Abstract
Background:
The left atrial end-systolic volume index (LAESVI) is a predictor of cardiovascular (CV) outcomes and is the recommended measurement of left atrial size. The left atrial end-diastolic volume index (LAEDVI), representing the minimum or residual left atrial volume, has not been fully evaluated as a predictor of CV events. This study evaluated the predictive power of LAEDVI compared to LAESVI for heart failure (HF) hospitalizations, a composite of HF hospitalizations, myocardial infarction (MI), stroke, and heart disease death, and all-cause mortality.
Methods:
We measured LAESVI and LAEDVI in subjects without atrial fibrillation or flutter or significant mitral valve disease. Using Cox Proportional Hazard models, the association of LAESVI and LAEDVI with the stated outcomes was examined.
Results:
After a mean of 7.3 ± 2.6 years of follow-up there were 147 HF hospitalizations, 118 MIs, 45 strokes, 96 heart disease deaths, and 351 deaths from all causes in 938 subjects. When comparing the highest and lowest quartiles of LAEDVI, there was a near 6-fold increase in the hazard ratio for heart failure hospitalization (HR 5.96; p<0.001). This was higher than what was seen with LAESVI (HR 4.85; p<0.001). Similar associations were noted for the composite CV outcome (HR for LAEDVI of 2.97; p<0.001) and for all-cause mortality (HR for LAEDVI 2.08; p<0.001). In adjusted models, LAEDVI demonstrated equal or better predictive power than LAESVI for HF hospitalization and the composite CV outcome.
Conclusions:
LAEDVI is a strong predictor of cardiovascular events in ambulatory patients with stable coronary heart disease and may merit routine use.
Keywords: Left atrial volume, echocardiography, heart failure, myocardial infarction, cardiovascular disease
INTRODUCTION
The left atrial end-systolic volume index (LAESVI), representing the largest left atrial volume, is a known predictor of cardiovascular outcomes and is the recommended measure of left atrial (LA) size by the American Society of Echocardiography (ASE) 1. The left atrial residual volume index or the left atrial end-diastolic volume index (LAEDVI) is the smallest LA volume, measured at the end of ventricular diastole, and has been shown to correlate more closely with left ventricular (LV) filling pressures than LAESVI 2. However, LAEDVI is not routinely measured in clinical practice and its predictive value for clinical outcomes is largely unknown. We hypothesized that LAEDVI has stronger predictive value than LAESVI in an ambulatory population with coronary heart disease (CHD).
METHODS
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Participants
The Heart and Soul Study is a prospective cohort study of psychosocial factors and health outcomes in patients with CHD. Study design has been described previously 3. Briefly, participants were enrolled if they met one or more of the following criteria: 1) history of myocardial infarction (MI); 2) presence of at least 50% stenosis in one or more coronary arteries on angiography; 3) evidence of inducible ischemia by stress testing; or 4) history of coronary revascularization. Furthermore, subjects had a baseline LV ejection fraction (LVEF) of at least 50%. Subjects were excluded if they were unable to walk one block, were within six months of an acute coronary syndrome, or were planning to move out of the local area within two years. Study participants provided informed consent for baseline echocardiography and review of their medical records. The institutional review board at each enrolling center approved the study protocol.
Between September 2000 and December 2002, a total of 1,024 subjects were enrolled. Of these, 54 subjects were excluded for atrial fibrillation or flutter or significant mitral valve disease (moderate or severe mitral regurgitation or mitral stenosis) as determined using the American Society of Echocardiography reference criteria 4. An additional 32 patients were excluded due to incomplete or missing echocardiographic data. The remaining 938 participants are the subjects of this analysis. Figure 1 in the Data Supplement is a CONSORT diagram that shows the inclusions and exclusions.
Measurements
Transthoracic echocardiography, using a standardized protocol, was performed using a commercially available ultrasound system (Acuson Sequoia; Siemens Medical Solutions, Mountain View, California). LA volumes were measured using the biplane method of disks from standard apical 2- and 4-chamber views 4 at end-systole (LAESVI) and end-diastole (LAEDVI). LA borders, traced by planimetry, consisted of the walls of the LA and a line drawn across the mitral annulus (Figure 1). Pulmonary vein ostia and the LA appendage were excluded from the measurement. The moments of first mitral valve opening and closing were used to determine end-systole and end-diastole, respectively. Volumes were indexed using the body surface area (BSA). A single experienced cardiologist blinded to clinical information (N.B.S.) interpreted all echocardiography studies and verified all measurements. The reproducibility of both LAESVI and LAEDVI was studied using a Bland-Altman comparison of the two measures with the coefficient of variability (within-subject standard deviation divided by the mean) and mean difference in a randomly selected group of 18 subjects in the Health eHeart Study, a global, Internet-based study of healthy volunteers, who underwent echocardiograms measured by the same experienced cardiologist (N.B.S). The reproducibility of LA volume measurements by this reader was previously evaluated using the left atrial function index (LAFI), which incorporates LAESVI and LAEDVI using the following equation: [(LAESVI-LAEDVI)/LAESVI x LV outflow tract-velocity time integral]/[LAESVI]5,6.
Figure 1. Left atrial volume measurements in apical 4-chamber and 2-chamber views.
