Abstract
Diisocyanates are well recognized to cause occupational asthma, yet diisocyanate asthma can be challenging to diagnose and differentiate from asthma induced by other allergens. The present study assesses the potential contribution of methylene diphenyl diisocyanate (MDI) to a workplace fatality.
Examination of medical records, tissue, and blood from the deceased worker were undertaken. Formalin fixed paraffin-embedded lung tissue sections were assessed through histologic and immunochemical stains. Serum MDI-specific IgE and IgG and total IgE, were measured by ELISA and/or Western blot. Information about potential chemical exposures and industrial processes in the workplace were provided by the employer and through interviews with co-workers.
Review of the worker’s medical records, occupational history, and autopsy findings were consistent with severe asthma as the cause of death, and ruled out cardiac disease, pulmonary embolism, or stroke. Lung pathology revealed hallmarks of asthma including smooth muscle hypertrophy, eosinophilia, basement membrane thickening, and mucus plugging of bronchioles. Immunochemical staining for MDI was positive in the thickened basement membrane of inflamed airways. MDI-specific serum IgE and IgG were significantly elevated and demonstrated specificity for MDI vs. other diisocyanates, however, total serum IgE was normal (24 IU/mL). The workplace had recently introduced MDI into the foundry as part of a new process, but MDI air levels had not been measured. Respirators were not required.
In summary, post-mortem findings support the diagnosis of diisocyanate asthma and a severe asthma attack at work as the cause of death in a foundry worker.
Keywords: methylene diphenyl diisocyanate (MDI), fatal, asthma, foundry, immunocytochemistry, occupational, allergen, IgE, IgG, chemical
INTRODUCTION
Exposure to diisocyanate, a small molecular weight chemical used industrially to cross-link polymer chains in manufacturing an array of products, is well known to cause occupational asthma.1 However diagnosis of isocyanate asthma is challenging as asthma is a common condition in working age adults and isocyanate asthma can be difficult to differentiate from other causes of airway inflammation and reversible hyper-reactivity.1 Once a worker becomes immunologically sensitized to diisocyanate, extremely low airborne levels of chemical, below permissible exposure levels (PELs) established by the United States’ Occupational Health and Safety Administration (OSHA) or the lower recommended exposure limits (RELs) defined by the National Institute for Occupational Safety and Health (NIOSH), can trigger severe asthmatic responses.2–4
Methylene diphenyl diisocyanate (MDI) is now the most abundantly produced and utilized diisocyanate world-wide.5–7 Major uses for MDI are in the production of polyurethane-based products and as a binding agent for various industrial processes.8–10 MDI is generally considered safer than other commonly used diisocyanates because its low vapor pressure (volatility) at room temperature produces less inhalable chemical in gas phase.5 However, industrial processes or occupational tasks that utilize MDI frequently involve spraying or heating, thereby creating potential for respiratory tract exposure to MDI aerosols and vapor.11–13
Severe asthma from occupational diisocyanate exposure is not uncommon, and while death is rare, specific cases have been well documented in published reports; since 1985, eight fatal cases have been described (see Table 1). Earlier cases (1985–1989) were caused by toluene diisocyanate (TDI), but more recent deaths have been attributed to MDI, including one in a work setting (foundry) similar to the present.14 As new uses evolve for MDI and other diisocyanates (e.g. spray foam insulation, mattresses, stabilization of underground mines, propeller blades for wind turbines)12, 13, 15–18, opportunities for occupational exposure and asthmatic responses are likely to continue. The present case of death in a subject with a prior history of work-related asthma emphasizes the importance of recognizing diisocyanate as the cause of airways disease, and exposure avoidance if chemical hypersensitivity develops.
Table 1:
Asthma Deaths from Diisocyanate Exposure
| Cause | Sex | Year of Death | Sensitizing Agent | Occupation | Reference |
|---|---|---|---|---|---|
| 1 | M | 1985 | TDI | car painter | Anonymous: Incident reports: Car paint death. Toxic Subst Bull 1985:4:719 (also cited/reviewed in Carino et al.)14 |
| 2 | M | 1987 | TDI | car painter | Fabbri et al.20 |
| 3 | M | 1989 | TDI | flame lamination | CCINFO fatality reports, record No. 1710, (also cited/reviewed in Carino et al.)14 |
| 4 | M | 1992 | MDI | foundry | Carino et al.14 |
| 5 | M | 2003 | MDI | truck bed liner | Chester et al.21 |
| 6 | M | 2005 | MDI and TDI | adhesive manufacture | Project SENSOR News. Asthma Mortality. Volume 19, No. 3 (2008)22 |
| 7 | F | 2006 | TDI | machine operator | Reilly et al.23 |
| 8 | M | 2015 | diisocyanate | casting room machine operator | Reilly et al.23 |
MATERIALS & METHODS
Human subject: The study subject’s available medical records were obtained and reviewed after OSHA received a referral from the county medical examiner suggesting a work-related death because of enlarged chest lymph nodes (the employer had not reported the fatality as work-related). Forensic pathology, including acquisition of lung tissue and serum samples was performed by the local medical examiner. Commercially available pooled human male AB+ serum was used as a negative control in serology studies as previously described.24 Permission was obtained from the subject’s next-of-kin and all studies were approved by Yale University’s Human Investigation Committee’s Institutional Review Board.
