, Henlon S, Bigby J, Community Health Workers’ Support for Cancer Clinical Trials: Description and Explanation, J. Community Health 35 (2010) 417–422. doi: 10.1007/s10900-010-9267-0. [DOI] [PubMed] [Google Scholar]
- [43].Corbie-Smith G, Thomas SB, St George DMM, Distrust, race, and research, Arch. Intern. Med 162 (2002) 2458–2463. [DOI] [PubMed] [Google Scholar]
- [44].Ding EL, Powe NR, Manson JE, Sherber NS, Braunstein JB, Sex Differences in Perceived Risks, Distrust, and Willingness to Participate in Clinical Trials: A Randomized Study of Cardiovascular Prevention Trials, Arch. Intern. Med 167 (2007) 905–912. doi: 10.1001/archinte.167.9.905. [DOI] [PubMed] [Google Scholar]
- [45].Collins D, Pretesting survey instruments: an overview of cognitive methods, Qual. Life Res. Int. J. Qual. Life Asp. Treat. Care Rehabil 12 (2003) 229–238. [DOI] [PubMed] [Google Scholar]
- [46].Peterson NA, Powell KG, Cognitive Interviewing for Item Development: Validity Evidence Based on Content and Response Processes AU - Peterson, Christina Hamme, Meas. Eval. Couns. Dev 50 (2017) 217–223. doi: 10.1080/07481756.2017.1339564. [DOI] [Google Scholar]
- [47].Islam NS, Patel S, Wyatt LC, Sim S-C, Mukherjee-Ratnam R, Chun K, Desai B, Tandon SD, Trinh-Shevrin C, Pollack H, Kwon SC, Sources of Health Information Among Select Asian American Immigrant Groups in New York City, Health Commun. 31 (2016) 207–216. doi: 10.1080/10410236.2014.944332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Wong CKM, Yeung DY, Ho HCY, Tse K-P, Lam C-Y, Chinese older adults’ Internet use for health information, J. Appl. Gerontol. Off. J. South. Gerontol. Soc 33 (2014) 316–335. doi: 10.1177/0733464812463430. [DOI] [PubMed] [Google Scholar]
- [49].Jiang S, Street RL, Pathway Linking Internet Health Information Seeking to Better Health: A Moderated Mediation Study, Health Commun. 32 (2017) 1024–1031. doi: 10.1080/10410236.2016.1196514. [DOI] [PubMed] [Google Scholar]
- [50].Paterniti DA, Chen MS Jr, Chiechi C, Beckett LA, Horan N, Turrell C, Smith L, Morain C, Montell L, Luis Gonzalez J, Davis S, Lara PN Jr, Asian Americans and cancer clinical trials: a mixed-methods approach to understanding awareness and experience, Cancer. 104 (2005) 3015–3024. doi: 10.1002/cncr.21522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Fischer SM, Kline DM, Min S-J, Okuyama S, Fink RM, Apoyo con Cariño: Strategies to Promote Recruiting, Enrolling, and Retaining Latinos in a Cancer Clinical Trial, J. Natl. Compr. Canc. Netw 15 (2017) 1392–1399. doi: 10.6004/jnccn.2017.7005. [DOI] [PubMed] [Google Scholar]
- [52].Salman A, Nguyen C, Lee Y-H, Cooksey-James T, A Review of Barriers to Minorities’ Participation in Cancer Clinical Trials: Implications for Future Cancer Research, J. Immigr. Minor. Health 18 (2016) 447–453. doi: 10.1007/s10903-015-0198-9. [DOI] [PubMed] [Google Scholar]
- [53].Fam E, Ferrante JM, Lessons Learned Recruiting Minority Participants for Research in Urban Community Health Centers, J. Natl. Med. Assoc 110 (2018) 44–52. doi: 10.1016/j.jnma.2017.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Burke NJ, Stuck in the Middle: Patient navigation and clinical trials recruitment in the safety net., in: Armin J Burke NJ Eichelberger Eds Negot. Struct. Vulnerability Cancer Control Contemp. Chall. Appl. Anthropol, School for Advanced Research, in press. [Google Scholar]