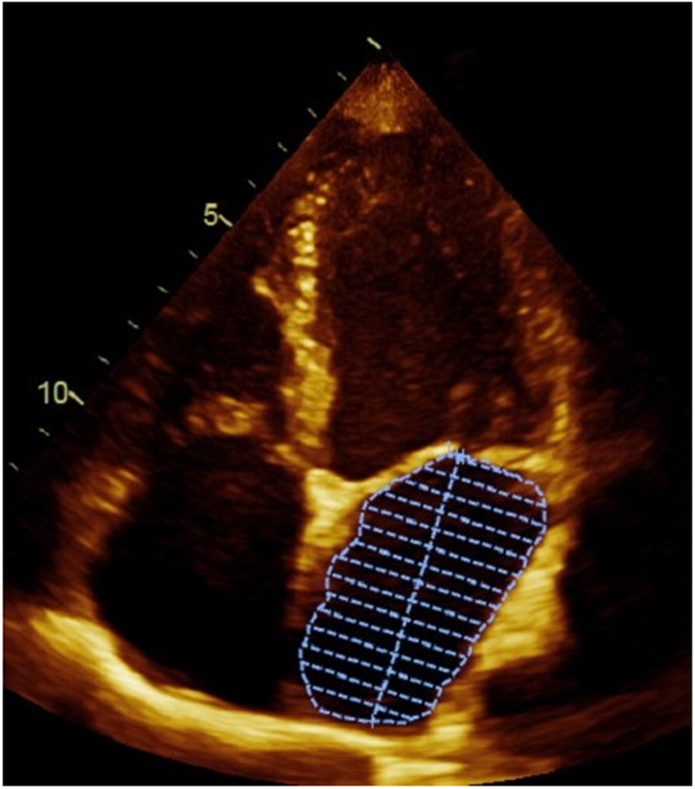
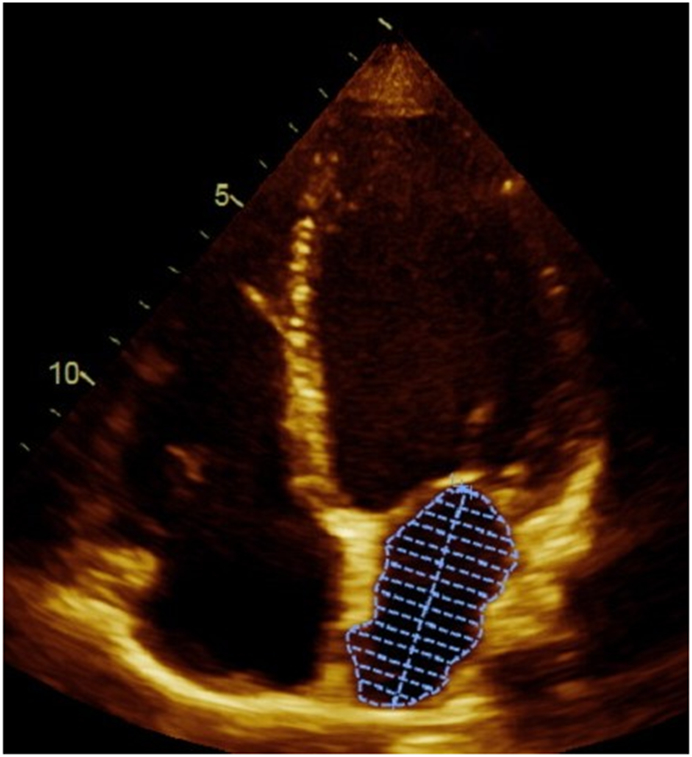
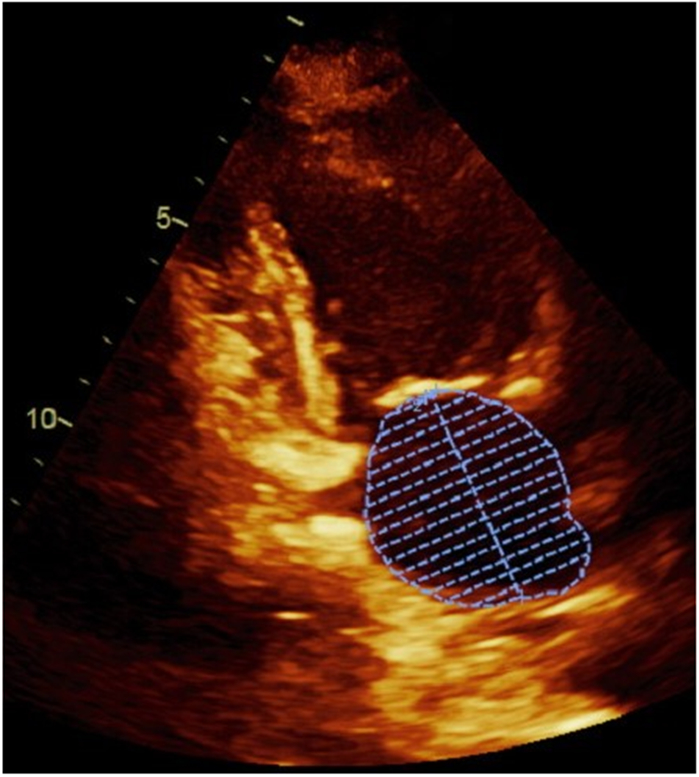
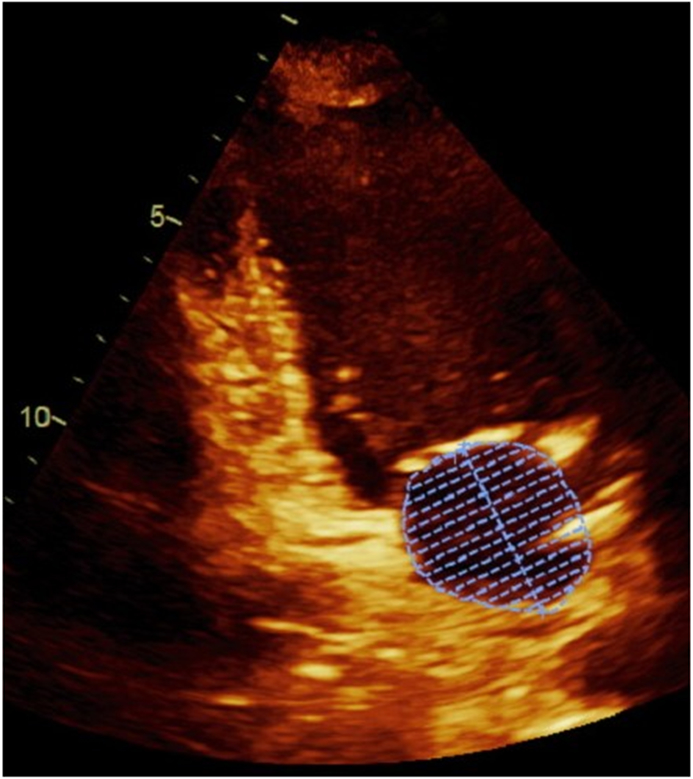
Panel A demonstrates the measurement of the largest left atrial volume at end-systole (LAESVI) while panel B demonstrates the measurement of the smallest left atrial volume at end-diastole (LAEDVI) in the apical 4-chamber view. Panel C demonstrates the measurement of LAESVI and panel D demonstrates the measurement of LAEDVI in the apical 2-chamber view.
Outcomes
There were three main outcomes: 1) heart failure (HF) hospitalizations; 2) a composite cardiovascular (CV) outcome consisting of HF hospitalizations, MI, stroke, and heart disease death; and 3) all-cause mortality.
We conducted telephone follow-up interviews with subjects or their proxies regarding recent emergency department visits, hospitalizations, or death. For any reported event, medical records, death certificates, and coroner’s reports were retrieved. Two blinded adjudicators reviewed each event, and if there was agreement, the outcome classification was binding. If there was disagreement, a third blinded adjudicator reviewed the event and determined the outcome classification. Outcome adjudications were available for all except 5 subjects. Mortality adjudications were based on hospital records, death certificates, and autopsy results.
Hospitalization for HF was defined as a clinical syndrome requiring a minimum one-night hospital stay with a change in two of the following clinical signs or symptoms: paroxysmal nocturnal dyspnea; orthopnea; elevated jugular venous pressure; pulmonary rales; a third heart sound; cardiomegaly on chest radiograph; or pulmonary edema on chest radiograph.
The outcome of MI was determined using standard diagnostic criteria developed by the American Heart Association Council on Epidemiology and Prevention 7. Stroke was considered an acute neurologic deficit of ischemic or hemorrhagic origin requiring supportive clinical and imaging documentation. Death was considered caused by heart disease if the subject died during the same hospitalization in which an acute MI was documented or the subject experienced sudden death, defined as unexpected otherwise unexplained death within one hour of the onset of terminal symptoms.
Other Measurements
The Data Supplement contains details regarding the collection and measurement of demographic, clinical, laboratory, echocardiographic, and other data.