Workplace evaluation: The employer was an aluminum parts foundry employing 97 individuals. The employer had started using MDI as a binding agent in the mold-making process. During the mold assembly process, a driving auger received and mixed sand from an overhead hopper along with the 3-part binder components, including MDI. The auger poured the mixture through an outflow tract into the mold forms (cope and drag). The sand/binder mixture was mechanically and manually pressed into the mold forms. The binder and sand would chemically set at ambient temperature. An electric arc induction furnace melted metal which was eventually poured into the set sand molds. Although the work was physically demanding, it did not represent a “hot” job with thermal loading. Work included exposure to known hazardous agents including free, crystalline silica and MDI. The employer had recently switched from using a phenol-formaldehyde binder (“hot box”) to MDI (“cold box”). Respirators were not required, and only some workers wore gloves voluntarily. The decedent was not wearing gloves. In late 2013 and early 2014, equipment and trial runs of the MDI binder chemicals were initiated. On April 1, 2014 full production utilizing MDI started. The employee was found unconscious and taken to the hospital on May 6, 2014. He passed away the same day.
Enzyme-linked immunosorbant assays (ELISA). ELISAs for diisocyanate specific IgG and/or IgE were performed as previously described.24, 25 Nunc MaxiSorp (Thermo Scientific; Rockford, IL) 96-well microtiter plates were coated with 100 μL/well of different diisocyanate or control (mock exposed) albumin conjugates, diluted to 10 μg/ml in 0.1 M carbonate buffer (pH 9.5), by overnight incubation at 4°C. Low endotoxin certified human albumin from Sigma Chemical (St. Louis, MO, USA) was conjugated with either 4,4’-methylene diphenyl diisocyanate, toluene diisocyanate (80:20 ratio of 2,4 to 2,6-isomers) or 1,6-hexamethylene diisocyanate from Aldrich (St. Louis, MO) and characterized as previously described.24–26 IgE and IgG ELISAs plates were blocked with 1% bovine serum albumin or 3% dry milk in PBS respectively. IgE tests were developed with biotinylated mouse monoclonal antibody specific for the human IgE-Fc region (SouthernBiotech, Birmingham, AL), followed by peroxidase-conjugated streptavidin (BD Pharmingen, San Jose, CA). IgG ELISAs were developed with peroxidase-conjugated mouse monoclonal anti-human IgG-Fc from BD Pharmingen (San Jose, CA). All ELISAs were developed for 20 min with TMB substrate from BD Bioscience (San Jose, CA), and the final reactions were terminated by the addition of 0.1 M HCl from Sigma. Optical density (OD) measurements were obtained on a Benchmark microtiter plate reader from Bio-Rad using dual wavelength absorbance (450 – 550 nm). An MDI-IgE binding ratio (IgE binding to MDI-albumin vs. control “mock” exposed albumin) and end titer for MDI-IgG were calculated as previously described.25 All samples were tested in triplicate to obtain mean and standard error values.
Western blot: Diisocyanate-conjugated or mock exposed albumin conjugates (2 μg/lane) were separated by reducing SDS-PAGE and transferred to nitrocellulose membrane as previously described.24, 27 Membranes were blocked with 3% dry milk in PBS and incubated overnight with a 1:500 dilution of the study subject’s serum IgG fraction prepared using a commercially available serum “clean-up” column that depletes albumin and other major serum non-immunoglobulin proteins (Melon Gel IgG Spin Purification Kit from Thermo Scientific; Rockford, IL). Western blots were developed with peroxidase conjugated mouse anti-human IgG-Fc specific monoclonal antibody using TMB substrate and exposure to photographic film (GE Healthcare, Buckinghamshire, UK).
Histology and immunocytochemistry: Formalin fixed, paraffin-embedded lung tissue sections were stained with hematoxylin-eosin (H+E) and periodic acid-Schiff (PAS) reagents as previously described.28 Immunocytochemical staining for MDI was performed as previously described29 with minor modifications. Briefly, airway tissue sections were incubated with 2 μg/mL of affinity-purified polyclonal anti-MDI rabbit IgG diluted in 20% goat serum, followed by development with biotin-labeled mouse monoclonal antibody specific for rabbit (and not other species, including human) IgG (ProSci Inc, Poway, CA), peroxidase conjugated streptavidin, and 3,3’-Diaminobenzidine (DAB) substrate from HistoMouse-MAX staining Kit (Invitrogen, Frederick, MD). Endogenous peroxidase and biotin were quenched with H2O2 and biotin blocking solution (Life Technologies, Eugene, OR). Negative control staining was performed with 2 μg/mL of affinity-purified polyclonal anti-keyhole limpet hemocyanin (KLH) rabbit IgG (Rockland Chemicals; Limerick, PA).
CASE REPORT
CASE HISTORY
The subject, a 55 year old male, was one of 97 workers employed in a foundry that makes aluminum products. The subject had worked for the same employer for 38 years, but his specific job duties had changed over time. His most recent position involved the production of molds, a process in which sand and a resin binder form a mold into which molten aluminum is poured. Previously the foundry had a formaldehyde-based resin, but a recent processing change replaced it with MDI three months earlier. While the employer offered respirators, it had not established a written respiratory protection plan and did not require their use. Some workers, though not the decedent, used gloves voluntarily. There were no available records of diisocyanate use prior to this time. An OSHA inspection of the facility spurred by the incident resulted in citation for one willful violation for failing to train workers in chemical hazards, as outlined under the National Emphasis Program for Occupational Exposure to Isocyanates.30 The company has since ceased MDI use.
The day the worker died he reported to a co-worker that he was not feeling well shortly after his shift started and was later found unconscious in the workplace restroom. The workplace called emergency medical services, who administered cardiopulmonary resuscitation, beginning in the workplace; however, EMT records are not available. The worker was pronounced dead a short time later at a nearby hospital.
REVIEW OF PAST MEDICAL RECORDS
A review of the decedents past medical records revealed a history of adult-onset asthma that had been diagnosed as occupational over 30 years previously, although he had been symptom-free for almost 20 years. He had first developed asthma symptoms in 1984 at age 25, prompting several acute visits to his primary care physician over a 6-month period, and a chest X-ray, which was normal. At that time, his job involved spraying a material on castings. The worker experienced episodes of cough and wheezing that forced him to leave work. His symptoms improved on weekends and with time away from work. His records note that he was diagnosed with asthma and treated with epinephrine, theophylline, and an inhaled bronchodilator, which provided varying degrees of symptom relief. In February of 1984, his physician had diagnosed an “allergic reaction to the spray that he was using” and suggested a respirator, both to the patient and in a letter to the employer, which he used with only limited success in alleviating symptoms. The worker reported more notable improvement in symptoms by avoiding the “fumes that give me trouble”.
In 1988, the worker again became symptomatic with emergency room visits and further treatment with theophylline and inhaled bronchodilators. After a 2 month period of symptoms, treatment, and work-up for asthma, the worker “changed away from the job” that was causing him trouble and reported improvements in breathing. Medical records note prescriptions for theophylline and bronchodilators in August 1989 and again in 1995, with a recommendation to identify and avoid the causative exposure. From 1995 until a week before his death, the worker had no ongoing asthma treatment noted. Diabetes mellitus was diagnosed in 1998.
One month prior to the worker’s death, two months after initiation of MDI use, he started to re-experience occupational asthma symptoms and eight days before death had visited his primary physician (not the same physician he had seen for his earlier asthma symptoms) for respiratory complaints. Earlier records documenting occupational asthma were unclear, however a new chest X-ray was read as normal and the patient was prescribed inhaled corticosteroids and bronchodilators.
AUTOPSY AND PATHOLOGY
Autopsy and review of medical records found no evidence of cardiac disease, pulmonary embolism or stroke. Cardiac laceration from the cardiopulmonary resuscitation leading to pericardial tamponade was considered the terminal event. Examination of the lungs showed pulmonary edema and inflammatory changes consistent with severe asthma including smooth muscle hypertrophy, eosinophilic inflammation, basement membrane thickening, increased mucous deposition and mucus airway plugging (Fig 1). Further immunostaining for MDI identified the chemical localized to the thickened basement membrane surrounding inflamed airways (Fig 2).
Figure 1.