Statistical Analysis
Subjects were divided into quartiles by LAEDVI and LAESVI. Baseline characteristics were reported as mean +/− SD for continuous variables and percentage for categorical variables. Differences in baseline characteristics between quartiles were determined using chi-square tests for categorical variables and 2-way analysis of variance for continuous variable. We used Kaplan-Meier analysis to examine cumulative event free survival, HF, and the composite CV endpoints for LAESVI and LAEDVI, using the log-rank test to evaluate these unadjusted outcomes. Cox Proportional Hazard models were developed to evaluate the unique association of LAEDVI and LAESVI with each outcome. Comparisons were made between the highest and lowest quartile of each of these predictors. For comparability between the two metrics, we treated each predictor as a continuous variable and estimated hazard ratios per 1 ml/m2. To determine the independent prognostic value of both LAEDVI and LAESVI, incremental Cox models were built using progressive combinations of baseline factors, adjusting for covariates with baseline differences. We adjusted for demographics (age, sex, and race) (model 1); plus clinical risk factors (prior revascularization, heart failure, LDL, and eGFR), medication use (beta blockers, angiotensin inhibitors, diuretics, antiarrhythmic drugs), systolic blood pressure, and heart rate (model 2); plus baseline inducible ischemia (model 3); plus NT-proBNP (model 4); plus echo parameters ( LVEDVI, LVEDSVI, pseudonormal or restrictive LV diastolic function, LVEF, and LV mass index) (model 5). Cox model assumptions were validated via the Schoenfeld Residuals Test. For each of the outcomes, using both the unadjusted and adjusted models, the area under the receiver operating characteristic curves (AUC) was evaluated for LAEDVI and LAESVI (segmented by quartiles) using Harrell’s c-statistic. In addition to Cox models, spline nonlinear regression analysis and Net Reclassification Improvement (NRI), were used to further evaluate the performance of LAEDVI and LAESVI in comparison to each other. For the HF hospitalizations outcome, a competing risk analysis was also performed using the Fine-Gray model of the cumulative incidence function. All analyses were done using the SPSS version 19.0 (IBM/SPSS, Chicago, IL) and STATA version13 (StataCorp, College Station, Texas) statistical software packages.
RESULTS
After a mean of 7.3 ± 2.6 years of follow-up there were 147 HF hospitalizations, 118 MIs, 45 strokes, 96 heart disease deaths, and 351 deaths from all causes in 938 subjects. Baseline characteristics and echocardiographic parameters of the participants segmented by quartiles of LAEDVI are shown in Tables 1 and 2, respectively. With increasing quartiles of LAEDVI, subjects were older and more likely to have a history of HF or revascularization, used more cardiac medications, had higher systolic blood pressures, lower heart rates, higher levels of NT-proBNP, and lower eGFR. LAEDVI was also significantly associated with each of the echo parameters examined.
Table 1.
Baseline Characteristics of participants stratified by quartile of left atrial end diastolic volume index
| Quartile 1 (1.85-9.93 ml/m2) (n = 243) |
Quartile 2 (9.95- 13.36 ml/m2) (n = 248) |
Quartile 3 (13.37 – 18.54 ml/m2) (n = 242) |
Quartile 4 (18.60 – 89.03 ml/m2) (n = 205) |
p-value | |
|---|---|---|---|---|---|
| Demographics | |||||
| Age, years | 64.9±11 | 64.9±11 | 67.0±10 | 69.7±11 | <0.0001 |
| Male, % | 84.6 | 77.9 | 82.1 | 81.0 | 0.293 |
| Caucasian, % | 54.5 | 61.4 | 61.0 | 62.1 | 0.276 |
| Medical History | |||||
| MI, % | 56.7 | 49.4 | 57.0 | 54.1 | 0.297 |
| CHF, % | 13.1 | 13.0 | 17.6 | 28.7 | <0.0001 |
| Hypertension, % | 69.1 | 68.5 | 69.8 | 76.1 | 0.217 |
| Diabetes Mellitus, % | 26.4 | 27.0 | 26.5 | 24.7 | 0.941 |
| Stroke, % | 13.5 | 10.9 | 14.3 | 18.5 | 0.108 |
| Revascularization*, % | 59.8 | 48.8 | 60.4 | 66.4 | 0.001 |
| Angina, % | 39.8 | 35.4 | 34.2 | 40.1 | 0.439 |
| Current tobacco use, % | 24.8 | 21.4 | 18.4 | 14.9 | 0.144 |
| Medications | |||||
| Aspirin, % | 75.4 | 71.4 | 75.8 | 69.6 | 0.327 |
| Beta-blocker, % | 49.2 | 54.4 | 66.4 | 64.0 | <0.0001 |
| ACE-I or ARB, % | 22.3 | 22.1 | 25.8 | 29.8 | 0.002 |
| Diuretic, % | 26.2 | 20.7 | 32.0 | 34.2 | <0.0001 |
| Antiarrhythmic Drug, % | 2.5 | 3.6 | 6.6 | 13.0 | <0.0001 |
| Statin, % | 63.9 | 64.3 | 68.4 | 63.2 | 0.613 |
| Alcohol use, drinks/week | 2.3±3 | 2.4±3 | 2.4±3 | 2.1±2 | 0.390 |
| Physical Exam | |||||
| BMI, kg/m2 | 28.1±5 | 29.1±5 | 28.3±5 | 28.3±6 | 0.169 |
| BSA, m2 | 1.95±0.2 | 1.97±0.2 | 1.95±0.2 | 1.94±0.2 | 0.326 |
| SBP, mm Hg | 130.0±17 | 134.2±22 | 133.6±22 | 135.3±22 | 0.015 |
| DBP, mm Hg | 74.5±11 | 75.6±11 | 74.5±11 | 74.6±12 | 0.633 |
| Heart rate, beats/min | 71.2±12 | 68.5±11 | 66.2±11 | 66.1±12 | <0.0001 |
| Laboratory | |||||
| Troponin, ng/ml | 0.0012±.01 | 0.0004±.03 | 0.0044±.05 | 0.0064±.03 | 0.086 |
| NT-proBNP, pg/ml | 174± 425 | 216± 327 | 428± 664 | 1275± 3202 | <0.0001 |
| HDL, mg/dl | 46±13 | 46±14 | 45±14 | 46±14 | 0.833 |
| LDL, mg/dl | 108±31 | 105±36 | 105±35 | 99±30 | 0.022 |
| eGFR†, mg/dl | 63±4 | 64±3 | 63±4 | 62±6 | <0.0001 |
Surgical or percutaneous
Estimated glomerular filtration rate (eGFR) by Modification of Diet in Renal Disease equation
MI = myocardial infarction; CHF = congestive heart failure; ACE-I = angiotensin converting enzyme inhibitors; ARB = angiotensin receptor blockers; BMI = body mass index; BSA = body surface area; SBP = systolic blood pressure; DBP = diastolic blood pressure; NT-proBNP = N-terminal pro–B-type natriuretic peptide; HDL = high density lipoprotein; LDL = low density lipoprotein; eGFR = estimated glomerular filtration rate
Table 2.