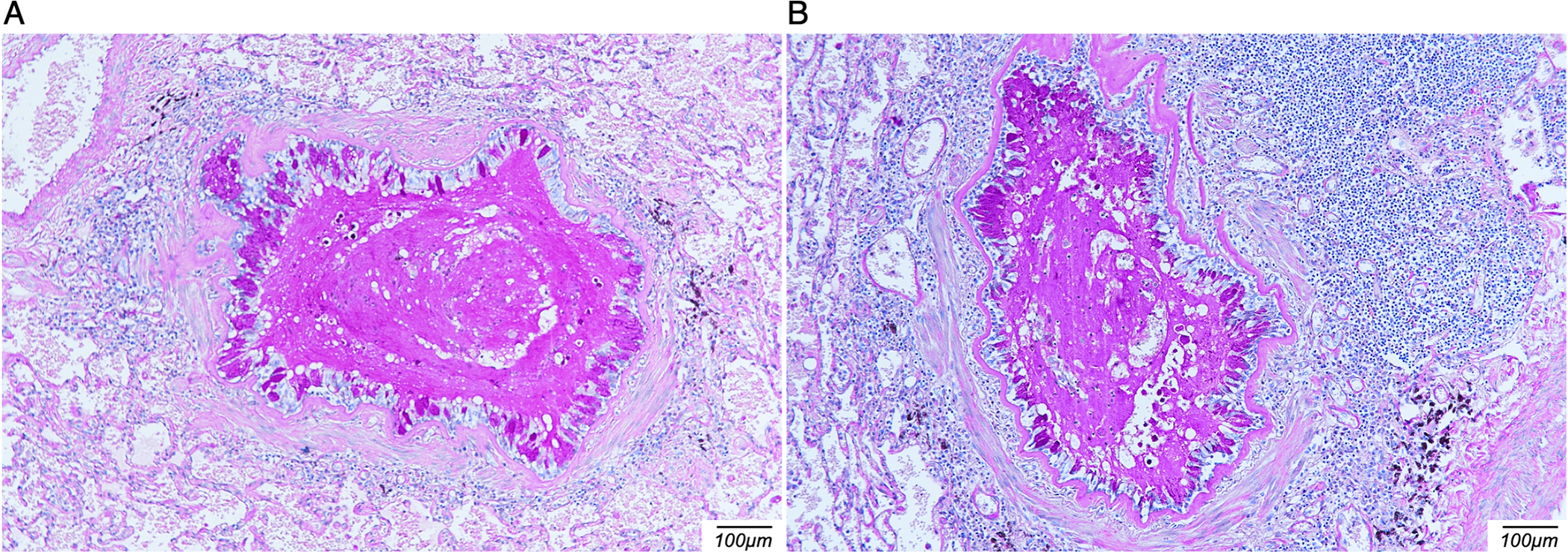
Periodic acid-Schiff (PAS) stain of lung tissue sections. Lung tissue sections obtained during autopsy and stained with PAS highlight mucus plugging, thickening of the basement membrane and intense inflammation surrounding airways/bronchioles.
Figure 2.
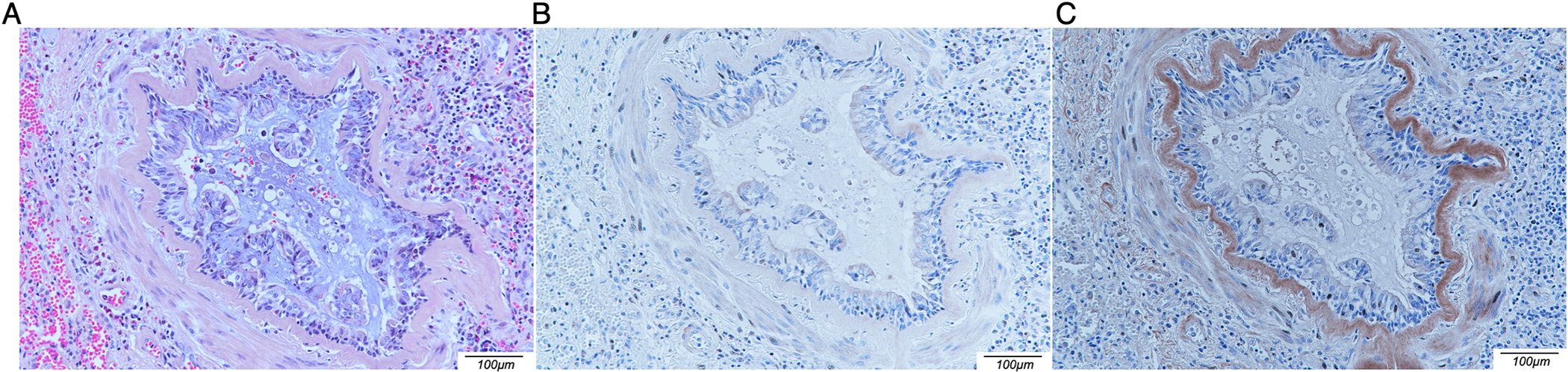
Immunochemical detection of MDI in situ. Lung tissue sections obtained during autopsy were stained with hematoxylin-eosin (A), negative control affinity-purified anti-KLH polyclonal rabbit IgG (B) or affinity-purified anti-MDI polyclonal rabbit IgG (C). Note brown DAB staining detecting MDI in the thickened basement membrane surrounding a highly inflamed bronchiole.
SEROLOGY STUDIES
Serum obtained from the decedent during autopsy had been saved and stored at −20°C. After the question of MDI asthma was raised, total IgE, and MDI-specific IgE and IgG were measured by ELISA. Total IgE was normal (24 IU/mL). However, the MDI-specific IgE (binding ratio to MDI-albumin vs. control albumin) was noticeably elevated with a ratio of 4.0 (ratio >2.0 positive based on prior studies)26, 31, and significantly higher than that observed with control serum pooled from healthy adult males, or ELISAs with other diisocyanate antigens, hexamethylene diisocyanate (HDI) or toluene diisocyanate (TDI) (Fig 3A). The MDI-specific IgG end titer was >1:6,400 (normal < 1:16) and demonstrated specificity for MDI vs. other diisocyanates (HDI and TDI) by Western blot (Fig 3B).
Figure 3.
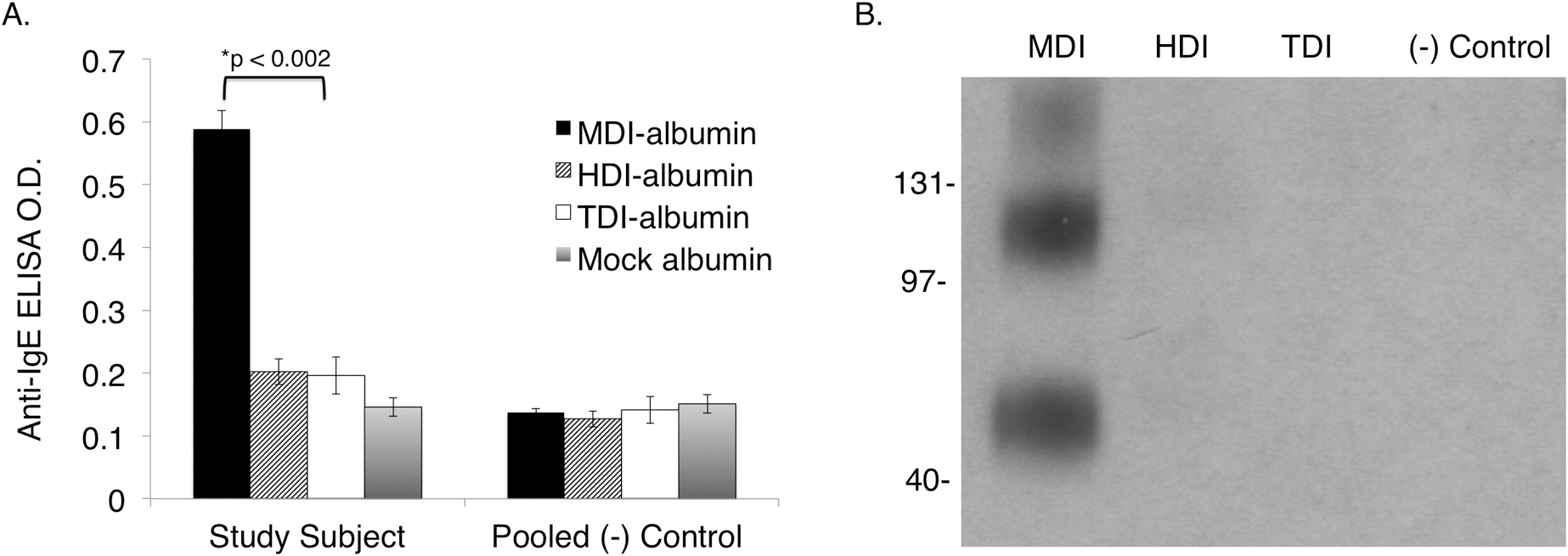
Serologic detection of MDI-specific IgE and IgG post-mortem. Serum samples obtained at autopsy were tested for the presence of IgE with specificity for MDI by ELISA (A). Study subject IgE binding to MDI (albumin conjugates) is significantly elevated compared to other diisocyanates and results obtained with control sera pooled from (non-diisocyanate asthmatic) healthy adult male subjects. Western blots of the study subject’s serum (B) demonstrate the presence of IgG with specificity for MDI (albumin conjugates) vs. other diisocyanates commonly used commercially, as labeled. Note the triple banding pattern reflective of IgG binding to monomeric, dimerized and trimerized MDI-albumin conjugates, as described in prior studies.25
DISCUSSION
In this case report, post-mortem analysis of autopsy samples, along with review past medical records and employer information, provide strong evidence supporting MDI exposure as the cause of a severe asthma attack leading to death of a foundry worker with a distant history of occupational asthma. The case highlights important features of diisocyanate exposure and the challenges of diagnosing and clinical management of diisocyanate asthma. The data also demonstrate utility of immunoassays (ELISA, Western blot and tissue immunocytochemistry) in post-mortem confirmation of exposure and immune sensitization.