Baseline Echocardiographic parameters of participants stratified by quartile of left atrial end diastolic volume index
| LAEDVI – Quartile 1 (1.85-9.93 ml/m2) (n=243) |
LAEDVI – Quartile 2 (9.95- 13.36 ml/m2) (n=248) |
LAEDVI – Quartile 3 (13.37 - 18.54 ml/m2)(n= 242) |
LAEDVI – Quartile 4 (18.60 - 89.03 ml/m2)(n= 205) |
p-value | |
|---|---|---|---|---|---|
| LVEF, % | 65±7 | 63±9 | 61±10 | 57±11 | <0.0001 |
| LAESVI, g/m2 | 23.1±5 | 28.3±5 | 33.6±6 | 47.2±13 | <0.0001 |
| LAFI | 61.8±16 | 45.0±10 | 34.5±8 | 22.1±9 | <0.0001 |
| LV End Diastolic Volume Index, ml/m2 | 44.0±13 | 47.8±15 | 54.1±18 | 61.5±21 | <0.0001 |
| LV End Systolic Volume Index, ml/m2 | 16.1±8 | 18.4±11 | 22.9±19 | 29.0±20 | <0.0001 |
| LV mass index, g/m2 | 88.5±3 | 93.4±23 | 100.7±27 | 109.7±29 | <0.0001 |
| LV diastolic dysfunction (pseudonormal or restrictive), % | 6.0 | 6.1 | 11.4 | 29.2 | <0.0001 |
| Inducible ischemia, % | 20.8 | 20.9 | 23.7 | 32.9 | 0.008 |
LVEF = left ventricular ejection fraction; LAESVI = left atrial end-systolic volume index; LAFI = left atrial function index; LV = left ventricular
HF Hospitalization
When comparing the highest and lowest quartiles of LAEDVI, there was a near 6-fold increase in the unadjusted hazard ratio for HF hospitalization (HR 5.96, 95% CI: 3.45-10.23; p<0.001). This was higher than the similar association between the highest and lowest quartiles of LAESVI (HR 4.85, 95% CI: 2.84-8.37; p<0.001). None of the other quartile comparisons reached statistical significance. Similarly, for every 1 ml/m2 increase in LAEDVI in the unadjusted model, there was a significant increase in the hazard of HF hospitalization (HR 1.05, 95% CI: 1.03-1.07; p<0.001) which was similar to what was seen for LAESVI (HR 1.03, 95% CI: 1.00-1.05; p=0.02) (Table 3).
Table 3.
Association of LAEDVI and LAESVI with HF Hospitalization
| LAEDVI | LAESVI | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Quartile IV vs. I | Per 1 ml/m2 Increase | Quartile IV vs. I | Per 1 ml/m2 Increase | ||||||
| HR (95% CI) |
p-value | HR (95% CI) |
p-value | HR (95% CI) |
p-value | HR (95% CI) |
p-value | ||
| Unadjusted | 5.96 (3.45-10.23) | <0.001 | 1.05 (1.03-1.07) | <0.001 | 4.85 (2.84-8.37) | <0.001 | 1.04 (1.03-1.06) | <0.001 | |
| Model 1 | 5.11 (2.93-8.92) | <0.001 | 1.05 (1.03-1.07) | <0.001 | 4.21 (2.44-7.30) | <0.001 | 1.04 (1.03-1.06) | <0.001 | |
| Model 2 | 3.44 (1.83-6.46) | <0.001 | 1.03 (1.01-1.05) | <0.001 | 3.09 (1.66-5.78) | <0.001 | 1.03 (1.00-1.04) | <0.001 | |
| Model 3 | 3.76 (1.84-7.71) | <0.001 | 1.03 (1.00-1.05) | =0.02 | 2.74 (1.37-5.47) | <0.001 | 1.02 (1.00-1.04) | <0.001 | |
| Model 4 | 3.20 (1.53-6.69) | =0.002 | 1.02 (1.00-1.04) | =0.11 | 2.73 (1.33-5.62) | =0.002 | 1.02 (1.00-1.04) | =0.05 | |
| Model 5 | 2.07 (0.91-4.68) | =0.08 | 1.05 (1.01-1.09) | <0.001 | 2.14 (0.98-4.65) | =0.08 | 1.03 (1.00-1.05) | =0.02 | |
Model 1 – age, sex, race
Model 2 – Model 1 plus CV risk factors (prior revascularization or heart failure), medications (beta blockers, angiotensin inhibitors, diuretics, and antiarrhythmic drugs), systolic blood pressure, heart rate, and basic labs (LDL and eGFR)
Model 3 – Model 2 plus inducible ischemia
Model 4 – Model 3 plus N-terminal pro–B-type natriuretic peptide
Model 5 – Model 4 plus echocardiographic parameters (left ventricular end-diastolic and end-systolic volume indices, pseudonormal or restrictive left ventricular diastolic function, left ventricular ejection fraction, and left ventricular mass index)
After adjusting for demographics, clinical risk factors, medication use, systolic blood pressure, and heart rate, the association between LAEDVI and HF hospitalization persisted. This association remained after adjusting for inducible ischemia on stress testing and NT-proBNP. In the fully adjusted model, which included echo parameters, the per 1 ml/m2 increase in hazard persisted for LAEDVI and LAESVI but not the Quartile IV vs I comparison (Table 3). As shown in Supplemental Table 1, there was no significant difference in the c-statistic when comparing models with LAEDVI to models with LAESVI. However, adding the LAEDVI to the fully adjusted model (model D) resulted in a significant increase in the c-statistic (0.85, 95% CI: 0.81-0.89 vs 0.83, 95% CI: 0.79-0.88; p=0.043) while the addition of LAESVI to model D did not. A similar increase in the c-statistic was seen when LAEDVI was added to model A (0.84, 95% CI: 0.80-0.88 vs 0.82, 95% CI: 0.78-0.87; p=0.032) while adding LAESVI to model A was not significant. In a competing risk analysis (Supplemental Table 2), the sub-distribution hazard ratios remained significant for both LAEDVI and LAESVI.
Kaplan-Meier survival estimates (Figure 2) revealed early separation of the event-free survival curves across quartiles of LAEDVI, which persisted throughout follow-up. A similar, though less pronounced, trend was observed for LAESVI.
Figure 2. Survival Free of Heart Failure Hospitalizations.