Diagnosis of diisocyanate asthma was not initially obvious in the present case. MDI had only recently (~3 months) been introduced into the plant. Although MDI has a low vapor pressure, the unreacted resin likely attached to fine sand particles and was inhaled as the auger drove sand into the molds. These findings highlight the importance of industrial hygiene (IH) and judicious use of personal protective equipment (PPE) at all worksites that use MDI. A recent report by Liljelind et al.32 found that exposure to MDI can be quantified on workers’ skin even if air levels are close to unquantifiable. Since skin exposure may initiate or potentiate immunologic sensitization and exacerbate subsequent responses to re-exposure, PPE against MDI should target both the respiratory tract and skin exposure. In the present case, gloves were apparently not used, which may have promoted immune sensitization via direct skin contact.
The decedent’s long history of occupational asthma, dating back 30-years prior to death, is an important feature of the present case. Physicians’ notes and medical records clearly document prior work-related asthma due to chemical fumes (likely from the mold release spray or the phenol-formaldehyde binders often used in mold and core making). Respiratory symptoms were abated, at least partially, through respirator use. Although the worker had reportedly been asthma symptom-free for almost 20 years, the cause of prior asthma attacks was never identified. The present data support the possibility of MDI as an initiating and ongoing source of airway inflammation, which may have resolved during periods when MDI was not used in the workplace, or when the worker was sufficiently distanced from the exposure source. The post-mortem serum levels of MDI-specific IgG were quite high (1:6,400) given the short (3-month) time period of documented MDI exposure, and are more consistent with a recall response. Furthermore, the relatively low total serum IgE argue against an atopic disposition, with its associated tendency to develop multiple sensitivities. Unfortunately, information of work processes from prior decades in different parts of the workplace were limited (there were no available records of prior MDI use); thus, the data cannot rule out the possibility that MDI sensitivity was newly acquired, or perhaps an exaggerated response due to prior occupational asthma.
A unique aspect of the present study was post-mortem confirmation of immune sensitization to an occupational allergen and identification of the allergen in situ in the airways of the affected worker. The presence of MDI-specific IgE, an indicator of immune hypersensitivity, is consistent with the subject’s clinical history, including the immediate-type reaction to exposure and the recent introduction of MDI into the workplace. The decedent’s serum also contained high levels of IgG that recognized MDI conjugated protein and demonstrated binding specificity for MDI vs. other diisocyanates (HDI and TDI). In addition to post-mortem serology, immunocytochemical staining of lung tissue sections obtained during autopsy revealed the presence of MDI, specifically localized within the thickened basement membrane of highly inflamed airways, a pattern similar to that reported in endobronchial biopsies from non-fatal cases of HDI asthma, and animal studies of MDI asthma.29, 33 Thus, post-mortem analysis of autopsy-derived tissue samples provided important information that corroborated conclusions based upon forensic pathology, past medical records and employer-provided information about potential chemical exposures.
In summary, multipronged analysis including forensic pathology, post-mortem studies of autopsy samples, review of medical records, employer-provided information, and interviews with co-workers was used to investigate severe asthma and death of a foundry worker. The findings (1) positive immunochemical staining for MDI and asthmatic pathology with mucus plugging in lung tissue sections obtained during autopsy, (2) post-mortem serology positive for MDI-specific IgE, and (3) employer confirmation of recent introduction of MDI into the workplace, support the clinical diagnosis of occupational asthma, with MDI as the causative agent. Ultimately, death was attributed to complications of acute asthma exacerbation due to isocyanate exposure. The case highlights the importance of PPE, IH, and engineering controls to prevent exposure (skin as well as respiratory tract), and the importance of employee information/training (OSHA regulation 29 CFR 1910.1200(h)(1)) given that preventative measures may never be 100% protective, and that sensitization may occur despite PPE use, IH, and engineering controls. The case also cautions against continued risk of exposure for workers with recognized immunologic sensitivity / asthma due to chemicals encountered in the workplace. Finally, the data set precedence for serologic and immunocytochemical analyses of autopsy samples under circumstances where occupational allergens may have contributed to a worker’s death.
Acknowledgements:
The authors thank the worker’s family for willingness to share medical and occupational history.