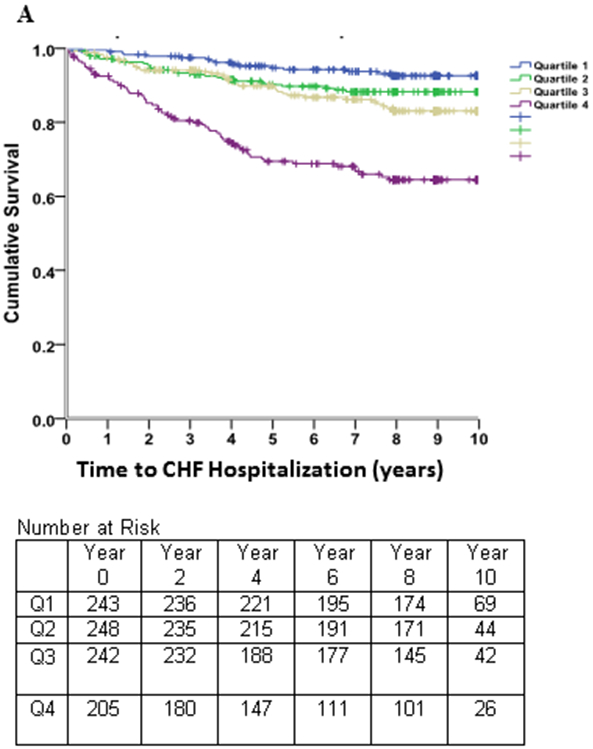
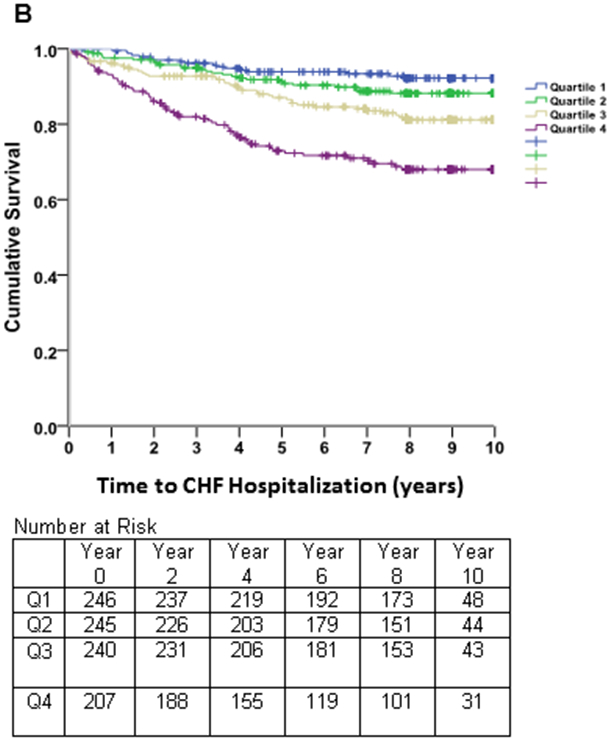
Kaplan-Meier curves of time to heart failure hospitalization in subjects with stable coronary heart disease and preserved left ventricular systolic function, stratified by quartiles of the left atrial end-diastolic volume index (LAEDVI; Panel A) and left atrial end-systolic volume index (LAESVI; Panel B). The rate of heart failure hospitalization was highest in subjects in the highest quartile and lowest in subjects in the lowest quartile. The separation of curves is less pronounced for LAESVI compared to LAEDVI.
Spline nonlinear regression with the continuous variable LAEDVI was significant (beta=.318; p=0.005) with a spline knot at 20 ml/m2. NRI analysis of the net contribution of LAEDVI to LAESVI was 0.047, indicating a very small reclassification effect for LAEDVI.
Composite CV Outcome
In evaluating the association of LAEDVI with the composite CV outcome (HF hospitalization, MI, stroke, or heart disease death), there was a three-fold increase in hazard between Quartile IV and Quartile I (HR 2.97, 95% CI: 2.07-4.22; p<0.001) and a significant per 1 ml/m2 increase in hazard (HR 1.04, 95% CI: 1.03-1.05; p<0.001). This was higher than the comparison between the highest and lowest quartiles of LAESVI (HR 2.17, 95% CI: 1.56-3.07; p<0.001) and similar to the per 1 ml/m2 increase (HR 1.03, 95% CI 1.02-1.04; p<0.001) (Table 4).
Table 4.
Association of LAEDVI and LAESVI with the Composite CV Outcome (HF hospitalization, MI, stroke, heart disease death)
| LAEDVI | LAESVI | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Quartile IV vs. I | Per 1 ml/m2 Increase | Quartile IV vs. I | Per 1 ml/m2 Increase | ||||||
| HR (95% CI) |
p-value | HR (95% CI) |
p-value | HR (95% CI) |
p-value | HR (95% CI) |
p-value | ||
| Unadjusted | 2.97 (2.07-4.22) | <0.001 | 1.04 (1.03-1.05) | <0.001 | 2.17 (1.56-3.07) | <0.001 | 1.03 (1.02-1.04) | <0.001 | |
| Model 1 | 2.71 (1.89-3.89) | <0.001 | 1.04 (1.03-1.05) | <0.001 | 1.95 (1.38-2.77) | <0.001 | 1.03 (1.02-1.04) | <0.001 | |
| Model 2 | 2.15 (1.41-3.28) | <0.001 | 1.02 (1.01-1.03) | <0.001 | 1.75 (1.16-2.65) | =0.01 | 1.02 (1.00-1.03) | <0.001 | |
| Model 3 | 2.04 (1.29-3.22) | =0.002 | 1.02 (1.01-1.03) | <0.001 | 1.50 (0.96-2.34) | =0.07 | 1.01 (1.00-1.03) | =0.02 | |
| Model 4 | 1.73 (1.07-2.80) | =0.03 | 1.01 (1.00-1.03) | =0.10 | 1.42 (0.89-2.27) | =0.14 | 1.00 (1.00-1.02) | =0.42 | |
| Model 5 | 1.33 (0.79-2.25) | =0.28 | 1.01 (1.03-1.04) | =0.21 | 1.23 (0.75-2.02) | =0.42 | 1.00 (0.99-1.02) | =0.75 | |
Model 1 – age, sex, race
Model 2 – Model 1 plus CV risk factors (prior revascularization or heart failure), medications (beta blockers, angiotensin inhibitors, diuretics, and antiarrhythmic drugs), systolic blood pressure, heart rate, and basic labs (LDL and eGFR)
Model 3 – Model 2 plus inducible ischemia
Model 4 – Model 3 plus N-terminal pro–B-type natriuretic peptide
Model 5 – Model 4 plus echocardiographic parameters (left ventricular end-diastolic and end-systolic volume indices, pseudonormal or restrictive left ventricular diastolic function, left ventricular ejection fraction, and left ventricular mass index)
After adjusting for demographics, clinical risk factors, medication use, systolic blood pressure, heart rate, and inducible ischemia on stress testing the association between LAEDVI and the composite CV outcome persisted. When adjusting for NT proBNP, the increase in hazard persisted for the Quartile IV vs I comparison but not for the per 1 ml/m2 increase in LAEDVI. The association of LAEDVI and the composite CV outcome was no longer significant when adjusting for echo parameters. In contrast, the association between the highest and lowest quartiles of LAESVI and the composite CV outcome was no longer significant when adding inducible ischemia, NT-proBNP, or echo parameters to the basic model (Table 4). As shown in Supplemental Table 1, there was no significant difference in the c-statistic for models that included either LAEDVI or LAESVI and models that did not include these.
Kaplan-Meier survival estimates (Figure 3) revealed early separation of the event-free survival curve for the fourth quartile of LAEDVI compared to the remaining quartiles, persisting throughout follow-up. A similar, though less pronounced, trend was observed for LAESVI.
Figure 3. Survival Free of Combined CV Endpoint.
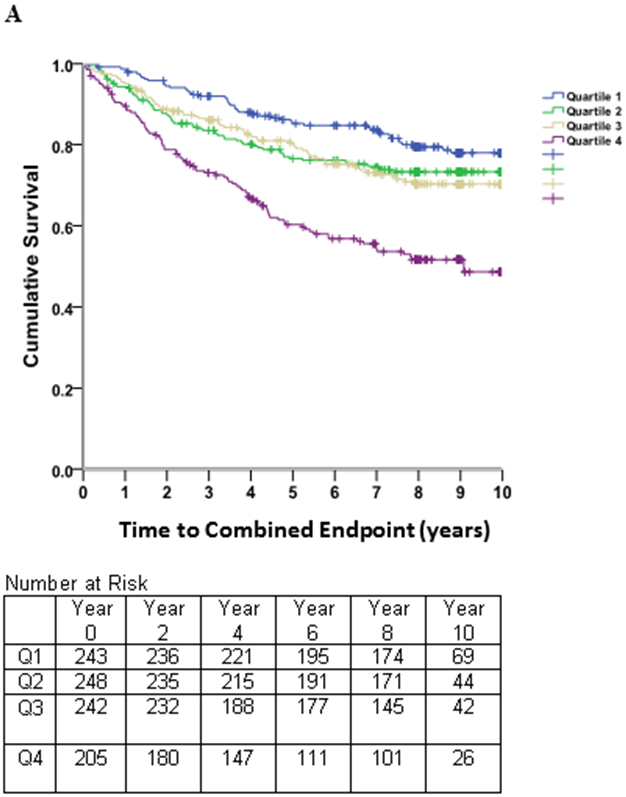
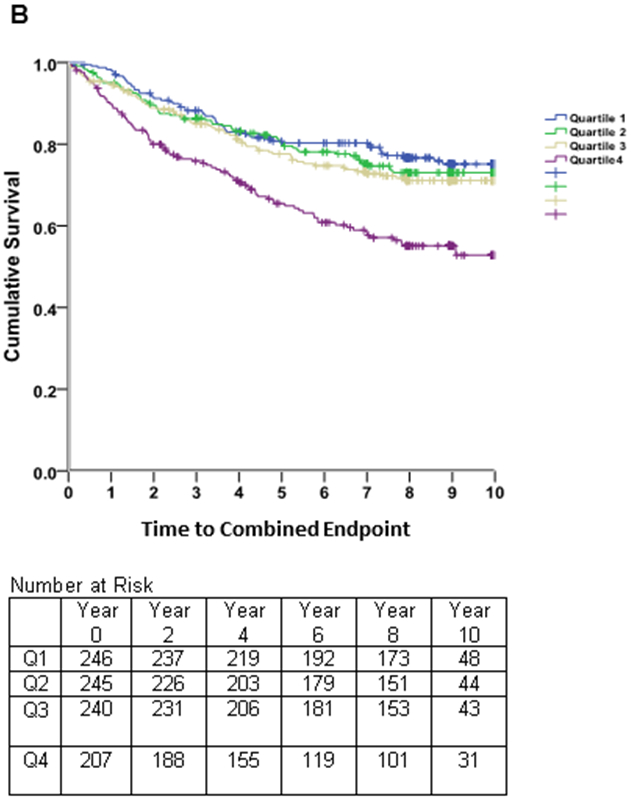
Kaplan-Meier curves of time to the combined cardiovascular (CV) endpoint (heart failure hospitalization, myocardial infarction, stroke, or heart disease death) in subjects with stable coronary heart disease and preserved left ventricular systolic function, stratified by quartiles of the left atrial residual volume index (LAEDVI; Panel A) and left atrial volume index (LAESVI; Panel B). The rate of the combined CV endpoint was highest in subjects in the highest quartile and lowest in subjects in the lowest quartile. The separation of curves is less pronounced for LAESVI compared to LAEDVI.
Spline non-linear regression showed a strong linear effect for LAEEDVI (beta=.402; p=001) and the NRI contribution of LAEDVI over LAESVI was 0.202.
All-Cause Mortality
LAEDVI was significantly associated with all-cause mortality (Quartile IV vs Quartile I: HR 2.08, 95% CI: 1.54-2.83; p<0.001; per 1 ml/m2 increase in LAEDVI: HR 1.03, 95% CI: 1.02-1.04; p<0.001). This was higher than the similar comparison between the highest and lowest quartiles of LAESVI (HR 1.90, 95% CI: 1.41-2.58; p<0.001) and the per 1 ml/m2 increase in LAESVI (HR 1.02, 95% CI: 1.01-1.03; p<0.001) (Table 5).
Table 5.
Association of LAEDVI and LAESVI with All-Cause Mortality
| LAEDVI | LAESVI | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Quartile IV vs. I | Per 1 ml/m2 Increase | Quartile IV vs. I | Per 1 ml/m2 Increase | ||||||
| HR (95% CI) |
p-value | HR (95% CI) |
p-value | HR (95% CI) |
p-value | HR (95% CI) |
p-value | ||
| Unadjusted | 2.08 (1.54-2.83) | <0.001 | 1.03 (1.02-1.04) | <0.001 | 1.90 (1.41-2.58) | <0.001 | 1.02 (1.01-1.03) | <0.001 | |
| Model 1 | 1.86 (1.36-2.54) | <0.001 | 1.02 (1.01-1.04) | <0.001 | 1.58 (1.16-2.16) | =0.004 | 1.02 (1.00-1.03) | <0.001 | |
| Model 2 | 1.71 (1.20-2.43) | =0.003 | 1.01 (1.00-1.03) | =0.01 | 1.60 (1.13-2.28) | =0.01 | 1.01 (1.00-1.02) | =0.02 | |
| Model 3 | 1.60 (1.08-2.36) | =0.02 | 1.01 (1.00-1.02) | =0.08 | 1.46 (1.01-2.15) | =0.05 | 1.00 (1.00-1.02) | =0.14 | |
| Model 4 | 1.42 (0.94-2.14) | =0.10 | 1.00 (1.00-1.02) | =0.58 | 1.36 (0.92-2.04) | =0.13 | 1.00 (1.00-1.01) | =0.64 | |
| Model 5 | 1.41 (0.91-2.21) | =0.13 | 1.01 (1.00-1.03) | =0.27 | 1.32 (0.87-2.01) | =0.20 | 1.00 (0.99-1.02) | =0.53 | |
Model 1 – age, sex, race
Model 2 – Model 1 plus CV risk factors (prior revascularization or heart failure), medications (beta blockers, angiotensin inhibitors, diuretics, and antiarrhythmic drugs), systolic blood pressure, heart rate, and basic labs (LDL and eGFR)
Model 3 – Model 2 plus inducible ischemia
Model 4 – Model 3 plus N-terminal pro–B-type natriuretic peptide
Model 5 – Model 4 plus echocardiographic parameters (left ventricular end-diastolic and end-systolic volume indices, pseudonormal or restrictive left ventricular diastolic function, left ventricular ejection fraction, and left ventricular mass index)
After adjusting for demographics, clinical risk factors, medication use, systolic blood pressure, heart rate, and inducible ischemia on stress testing the association between LAEDVI (Quartile IV vs Quartile I) and all-cause mortality persisted. However, when adjusting for NT-proBNP or echo parameters the association was no longer significant. For LAESVI (Quartile IV vs Quartile I), the association was only borderline significant at the point inducible ischemia was added to the model. When either LAEDVI or LAESVI were evaluated as continuous variables, the association was no longer significant when inducible ischemia was added to the model (Table 5). As shown in Supplemental Table 1, there was no significant difference in the c-statistic for models that included either LAEDVI or LAESVI and models that did not include these. After about one year, Kaplan-Meier survival estimates (Figure 4) revealed separation of the event-free survival curve for the fourth quartile of LAEDVI compared to the remaining quartiles, persisting throughout follow-up. A similar trend was observed for LAESVI, though the separation seemed to occur later.