Funding:
Provided by grants from the National Institute of Environmental Health Sciences: R42-ES 018021 and the National Institute of Occupational Safety and Health: OH010494 and OH010438.
ABBREVIATIONS:
- DAB
3,3’-Diaminobenzidine
- ELISA
Enzyme-linked immunosorbant assays
- H+E
hematoxylin-eosin
- HDI
hexamethylene diisocyanate
- KLH
keyhole limpet hemocyanin
- MDI
methylene diphenyl diisocyanate
- PPE
personal protective equipment
- OSHA
Occupational Health and Safety Administration
- PAS
periodic acid-Schiff
- PELs
permissible exposure levels
- TDI
toluene diisocyanate
Footnotes
Disclosure (Authors): The authors declare no conflicts of interest.
Institution and Ethics approval and informed consent: The work was performed at Yale University under the Human Investigation Committee’s Institutional Review Board approved protocols 1103008166 and 0603001242. Verbal consent was obtained from next-of-kin.
Disclaimer: The opinions are those of the author and not the funding or regulatory agencies. This paper sets no new standards or regulations, creates no new legal obligations, and makes no changes to existing OSHA policies.
REFERENCES:
- 1.Redlich CA, Karol MH. Diisocyanate asthma: clinical aspects and immunopathogenesis. Int Immunopharmacol 2002; 2:213–24. [DOI] [PubMed] [Google Scholar]
- 2.Sastre J, Fernandez-Nieto M, Novalbos A, De Las Heras M, Cuesta J, Quirce S. Need for monitoring nonspecific bronchial hyperresponsiveness before and after isocyanate inhalation challenge. Chest 2003; 123:1276–9. [DOI] [PubMed] [Google Scholar]
- 3.Meca O, Cruz MJ, Sanchez-Ortiz M, Gonzalez-Barcala FJ, Ojanguren I, Munoz X. Do Low Molecular Weight Agents Cause More Severe Asthma than High Molecular Weight Agents? PLoS One 2016; 11:e0156141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vandenplas O, D’Alpaos V, Evrard G, Jamart J. Incidence of severe asthmatic reactions after challenge exposure to occupational agents. Chest 2013; 143:1261–8. [DOI] [PubMed] [Google Scholar]
- 5.Allport DC, Gilbert DS, Outterside SM. MDI and TDI : a safety, health and the environment : a source book and practical guide. New York, NY: J. Wiley; 2003. [Google Scholar]
- 6.Global Insulation Staff; 2018. Available from http://www.globalinsulation.com/news/item/1359-basf-to-build-new-methylene-diphenyl-diisocyanate-unit-in-louisiana. (last accessed 10/06/2021)
- 7.Statistica; 2018. Available from https://www.statista.com/statistics/750809/mdi-demand-worldwide/. (last accessed 10/06/2021)
- 8.Zhang C, Hu J, Chen S, Ji F. Theoretical study of hydrogen bonding interactions on MDI-based polyurethane. J Mol Model 2010; 16:1391–9. [DOI] [PubMed] [Google Scholar]
- 9.Zou X, Qin T, Wang Y, Huang L, Han Y, Li Y. Synthesis and properties of polyurethane foams prepared from heavy oil modified by polyols with 4,4’-methylene-diphenylene isocyanate (MDI). Bioresour Technol 2012; 114:654–7. [DOI] [PubMed] [Google Scholar]
- 10.Lofgren DJ, Walley TL, Peters PM, Weis ML. MDI Exposure for Spray-On Truck Bed Lining. Appl Occup Environ Hyg 2003; 18:772–9. [DOI] [PubMed] [Google Scholar]
- 11.Crespo J, Galan J. Exposure to MDI during the process of insulating buildings with sprayed polyurethane foam. Ann Occup Hyg 1999; 43:415–9. [PubMed] [Google Scholar]
- 12.Kaaria K, Hirvonen A, Norppa H, Piirila P, Vainio H, Rosenberg C. Exposure to 4,4’-methylenediphenyl diisocyanate (MDI) during moulding of rigid polyurethane foam: determination of airborne MDI and urinary 4,4’-methylenedianiline (MDA). Analyst 2001; 126:476–9. [DOI] [PubMed] [Google Scholar]
- 13.Lesage J, Stanley J, Karoly WJ, Lichtenberg FW. Airborne methylene diphenyl diisocyanate (MDI) concentrations associated with the application of polyurethane spray foam in residential construction. J Occup Environ Hyg 2007; 4:145–55. [DOI] [PubMed] [Google Scholar]