Figure 4. Survival Free of Death.
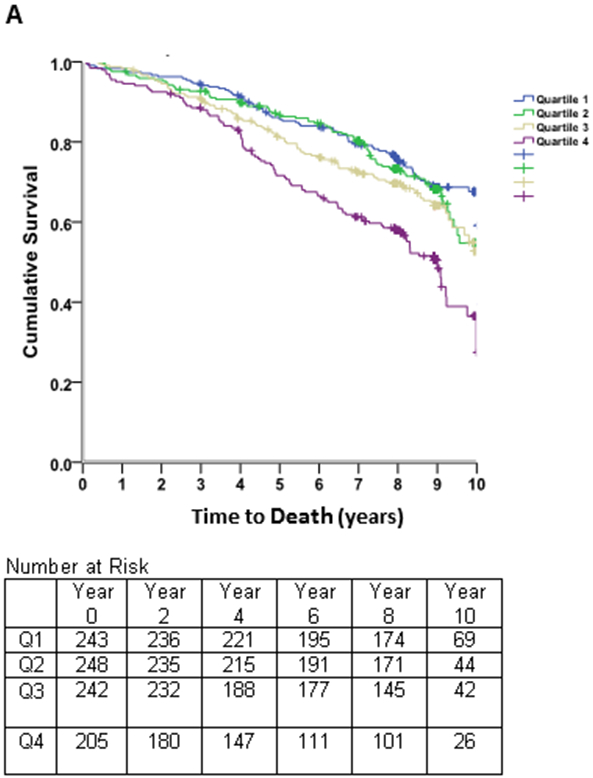
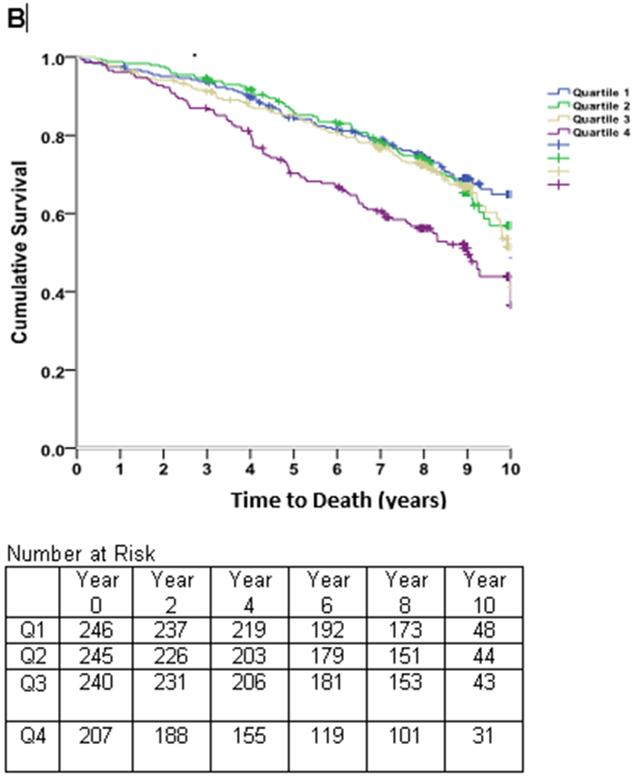
Kaplan-Meier curves of time to death in subjects with stable coronary heart disease and preserved left ventricular systolic function, stratified by quartiles of the left atrial end-diastolic volume index (LAEDVI; Panel A) and left atrial volume index (LAESVI; Panel B). The rate of death was highest in subjects in the highest quartile and lowest in subjects in the lowest quartile. The separation of curves occurred earlier for LAEDVI compared to LAESVI.
The NRI contribution of LAEDVI was very low (0.004).
Reproducibility
For the randomly selected group of 18 subjects in a separate cohort, the coefficient of variability was 9.1% for LAEDVI with a mean difference of 0.4 (95% confidence interval [CI]: −0.6 to 1.3; SD of 1.8) and 8.0% for LAESVI with a mean difference of 1.4 (95% CI: −0.2 to 3.0; SD of 3.2). A Bland-Altman analysis revealed no significant variation in LAFI (intraobserver reproducibility: mean difference 0.0059, 95% CI: 0.015 to −0.012; interobserver reproducibility: mean difference 0.0017, 95% CI: 0.025 to −0.013) 5.
DISCUSSION
In a cohort of 938 ambulatory patients with stable coronary artery disease and preserved baseline left ventricular systolic function, LAEDVI was associated with HF hospitalization, a composite of HF hospitalization, MI, stroke, or heart disease death, and all-cause mortality. This association was equal to or better than that of LAESVI and persisted after adjusting for age, sex, race, traditional cardiovascular risk factors, heart rate, systolic blood pressure, and inducible ischemia as well as NT-proBNP (for HF hospitalization and the composite CV outcome).
The LA serves to regulate LV filling by serving as a reservoir for pulmonary venous return in systole, a conduit of stored blood flow in early diastole, and an active contractile pump in late diastole 8. Measuring LA size is a challenge due to its irregular geometry and variable sized appendage and pulmonary veins. LAESVI has been shown previously to be a strong predictor of cardiovascular outcomes 9-12. Based on these data, the current ASE guidelines recommend LAESVI as the measure of LA size 1. However, there are some limitations of LAESVI. It is significantly influenced by the LV longitudinal contractile function and the descent of the mitral annular plane 13. Thus, although it is a marker of LV diastolic function, it is influenced by events that occur during ventricular systole.
LAEDVI, or the minimal LA volume measured at end-diastole, represents atrial afterload and is more closely related to LVEDP and LV filling pressures. This was demonstrated in a study of patients undergoing cardiac catheterization in which a minimal LA volume more than 40 mL predicted a pulmonary capillary wedge pressure of more than 12 mmHg with 82% sensitivity and 98% specificity 2. These findings suggest a mechanistic analogy between LAEDVI for the atrium and LV end-systolic volume index for the ventricle. Finally, in a prospective study of nearly 600 patients, LAEDVI was shown to be a better predictor of first episode of atrial fibrillation or flutter compared to LAESVI 14. The findings in our study build upon these studies by providing more robust outcomes data analogous to what has previously been demonstrated with LAESVI.
Study Limitations
Most participants were urban men therefore the results may not generalize to others. Additionally, all patients had stable coronary heart disease, so the results may not apply in healthy patients or those with unstable CHD. Although LAEDVI was a strong predictor of outcomes, this association was not independent of other echocardiographic parameters. This is largely driven by the fact that parameters such as LVEF and LV diastolic function are known to be strong predictors of cardiovascular events and the latter is highly correlated with LA volume. Finally, reproducibility data are not directly available within this cohort. Instead, these data were reported for a separate cohort and for LAFI, of which LAEDVI is one component. In a study done by another group, interobserver reproducibility was higher with LAESVI compared to LAEDVI 14. Of note, the mean differences for both LAEDVI and LAESVI in that study were significantly higher than what we report.
Conclusions
The minimum or residual left atrial volume index (left atrial end-diastolic volume index), is at least equivalent to and possibly a better predictor of cardiovascular events than the maximum left atrial volume index (left atrial end-systolic volume index) in ambulatory patients with stable coronary heart disease. This association remained significant after adjusting for demographics and traditional cardiovascular risk factors. Further outcomes studies in healthier and sicker populations are needed to assess whether LAEDVI should be added to LAESVI or even replace it as part of a standard echocardiography exam.
Supplementary Material
CLINICAL SUMMARY.
We demonstrate that a little-used measurement, the left atrial end-diastolic volume index (LAEDVI), representing the minimal or residual left atrial volume, is as strong as and possibly a better predictor of cardiovascular outcomes than the standard measurement, the left atrial end-systolic volume index (LAESVI), representing the maximum left atrial volume. Our study is the largest to demonstrate a significant relationship between LAEDVI and cardiovascular events. These findings raise serious questions as to whether we should be measuring and reporting LAEDVI instead of or in addition to LAESVI.
Acknowledgments
The authors wish to thank Mathilda Regan for assisting with data extraction and analysis.
Sources of Funding
The Heart and Soul Study was supported by the Department of Veterans Affairs (Washington, DC), the National Heart, Lung, and Blood Institute (Bethesda, MD), the American Federation for Aging Research (New York, NY), the Robert Wood Johnson Foundation (Princeton, NJ), and the Ischemia Research and Education Foundation (San Bruno, CA).
Footnotes
Disclosures:
ST – none
RES – none
QF - none
MAW – none
NBS - none
REFERENCES
- 1.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the american society of echocardiography and the European association of cardiovascular imaging. J Am Soc Echocardiogr. 2015;28:1–39 e14. DOI: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 2.Appleton CP, Galloway JM, Gonzalez MS, Gaballa M and Basnight MA. Estimation of left ventricular filling pressures using two-dimensional and Doppler echocardiography in adult patients with cardiac disease. Additional value of analyzing left atrial size, left atrial ejection fraction and the difference in duration of pulmonary venous and mitral flow velocity at atrial contraction. J Am Coll Cardiol. 1993;22:1972–82. DOI: 10.1016/0735-1097(93)90787-2 [DOI] [PubMed] [Google Scholar]
- 3.Ruo B, Rumsfeld JS, Hlatky MA, Liu H, Browner WS and Whooley MA. Depressive symptoms and health-related quality of life: the Heart and Soul Study. JAMA; 2003: 215–21. DOI: 10.1001/jama.290.2.215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005: 1440–63. DOI: 10.1016/j.echo.2005.10.005 [DOI] [PubMed] [Google Scholar]
- 5.Thomas L, Hoy M, Byth K and Schiller NB. The left atrial function index: a rhythm independent marker of atrial function. Eur J Echocardiogr. 2008;9:356–62. DOI: 10.1016/j.euje.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 6.Welles CC, Ku IA, Kwan DM, Whooley MA, Schiller NB and Turakhia MP. Left atrial function predicts heart failure hospitalization in subjects with preserved ejection fraction and coronary heart disease: longitudinal data from the Heart and Soul Study. J Am Coll Cardiol. 2012;59:673–80. DOI: 10.1016/j.jacc.2011.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luepker RV, Apple FS, Christenson RH, Crow RS, Fortmann SP, Goff D, Goldberg RJ, Hand MM, Jaffe AS, Julian DG, et al. Case definitions for acute coronary heart disease in epidemiology and clinical research studies: a statement from the AHA Council on Epidemiology and Prevention; AHA Statistics Committee; World Heart Federation Council on Epidemiology and Prevention; the European Society of Cardiology Working Group on Epidemiology and Prevention; Centers for Disease Control and Prevention; and the National Heart, Lung, and Blood Institute. Circulation. 2003;108:2543–9. DOI: 10.1161/01.CIR.0000100560.46946.EA. [DOI] [PubMed] [Google Scholar]
- 8.Hoit BD. Left atrial size and function: role in prognosis. J Am Coll Cardiol. 2014;63:493–505. DOI: 10.1016/j.jacc.2013.10.055. [DOI] [PubMed] [Google Scholar]
- 9.Moller JE, Hillis GS, Oh JK, Seward JB, Reeder GS, Wright RS, Park SW, Bailey KR and Pellikka PA. Left atrial volume: a powerful predictor of survival after acute myocardial infarction. Circulation. 2003;107:2207–12. DOI: 10.1161/01.CIR.0000066318.21784.43. [DOI] [PubMed] [Google Scholar]
- 10.Osranek M, Fatema K, Qaddoura F, Al-Saileek A, Barnes ME, Bailey KR, Gersh BJ, Tsang TS, Zehr KJ and Seward JB. Left atrial volume predicts the risk of atrial fibrillation after cardiac surgery: a prospective study. J Am Coll Cardiol. 2006;48:779–86. DOI: 10.1016/j.jacc.2006.03.054. [DOI] [PubMed] [Google Scholar]
- 11.Tsang TS, Abhayaratna WP, Barnes ME, Miyasaka Y, Gersh BJ, Bailey KR, Cha SS and Seward JB. Prediction of cardiovascular outcomes with left atrial size: is volume superior to area or diameter? J Am Coll Cardiol. 2006;47:1018–23. DOI: 10.1016/j.jacc.2005.08.077. [DOI] [PubMed] [Google Scholar]
- 12.Tsang TS, Barnes ME, Gersh BJ, Takemoto Y, Rosales AG, Bailey KR and Seward JB. Prediction of risk for first age-related cardiovascular events in an elderly population: the incremental value of echocardiography. J Am Coll Cardiol. 2003;42:1199–205. DOI: 10.1016/S0735-1097(03)00943-4. [DOI] [PubMed] [Google Scholar]
- 13.Russo C, Jin Z, Homma S, Rundek T, Elkind MS, Sacco RL and Di Tullio MR. Left atrial minimum volume and reservoir function as correlates of left ventricular diastolic function: impact of left ventricular systolic function. Heart. 2012;98:813–20. DOI: 10.1136/heartjnl-2011-301388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fatema K, Barnes ME, Bailey KR, Abhayaratna WP, Cha S, Seward JB and Tsang TS. Minimum vs. maximum left atrial volume for prediction of first atrial fibrillation or flutter in an elderly cohort: a prospective study. Eur J Echocardiogr. 2009;10:282–6. DOI: 10.1093/ejechocard/jen235. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