- 14.Carino M, Aliani M, Licitra C, Sarno N, Ioli F. Death due to asthma at workplace in a diphenylmethane diisocyanate-sensitized subject. Respiration 1997; 64:111–3. [DOI] [PubMed] [Google Scholar]
- 15.News CE. Pushing wind turbine blades toward 200 meters. C&EN Global Enterprise 2017; 95:20–1. [Google Scholar]
- 16.Bertrand JP, Simon V, Chau N. Associations of symptoms related to isocyanate, ureaformol, and formophenolic exposures with respiratory symptoms and lung function in coal miners. Int J Occup Environ Health 2007; 13:181–7. [DOI] [PubMed] [Google Scholar]
- 17.Nemery B, Lenaerts L. Exposure to methylene diphenyl diisocyanate in coal mines. Lancet 1993; 341:318. [DOI] [PubMed] [Google Scholar]
- 18.Arnold SM, Collins MA, Graham C, Jolly AT, Parod RJ, Poole A, et al. Risk assessment for consumer exposure to toluene diisocyanate (TDI) derived from polyurethane flexible foam. Regul Toxicol Pharmacol 2012; 64:504–15. [DOI] [PubMed] [Google Scholar]
- 19.Occupational/Work Related Asthma Medical Treatment Guideline; 2016. Available from https://www.dir.ca.gov/dwc/MTUS/ACOEM_Guidelines/Occupational-Work-Related-Asthma-Guideline.pdf. (last accessed 10/06/2021)
- 20.Fabbri LM, Danieli D, Crescioli S, Bevilacqua P, Meli S, Saetta M, et al. Fatal asthma in a subject sensitized to toluene diisocyanate. Am Rev Respir Dis 1988; 137:1494–8. [DOI] [PubMed] [Google Scholar]
- 21.Chester DA, Hanna EA, Pickelman BG, Rosenman KD. Asthma death after spraying polyurethane truck bedliner. Am J Ind Med 2005; 48:78–84. [DOI] [PubMed] [Google Scholar]
- 22.Project SENSOR News. Asthma Mortality; Volume 19, No. 3. 2008. Available from http://www.oem.msu.edu/images/newsletter/ProjectSensor/v19n3.pdf. (last accessed 10/06/2021) [Google Scholar]
- 23.Reilly MJ, Wang L, Rosenman KD. The Burden of Work-related Asthma in Michigan, 1988–2018. Ann Am Thorac Soc 2020; 17:284–92. [DOI] [PubMed] [Google Scholar]
- 24.Wisnewski AV, Stowe MH, Cartier A, Liu Q, Liu J, Chen L, et al. Isocyanate vapor-induced antigenicity of human albumin. J Allergy Clin Immunol 2004; 113:1178–84. [DOI] [PubMed] [Google Scholar]
- 25.Wisnewski AV, Liu J, Redlich CA. Antigenic changes in human albumin caused by reactivity with the occupational allergen diphenylmethane diisocyanate. Anal Biochem 2010; 400:251–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ye YM, Kim CW, Kim HR, Kim HM, Suh CH, Nahm DH, et al. Biophysical determinants of toluene diisocyanate antigenicity associated with exposure and asthma. J Allergy Clin Immunol 2006; 118:885–91. [DOI] [PubMed] [Google Scholar]
- 27.Wisnewski AV, Srivastava R, Herick C, Xu L, Lemus R, Cain H, et al. Identification of human lung and skin proteins conjugated with hexamethylene diisocyanate in vitro and in vivo. Am J Respir Crit Care Med 2000; 162:2330–6. [DOI] [PubMed] [Google Scholar]
- 28.Lillie RD. Histopathologic technic and practical histochemistry. New York: McGraw-Hill; 1965. [Google Scholar]
- 29.Wisnewski AV, Liu J. Immunochemical detection of the occupational allergen, methylene diphenyl diisocyanate (MDI), in situ. J Immunol Methods 2016; 429:60–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.OSHA News Release - Region 5; 2014. Available from https://www.osha.gov/news/newsreleases/region5/11062014. (last accessed 10/06/2021)
- 31.Karol MH, Kramarik JA, Ferguson J. Methods to assess RAST results in patients exposed to chemical allergens. Allergy 1995; 50:48–54. [DOI] [PubMed] [Google Scholar]
- 32.Liljelind I, Norberg C, Egelrud L, Westberg H, Eriksson K, Nylander-French LA. Dermal and inhalation exposure to methylene bisphenyl isocyanate (MDI) in iron foundry workers. Ann Occup Hyg 2010; 54:31–40. [DOI] [PubMed] [Google Scholar]
- 33.Redlich CA, Karol MH, Graham C, Homer RJ, Holm CT, Wirth JA, et al. Airway isocyanate-adducts in asthma induced by exposure to hexamethylene diisocyanate. Scand J Work Environ Health 1997; 23:227–31. [DOI] [PubMed] [Google Scholar]


